Abstract
Schistosoma mansoni cercariae display specific behavioral responses to abiotic/biotic stimuli enabling them to locate and infect the definitive human host. Here we report the effect of such stimulants on signaling pathways of cercariae in relation to host finding and invasion. Cercariae exposed to various light/temperature regimens displayed modulated protein kinase C (PKC), extracellular signal–regulated kinase (ERK) and p38 mitogen-activated protein kinase (p38 MAPK) activities, with distinct responses at 37°C and intense light/dark, when compared to 24°C under normal light. Kinase activities were localized to regions including the oral sensory papillae, acetabular ducts, tegument, acetabular glands, and nervous system. Furthermore, linoleic acid modulated PKC and ERK activities concurrent with the temporal release of acetabular gland components. Attenuation of PKC, ERK, and p38 MAPK activities significantly reduced gland component release, particularly in response to linoleic acid, demonstrating the importance of these signaling pathways to host penetration mechanisms.
Keywords: Schistosoma mansoni, schistosomiasis, protein kinase C, extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, cell signaling, linoleic acid, temperature, light, cercariae
The cercarial stage of Schistosoma mansoni is exquisitely adapted, anatomically and behaviorally, to locate and penetrate humans and other mammalian definitive hosts [1]. On leaving the snail intermediate host, the cercaria senses and responds to fluctuations in light and temperature [2–6], and it is particularly attracted to human skin surface molecules, such as fatty acids [2, 3, 5, 7–9]. Once attached to skin, cercariae creep to find a suitable entry site, where they secrete acetabular gland contents aiding penetration [7, 10] and are exposed to increased temperature at the body surface. Once inside the skin, the parasite transforms to a schistosomule that migrates within the epidermis before penetrating the stratum basal [11]. The schistosomule next locates/enters the vasculature, migrates, and develops into an adolescent worm, which pairs with an opposite-sex worm. The male and female worms mature to produce large quantities of eggs for lifecycle transmission [1]; these eggs are responsible for the debilitating disease human schistosomiasis [12].
Protein kinases play important roles as signal transducers within cells and orchestrate biological responses of organisms, but, as yet, little is known of the role of protein kinases in sensory perception in cercariae. Advances in S. mansoni genomics and kinomics [13–16] have considerably supported recent work on schistosome protein kinases [16–24]. However, identifying functional roles for these enzymes remains one of the great challenges of the schistosome postgenomic era [25]. The current work aimed to determine effects of abiotic and biotic stimuli on protein kinase signaling in cercariae in the context of human host infection, through multiplexing and functionally mapping the activities of 3 signaling pathways: extracellular signal–regulated kinase (ERK), p38 mitogen-activated protein kinase (MAPK), and protein kinase C (PKC). In animals, these pathways help coordinate diverse cellular responses, including development and differentiation, motility, survival, and apoptosis; they also mediate stress responses and light adaptation [26–30]. We hypothesized that ERK, p38 MAPK, and PKC act as responders to light and temperature cues and to the skin fatty acid linoleic acid (LA) and that these pathways play a role in LA-induced acetabular gland release.
MATERIALS AND METHODS
Parasites
Biomphalaria glabrata snails exposed to S. mansoni (strain NMRI) were supplied by the National Institute of Allergy and Infectious Diseases Schistosomiasis Resource Center, distributed via BEI Resources. When patent, snails were placed in filtered tap water (Brimac filter, Silverline, United Kingdom) under light for 2 hours to induce cercarial emergence; cercariae were collected and counted.
Exposure of S. mansoni Cercariae to Light and Temperature Regimens and Effects on Kinase Signaling
Cercariae, equilibrated for 1 hour at 24°C under normal laboratory lighting, were transferred into clear microfuge tubes (approximately 800 cercariae/tube in filtered tap water) and incubated at 24°C or 37°C for 15, 30, or 60 minutes in the dark, under normal laboratory lighting (approximately 400 lux, as determined with the Robin Illuminometer 5200), or under intense direct light (approximately 5000 lux) from a nonheating light source. Cercariae were then cooled on ice for 1 minute and either (1) pulse centrifuged and homogenized in radioimmunoprecipitation assay buffer containing Halt protease/phosphatase inhibitors (Life Technologies), followed by addition of sodium dodecyl sulfate–polyacrylamide gel electrophoresis sample buffer, and heated to 90°C for 5 minutes for Western blotting; or (2) fixed in 80% ice-cold acetone for immunohistochemical analysis.
To determine the effects of light on protein kinase activation within tails, cercariae were transformed mechanically by vortexing a concentrated cercarial suspension in Eagle's basal medium, and detached tails were separated from heads by centrifugation (at 95 × g) in Hank's basal salt solution. Tails were transferred to clear tubes (approximately 1500 tails/tube) and exposed to intense direct light or dark for 1 minute at 24°C before processing for Western blotting or immunohistochemical analysis.
Western blotting and immunohistochemical analysis with anti-phospho PKC (ζ Thr410 and βII Ser660), anti-phospho p44/42 MAPK (ERK1/2; Thr202/Tyr204), or anti-phospho p38 MAPK (Thr180/Tyr182) antibodies (Cell Signaling Technology, New England Biolabs) were performed and findings were analyzed using our published methods [17–20, 31]. For blotting, anti-actin antibodies (Sigma; dilution, 1:3000) were used to assess protein-loading differences. This was important because of difficulties in obtaining equal numbers of cercariae in each sample, owing to their motile behavior; phosphorylation levels were then normalized against differences in actin signals between samples [18, 20]. For immunohistochemical analysis, actin was stained with anti-actin cy3 conjugated antibodies. Cercariae were visualized using a Leica SP2 AOBS laser scanning confocal microscope (objectives, 40× or 63× original magnification), and images were captured; photomultiplier tube voltages were equal for each comparative experiment.
Exposure of S. mansoni Cercariae to LA and Effect on Protein Kinase Signaling
Individual wells of 24-well tissue culture plates (Nunc; well area, 1.9 cm2) were coated with 500 µL of 26.6 µg/mL LA in methanol and air-dried overnight at 4°C to provide LA at approximately 7 µg/cm2, a concentration that induces acetabular gland release [32]. Uncoated or LA-coated plates were equilibrated at 24°C for 1 hour, and cercariae (approximately 1000 in 1 mL of filtered tap water) were loaded into wells and incubated for 0–120 minutes. Cercariae were then prepared for Western blotting or immunohistochemical analysis.
Release of CFDA-Labeled Acetabular Gland Components in Response to LA and Effect of Kinase Inhibition
Cercariae were incubated in 20 µM of carboxyfluorescein diacetate succinimidyl ester (CFDA-SE; Molecular Probes, Invitrogen) for 45 minutes in Falcon tubes at 24°C to allow CFDA-SE to label the acetabular gland contents; this CFDA-SE concentration provides optimal labeling and is nontoxic to the parasite [33]. Next, tubes were placed on ice for 15 minutes, and cercariae were concentrated by centrifugation (at 200g for 30 seconds) prior to resuspension in filtered tap water. Control cercariae without CFDA-SE were manipulated similarly.
Cercariae (approximately 700) were loaded into wells of uncoated or LA-coated plates and incubated for 0–120 minutes at 24°C. At appropriate times, cercarial suspensions were transferred to microfuge tubes, placed on ice for 1 minute, and pulse centrifuged. Aliquots (25 µL) of supernatants were transferred to 96-well plates (Nunc) for quantification of released fluorescent gland contents, using a Fluostar Optima reader (excitation, 492 nm; emission, 520 nm; BMG Labtech); wells were also inspected to ensure no cercariae remained. Negative controls included samples without CFDA-SE and aliquots obtained at minute 0 from a CDFA–labeled cercarial suspension. Confocal laser scanning microscopy was also performed on LA-exposed CFDA-SE–labeled cercariae, to visualize remaining fluorescence in the glands. Aliquots containing cercariae were removed from wells, fixed in 2% paraformaldehyde, mounted under coverslips, and visualized with a Leica SP2 AOBS laser scanning confocal microscope.
Cercariae (approximately 1000/treatment) were incubated in 1 µM/10 µM GF109203X, 1 µM U0126, 1 µM SB203580, or 0.1% dimethyl sulfoxide (DMSO; vehicle for U0126) in 24-well culture plates for 15, 30, or 60 minutes at 24°C, and Western blotting was performed to determine inhibition of PKC, ERK, and p38 MAPK, respectively. Thereafter, the inhibitors (or DMSO) were preincubated with cercariae during CFDA-SE labeling, and the effects of pathway inhibition on acetabular gland release were determined as described above, while maintaining inhibitor concentrations.
Statistical Analysis
One-way analysis of variance (ANOVA) was performed using Minitab (version 16); the statistical significance of differences between means was determined by Fisher multiple pair-wise comparison tests.
RESULTS
Phosphorylation of S. mansoni Cercaria PKC, ERK, and p38 MAPK is Modulated by Light and Temperature
Previously, we validated anti-phospho antibodies against PKCs (anti-phospho PKC [ζ Thr410] and anti-phospho PKC [βII Ser660]), ERK (anti-phospho p44/42 MAPK), and p38 MAPK (anti-phospho p38 MAPK) to detect exclusively activated (phosphorylated) forms of these kinases in S. mansoni [16, 18, 20, 31]; approximately 78- and 81-kDa PKC, 116-kDa PKC, 43-kDa ERK, and 42-kDa p38 MAPK bands are recognized by the respective antibodies in cercariae [20]. Importantly, the antibodies react specifically with their activated kinases because of the high sequence homology in key phosphorylation sites that exist within each kinase between S. mansoni, humans, and other organisms [18, 20, 31] and because phosphorylation at these sites is vital to enzyme activation [16]. Here, these antibodies have been used to multiplex kinase activation. S. mansoni cercariae were exposed to 6 light and temperature regimens for different durations, and PKC, ERK, and p38 MAPK activation was determined by Western blotting; additionally, immunohistochemical analysis was used to map the location of the functionally activated kinases within intact cercariae when phosphorylation changes were observed.
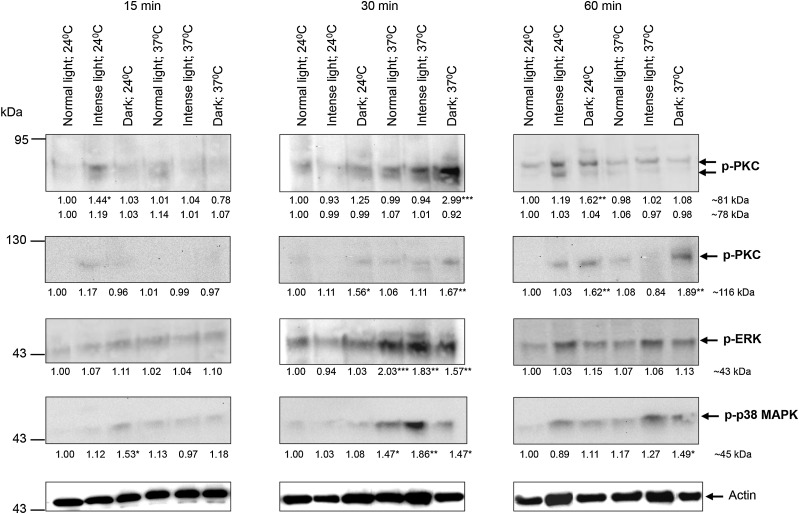
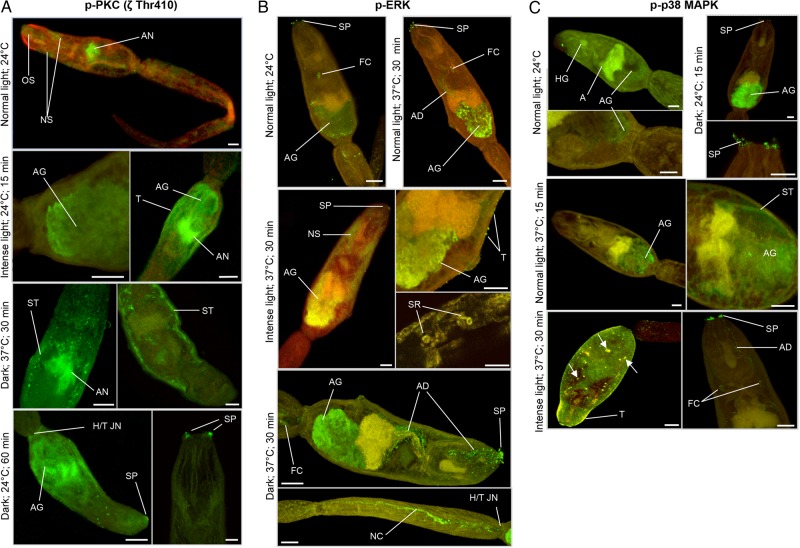
Relative to control conditions (defined as a temperature of 24°C under normal light), intense light induced a transient increase of approximately 40% (P ≤ .05) in 81-kDa PKC activation at 15 minutes when cercariae were maintained at 24°C (Figure 1). Activation of this PKC was also triggered by dark (P ≤ .01, for 60 minutes and 24°C), particularly at 37°C, when phosphorylation increased by approximately 200% after 30 minutes (P ≤ .001; Figure 1). No significant changes were detected for the 78-kDa PKC. Immunolocalization with anti-phospho PKC (ζ Thr410) antibodies that detect these PKCs and analysis of individual confocal z-sections through intact parasites revealed that, at 24°C and under normal light, activated PKC associated with the nervous system, including the acetabular nerves and oral sucker (anterior cone; Figure 2A); negative controls incubated with secondary antibodies alone displayed no fluorescence throughout the parasite (not shown). However, under intense light at 24°C for 15 minutes, activated PKC associated not only with acetabular nerves, but also with the acetabular gland region and sometimes the tegument (Figure 2A); activated PKC was also in the subtegument region when cercariae were exposed to dark for 30 minutes at 37°C. After 60 minutes in the dark at 24°C, activated PKC localized to the oral sensory papillae and the acetabular gland region and was diffusely distributed throughout the anterior of the head and sometimes at the head-tail junction (Figure 2A). Contrary to the 81-kDa PKC, activation of the 116-kDa PKC detected by anti-phospho PKC (βII Ser660) antibodies was unaffected by intense light; however, dark stimulated activation at 30 and 60 minutes at 24°C or 37°C (P ≤ .05; Figure 1). In situ localization revealed that this activated PKC associated with the acetabular gland region, acetabular nerves, and head gland region in control cercariae, with activation observed at the acetabular glands and oral sensory papillae under dark conditions (30 and 60 minutes at 24°C and 37°C; data not shown).
Figure 1.
Effect of different temperature and illumination regimens on protein kinase C (PKC), extracellular signal–regulated kinase (ERK), and p38 mitogen-activated protein kinase (p38 MAPK) phosphorylation (activation) in Schistosoma mansoni cercariae. Cercariae were maintained for 15, 30, or 60 minutes under normal light, intense light, or dark at either 24°C or 37°C, and proteins were processed for Western blotting with anti-phospho PKC (ζ Thr410), anti-phospho PKC (βII Ser660), anti-phospho p44/42 MAPK (ERK), or anti-phospho p38 MAPK antibodies; anti-actin antibodies were used to ascertain protein-loading levels. Blots are representative of those from 3 independent experiments that were analyzed for band intensity; intensity values were normalized with reference to actin. Mean change in phosphorylation (shown under each blot) was determined for each regimen relative to normal light at 24°C, which was assigned a value of 1. *P ≤ .05, **P ≤ .01, and ***P ≤ .001, by analysis of variance.
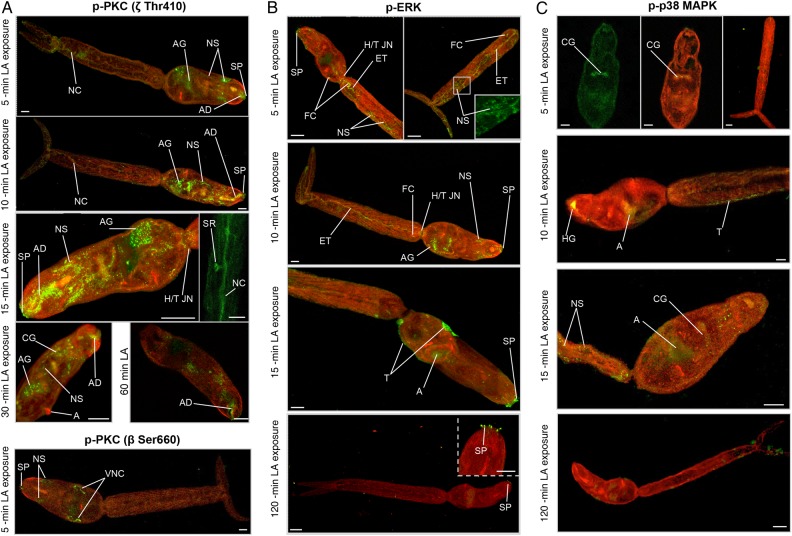
Figure 2.
In situ localization of activated protein kinase C (PKC), extracellular signal–regulated kinase (ERK), and p38 mitogen-activated protein kinase (p38 MAPK) in Schistosoma mansoni cercariae maintained under different illumination and temperature regimens. Cercariae were maintained under normal light, intense light, or dark at either 24°C or 37°C (according to phosphorylation changes presented in Figure 1) and processed for immunohistochemical analysis with anti-phospho PKC (ζ Thr410; A), anti-phospho p44/42 MAPK (ERK; B), and anti-phospho p38 MAPK antibodies (green; C). Red (cy3) fluorescence (where shown) reveals actin. Images are maximum projections of approximately 100 z-sections, except when deep scanning was done of specific structures (10–25 z-sections). The scale bar denotes 10 µm. Results are representative of those observed in cercarial populations from 2 independent experiments. Arrows show activation at surface structures of unknown identity. Abbreviations: A, acetabulum; AD, acetabular gland duct; AG, acetabular gland; AN, acetabular nerves; FC, flame cell; HG, head gland; H/T JN, head/tail junction; NC, nerve cord; NS, nervous system; OS, oral sucker (anterior cone); SP, sensory papillae; SR, sensory receptors; ST, subtegument; T, tegument. This figure is available in black and white in print and in color online.
ERK activation increased at 30 minutes and only at 37°C, an effect independent of light intensity (P ≤ .01; Figure 1). While activated ERK localized to oral sensory papillae, flame cells, and the acetabular gland region in all samples (Figure 2B), an apparent increase in ERK activation was seen in the acetabular gland region at 37°C (Figure 2B). Additionally, neural structures displayed activated ERK when cercariae were under intense light or dark, with putative sensory structures and the nerve cord stained in the tail; ERK activation was also seen at the tegument under intense light at 37°C (Figure 2B). Finally, acetabular ducts displayed ERK activation that was particularly apparent in the dark (Figure 2B).
Increased p38 MAPK activation occurred at 30 minutes through the temperature shift from 24°C to 37°C under any light intensity (P ≤ .01), except dark conditions, which also caused activation at 24°C at 15 minutes (Figure 1). Cercariae exposed to normal light at 24°C displayed activated p38 MAPK at the acetabular gland region, sometimes the oral sensory papillae (data not shown), and head gland (Figure 2C). After 15 minutes under dark conditions at 24°C, there was pronounced fluorescence associated with the acetabular gland region and sensory papillae. Cercariae maintained at 37°C in either normal light (or dark) displayed activated p38 MAPK in the subtegument and acetabular gland regions but not the sensory papillae; however, exposure to intense light resulted in activity at the sensory papillae, acetabular ducts, flame cells, and tegument (Figure 2C).
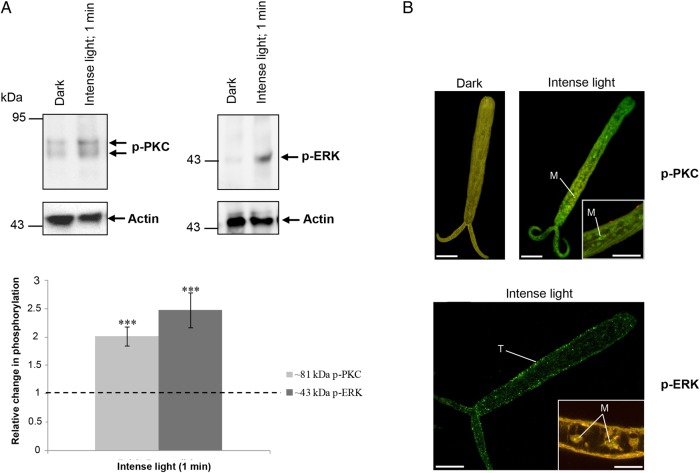
When cercariae transform into schistosomules, tail detachment occurs. We observed that detached tails sustained contractile movements and responded rapidly to light (demonstrating apparent increased movement). Consequently, we exposed detached tails to intense light or dark for 1 minute and determined kinase activation and localization. Strikingly, ERK and 81-kDa PKC activation increased approximately 150% and approximately 100% (P ≤ .001), respectively, under intense light (Figure 3A). This was concurrent with PKC activation at the tail surface and in cells resembling myocytes and with ERK activation in the tail surface tegument and possibly the nuclear region of myocytes (Figure 3B). No significant changes in phosphorylation of p38 MAPK or the other PKC isotypes were observed (data not shown).
Figure 3.
Illumination rapidly activates protein kinase C (PKC) and extracellular signal–regulated kinase (ERK) in isolated tails of Schistosoma mansoni cercariae. Tails were incubated in dark or intense light for 1 minute at 24°C. A, Proteins were processed for Western blotting with anti-phospho PKC (ζ Thr410) or anti-phospho p44/42 MAPK (ERK) antibodies. Immunoreactive bands were quantified and mean change (±standard error of the mean) in phosphorylation calculated (bottom; n = 6; ***P ≤ .001, by analysis of variance), after normalization for actin, with respect to phosphorylation levels of tails in the dark, which were assigned a value of 1. B, Immunohistochemical analysis of tails with anti-phospho PKC (ζ Thr410) or anti-phospho p44/42 MAPK (green) antibodies. Red (cy3) fluorescence (where shown) reveals actin. Images are maximum projections of approximately 100 z-sections, except when deep scanning was done of specific structures (10–25 z-sections). The scale bar denotes 10 µm. Results are representative of those observed in cercarial populations from 2 independent experiments. Abbreviations: M, myocytes; T, tegument. This figure is available in black and white in print and in color online.
PKC, ERK, and p38 MAPK Coordinate Acetabular Gland Release in Response to LA
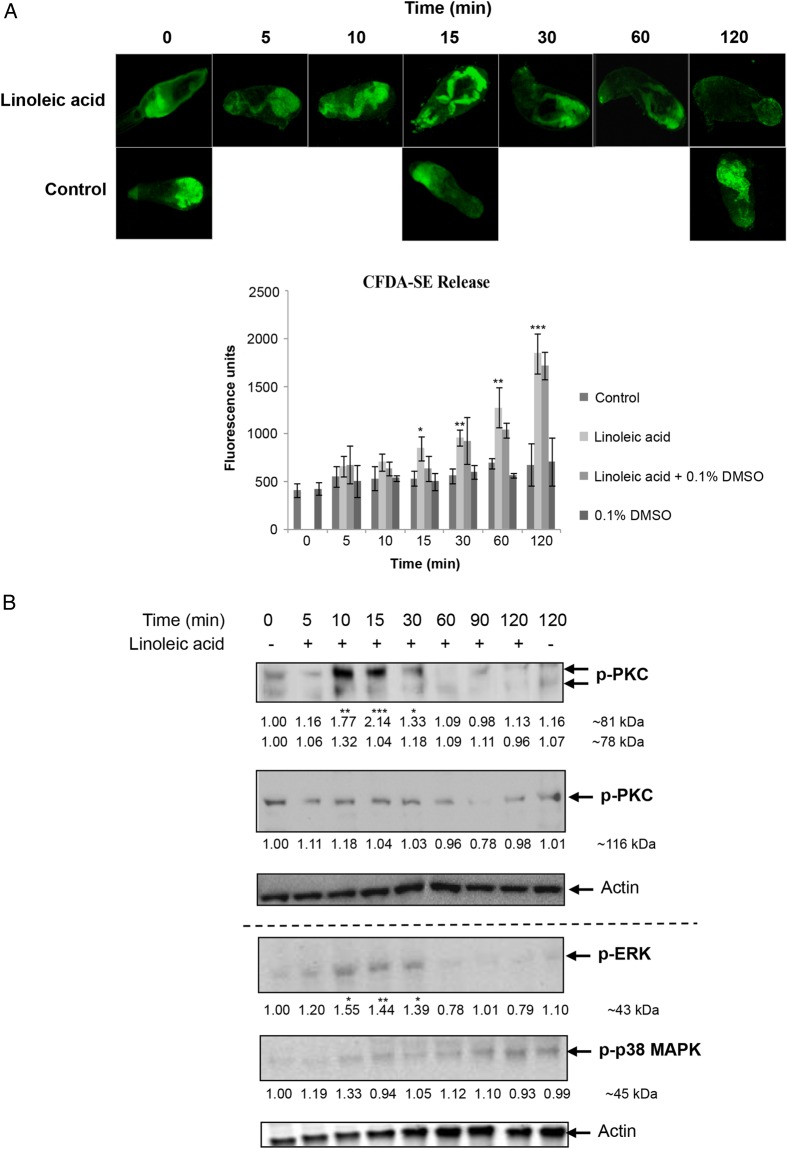
Given the locations of activated PKC, ERK, and p38 MAPK in cercariae, we hypothesized that these kinases play an important role in sensing host skin molecules and coordinating acetabular gland release. An assay was designed in which the cell permeable amine reactive tracer CFDA-SE was used to label cercariae acetabular gland contents [33], and LA, a polyunsaturated fatty acid of human skin, was used to induce their release [32]. Only cercariae that experienced LA released CFDA-SE–labeled gland contents over 120 minutes, with significant release detected as early as 15 minutes (P ≤ .05; Figure 4A); these kinetics of release concur with those observed when S. mansoni cercariae experience mammalian skin [33]. Confocal microscopy further revealed that the gland contents were essentially exhausted within 120 minutes (Figure 4A). DMSO (discussed below) did not affect gland release (Figure 4A).
Figure 4.
Effect of linoleic acid (LA) on release of acetabular gland components and protein kinase C (PKC), extracellular signal–regulated kinase (ERK), and p38 mitogen-activated protein kinase (p38 MAPK) signaling in Schistosoma mansoni cercariae. A, Release of cercariae acetabular gland components (labeled with carboxyfluorescein diacetate succinimidyl ester [CFDA-SE]; green) following LA exposure. Images are representative of cercariae populations obtained from 2 independent experiments. Cercariae preincubated with CFDA-SE were or were not exposed to LA, with or without 0.1% dimethyl sulfoxide (DMSO), and mean fluorescence (±SD) was determined over 120 minutes (bottom; n = 3). *P ≤ .05, **P ≤ .01, and ***P ≤ .001, by analysis of variance (ANOVA), when compared to control or 0.1% DMSO values. B, Cercariae were (for 5–120 minutes) or were not (data are from 0 and 120 minutes; controls) exposed to LA, and proteins were processed for Western blotting with anti-phospho PKC (ζ Thr410), anti-phospho PKC (βII Ser660), anti-phospho p44/42 MAPK (ERK), anti-phospho p38 MAPK, and anti-actin antibodies. Blots from at least 3 independent experiments were analyzed for band intensity, and intensity values were normalized for actin. Mean change in phosphorylation (shown under each blot) was determined relative to 0-minute controls, which were assigned a value of 1. *P ≤ .05, **P ≤ .01, and ***P ≤ .001, by ANOVA. This figure is available in black and white in print and in color online.
Next the temporal activation profiles of protein kinases were determined after cercariae experienced LA. Of the PKCs, only the 81-kDa isotype displayed increased activation, peaking with a approximately 110% stimulation at 15 minutes (P ≤ .001; Figure 4B). ERK activation followed a similar temporal pattern (P ≤ .05), whereas p38 MAPK activation was unaffected in cercariae exposed to LA (Figure 4B). Immunolocalization was next performed to define potential effects of LA on the protein kinase pathways in situ. Control cercariae (data not shown) appeared similar to those for controls (under normal light at 24°C) presented in Figure 2. Staining with anti-phospho PKC (ζ Thr410) antibodies revealed that, after 5-minute exposure to LA, activated PKC was evident in the nervous system, sensory papillae, acetabular ducts, and acetabular gland region (Figure 5A). Between 10 and 30 minutes, PKC activation was pronounced in the acetabular nerves and ducts and was sometimes seen within the tail in putative sensory receptors; activation generally decreased after 60 minutes of LA exposure (Figure 5A). Staining of LA-exposed cercariae with anti-phospho PKC (βII Ser660) antibodies revealed activated PKC at the ventral nerve cords, in addition to the sensory papillae and other neural structures that were seen in control cercariae (described above). Activated ERK was seen in structures such as the oral sensory papillae, flame cells, neural structures, head-tail junction, and excretory tubules early (5 and 10 minutes) after exposure to LA, whereas after 15 minutes, activation was observed at the acetabulum and tegument, and, at 120 minutes, was only observed at the sensory papillae (Figure 5B). Finally, activated p38 MAPK associated with the cephalic ganglia after 5 minutes of LA exposure, with the head gland and tegument after 10 minutes, and with neural structures within the tail after 15 minutes; activation generally was associated with the acetabulum at other exposure times (data not shown), except for 120 minutes, when negligible p38 MAPK activity was observed (Figure 5C).
Figure 5.
In situ localization of activated protein kinase C (PKC), extracellular signal–regulated kinase (ERK), and p38 mitogen-activated protein kinase (p38 MAPK) in Schistosoma mansoni cercariae exposed to linoleic acid (LA). Cercariae were exposed to LA for increasing durations and processed for immunohistochemical analysis with anti-phospho PKC (ζ Thr410) or anti-phospho PKC (β Ser660; A), anti-phospho p44/42 MAPK (ERK; B), and anti-phospho 38 MAPK antibodies (green; C). Red (cy3) fluorescence (where shown) reveals actin. Images are maximum projections of approximately 100 z-sections, except when deep scanning was done of specific structures (10–25 z-sections). The scale bar denotes 10 µm. Results are representative of those observed in cercarial populations from 2 independent experiments. Abbreviations: A, acetabulum; AD, acetabular gland duct; AG, acetabular gland; CG, cephalic ganglia; ET, excretory tubules; FC, flame cell; HG, head gland; H/T JN, head/tail junction; NC, nerve cord; NS, nervous system; SP, sensory papillae; SR, sensory receptors; T, tegument; VNC, ventral nerve cord. This figure is available in black and white in print and in color online.
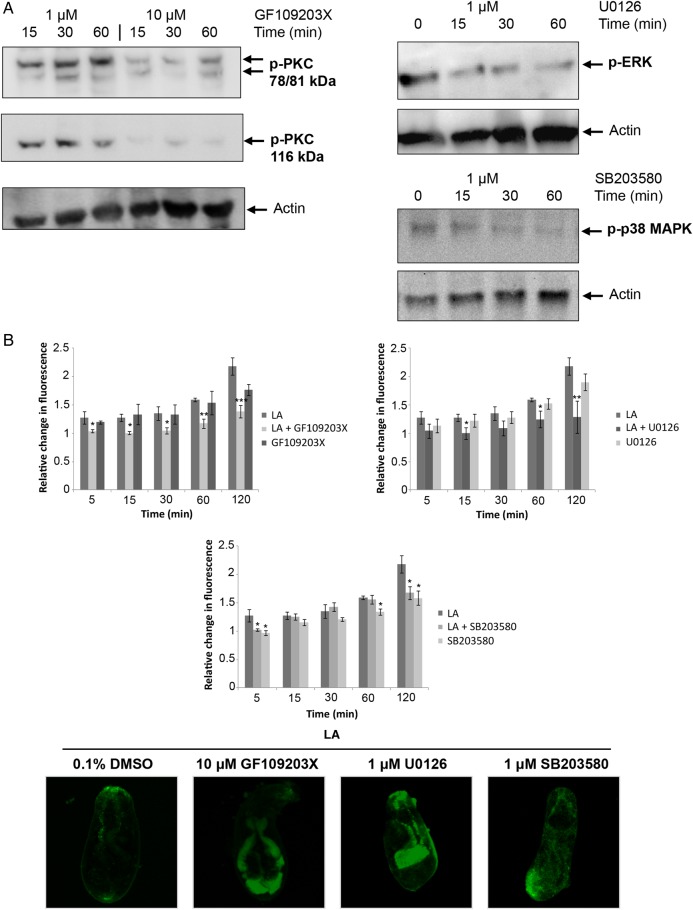
We have previously used GF109203X, U0126, and SB203580 to block PKC, ERK, and p38 MAPK activity, respectively, in S. mansoni adults and miracidia [16–18, 20, 31]. To demonstrate that these compounds are effective in cercariae, cercariae were incubated with inhibitors and proteins processed for Western blotting. GF109203X (10 µM), U0126 (1 µM), and SB203580 (1 µM) were capable of suppressing the phosphorylation (activation) of their respective target kinases (Figure 6A). Finally, effects of pathway perturbation on LA-induced release of acetabular gland components were investigated. In the presence of LA, 10 µM GF109203X attenuated the release of cercarial acetabular gland components as early as 5 minutes (P ≤ .05), with 80% (P ≤ .01) and 67% (P ≤ .001) inhibition at 60 and 120 minutes, respectively (Figure 6B). A similar overall effect occurred following suppression of ERK signaling (Figure 6B). Finally, cercariae treated with the p38 MAPK inhibitor SB203580 showed impeded gland release after 5, 60, and 120 minutes (P ≤ .05). Confocal microscopy revealed that gland contents remained within cercariae treated with either inhibitor, compared with DMSO controls (Figure 6B).
Figure 6.
Role for protein kinase C (PKC), extracellular signal–regulated kinase (ERK), and p38 mitogen-activated protein kinase (p38 MAPK) in the release of Schistosoma mansoni cercarial acetabular gland components in response to linoleic acid (LA). A, Cercariae were exposed to 1 µM/10 µM GF109203X, 1 µM U0126, or 1 µM SB203580, and proteins were detected with anti-phospho PKC (ζ Thr410), anti-phospho PKC (βII Ser660), anti-phospho p44/42 MAPK, anti-phospho p38 MAPK, and anti-actin antibodies by Western blotting. Results are representative of 2 independent experiments. B, Cercariae preincubated with carboxyfluorescein diacetate succinimidyl ester with GF109203X (10 µM), U0126 (1 µM), SB203580 (1 µM), or dimethyl sulfoxide (DMSO; vehicle, 0.1%) were or were not exposed to LA, keeping inhibitors present. Mean fluorescence (±standard error of the mean) of the released gland components was then measured over 120 minutes (bottom; n = 3), relative to 0-minute values (control with no LA), which were assigned a value of 1. *P ≤ .05, **P ≤ .01, and ***P ≤ .001, by analysis of variance, when compared to LA. Micrographs (maximum projections of 20 confocal z-sections) show acetabular gland components (green) remaining after 120 minutes of LA exposure in the presence of inhibitors or DMSO and represent results seen in cercariae populations obtained from 3 independent experiments. This figure is available in black and white in print and in color online.
DISCUSSION
Host finding and invasion by schistosome cercariae are complex adaptations intrinsically linked to the sensory physiology of the parasite. There are approximately 76 sensory surface papillae (nerve termini) on the S. mansoni cercarial body/anterior organ, of which 14 are associated with 7 gland duct openings at the oral tip; fewer papillae exist on the tail [34]. While these papillae potentially mediate responses to light, temperature, and/or host molecules [35, 36], we lack knowledge of cellular sensory transduction mechanisms in cercariae. To begin to address this in the context of host infection, we multiplexed PKC, ERK, and p38 MAPK activities in response to light, temperature, and LA cues and mapped the functionally activated kinases in situ. In addition the importance of these protein kinases to acetabular gland release, critical for host-skin penetration, were investigated. Cercariae PKC, ERK, and p38 MAPK activities were affected by light and temperature and exposure to LA and were associated with various sensory and neural structures, and attenuation of these kinase activities blocked LA-mediated acetabular gland release.
At 24°C, the 81-kDa PKC, likely an aPKC ι-type [20], was transiently activated by intense light at 15 minutes and by dark at 60 minutes, although with a pronounced/earlier response at 37°C. Activated PKC associated with the nervous system/acetabular nerves (similar to that stained with antisynapsin antibodies [37]) under light, as well as with the sensory papillae under dark. PKC also associates with neural structures in adult S. mansoni [20] and is involved in temperature-sensitive neural signaling in C. elegans [38] and photoreceptor light adaptation in Drosophila [39], suggesting a photoadaptive/thermoadaptive role for PKC in cercariae. That rhodopsin-like guanine protein-coupled receptors (GPCRs) exist in S. mansoni [40, 41] further supports a role for PKC in phototransduction in cercariae, as PKC activation is coordinated through this GPCR. Furthermore, increased PKC activation at the subtegument in response to dark might help drive tegument remodeling, which is critical during schistosomule transformation.
The 81-kDa PKC was also activated by exogenous LA as early as 10 minutes after exposure, with kinetics concomitant with initial gland release. Simultaneously, PKC activation was rapidly observed at the oral sensory papillae, within the acetabular ducts, nervous system, and acetabular gland region. LA is a known PKC activator [42], and although the mechanism of interaction between LA and oral papillae remains unknown, a hypothesis is that skin LA acts as a second messenger in papillae by intercalating with their membranes to activate PKC downstream. The temporal patterns observed are consistent with early (at 5 minutes) LA-mediated PKC activation (seen with both anti-PKC antibodies) at the oral sensory papillae, structures that provide initial contact with the host, inducing neuronal signaling to the acetabular glands and ducts that, in turn, stimulates release of acetabular gland contents through muscular contraction to enable host infection. This is supported further by PKC deactivation (presumably through feedback mechanisms) occurring in these structures when gland contents are exhausted and by GF109203X blunting gland content release. Moreover, because acetabular nerves have possible roles in neuromuscular function of the acetabulum for attachment/creeping on host skin [35], and because PKC regulates schistosome muscular activity [20], PKC activation in response to LA might facilitate further mechanosensory interactions between parasite and host.
With the exception of dark exposure at 24°C at 15 minutes, p38 MAPK responses appeared to be largely positively thermokinetic (stimulated by temperature increase) at 30 minutes. A similar thermokinetic response was observed for ERK. Although ERK can act downstream of PKC in adult S. mansoni [20], the differential responses of ERK and PKC to light/temperature suggest that they are not exclusively coupled in cercariae. In animal parasitic nematodes, thermosensory organs are often found at the tip of the head, close to the mouth [43]. While this might be the case in S. mansoni, the association of activated p38 MAPK and ERK with the oral sensory papillae under a variety of light and temperature regimens (and ERK with LA) suggests that p38 MAPK and ERK might not act as sensory responders to these cues in the oral papillae. Nevertheless, ERK (but not p38 MAPK) was activated in response to LA, with temporal kinetics similar to those for PKC, and inhibition of ERK or p38 MAPK blunted acetabular gland release. In addition, striking ERK activation was seen in the acetabular ducts under dark conditions at 37°C. These findings suggest complex protein kinase signaling mechanisms governing cercarial thermosensation and gland release. Importantly, in addition to driving immediate cellular responses that change cercariae behavior, such as acetabular gland release, ERK and p38 MAPK regulate downstream transcription factor activity; thus, thermosensory kinase activation may drive transcriptional changes required for the transformation and survival of schistosomules in the warm-blooded host. Similar to adult S. mansoni [20], activated ERK was also detected in flame cells and the excretory system of cercariae, demonstrating conserved functionality in schistosome excretory processes, likely analogous to that of planarians, in which epidermal growth factor signaling is essential for excretory system maintenance [44].
Finally, localization of activated PKC and ERK to putative sensory receptors in the tail in response to LA and to intense light at 37°C, respectively, is curious. However, we observed upregulation of ERK and PKC activity within tails after only 1 minute of exposure to intense light as compared to findings during dark exposure, supporting the notion that photoreceptive structures and mechanisms exist in cercariae tails that might be critical to the coordination of swimming and infection behavior.
In summary, this research provides the first insights into sensory cell signaling in schistosome cercariae and a valuable functional atlas of activated PKC, ERK, and p38 MAPK in situ. Furthermore, the importance of these pathways to acetabular gland release, a critical step in host skin invasion, is demonstrated.
Notes
Acknowledgments. The following reagent was provided by the National Institutes of Allergy and Infectious Diseases (NIAID) Schistosomiasis Resource Center, for distribution through BEI Resources, NIAID, National Institutes of Health: Schistosoma mansoni, Strain NMRI Exposed Biomphalaria glabrata, Strain NMRI (catalog no. NR-21962).
Disclaimer. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by Kingston University (research studentship to M. R.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Walker AJ. Insights into the functional biology of schistosomes. Parasit Vectors 2011; 4:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saladin KS. Schistosoma mansoni: cercarial responses to irradiance changes. J Parasitol 1982; 68:120–4. [PubMed] [Google Scholar]
- 3.Haeberlein S, Haas W. Chemical attractants of human skin for swimming Schistosoma mansoni cercariae. Parasitol Res 2008; 102:657–62. [DOI] [PubMed] [Google Scholar]
- 4.Brachs S, Haas W. Swimming behaviour of Schistosoma mansoni cercariae: responses to irradiance changes and skin attractants. Parasitol Res 2008; 102:685–90. [DOI] [PubMed] [Google Scholar]
- 5.Lee VST, Burgess JL, Sterling CR, Lutz EA. Schistosoma mansoni: assessment of effects of oleic acid, cercarial age and water temperature on parasite-host attraction. Int J Parasitol 2013; 43:837–42. [DOI] [PubMed] [Google Scholar]
- 6.Cohen LM, Neimark H, Eveland LK. Schistosoma mansoni: response of cercariae to a thermal gradient. J Parasitol 1980; 66:362–4. [PubMed] [Google Scholar]
- 7.Haas W, Diekhoff D, Koch K, Schmalfuss G, Loy C. Schistosoma mansoni cercariae: stimulation of acetabular gland secretion is adapted to the chemical composition of mammalian skin. J Parasitol 1997; 83:1079–85. [PubMed] [Google Scholar]
- 8.Haas W, Haeberlein S, Behring S, Zoppelli E. Schistosoma mansoni: human skin ceramides are a chemical cue for host recognition of cercariae. Exp Parasitol 2008; 120:94–7. [DOI] [PubMed] [Google Scholar]
- 9.Shiff CJ, Graczyk TK. A chemokinetic response in Schistosoma mansoni cercariae. J Parasitol 1994; 80:879–83. [PubMed] [Google Scholar]
- 10.Knudsen GM, Medzihradszky KF, Lim K-C, Hansell E, McKerrow JH. Proteomic analysis of Schistosoma mansoni cercarial secretions. Mol Cell Proteomics 2005; 4:1862–75. [DOI] [PubMed] [Google Scholar]
- 11.Grabe K, Haas W. Navigation within host tissues: Schistosoma mansoni and Trichobilharzia ocellata schistosomula respond to chemical gradients. Int J Parasitol 2004; 34:927–34. [DOI] [PubMed] [Google Scholar]
- 12.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet 2006; 368:1106–18. [DOI] [PubMed] [Google Scholar]
- 13.Berriman M, Haas BJ, LoVerde PT et al. The genome of the blood fluke Schistosoma mansoni. Nature 2009; 460:352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Protasio AV, Tsai IJ, Babbage A et al. A systematically improved high quality genome and transcriptome of the human blood fluke Schistosoma mansoni. PLoS Negl Trop Dis 2012; 6:e1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrade LF, Nahum LA, Avelar LGA et al. Eukaryotic protein kinases (ePKs) of the helminth parasite Schistosoma mansoni. BMC Genomics 2011; 12:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker AJ, Ressurreição M, Rothermel R. Exploring the function of protein kinases in schistosomes: perspectives from the laboratory and from comparative genomics. Front Genet 2014; 5:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ressurreição M, Rollinson D, Emery AM, Walker AJ. A role for p38 mitogen-activated protein kinase in early post-embryonic development of Schistosoma mansoni. Mol Biochem Parasitol 2011; 180:51–5. [DOI] [PubMed] [Google Scholar]
- 18.Ressurreição M, Rollinson D, Emery AM, Walker AJ. A role for p38 MAPK in the regulation of ciliary motion in a eukaryote. BMC Cell Biol 2011; 12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Saram PSR, Ressurreição M, Davies AJ, Rollinson D, Emery AM, Walker AJ. Functional mapping of protein kinase A reveals its importance in adult Schistosoma mansoni motor activity. PLoS Negl Trop Dis 2013; 7:e1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ressurreição M, De Saram P, Kirk RS et al. Protein kinase C and extracellular signal-regulated kinase regulate movement, attachment, pairing and egg release in Schistosoma mansoni. PLoS Negl Trop Dis 2014; 8:e2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long T, Vanderstraete M, Cailliau K et al. SmSak, the second Polo-like kinase of the helminth parasite Schistosoma mansoni: conserved and unexpected roles in meiosis. PLoS One 2012; 7:e40045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanderstraete M, Gouignard N, Cailliau K et al. Dual targeting of insulin and venus kinase receptors of Schistosoma mansoni for novel anti-schistosome therapy. PLoS Negl Trop Dis 2013; 7:e2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrade LF, Mourão MDM, Geraldo JA et al. Regulation of Schistosoma mansoni development and reproduction by the mitogen-activated protein Kinase signaling pathway. PLoS Negl Trop Dis 2014; 8:e2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beckmann S, Buro C, Dissous C, Hirzmann J, Grevelding CG. The Syk kinase SmTK4 of Schistosoma mansoni is involved in the regulation of spermatogenesis and oogenesis. PLoS Pathog 2010; 6:e1000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dissous C, Grevelding CG. Piggy-backing the concept of cancer drugs for schistosomiasis treatment: a tangible perspective? Trends Parasitol 2011; 27:59–66. [DOI] [PubMed] [Google Scholar]
- 26.Krishna M, Narang H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell Mol Life Sci 2008; 65:3525–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearson G, Robinson F, Beers Gibson T et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 2001; 22:153–83. [DOI] [PubMed] [Google Scholar]
- 28.Newton AC. Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab 2010; 298:E395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roffey J, Rosse C, Linch M, Hibbert A, Mcdonald NQ, Parker PJ. Protein kinase C intervention — the state of play. Cur Opin Cell Biol 2009; 21:268–79. [DOI] [PubMed] [Google Scholar]
- 30.Hardie RC, Peretz A, Suss-Toby E et al. Protein kinase C is required for light adaptation in Drosophila photoreceptors. Nature 1993; 363:634–7. [DOI] [PubMed] [Google Scholar]
- 31.Ludtmann MHR, Rollinson D, Emery AM, Walker AJ. Protein kinase C signalling during miracidium to mother sporocyst development in the helminth parasite, Schistosoma mansoni. Int J Parasitol 2009; 39:1223–33. [DOI] [PubMed] [Google Scholar]
- 32.Stirewalt M. Quantitative collection and proteolytic activity of preacetabular gland enzyme(s) of cercariae of Schistosoma mansoni. Am J Trop Med Hyg 1978; 27:548–53. [DOI] [PubMed] [Google Scholar]
- 33.Paveley RA, Aynsley SA, Cook PC, Turner JD, Mountford AP. Fluorescent imaging of antigen released by a skin-invading helminth reveals differential uptake and activation profiles by antigen presenting cells. PLoS Negl Trop Dis 2009; 3:e528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Short RB, Cartrett ML. Argentophilic “papillae” of Schistosoma mansoni cercariae. J Parasitol 1973; 59:1041–59. [PubMed] [Google Scholar]
- 35.Dorsey CH, Cousin CE, Lewis FA, Stirewalt MA. Ultrastructure of the Schistosoma mansoni cercaria. Micron 2002; 33:279–323. [DOI] [PubMed] [Google Scholar]
- 36.Short RB, Gagné HT. Fine structure of possible photoreceptor in cercariae of Schistosoma mansoni. J Parasitol 1975; 61:69–74. [PubMed] [Google Scholar]
- 37.Collins JJ, King RS, Cogswell A, Williams DL, Newmark PA. An atlas for Schistosoma mansoni organs and life-cycle stages using cell type-specific markers and confocal microscopy. PLoS Negl Trop Dis 2011; 5:e1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okochi Y, Kimura KD, Ohta A, Mori I. Diverse regulation of sensory signaling by C. elegans nPKC-epsilon/eta TTX-4. EMBO J 2005; 24:2127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cronin MA, Lieu M-H, Tsunoda S. Two stages of light-dependent TRPL-channel translocation in Drosophila photoreceptors. J Cell Sci 2006; 119:2935–44. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann KF, Davis EM, Fischer ER, Wynn TA. The guanine protein coupled receptor rhodopsin is developmentally regulated in the free-living stages of Schistosoma mansoni. Mol Biochem Parasitol 2001; 112:113–23. [DOI] [PubMed] [Google Scholar]
- 41.Campos TDL, Young ND, Korhonen PK et al. Identification of G protein-coupled receptors in Schistosoma haematobium and S. mansoni by comparative genomics. Parasit Vectors 2014; 7:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lester DS. In vitro linoleic acid activation of protein kinase C. Biochim Biophys Acta 1990; 1054:297–303. [DOI] [PubMed] [Google Scholar]
- 43.Ashton FT, Li J, Schad GA. Chemo- and thermosensory neurons: structure and function in animal parasitic nematodes. Vet Parasitol 1999; 84:297–316. [DOI] [PubMed] [Google Scholar]
- 44.Rink JC, Vu HT-K, Sánchez Alvarado A. The maintenance and regeneration of the planarian excretory system are regulated by EGFR signaling. Development 2011; 138:3769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]