Abstract
The development and widespread use of serum creatinine concentration–based prediction equations to calculate eGFR have been major advances for detection of patients with CKD and the epidemiologic study of CKD and its outcomes. However, these equations as well as those that also incorporate serum cystatin C concentration provide GFR estimates that, although reasonably precise on average, can differ markedly and in clinically important ways from actual GFR. Thus, it is important that clinicians who use these equations for clinical decision-making be familiar with their strengths and weaknesses and have an appreciation of their potential for error. More precise knowledge of actual GFR is important in certain clinical circumstances, including, as presented in this Attending Rounds, patients with stage 5 CKD, in whom decisions regarding dialysis initiation are necessary. Nephrologists should have the ability to accurately determine GFR when needed if clinical circumstances suggest inaccuracy of the calculated eGFR reported by the clinical laboratory.
Keywords: CKD, GFR, ESRD, Cockcroft–Gault
Introduction
Mrs. J. J. is a 63-year-old woman with hypertension, obesity, proteinuria, and CKD. A kidney biopsy in the past revealed diffuse global glomerulosclerosis and hypertensive arteriolosclerosis with moderate to severe tubular atrophy and interstitial fibrosis. Her serum creatinine had been stable for several years in the range of 2.42–3.41 mg/dl, with spot urine protein/creatinine ratio of 1.0–1.4. She missed several office appointments; then, she returned for follow-up reporting mild fatigue and slightly diminished appetite but was asymptomatic otherwise. Medications included amlodipine, furosemide, losartan, spironolactone, and ergocalciferol.
Her weight had declined about 2 kg to 79 kg (body mass index [BMI]=31.8). BP was 134/70 mmHg. Physical examination was normal, with no edema or asterixis. Serum creatinine was 4.96 mg/dl, BUN was 37 mg/dl, potassium was 3.7 mmol/L, bicarbonate was 21 mmol/L, calcium was 8.7 mg/dl, phosphorous was 3.7 mg/dl, albumin was 3.7 g/dl, hemoglobin was 10.3 g/dl, urine protein/creatinine ratio was 1.8, and immunoreactive parathyroid hormone was 26.4 pmol/L. Laboratory-reported eGFR was 11 ml/min per 1.73 m2.
Discussion
Some measured or calculated assessment of GFR is our standard determination of kidney function for purposes of assessing and categorizing both AKI and CKD. Whole-kidney GFR represents the summation of the single-nephron GFR of all functioning glomeruli. Single-nephron GFR is a function of the balance of transcapillary hydrostatic and oncotic pressures, an ultrafiltration coefficient reflecting the product of glomerular capillary surface area and hydraulic permeability, and renal plasma flow (1). Despite the well known limitations of creatinine-based assessments of GFR, which date back to the 1920s, their use persists in the absence of a generally acceptable alternative. Contemporary clinical practice guidelines use serum creatinine–based formulas, discussed below, to define eGFR≥90 ml/min per 1.73 m2 as normal and eGFR<60 ml/min per 1.73 m2 when confirmed with at least two determinations over >3 months as CKD (2).
On the basis of her serum creatinine, age, and race, this patient has stage 5 CKD or, more precisely, stage G5A3 CKD (2) accounting for both eGFR and albuminuria. In this Attending Rounds, I will address eGFR-based clinical decision-making in adults with advanced (stage 5) CKD. GFR determination in children and adolescents is reviewed elsewhere (3).
Measured GFR—Inulin and Other Exogenous Markers
Inulin is a 5200-D fructose polymer derived originally from roots of the Inula helenium plant and more recently, the Jerusalem artichoke (oddly named, because it is a sunflower and is native to certain parts of the United States and Canada but not Jerusalem), chicory, and other plants. It is not protein bound. It is freely filtered at the glomerulus. It is not metabolized, synthesized, secreted, or reabsorbed in the kidneys, and aside from a trivial degree of biliary excretion, it is entirely excreted in the urine (4). The renal or urinary clearance of exogenously administered inulin has been the gold standard for measurement of whole-kidney GFR since its first description by Smith and colleagues (5,6) in 1938.
When asked to define clearance, many physicians will be able to recite the formula for calculating clearance (“It’s UV/P”) but will not be able to precisely describe what it is. Renal clearance is a concept derived from mass balance principles. For substances that are not synthesized or metabolized by the kidneys, the rate at which a filtered substance is excreted in the urine equals its plasma removal rate. Thus, renal clearance of a substance is conceptualized as the theoretical or virtual volume of plasma from which the substance would need to be completely removed so as to appear in the urine during a defined time period, typically expressed in milliliters per minute. It is calculated as Cx=UxV/Px, where Cx is renal clearance of substance x, Ux is its urinary concentration, Px is its plasma concentration, and V is the timed urinary flow rate. For a substance, such as inulin, with the characteristics noted above, it can be shown that its clearance equals GFR, because its filtered load, (GFR)Pinulin, equals its urinary excretion over time (UinulinV); hence, GFR=UinulinV/Pinulin (1,4). Normal levels of GFR on the basis of inulin clearance studies have been reported to be approximately 130 and 120 ml/min per 1.73 m2 in young healthy men and women, respectively. Although the ideal measure of GFR in my patient might be urinary inulin clearance, such studies are cumbersome, they require intravenous infusion of inulin, inulin is not widely available and is expensive, and inulin assays are not standardized.
Other noncreatinine exogenous markers have also been studied to measure GFR on the basis of either renal (urinary) or plasma clearance. The former requires measurement of the substance in both urine and plasma, ideally while at steady-state plasma concentration, whereas the latter measures the disappearance of a substance from plasma over time, typically after a bolus intravenous infusion, without the need for timed urine collections. A recent systematic review analyzed the accuracy of common alternative approaches to measuring GFR using the radioisotopes EDTA (51Cr-EDTA) and diethylentriaminepenta-acetic acid (99Tc-DTPA) and the iodinated contrast agents iohexol and iothalamate (measured by chemical assay or as the isotope 125I-iothalamate) with inulin clearance as the reference test. Using criteria of median bias (systematic difference between measured GFR and eGFR) ≤5%, mean bias ≤10%, at least 80% of measurements within ±30% of reference measurements (P30), and at least 50% of measurements within ±10% of reference measurements, renal clearance of iothalamate, renal and plasma clearance of 51Cr-EDTA, plasma clearance of iohexol, and renal clearance of DTPA and iohexol were determined to be of sufficient accuracy (7). Endogenous creatinine clearance, discussed next, and plasma DTPA clearance, were not. Test performance in patients with very low GFR, such as the patient discussed here, was not evaluated.
One recent systematic review stated that, “In usual practice, an eGFR equation is defined as having sufficient accuracy when at least 75% of the estimates fall within ±30% of the measured GFR” (8). However, when important clinical decisions require knowledge of a patient’s GFR, knowing the actual (measured) GFR as accurately as possible seems preferable to having a ballpark estimate. Unfortunately, if one were to insist on a higher degree of accuracy from these inulin “wannabes”, such as having 90% or more determinations being within 10% of inulin clearance, none would be satisfactory.
Creatinine-Based GFR Measurement
Creatinine is a metabolic product of creatinine and phosphocreatine, both of which are found primarily in muscle (9). Despite the historical tenacity of its use, it is now well known that endogenous urinary creatinine clearance does not have acceptable precision for most purposes in clinical practice. Although creatinine shares most of the features that makes inulin an ideal marker of GFR, it fails in one critically important aspect, in that a significant fraction of its urinary excretion results from secretion in the proximal tubule. In normal individuals, creatinine clearance thus exceeds inulin clearance by 10%–20% and in patients with advanced kidney disease, >50%–60% of urinary creatinine may be caused by tubular secretion. Creatinine reabsorption may also occur at very low urine flow rates (9). One interesting recent report actually indicated that fractional excretion of creatinine (using inulin-measured GFR) varied from 0.7 (indicating substantial net creatinine reabsorption) to 1.4 (indicating net secretion) in a kidney transplant cohort (10). Creatinine is also subject to variable elimination through the gastrointestinal tract in individuals with advanced kidney disease. Cimetidine, an H2-receptor antagonist that inhibits tubular secretion of creatinine, was used in the past to accurately assess GFR using creatinine clearance with reasonable success (11–13). This has fallen out of favor, however, because of, in part, side effects associated with the high doses of cimetidine that were used.
Creatinine use as a GFR marker is further complicated by variability in the amount of creatinine generated and its turnover as a function of muscle mass, age, diet, and concurrent illnesses and conditions as well as the inherent inaccuracy of collecting a timed (usually 24-hour) urine specimen (9). Variability in serum creatinine measurement has been improved with development and implementation of an isotope dilution mass spectrometry reference standard for creatinine measurement and reporting (14), although other laboratory and biologic variability remains.
Serum Creatinine–Based Estimating Equations
Given the difficulty with measuring and accurately knowing GFR, a variety of formulas have been derived to estimate either creatinine clearance, as a GFR surrogate, or GFR itself. Use of these estimating equations requires a number of assumptions about creatinine production and excretion, their relationship to age, sex, weight, and in some cases, race or ethnicity, and the presence of steady-state kidney function and serum creatinine concentration.
Probably the best known and most used until relatively recently is the simple Cockcroft–Gault formula for estimation of creatinine clearance (not GFR) (15) (Table 1). This formula was derived nearly 40 years ago from examination of 249 hospitalized men (all white) without known liver or kidney disease between the ages of 18 and 82 years old with mean serum creatinine values of 0.99–1.78 mg/dl. A 15% reduction in the calculated creatinine clearance in women to adjust for their lower creatinine generation per kilogram of body weight was proposed on the basis of the assumption that creatinine generation in women was 10%–20% less on a weight basis compared with men, but this figure was not derived from study of individual subjects. If it were not for its simplicity and continued use as the basis of many medication dosing adjustments, this formula would likely have been relegated to the trash heap long ago, because it has generally been found to be the least accurate of commonly used prediction equations.
Table 1.
Cockcroft–Gault, Modification of Diet in Renal Disease, and CKD Epidemiology Collaboration GFR-estimating equations
| Estimating Equation Name | Estimating Equation Formula |
|---|---|
| Cockcroft–Gault | (140− age) × weight/72×SCr×0.85 (if a woman) |
| MDRD (four-variable) | 175×SCr−1.154× age−0.203 ×0.742 (if a woman) ×1.212 (if black) |
| CKD-EPI (creatinine) | 141× min(SCr/κ, 1)α × max(SCr/κ, 1)−1.209 ×0.993age×1.018 (if a woman) ×1.159 (if black), where κ is 0.7 for women and 0.9 for men, α is −0.329 for women and −0.411 for men, min indicates the minimum of SCr/κ or 1, and max indicates the maximum of SCr/κ or 1 |
| CKD-EPI (cystatin) | 133× min(Scys/0.8, 1)−0.499 × max(Scys/0.8, 1)−1.328 ×0.996age ×0.932 (if a woman), where min indicates the minimum of SCr/κ or 1, and max indicates the maximum of SCr/κ or 1 |
| CKD-EPI (cystatin-creatinine) | 135× min(SCr/κ, 1)α × max(SCr/κ, 1)−0.601 × min(Scys/0.8, 1)−0.375 × max(Scys/0.8, 1)−0.711 ×0.995age ×0.969 (if a woman) ×1.08 (if black); where κ is 0.7 for women and 0.9 for men, α is −0.248 for women and −0.207 for men, min indicates the minimum of SCr/κ or 1, and max indicates the maximum of SCr/κ or 1 |
MDRD, Modification of Diet in Renal Disease; CKD-EPI, CKD Epidemiology Collaboration; SCr, serum creatinine; Scys, serum cystatin C.
The Modification of Diet in Renal Disease Study and a New Era of GFR-Estimating Equations
A turning point in how clinicians assess GFR occurred in 1999, when Levey et al. (16) published a GFR-estimating equation derived from baseline data of subjects enrolled in the Modification of Diet in Renal Disease (MDRD) Study, a randomized trial that assessed effects of BP lowering and dietary protein restriction on progression of what we now call CKD (16). GFR was measured using renal clearance of 125I-iothalamate in 1628 subjects and compared with creatinine clearance, urea clearance, the mean of these two clearances, the reciprocal of the serum creatinine concentration, the Cockcroft–Gault formula, a formula on the basis of creatinine, urea clearance, and race (black or not), and two regression equations using serum creatinine, age, sex, race (black or not), serum urea nitrogen (SUN) concentration, and either serum albumin concentration or urine urea nitrogen (UUN) concentration. The MDRD cohort was 60% men, 88% white, ages 18–70 years old (mean=50.6±12.7 years old) and included only 6% patients with diabetes, with an overall mean serum creatinine of 2.3 mg/dl, GFR of 39.8 ml/min per 1.73 m2, and creatinine clearance of 48.6 ml/min per 1.73 m2. Creatinine clearance and the Cockcroft–Gault equation systematically overestimated GFR, and urea clearance underestimated GFR, whereas the mean of the creatinine and urea nitrogen clearances was much more accurate than either. Even more accurate was a prediction equation using serum creatinine, demographic variables, SUN, and UUN. An equation with serum creatinine, demographic variables, SUN, and albumin was less accurate than the UUN-containing equation and also slightly less accurate than the creatinine clearance/urea clearance mean, but it was proposed as being the most practical for clinical use. A further simplified four-variable equation (including only serum creatinine, age, sex, and race [black or not]) (A.S. Levey et al., unpublished data) was modified on the basis of use of standardized serum creatinine assays (17) (Table 1) is now used by the majority of clinical chemistry laboratories in this country for eGFR reporting. The MDRD equation expresses GFR normalized for body surface area (BSA) in milliliters per minute per 1.73 m2, whereas the Cockcroft–Gault formula and most clearance measurements are expressed as milliliters per minute. Whether BSA normalization of GFR is physiologically or clinically correct remains a matter of debate (18–22).
Although most clinical laboratories report eGFR using the modified four-variable equation, other equations, most notably the CKD Epidemiology Collaboration (CKD-EPI) equations (23), have been derived using serum creatinine, serum cystatin C (24), or both (1,24,25) (Table 1). Additional equations include the Lund–Malmö (26), Mayo Clinic (27), the Berlin Initiative Study (28), and the Chronic Renal Insufficiency Cohort (29) formulas, among others, some of which were developed for specific ethnic/racial groups, patient populations, or disease states (30,31). Cystatin C is a cysteine proteinase inhibitor produced by all nucleated cells. It is freely filtered across the glomerular filtration barrier, and then, it is virtually completely reabsorbed and catabolized in the proximal tubule. Plasma cystatin C concentrations have been shown to correlate with GFR. Some estimating equations using both serum creatinine and cystatin C seem to be more accurate than formulas using either alone (24,25).
The CKD-EPI equation, developed and validated through a collaborative effort with data from 26 research studies and clinical populations, provides eGFRs that are a bit higher than those calculated with the modified MDRD equation, with slightly less bias and slightly better accuracy (23). It is more accurate at eGFR≥60 ml/min per 1.73 m2, whereas the MDRD equation may be more accurate at lower levels of GFR (32,33). However, neither equation has a P30>80% in patients with eGFR<60 ml/min per 1.73 m2, in whom the interquartile (25th to 75th percentile) range of difference from measured GFR is approximately 12 ml/min per 1.73 m2 (and twice that in patients with eGFR≥60 ml/min per 1.73 m2). As I have said many times to my fellows, if measurement of serum sodium concentration had this degree of precision, we would stop measuring it. Nonetheless, the CKD-EPI equation is now generally preferred over the MDRD equation because of, at least in part, its lower misclassification of patients with relatively well preserved kidney function as having CKD, although the performance of each varies depending on the population studied (34).
eGFRs from the MDRD and CKD-EPI equations using serum creatinine, cystatin C, and both have been extensively studied as tools to assess relative risks in populations of diverse patients with CKD for clinically important outcomes, including AKI, complications of CKD, development of ESRD, cardiovascular morbidity, and all-cause and cardiovascular mortality (2). What has not been as well studied is how to best know the actual GFR in individual patients, such as the patient presented here, with rather advanced CKD who may (or may not) have symptoms consistent with uremia and may (or may not) benefit from starting dialysis without resorting to inulin or other exogenous substance–based clearances. In fact, in the only prospective, randomized, controlled trial evaluating the timing of starting dialysis, GFR was estimated with the Cockcroft–Gault equation (35). There are also potentially important differences in assessment of CKD progression on the basis of slopes of eGFR compared with measured GFR (36–38).
A recent report from the Swedish Renal Registry CKD (SRR-CKD) compared measured GFR using iohexol plasma clearance with eGFRs derived from the Lund–Malmö, CKD-EPI, Mayo, MDRD, and Cockcroft–Gault equations (39). Because patients were from clinics in Sweden, the sample was demographically very different from my patient and many others in the United States. Because the MDRD and CKD-EPI equations are most commonly used in the United States, only results with these equations will be reviewed here. For patients with measured GFR=11–20 ml/min per 1.73 m2, the median bias values were 1.3 and 1.8 ml/min per 1.73 m2 (9.4% and 12.9%) for the CKD-EPI and MDRD equations, respectively. For patients with measured GFR ≤10 ml/min per 1.73 m2, the median bias values were 1.2 and 1.7 ml/min per 1.73 m2 (13.2% and 19.3%) for the CKD-EPI and MDRD equations, respectively, indicating slight systematic overestimation of GFR. The accuracy of these equations in absolute terms (milliliters per minute per 1.73 m2 difference between eGFR and measured GFR) was modest, with as many as 20% of patients with measured GFR ≤10 ml/min per 1.73 m2 having a difference >5 ml/min per 1.73 m2 and as many as 6.5% of patients having a difference >10 ml/min per 1.73 m2. For patients with measured GFR of 11–20 ml/min per 1.73 m2, nearly one third of patients had a difference >5 ml/min per 1.73 m2, and about 10% had a difference >10 ml/min per 1.73 m2.
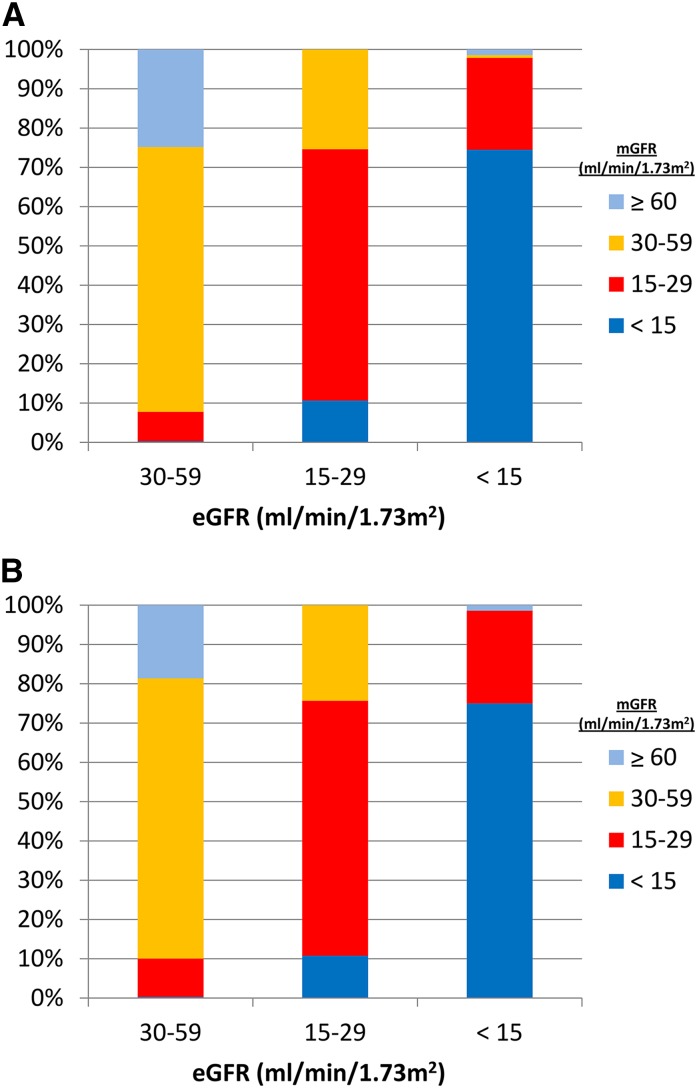
Additional information is available from the MDRD and CKD-EPI datasets (23). There were 141 and 144 patients with eGFR<15 ml/min per 1.73 m2 in the MDRD and CKD-EPI validation cohorts, respectively. In both cohorts, 25% of subjects with eGFR<15 ml/min per 1.73 m2 had measured GFR values >15 ml/min per 1.73 m2. Most of these were in the 15–29 ml/min per 1.73 m2 range, but two patients had measured GFR values in the 60–89 ml/min per 1.73 m2 range (Figure 1). In practical terms, this means that one of four patients urged by their nephrologists to accept a kidney transplant or start dialysis when eGFR falls below 15 ml/min per 1.73 m2 will, in fact, have a measured GFR above and in some patients much above, this range.
Figure 1.
Significant differences exist between mGFR and eGFR with both the MDRD and CKD Epidemiology Collaborative equations. Comparison of eGFR using (A) the MDRD and (B) the CKD Epidemiology Collaboration equations compared with mGFR (iothalamate). MDRD, Modification of Diet in Renal Disease; mGFR, measured GFR. Reference 23.
Several other studies have similarly addressed comparisons between eGFR and measured GFR with advanced CKD (GFR<15 ml/min per 1.73 m2), most using inulin or radioisotope clearances. Each of these studies corroborates findings of the SRR-CKD Study; regardless of the population statistics on bias and accuracy, some patients with eGFR around 10 ml/min per 1.73 m2 will have measured GFR values of 20 ml/min per 1.73 m2 or higher, and some will have measured GFR values near 5 ml/min per 1.73 m2 (40–47). Few if any blacks were included in any of these reports, with the exception of a report from the African-American Study of Hypertension and Kidney Disease Trial, which included derivation of its own formula for eGFR calculation in blacks (48). A recent report identified differences in eGFR and CKD staging on the basis of genetic determinants of the proportion of African ancestry in self-reported blacks and Hispanic/Latino Americans, further confounding GFR assessments in these populations (49).
A quote from Soveri et al. (7) from the SRR-CKD Study cited above is worth considering:
Although our results compared well with others, the Acc30 (percent of estimated within 30% of measured) is still strikingly bad on an individual basis. Even when using the best possible equation, almost 24% of the individuals with an eGFR of 10 ml/min/1.73 m2 will have true GFR values of <5 or >15 ml/min/1.73 m2. Thus, our results stress the importance not to make important decisions regarding the timing of dialysis initiation or timing of vascular access surgery based on eGFRs. In fact, for patients with higher age and diabetic nephropathy, there is even greater overestimation of true GFR and worse accuracy (7).
Summary
Returning to my patient, the MDRD eGFR was 11 ml/min per 1.73 m2, and the CKD-EPI eGFR was 10 ml/min per 1.73 m2. Calculated BSA values ranged from 1.80 to 1.91 m2 depending the formula used, leading to an eGFR of 11.4–12.1 ml/min not normalized to BSA. Twenty-four hour urinary creatinine and urea nitrogen clearances were 16.8 and 10.3 ml/min, respectively (mean=13.6 ml/min). We discussed starting dialysis (she was interested in doing peritoneal dialysis), but because her symptoms improved spontaneously over a few weeks and she was without significant metabolic or laboratory complications of CKD, we together opted for a watch and wait approach before placing a peritoneal dialysis catheter.
Conclusions
GFR-estimating equations have clearly been a major advance in detection of patients with CKD and the epidemiologic study of CKD and its outcomes, although concerns remain about their imperfections and risk of misdiagnosis (50–53). However, they are just estimating equations; clinicians who use them should be familiar with their strengths and weaknesses and understand their potential for significant inaccuracy. Perhaps new methods for determining GFR will eventually make their way into clinical practice (54–56).
Precise knowledge of GFR might be of value in patients, such as mine, who may have symptoms suggestive of uremia given that symptoms of uremia can be subtle and are nonspecific, medication dosing (or avoidance) (57–59), use of contrast media, assessing individuals considering living kidney donation (60–62) or who are transplant recipients (63), and planning timing of dialysis access or the start of dialysis and transplantation. Knowing the measured GFR might be important also when trying to assess kidney function in patients who are elderly (64), are morbidly obese, have significant nonrenal acute or chronic illness, have reduced muscle mass, have liver disease, have malnutrition, or had an amputation. Available estimating equations do not have verified accuracy in various non–United States and European white and black racial/ethnic groups (30) or those at extremes of muscle mass or body size or who consume vegetarian or vegan diets. We still have much to learn about relationships between BMI, muscle mass, and creatinine excretion (21,65,66). Furthermore, the serum creatinine concentration on which these formulas are based may be influenced by diet, creatine supplements, and certain medications (i.e., trimethoprim and cimetidine). Cystatin C may be influenced by thyroid function, adiposity, inflammation, and other factors (67).
Although I have focused, given the patient presented, on low levels of GFR and stage 5 CKD, the accuracy of GFR-estimating equations is much worse at higher GFR levels (Figure 2), although better for the CKD-EPI compared with MDRD equation (32). As such, nephrologists should probably arrange to have more accurate means of measuring GFR available when needed until more consistently accurate estimating equations become available, which has also been suggested by others (68). Averaging the urea nitrogen and creatinine clearances may be helpful but is, by no means, ideal. Decisions regarding starting dialysis can be complex and must take into consideration not just GFR estimates but also, careful review of patient symptoms and other laboratory data. As should be clear from the above discussion, using an isolated eGFR as a trigger for starting dialysis is overly simplistic and likely to often lead to erroneous clinical decision-making.
Figure 2.
Difference between mGFR and eGFR (MDRD equation) from the combined dataset derived from 10 studies. mGFR, measured GFR; MDRD, Modification of Diet in Renal Disease. Reprinted from reference 47, with permission.
One final thought—perhaps clinical laboratories could report not just calculated eGFR but also, a 95% confidence interval, interquartile (25th to 75th percentile) range, other indicator of the range of potential actual GFR levels that the eGFR might represent, or even some simple explanatory text, which is now often done when laboratories report lipid panel results. This might help clinicians to better appreciate the range of potential actual measured GFRs around a reported eGFR in their patients when a difference between 10 and 30 ml/min per 1.73 m2 is clinically important.
Questions
Jordana Cohen, MD (Research Fellow)
In the patient who you presented, if an inulin clearance showed a measured GFR of 5 ml/min per 1.73 m2, would that change your management decision given that she is now asymptomatic with no metabolic derangements?
Answer
In essence, the issue here is how low can you go, whether using measured GFR or eGFR, in waiting to start dialysis in an asymptomatic or minimally symptomatic patient. Although the target for late-start dialysis in the only prospective, randomized clinical trial of early- versus late-start dialysis, the Initiating Dialysis Early and Late (IDEAL) Study, was eGFR of 5.0–7.0 ml/min, most patients started with eGFR>7.0 ml/min because of symptoms or other indications (35); therefore, this study was not able to directly answer this question. Several retrospective studies suggest that dialysis initiation with eGFR<5 ml/min per 1.73 m2 is associated with lower mortality than higher eGFR dialysis starts (69–72), although such studies are limited by survival bias, confounding by indication, and other issues. At least one prospective cohort study, however, found a significantly higher mortality (hazard ratio, 4.65; 95% confidence interval, 2.28–9.49) among patients not yet on dialysis with eGFR<7.5 ml/min per 1.73 m2 (73). A recent Canadian clinical practice guideline recommends deferring dialysis until the eGFR is 6 ml/min per 1.73 m2 (74), whereas another indicates that clinical indications for starting dialysis “often but not invariably” occur when eGFR is 5–10 ml/min per 1.73 m2 (2). Although seemingly safe in the absence of symptoms or other indications for dialysis, such late starts necessitate close patient follow-up.
Jamie Lin, MD (Nephrology Fellow)
Knowing that these equations are by no means perfect in estimating renal function, are there any studies that assess whether certain comorbidities should influence dialysis initiation timing?
Answer
Diabetes and cardiovascular disease are often cited as indicators for earlier start of RRT and are more common in patients who start dialysis at higher eGFR levels (69). In the IDEAL Trial, however, age, diabetes, BMI, history of cardiovascular disease, and serum albumin concentration did not significantly influence the primary study finding of no benefit of a planned dialysis start at the higher eGFR level (35).
Rebecca Seshasai, MD (Research Fellow)
Why do we still dose medications on the basis of the Cockcroft–Gault estimation of creatinine clearance when better estimating equations are now available?
Answer
Use of the Cockcroft–Gault formula was recommended by the US Food and Drug Administration (FDA) in 1998, long before other GFR-estimating equations were available. Stevens et al. (59) compared measured GFR with eGFR using the MDRD equation and the Cockcroft–Gault equation. Discordance in FDA-assigned kidney function categories was found in 22%–34% of comparisons and recommended drug dosages in 12%–18% of comparisons, with 82%–89% concordance between the estimating equations. Of note is that eGFR in this analysis was adjusted to remove BSA normalization, something that is not typically considered in practice but is probably appropriate for this use (75). There also seems to be substantial drug-dosing discordance in older individuals comparing MDRD and CKD-EPI equations (76). It is not clear which formula is superior in terms of maximizing drug efficacy and minimizing drug toxicity (58,75).
Disclosures
J.S.B. is the President of the National Kidney Foundation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Levey AS, Inker LA, Coresh J: GFR estimation: From physiology to public health. Am J Kidney Dis 63: 820–834, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group: KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 3.Schwartz GJ, Work DF: Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 4: 1832–1843, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Israni AK, Kasiske BL: Laboratory assessment of kidney disease: Glomerular filtration rate, urinalysis, and proteinuria. In: Brenner and Rector's The Kidney, edited by Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu ASL, Brenner BM, Philadelphia, PA, Elsevier, 2012, pp 868–896 [Google Scholar]
- 5.Smith HW, Goldring W, Chasis H: The measurement of the tubular excretory mass, effective blood flow and filtration rate in the normal human kidney. J Clin Invest 17: 263–278, 1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith HW: Measurement of the Filtration Rate. The Kidney: Structure and Function, Oxford, NY, Oxford University Press, 1951, pp 39–62 [Google Scholar]
- 7.Soveri I, Berg UB, Björk J, Elinder CG, Grubb A, Mejare I, Sterner G, Bäck SE; SBU GFR Review Group: Measuring GFR: A systematic review. Am J Kidney Dis 64: 411–424, 2014 [DOI] [PubMed] [Google Scholar]
- 8.SBU: Methods to Estimate and Measure Kidney Function (Glomerular Filtration Rate). A Systematic Review, 2013. Available at: http://www.sbu.se/upload/Publikationer/Content1/1/Renal_%20Function.pdf. Accessed January 3, 2015 [PubMed]
- 9.Perrone RD, Madias NE, Levey AS: Serum creatinine as an index of renal function: New insights into old concepts. Clin Chem 38: 1933–1953, 1992 [PubMed] [Google Scholar]
- 10.Reznichenko A, Sinkeler SJ, Snieder H, van den Born J, de Borst MH, Damman J, van Dijk MC, van Goor H, Hepkema BG, Hillebrands JL, Leuvenink HG, Niesing J, Bakker SJ, Seelen M, Navis G: SLC22A2 is associated with tubular creatinine secretion and bias of estimated GFR in renal transplantation. Physiol Genomics 45: 201–209, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Burgess E, Blair A, Krichman K, Cutler RE: Inhibition of renal creatinine secretion by cimetidine in humans. Ren Physiol 5: 27–30, 1982 [DOI] [PubMed] [Google Scholar]
- 12.Hilbrands LB, Artz MA, Wetzels JF, Koene RA: Cimetidine improves the reliability of creatinine as a marker of glomerular filtration. Kidney Int 40: 1171–1176, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Serdar MA, Kurt I, Ozcelik F, Urhan M, Ilgan S, Yenicesu M, Kenar L, Kutluay T: A practical approach to glomerular filtration rate measurements: Creatinine clearance estimation using cimetidine. Ann Clin Lab Sci 31: 265–273, 2001 [PubMed] [Google Scholar]
- 14.Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, Hostetter T, Levey AS, Panteghini M, Welch M, Eckfeldt JH; National Kidney Disease Education Program Laboratory Working Group: Recommendations for improving serum creatinine measurement: A report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem 52: 5–18, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41, 1976 [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Geddes CC, Woo YM, Brady S: Glomerular filtration rate—what is the rationale and justification of normalizing GFR for body surface area? Nephrol Dial Transplant 23: 4–6, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Milic R, Colombini A, Lombardi G, Lanteri P, Banfi G: Estimation of glomerular filtration rate by MDRD equation in athletes: Role of body surface area. Eur J Appl Physiol 112: 201–206, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Peters AM, Snelling HL, Glass DM, Love S, Bird NJ: Estimated lean body mass is more appropriate than body surface area for scaling glomerular filtration rate and extracellular fluid volume. Nephron Clin Pract 116: c75–c80, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Fotheringham J, Weatherley N, Kawar B, G Fogarty D, Ellam T: The body composition and excretory burden of lean, obese, and severely obese individuals has implications for the assessment of chronic kidney disease. Kidney Int 86: 1221–1228, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Hsu CY, Chertow GM, Curhan GC: Methodological issues in studying the epidemiology of mild to moderate chronic renal insufficiency. Kidney Int 61: 1567–1576, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators: Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyman U, Grubb A, Larsson A, Hansson LO, Flodin M, Nordin G, Lindström V, Björk J: The revised Lund-Malmö GFR estimating equation outperforms MDRD and CKD-EPI across GFR, age and BMI intervals in a large Swedish population. Clin Chem Lab Med 52: 815–824, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG: Using serum creatinine to estimate glomerular filtration rate: Accuracy in good health and in chronic kidney disease. Ann Intern Med 141: 929–937, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Schaeffner ES, Ebert N, Delanaye P, Frei U, Gaedeke J, Jakob O, Kuhlmann MK, Schuchardt M, Tölle M, Ziebig R, van der Giet M, Martus P: Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med 157: 471–481, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI; CRIC Study Investigators: Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 60: 250–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens LA, Claybon MA, Schmid CH, Chen J, Horio M, Imai E, Nelson RG, Van Deventer M, Wang HY, Zuo L, Zhang YL, Levey AS: Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int 79: 555–562, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee CS, Cha RH, Lim YH, Kim H, Song KH, Gu N, Yu KS, Lim CS, Han JS, Kim S, Kim YS: Ethnic coefficients for glomerular filtration rate estimation by the Modification of Diet in Renal Disease study equations in the Korean population. J Korean Med Sci 25: 1616–1625, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M, Hamm LL, Lewis JB, Mauer M, Navis GJ, Steffes MW, Eggers PW, Coresh J, Levey AS: Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis 56: 486–495, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Earley A, Miskulin D, Lamb EJ, Levey AS, Uhlig K: Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med 156: 785–795, W-270, W-271, W-272, W-273, W-274, W-275, W-276, W-277, W-278, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Murata K, Baumann NA, Saenger AK, Larson TS, Rule AD, Lieske JC: Relative performance of the MDRD and CKD-EPI equations for estimating glomerular filtration rate among patients with varied clinical presentations. Clin J Am Soc Nephrol 6: 1963–1972, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, Harris A, Johnson DW, Kesselhut J, Li JJ, Luxton G, Pilmore A, Tiller DJ, Harris DC, Pollock CA; IDEAL Study: A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 363: 609–619, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Xie D, Joffe MM, Brunelli SM, Beck G, Chertow GM, Fink JC, Greene T, Hsu CY, Kusek JW, Landis R, Lash J, Levey AS, O’Conner A, Ojo A, Rahman M, Townsend RR, Wang H, Feldman HI: A comparison of change in measured and estimated glomerular filtration rate in patients with nondiabetic kidney disease. Clin J Am Soc Nephrol 3: 1332–1338, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis J, Greene T, Appel L, Contreras G, Douglas J, Lash J, Toto R, Van Lente F, Wang X, Wright JT, Jr.; AASK Study Group: A comparison of iothalamate-GFR and serum creatinine-based outcomes: Acceleration in the rate of GFR decline in the African American Study of Kidney Disease and Hypertension. J Am Soc Nephrol 15: 3175–3183, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Rossing P, Rossing K, Gaede P, Pedersen O, Parving HH: Monitoring kidney function in type 2 diabetic patients with incipient and overt diabetic nephropathy. Diabetes Care 29: 1024–1030, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Evans M, van Stralen KJ, Schön S, Prütz KG, Stendahl M, Rippe B, Jager KJ; ERA-EDTA Registry; Swedish Renal Registry Collaboration: Glomerular filtration rate-estimating equations for patients with advanced chronic kidney disease. Nephrol Dial Transplant 28: 2518–2526, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Botev R, Mallié JP, Couchoud C, Schück O, Fauvel JP, Wetzels JF, Lee N, De Santo NG, Cirillo M: Estimating glomerular filtration rate: Cockcroft-Gault and Modification of Diet in Renal Disease formulas compared to renal inulin clearance. Clin J Am Soc Nephrol 4: 899–906, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fontseré N, Bonal J, Navarro M, Riba J, Fraile M, Torres F, Romero R: A comparison of prediction equations for estimating glomerular filtration rate in adult patients with chronic kidney disease stages 4-5. Effect of nutritional status and age. Nephron Clin Pract 104: c160–c168, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P: Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol 16: 763–773, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Kuan Y, Hossain M, Surman J, El Nahas AM, Haylor J: GFR prediction using the MDRD and Cockcroft and Gault equations in patients with end-stage renal disease. Nephrol Dial Transplant 20: 2394–2401, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Lee D, Levin A, Roger SD, McMahon LP: Longitudinal analysis of performance of estimated glomerular filtration rate as renal function declines in chronic kidney disease. Nephrol Dial Transplant 24: 109–116, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Poggio ED, Wang X, Greene T, Van Lente F, Hall PM: Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol 16: 459–466, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT: Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol 5: 1003–1009, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevens LA, Coresh J, Feldman HI, Greene T, Lash JP, Nelson RG, Rahman M, Deysher AE, Zhang YL, Schmid CH, Levey AS: Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol 18: 2749–2757, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Lewis J, Agodoa L, Cheek D, Greene T, Middleton J, O’Connor D, Ojo A, Phillips R, Sika M, Wright J, Jr.; African-American Study of Hypertension and Kidney Disease: Comparison of cross-sectional renal function measurements in African Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. Am J Kidney Dis 38: 744–753, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Udler MS, Nadkarni GN, Belbin G, Lotay V, Wyatt C, Gottesman O, Bottinger EP, Kenny EE, Peter I: Effect of genetic African ancestry on eGFR and kidney disease [published online ahead of print October 27, 2014]. J Am Soc Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalantar-Zadeh K, Amin AN: Toward more accurate detection and risk stratification of chronic kidney disease. JAMA 307: 1976–1977, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Glassock RJ: Estimated glomerular filtration rate: Time for a performance review? Kidney Int 75: 1001–1003, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Glassock RJ, Winearls C: The global burden of chronic kidney disease: How valid are the estimates? Nephron Clin Pract 110: c39–c46, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Rule AD, Glassock RJ: GFR estimating equations: Getting closer to the truth? Clin J Am Soc Nephrol 8: 1414–1420, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng MH, Zeng FW, Xie LJ, Li JF, Zhang F, Jiang H: A new quantitative method for estimating glomerular filtration rate and its clinical value [published online ahead of print November 21, 2014]. Clin Physiol Funct Imaging [DOI] [PubMed] [Google Scholar]
- 55.Wang E, Meier DJ, Sandoval RM, Von Hendy-Willson VE, Pressler BM, Bunch RM, Alloosh M, Sturek MS, Schwartz GJ, Molitoris BA: A portable fiberoptic ratiometric fluorescence analyzer provides rapid point-of-care determination of glomerular filtration rate in large animals. Kidney Int 81: 112–117, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu W, Sandoval RM, Molitoris BA: Rapid determination of renal filtration function using an optical ratiometric imaging approach. Am J Physiol Renal Physiol 292: F1873–F1880, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Hermsen ED, Maiefski M, Florescu MC, Qiu F, Rupp ME: Comparison of the Modification of Diet in Renal Disease and Cockcroft-Gault equations for dosing antimicrobials. Pharmacotherapy 29: 649–655, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Hudson JQ, Nyman HA: Use of estimated glomerular filtration rate for drug dosing in the chronic kidney disease patient. Curr Opin Nephrol Hypertens 20: 482–491, 2011 [DOI] [PubMed] [Google Scholar]
- 59.Stevens LA, Nolin TD, Richardson MM, Feldman HI, Lewis JB, Rodby R, Townsend R, Okparavero A, Zhang YL, Schmid CH, Levey AS; Chronic Kidney Disease Epidemiology Collaboration: Comparison of drug dosing recommendations based on measured GFR and kidney function estimating equations. Am J Kidney Dis 54: 33–42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Issa N, Meyer KH, Arrigain S, Choure G, Fatica RA, Nurko S, Stephany BR, Poggio ED: Evaluation of creatinine-based estimates of glomerular filtration rate in a large cohort of living kidney donors. Transplantation 86: 223–230, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Lujan PR, Chiurchiu C, Douthat W, de Arteaga J, de la Fuente J, Capra RH, Massari PU: CKD-EPI instead of MDRD for candidates to kidney donation. Transplantation 94: 637–641, 2012 [DOI] [PubMed] [Google Scholar]
- 62.Macías LB, Poblet MS, Jerez RI, Roncero FM, Blanco GB, Palomo PP, Govantes MA: Study of renal function in living kidney donors: Estimated or measured glomerular filtration. Transplant Proc 45: 3612–3615, 2013 [DOI] [PubMed] [Google Scholar]
- 63.Shaffi K, Uhlig K, Perrone RD, Ruthazer R, Rule A, Lieske JC, Navis G, Poggio ED, Inker LA, Levey AS: Performance of creatinine-based GFR estimating equations in solid-organ transplant recipients. Am J Kidney Dis 63: 1007–1018, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fan L, Levey AS, Gudnason V, Eiriksdottir G, Andresdottir MB, Gudmundsdottir H, Indridason OS, Palsson R, Mitchell G, Inker LA: Comparing GFR estimating equations using cystatin C and creatinine in elderly individuals [published online ahead of print December 19, 2014]. J Am Soc Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gerchman F, Tong J, Utzschneider KM, Zraika S, Udayasankar J, McNeely MJ, Carr DB, Leonetti DL, Young BA, de Boer IH, Boyko EJ, Fujimoto WY, Kahn SE: Body mass index is associated with increased creatinine clearance by a mechanism independent of body fat distribution. J Clin Endocrinol Metab 94: 3781–3788, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson FP, Xie D, Anderson AH, Leonard MB, Reese PP, Delafontaine P, Horwitz E, Kallem R, Navaneethan S, Ojo A, Porter AC, Sondheimer JH, Sweeney HL, Townsend RR, Feldman HI; CRIC Study Investigators: Urinary creatinine excretion, bioelectrical impedance analysis, and clinical outcomes in patients with CKD: The CRIC study. Clin J Am Soc Nephrol 9: 2095–2103, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE: Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 65: 1416–1421, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Stevens LA, Levey AS: Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol 20: 2305–2313, 2009 [DOI] [PubMed] [Google Scholar]
- 69.Kazmi WH, Gilbertson DT, Obrador GT, Guo H, Pereira BJ, Collins AJ, Kausz AT: Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis 46: 887–896, 2005 [DOI] [PubMed] [Google Scholar]
- 70.Rosansky SJ, Eggers P, Jackson K, Glassock R, Clark WF: Early start of hemodialysis may be harmful. Arch Intern Med 171: 396–403, 2011 [DOI] [PubMed] [Google Scholar]
- 71.Stel VS, Dekker FW, Ansell D, Augustijn H, Casino FG, Collart F, Finne P, Ioannidis GA, Salomone M, Traynor JP, Zurriaga O, Verrina E, Jager KJ: Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant 24: 3175–3182, 2009 [DOI] [PubMed] [Google Scholar]
- 72.Susantitaphong P, Altamimi S, Ashkar M, Balk EM, Stel VS, Wright S, Jaber BL: GFR at initiation of dialysis and mortality in CKD: A meta-analysis. Am J Kidney Dis 59: 829–840, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Evans M, Tettamanti G, Nyrén O, Bellocco R, Fored CM, Elinder CG: No survival benefit from early-start dialysis in a population-based, inception cohort study of Swedish patients with chronic kidney disease. J Intern Med 269: 289–298, 2011 [DOI] [PubMed] [Google Scholar]
- 74.Nesrallah GE, Mustafa RA, Clark WF, Bass A, Barnieh L, Hemmelgarn BR, Klarenbach S, Quinn RR, Hiremath S, Ravani P, Sood MM, Moist LM; Canadian Society of Nephrology: Canadian Society of Nephrology 2014 clinical practice guideline for timing the initiation of chronic dialysis. CMAJ 186: 112–117, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jones GR: Estimating renal function for drug dosing decisions. Clin Biochem Rev 32: 81–88, 2011 [PMC free article] [PubMed] [Google Scholar]
- 76.Dowling TC, Wang ES, Ferrucci L, Sorkin JD: Glomerular filtration rate equations overestimate creatinine clearance in older individuals enrolled in the Baltimore Longitudinal Study on Aging: Impact on renal drug dosing. Pharmacotherapy 33: 912–921, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]