Abstract
Background and objectives
Endovascular repair (EVAR) is a common treatment for abdominal aortic aneurysm (AAA). However, its long-term effects on renal function remain unclear. We aimed to assess long-term renal dysfunction after EVAR using a contemporary estimate of GFR and to compare long-term renal outcomes in patients after EVAR with open aneurysm repair (OAR) and in patients without an AAA.
Design, settings, participants, & measurements
We performed a nested case-matched analysis of 726 patients (using a prospectively maintained database for repairs that took place between January 2000 and May 2010 in a tertiary center): 121 patients undergoing OAR (with data at baseline and 5 years postrepair) were case matched (age, sex, smoking, diabetes, baseline eGFR) to patients undergoing suprarenal and infrarenal fixation EVAR (242 in each group) and to 121 patients undergoing carotid endarterectomy (CEA) without AAA. Changes in eGFR were compared (1 and 5 years).
Results
The OAR patients lost an average of 7.4 ml/min per 1.73 m2 at 5 years (95% confidence interval [95% CI], 4.8 to 10.6), compared with 8.2 ml/min per 1.73 m2 (95% CI, 6.5 to 10.8; P<0.001) for infrarenal-fixation EVAR, 16.9 ml/min per 1.73 m2 (95% CI, 13.0 to 21.9, P<0.001) for suprarenal-fixation EVAR, and 5.4 ml/min per 1.73 m2 (95% CI, 1.7 to 7.5; P<0.001) for CEA. The decrease in eGFR was steeper during the first postoperative year, with each group losing −2.2 ml/min per 1.73 m2 (infrarenal-fixation EVAR), −10.7 ml/min per 1.73 m2 (suprarenal-fixation EVAR), and −4.6 ml/min per 1.73 m2 (OAR), compared with −1.9 ml/min per 1.73 m2 for CEA.
Conclusions
Elective EVAR is associated with a significant decline in eGFR after 5 years, which is steeper in the first postoperative year and more pronounced compared with a similar population with atherosclerotic disease.
Keywords: chronic kidney disease; cardiovascular disease; endovascular aneurysm repair; renal injury; aortic aneurysm, abdominal; endarterectomy, carotid; endovascular procedures; glomerular filtration rate; humans
Introduction
Abdominal aortic aneurysm (AAA) constitutes an important cause of cardiovascular death (1,2). Endovascular repair (EVAR) is considered the first-line treatment modality, because medium-term morbidity and mortality have proven superior to open aneurysm repair (OAR) (3–5). Both OAR and EVAR may adversely affect renal function, in the short and medium term, as we have previously suggested (6). Hypovolemia and clamping of the aorta are traditionally perceived as the most likely main renal insults during OAR. Renal damage mechanisms in EVAR may be more complex and theoretically consist of contrast-nephropathy, renal microembolization, ischemia-reperfusion injury, and postoperative contrast-injury as a result of reintervention and diagnostic imaging (7). The exact pathophysiologic mechanisms remain understudied. We recently demonstrated that patients undergoing elective EVAR are at significant risk of developing AKI, with a prevalence of 19% (8). A meta-analysis showed that 18% of patients undergoing EVAR will have a clinically significant increase in their serum creatinine (SCr) after 1 year (9). Previous studies have also demonstrated a greater decrease in eGFR for patients who undergo EVAR for the first year compared with the general population (6,10). EVAR is now offered electively in younger patients and it is therefore important to be aware of the long-term renal implications. However, there is a paucity of data regarding long-term renal injury after EVAR. The majority of the literature does not extend beyond the first postoperative year and consists of retrospective studies reporting SCr. The latter will deviate from normal values only after at least one-half of the functioning renal mass has been affected (11). Two studies offer sufficiently long follow-up but only included a small number of patients after the first 2 years (12,13) and did not differentiate between suprarenal and infrarenal device fixation during EVAR; suprarenal fixation may adversely affect renal function (10). In addition, there are no high-quality comparisons between EVAR, OAR, and similar populations without aneurysmal disease. As a result, the long-term renal implications of EVAR remain unclear.
Thus, this study aimed to assess the changes in kidney function after elective AAA repair (EVAR and OAR) at 1 and 5 years and to compare these to a similar population without aneurysmal disease. A nested case-matched analysis was performed including patients undergoing OAR, patients underdoing EVAR with suprarenal or infrarenal fixation, and patients undergoing carotid surgery. The main outcome measure was change in eGFR derived from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (11,14).
Materials and Methods
Study Design and Population
Patients undergoing repair of an infrarenal AAA between January 2000 and May 2010 were included, from a single tertiary referral center, using a prospectively maintained database. Patients underwent repair if they had an AAA diameter >5.5 cm or an AAA <5.5 cm with a rapidly increasing sac (>1 cm per year). EVAR was offered as the first-line procedure, unless the patient opted for open repair after consultation. Open repair may be considered by younger patients because it is associated with less reintervention and may be a more durable method, not requiring follow-up with serial imaging. Regarding EVAR devices, devices with suprarenal or infrarenal fixation were used, adhering to the manufacturer’s anatomic instructions for use. Suprarenal fixation devices may provide better proximal fixation (15) and were therefore chosen in patients with shorter proximal necks. All AAAs were infrarenal without excessive thrombus, defined as per reporting criteria by Chaikof et al. (16), at the proximal neck. Patients with symptomatic, leaking, ruptured, infected, inflammatory aneurysms or already receiving RRT were excluded. To compare 5-year changes in eGFR between EVAR (suprarenal and infrarenal fixation analyzed separately), OAR, and patients with carotid disease who underwent carotid-endarterectomy (CEA) and had no history of AAA, a cohort of OAR patients with available GFR estimates and follow-up data was initially identified (overall 121 patients with 5-year data available) using the departmental OAR database, composed of 248 individuals. These individuals were then case matched (for age within 2 years, sex, smoking habits, diabetes, and baseline eGFR) with patients undergoing EVAR (suprarenal and infrarenal fixation EVAR matched separately; 1:2 ratio) and patients undergoing CEA. EVAR patients were derived from a prospectively maintained departmental database of 1183 individuals; 5-year data were available for 624 patients (281 deaths and 278 patients lost to follow-up).
Study and Follow-Up Protocol
Baseline demographics and comorbidities were stored electronically. All patients with an AAA underwent a computed tomographic angiography (CTA) with reconstruction at baseline, performed at least 2 weeks before the operation. Patients with an eGFR <60 ml/min per 1.73 m2 received hydration (1 L Hartmann’s solution in 12 hours) before the CTA, both at baseline and throughout follow-up. Blood samples were obtained before imaging and immediately before surgery. Routine follow-up visits took place at 30 days, at 3, 6, and 12 months after the operation, and annually thereafter. Imaging at these intervals included plain abdominal radiography and a CTA at 3, 6, and 12 months and annually thereafter. This imaging protocol was used until 2011, when ultrasonography-based follow-up replaced computed tomography. After that time, angiography, either in the form of computed tomography or conventional angiogram, was reserved for patients with a possible endoleak. For those undergoing OAR, no follow-up cross-sectional imaging was obtained routinely. None of the patients with carotid disease were found to have an AAA. Written informed consent was obtained for the repairs and participation in this study; institutional guidance for ethical approval was adhered to as per the Declaration of Helsinki.
Procedures
The following EVAR stent grafts were used: Anaconda (Vascutek, Scotland, UK), Gore Excluder (Gore, Flagstaff, AZ), Endurant (Medtronic, Minneapolis, MN), and Zenith (Cook Medical, Bloomington, IN). Indications and specifications are described elsewhere (15,17–20). Procedures were performed in a surgical operating theater under general anesthesia via femoral-artery exposure with low-osmolality nonionic contrast. Nonsteroidal anti-inflammatory drugs were avoided and nephrotoxic medications were withheld for at least 24 hours before and after the procedure. Those undergoing EVAR or OAR with a preoperative eGFR <60 ml/min per 1.73 m2 were admitted 1 day before and received intravenous fluids (Hartmann’s solution, 1.5 L/24 hours) for 24 hours, until oral foods and fluids were withheld, when intravenous fluids were given (2 ml/kg per hour). Urinary catheterization and hourly urine output measurements were used. Aspirin (75 mg) was administered on the day of the procedure and was continued as a lifelong treatment unless it was not tolerated, in which case, clopidogrel (75 mg) was used (21). A statin was prescribed as lifelong treatment. The patient was ambulated as soon as possible and was usually discharged the second day.
For patients undergoing OAR, the procedure was performed in an operating theater under general anesthesia. A Dacron prosthetic graft was used in standard fashion. None of the patients required suprarenal clamping. Patients remained in an intensive care unit for at least 24 hours. Urinary catheterization and hourly urine output measurements were routinely utilized. All patients were already taking aspirin (75 mg) and a statin preoperatively, which was continued lifelong.
Carotid endarterectomy was performed in an operating theater under general anesthesia, as previously reported (22). Of note, all patients had presented with anterior circulation symptoms and an ipsilateral internal carotid artery stenosis exceeding 50%. Shunting was routinely utilized. All patients received intravenous heparin (40 U/kg) before cross-clamping. A bovine arterial patch was used. All patients were already taking aspirin (75 mg) and a statin preoperatively, which was continued lifelong.
Outcome Measures
The primary outcome measure was change in eGFR (CKD-EPI equation) at 5 years compared with baseline. Secondary study measures included change in eGFR at 1 year and stages of CKD at 1 and 5 years. The Modification of Diet in Renal Disease (MDRD) eGFR equation (23) was also used to report on renal outcomes at baseline and 5 years (however, the primary outcome was based on CKD-EPI calculations).
Definitions
Complications were defined according to the reporting standards for EVAR by Chaikof et al. (16). The Kidney Disease Outcomes Quality Initiative clinical practice guidelines for evaluation, classification, and stratification of CKD (24) were used to classify stages of renal function. The diagnosis of AKI was defined as an increase in SCr ≥0.30 mg/dl, or >1.5-fold from baseline, within 48 hours, per the biochemical Acute Kidney Injury Network guidance (25).
Statistical Analyses
Analyses were performed using SPSS 21.0 software (SPSS, Chicago, IL). Continuous parametric data are presented as means ± SDs or 95% confidence intervals (95% CIs) and categorical data are presented as percentages. Normality of distributions was confirmed using the Kolmogorov–Smirnov test, in conjunction with an assessment of the distribution based on skewness and SD. eGFRs were compared using ANOVA. Further comparisons between groups were made using unpaired or paired samples t tests, where applicable. The chi-squared test was used to compare categorical variables. Multivariate linear regression was applied to assess the effect of baseline eGFR, AKI, and other parameters that differed (cardiovascular disease, use of statins, use of angiotensin enzyme inhibitors) on changes in eGFR (Table 1). A P value of <0.05 was considered significant.
Table 1.
Characteristics of the case-matched groups at baseline
| Characteristic | Infrarenal EVAR | Suprarenal EVAR | OAR | CEA | P Value |
|---|---|---|---|---|---|
| Women | 8.3 | 8.3 | 8.3 | 8.3 | NA |
| Age (yr) | 69±3 | 71±8 | 68±6 | 73±2 | 0.03 |
| AAA diameter (cm) | 6.2±1.1 | 6.7±1.9 | 6.6±1.0 | NA | 0.09 |
| Proximal neck (mm) | |||||
| Length | 21±3 | 18±4 | 16±6 | NA | 0.27 |
| Neck | 23±2 | 21±3 | 24±3 | NA | 0.09 |
| Hypertension | 65 | 65 | 65 | 65 | NA |
| Current smoker | 24 | 24 | 24 | 24 | NA |
| Ex-smoker | 53 | 53 | 53 | 53 | NA |
| Cholesterolemia | 29 | 29 | 29 | 29 | NA |
| Statin use | 68 | 54 | 71 | 79 | <0.001 |
| ACEi use | 32 | 24 | 22 | 44 | <0.001 |
| Previous MI | 22 | 19 | 11 | 34 | <0.001 |
| Lower limb PAD | 12 | 19 | 11 | 7 | <0.001 |
| Diabetes | 14 | 14 | 14 | 14 | NA |
| Previous stroke or TIA | 7 | 10 | 4 | 100 | <0.001 |
| SCr (mg/dl) | 0.98 (0.82 to 1.04) | 1.03 (0.88 to 1.10) | 1.05 (0.90 to 1.14) | 0.94 (0.81 to 1.02) | 0.01 |
| eGFR (ml/min per 1.73 m2) | |||||
| CKD-EPI equation | 77.4 (75.6 to 79.7) | 73.4 (72.8 to 75.7) | 73.0 (71.4 to 75.6) | 79.3 (77.0 to 80.5) | 0.01 |
| MDRD equation | 76.2 (74.6 to 79.1) | 71.2 (69.8 to 74.1) | 70.1 (68.3 to 73.2) | 79.6 (77.3 to 79.9) | 0.03 |
| Contrast (ml) | 118±21 | 121±32 | NA | NA | 0.63 |
Data are presented as percentages, means ± SDs, or values with 95% confidence intervals. EVAR, endovascular aneurysm repair; OAR, open aneurysm repair; CEA, carotid endarterectomy (all patients were symptomatic); NA, not applicable; AAA, abdominal aortic aneurysm; ACEi, angiotensin-converting enzyme inhibitor; MI, myocardial infarction; PAD, peripheral artery disease; TIA, transient ischemic attack; SCr, serum creatinine; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; MDRD, Modification of Diet in Renal Disease.
Results
Baseline and Procedural Characteristics
A total of 121 patients undergoing elective OAR (10 women (8%); mean age 72±6 years) with available eGFR estimates at 5 years were identified and case matched with 242 suprarenal and 242 infrarenal fixation EVAR patients for age, sex, smoking habits, diabetes, and eGFR at baseline (1:2 ratio) as well as 121 patients undergoing CEA. None of the EVARs were immediately converted to OAR; all aneurysms were successfully excluded. Table 1 summarizes baseline characteristics. Mean follow-up was 68±8 months, 79±14 months, and 79±11 months for the OAR, EVAR, and CEA cohorts, respectively. None of the EVAR patients developed renal artery occlusion during follow-up. Data regarding significant events during follow-up are summarized in Table 2. None of the patients underwent major urinary tract intervention.
Table 2.
Events of interest and renal outcomes during follow-up for the case-matched groups
| Event | Infrarenal EVAR | Suprarenal EVAR | OAR | CEA | P Value |
|---|---|---|---|---|---|
| AKI | 11 | 13 | 9 | — | 0.57 |
| Endoleak | |||||
| Type 1 | 4 | 5 | — | — | — |
| Type 2 | 11 | 11 | — | — | — |
| Endovascular reintervention | 9 | 8 | 1 | 0 | <0.001 |
| Nonfatal MI | 7 | 5 | 14 | 8 | 0.01 |
| Nonfatal stroke or TIA | 1.7 | 2.3 | 1 | 3.2 | 0.08 |
| Nonfatal peripheral vascular complication | 4.8 | 3.2 | 1 | 8.7 | <0.001 |
| Renal artery occlusion | 0 | 0 | 0 | N/A | — |
| Median number of CTA scans during follow-up | 7 (2–14) | 6 (1–11) | 0 (0–3) | 0 (0–2) | <0.001 |
| eGFR decrease (ml/min per 1.73 m2) | |||||
| At 1 yr (CKD-EPI equation) | −2.2 (0.8 to 3.5) | −10.7 (4.4 to 14.3) | −4.6 (2.8 to 7.2) | −1.9 (0.9 to 2.4) | <0.001 |
| At 5 yr (CKD-EPI equation) | −8.2 (6.6 to 11.8) | −16.9 (13.0 to 21.9) | −7.4 (4.9 to 10.6) | −5.4 (1.8 to 8.5) | <0.001 |
| At 5 yr (MDRD equation) | −4.9 (3.0 to 7.2) | −10.8 (8.2 to 14.9) | −3.5 (2.3 to 6.8) | −4.4 (1.6 to 7.2) | <0.001 |
| >30% eGFR decrease (ml/min per 1.73 m2) | |||||
| At 1 yr | 19 | 27 | 22 | 4 | <0.001 |
| At 5 yr | 32 | 47 | 33 | 14 | <0.001 |
Data are presented as percentages, medians with ranges, or values with 95% confidence intervals. EVAR, endovascular aneurysm repair; OAR, open aneurysm repair; CEA, carotid endarterectomy (all patients were symptomatic); MI, myocardial infarction; TIA, transient ischemic attack; CTA, computed tomographic angiography; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; MDRD, Modification of Diet in Renal Disease.
Renal Outcomes
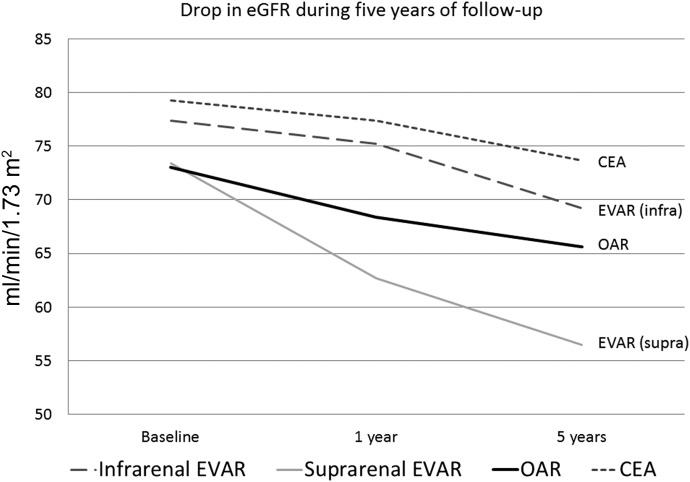
The OAR patients lost an average of 7.4 ml/min per 1.73 m2 at 5 years (95% CI, 4.8–10.6)], compared with 8.2 ml/min/1.73m^2 (95% CI: 6.5–10.8,p<0.001) for infrarenal-fixation EVAR, 16.9 ml/min per 1.73 m2 (95% CI: 13.0–21.9, P<0.001) for suprarenal-fixation EVAR and 5.4 ml/min per 1.73 m2 (95% CI: 1.7–7.5, P<0.001) for those undergoing CEA (Figure 1, Tables 2 and 3). No patient in the case-matched groups progressed to end stage renal failure requiring dialysis at 5 years. The aneurysm repair groups had a steeper drop in renal function during the first year, with −2.2 ml/min per 1.73 m2 for infrarenal fixation EVAR, −10.7 ml/min per 1.73 m2 for suprarenal fixation EVAR, and −4.6 ml/min per 1.73 m2 for OAR, compared with −1.9 ml/min per 1.73 m2 for the CEA patients. The suprarenal fixation EVAR patients were significantly more likely to have their eGFR decrease by >30% at 1 and 5 years compared with the other populations (27% and 47%, respectively; P<0.001). Multivariate linear regression assessed use of angiotensin-converting enzyme inhibitors (β coefficient, 0.11; 95% CI, −0.57 to 11.10; P=0.10), development of AKI (β coefficient, 0.04; 95% CI, −4.25 to 8.54; P=0.06), history of cardiovascular disease (β coefficient, 0.01; 95% CI, −3.52 to 4.43; P=0.52), and baseline eGFR (β coefficient, −0.13; 95% CI, −0.29 to −0.01; P=0.03). The regression results confirmed that treatment group was significantly associated with change in eGFR at 5 years, after adjusting for other covariates (β coefficient, 0.12; 95% CI, 0.03 to 2.75; P=0.04) (Table 4).
Figure 1.
Change in average eGFR (CKD-EPI equation) after 5 years for the case-matched groups. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; EVAR, endovascular repair; OAR, open aneurism repair.
Table 3.
Changes in eGFR for CKD stages 3 and 4 (at baseline) compared with patients with an eGFR of ≥60 ml/min per 1.73 m2 (at baseline)
| Group | 1 yr | 5 yr | ||
|---|---|---|---|---|
| eGFR ≥60 ml/min per 1.73 m2 | CKD Stage ≥3 | eGFR ≥60 ml/min per 1.73 m2 | CKD Stage ≥3 | |
| Suprarenal endovascular aneurysm repair | −10.2 | −11.8 | −15.7 | −18.8 |
| Infrarenal endovascular aneurysm repair | −1.2 | −5.4 | −5.1 | −14.4 |
| Open aneurysm repair | −4.3 | −5.5 | −6.2 | −9.8 |
| Carotid endarterectomy | −1.4 | −3.2 | −4.5 | −7.4 |
Table 4.
Multivariate regression to assess the effect of various factors on change in eGFR at 5 years
| Parameter | Standardized Regression Coefficient (95% CI) | P Value |
|---|---|---|
| ACEi use | 0.11 (−0.57 to 11.10) | 0.10 |
| AKI | 0.04 (−4.26 to 8.54) | 0.06 |
| Cardiovascular disease | 0.01 (−3.52 to 4.43) | 0.52 |
| eGFR baseline | −0.13 (−0.29 to −0.01) | 0.03 |
| Type of surgery | 0.12 (0.03 to 2.75) | 0.04 |
Regression coefficients represent the change in eGFR (ml/min per 1.73 m2) by units of change in the relevant risk factor. ACEi, angiotensin enzyme inhibitor; 95% CI, 95% confidence interval.
Discussion
This analysis used a precise estimate of GFR (11,26) to assess effects of AAA repair on renal function. To the authors’ knowledge, this represents the first attempt to compare long-term renal function after AAA repair (EVAR or OAR) with a population without aneurysmal disease. Elective AAA repair, especially EVAR, seems to be associated with a significant decline in renal function at 5 years.
Epidemiologic data from the National Health and Nutrition Examination Survey (27) suggest that for a population aged >70 years, an average of 24.6% of patients would have an eGFR between 30 and 59 ml/min per 1.73 m2 (stage 3 CKD) and an annual decrease of approximately 1% would be considered average. The maximum decrease in our analysis (suprarenal fixation EVAR) was 17 ml/min per 1.73 m2 in 5 years. The decline in the EVAR population exceeded that seen in the National Health and Nutrition Examination Survey and needs to be taken into account when offering EVAR to young patients, because it is established that renal impairment leads to increased morbidity (26,28). As a result, renoprotection should be addressed more aggressively in patients undergoing AAA repair.
Whether the procedure or other patient-related factors is the main driver behind the renal decline is hard to prove, given the lack of mechanistic evidence.
Mechanisms leading to renal damage in EVAR may include the following: contrast administration, because contrast (29) leads to decreased local PG and nitric oxide–mediated vasodilation, a direct toxic effect on tubular cells by oxygen free radicals; increased oxygen consumption, intratubular pressure, and urinary viscosity, and tubular obstruction, culminating in medullary ischemia; renal microembolization during device deployment (30), leading to localized ischemia of the renal parenchyma; possible renal artery occlusion (31); lower limb ischemia and subsequent ischemia-reperfusion (32); hypovolemia; the presence of an inflammatory infiltrate, the aneurysmal sac, that is not excised such as in OAR (33); and the presence of premorbid risk factors (34).
In this study, the loss of renal function was steeper during the first year after EVAR. After that time, decline was more in line with that of the general population (26). Our recent analysis assessing AKI after EVAR showed an incidence of 19% and a meta-analysis of 1813 elective EVARs showed that 18% of patients will develop an increase in SCr >30% compared with baseline at 1 year (9). It would therefore be rational to suggest that some form of renoprotection should be routinely utilized perioperatively in EVAR; however, current practice varies significantly (7). Although we did not detect a direct association between AKI development and eGFR change at 5 years in this study, our analysis was not powered to detect this. Regarding the type of renoprotection, existing strategies that are applicable to other interventions cannot be fully extrapolated because of the complex pathophysiology of EVAR-associated renal damage. Strategies that have been studied in EVAR include various hydration regimes (35), ischemic preconditioning (36), regional anesthesia (37,38), some pharmacologic agents (7), and targeted renal therapy (39). These have been predominantly assessed in small underpowered studies. Better adequately powered randomized studies investigating renoprotection specifically in EVAR are therefore required. Until then, hydration remains the mainstay of kidney damage prevention, because it is safe and alleviates the effects of contrast, hypovolemia, and ischemia-reperfusion injury (7). Bicarbonate administration, which prevents contrast-associated and inflammatory tubular ischemia, has been shown to be beneficial in coronary intervention AKI (40), but evidence in EVAR is lacking.
Regarding long-term renal dysfunction, two previous subanalyses of randomized EVAR trials have attempted to analyze long-term renal effects (12,13). Data from the UK Endovascular Aneurysm Repair (EVAR-1 and EVAR-2) trials were used to calculate eGFR after a mean of 3.6 years (12). The average annual decrease was 1.13±1.43 ml/min per 1.73 m2. However, the EVAR-1 and EVAR-2 trials used the MDRD equation (23), which is known to underestimate renal function in groups with cardiovascular morbidity (13,41). In addition, no distinction was made between infrarenal and suprarenal fixation devices and patients were treated with older-generation devices. A similar decrease was documented in a subanalysis of 95 patients from the Dutch Randomized Endovascular Aneurysm Management (DREAM) trial with available eGFRs (CKD-EPI equation) at 5 years (13); patients lost 0.9±3.9 ml/min per 1.73 m2 annually and 35% were at CKD stage 3 at 5 years. These changes did not reach significance and there was no division between suprarenal and infrarenal fixation. Our EVAR cohort intratubularhad a mean eGFR close to 70 ml/min per 1.73 m2, compared with 80±7.6 ml/min per 1.73 m2 for DREAM participants, which may explain this discrepancy. The number of patients in the DREAM study surviving at 5 years was also small; therefore selection bias could have been introduced.
An interesting observation from our series is the significant effect of suprarenal fixation on eGFR change. Suprarenal fixation may lead to microembolization, both during deployment, especially if the neck is calcified and/or has excessive thrombus, or over the long term (42). In a previous EVAR study with CTA follow-up, 5.9% of patients had bilateral microembolic cortical defects and those with more AAA thrombus were more likely to have microemboli (30). In this series, we have not specifically looked at perfusion defects and our CTAs have not been timed in a way that would allow such an assessment. We cannot, therefore, report on subclinical microembolization. A recent meta-analysis has not shown any significant differences compared with infrarenal fixation (43); however, significant heterogeneity was found among the retrospective reports included and renal dysfunction measures were not uniform. Overall, 6.4% of patients with a suprarenal fixation device had cortical renal infarcts in this review. Suprarenal fixation does confer more aortic fixation force (15), and more prospective data together with pathophysiologic analyses are required to define its effect on renal parenchyma before coming to definite conclusions.
The drop in renal function seen in this population undergoing aneurysm repair has multiple implications. Younger patients are now offered EVAR; hence, long-term renal dysfunction becomes an issue. Current EVAR follow-up protocols do not include any renal outcome measurements and this may need to be changed. The use of renoprotective medication, such as statins and angiotensin-converting enzyme inhibitors (44), may also need to become more liberal. Overall, loss of renal function is known to affect cardiovascular outcomes (45); hence, we consider it of major clinical relevance in this population with a high incidence of cardiovascular disease.
Our study has some limitations that must be mentioned. First, this is a retrospective analysis. Another limitation is the lack of information on change in body weight over time, which could have affected GFR estimation. The CKD-EPI equation does not include weight as a parameter; however, the equation uses SCr, which is directly affected by body mass. This could have been addressed by measuring sensitive subclinical markers of kidney damage such as cystatin-C; however, this is costly and not routinely utilized in clinical practice. In addition, we did not use urine output measurements when reporting AKI because they were not available; hence, AKI incidence may be under-reported in this study. Our study was not adequately powered to detect an association between AKI and eGFR change at 5 years. Patients receiving infrarenal or suprarenal fixation devices have not been randomly allocated into groups, which may introduce selection bias; it would be unethical to randomize individuals because device selection depends on anatomy. Finally, patients undergoing EVAR did not have challenging proximal neck anatomy. Certain centers perform infrarenal EVAR in patients with adverse neck anatomy. The results of this study cannot be extrapolated for this population.
Overall, we documented that EVAR is associated with a significant long-term decline in renal function. This is more pronounced compared with a similar population of arteriopathy and is also steeper during the first postoperative year. The latter provides a window of opportunity to apply more aggressive perioperative renoprotective strategies, which need to be assessed in well designed studies.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Long–Term Renal Function after Abdominal Aortic Aneurysm Repair,” on pages 1889–1891.
References
- 1.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM: Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol 8: 92–102, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Cosford PA, Leng GC: Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev 2: CD002945, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Lovegrove RE, Javid M, Magee TR, Galland RB: A meta-analysis of 21,178 patients undergoing open or endovascular repair of abdominal aortic aneurysm. Br J Surg 95: 677–684, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Greenhalgh RM: Commentary: Impact of EVAR and DREAM trials on clinical practice. J Endovasc Ther 14: 541–543, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Greenhalgh RM, Brown LC, Powell JT, Thompson SG, EVAR Trial Participants : Current interpretation of the UK EVAR Trials. Acta Chir Belg 106: 137–138, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Saratzis A, Sarafidis P, Melas N, Khaira H: Comparison of the impact of open and endovascular abdominal aortic aneurysm repair on renal function. J Vasc Surg 60: 597–603, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Saratzis AN, Goodyear S, Sur H, Saedon M, Imray C, Mahmood A: Acute kidney injury after endovascular repair of abdominal aortic aneurysm. J Endovasc Ther 20: 315–330, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Saratzis A, Melas N, Mahmood A, Sarafidis P: Incidence of acute kidney injury (AKI) after endovascular abdominal aortic aneurysm repair (EVAR) and impact on outcome. Eur J Vasc Endovasc Surg 49: 534–540, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Karthikesalingam A, Bahia SS, Patel SR, Azhar B, Jackson D, Cresswell L, Hinchliffe RJ, Holt PJ, Thompson MM: A systematic review and meta-analysis indicates underreporting of renal dysfunction following endovascular aneurysm repair. Kidney Int 87: 442–451, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saratzis A, Sarafidis P, Melas N, Hunter JP, Saratzis N, Kiskinis D, Kitas GD: Suprarenal graft fixation in endovascular abdominal aortic aneurysm repair is associated with a decrease in renal function. J Vasc Surg 56: 594–600, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown LC, Brown EA, Greenhalgh RM, Powell JT, Thompson SG, UK EVAR Trial Participants : Renal function and abdominal aortic aneurysm (AAA): The impact of different management strategies on long-term renal function in the UK EndoVascular Aneurysm Repair (EVAR) Trials. Ann Surg 251: 966–975, 2010 [DOI] [PubMed] [Google Scholar]
- 13.de Bruin JL, Vervloet MG, Buimer MG, Baas AF, Prinssen M, Blankensteijn JD, DREAM Study Group : Renal function 5 years after open and endovascular aortic aneurysm repair from a randomized trial. Br J Surg 100: 1465–1470, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, Polkinghorne KR, Shankar A, Smith DH, Tonelli M, Warnock DG, Wen CP, Coresh J, Gansevoort RT, Hemmelgarn BR, Levey AS, Chronic Kidney Disease Prognosis Consortium : Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 307: 1941–1951, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melas N, Saratzis A, Saratzis N, Lazaridis J, Psaroulis D, Trygonis K, Kiskinis D: Aortic and iliac fixation of seven endografts for abdominal-aortic aneurysm repair in an experimental model using human cadaveric aortas. Eur J Vasc Endovasc Surg 40: 429–435, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Chaikof EL, Blankensteijn JD, Harris PL, White GH, Zarins CK, Bernhard VM, Matsumura JS, May J, Veith FJ, Fillinger MF, Rutherford RB, Kent KC, Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery : Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg 35: 1048–1060, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Lazaridis J, Melas N, Saratzis A, Saratzis N, Sarris K, Fasoulas K, Kiskinis D: Reporting mid- and long-term results of endovascular grafting for abdominal aortic aneurysms using the aortomonoiliac configuration. J Vasc Surg 50: 8–14, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Saratzis N, Antonitsis P, Melas N, Lazaridis I, Ginis G, Lykopoulos D, Lioupis A, Kiskinis D: Midterm results of endovascular abdominal aortic aneurysm repair with Talent stent graft in a single center. Int Angiol 25: 197–203, 2006 [PubMed] [Google Scholar]
- 19.Saratzis N, Melas N, Saratzis A, Lazarides J, Ktenidis K, Tsakiliotis S, Kiskinis D: Anaconda aortic stent-graft: Single-center experience of a new commercially available device for abdominal aortic aneurysms. J Endovasc Ther 15: 33–41, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Stather PW, Sayers RD, Cheah A, Wild JB, Bown MJ, Choke E: Outcomes of endovascular aneurysm repair in patients with hostile neck anatomy. Eur J Vasc Endovasc Surg 44: 556–561, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Saratzis A, Saratzis N, Melas N, Kiskinis D: Pharmacotherapy before and after endovascular repair of abdominal aortic aneurysms. Curr Vasc Pharmacol 6: 240–249, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Naylor AR, Hayes PD, Allroggen H, Lennard N, Gaunt ME, Thompson MM, London NJ, Bell PR: Reducing the risk of carotid surgery: a 7-year audit of the role of monitoring and quality control assessment. J Vasc Surg 32: 750–759, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 24.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 25.Ftouh S, Thomas M, Acute Kidney Injury Guideline Development Group : Acute kidney injury: Summary of NICE guidance. BMJ 347: f4930, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Shafi T, Matsushita K, Selvin E, Sang Y, Astor BC, Inker LA, Coresh J: Comparing the association of GFR estimated by the CKD-EPI and MDRD study equations and mortality: The Third National Health and Nutrition Examination Survey (NHANES III). BMC Nephrol 13: 42, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Saratzis A, Sarafidis P, Melas N, Saratzis N, Kitas G: Impaired renal function is associated with mortality and morbidity after endovascular abdominal aortic aneurysm repair. J Vasc Surg 58: 879–885, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Barrett BJ, Parfrey PS: Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med 354: 379–386, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Boules TN, Stanziale SF, Chomic A, Selzer F, Tublin ME, Makaroun MS: Predictors of diffuse renal microembolization following endovascular repair of abdominal aortic aneurysms. Vascular 15: 18–23, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Karmacharya J, Parmer SS, Antezana JN, Fairman RM, Woo EY, Velazquez OC, Golden MA, Carpenter JP: Outcomes of accessory renal artery occlusion during endovascular aneurysm repair. J Vasc Surg 43: 8–13, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Edrees WK, Lau LL, Young IS, Smye MG, Gardiner KR, Lee B, Hannon RJ, Soong CV: The effect of lower limb ischaemia-reperfusion on intestinal permeability and the systemic inflammatory response. Eur J Vasc Endovasc Surg 25: 330–335, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Rowlands TE, Homer-Vanniasinkam S: Pro- and anti-inflammatory cytokine release in open versus endovascular repair of abdominal aortic aneurysm. Br J Surg 88: 1335–1340, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Brooks MJ, Brown LC, Greenhalgh RM: Defining the role of endovascular therapy in the treatment of abdominal aortic aneurysm: Results of a prospective randomized trial. Adv Surg 40: 191–204, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Moore NN, Lapsley M, Norden AG, Firth JD, Gaunt ME, Varty K, Boyle JR: Does N-acetylcysteine prevent contrast-induced nephropathy during endovascular AAA repair? A randomized controlled pilot study. J Endovasc Ther 13: 660–666, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Walsh SR, Boyle JR, Tang TY, Sadat U, Cooper DG, Lapsley M, Norden AG, Varty K, Hayes PD, Gaunt ME: Remote ischemic preconditioning for renal and cardiac protection during endovascular aneurysm repair: A randomized controlled trial. J Endovasc Ther 16: 680–689, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Parra JR, Crabtree T, McLafferty RB, Ayerdi J, Gruneiro LA, Ramsey DE, Hodgson KJ: Anesthesia technique and outcomes of endovascular aneurysm repair. Ann Vasc Surg 19: 123–129, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Verhoeven EL, Cinà CS, Tielliu IF, Zeebregts CJ, Prins TR, Eindhoven GB, Span MM, Kapma MR, van den Dungen JJ: Local anesthesia for endovascular abdominal aortic aneurysm repair. J Vasc Surg 42: 402–409, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Allie DE, Lirtzman MD, Wyatt CH, Keller VA, Mitran EV, Hebert CJ, Patlola R, Veerina KK, Walker CM: Targeted renal therapy and contrast-induced nephropathy during endovascular abdominal aortic aneurysm repair: Results of a feasibility pilot trial. J Endovasc Ther 14: 520–527, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Jang JS, Jin HY, Seo JS, Yang TH, Kim DK, Kim TH, Urm SH, Kim DS, Kim DK, Seol SH, Kim DI, Cho KI, Kim BH, Park YH, Je HG, Ahn JM, Kim WJ, Lee JY, Lee SW: Sodium bicarbonate therapy for the prevention of contrast-induced acute kidney injury – a systematic review and meta-analysis –. Circ J 76: 2255–2265, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Sarafidis PA, Stafylas PC, Georgianos PI, Saratzis AN, Lasaridis AN: Effect of thiazolidinediones on albuminuria and proteinuria in diabetes: A meta-analysis. Am J Kidney Dis 55: 835–847, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Wald R, Waikar SS, Liangos O, Pereira BJ, Chertow GM, Jaber BL: Acute renal failure after endovascular vs open repair of abdominal aortic aneurysm. J Vasc Surg 43: 460–466, discussion 466, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Miller LE, Razavi MK, Lal BK: Suprarenal versus infrarenal stent graft fixation on renal complications after endovascular aneurysm repair. J Vasc Surg 61: 1340.e1–1349.e1, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Agrawal H, Aggarwal K, Littrell R, Velagapudi P, Turagam MK, Mittal M, Alpert MA: Pharmacological and non pharmacological strategies in the management of coronary artery disease and chronic kidney disease. Curr Cardiol Rev 11: 261–269, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santoro A, Mandreoli M: Chronic renal disease and risk of cardiovascular morbidity-mortality. Kidney Blood Press Res 39: 142–146, 2014 [DOI] [PubMed] [Google Scholar]