Abstract
Background and objectives
Although a peritoneal equilibration test yields data on three parameters (4-hour dialysate/plasma creatinine, 4- to 0-hour dialysate glucose, and 4-hour ultrafiltration volume), all studies have focused on the prognostic value of dialysate/plasma creatinine for patients undergoing peritoneal dialysis. Because dialysate 4- to 0-hour glucose and ultrafiltration volume may be superior in predicting daily ultrafiltration, the likely mechanism for the association of peritoneal equilibration test results with outcomes, we hypothesized that they are superior to dialysate/plasma creatinine for risk prediction.
Design, setting, participants, & measurements
We examined unadjusted and adjusted associations of three peritoneal equilibration test parameters with all-cause mortality, technique failure, and hospitalization rate in 10,142 patients on peritoneal dialysis treated between January 1, 2007 and December 31, 2011 in 764 dialysis facilities operated by a single large dialysis organization in the United States, with a median follow–up period of 15.8 months; 87% were treated with automated peritoneal dialysis.
Results
Demographic and clinical parameters explained only 8% of the variability in dialysate/plasma creatinine. There was a linear association between dialysate/plasma creatinine and mortality (adjusted hazards ratio per 0.1 unit higher, 1.07; 95% confidence interval, 1.02 to 1.13) and hospitalization rate (adjusted incidence rate ratio per 0.1 unit higher, 1.05; 95% confidence interval, 1.03 to 1.06). Dialysate/plasma creatinine and dialysate glucose were highly correlated (r=−0.84) and yielded similar risk prediction. Ultrafiltration volume was inversely related with hospitalization rate but not with all-cause mortality. None of the parameters were associated with technique failure. Adding 4- to 0-hour dialysate glucose, ultrafiltration volume, or both did not result in any improvement in risk prediction with dialysate/plasma creatinine alone.
Conclusions
This analysis from a large contemporary cohort treated primarily with automated peritoneal dialysis validates dialysate/plasma creatinine as a robust predictor of outcomes in patients treated with peritoneal dialysis.
Keywords: peritoneal dialysis, mortality, creatinine, dialysis solutions, follow-up studies, hospitalization, humans, renal dialysis, ultrafiltration
Introduction
Patients treated with peritoneal dialysis (PD) generally undergo a peritoneal equilibration test (PET) to characterize the rate of transfer of solute and water across the peritoneal barrier (1). The test yields three parameters—4-hour dialysate to plasma ratio of creatinine (D/P creatinine), 4- to 0-hour dialysate glucose ratio (D/D0 glucose), and 4-hour ultrafiltration volume (UFV) (1). Although the results of the PET can help optimize solute clearances, they are more often used to individualize PD prescriptions to maximize daily peritoneal ultrafiltration (2,3). Indeed, the higher risk for death or transfer to hemodialysis in individuals with faster solute transfer rate is thought to result from volume overload from challenges with fluid removal with continuous ambulatory PD in such patients (4).
Although the superiority of D/P creatinine over D/D0 glucose or UFV for risk prediction has never been established, it is the parameter used almost exclusively for these purposes (5–10). The utility of PET in optimizing PD prescription is primarily related to prediction of daily ultrafiltration; however, D/P creatinine is only modestly related to UFV (11,12). Given the importance of maintaining an osmotic gradient to ensure adequate ultrafiltration, D/D0 glucose may be a stronger predictor of daily UFV and hence, patient prognosis (13). Furthermore, it could be posited that a direct estimation of UFV during the PET may provide even better risk prediction than D/P creatinine or D/D0 glucose. However, this premise has never been tested. The inability of some recent studies to show an association between PET parameters and patient outcomes may have been secondary to the use of a potentially insensitive marker of peritoneal ultrafiltration: D/P creatinine (9,14–16).
We undertook this study to test the hypothesis that D/D0 glucose and UFV from the PET are superior to D/P creatinine in identifying patients at higher risk for adverse outcomes.
Materials and Methods
Study Population and Data Source
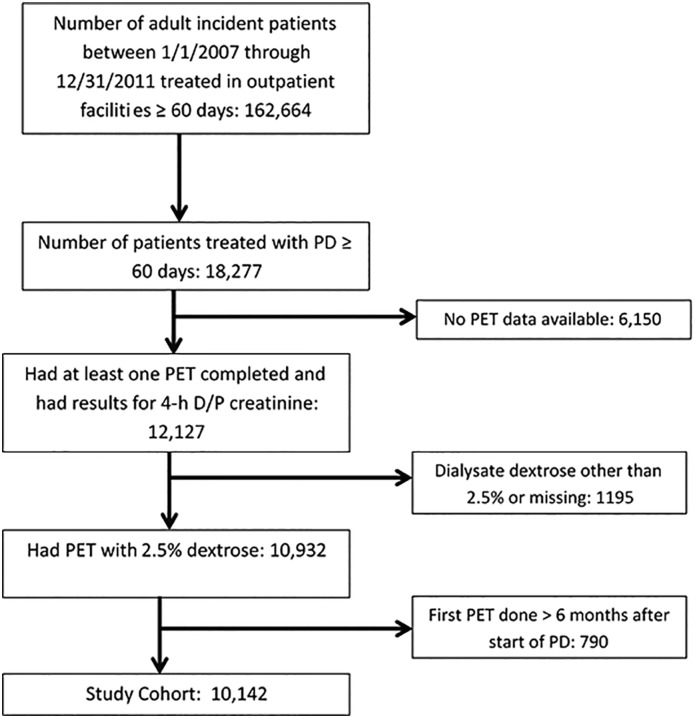
This observational cohort study comprised all patients who started maintenance dialysis in calendar years 2007–2011 with follow-up through December 31, 2011 and underwent a PET with 2.5% dextrose within 6 months of start of PD in one of the facilities operated by a large dialysis organization (Figure 1). The characteristics of patients included in the analysis compared with those excluded are summarized in Supplemental Table 1. All data were obtained from electronic records at the dialysis organization.
Figure 1.
Consort diagram summarizing the criteria used to constitute the analytic cohort. D/P creatinine, dialysate to plasma ratio of creatinine; PD, peritoneal dialysis; PET, peritoneal equilibration test.
A standard PET was performed; dialysate samples were collected at times 0, 120, and 240 minutes, and a blood sample was collected at 120 minutes. All blood and dialysate samples were shipped to a central laboratory in Deland, Florida and analyzed within 24 hours. Dialysate concentration of creatinine was corrected for interference with glucose in the effluent. The D/P creatinine was calculated as the ratio of the dialysate concentration of creatinine at 240 minutes to the serum concentration, the D/D0 glucose was calculated as the ratio of dialysate concentration of glucose at 240 minutes to time 0, and the UFV was calculated as the difference between the 4-hour drain and instillation volumes. Only results within the following ranges were included: D/P creatinine, 0.30–1.15; D/D0 glucose, 0.00–1.00; and 4-hour drain volume, −500–4000 ml; they comprised >99% of results. Of 10,142 patients, data on D/D0 glucose were not available for 408 (4%) patients, and data on UFV were not available for 269 (3%) patients.
Patients were assigned as treated with continuous ambulatory or automated PD (APD) at any point of time if they performed the therapy for at least 60 consecutive days. The entire follow–up period of each patient was divided into 91-day periods starting with the date of first dialysis, and each such 91-day period was assigned the modality used for at least 45 days of that period. The initial modality (continuous ambulatory or automated PD) was the therapy assigned for the first 91-day period when the modality was PD. Patients were assigned to the ever-treated group with APD if this submodality was assigned for at least one 91-day period on follow-up.
All other laboratory tests were also performed in a single central laboratory, and the results of all tests within each 3-month period starting from the date of first dialysis were averaged. Similarly, summary values of each parenteral medication were computed for every 3-month period. Data on sex, cause of ESRD, and laboratory parameters were missing for <1%, data on total weekly Kt/Vurea in the first 91-day period were missing for 12%, and data on geographic location were missing for 3%.
Statistical Analyses
Data are presented as means ± SDs, medians with interquartile ranges, or proportions as appropriate. Correlations between PET parameters were examined using Pearson’s correlation coefficient. Patients were divided into three groups (low/slow, average, and high/fast) using each one of three PET parameters, with individuals with values >1 SD below or above the mean assigned as slow and fast groups, respectively, and individuals with values within 1 SD of the mean assigned as the average group (D/P creatinine: slow, ≤0.52; average, 0.53–0.77; fast, ≥0.78; D/D0 glucose: slow, ≥0.50; average, 0.31–0.49; fast, ≤0.30; UFV: slow, ≥540 ml; average, 30–530 ml; fast ≤25 ml). Linear regression analysis was used to determine the variability in each PET parameter explained by demographic and clinical variables.
The association of PET parameters was examined for three separate outcomes—death, transfer from PD to another dialysis modality (technique failure), and hospitalization rate. Follow-up began on the day of first treatment with PD, and patients were censored at death, transfer to another dialysis modality, kidney transplantation, or transfer to a facility operated by another dialysis provider. All models were fit to imputed data, and point estimates were determined using Rubin’s rules (17). Cox proportional hazards model was used to determine the risk for death or technique failure associated with 0.1 unit higher D/P creatinine, 0.1 unit lower D/D0 glucose, and 250 ml lower UFV using two different levels of adjustment: (1) unadjusted and (2) adjusted for demographics (age, sex, race, health insurance, geographic region, and year of incidence), case mix (cause of ESRD, previous transplant, previous treatment with hemodialysis, and presence of diabetes, hypertension, congestive heart failure, atherosclerotic heart disease, other cardiovascular disease, or dyslipidemia), residual kidney function (mean of 24-hour urinary urea and creatinine clearances), and PD-related variables (peritoneal Kt/Vurea and initial or ever treatment with APD). Unadjusted and adjusted Poisson regression analyses were used to test the association of each parameter with hospitalization rate. Additionally, for all three outcomes, we tested if the association of any PET parameter with the outcomes was affected by (1) center experience defined as the cumulative number of 91-day periods of treatment with PD in the facility for all patients who initiated dialysis between 2007 and 2011, (2) treatment with APD at the time of initiation of PD, or (3) treatment with APD at any follow-up period. This was determined by including a multiplicative interaction term in statistical models, and it was deemed to vary by exposure if the P value for the interaction term was <0.05. Restricted cubic splines with three knots were constructed to explore nonlinear trends for the association of PET parameters with outcomes.
Sensitivity analysis was done to assess the consistency of associations by fitting a Cox proportional hazards or Poisson regression model for each of three outcomes by ranking patients on the basis of their percentile rank for each parameter and test association with each percentile higher variable.
The strength of association of each parameter with each of three outcomes was compared using a modification of the approach suggested by Lin et al. (18). For each pairwise comparison, two records were created for each patient—one for each PET parameter—and all other covariates were held constant. Cox proportional hazards model, stratified on the record indicator, was used with robust variance–covariance estimation and clustering on the identification numbers of the individuals. The coefficients of the two PET parameters were tested for equality using a Wald test. The significance of change in likelihood ratios was used to determine if the addition of D/D0 glucose, UFV, or both improved the risk prediction of the baseline-adjusted model for the association of D/P creatinine with outcomes.
All analyses were performed using SAS, version 9.3 (SAS Institute Inc., Cary, NC), and splines were constructed using Stata, version 13 (StatCorp LP, College Station, TX).
Results
Study Population and PET Characteristics
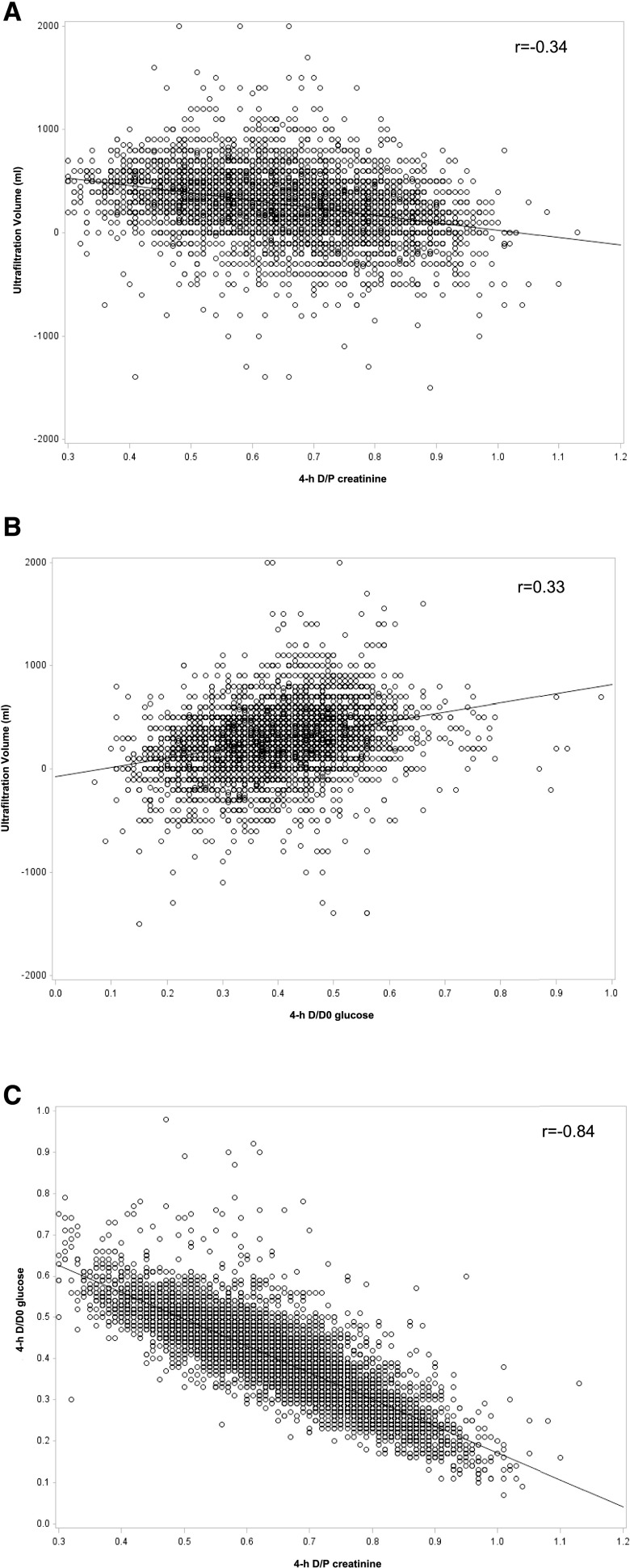
Of 162,664 patients who started maintenance dialysis between January 1, 2007 and December 31, 2011 and were treated in DaVita facilities, 18,277 patients underwent PD for at least 60 days. Of these, 10,142 individuals treated in 764 facilities had a PET with 2.5% dextrose within 6 months of the start of PD and comprised the analytic cohort (Figure 1). The summary statistics for data from the PET are summarized in Supplemental Table 2. The test was performed at a median of 39 days from the start of PD, and the mean D/P creatinine, D/D0 glucose, and UFV were 0.65±0.12, 0.40±0.09, and 281±254 ml, respectively. There was a strong inverse correlation between D/P creatinine and D/D0 glucose (r=−0.84; P<0.001) but a modest correlation between these parameters and UFV (D/P creatinine and UFV, r=−0.34; D/D0 glucose and UFV, r=0.33; both P<0.001) (Figure 2). Given the strong correlation between D/P creatinine and D/D0 glucose, the primary comparisons were limited to D/P creatinine and UFV.
Figure 2.
Scatterplots showing the association between the three peritoneal equilibration test (PET) parameters. (A) 4-hour dialysate to plasma ratio of creatinine (D/P creatinine) and ultrafiltration volume, (B) 4- to 0-hour dialysate glucose ratio (D/D0 glucose) and ultrafiltration volume, and (C) 4-hour D/P creatinine and 4-hour D/D0 glucose. The results were obtained from 10,142 individuals who underwent a peritoneal equilibration test with 2.5% dextrose within 6 months of start of treatment with peritoneal dialysis.
Demographic and Clinical Predictors of PET parameters
The differences in demographic, clinical, and laboratory parameters between slow, average, and fast groups defined by D/P creatinine or UFV are summarized in Tables 1 and 2, respectively. Patients with faster solute transfer rate were older; were more likely to be men; had prior treatment with hemodialysis; had diabetes, hypertension, or congestive heart failure; and had a lower residual kidney function. Put together, the demographic and clinical variables explained 8% and 3%, respectively, of the total variability in D/P creatinine and UFV. Faster solute transfer rate was also associated with lower serum albumin levels. However, there was no association between peritoneal solute transfer rate and the use of automated PD.
Table 1.
Patient characteristics stratified by 4-hour dialysate to plasma ratio of creatinine
| Variable | Low/Slow (n=1634) | Average (n=6954) | High/Fast (n=1555) | All (n=10,142) |
|---|---|---|---|---|
| D/P creatinine, mean [range] | 0.46±0.05 [0.30–0.52] | 0.65±0.07 [0.53–0.77] | 0.84±0.05 [0.78–1.13] | 0.65±0.12 [0.30–1.13] |
| Interval from start of PD to PET, da | 39 (27–59) | 39 (28–61) | 41 (28–65) | 39 (28–61) |
| Interval from first dialysis to PET, da | 83 (52–159) | 98 (57–194) | 118 (63–240) | 96 (56–188) |
| D/D0 glucose, mean [range] | 0.51±0.06 [0.3–0.98] | 0.40±0.07 [0.2–0.92] | 0.27±0.06 [0.07–0.6] | 0.40±0.09 [0.07–0.98] |
| 4-h Ultrafiltration volume, ml [range] | 405±240 [−1400–2000] | 285±240 [−1400–2000] | 137±257 [−1500–1100] | 281±254 [−1500–2000] |
| Age, yr | 54±16 | 56±15 | 55±16 | 56±15 |
| Sex, % men | 44 | 58 | 66 | 57 |
| Race, % | ||||
| White | 58 | 58 | 56 | 58 |
| Black | 22 | 23 | 24 | 23 |
| Hispanic | 13 | 12 | 13 | 12 |
| Others | 7 | 7 | 7 | 7 |
| Cause of ESRD, % | ||||
| Diabetes | 39 | 40 | 40 | 40 |
| Hypertension | 25 | 28 | 26 | 27 |
| Others | 36 | 32 | 34 | 33 |
| H/o previous transplant, % | 2 | 2 | 4 | 3 |
| H/o prior treatment with hemodialysis, % | 31 | 40 | 46 | 39 |
| Comorbidities, % | ||||
| Diabetes | 61 | 64 | 64 | 63 |
| Hypertension | 49 | 53 | 55 | 53 |
| Congestive heart failure | 17 | 20 | 24 | 20 |
| Atherosclerotic heart disease | 16 | 18 | 17 | 17 |
| Other cardiovascular | 13 | 15 | 15 | 15 |
| Dyslipidemia | 48 | 48 | 45 | 47 |
| Weekly Kt/Vureaa | ||||
| Peritoneal | 1.39 (1.14–1.68) | 1.47 (1.23–1.73) | 1.51 (1.27–1.78) | 1.46 (1.22–1.73) |
| Residual renal | 0.99 (0.54–1.65) | 0.86 (0.40–1.41) | 0.68 (0.26–1.24 | 0.86 (0.39–1.43) |
| Total | 2.43 (2.01–3.02) | 2.37 (1.90–2.90) | 2.27 (1.89–2.79) | 2.36 (1.95–2.90) |
| Residual kidney function, L/wk per 1.73 m2b | 76±54 | 64±49 | 52±47 | 64±50 |
| Median weekly ESA dose, unitsa | 4474 (1323–10,766) | 4830 (1435–11,157) | 5280 (1554–11,942) | 4864 (1430–11,190) |
| Laboratory parameters | ||||
| Hemoglobin, g/dl | 11.8±1.28 | 11.6±1.3 | 11.3±1.4 | 11.6±1.3 |
| Albumin, g/dl | 3.8±0.4 | 3.7±0.4 | 3.4±0.5 | 3.6±0.5 |
| Calcium, mg/dl | 9.2±0.6 | 9.1±0.6 | 9.0±0.7 | 9.1±0.6 |
| Phosphorus, mg/dl | 5.0±1.3 | 5.0±1.2 | 5.2±1.3 | 5.0±1.3 |
| Parathyroid hormone, pg/mla | 281 (176–455) | 291 (182–463) | 315 (192–516) | 292 (183–469) |
| Creatinine, mg/dl | 6.5±3.4 | 7.1±3.6 | 7.9±3.7 | 7.2±3.6 |
| Potassium, mEq/L | 4.2±0.5 | 4.2±0.5 | 4.2±0.5 | 4.2±0.5 |
| Use of APD, % | ||||
| Initial | 58 | 52 | 48 | 52 |
| Ever through follow-up | 87 | 88 | 87 | 88 |
| Geographic location, % | ||||
| Northeast | 9 | 8 | 8 | 8 |
| Midwest | 18 | 20 | 19 | 20 |
| West | 48 | 47 | 47 | 47 |
| South | 25 | 25 | 26 | 25 |
| Year of incidence, % | ||||
| 2007 | 14 | 16 | 20 | 17 |
| 2008 | 24 | 22 | 23 | 22 |
| 2009 | 22 | 25 | 23 | 24 |
| 2010 | 26 | 24 | 22 | 24 |
| 2011 | 14 | 13 | 12 | 13 |
| Outcomes | ||||
| Died, % | 10 | 12 | 13 | 12 |
| Transfer to in-center hemodialysis, % | 20 | 22 | 25 | 22 |
| No. of hospitalizations per patient-year | 1.06 | 1.18 | 1.47 | 1.20 |
| Transplant, % | 10 | 8 | 8 | 9 |
Data are presented as means ± SDs, except where indicated. D/P creatinine, 4-hour dialysate to plasma ratio of creatinine; PD, peritoneal dialysis; PET, peritoneal equilibration test; D/D0 glucose, 4- to 0-hour dialysate glucose ratio; H/o, history of; ESA, erythropoiesis stimulating agent; APD, automated peritoneal dialysis.
Data are presented as medians with interquartile ranges.
Mean of 24-hour urinary urea and creatinine clearance.
Table 2.
Patient characteristics stratified by 4-hour ultrafiltration volume with PET
| Variable | Low/Slow (n=1045) | Average (n=7328) | High/Fast (n=1500) | All (n=10,142) |
|---|---|---|---|---|
| 4-h Ultrafiltration volume, ml [range] | 710±182 [540–2000] | 300±132 [30–530] | −110±184 [−1500–25] | 281±254 [−1500–2000] |
| Interval from start of PD to PET, da | 41 (28–64) | 39 (28–60) | 40 (27–63) | 39 (28–61) |
| Interval from first dialysis to PET, da | 97 (57–194) | 97 (56–191) | 108 (59–209) | 96 (56–188) |
| D/P creatinine, mean | 0.58±0.11 | 0.64±0.12 | 0.72±0.12 | 0.65±0.12 |
| D/D0 glucose, mean | 0.45±0.09 | 0.40±0.09 | 0.35±0.10 | 0.40±0.09 |
| Age, yr | 53±15 | 56±15 | 58±15 | 56±15 |
| Sex, % men | 58 | 55 | 63 | 57 |
| Race, % | ||||
| White | 51 | 57 | 63 | 58 |
| Black | 29 | 23 | 19 | 23 |
| Hispanic | 13 | 13 | 11 | 12 |
| Others | 7 | 7 | 7 | 7 |
| Cause of ESRD, % | ||||
| Diabetes | 36 | 41 | 40 | 40 |
| Hypertension | 30 | 27 | 26 | 27 |
| Others | 34 | 32 | 34 | 33 |
| H/o previous transplant, % | 2 | 3 | 2 | 3 |
| H/o prior treatment with hemodialysis, % | 37 | 39 | 43 | 39 |
| Comorbidities, % | ||||
| Diabetes | 61 | 64 | 64 | 63 |
| Hypertension | 56 | 52 | 53 | 53 |
| Congestive heart failure | 22 | 19 | 21 | 20 |
| Atherosclerotic heart disease | 17 | 17 | 20 | 17 |
| Other cardiovascular | 16 | 15 | 16 | 15 |
| Dyslipidemia | 46 | 47 | 47 | 47 |
| Weekly Kt/Vureaa | ||||
| Peritoneal | 1.49 (1.21–1.77) | 1.47 (1.23–1.73) | 1.42 (1.19–1.70) | 1.46 (1.22–1.73) |
| Residual renal | 0.77 (0.35–1.33) | 0.86 (0.40–1.41) | 0.9 (0.42–1.55) | 0.86 (0.39–1.43) |
| Total | 2.29 (1.94–2.86) | 2.37 (1.95–2.90) | 2.37 (1.95–3.00) | 2.36 (1.95–2.90) |
| Residual kidney function, L/wk per 1.73 m2b | 61±50 | 64±50 | 67±52 | 64±50 |
| Median weekly ESA dose, unitsa | 4669 (1250–11,000) | 4918 (1466–11,440) | 4880 (1408–10,939) | 4864 (1430–11,190) |
| Laboratory parameters | ||||
| Hemoglobin, g/dl | 11.6±1.3 | 11.6±1.3 | 11.5±1.4 | 11.6±1.3 |
| Albumin, g/dl | 3.7±0.5 | 3.6±0.5 | 3.6±0.5 | 3.6±0.5 |
| Calcium, mg/dl | 9.1±0.6 | 9.1±0.6 | 9.0±0.7 | 9.1±0.6 |
| Phosphorus, mg/dl | 5.3±1.4 | 5.0±1.2 | 4.9±1.2 | 5.0±1.3 |
| Parathyroid hormone, pg/mla | 316 (194–499) | 291 (183–468) | 282 (175–453) | 292 (183–469) |
| Creatinine, mg/dl | 7.8±3.9 | 7.1±3.5 | 6.8±3.4 | 7.2±3.6 |
| Potassium, mEq/L | 4.2±0.5 | 4.2±0.5 | 4.2±0.6 | 4.2±0.5 |
| Use of APD, % | ||||
| Initial | 57 | 52 | 52 | 52 |
| Ever through follow-up | 87 | 88 | 87 | 88 |
| Geographic location, % | ||||
| Northeast | 10 | 8 | 8 | 8 |
| Midwest | 20 | 19 | 20 | 20 |
| West | 49 | 48 | 47 | 47 |
| South | 21 | 25 | 25 | 25 |
| Year of incidence, % | ||||
| 2007 | 18 | 16 | 17 | 17 |
| 2008 | 22 | 22 | 22 | 22 |
| 2009 | 23 | 24 | 26 | 24 |
| 2010 | 23 | 24 | 24 | 24 |
| 2011 | 14 | 14 | 11 | 13 |
| Outcomes | ||||
| Died, % | 9 | 12 | 13 | 12 |
| Transfer to in-center hemodialysis, % | 24 | 21 | 23 | 22 |
| No. of hospitalizations per patient-year | 1.29 | 1.19 | 1.22 | 1.20 |
| Transplant, % | 10 | 8 | 8 | 9 |
Data are presented as means ± SDs, except where indicated. PD, peritoneal dialysis; PET, peritoneal equilibration test; D/P creatinine, 4-hour dialysate to plasma ratio of creatinine; D/D0 glucose, 4- to 0-hour dialysate glucose ratio; H/o, history of; ESA, erythropoiesis stimulating agent; APD, automated peritoneal dialysis.
Data are presented as medians with interquartile ranges.
Mean of 24-hour urinary urea and creatinine clearance.
PET Parameters and Patient Outcomes
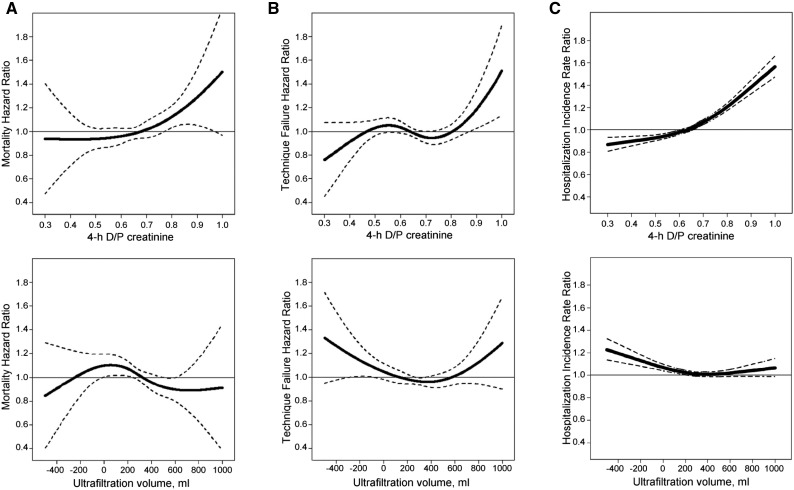
Over a median follow–up period of 15.8 months, 1178 individuals died, 2207 patients transferred to another dialysis modality, and 869 patients received a kidney transplant. While undergoing PD, patients were hospitalized at a rate of 1.20 per patient-year. There was a significant association of D/P creatinine with all-cause mortality and hospitalization rate in both unadjusted and adjusted models (adjusted hazards ratio for all-cause mortality per 0.1 unit higher, 1.07; 95% confidence interval, 1.02 to 1.13 and adjusted incidence rate ratio for hospitalization per 0.1 unit higher, 1.05; 95% confidence interval, 1.03 to 1.06) (Figure 3, Table 3). In contrast, UFV was significantly associated only with hospitalization rate and not all-cause mortality (Figure 3, Table 3). The same trends were evident in the sensitivity analyses, in which patients were ranked by the percentile rank for D/P creatinine and UFV (Supplemental Figures 1–3). Neither of the two parameters was associated with technique failure (Figure 3).
Figure 3.
Association of PET parameters with patient-centered outcomes. Restricted cubic splines illustrating the relationship between 4-hour dialysate to plasma ratio of creatinine (D/P creatinine) and ultrafiltration volume from the peritoneal equilibration test and (A) all-cause mortality, (B) technique failure, and (C) hospitalization rate. The bold line reflects the summary effect, and the dotted lines represent 95% confidence intervals.
Table 3.
Association of different peritoneal equilibration test parameters with clinical outcomes
| Outcome | 4-h D/P Creatinine per 0.1 Unit Higher | 4-h Ultrafiltration Volume per 250 ml Lower | ||
|---|---|---|---|---|
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| All–cause mortality HR (95% CI) | 1.13 (1.07 to 1.18) | 1.07 (1.02 to 1.13) | 1.14 (1.08 to 1.21) | 1.05 (0.99 to 1.11) |
| Technique failure HR (95% CI) | 1.02 (0.98 to 1.06) | 1.01 (0.98 to 1.05) | 1.03 (0.99 to 1.07) | 1.02 (0.98 to 1.07) |
| Hospitalization IRR (95% CI) | 1.09 (1.08 to 1.11) | 1.05 (1.03 to 1.06) | 1.02 (1.01 to 1.04) | 1.02 (1.00 to 1.04) |
D/P creatinine, dialysate to plasma ratio of creatinine; HR, hazard ratio; 95% CI, 95% confidence interval; IRR, incidence rate ratio; h/o, history of; APD, automated peritoneal dialysis.
Adjusted for demographics (age, sex, race, health insurance, geographic region, and year of incidence), case mix (cause of ESRD, h/o previous transplant, h/o previous treatment with hemodialysis, comorbidities, and residual kidney function), peritoneal dialysis–related variables (peritoneal Kt/Vurea and treatment with APD), and selected laboratory variables (hemoglobin, serum calcium, phosphorus, parathyroid hormone, creatinine, and potassium).
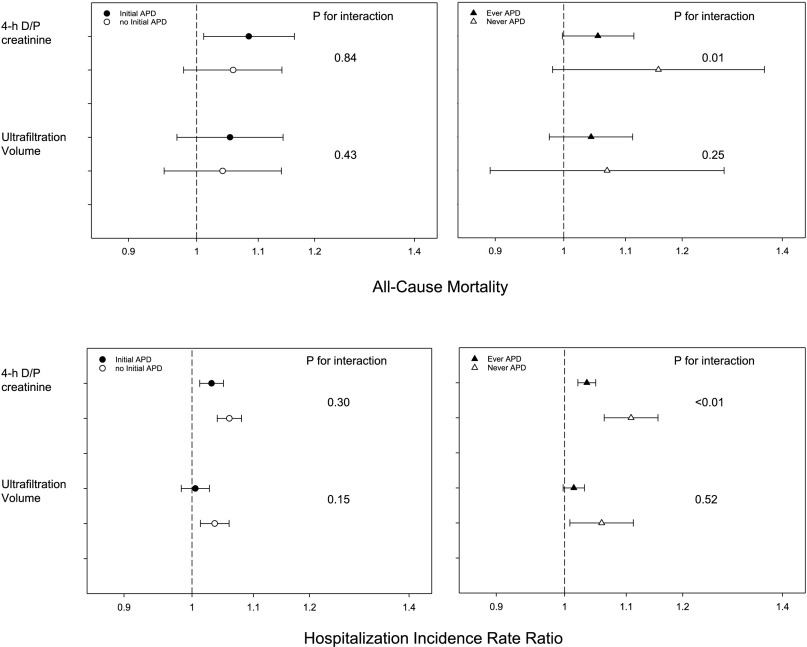
There was no significant effect modification with the cumulative center experience or initial treatment with APD (P value for interaction term >0.05). There was a significant interaction of treatment with APD at any time during follow-up with the association of D/P creatinine with mortality (P=0.01) and hospitalization rate (P<0.01) (Figure 4). This indicates that there was a significantly higher risk for death or hospitalization in patients with higher D/P creatinine who were treated with continuous ambulatory PD compared with those treated with automated PD at any time during follow-up. There was no significant interaction of this variable for the association of any parameter with technique failure.
Figure 4.
Difference in association of dialysate to plasma ratio of creatinine (D/P creatinine) and ultrafiltration volume from peritoneal equilibration test by use of automated PD. Association of 4-hour dialysate to plasma ratio of creatinine and ultrafiltration volume from the peritoneal equilibration test with all-cause mortality and hospitalization rate stratified by initial (left panels) or ever treatment (right panels) with automated peritoneal dialysis (APD). There was a significant interaction of treatment with APD at any time during follow-up with the association of D/P creatinine with mortality (P=0.01) and hospitalization rate (P<0.01 each). This indicates that there was a significantly higher risk for death or hospitalization in patients with higher D/P creatinine who were treated with continuous ambulatory PD compared with those treated with automated PD at any time during follow-up.
Comparison of Risk Prediction with Three PET Parameters
Using the Wald test, there was no significant difference in the hazards ratio or incidence rate ratio for the association of D/P creatinine, D/D0 glucose, or UFV with any outcome. With the adjusted survival model using D/P creatinine as the exposure as the base model, there was no improvement in risk prediction for all-cause mortality, technique failure, or hospitalization rate with the addition of D/D0 glucose, UFV, or both as tested using change in likelihood ratios (P values =0.64, 0.15, and 0.31, respectively).
Discussion
In this large and diverse cohort of patients undergoing PD, D/P creatinine was a robust predictor of all-cause mortality and hospitalization from among three PET parameters. To our knowledge, this study provides the first validation for the use of D/P creatinine to characterize the peritoneal barrier for risk prediction in individuals undergoing PD. Furthermore, unlike other studies, it shows that peritoneal solute transfer rate is consistently associated with all-cause mortality and hospitalization rate, even in patients undergoing automated PD.
It has long been recognized that there is a large interindividual variability in the rate of transfer of solutes and water across the peritoneal barrier and that, in some individuals, these characteristics change over time (1,11,19,20). The variability in peritoneal solute (or water) transfer rate is important for a variety of reasons and includes individualizing PD prescription to improve patient outcomes and enhancing our understanding of peritoneal biology and pathobiology. Hence, it is critically important to determine which measure of peritoneal solute or water transfer should be used in population-based studies of patients on PD, such as those examining genetic associations or practice patterns. In this context, this study compared risk prediction with three different parameters obtained from the PET with 2.5% dextrose, the most widely used test in clinical practice. There was no significant difference in the risk prediction with the three parameters in the primary analyses, but the association of D/P creatinine with all-cause mortality and hospitalization rate was the most consistent across different analyses. Furthermore, adding D/D0 glucose, UFV, or both did not improve risk prediction with D/P creatinine alone. These analyses provide the first validation for using D/P creatinine from the PET for risk prediction.
There are at least two potential reasons for the superiority of D/P creatinine for risk prediction. First, of the three parameters, D/P creatinine can be measured with the greatest precision, and UFV with PET is likely to have the greatest measurement error. The latter is, in part, because commercially available PD bags contain a larger volume of dialysate (overfill) than stated for flush before fill that is variable, which hence, contributes to the imprecision in measurement (21,22). Second, it is possible that the three PET parameters capture different biologic processes with different implications for patient health. It is important to acknowledge that, on the basis of the opinion of experts, the International Society for Peritoneal Dialysis recommends the use of 4.25% dextrose for the PET (2). It is possible that UFV obtained from a PET done using 4.25% dextrose may provide better risk prediction. However, these issues were not examined in this study, have not been tested thus far, and remain speculative.
In addition to validating the use of D/P creatinine, our study showed that D/P creatinine is associated with meaningful patient–centered outcomes (all-cause mortality and hospitalization rate), even in individuals undergoing automated PD. This is in contrast to, among others, a metaregression of published studies and the findings from the recently published Global Fluid Study (15,16). In a meta-analysis of 19 published studies, the summary mortality relative risk for each 0.1 higher D/P creatinine was 1.15, similar to the adjusted hazards ratio for this study. However, metaregression of published studies showed an inverse association between the mortality risk and the proportion of patients in the study undergoing automated PD (16). Indeed, there was no demonstrable association between D/P creatinine and mortality in studies that included primarily patients undergoing automated PD (16). Similarly, in the recently published Global Fluid Study (959 patients from 10 centers in the United Kingdom, South Korea, and Canada), there was demonstrable association between peritoneal solute transfer rate as measured by D/P creatinine and all-cause mortality only in prevalent patients; unlike this study, no such association was demonstrable in incident patients (15). The difference between our results and the results of these studies and others is most likely related to a substantially larger sample size in these analyses that provided us with much greater statistical power. In that the sample size of this study is over 10-fold larger than that of the Global Fluid Study, data were obtained from 764 centers, and all measurements were made in a single central laboratory, our study also has greater external validity.
Several different mechanisms have been proposed to explain the association of peritoneal solute transport rate with patient-centered outcomes. They include a higher prevalence of systemic inflammation, higher protein energy wasting, greater burden of comorbidity, or inadequate fluid removal with long dwells of continuous ambulatory PD in patients with faster peritoneal solute transfer rate (23). The evidence for these postulated mechanisms has been inconsistent, and the emerging consensus seems to support the centrality of inadequate fluid removal in individuals with faster peritoneal solute transfer rate treated with continuous ambulatory PD (4). This argument has been bolstered by studies that have not been able to show association of D/P creatinine with all-cause mortality in patients treated with automated PD. Although our study may seemingly question that premise, such a conclusion would be premature. There was a significant interaction between treatment with automated PD and the association of peritoneal solute transfer rate for some of the outcomes. This suggests that the higher risk for adverse outcomes with faster peritoneal solute transfer rate could be partially mitigated with automated PD. Moreover, there is heterogeneity in automated PD prescriptions, particularly with management of the long-day dwell (such as number of exchanges or use of icodextrin), which has not been considered in this study or others. During the period of study, only 5%–8% of patients undergoing PD were treated with icodextrin. Although this could explain the differences between the results of our study and those from the Global Fluid Study, it is highly unlikely to be the cause for the difference with results of studies before the availability of icodextrin.
Despite its considerable strengths, the results of our study need to be interpreted in light of its limitations. The study included limited data on clinical characteristics at the time of start of PD, and as in all observational studies, there remains risk for residual confounding. However, like previous studies, demographic and clinical variables explain only a very small part of the variability in peritoneal solute transport rate, and the risk for residual confounding is low (24). Data on measures of inflammation and measures of protein-energy wasting, such as subjective global assessment, were not available, and hence, we cannot exclude these mechanisms from the causal pathway for risk associated with faster peritoneal solute transfer rate. Finally, the analyses included data on D/P creatinine and UFV from the time of start of PD, because data for change in these parameters over time were not available for most patients.
In conclusion, data from a large contemporary cohort treated primarily with automated PD validate D/P creatinine as a robust predictor of outcomes in patients undergoing PD. These results make it imperative for us to better understand the biologic basis for interindividual variability and mitigate the change in peritoneal solute transport rate over time. Furthermore, it underscores the continued importance of identifying interventions to improve outcomes in patients on PD with faster peritoneal solute transport rate.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health: R01DK95668 (to R.M. and K.K.-Z.), R21AG047306 (to R.M., M.Z.M., and K.K.-Z.), and R01DK099165 (to R.M.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Predicting Risk in Peritoneal Dialysis: Is Membrane Biology Destiny?,” on pages 1895–1896.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03470315/-/DCSupplemental.
References
- 1.Twardowski ZJ, Nolph KD, Khanna R, Prowant BF, Ryan LR, Moore HL, Nielsen MP: Peritoneal equilibration test. Perit Dial Bull 7: 138–148, 1987 [Google Scholar]
- 2.Mujais S, Nolph K, Gokal R, Blake P, Burkart J, Coles G, Kawaguchi Y, Kawanishi H, Korbet S, Krediet R, Lindholm B, Oreopoulos D, Rippe B, Selgas R, International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis : Evaluation and management of ultrafiltration problems in peritoneal dialysis. Perit Dial Int 20[Suppl 4]: S5–S21, 2000 [PubMed] [Google Scholar]
- 3.Akonur A, Guest S, Sloand JA, Leypoldt JK: Automated peritoneal dialysis prescriptions for enhancing sodium and fluid removal: A predictive analysis of optimized, patient-specific dwell times for the day period. Perit Dial Int 33: 646–654, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies SJ: Mitigating peritoneal membrane characteristics in modern peritoneal dialysis therapy. Kidney Int Suppl 103: S76–S83, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Churchill DN, Thorpe KE, Nolph KD, Keshaviah PR, Oreopoulos DG, Pagé D, The Canada-USA (CANUSA) Peritoneal Dialysis Study Group : Increased peritoneal membrane transport is associated with decreased patient and technique survival for continuous peritoneal dialysis patients. J Am Soc Nephrol 9: 1285–1292, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Davies SJ, Phillips L, Griffiths AM, Russell LH, Naish PF, Russell GI: What really happens to people on long-term peritoneal dialysis? Kidney Int 54: 2207–2217, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Szeto CC, Law MC, Wong TY, Leung CB, Li PK: Peritoneal transport status correlates with morbidity but not longitudinal change of nutritional status of continuous ambulatory peritoneal dialysis patients: A 2-year prospective study. Am J Kidney Dis 37: 329–336, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Bhaskaran S, Schaubel DE, Jassal SV, Thodis E, Singhal MK, Bargman JM, Vas SI, Oreopoulos DG: The effect of small solute clearances on survival of anuric peritoneal dialysis patients. Perit Dial Int 20: 181–187, 2000 [PubMed] [Google Scholar]
- 9.Brown EA, Davies SJ, Rutherford P, Meeus F, Borras M, Riegel W, Divino Filho JC, Vonesh E, van Bree M, EAPOS Group : Survival of functionally anuric patients on automated peritoneal dialysis: The European APD Outcome Study. J Am Soc Nephrol 14: 2948–2957, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Rumpsfeld M, McDonald SP, Johnson DW: Higher peritoneal transport status is associated with higher mortality and technique failure in the Australian and New Zealand peritoneal dialysis patient populations. J Am Soc Nephrol 17: 271–278, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Davies SJ: Monitoring of long-term peritoneal membrane function. Perit Dial Int 21: 225–230, 2001 [PubMed] [Google Scholar]
- 12.Wang T, Heimbürger O, Waniewski J, Bergström J, Lindholm B: Increased peritoneal permeability is associated with decreased fluid and small-solute removal and higher mortality in CAPD patients. Nephrol Dial Transplant 13: 1242–1249, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Mujais S, Vonesh E: Profiling of peritoneal ultrafiltration. Kidney Int Suppl 81: S17–S22, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Chang TI, Park JT, Lee DH, Lee JH, Yoo TH, Kim BS, Kang SW, Lee HY, Choi KH: High peritoneal transport status is not an independent risk factor for high mortality in patients treated with automated peritoneal dialysis. J Korean Med Sci 25: 1313–1317, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambie M, Chess J, Donovan KL, Kim YL, Do JY, Lee HB, Noh H, Williams PF, Williams AJ, Davison S, Dorval M, Summers A, Williams JD, Bankart J, Davies SJ, Topley N, Global Fluid Study Investigators : Independent effects of systemic and peritoneal inflammation on peritoneal dialysis survival. J Am Soc Nephrol 24: 2071–2080, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brimble KS, Walker M, Margetts PJ, Kundhal KK, Rabbat CG: Meta-analysis: Peritoneal membrane transport, mortality, and technique failure in peritoneal dialysis. J Am Soc Nephrol 17: 2591–2598, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Rubin DB: Multiple Imputation for Non-Response in Surveys, New York, John Wiley and Sons, 2004 [Google Scholar]
- 18.Lin DY, Fleming TR, De Gruttola V: Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med 16: 1515–1527, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Davies SJ: Longitudinal relationship between solute transport and ultrafiltration capacity in peritoneal dialysis patients. Kidney Int 66: 2437–2445, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Davies SJ, Brown EA, Frandsen NE, Rodrigues AS, Rodriguez-Carmona A, Vychytil A, Macnamara E, Ekstrand A, Tranaeus A, Filho JC, EAPOS Group : Longitudinal membrane function in functionally anuric patients treated with APD: Data from EAPOS on the effects of glucose and icodextrin prescription. Kidney Int 67: 1609–1615, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Davies SJ: Overfill or ultrafiltration? We need to be clear. Perit Dial Int 26: 449–451, 2006 [PubMed] [Google Scholar]
- 22.La Milia V, Pozzoni P, Crepaldi M, Locatelli F: Overfill of peritoneal dialysis bags as a cause of underestimation of ultrafiltration failure. Perit Dial Int 26: 503–505, 2006 [PubMed] [Google Scholar]
- 23.Pecoits-Filho R, Araújo MR, Lindholm B, Stenvinkel P, Abensur H, Romão JE, Jr., Marcondes M, De Oliveira AH, Noronha IL: Plasma and dialysate IL-6 and VEGF concentrations are associated with high peritoneal solute transport rate. Nephrol Dial Transplant 17: 1480–1486, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Rumpsfeld M, McDonald SP, Purdie DM, Collins J, Johnson DW: Predictors of baseline peritoneal transport status in Australian and New Zealand peritoneal dialysis patients. Am J Kidney Dis 43: 492–501, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.