Abstract
Background and objective
Serum uric acid may predict the onset and progression of kidney disease, but it is unclear whether uric acid is an independent risk factor for diabetic nephropathy. Our aim was to study the relationship between uric acid levels and the development of CKD components in patients with type 2 diabetes.
Design, setting, participants, & measurements
Longitudinal study of a cohort of patients with type 2 diabetes from the database of the Italian Association of Clinical Diabetologists network. From a total of 62,830 patients attending the diabetes centers between January 1, 2004, and June 30, 2008, we considered those with baseline eGFR values ≥60 ml/min per 1.73 m2 and normal albumin excretion (n=20,142). Urinary albumin excretion, GFR, and serum uric acid were available in 13,964 patients. We assessed the association of serum uric acid quintiles with onset of CKD components by multinomial logistic regression model adjusting for potential confounders. We calculated the relative risk ratios (RRRs) for eGFR <60 ml/min per 1.73 m2, albuminuria, and their combination at 4 years.
Results
At 4-year follow-up, 1109 (7.9%) patients developed GFR <60 ml/min per 1.73 m2 with normoalbuminuria, 1968 (14.1%) had albuminuria with eGFR ≥60 ml/min per 1.73 m2, and 286 (2.0%) had albuminuria with eGFR <60 ml/min per 1.73 m2. The incidence of eGFR <60 ml/min per 1.73 m2 increased in parallel with uric acid quintiles: Compared with the lowest quintile, RRRs were 1.46 (95% confidence interval [CI], 1.14 to 1.88; P=0.003), 1.44 (95% CI, 1.11 to 1.87; P=0.006), 1.95 (95% CI, 1.48 to 2.58; P<0.001), and 2.61 (95% CI, 1.98 to 3.42; P<0.001) for second, third, fourth, and fifth quintiles, respectively. Serum uric acid was significantly associated with albuminuria only in presence of eGFR <60 ml/min per 1.73 m2.
Conclusions
Mild hyperuricemia is strongly associated with the risk of CKD in patients with type 2 diabetes.
Keywords: diabetes mellitus, glomerular filtration rate, albuminuria
Introduction
Diabetes, a major health problem affecting global estimates exceeding 387 million people (1), is the leading cause of CKD (2). In fact, approximately 40% of patients with diabetes develop diabetic kidney disease (DKD) resulting in albuminuria, reduction of GFR, or both (3). Several studies have shown that increased urinary albumin excretion (4) and reduced GFR (5) are both associated with cardiovascular risk factors and are independent predictors of cardiovascular events among patients with diabetes.
In addition to primary risk factors, such as hyperglycemia and increased BP (6), genetic determinants are also thought to play a relevant role in the pathophysiology of DKD (7). A better knowledge of modifiable risk factors for kidney complications in diabetic patients is definitively needed to develop more effective prevention strategies.
Hyperuricemia is largely prevalent in patients with CKD, and in many studies it independently predicted the development of type 2 diabetes mellitus (T2DM) (8–10) and cardiovascular events (11). So far, only one longitudinal study has evaluated the role of serum uric acid (SUA) in the development of CKD in patients with T2DM (12). Cross-sectional studies have reported an association between hyperuricemia and reduction of GFR and/or albuminuria; some of these have had conflicting results, especially after adjustment for confounders (13–19). Furthermore, the role for SUA as an independent predictor of progression once DKD is established is uncertain (20). In a post hoc analysis of 1342 patients with T2DM and albuminuria participating in the Reduction of End Points in Non-Insulin-Dependent Diabetes Mellitus With the Angiotensin II Antagonist Losartan (RENAAL) Trial, the authors observed that the risk of unfavorable renal outcomes was decreased by 6% for each 0.5-mg/dl decrement in SUA levels during the first 6 months of treatment (21). Given the paucity and relatively discordant nature of data on this issue, we designed the current observational study to investigate whether SUA can be considered an independent predictor of incident CKD in a large cohort of patients with T2DM who have preserved renal function at baseline.
Materials and Methods
Study Setting and Data Sources
In Italy, diabetes care is mainly provided by a public network of about 700 diabetes clinics, where a team of specialists provide diagnostic confirmation and prevention and treatment of diabetes and its complications through close patient follow-up and regular check-ups (22,23).
In the present report we analyzed a large cohort of patients with T2DM followed up at 207 diabetes centers in Italy that were affiliated with the Italian Association of Clinical Diabetologists (Associazione Medici Diabetologi [AMD]) initiative. The centers participated in the study voluntarily and include about one third of all the Italian centers for diabetes. They are homogeneously distributed throughout the country and thus may be representative of the Italian population with T2DM. The analysis was performed using the data set of electronic medical records collected between January 1, 2004, and June 30, 2008. For the purpose of the analysis, we considered only patients who were age ≥18 years and had a follow-up evaluation within 48±6 months that was complete for data on eGFR and albuminuria. In the case of multiple records, we considered the evaluation closest to 48 months after study entry for each patient.
Study Patients
Of 62,830 patients identified, we excluded those with albuminuria, eGFR ≤60 ml/min per 1.73 m2 or a second eGFR value that was discordant (<60 ml/min per 1.73 m2) from the first value within the first 6 months of the follow-up period and those with missing data on SUA levels or antidiabetic treatment. The study population consisted of 13,964 patients (see Supplemental Figure 1).
Data Collection
The Italian AMD initiative analyzed the database to identify a set of indicators that can be used in the context of continuous quality improvement. Participating centers adopted the same software systems for the everyday management of outpatients, while a specially developed software package allowed us to extract the information we intended to analyze from all the clinical databases (AMD data file). Moreover, data from all participating centers were collected and centrally analyzed anonymously (22). The results were internally approved by the AMD Annals scientific committee. T2DM was diagnosed at participating diabetes centers according to the American Diabetes Association 2003 criteria. The biochemical measurements were performed at clinical laboratories at each participating center. This initiative includes measuring and monitoring hemoglobin A1c (HbA1c), BP, lipid profile (LDL cholesterol or total and HDL cholesterol and triglycerides) and SUA. The use of specific classes of drugs (insulin, statins and two or more antihypertensive agents) was also evaluated. Because normal ranges for HbA1c varied among centers, the percentage change with respect to the upper normal value (measured value/upper normal limit) was estimated and multiplied by 6.0 to allow comparisons among the centers. Kidney function was assessed by serum creatinine and urinary albumin excretion measurements. GFR was estimated for each patient using a standardized serum creatinine assay and the CKD-Epidemiology Collaboration equation (24).
To be included in the study, the patients had to have at least one measurement of serum creatinine, with concordant eGFR values, in the 3 months before study entry. Increased urinary albumin excretion was diagnosed and defined as albuminuria if urinary albumin concentration was >30 mg/L, urinary albumin excretion rate was >20 µg/min, or urinary albumin-to-creatinine ratio was >2.5 mg/mmol in men and >3.5 mg/mmol in women. CKD was defined as diabetes with albuminuria or low eGFR (<60 ml/min per 1.73 m2) or both. The development of eGFR <45 ml/min per 1.73 m2 was considered as a secondary outcome. SUA was measured by enzymatic method.
Exposure
The exposure of interest was SUA. The association of SUA with renal outcomes was explored by dividing the whole cohort into sex-specific SUA quintiles (<3.7, 3.7–4.3, 4.4–4.9, 5.0–5.7, ≥5.8 mg/dl in women and <4.3, 4.3–4.8, 4.9–5.5, 5.6–6.3, ≥6.4 mg/dl in men) or taking SUA values as a continuous trait.
Outcomes
The primary outcomes were as follows: (1) eGFR <60 ml/min per 1.73 m2 and normoalbuminuria, (2) albuminuria and eGFR ≥60 ml/min per 1.73 m2, and (3) eGFR <60 ml/min per 1.73 m2 and albuminuria. Secondary outcomes were (1) eGFR <60 ml/min per 1.73 m2, (2) eGFR <45 ml/min per 1.73 m2, and (3) albuminuria.
Statistical Analyses
The data are given as mean values±SD; categorical variables are described as frequencies and percentages. The relationship between SUA and patients' characteristics was evaluated using a multilevel mixed-effects linear regression model with diabetes clinics fitted as random. The main analysis was performed using a multinomial logistic regression model considering four outcome categories: (1) eGFR ≥60 ml/min per 1.73 m2 and normoalbuminuria, (2) eGFR <60 ml/min per 1.73 m2 and normoalbuminuria, (3) eGFR ≥60 ml/min per 1.73 m2 and albuminuria, and (4) eGFR <60 ml/min per 1.73 m2 and albuminuria. A multilevel mixed-effects logistic regression model was used to evaluate single outcome (eGFR <60 ml/min per 1.73 m2, eGFR <45 ml/min per 1.73 m2, and albuminuria). Data were analyzed considering diabetes clinics as clusters of observations, so that possible differences in data across centers could be considered. Relative risk ratios (RRRs) and odds ratios (ORs) were reported with their 95% confidence intervals (95% CIs). The multivariate model was fitted, including a missing indicator variable for patients with missing data. A complete-case analysis was performed, including patients for whom all data were observed. Multivariate analyses adjusted for all baseline clinical characteristics (sex, age, duration of diabetes, body mass index (BMI), HbA1c, SUA, lipid profile, BP, eGFR, albuminuria, retinopathy, smoking habits, and treatment). For each baseline clinical characteristic, a subgroup analysis that took into account sex, age, duration of disease, BMI, HbA1c, triglycerides, HDL cholesterol, BP, eGFR, and retinopathy was performed to evaluate the influence of baseline SUA levels on the development of eGFR <60 ml/min per 1.73 m2 at 4 years. Likelihood ratio test was used to evaluate interactions between variables. The goodness-of-fit of models was evaluated in terms of calibration between observed and expected frequencies. Discrimination was estimated by c-statistic computed for each prediction. The analyses were performed by using Stata software, version 12 (Stata Corp., College Station, Texas). P values <0.05 were considered to indicate statistically significantly findings.
Results
The main clinical features of the study cohort at baseline are summarized in Table 1. Overall, the mean age was 64±10 years, 56.0% of patients were male, and the mean duration of diabetes was 10±8 years. Thirty-nine percent of patients were obese. The glycemic, lipid, and BP control of participants was fairly good; mean HbA1c was 7.2%, mean LDL cholesterol was 110 mg/dl, and mean BP was 138/80 mmHg. The eGFR was 85±13 ml/min per 1.73 m2 (Table 1). By study design, all patients had normal urine albumin excretion and eGFR >60 ml/min per 1.73 m2. In the whole cohort, SUA levels were 5.1±1.6 mg/dl and significantly differed between women and men (4.8±1.6 mg/dl versus 5.4±1.5 mg/dl, respectively; P<0.001). Urine albumin excretion was evaluated by means of urine albumin concentration in most study patients (88% at baseline and 83% at follow-up) and by albumin-to-creatinine ratio (spot morning samples) in fewer patients (11% at baseline and 16% at follow-up). Timed urine collection (24 hours) was used in a minority of patients (1% at baseline and follow-up). There was good concordance between baseline and follow-up regarding method used in each patient.
Table 1.
Baseline characteristics of study patients as a whole and stratified by sex-specific serum uric acid quintiles
| Characteristic | Whole Cohort (n=13,964) | Serum Uric Acid Quintiles | P Value | ||||
|---|---|---|---|---|---|---|---|
| 1st (Lowest) n=3,005 | 2nd (n=2712) | 3rd (n=2877) | 4th (n=2830) | 5th (Highest) n=2540) | |||
| Men | 7814 (56.0) | 1690 (56.2) | 1443 (53.2) | 1707 (59.3) | 1566 (55.3) | 1408 (55.4) | – |
| Age (yr) | 64±10 | 63±11 | 64±10 | 64±10 | 64±9 | 65±9 | <0.001 |
| Duration of diabetes (yr) | 10±8 | 11±9 | 10±8 | 10±8 | 9±8 | 9±8 | <0.001 |
| BMI (kg/m2) | 29.3±5.1 | 27.5±4.4 | 28.5±4.6 | 29.4±4.9 | 30.2±5.1 | 31.2±5.5 | <0.001 |
| HbA1c (%) | 7.2±1.3 | 7.6±1.4 | 7.2±1.3 | 7.2±1.2 | 7.0±1.1 | 7.0±1.1 | <0.001 |
| Serum uric acid (mg/dl) | 5.1±1.6 | 3.5±0.6 | 4.4±0.3 | 5.1±0.3 | 5.8±0.4 | 7.1±2.2 | – |
| Total cholesterol (mg/dl) | 187±36 | 187±36 | 186±36 | 186±37 | 186±36 | 188±37 | 0.34 |
| Triglycerides (mg/dl) | 132±90 | 117±88 | 121±81 | 134±92 | 138±82 | 154±102 | <0.001 |
| HDL cholesterol (mg/dl) | 52±15 | 55±16 | 53±15 | 51±14 | 50±14 | 49±14 | <0.001 |
| LDL cholesterol (mg/dl) | 110±32 | 110±32 | 110±32 | 110±33 | 110±32 | 111±33 | 0.72 |
| Systolic BP (mmHg) | 138±17 | 136±18 | 138±18 | 138±17 | 139±17 | 139±18 | <0.001 |
| Diastolic BP (mmHg) | 80±9 | 79±8 | 79±8 | 80±9 | 80±9 | 80±9 | <0.001 |
| Serum creatinine (mg/dl) | 0.84±0.16 | 0.81±0.16 | 0.82±0.15 | 0.85±0.16 | 0.86±0.16 | 0.89±0.17 | <0.001 |
| eGFR (ml/min per 1.73 m2) | 85±13 | 89±13 | 87±13 | 85±13 | 83±13 | 80±13 | <0.001 |
| Retinopathy | 1418 (10.2) | 367 (12.2) | 287 (10.6) | 305 (10.6) | 235 (8.3) | 224 (8.8) | 0.004 |
| Smokers | 1116 (16.8) | 279 (19.2) | 253 (19.5) | 229 (17.0) | 203 (14.9) | 152 (12.9) | <0.001 |
| Lipid-lowering treatment | 5985 (42.9) | 1237 (41.2) | 1162 (42.8) | 1254 (43.6) | 1223 (43.2) | 1109 (43.7) | 0.225 |
| Treatment with statins | 5485 (39.3) | 1112 (37.0) | 1081 (39.9) | 1163 (40.4) | 1131 (40.0) | 998 (39.3) | 0.29 |
| Treatment with fibrates | 283 (2.0) | 98 (3.3) | 47 (1.7) | 47 (1.6) | 47 (1.7) | 44 (1.7) | 0.002 |
| Antihypertensive treatment | 7978 (57.1) | 1391 (46.3) | 1435 (52.9) | 1651 (57.4) | 1781 (62.9) | 1720 (67.7) | <0.001 |
| Treatment with ACE inhibitors/ARBs | 6639 (47.5) | 1139 (37.9) | 1173 (43.3) | 1393 (48.4) | 1488 (52.6) | 1446 (56.9) | <0.001 |
| Aspirin | 2689 (19.3) | 526 (17.5) | 531 (19.6) | 570 (19.8) | 554 (19.6) | 508 (20.0) | 0.04 |
| Antidiabetic therapy | |||||||
| Diet | 1383 (9.9) | 210 (7.0) | 303 (11.2) | 307 (10.7) | 281 (9.9) | 282 (11.1) | <0.001 |
| Oral antidiabetic drugs | 9247 (66.2) | 1795 (59.7) | 1714 (63.2) | 1962 (68.2) | 1993 (70.4) | 1783 (70.2) | <0.001 |
| Oral antidiabetic drugs and insulin | 1747 (12.5) | 437 (14.5) | 357 (13.2) | 339 (11.8) | 336 (11.9) | 278 (10.9) | 0.003 |
| Insulin | 1587 (11.4) | 563 (18.7) | 338 (12.5) | 269 (9.4) | 220 (7.8) | 197 (7.8) | <0.001 |
Values are expressed as mean±SD or absolute frequency (percentage). Patients' baseline missing data: known duration of diabetes in 389 (2.8%), BMI in 1430 (10.2%), HbA1c in 71 (0.5%), total cholesterol in 143 (1.0%), triglycerides in 191 (1.4%), HDL cholesterol in 326 (2.3%), LDL cholesterol in 438 (3.1%), BP in 1608 (11.5%), and smoking status in 7327 (52.5%). The P values refer to the regression coefficient between each characteristic and serum uric acid as dependent variable in a linear regression model adjusting for patient's sex. BMI, body mass index; HbA1c, hemoglobin A1c; ACE, angiotensin-converting enzyme; ARBs, angiotensin II receptor antagonists.
At 4-year evaluation, lipid profile and BP further improved on average, along with a parallel increase in hypolipidemic and antihypertensive treatment over the study period in the whole cohort. HbA1c and SUA levels remained almost unchanged.
Baseline clinical features of patients grouped by renal outcome at 4-year follow-up are reported in Supplemental Table 1. Baseline clinical characteristics of study patients across SUA quintiles are reported in Table 1. Patients in the highest quintile were heavier and had higher triglyceride and lower HDL cholesterol levels. Furthermore, there was a trend toward a greater use of cardiac and renoprotective therapies among patients with progressively higher SUA values.
At 4-year follow-up, 1109 (7.9%) patients developed an eGFR <60 ml/min per 1.73 m2 with normoalbuminuria, 1968 (14.1%) had albuminuria with an eGFR ≥60 ml/min per 1.73 m2, and 286 (2.0%) had an eGFR <60 ml/min per 1.73 m2 and albuminuria. Table 2 reports renal outcomes on the basis of sex-specific quintiles of SUA. The incidence of eGFR <60 ml/min per 1.73 m2 increased progressively across quintiles (from 4.1% in the first quintile to 13.7% in the fifth quintile).
Table 2.
Renal outcomes at 4-year follow-up by sex-specific serum uric acid quintiles
| Variable | Serum Uric Acid Quintiles | ||||
|---|---|---|---|---|---|
| 1st (Lowest) (n=3005) | 2nd (n=2712) | 3rd (n=2877) | 4th (n=2830) | 5th (Highest) (n=2540) | |
| eGFR <60 ml/min per 1.73 m2 and normoalbuminuria | 123 (4.1) | 170 (6.3) | 196 (6.8) | 273 (9.6) | 347 (13.7) |
| eGFR ≥60 ml/min per 1.73 m2 and albuminuria | 406 (13.5) | 380 (14.0) | 382 (13.3) | 419 (14.8) | 381 (15.0) |
| eGFR <60 ml/min per 1.73 m2and albuminuria | 46 (1.5) | 42 (1.5) | 62 (2.2) | 57 (2.0) | 79 (3.1) |
| eGFR <60 ml/min per 1.73 m2 | 169 (5.6) | 212 (7.8) | 258 (9.0) | 330 (11.7) | 426 (16.8) |
| Albuminuria | 452 (15.0) | 422 (15.6) | 444 (15.4) | 476 (16.8) | 460 (18.1) |
Values are expressed as absolute frequency (percentage).
The association of SUA levels with different combinations of kidney dysfunction traits was further investigated by logistic multivariate analysis. SUA levels strongly and significantly predict the development of low eGFR (eGFR <60 ml/min per 1.73 m2), independently of several potential confounders, including baseline eGFR (Table 3). In fact, the risk of developing low eGFR gradually and linearly increased across SUA quintiles up to 2.6 times for patients in the top compared with those in the lowest quintile. The risk of developing albuminuria remained flat except for patients with higher baseline SUA levels (i.e., those in the fifth quintile) and concomitant eGFR <60 ml/min per 1.73 m2. Finally, the risk of developing low eGFR increases by 10% for each 1-mg/dl higher baseline SUA, while there seems to be no relationship with the onset of albuminuria (Supplemental Table 2).
Table 3.
Multivariate relative risk ratios for 4-year renal outcomes
| Variable | RRR for Alb−/eGFR+ n=1,109 | P Value | RRR for Alb+/eGFR− n=1,968 | P Value | RRR for Alb+/eGFR+ n=286 | P Value |
|---|---|---|---|---|---|---|
| Serum uric acid | ||||||
| 2nd quintile | 1.46 (1.14 to 1.88) | 0.003 | 1.04 (0.90 to 1.21)a | 0.576 | 0.99 (0.69 to 1.42)a | 0.94 |
| 3rd quintile | 1.44 (1.11 to 1.87) | 0.006 | 0.93 (0.75 to 1.15)a | 0.490 | 1.20 (0.80 to 1.80) | 0.37 |
| 4th quintile | 1.95 (1.48 to 2.58) | <0.001 | 1.09 (0.89 to 1.32)a | 0.417 | 1.08 (0.76 to 1.52)a | 0.67 |
| 5th quintile | 2.61 (1.98 to 3.42) | <0.001 | 1.14 (0.94 to 1.38)a | 0.184 | 1.54 (1.13 to 2.09)a | 0.006 |
| Male sex | 0.83 (0.72 to 0.96) | 0.010 | 1.55 (1.39 to 1.72)a | <0.001 | 1.02 (0.76 to 1.38)b | 0.88 |
| Age (by 10 yr) | 2.06 (1.87 to 2.28) | <0.001 | 1.22 (1.07 to 1.40)a | 0.003 | 1.87 (1.45 to 2.40)b | <0.001 |
| Known duration of diabetes | ||||||
| 5–10 yr | 1.12 (0.91 to 1.38) | 0.277 | 0.98 (0.86 to 1.12) | 0.754 | 0.96 (0.66 to 1.40) | 0.84 |
| >10 yr | 1.13 (0.89 to 1.45) | 0.322 | 0.91 (0.80 to 1.04) | 0.170 | 1.05 (0.78 to 1.41) | 0.73 |
| BMI | ||||||
| 27–30 kg/m2 | 1.36 (1.09 to 1.70) | 0.006 | 1.16 (1.00 to 1.35) | 0.053 | 0.93 (0.63 to 1.38) | 0.72 |
| >30 kg/m2 | 1.33 (1.09 to 1.63) | 0.006 | 1.20 (1.04 to 1.39) | 0.012 | 0.95 (0.66 to 1.39) | 0.81 |
| HbA1c ≥7% | 1.15 (0.97 to 1.36) | 0.104 | 1.17 (0.98 to 1.39) | 0.083 | 1.41 (1.02 to 1.95) | 0.04 |
| Triglycerides ≥150 mg/dl | 1.20 (1.02 to 1.41) | 0.028 | 1.11 (0.96 to 1.28) | 0.155 | 1.56 (1.25 to 1.96)ab | <0.001 |
| HDL cholesterol <40 (men) or <50 mg/dl (women) | 1.08 (0.90 to 1.28) | 0.418 | 1.23 (1.06 to 1.44) | 0.008 | 1.37 (1.05 to 1.80) | 0.02 |
| LDL cholesterol ≥100 mg/dl | 0.72 (0.63 to 0.83) | <0.001 | 0.95 (0.86 to 1.04)a | 0.268 | 0.87 (0.70 to 1.09) | 0.23 |
| BP ≥140/85 mmHg | 1.16 (0.98 to 1.36) | 0.081 | 1.05 (0.91 to 1.20) | 0.533 | 1.08 (0.82 to 1.43) | 0.58 |
| eGFR (by 10 below 90 ml/min per 1.73 m2) | 2.16 (1.90 to 2.46) | <0.001 | 1.03 (0.94 to 1.13)a | 0.471 | 2.33 (1.95 to 2.78)b | <0.001 |
| Retinopathy | 1.11 (0.93 to 1.33) | 0.253 | 1.38 (1.08 to 1.76) | 0.011 | 1.15 (0.78 to 1.71) | 0.49 |
| Smokers | 0.85 (0.65 to 1.11) | 0.246 | 1.23 (1.02 to 1.48)a | 0.028 | 1.61 (1.00 to 2.59)b | 0.05 |
| Treatment with statins | 1.08 (0.94 to 1.25) | 0.287 | 0.95 (0.80 to 1.13) | 0.573 | 0.80 (0.57 to 1.12) | 0.20 |
| Treatment with fibrates | 1.32 (0.90 to 1.94) | 0.156 | 0.85 (0.51 to 1.44) | 0.551 | 0.74 (0.32 to 1.73) | 0.49 |
| Antihypertensive treatment | 1.04 (0.73 to 1.50) | 0.820 | 1.14 (0.83 to 1.57) | 0.406 | 1.21 (0.83 to 1.77) | 0.32 |
| Treatment with ACE inhibitor/ARBs | 1.17 (0.92 to 1.48) | 0.200 | 1.15 (0.93 to 1.42) | 0.186 | 1.36 (0.84 to 2.21) | 0.21 |
| Aspirin | 0.90 (0.71 to 1.14) | 0.364 | 1.06 (0.84 to 1.33) | 0.644 | 1.10 (0.67 to 1.82) | 0.70 |
| Antidiabetic therapy | ||||||
| Diet | 0.68 (0.50 to 0.94) | 0.019 | 0.84 (0.58 to 1.23) | 0.368 | 0.28 (0.08 to 1.01) | 0.05 |
| Oral antidiabetic drugs | Reference | – | Reference | – | Reference | – |
| Oral antidiabetic drugs and insulin | 1.45 (1.21 to 1.73) | <0.001 | 1.16 (0.83 to 1.61) | 0.389 | 1.39 (0.95 to 2.04) | 0.09 |
| Insulin | 1.30 (1.08 to 1.56) | 0.006 | 0.87 (0.67 to 1.11)a | 0.259 | 1.57 (0.96 to 2.55)b | 0.07 |
Values in parentheses are 95% confidence intervals. Multinomial logistic regression analysis performed with the missing indicator method for each incomplete variable. Reference category for the variable known duration of diabetes was <5 years; for BMI, <27 kg/m2; and for serum uric acid, the first quintile. Goodness-of-fit test: P=0.20 according to observed and expected frequencies by quartiles of predictions. Model discrimination by c-statistic was 0.82 for Alb−/eGFR+, 0.61 for Alb+/eGFR− and 0.83 for Alb+/eGFR+. BMI, body mass index; HbA1c, hemoglobin A1c; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; RRR, relative risk ratios with eGFR−/Alb− as reference group; Alb−, normoalbuminuria; Alb+, albuminuria; eGFR−, eGFR ≥60 ml/min per 1.73 m2; eGFR+, eGFR <60 ml/min per 1.73 m2.
P<0.05 versus Alb−/eGFR+.
P<0.05 versus Alb+/eGFR−.
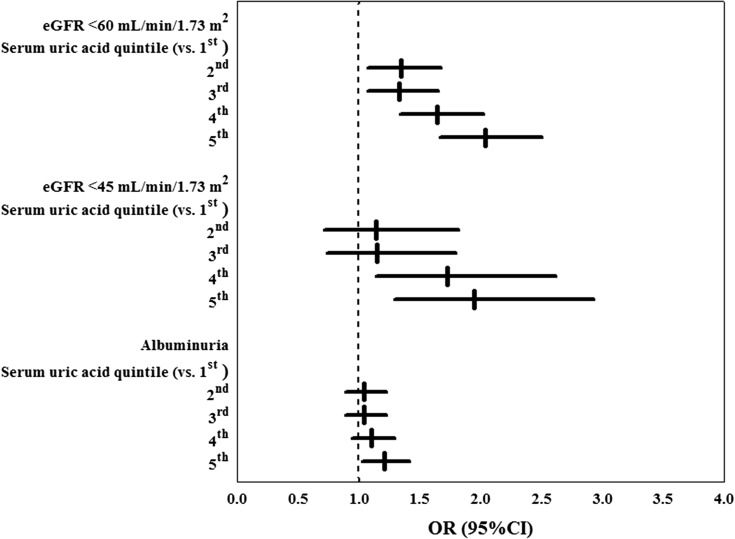
Figure 1 depicts the association of SUA levels with the development of low eGFR (<60 or <45 ml/min per 1.73 m2) and albuminuria. It shows a significant association between SUA levels and eGFR <60 ml/min per 1.73 m2, which is consistent even for lower eGFR levels and after adjustment for baseline eGFR. On the other hand, the association with the development of albuminuria appears to be weaker.
Figure 1.
The graded relationship of serum uric acid (SUA) with the development of incident low eGFR and albuminuria in type 2 diabetes. Odds ratios (ORs) with their 95% confidence intervals (95% CIs) for eGFR <60 or <45 ml/min per 1.73 m2 and albuminuria onset by sex-specific quintile of serum uric acid adjusted for baseline eGFR. The first serum uric acid quintile was considered as reference category.
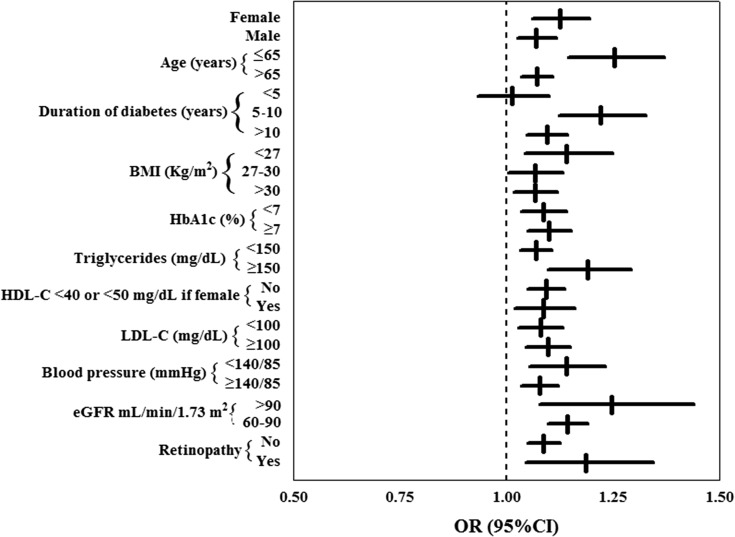
Furthermore, the significant association between SUA levels and the risk of developing eGFR <60 ml/min per 1.73 m2 was consistent in all subgroups evaluated (Figure 2) with the exception of patients who had had diabetes for <5 years. A significant interaction was detected between SUA levels and diabetes duration (likelihood ratio test for the interaction, P=0.005), with a weaker association of SUA values with incident low eGFR at 4 years in patients with short disease duration.
Figure 2.
Serum uric acid (SUA) levels are independent predictors of low eGFR in type 2 diabetes. Association of sex- and eGFR-adjusted serum uric acid levels analyzed continuously with the incidence of eGFR <60 ml/min per 1.73 m2 within subgroups. In each subgroup, the odds ratio estimates the association between 1-unit change and the risk of eGFR <60 ml/min per 1.73 m2 at 4-year evaluation. Data are expressed as odds ratios (ORs) and 95% confidence intervals (95% CIs). BMI, body mass index. HDL-C, HDL cholesterol; LDL-C, LDL cholesterol.
Finally, when analysis was repeated by applying less stringent selection criteria (i.e., including patients with only one GFR measurements at baseline [n=601, 3.2%] and those with a eGFR value <60 ml/min per 1.73 m2 preceding baseline [n=4035, 21.7%]), results were almost superimposable (data not shown).
Discussion
We found that SUA is a strong independent predictor of development of eGFR <60 ml/min per 1.73 m2 in a large, real-life cohort of patients with T2DM. During a 4-year follow-up period, SUA levels in the top sex-specific quintile entailed a greater risk of developing eGFR <60 ml/min per 1.73 m2 alone (OR, 2.6) and in association with albuminuria (OR, 1.5) compared with the lowest SUA quintile. The association with baseline SUA values and subsequent development of renal damage seems to be particularly strong with respect to de novo eGFR <60 ml/min per 1.73 m2, even after adjustment for confounders such as BP, BMI, glycometabolic control, and lipid profile. Accordingly, a significant association of SUA levels with subsequent development of albuminuria was detected only for patients in the highest SUA quintile (OR, 1.2) (Figure 1).
There are controversial reports on the association between SUA levels and CKD in the literature, both in the general population (17,18,25) and in at-risk cohorts (14,16,19,26,27). Similarly, an independent role of mild hyperuricemia on the progression of renal disease is at present uncertain (13). As for T2DM, so far only one longitudinal study has shown an association between SUA and development of DKD (12). Thus, our data confirm and extend previous findings (12) on a much larger and more representative real-life clinical practice cohort. In fact, while in Zoppini and colleagues' study a significant proportion of patients already showed abnormal urine albumin excretion at baseline, by selecting a subset of patients with normal renal function and no albuminuria we provided more detailed insights on the predictive role of SUA on both DKD and each one of its individual components (GFR and albuminuria).
The incidence of renal events should be interpreted in the context of clinical characteristics of our study patients and of selection criteria used. In fact, patients' risk profile was, on average, only mildly elevated at baseline, with fair BP control and slightly elevated LDL cholesterol and HbA1c. During the observation period, the intensity of pharmacologic treatment increased significantly with parallel improvement in several of the above-cited parameters.
For each progressively greater sex-specific SUA quintile, there was a significantly higher risk of reaching a GFR <60 ml/min per 1.73 m2, while the association with albuminuria was somewhat weaker: Only patients in the top SUA quintile showed a significantly greater risk of albuminuria development. The independent role of SUA as a predictor of renal damage is consistent with further analysis of data from the small subgroup of patients (2%) whose GFR dropped to <45 ml/min per 1.73 m2 during follow-up. Again, with the exception of patients with a very short disease duration (<5 years), SUA was strongly and independently related to the development of eGFR <60 ml/min per 1.73 m2 in each subgroup we investigated, a finding that strengthens and is consistent with a role as a promoter of renal damage.
SUA was strongly related to features of the metabolic syndrome at baseline. In fact, BMI, triglycerides, systolic BP, and the burden of treatment showed a growing trend along with SUA quintiles, while HDL cholesterol showed parallel reduction. These factors proved to be independent determinants of changes in renal function in our study, while the predictive power of other, more traditional risk factors, such as disease duration and HbA1c, was less evident. Furthermore, the development of low eGFR was related to female sex and features of metabolic syndrome, while development of albuminuria was more strictly associated with male sex, age, HbA1c, and retinopathy.
The stronger association we found between SUA and the development of low eGFR compared with the development of albuminuria is intriguing in light of previous findings suggesting that albuminuric and nonalbuminuric DKD phenotypes may bear different prognostic power toward different end points (28).
Mild hyperuricemia is relatively common in patients with CKD because of the well known clustering of traditional risk factors, such as insulin resistance and hypertension in renal patients and because of progressive impairment in uric acid excretion along with GFR deterioration. Experimental evidence linking SUA to initiation and progression of renal damage by several pathogenetic mechanisms is well established. In fact, increased SUA levels stimulate renin-angiotensin-aldosterone system activity and promote endothelial damage and oxidative stress at the cellular and tissue level (29–31).
Lowering SUA has also been reported to reduce renal injury and improve renal function in mice with T2DM (32). So far, however, evidence that pharmacologic reduction of SUA may affect the course of DKD is missing despite some preliminary and encouraging evidence from pilot studies (33–35). Interestingly, with the exception of patients with a relatively short duration of disease, the role of SUA as independent risk factor for the development of CKD was evident in all patient subgroups. In particular higher SUA was significantly associated to eGFR <60 ml/min per 1.73 m2 at 4-year follow-up independently of sex, BMI, glycometabolic profile, BP, and microvascular damage (such as hyperfiltration or retinopathy), suggesting that the data presenting herein are consistent with a possible pathogenetic pathway linking SUA to the development of DKD in several clinical condition.
Our study has some limitations as well as several strengths that should be mentioned. We must acknowledge that laboratory variables, including serum creatinine, were not measured in a single centralized laboratory, and this may have led to some variability, especially in GFR estimation. In addition, we have information on albuminuria only as a categorical trait; this, together with some heterogeneity in the techniques used to measure urine albumin concentration in different laboratories, may have contributed to variability in the outcome measure. Moreover, data from the entire 4-year follow-up period were available for most but not all patients, and therefore caution should be taken not to generalize our findings because mortality from competitive risk was not positively collected in the missing subgroup. Baseline clinical characteristics of the subgroup with missing values, however, were similar to those of the entire cohort. Furthermore, even when we applied less stringent selection criteria for our study cohort, our main study results remained unchanged. We did not gather specific information on urate-lowering treatment. This, however, does not lessen the core message of our study, which deals with the prognostic power of SUA values independent of confounding variables. Finally, our data may not be applicable to the T2DM population at large because most participants were white and ethnicity has some effect on the risk of developing renal complications (36). On the other hand, we should mention the large size of the study cohort and the homogeneous geographic distribution of the recruiting centers as well as the relatively long follow-up period. These factors certainly help make the study cohort and our results representative of real-life clinical condition.
In conclusion, SUA is a strong independent predictor of low eGFR in patients with T2DM. Higher SUA levels entail an almost two-fold increased risk of developing DKD. Our data support the rationale to complete ad hoc randomized controlled trials to assess the benefit of specific pharmacologic intervention to prevent and slow down renal damage by reducing SUA in at-risk population, such as patients with T2DM.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Dr. E. Mannucci (Firenze, Italy) for reading the manuscript and providing useful suggestions.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03140315/-/DCSupplemental.
References
- 1.International Diabetes Foundation: IDF Diabetes Atlas. Sixth edition. 2014. Available at: http://www.idf.org/diabetesatlas/5e/Update2013
- 2.The US Renal Data System: USRDS 2014 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the U.S. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2014 [Google Scholar]
- 3.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR, UKPDS Group : Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 63: 225–232, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX: Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol 17: 2034–2047, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Macmahon S, Chalmers J, ADVANCE Collaborative Group : Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 20: 1813–1821, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reidy K, Kang HM, Hostetter T, Susztak K: Molecular mechanisms of diabetic kidney disease. J Clin Invest 124: 2333–2340, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pezzolesi MG, Krolewski AS: The genetic risk of kidney disease in type 2 diabetes. Med Clin North Am 97: 91–107, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lv Q, Meng XF, He FF, Chen S, Su H, Xiong J, Gao P, Tian XJ, Liu JS, Zhu ZH, Huang K, Zhang C: High serum uric acid and increased risk of type 2 diabetes: A systemic review and meta-analysis of prospective cohort studies. PLoS One 8: e56864, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viazzi F, Leoncini G, Vercelli M, Deferrari G, Pontremoli R: Serum uric acid levels predict new-onset type 2 diabetes in hospitalized patients with primary hypertension: The MAGIC study. Diabetes Care 34: 126–128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Shafiu M, Sundaram S, Le M, Ishimoto T, Sautin YY, Lanaspa MA: Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 62: 3307–3315, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feig DI, Kang DH, Johnson RJ: Uric acid and cardiovascular risk. N Engl J Med 359: 1811–1821, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zoppini G, Targher G, Chonchol M, Ortalda V, Abaterusso C, Pichiri I, Negri C, Bonora E: Serum uric acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function. Diabetes Care 35: 99–104, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viazzi F, Leoncini G, Ratto E, Falqui V, Parodi A, Conti N, Derchi LE, Tomolillo C, Deferrari G, Pontremoli R: Mild hyperuricemia and subclinical renal damage in untreated primary hypertension. Am J Hypertens 20: 1276–1282, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Pinelli M, Bindi M, Moroni F, Castiglioni M: Relationship between serum uric acid levels and urinary albumin excretion in patients with heart failure. Acta Cardiol 63: 191–195, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Rodilla E, Pérez-Lahiguera F, Costa JA, González C, Miralles A, Moral D, Pascual JM: Association between serum uric acid, metabolic syndrome and microalbuminuria in previously untreated essential hypertensive patients. Med Clin (Barc) 132: 1–6, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Kim ES, Kwon HS, Ahn CW, Lim DJ, Shin JA, Lee SH, Cho JH, Yoon KH, Kang MI, Cha BY, Son HY: Serum uric acid level is associated with metabolic syndrome and microalbuminuria in Korean patients with type 2 diabetes mellitus. J Diabetes Complications 25: 309–313, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Suzuki K, Konta T, Kudo K, Sato H, Ikeda A, Ichikawa K, Ueno Y, Kato T, Kayama T, Kubota I: The association between serum uric acid and renal damage in a community-based population: the Takahata study. Clin Exp Nephrol 17: 541–548, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Oh CM, Park SK, Ryoo JH: Serum uric acid level is associated with the development of microalbuminuria in Korean men. Eur J Clin Invest 44: 4–12, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Bo S, Cavallo-Perin P, Gentile L, Repetti E, Pagano G: Hypouricemia and hyperuricemia in type 2 diabetes: Two different phenotypes. Eur J Clin Invest 31: 318–321, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Johnson RJ, Nakagawa T, Jalal D, Sánchez-Lozada LG, Kang DH, Ritz E: Uric acid and chronic kidney disease: Which is chasing which? Nephrol Dial Transplant 28: 2221–2228, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miao Y, Ottenbros SA, Laverman GD, Brenner BM, Cooper ME, Parving HH, Grobbee DE, Shahinfar S, de Zeeuw D, Lambers Heerspink HJ: Effect of a reduction in uric acid on renal outcomes during losartan treatment: A post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension 58: 2–7, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Nicolucci A, Rossi MC, Arcangeli A, Cimino A, de Bigontina G, Fava D, Gentile S, Giorda C, Meloncelli I, Pellegrini F, Valentini U, Vespasiani G, AMD-Annals Study Group : Four-year impact of a continuous quality improvement effort implemented by a network of diabetes outpatient clinics: The AMD-Annals initiative. Diabet Med 27: 1041–1048, 2010 [DOI] [PubMed] [Google Scholar]
- 23.De Cosmo S, Rossi MC, Pellegrini F, Lucisano G, Bacci S, Gentile S, Ceriello A, Russo G, Nicolucci A, Giorda C, Viazzi F, Pontremoli R, AMD-Annals Study Group : Kidney dysfunction and related cardiovascular risk factors among patients with type 2 diabetes. Nephrol Dial Transplant 29: 657–662, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS: Uric acid and incident kidney disease in the community. J Am Soc Nephrol 19: 1204–1211, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Syrjänen J, Mustonen J, Pasternack A: Hypertriglyceridaemia and hyperuricaemia are risk factors for progression of IgA nephropathy. Nephrol Dial Transplant 15: 34–42, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Haririan A, Metireddy M, Cangro C, Nogueira JM, Rasetto F, Cooper M, Klassen DK, Weir MR: Association of serum uric acid with graft survival after kidney transplantation: A time-varying analysis. Am J Transplant 11: 1943–1950, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Penno G, Solini A, Bonora E, Fondelli C, Orsi E, Zerbini G, Trevisan R, Vedovato M, Gruden G, Cavalot F, Cignarelli M, Laviola L, Morano S, Nicolucci A, Pugliese G, Renal Insufficiency And Cardiovascular Events (RIACE) Study Group : Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J Hypertens 29: 1802–1809, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, Truong L, Harris R, Johnson RJ: A role for uric acid in the progression of renal disease. J Am Soc Nephrol 13: 2888–2897, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Sánchez-Lozada LG, Tapia E, Santamaría J, Avila-Casado C, Soto V, Nepomuceno T, Rodríguez-Iturbe B, Johnson RJ, Herrera-Acosta J: Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int 67: 237–247, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Ryu ES, Kim MJ, Shin HS, Jang YH, Choi HS, Jo I, Johnson RJ, Kang DH: Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. Am J Physiol Renal Physiol 304: F471–F480, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Kosugi T, Nakayama T, Heinig M, Zhang L, Yuzawa Y, Sanchez-Lozada LG, Roncal C, Johnson RJ, Nakagawa T: Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice. Am J Physiol Renal Physiol 297: F481–F488, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincón A, Arroyo D, Luño J: Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol 5: 1388–1393, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siu YP, Leung KT, Tong MK, Kwan TH: Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis 47: 51–59, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Goicoechea M, Garcia de Vinuesa S, Verdalles U, Verde E, Macias N, Santos A, Pérez de Jose A, Cedeño S, Linares T, Luño J. Allopurinol and progression of CKD and cardiovascular events: Long-term follow-up of a randomized clinical trial. Am J Kidney Dis 65: 543–549, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Bhalla V, Zhao B, Azar KM, Wang EJ, Choi S, Wong EC, Fortmann SP, Palaniappan LP: Racial/ethnic differences in the prevalence of proteinuric and nonproteinuric diabetic kidney disease. Diabetes Care 36: 1215–1221, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.