Abstract
Traditionally, T cells were CD4+ helper or CD8+ cytotoxic T cells, and with antibodies, they were the soldiers of immunity. Now, many functionally distinct subsets of activated CD4+ and CD8+ T cells have been described, each with distinct cytokine and transcription factor expression. For CD4+ T cells, these include Th1 cells expressing the transcription factor T-bet and cytokines IL-2, IFN-γ, and TNF-β; Th2 cells expressing GATA-3 and the cytokines IL-4, IL-5, and IL-13; and Th17 cells expressing RORγt and cytokines IL-17A, IL-17F, IL-21, and IL-22. The cytokines produced determine the immune inflammation that they mediate. T cells of the effector lineage can be naïve T cells, recently activated T cells, or memory T cells that can be distinguished by cell surface markers. T regulatory cells or spies were characterized as CD8+ T cells expressing I-J in the 1970s. In the 1980s, suppressor cells fell into disrepute when the gene for I-J was not present in the mouse MHC I region. At that time, a CD4+ T cell expressing CD25, the IL-2 receptor-α, was identified to transfer transplant tolerance. This was the same phenotype of activated CD4+CD25+ T cells that mediated rejection. Thus, the cells that could induce tolerance and undermine rejection had similar badges and uniforms as the cells effecting rejection. Later, FOXP3, a transcription factor that confers suppressor function, was described and distinguishes T regulatory cells from effector T cells. Many subtypes of T regulatory cells can be characterized by different expressions of cytokines and receptors for cytokines or chemokines. In intense immune inflammation, T regulatory cells express cytokines characteristic of effector cells; for example, Th1-like T regulatory cells express T-bet, and IFN-γ–like Th1 cells and effector T cells can change sides by converting to T regulatory cells. Effector T cells and T regulatory cells use similar molecules to be activated and mediate their function, and thus, it can be very difficult to distinguish soldiers from spies.
Keywords: Treg, T cell, cytokine, transplantation, GN
Introduction
In this review, effector T cells are referred to as soldiers, because they mediate immunity and destroy cells with the specific antigen. Until recently, the role of T regulatory cells (Tregs) in monitoring and limiting every step of the effector immune response has been underappreciated. Although they are referred to as spies, their function is to not only monitor immunity but also, actively control immunity. Tregs prevent uncontrolled immunity, unnecessarily inflicting injury that, in its own right, may kill the host.
Whereas an antibody identifies extracellular structures, such as soluble antigen or antigen on surfaces of cells or organisms, T cells monitor the intracellular compartment of the host. They do this so that they can kill cells infected with a pathogen or cells that are allogeneic, xenogeneic, or malignant cells expressing new tumor-associated antigens. In autoimmunity, they kill normal cells. To deal with the vast array of pathogens, T cell responses, such as Th1, Th2, and Th17, have evolved to allow protective responses that are adapted to better eliminate the different types of pathogens.
Tregs are generated in all immune responses and limit response to pathogens, transplant tissue, and tumor cells. Normally, Tregs control autoimmune responses, and autoimmunity occurs when Treg responses fail. Tregs are beneficial in patients with transplants, because they can promote tolerance, whereas they are undesirable in cancer, where they prevent elimination of malignant cells by T cells. Thus, promoting Tregs in patients with transplants or autoimmunity is desirable, whereas in chronic infection and malignancy, it may be undesirable.
What Is a T Cell?
T cells are mainly produced in the thymus and were first recognized as lymphocytes that do not express surface Ig or genes for Ig (1). The hallmark of a T cell is expression of an antigen-recognizing T cell receptor (TCR) (2). There are two forms of TCRs: an α- and β-chain TCR (TCRα,β) expressed by 95% of peripheral T cells (3) and a γ- and δ-chain TCR (TCRγ,δ) (4). TCRγ,δ T cells will not be discussed further. Each T cell has a unique TCR with the potential to recognize a unique antigen. Progeny of T cells express the same TCR and are clonally expanded to effect antigen-specific immunity.
The TCR is coexpressed with CD3, a complex of heterodimers of CD3ε,γ and CD3ε,δ with a homodimer of ζ-chain. The negatively charged transmembrane regions of the CD3 associate with positively charged transmembrane regions of TCR. TCR has a small intracellular domain; thus, signaling after contact with a specific antigen is by CD3. CD3 is phosphorylated on an immunoreceptor tyrosine-based activation motif that allows ζ-associated protein 70 to activate the intracellular pathway that releases calcium from the endoplasmic reticulum. Calcium binds to calmodulin to activate phosphatase activity of calcineurin to activated nuclear factor of activated T cells (NFAT). NFAT, a transcription factor, activates a series of genes, especially IL-2. Calcineurin inhibitors, such as cyclosporin and tacrolimus, block activation of T cells by inhibiting calcineurin activation.
Other molecules unique to T cells are CD2 and some isoforms of CD45. All other cell surface markers are differentially expressed on T cell subpopulations or non–T cells.
Presentation of Antigen to TCR
TCR, unlike antibody, does not directly bind to unprocessed antigen. TCR recognizes peptides of antigen presented by MHC present on cell membrane. The role of MHC molecules as presenters of antigen was first recognized when the crystal structure of human HLA-2 identified a peptide not encoded by the HLA gene in a groove created by the variant α1- and α2-domains (3,5,6).
Antigenic peptides presented by class I MHC molecules, such as HLA-A,B,C, are usually from proteins synthesized within a cell and bind to class I MHC before its expression on the cell surface (7).
The antigenic peptide in a class I MHC groove is usually nine amino acids. Each class I MHC only presents peptides with a consensus motif, usually at p2, p3, and p5 amino acids, that fits its groove. The antigenicity is generated by the amino acids at the other positions. Humans have six class I MHCs, two HLA-A, two HLA-B, and two HLA-C, with different consensus motifs that each can present thousands of different peptides. Thus, a cell can display many thousands of intracellular peptides in class I MHC, like a chip array (8). CD8 binds to the invariant α3-domain of class I MHC (9), facilitating TCR on CD8+ T cells surveying antigen presented by class I MHC.
Antigenic peptides presented by class II MHCs (in humans, HLA-DR, HLA-DP, and HLA-DQ) are usually from proteins produced outside the cell. These foreign proteins are ingested and processed by class II MHC-expressing cells, such as dendritic cells, monocytes, macrophages, oligodendrocytes, Langerhans cells, and B cells. TCR recognizes antigenic peptides of ≥15 amino acids entrapped in a groove created by the variant α1- and β1-domains (10). CD4 binds to the invariant β2 of class II MHC to facilitate TCR recognition of antigenic peptides presented by class II MHC (11,12).
The TCR antigen recognition site interacts with both the peptide and the surrounding MHC structure. This explains MHC restriction of cytotoxic T cells, which only kills virally infected cells expressing the same class I MHC that activated the T cell (13).
The pathways for presentation of antigen by MHC are complex (7). To activate T cells, antigen-presenting cells (APCs) must first be activated by the antigen and induced to express MHC and costimulatory molecules. APCs are activated by bacterial wall molecules or virus materials, such as double-stranded DNA, that bind to Toll-like receptors (7). This leads to production of inflammatory mediators, such as TNF-α, IL-1β, and PGE2, which further activate APCs (7).
In nonimmune situations, MHC class II is only expressed by APCs and B cells. During immune inflammation, IFN-γ induces expression of class II MHC on somatic cells and increases class I MHC expression (14). Activated Tregs are the only T cells that express class II MHC (15), but its function on Treg is unknown.
Generation of Diversity in TCR for T Cells in Thymus—Clonal Deletion and Selection for Ability to React to Self-MHC
A massive number of different TCRs are generated when CD4+CD8+ thymocytes are produced. This occurs by random selection of different combinations of variable and junctional genes for α- and β-chains and diversity genes for β-chain. These form three hypervariable or complementarity determining regions that are the sites where TCRs interact with antigenic peptide and the MHC (6). The antigen recognition site of TCR interacts with the peptide and the surrounding self-MHC structure.
There is negative selection by clonal deletion of thymocytes with TCR that strongly recognize self-antigen (16–18), which leads to tolerance to self. Autoimmune regulator, a transcription factor, induces expression of a large number of proteins found in peripheral tissues in thymic medullary epithelial cells (19). Peptides from these normal proteins expressed in peripheral tissues are presented on self-MHC to thymocytes and promote deletion of autoreactive clones. Mutations in autoimmune regulator cause Autoimmune Polyendocrinopathy Syndrome type 1 with hypoparathyroidism, primary adrenocorticoid failure, and chronic mucocutaneous candidiasis (20). The mechanisms for deletion of autoreactive clones are not perfect, and surviving autoreactive T cells are normally controlled by peripheral mechanisms that prevent their activation, including by Treg.
In the thymus, there is also positive selection of T cells with TCR that can bind to antigen associated with self-MHC (21). If a TCR does not bind to self-MHC, the thymocyte dies. If a thymocytes TCR recognizes class II MHC, CD4 expression is retained, and CD8 expression lost. If the TCR recognizes class I MHC, the thymocyte continues to express CD8 but not CD4. Nearly all T cells released from the thymus express either CD4 or CD8.
The majority of peripheral TCRα,β T cells is effector programmed to become soldiers. A minority of peripheral CD4+ TCRα,β Τ cells released from the thymus expresses CD25 and FOXP3, and they are professional Tregs or spies. Both effector T cells and Tregs have a vast array of TCR to recognize a broad repertoire of specific antigen.
Nonantigen-Specific Adhesion Molecules Required for Signal 1 to Activate T Cells
LFA1, LFA2(CD2), and LFA3(CD58) were identified to facilitate cytotoxic T cells interaction with target cells (22) (Figure 1). CD2 binds to LFA3 expressed on APCs and other cells (23) and is widely expressed in the kidney (24). LFA1, an integrin heterodimer of CD11a and CD18, binds to intercellular adhesion molecule 1 (ICAM1) and is the initial contact of T cells with APCs. LFA1 is also expressed by B cells, macrophages, and neutrophils. ICAM1, although constitutively expressed by APCs, can be induced on other cells by IFN-γ (25). Antibodies to LFA1, LFA2, and LFA3 can delay or prevent rejection and are potential therapeutic targets in transplantation and autoimmunity.
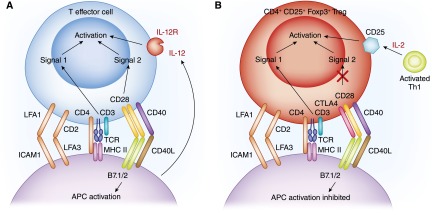
Figure 1.
Activation of effector and regulatory T cells by antigen presenting cells. Key surface molecules in activation of (A) T effector cells and (B) T regulatory cells (Tregs). The key molecules required for both cells are similar. The T cell receptor complex includes CD3, CD2, CD4 or CD8, LFA1, and CD45R, and activation of T cell receptor (TCR) by antigen results in Signal 1 for T effector cells and Tregs. In effector T cell–lineage T cells, CD28 on the T cells is activated by B7.1 and B7.2 on antigen-presenting cells (APCs) and generates Signal 2, which combined with Signal 1, initiates effector T-cell activation. The activation of effector T cells is augmented by CD40L binding to CD40 and cytokines, such as IL-2 and IL-12, for generation of Th1 cells. With Tregs, CTLA4 binds to B7.1 and B7.2 and limits activation through CD28. Thus, the effector T cells Signal 2 pathway is not required for Treg activation. The second signal for Treg activation is generated by IL-2 binding to the IL-2 receptor, which includes CD25.
These molecules form an immunologic synapse around the TCR/MHC interaction (26). The synapse includes TCR, CD3, CD4 or CD8, CD2, LFA1, and CD45 that collectively produce Signal 1 for T-cell activation (Figure 1). Signal 1 is blocked by calcineurin inhibitors, such as cyclosporin, which complexes with cyclophilin, or tacrolimus (FK506), which complexes with FK506 binding protein (FKBP). Both complexes inhibit calcium binding to calcineurin and the induction of phosphatase activity required to release NFAT.
The molecules and mechanisms of antigen recognition and generation of Signal 1 required to activate antigen-specific T cells are common to effector T cells and Tregs (Figure 1).
Signal 2 for T Cell Activation
CD28 expressed by naïve T cells binds to B7.1(CD80) or B7.2(CD86) on APCs and generates Signal 2 (27). B7.1 and B7.2 are normally only expressed by specialized APCs, such as dendritic cells and Langerhan’s cells. These APCs need to be activated by a pathogen binding to Toll-like receptors to induce the inflammasome and production of IL-1β, IL-6, and TNF-α. This increases expressions of MHC and ligands on APCs that are required for T cells to bind. Normal healthy somatic cells cannot activate T cells, because they do not express B7.1 and B7.2. CTLA4 from Tregs preferentially binds and blocks to B7.1 and B7.2, preventing induction of Signal 2. mAbs to block costimulation have been used to prevent rejection. CTLA4-Ig (abatacept, and belatacept) blocks T-cell activation and prevents renal transplant rejection and some autoimmunity. Antagonists of CTLA4 (ipilimumab) block Treg function and allow immune destruction of tumors, such as melanoma.
Signal 2 activates a separate intracellular pathway in T cells that is blocked by target of rapamycin (mTOR) inhibitors, such as rapamycin, that also bind to FKBP. This complex of rapamycin/FKBP blocks activation of mTOR but not calcineurin. mTOR inhibitors act by blocking signal 2 and prevent rejection.
The combination of Signal 1 and Signal 2 induces expression of genes required for T cell activation and promotes T cell proliferation to produce effector T cells (Figure 1A). In vivo natural T regulatory cells (nTregs) cannot active Signal 2 (Figure 1B), albeit in vitro, this pathway is activated by anti-CD28 to polyclonally expand nTreg.
CD40L is expressed by T cells and binds to CD40 on APCs, B cells, and macrophages as well as other cells. CD40L binding to CD40 activates the APCs that, in turn, activate T cells. Other T cell surface molecules promote APC activation, including inducible T cell costimulatory (CD278), a member of the CD28, CTLA4 family (28).
Naïve, Activated, and Memory T Cells
The T cells that have not previously contacted their relevant antigen are naïve. The normal immune system has a massive reservoir of naïve T cells, with the potential to respond to millions of different antigens presented by self-MHC. For a specific virus, <0.01% of naïve T cells have a specific TCR, whereas 1%–9% of naïve T cells have TCRs that recognize MHC on incompatible allografts.
Naïve T cells are programmed to recirculate from blood into peripheral lymphoid tissues and then back to blood by the lymphatics to facilitate contact with their specific antigen (29). They traffic past APCs activated by antigen in tissues that migrated through the afferent lymphatics to lymphoid tissues. Naïve T cells that recognize antigen are arrested and activated by these activated APCs (30).
CD62L expressed on naïve T cells binds to ligands on high endothelial venules to facilitate this migration into lymphoid tissues (31). The chemokine receptor CCR7 on naïve T cells is bound by CCL21 and CCL19 from the lymphoid tissues to attract them (32). CD62L and CCR7 distinguish naïve from effector and memory T cells, which express other integrins, such as VLA4, and chemokine receptors that promote migration into inflamed tissue (Table 1). Activated T cells and effector memory T cells migrate through normal tissues (33) to survey for cells expressing specific antigen. Central memory cells express CD62L and CCR7 and migrate through lymphoid tissues, like naïve T cells. Other markers of memory T cells are expression of CD45RO, CD44, and higher expression of CD2 than naïve T cells.
Table 1.
Comparison of phenotype of Th effector lines and Th-like T regulatory cells
| Marker | Th1 Effector | Th1-Like Treg | Th2 Effector | Th2-Like Treg | Th17 Effector | Th17-Like Treg |
| CD4 | +++ | +++ | +++ | +++ | +++ | ++ |
| TCR/CD3 | +++ | +++ | +++ | +++ | +++ | +++ |
| CD2 | +++ | +++ | +++ | +++ | +++ | +++ |
| CD45 | RO | RO | RO | RO | RO | RO |
| CD25 | ++ | +++ | − | +++ | − | +++ |
| FOXP3 | − | +++ | − | +++ | − | +++ |
| T-bet | +++ | +++ | − | − | − | − |
| IRF4 | − | − | ++ | +++ | − | − |
| RORγt | − | − | − | − | +++ | ++ |
| Stat | 1 | 1 | 4, 5 | 4 | 3 | 3 |
| Chemokine receptor | CXCR3 | CXCR3 | CCR8 | CCR8 | CCR6 | CCR6 |
| IFN-γ | ++++ | +++ | − | − | − | − |
| IL-5 | − | − | ++++ | +++ | − | − |
| IL-17A | − | − | − | − | +++ | + |
| IL-2 | +++ | − | − | − | − | − |
| IFNGR | ++ | +++ | − | − | − | − |
| IL-12Rβ2 | +++ | +++ | − | − | − | − |
| IL-5Rα | − | − | − | +++ | − | − |
Treg, T regulatory cell; TCR, T cell receptor.
Effector T cells and Tregs express the same markers and traffic in the same way (Table 1).
CD45, a Marker of T-Cell Activation.
CD45 is expressed by all leukocytes but not expressed by other cells. CD45 is encoded in 34 exons that are fully transcribed and glycosylated in a gp220 expressed by B cells and other leukocytes but not T cells. The intracytoplasmic domain of CD45 contains two tyrosine phosphatases that associate with kinase associated with TCR/CD3 and Ig signaling. CD45 is essential for antigen-driven activation of B and T cells and promotes their differentiation and proliferation. The ligand for CD45 is unknown.
On T cells, exons 4–6, which encoded CD45RA, CD45RB, and CD45RC, respectively, are spliced out to generate potentially eight different protein products. CD45RA(gp200kd) is expressed by naïve T cells. On activation of T cells, CD45RA is replaced by CD45RB, CD45RC, and/or CD45RO. In alloimmune responses, naïve T cells express CD45RB (34), and blocking CD45RB prevents rejection. Memory T cells only express CD45RO(gp180) where exons 4–6 are spliced out. The major component of antithymocyte globulin is anti-CD45.
Soldier Versus Spy T Cells
The majority of T cells expressing TCRα,β are programmed to be effector cells and express either CD4 or CD8 but do not express the IL-2Rα(CD25) or FOXP3 (35). A minority (<5%) population of professional spies is CD4+CD8−CD25+FOXP3+ Tregs (35,36). Most early work on T cells focused on immune destruction of infected, malignant, transplanted, or normal self-cells in autoimmunity but not Tregs.
In the early 1970s, T cells that suppress immunity were described as CD8+I-J+ T cells. Thymocytes were suppressive, and removal of the thymus made animals prone to autoimmunity. When no gene for I-J was found in the murine MHC region, suppressor T cells fell into disrepute, and most work in the field was abandoned (37).
The revival of Tregs started when CD4+CD8− T cells and not CD8+ T cells were found to transfer antigen-specific tolerance and suppress naïve T effector cells (38). The CD4+ T cells that transferred tolerance expressed CD25, the IL-2 receptor-α (15). This created a paradox, because CD4+ T cells activated to mediate rejection expressed CD25 (39), and their depletion with mAbs to CD25 reduced rejection in animals (40,41) and humans (42). We now know that depletion of CD25+ T cells prevents induction of tolerance in transplant and autoimmunity. Thus, the soldiers and spies had the same markers.
Other observations supported the existence of CD4+ Tregs. First, transferred tolerant CD4+ T cells interacted with a second host’s CD4+ T cells to induce transplant tolerance (43). Second, autoimmunity in neonatal thymectomized mice was prevented by CD4+CD25+ T cells (44). Third, in the early 2000s, the transcription factor FOXP3 identified Tregs from activated CD4+CD25+ T effectors (35,36). FOXP3 prevents IL-2 production and induces CD25 expression.
Defects in the FOXP3 gene lead to immunodysregulation polyendocrinopathy enteropathy X–linked syndrome manifesting as enteropathy, dermatitis, nail dystrophy, autoimmune endocrinopathy, lymphoid enlargement, and infections (45). Scurfy mice have defects in FOXP3, widespread uncontrolled lymphoid hyperplasia of CD4+ T cells, T cell infiltration of organs, and overexpression of cytokines (46). Similar phenotypes to scurfy are found in CTLA4, IL-2, and CD25 knockout mice, indicating the key role that these molecules play in nTreg function.
The Survival and Maturation of T Cell Subpopulations Depends on the Cytokine Milieu
Different functional T cell subpopulations express different cytokine receptors and cytokines. Cytokine binding to its specific receptor induces Jaks, Stats, and cell line-specific transcription factors.
Expression of cytokine receptors distinguishes different subpopulations. Effector lineage T cells need IL-7 to survive and express IL-7Rα(CD127). CD4+CD25+FOXP3+ Tregs express IL-2R and need IL-2 to survive. Tregs have low expression of CD127, and depletion of CD127hi cells is used to enrich Tregs and eliminate activated effector CD4+CD25+ T cells (47). Memory T cells are maintained by IL-15 and express IL-15Rα.
Activated T effector cells and Tregs express different cytokine receptors and cytokines. These patterns of expression distinguish different subpopulations (Figures 2 and 3).
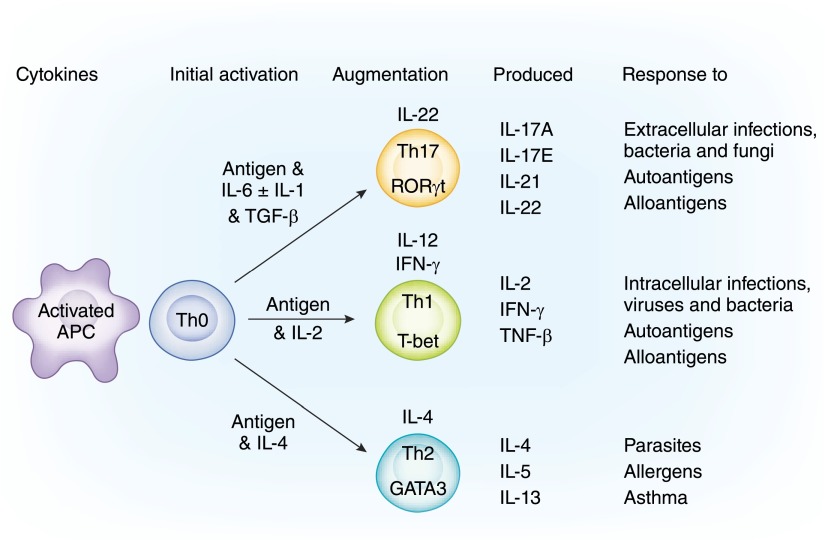
Figure 2.
Induction of soldiers into functionally distinct cell lines. On contact with antigen on APCs, Th0 cells can be activated to different Th subsets cells. The pathway of differentiation is driven by the nature of the antigen to which they are making an effector response. The most primitive is driven by inflammatory cytokines IL-6 and IL-1β to induce the transcription factor RORγt that produces Th17 cells producing a unique set of cytokines (top pathway). Th1 cells are initially activated by IL-2 to induce T-bet and a Th1 phenotype of cytokine expression (middle pathway). Th2 cells are induced by IL-4 to express GATA3 and Th2 cytokines (bottom pathway). The maturation of all cell lines is augmented by other cytokines: IL-22 for Th17 and IL-12 and IFN-γ for Th1. There are other lineages, such as Tfh and Th9 cells (not illustrated), that are induced by different cytokines, have a different transcription factor, and produce different cytokines.
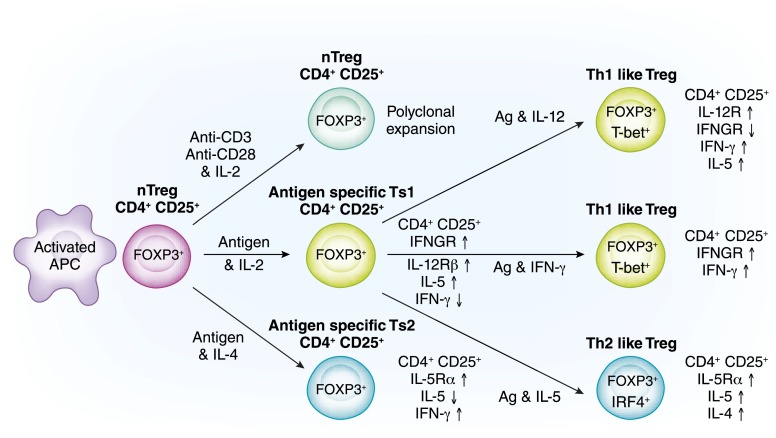
Figure 3.
Induction of cell lines of spy cells into functionally distinct Treg lines. The most common method of expanding natural T regulatory cells (nTregs) is polyclonal activation with anti-CD3 and anti-CD28 with high concentrations of IL-2 (top pathway). This expands nTregs that retain the nTreg phenotype and have no increased potency to suppress. In the last few years, it has been appreciated that nTregs (CD4+CD25+CD127loFOXP3+T) are not an end line of cells that only functions to suppress the initiation of immune cells in a nonantigen-specific manner. The pathways for activation of nTregs with T cell receptors that recognize the specific antigen are as complex as those described for activation of effector T cells, which are outlined in Figure 2. With antigen and high concentrations of IL-2, nTregs within days express receptors for Th1 cytokines, IFN-γ, and IL-12 (57) (middle pathway). They have enhanced capacity to suppress to specific antigen. This is a first step in activation of these cells, which we call Ts1 cells (57). If inflammation persists, Th1 cytokines IFN-γ and IL-12 (in the absence of IL-2) can further expand the antigen-specific Treg (58). These are highly potent Th1-like Tregs that express both FOXP3 and T-bet (the Th1 transcription factor) and produce IFN-γ but not IL-2 (58). Other cytokines induce separate pathways: Th2, Th17, and Tfh responses induced Th2-, Th17-, and Tfh-like Tregs, respectively. As an example, specific antigen and IL-4, in the absence of IL-2, activate antigen-specific Ts2 cells that express receptor for IL-4 and IL-5 but not for IFN-γ and IL-12 (57) (bottom pathway). They enhance antigen-specific suppressor capacity and can be further activated by specific antigen and IL-5 in the absence of IL-4 to Th2-like Tregs that have a very potent antigen–specific suppressor capacity.
Activation of Professional Soldiers—T Effector Cells
Effector lineage CD4+CD25−CD127hiFOXP3− T cells activated by antigen are clonally expanded and can develop into functionally different CD4+ T cell lines. Different pathways are driven by the cytokine milieu and the cytokine receptors induced during activation (Figure 2) and induce functional distinct T cells, such as Th17, Th1, Th2, or Tfh cells.
Th17 Cells
Th17 cells are induced if the inflammatory cytokine IL-6 (and IL-1β in humans) is present with TGF-β (48,49). The transcription factors Stat3 and RORγt are induced and regulate Th17 cytokine expression. TGF-β alone induces a regulatory cell, known as induced T regulatory cell (iTreg) (49).
Pathogens activate Toll-like receptors on APCs that induce IL-6 and IL-1β. The full maturation of Th17 cells requires IL-23 (50) and IL-21 produced by Th17 cells (51).
Th17 cells produce IL-17A and IL-17E and IL-21 and IL-22 but do not produce the Th1 cytokines IL-2, IFN-γ, or TGF-β or the Th2 cytokines IL-4, IL-5, or IL-13. The Th1 cytokine IFN-γ inhibits Th17 cells and promotes Tregs (52). Th17 cells express CCR6 to promote migration to tissue.
Th17 cells provide immunity to bacteria and fungi at epithelial and mucosal barriers. IL-17A and IL-17E recruit neutrophils. IL-22 stimulates epithelial cells to produce antimicrobial agents that destroy bacteria and fungi. Th17 cells can directly kill target cells and by release of cytokine, promote IgM production to kill pathogens. Th17 cells mediate many autoimmune responses (53), including multiple sclerosis, Crohn’s disease, psoriasis, rheumatoid arthritis, and uveitis. Th17 also plays a role in transplant rejection and GN models (54), but their full role in renal diseases remains to be elucidated.
Th1 Cells
Th1 cells were considered the central pathway of CD4+ T-cell activation for autoimmunity, intracellular infections, and allograft rejection. Activated CD4+ T cells express IL-2R, a complex of α-, β-, and γ-chains that induces Jak1, Jak3, and Stat5 (55). IL-2 produced by the activated T cells acts as a growth factor inducing proliferation to Th1 cells expressing the transcription factor T-bet, which induces IFN-γ and TNF-β but not Th17 or Th2 cytokines. Th1 expresses CXCR3, which promotes migration to sites of Th1 inflammation. Th1 cells directly mediate tissue injury by release of IFN-γ and TNF-α as well as cytotoxic mechanisms, such as perforin and granzymes. Th1 cells are the principal mediators of transplant rejection and also, mediate some forms of GN (56).
Th1 cells release cytokines that induce other inflammatory cells. Th1 cytokines, IL-2, IFN-γ, and IL-12p70 help activation of CD8+ T cells to cytotoxic Tc1, which expresses IFN-γ and cytolytic molecules, such as perforin and granzymes. These are cytotoxic/killer cells that destroy infected, malignant, and allografted cells. Tc1 mediates autoimmunity in type 1 diabetes and GN (56).
IFN-γ induces B cells to switch to complement-fixing Ig. Th1 cytokines IFN-γ and IL-12 activate macrophages to produce TNF-α and induce nitric oxide synthase to produce nitric oxide. These are the M1 subpopulations of macrophages that can kill bacteria and other pathogens. IFN-γ and TNF-α activate endothelial and other cells to express classes I and II MHC and ICAM1, and therefore, the T cells can interact with these cells (14).
Th1 cytokines promote antigen–specific CD4+CD25+FOXP3+ Tregs to express receptors for Th1 cytokines IFN-γ and IL-12, which are called Ts1 cells (57), and these Ts1 cells can be activated further to Th1-like Tregs (58).
Th1 responses together with Th17 are key to targeted destruction of cells with infection or malignant transformation as well as foreign cells with alloantigen in transplants. Th1 with Th17 cells mediate many forms of autoimmunity. Th1 and Tc1 cells are key to injury in models of nephritis (56,59,60).
Th2 Cells
Th2 cells are induced in responses to parasites and allergens and are driven by IL-4 (61,62), which binds to the IL-4Rα (63) and the common γ-chain to activate Jak1 and Jak3, the transcription factors Stat4 and GATA-3 (55). CD4+Th2 cells initially produce IL-4 and later, IL-5 and IL-13. Th2 cells were considered anti-inflammatory and protolerance induction (64). IFN-γ inhibits Th2 induction. Th2 expresses CCR8, which facilitates migration into tissues with Th2 inflammation.
Th2 cytokines affect other immune cells. IL-4 induces CD8+ T cells to a noncytolytic Tc2 phenotype that does not express perforin, granzyme, and IFN-γ. IL-4 and IL-5 (in mice but not humans) induce Ig isotype switches in B cells to produce noncomplement-fixing IgG and IgE. IL-4 and IL-13 convert macrophages to an M2 phenotype, which lacks the inflammatory activity of M1 cells. Th2 cytokines promote antigen-specific CD4+CD25+FOXP3+ Tregs to Ts2- and Th2-like Tregs (57,65).
Th2 responses are dominant in some forms of drug-induced interstitial nephritis, where there is eosinophilia, and can contribute to rejection. Treatment with IL-4 to promote Th2 responses reduces injury in models of nephritis (66,67) and allograft rejection (68) as does treatment with IL-5 (65,69) or IL-13 (70). These treatments reduce Th1 and macrophage activation, promote a Th2-dominant response, and induce Ts2- and Th2-like Tregs. Th2 dominance alone does not explain immune tolerance and is not necessary of tolerance induction (71,72).
Tfh Cells
Follicular helper T cells (Tfh) promote B cell maturation and activation in B cell follicles in secondary lymphoid tissues. They are induced by inducible T-cell costimulator on APCs. They function by secretion of IL-4 and IL-21 (73) and the expression of CD40L, which binds CD40 on follicular B cells and causes isotype switching of Ig, somatic hypermutation of Ig, and proliferation to germinal center B cells, thereby promoting their maturation to memory B cells and Ig-secreting plasma cells. Tfhs are regulated by the transcription factor bcl-6 (74) and express CXCR5.
Professional Spies—Tregs
The most important professional Tregs are CD4+CD25+CD127loFOXP3+ T cells produced by the thymus. CD4+CD8+ thymocytes interact with class II MHC-expressing cells in the medullar and Hassall’s corpuscles of thymus, where thymic stromal lymphopoietin promotes myeloid and plasmacytoid dendritic cells that induce Tregs (75). Treg induction requires TGF-β, stimulation of CD28 by B7.2, and IL-2 activating the IL-2R to induce Stat5 and FOXP3 (76). FOXP3 induces CD25 and inhibits IL-2 expression.
After a process of clonal deletion and selection, CD4+CD8−CD25+CD127loFOXP3+ T cells with a wide array of TCR specificity are released. These are naïve nTregs that are also known as thymic-derived T regulatory cells (tTregs) (77). tTregs have epigenetic demethylation of the T regulatory cell–specific demethylation region (TSDR), a promoter region of foxp3. This selective demethylation of TSDR makes it hard for foxp3 to be switched off and stabilizes the nTreg lineage (78), and therefore, they and their progeny cannot revert to effector T cells (79,80). Homeostatic regulation ensures that CD4+CD25+FOXP3+ Tregs remain as <10% of peripheral CD4+ T cells (81).
nTreg survival requires low levels of IL-2, whereas effector lineage T cells express high levels of IL-7α(CD127) and depend on IL-7 to survive. Depletion of CD127hi cells enriches CD4+CD25+FOXP3+ nTregs by removing activated effector lineage CD4+CD25+FOXP3− T cells. nTregs express helios, an ikaros transcription family member, that differentiates tTregs from periphery-induced iTregs.
Naïve nTregs express CD45RA, whereas activated effector and regulatory T cells express CD45RO. nTregs express CTLA4(CD152) and glucocorticoid-induced TNF receptor (GITR), which are also expressed by activated effector T cells. CTLA4 produced by nTregs binds B7.1(CD80) and B7.2(CD86) to block activation through CD28, sending a negative signal to TCR/CD3 (82). nTreg through CTLA4 downregulates B7.1 and B7.2 expression by APCs (83) (Figure 1B). Fusion molecules of CTLA4 with Ig(Belatacept) prevent rejection and autoimmunity. Ipilimumab, which blocks CTLA4 and Treg, is approved for melanoma treatment (84).
nTregs at physiologic ratios of <1:10 only partially suppress naïve T-cell responses, and full suppression requires ratios of ≥1:1 to naïve CD4+ T cells (85–88). Full suppression in vivo by nTregs requires marked depletion of effector T cells (85) or expansion of nTregs, which is transiently achieved in mice with an IL-2 and anti–IL-2 mAb complex (89). Depletion of nTregs after neonatal thymectomy (90) or anti-CD25 mAb therapy leads to autoimmunity and prevents induction of transplant tolerance.
There is a paradox, in that anti-CD25 mAb (daclizumab or basiliximab) is used as an induction therapy in patients with renal transplants and reduces rejection by depletion of activated effector CD25+ T cells. Although effective in reducing rejection, these antibodies also deplete CD25+ Tregs and may impede induction of transplant tolerance. The ONE Study, which is trialing immunoregulatory cells in recipients of renal transplants, excludes use of anti-CD25 mAb therapy, because it may prevent tolerance induction by host or therapeutically administered nTregs.
Activation of nTregs
CTLA4 binding to B7.1 and B7.2 blocks the activation of CD28 on nTregs, and therefore, in vivo, this second signal is not activated and not required for nTreg activation. The alternate second signal for nTreg activation requires high levels of IL-2 (91) compared with those required to activate effector T cells. Thus, both in vivo (92) and in vitro (93) nTreg activations are blocked by calcineurin inhibitors, which inhibit Signal 1. mTOR inhibitors, which inhibit Signal 2, block effector T-cell activation but spare Treg activation and allow preferential expansion of Tregs.
In Vitro Expansion of nTregs
Many groups propose to use nTregs to prevent graft-versus-host disease, graft rejection, and autoimmunity. To do this, they enrich nTregs and expand their number in vitro. The most common method for expansion of nTregs is culture with anti-CD3 and anti-CD28 mAbs with high concentrations of IL-2. Over a period of weeks, tens of thousandfold increases in nTreg numbers can be achieved. However, they remain nTregs in phenotype and function and need to be at ratios of ≥1:1 with effector lineage T cells to fully suppress an immune response. Expansion of nTreg with an mTOR inhibitor to selectively block effector T-cell activation can also be used (94). Tang and Lee (95) estimated that it will be impossible to prepare sufficient nTregs to induce tolerance for transplant or autoimmunity.
Marek-Trzonkowska et al. (96) in Poland were first to use nTregs as therapy in patients with type 1 diabetes and recipients of bone marrow with graft-versus-host disease (97). The ONE Study is examining a variety of in vitro–activated nTregs to promote tolerance in clinical renal transplants but will be combined with conventional immunosuppression, except that anti-CD25 will not be used, because it could deplete Tregs (98). Inducing antigen-specific Tregs has also been trialed (57,58,99). The use of Tregs as a therapy was recently reviewed (100).
Activated/Antigen-Specific CD4+CD25+FOXP3+ Tregs
There is a common misunderstanding that all CD4+CD25+FOXP3+ T cells are nTregs. CD4+CD25+FOXP3+ Tregs are a very heterogeneous population and include different subclasses of activated antigen-specific Tregs as well as nTregs, which have been recently reviewed (101,102). Activated antigen-specific Tregs are induced from nTregs or iTregs. The pathways for activation of antigen-specific Tregs are similar to those for activated effector T cells (Table 1).
The original examination of CD25 expression on tolerant CD4+ T cells was undertaken, because tolerance-transferring cells die in culture, even if stimulated with specific antigen (15,103) but did survive if a cocktail of T cell–derived cytokines or IL-2 was present (15,103). Because IL-2 alone was insufficient to maintain antigen-specific Tregs (15,103), we examined which other cytokines promoted antigen-specific Tregs.
Culture of nTregs with alloantigen and Th1 and Th2 cytokines identified that both IL-2 and IL-4 induced polyclonal activation of nTregs (57). We identified two pathways for activation of nTregs: one by Th1 cytokines and one by Th2 cytokines (57) (Figure 3). Others have described similar pathways with Th17 and Tfh cytokines, which are reviewed in references 101 and 102. These pathways parallel those for activation of Th1 and Th2 cells and use many of the same cytokines and activation pathways as effector T cells (Table 1).
IL-2 Promotes Antigen-Specific Tregs
Numerous studies activated nTregs with antigen and IL-2 and produced antigen-specific Tregs with increased suppression to specific antigen. In our studies, culture of nTregs with specific antigen and IL-2 induces antigen-specific Tregs that express the receptors for IFN-γ (57) and IL-12 (58) (Figure 3). These CD4+CD25+ T cells express FOXP3 and IL-5 but not IFN-γ or IL-2, and we named them Ts1 cells (57). They have increased antigen–specific suppressor potency in vivo and in vitro, suppressing at <1:10, whereas fresh nTregs only fully suppress at ≥1:1.
Additional activation of these cells with antigen and IL-12 without IL-2 induces a more potent antigen-specific Th1-like Treg (58) (Figure 3, Table 1). The Th1-like Tregs that we generated suppressed in vitro at 1:1000 and delayed fully allogeneic graft rejection in nonimmunosuppressed normal hosts (58). These Th1-like Tregs expressed FOXP3, T-bet, and the receptors for IL-12 and IFN-γ. They expressed IFN-γ but not IL-2. They are considered Th1-like Tregs, because they express T-bet and IFN-γ. Of note, the continued presence of IL-2 blocks generation of Th1-like Tregs, suggesting that the current methods of Treg expansion with repeated IL-2 exposure may select against growth of antigen-specific activated Tregs (58). Th1-like Tregs occur in humans, including recipients of renal transplants (104).
Th1-like Tregs can be generated by IL-12 (105,106), IFN-γ (107,108), or IL-27, but the final Treg induced by each pathway is probably different.
IL-4 Promotes Antigen-Specific Tregs
In our studies, culture of nTregs with specific antigen and IL-4 induces antigen-specific Tregs that express the specific IL-5 receptor (IL-5Rα CD125) (109). These CD4+CD25+ T cells express FOXP3 and IFN-γ but not IL-5 or IL-2 (57,65) and were named Ts2 cells. Ts2 cells have increased antigen–specific suppressor potency in vivo and in vitro, suppressing at <1:10, whereas fresh nTregs only fully suppress at ≥1:1. We found that treatment with IL-5 promoted these cells to control autoimmunity (65) and transplant rejection (57). Tregs controlling Th2 responses express the transcription factor IRF4 (110). Additional activation of these cells with antigen and IL-5 and without IL-4 induces a more potent antigen-specific Th2-like Treg (Figure 3).
Soldiers Become Spies
In Situations with No Inflammation
T effector lineage cells contacting antigen are not activated or converted to iTregs (Figure 4). Naïve CD4+CD25− T cells that contact an antigen that their TCR recognizes can convert to an antigen-specific iTreg if there is TGF-β but no IL-6. Thus, in normal tissue remodeling or after noninflammatory tissue injury, the autoantigens released do not activate effector T cells, because there are no inflammatory cytokines. TGF-β produced to promote tissue repair induces protective iTregs to prevent autoimmunity.
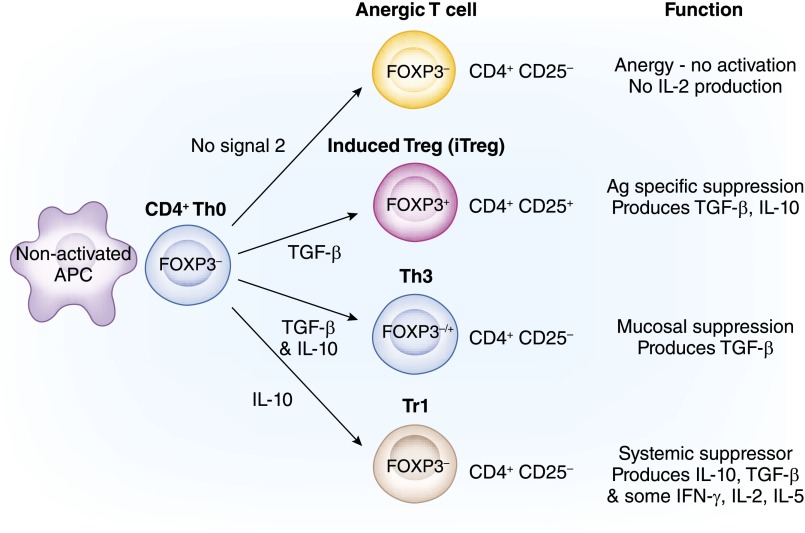
Figure 4.
Newly recruited CD4+ T-cell soldiers induced to be spies. Professional soldier lineage CD4+CD2−CD127hiFOXP3− T cells can fail to be activated into effector cells when they contact antigen. This figure illustrates four pathways that will be described in order from top to bottom. (1) Anergy is induced when there is no Signal 2, and these cells, when re-exposed to specific antigen with activated antigen-presenting cells (APCs), are not activated and do not proliferate or produce IL-2. This occurs in the absence of inflammation and activated APCs (pathway 1). (2) Induced T regulatory cells (iTregs) are induced by TGF-β and antigen when there is no IL-6 or IL-1β (pathway 2). These cells express FOXP3 and CD25 but can revert to effector T cells, because FOXP3 expressions are not stable. (3) In the mucosa, to induce oral tolerance, TGF-β and antigen can induce Th3 cells that can express FOXP3 (pathway 3). Th3 cells suppress by release of TGF-β. (4) Tr1 cells are induced by repeated culture with antigen and IL-10, which converts the APCs to DC-10 cells (pathway 4). They do not express FOXP3 or CD25 and suppress by release of TGF-β and IL-10.
Anergy
Effector lineage T cells that contact specific antigens through their TCR/CD3 and other ligands associated with Signal 1 that do not receive a second signal through CD28 become anergic (27). Anergic cells are not activated to proliferate or express IL-2 if re-exposed to the specific antigen with a Signal 2. They cannot be mobilized as a soldier.
Th3 Cells
The first CD4+ Tregs that were described to be induced from effector lineages were Th3. Th3 cells are induced in mucosa by specialized dendritic antigen presenting cells, known as CD103+DC (111). Th3 is suppressed in mucosa by release of IL-10 and TGF-β (112). Th3 cells induce oral tolerance induced by antigen exposure through the gut.
iTregs
CD4+CD25−FOXP3− T cells that are exposed to antigen in the presence of TGF-β (49), where there is no IL-6 or IL-1β by inflammation, are induced to express FOXP3 (113,114). TGF-β inhibits RORγT expression and development of Th17 cells (115). These iTregs have a CD4+CD25+ phenotype and express other markers of nTregs, such as CTLA4 and GITR, but they do not have demethylation of TSDR of FOXP3 and do not express helios. Thus, expression of FOXP3 is not stable (78). This process of generation of iTregs increases the number of antigen-specific Tregs when there is autoantigen released by normal tissue remodeling and noninflammatory tissue injury (101,102) that is associated with TGF-β release (116).
iTregs can control Th17 responses in autoimmunity (114) and rescue scurfy mice (113). iTreg induction is inhibited by the presence of IL-6 (117). The presence of IL-4 with TGF-β induces Th9 cells expressing IL-9 and IL-10 (118). iTreg survival depends on IL-2 (119,120); iTregs can revert to effector T cells (121).
Tr1 Cells
Tr1 cells are induced by repeated culture of naïve CD4+ T cells with antigen and IL-10, which induces APCs to DC-10 cells (122). Tr1 cells are CD4+CD25−Foxp3− T cells that produce IL-10 and TGF-β as well as some IL-5, IFN-γ, and IL-2 but no IL-4 (123). Tr1 cells suppress autoimmune and allograft responses by release of IL-10 and TGF-β and through perforin/granzyme B (124). Therapy with Tr1 cells is in clinical trials.
Activated Effector T Cells Fail to Fight or Become Spies
T Effectors Die from Exhaustion
T effectors die from exhaustion from ongoing activation and proliferation, leading to clonal pruning with a reduction in the number of antigen-reactive clones (125) (Figure 5). The mechanism of clonal exhaustion remains unclear but is driven by persistent antigen activation of TCRs (126). It may include Treg elimination of effector T cells.
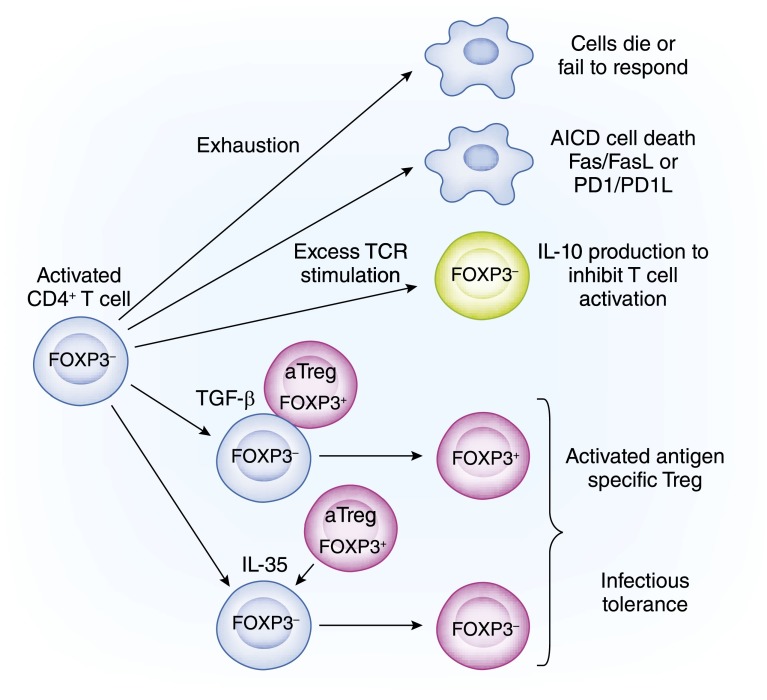
Figure 5.
Activated and aggressive CD4+ T cell soldiers convert to spies. Fully activated T cells programmed to mediate antigen-specific injury can be neutralized or change sides. Five pathways are illustrated, and described from top to bottom. (1) Neutralization or cell death occurs from repeated T cell receptor (TCR) activation and proliferation of effector T cells (pathway 1). The cells can die of exhaustion, leading to clonal pruning. (2) Excessive and repeated stimulation of TCRs on effector cells induces them to express surface molecules that, when they bind ligand, induces apoptosis (pathway 2). This leads to activation induced cell death (AICD). The best described pathways are Fas/FasL and programmed cell death protein-1 (PD1) /programmed cell death protein-1 ligand (PD-L1). (3) Induction of IL-10 expression by effector T cells (pathway 3). After repeated stimulation and expansion, activated effector T cells can be induced to express IL-10 usually by IL-27 binding to IL-27R. The release of IL-10 by these effector T cells reduces inflammation, especially the activation of APCs. The last two pathways involve T regulatory cells (Tregs) infection of effector T cells to convert them to Tregs. Direct contact with activated Tregs can infect effector T cells to make them regulatory. (4) TGF-β on Treg surface can, by cell-cell contact, induce FOXP3 and endow Treg function in effector T cells (pathway 4). (5) IL-35 released from activated Tregs binds the IL-35 receptor on activated T cells and converts them into FOXP3− iTr35 cells (pathway 5). AICD, activation induced cell death.
T Effectors Die of Activation-Induced Cell Death
Activation-induced cell death can be caused by Fas/FasL-mediated apoptosis after repeated stimulation of TCR inducing FasL (127). Fas/FasL also induced apoptosis in Tregs (128). The Fas/FasL pathway alone cannot induce immune tolerance (129).
Activated T cells as well as B cells and macrophages express programmed cell death protein-1 (PD1; CD279), a member of CD28 family. PD1 on binding to programmed cell death protein-1 ligand (PD-L1) or PD-L1/B7 complex blocks TCR signaling (130) and can lead to activated T-cell deaths. PD-L1 in normal tissue is expressed in kidney, heart, lung, thymus, and spleen and upregulated on dendritic cells and macrophages during inflammation. The second ligand for PD1 is PD-L2 that is restricted to dendritic and tumor cells.
PD1 knockout mice develop lupus nephritis and cardiomyopathy, suggesting that this pathway prevents autoimmunity in the kidney and heart. PD-L1 is expressed on many tumor cells, and treatment with mAbs against PD1 (nivolumab and pembrolizumab) is effective in some patients with melanoma, nonsmall cell lung cancer, or renal cell cancer. Treatment with PD1 antagonists can unmask autoimmunity and theoretically, may unmask rejection of renal transplants as may inhibitors of CTLA4.
Activated effector T cells during intense inflammation are induced to express IL-10 (131). IL-27 binds to the IL-27 receptor (132) on Th1, Th2, or Th17 cells and induces IL-10 (133,134). IL-10 is anti-inflammatory and prevents APC activation. IL-27 is a member of the IL-12 family, in which cytokines and their receptors are heterodimers formed by various combinations of proteins in the family (135). IL-12, IL-23, and IL-27 promote effector T cells and induce IFN-γ. IL-12p40, IL-27, and IL-35 inhibit activated T cells. IL-12 (58,105,106) and IL-27 promote Tregs to Th1-like Tregs (105).
Activated Tregs infect activated T cells to become Tregs by two mechanisms (43,136). First, TGF-β on the surface of Tregs binds to activated T effectors by a TGF-β receptor and induces expression of FOXP3 and the ability to suppress (137). Second, activated Tregs secrete IL-35 that binds to IL-35 receptor on activated effector T cells and converts them to iTr35. iTr35s are distinct from iTregs and Tr1 cells and are FOXP3− (138). iTr35s occur in humans and are potent suppressors (138).
Mechanisms of Action of Activated Tregs
A variety of mechanisms mediates suppression. With nTregs, CTLA4 binds to B7.1 and B7.2 to block these molecules and prevent T-cell activation. It is an oversimplification to attribute all suppression to IL-10 and TGF-β, which mainly suppress in mucosa (139).
With activated Tregs, IL-10 or IL-35 can suppress but is not essential (140). Activated Tregs can express CD39 and CD73 that metabolize extracellular ATP and ADP to adenosine, which suppresses activated effector T cells through the A2A adenosine receptor (141). IFN-γ, perforin, and granzyme B used by cytotoxic T cells also mediate suppression by some activated Tregs (142,143); thus, the main weapons of cytotoxic T cells are used by Tregs to suppress.
Activated Tregs can suppress the function of activated CD4+ T cells, CD8+ T cells, B cells, and macrophages. They control all aspects of immunity, albeit that memory CD4+ T cells are less responsive to control by Tregs (15).
Do Spies Become Soldiers?
There is concern that, if Tregs can change to effector lineage, their use as therapy may be unreliable, if not dangerous (144–147). At present, the consensus is that nTregs/tTregs that have demethylation of TSDR are stable, and their progeny remain Tregs (144,145). nTregs have demethylation of regions of other genes essential to their function, including CTLA4 and GITR (148). Transfer of nTregs to lymphopenic hosts, where there is inadequate IL-2, can lead to transient loss of FOXP3 (149). In uncontrolled immune inflammation, Tregs can be induced to the Th1-, Th2-, or Th17-like Tregs as described above. Whether these cells are effector or only suppressor is not resolved, but to survive, they depend on cytokines produced by the effector T cells (57,58,103).
Induced or peripherally generated Tregs (iTregs/pTregs) that develop from effector lineage T cells activated by antigen and TGF-β if there is no IL-6 or IL-1 express FOXP3 and become regulatory. These cells are plastic and readily revert to effector lineage if exposed to IL-6 in the absence of TGF-β (150).
Role of T Cells in Renal Diseases
Although antibodies are considered the main mediators of GN, T cells also play a central role. First, T cells provide help for isotype switching of antibody from IgM to IgG, IgA, and IgE. Th1 cells provide the cytokines to promote development of complement fixing antibodies, such as IgG1 and IgG3. Th2 cells promote noncomplement fixing antibodies IgG2 and IgG4. IgA induction requires TGF-β from T cells in the mucosa. Thf cells promote B-cell proliferation and the maturation of B-cell response in lymphoid follicles.
There is compelling evidence that T cells also contribute directly to glomerular injury (151), especially in nephritis, where there is no or little Ig and complement deposition. In experimental models, infiltration of T cells in glomeruli is associated with injury, especially Th1 and Th17 cells (54,60,66) but not Th2 cells, which tend to be protective (67,152). These are reviewed in an companion article by Holdsworth and Gan (153). Although Tregs can suppress nephritis in animal models (154), the potential of these cells as a therapy requires more investigation.
In drug-induced interstitial nephritis, there are Th2 and Th1 responses.
There is a T cell and macrophage interstitial infiltrate in the kidney in acute ischemia (155), with ureteric obstruction, and in many forms of GN and end stage renal failure. In AKI, Th1 responses are present (156), and injury can be reduced by Treg (157) depletion of T cells (158) and blocking T-cell migration into kidneys (159). Whether T cells contribute to injury or are a benign reaction (160) remains to be resolved.
Role of T Cells in Transplant Rejection
Acute cellular rejection is T cell–mediated (30,161) and involves CD4+ and CD8+ T cells (162). The CD4+ T cells mediating rejection are Th1 (163) and Th17 but can include Th2 cells (72). Alloantibody responses are also dependent on help from CD4+ T cells. Transplant tolerance is mediated by CD4+ T cells (15,164), and CD4+CD25+ T cells (15) are essential for induction and maintenance of tolerance. nTregs and alloantigen-activated Tregs are being trialed as therapy to reduce rejection with an ultimate aim of inducing tolerance (98).
There is much overlap in the molecules and pathways used by T cells that act as soldiers and spies. There is also considerable plasticity in that soldiers can fail to fight or become active spies. The presence of spies has benefits in controlling unwanted destructive immune response damaging vital tissues, such as the kidney. Inadequate Treg responses can lead to autoimmunity. Activation of Tregs induces tolerance to allografts, bone marrow grafts, and normal host tissues in autoimmunity. Destroying the spies may allow immune destruction of tumor cells. How to control T cell–mediated injury is of relevance to nephrologists caring for patients with renal transplants and immune-mediated diseases, such as GN, interstitial nephritis, and possibly, AKI.
Disclosures
B.M.H. holds patents related to the generation and production of antigen–specific T regulatory cells and the diagnosis of immune tolerance. B.M.H. owns and holds licenses for mAbs used to assess and monitor immune cells. Research funding is by the National Health and Medical Research Council of Australia, Multiple Sclerosis Research Australia, and in the past, Novartis Basle CH. B.M.H. is a full-time employee of UNSW Australia and Liverpool Hospital. In the last 5 years, B.M.H. held no consultancy agreements, received no honoraria, and had no scientific advisory roles or other interests related to science.
Acknowledgments
Rachael Hall assisted in preparing illustrations. Dr. Suzanne Hodgkinson and Prof. Michael Suranyi reviewed the manuscript.
The laboratory of B.M.H. was supported by the National Health and Medical Research Council of Australia of Australia, Bob and Jack Ingham, the South Western Sydney Local Health District, the Juvenile Diabetes Foundation, Novartis Basel CH, Multiple Sclerosis Research Australia, and anonymous donations. The author has received research funds from Novartis Pharma CH.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Kronenberg M, Davis MM, Early PW, Hood LE, Watson JD: Helper and killer T cells do not express B cell immunoglobulin joining and constant region gene segments. J Exp Med 152: 1745–1761, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chien YH, Gascoigne NR, Kavaler J, Lee NE, Davis MM: Somatic recombination in a murine T-cell receptor gene. Nature 309: 322–326, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Davis MM, Bjorkman PJ: T-cell antigen receptor genes and T-cell recognition. Nature 334: 395–402, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Loh EY, Lanier LL, Turck CW, Littman DR, Davis MM, Chien YH, Weiss A: Identification and sequence of a fourth human T cell antigen receptor chain. Nature 330: 569–572, 1987 [DOI] [PubMed] [Google Scholar]
- 5.Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC: Structure of the human class I histocompatibility antigen, HLA-A2. Nature 329: 506–512, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC: The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature 329: 512–518, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Guermonprez P, Valladeau J, Zitvogel L, Théry C, Amigorena S: Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol 20: 621–667, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Shastri N, Schwab S, Serwold T: Producing nature’s gene-chips: The generation of peptides for display by MHC class I molecules. Annu Rev Immunol 20: 463–493, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Salter RD, Benjamin RJ, Wesley PK, Buxton SE, Garrett TP, Clayberger C, Krensky AM, Norment AM, Littman DR, Parham P: A binding site for the T-cell co-receptor CD8 on the alpha 3 domain of HLA-A2. Nature 345: 41–46, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Brown JH, Jardetzky T, Saper MA, Samraoui B, Bjorkman PJ, Wiley DC: A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature 332: 845–850, 1988 [DOI] [PubMed] [Google Scholar]
- 11.Cammarota G, Scheirle A, Takacs B, Doran DM, Knorr R, Bannwarth W, Guardiola J, Sinigaglia F: Identification of a CD4 binding site on the beta 2 domain of HLA-DR molecules. Nature 356: 799–801, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Doyle C, Strominger JL: Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature 330: 256–259, 1987 [DOI] [PubMed] [Google Scholar]
- 13.Zinkernagel RM, Doherty PC: MHC-restricted cytotoxic T cells: Studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol 27: 51–177, 1979 [DOI] [PubMed] [Google Scholar]
- 14.Hall BM, Bishop GA, Duggin GG, Horvath JS, Philips J, Tiller DJ: Increased expression of HLA-DR antigens on renal tubular cells in renal transplants: Relevance to the rejection response. Lancet 2: 247–251, 1984 [DOI] [PubMed] [Google Scholar]
- 15.Hall BM, Pearce NW, Gurley KE, Dorsch SE: Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporine. III. Further characterization of the CD4+ suppressor cell and its mechanisms of action. J Exp Med 171: 141–157, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sprent J, Kishimoto H: The thymus and negative selection. Immunol Rev 185: 126–135, 2002 [DOI] [PubMed] [Google Scholar]
- 17.von Boehmer H, Aifantis I, Gounari F, Azogui O, Haughn L, Apostolou I, Jaeckel E, Grassi F, Klein L: Thymic selection revisited: How essential is it? Immunol Rev 191: 62–78, 2003 [DOI] [PubMed] [Google Scholar]
- 18.von Boehmer H, Kisielow P: Self-nonself discrimination by T cells. Science 248: 1369–1373, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC: Aire regulates negative selection of organ-specific T cells. Nat Immunol 4: 350–354, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Anderson MS, Su MA: Aire and T cell development. Curr Opin Immunol 23: 198–206, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kisielow P, Teh HS, Blüthmann H, von Boehmer H: Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature 335: 730–733, 1988 [DOI] [PubMed] [Google Scholar]
- 22.Krensky AM, Sanchez-Madrid F, Robbins E, Nagy JA, Springer TA, Burakoff SJ: The functional significance, distribution, and structure of LFA-1, LFA-2, and LFA-3: Cell surface antigens associated with CTL-target interactions. J Immunol 131: 611–616, 1983 [PubMed] [Google Scholar]
- 23.Bromberg JS: The biology of CD2: Adhesion, transmembrane signal, and regulatory receptor of immunity. J Surg Res 54: 258–267, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Suranyi MG, Bishop GA, Clayberger C, Krensky AM, Leenaerts P, Aversa G, Hall BM: Lymphocyte adhesion molecules in T cell-mediated lysis of human kidney cells. Kidney Int 39: 312–319, 1991 [DOI] [PubMed] [Google Scholar]
- 25.Bishop GA, Hall BM: Expression of leucocyte and lymphocyte adhesion molecules in the human kidney. Kidney Int 36: 1078–1085, 1989 [DOI] [PubMed] [Google Scholar]
- 26.Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML: The immunological synapse. Annu Rev Immunol 19: 375–396, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP: CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature 356: 607–609, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA: ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397: 263–266, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Gowans JL: The recirculation of lymphocytes from blood to lymph in the rat. J Physiol 146: 54–69, 1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall BM, Dorsch S, Roser B: The cellular basis of allograft rejection in vivo. I. The cellular requirements for first-set rejection of heart grafts. J Exp Med 148: 878–889, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Springer TA: Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell 76: 301–314, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Subramanian H, Grailer JJ, Ohlrich KC, Rymaszewski AL, Loppnow JJ, Kodera M, Conway RM, Steeber DA: Signaling through L-selectin mediates enhanced chemotaxis of lymphocyte subsets to secondary lymphoid tissue chemokine. J Immunol 188: 3223–3236, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Hall BM, Dorsch S, Roser B: The cellular basis of allograft rejection in vivo. II. The nature of memory cells mediating second set heart graft rejection. J Exp Med 148: 890–902, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aversa G, Waugh JA, Hall BM: A monoclonal antibody (A6) recognizing a unique epitope restricted to CD45RO and RB isoforms of the leukocyte common antigen family identifies functional T cell subsets. Cell Immunol 158: 314–328, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Hori S, Nomura T, Sakaguchi S: Control of regulatory T cell development by the transcription factor Foxp3. Science 299: 1057–1061, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Fontenot JD, Gavin MA, Rudensky AY: Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4: 330–336, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Möller G: Do suppressor T cells exist? Scand J Immunol 27: 247–250, 1988 [DOI] [PubMed] [Google Scholar]
- 38.Hall BM, Jelbart ME, Dorsch SE: Specific unresponsiveness to allografts induced by cyclosporine is not antibody dependent. Transplant Proc 17: 1650–1652, 1985 [PubMed] [Google Scholar]
- 39.Dallman MJ, Shiho O, Page TH, Wood KJ, Morris PJ: Peripheral tolerance to alloantigen results from altered regulation of the interleukin 2 pathway. J Exp Med 173: 79–87, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirkman RL, Barrett LV, Gaulton GN, Kelley VE, Ythier A, Strom TB: Administration of an anti-interleukin 2 receptor monoclonal antibody prolongs cardiac allograft survival in mice. J Exp Med 162: 358–362, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kupiec-Weglinski JW, Diamantstein T, Tilney NL, Strom TB: Therapy with monoclonal antibody to interleukin 2 receptor spares suppressor T cells and prevents or reverses acute allograft rejection in rats. Proc Natl Acad Sci U S A 83: 2624–2627, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soulillou JP, Peyronnet P, Le Mauff B, Hourmant M, Olive D, Mawas C, Delaage M, Hirn M, Jacques Y: Prevention of rejection of kidney transplants by monoclonal antibody directed against interleukin 2. Lancet 1: 1339–1342, 1987 [DOI] [PubMed] [Google Scholar]
- 43.Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, Waldmann H: “Infectious” transplantation tolerance. Science 259: 974–977, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Sakaguchi S, Toda M, Asano M, Itoh M, Morse SS, Sakaguchi N: T cell-mediated maintenance of natural self-tolerance: Its breakdown as a possible cause of various autoimmune diseases. J Autoimmun 9: 211–220, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD: The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 27: 20–21, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME: X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 27: 18–20, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B: Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 203: 1693–1700, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK: Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441: 235–238, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Korn T, Bettelli E, Oukka M, Kuchroo VK: IL-17 and Th17 cells. Annu Rev Immunol 27: 485–517, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA: IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature 454: 350–352, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, Oukka M, Kuchroo VK: IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 448: 484–487, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng G, Gao W, Strom TB, Oukka M, Francis RS, Wood KJ, Bushell A: Exogenous IFN-gamma ex vivo shapes the alloreactive T-cell repertoire by inhibition of Th17 responses and generation of functional Foxp3+ regulatory T cells. Eur J Immunol 38: 2512–2527, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jäger A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK: Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol 183: 7169–7177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Summers SA, Steinmetz OM, Li M, Kausman JY, Semple T, Edgtton KL, Borza DB, Braley H, Holdsworth SR, Kitching AR: Th1 and Th17 cells induce proliferative glomerulonephritis. J Am Soc Nephrol 20: 2518–2524, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moriggl R, Kristofic C, Kinzel B, Volarevic S, Groner B, Brinkmann V: Activation of STAT proteins and cytokine genes in human Th1 and Th2 cells generated in the absence of IL-12 and IL-4. J Immunol 160: 3385–3392, 1998 [PubMed] [Google Scholar]
- 56.Penny MJ, Boyd RA, Hall BM: Permanent CD8(+) T cell depletion prevents proteinuria in active Heymann nephritis. J Exp Med 188: 1775–1784, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verma ND, Plain KM, Nomura M, Tran GT, Robinson C, Boyd R, Hodgkinson SJ, Hall BM: CD4+CD25+ T cells alloactivated ex vivo by IL-2 or IL-4 become potent alloantigen-specific inhibitors of rejection with different phenotypes, suggesting separate pathways of activation by Th1 and Th2 responses. Blood 113: 479–487, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Verma ND, Hall BM, Plain KM, Robinson CM, Boyd R, Tran GT, Wang C, Bishop GA, Hodgkinson SJ: Interleukin-12 (IL-12p70) promotes induction of highly potent Th1-like CD4+CD25+ T regulatory cells that inhibit allograft rejection in unmodified recipients. Front Immunol 5: 190, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pearce EJ, M Kane C, Sun J, J Taylor J, McKee AS, Cervi L: Th2 response polarization during infection with the helminth parasite Schistosoma mansoni. Immunol Rev 201: 117–126, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Penny MJ, Boyd RA, Hall BM: Role of T cells in the mediation of Heymann nephritis. ii. Identification of Th1 and cytotoxic cells in glomeruli. Kidney Int 51: 1059–1068, 1997 [DOI] [PubMed] [Google Scholar]
- 61.Mosmann TR, Coffman RL: TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 7: 145–173, 1989 [DOI] [PubMed] [Google Scholar]
- 62.Mosmann TR, Sad S: The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today 17: 138–146, 1996 [DOI] [PubMed] [Google Scholar]
- 63.Swain SL, Weinberg AD, English M, Huston G: IL-4 directs the development of Th2-like helper effectors. J Immunol 145: 3796–3806, 1990 [PubMed] [Google Scholar]
- 64.Plain KM, Chen J, Merten S, He XY, Hall BM: Induction of specific tolerance to allografts in rats by therapy with non-mitogenic, non-depleting anti-CD3 monoclonal antibody: Association with TH2 cytokines not anergy. Transplantation 67: 605–613, 1999 [DOI] [PubMed] [Google Scholar]
- 65.Tran GT, Hodgkinson SJ, Carter NM, Verma ND, Plain KM, Boyd R, Robinson CM, Nomura M, Killingsworth M, Hall BM: IL-5 promotes induction of antigen-specific CD4+CD25+ T regulatory cells that suppress autoimmunity. Blood 119: 4441–4450, 2012 [DOI] [PubMed] [Google Scholar]
- 66.Kitching AR, Tipping PG, Mutch DA, Huang XR, Holdsworth SR: Interleukin-4 deficiency enhances Th1 responses and crescentic glomerulonephritis in mice. Kidney Int 53: 112–118, 1998 [DOI] [PubMed] [Google Scholar]
- 67.Spicer ST, Ha H, Boyd RA, He XY, Carter N, Tran G, Penny MJ, Hodgkinson SJ, Hall BM: Il-4 therapy prevents the development of proteinuria in active Heymann nephritis by inhibition of Tc1 cells. J Immunol 167: 3725–3733, 2001 [DOI] [PubMed] [Google Scholar]
- 68.He XY, Chen J, Verma N, Plain K, Tran G, Hall BM: Treatment with interleukin-4 prolongs allogeneic neonatal heart graft survival by inducing T helper 2 responses. Transplantation 65: 1145–1152, 1998 [DOI] [PubMed] [Google Scholar]
- 69.He XY, Verma N, Chen J, Robinson C, Boyd R, Hall BM: IL-5 prolongs allograft survival by downregulating IL-2 and IFN-gamma cytokines. Transplant Proc 33: 703–704, 2001 [DOI] [PubMed] [Google Scholar]
- 70.Davidson C, Verma ND, Robinson CM, Plain KM, Tran GT, Hodgkinson SJ, Hall BM: IL-13 prolongs allograft survival: Association with inhibition of macrophage cytokine activation. Transpl Immunol 17: 178–186, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Hall BM, Fava L, Chen J, Plain KM, Boyd RA, Spicer ST, Berger MF: Anti-CD4 monoclonal antibody-induced tolerance to MHC-incompatible cardiac allografts maintained by CD4+ suppressor T cells that are not dependent upon IL-4. J Immunol 161: 5147–5156, 1998 [PubMed] [Google Scholar]
- 72.Plain KM, Verma ND, Tran GT, Nomura M, Boyd R, Robinson CM, Hodgkinson SJ, Hall BM: Cytokines affecting CD4(+) T regulatory cells in transplant tolerance. Interleukin-4 does not maintain alloantigen specific CD4(+)CD25(+) Treg. Transpl Immunol 29: 51–59, 2013 [DOI] [PubMed] [Google Scholar]
- 73.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C: Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29: 138–149, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR: T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol 173: 68–78, 2004 [DOI] [PubMed] [Google Scholar]
- 75.Hanabuchi S, Ito T, Park WR, Watanabe N, Shaw JL, Roman E, Arima K, Wang YH, Voo KS, Cao W, Liu YJ: Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. J Immunol 184: 2999–3007, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheng G, Yu A, Dee MJ, Malek TR: IL-2R signaling is essential for functional maturation of regulatory T cells during thymic development. J Immunol 190: 1567–1575, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shevach EM, Thornton AM: tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev 259: 88–102, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huehn J, Polansky JK, Hamann A: Epigenetic control of FOXP3 expression: The key to a stable regulatory T-cell lineage? Nat Rev Immunol 9: 83–89, 2009 [DOI] [PubMed] [Google Scholar]
- 79.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY: Foxp3-dependent programme of regulatory T-cell differentiation. Nature 445: 771–775, 2007 [DOI] [PubMed] [Google Scholar]
- 80.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY: Stability of the regulatory T cell lineage in vivo. Science 329: 1667–1671, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A: Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat Immunol 3: 33–41, 2002 [DOI] [PubMed] [Google Scholar]
- 82.Krummel MF, Allison JP: CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 182: 459–465, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM: Trans-endocytosis of CD80 and CD86: A molecular basis for the cell-extrinsic function of CTLA-4. Science 332: 600–603, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pardoll DM: The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12: 252–264, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hall BM, Robinson CM, Plain KM, Verma ND, Carter N, Boyd RA, Tran GT, Hodgkinson SJ: Studies on naïve CD4+CD25+T cells inhibition of naïve CD4+CD25- T cells in mixed lymphocyte cultures. Transpl Immunol 18: 291–301, 2008 [DOI] [PubMed] [Google Scholar]
- 86.Nomura M, Plain KM, Verma N, Robinson C, Boyd R, Hodgkinson SJ, Hall BM: The cellular basis of cardiac allograft rejection. IX. Ratio of naïve CD4+CD25+ T cells/CD4+CD25- T cells determines rejection or tolerance. Transpl Immunol 15: 311–318, 2006 [DOI] [PubMed] [Google Scholar]
- 87.Thornton AM, Shevach EM: CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med 188: 287–296, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thornton AM, Shevach EM: Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol 164: 183–190, 2000 [DOI] [PubMed] [Google Scholar]
- 89.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J: In vivo expansion of T reg cells with IL-2-mAb complexes: Induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med 206: 751–760, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M: Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 155: 1151–1164, 1995 [PubMed] [Google Scholar]
- 91.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM: Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol 172: 6519–6523, 2004 [DOI] [PubMed] [Google Scholar]
- 92.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB: Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant 7: 1722–1732, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Battaglia M, Stabilini A, Roncarolo MG: Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 105: 4743–4748, 2005 [DOI] [PubMed] [Google Scholar]
- 94.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG: Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol 177: 8338–8347, 2006 [DOI] [PubMed] [Google Scholar]
- 95.Tang Q, Lee K: Regulatory T-cell therapy for transplantation: How many cells do we need? Curr Opin Organ Transplant 17: 349–354, 2012 [DOI] [PubMed] [Google Scholar]
- 96.Marek-Trzonkowska N, Myśliwiec M, Dobyszuk A, Grabowska M, Derkowska I, Juścińska J, Owczuk R, Szadkowska A, Witkowski P, Młynarski W, Jarosz-Chobot P, Bossowski A, Siebert J, Trzonkowski P: Therapy of type 1 diabetes with CD4(+)CD25(high)CD127-regulatory T cells prolongs survival of pancreatic islets - results of one year follow-up. Clin Immunol 153: 23–30, 2014 [DOI] [PubMed] [Google Scholar]
- 97.Trzonkowski P, Bieniaszewska M, Juścińska J, Dobyszuk A, Krzystyniak A, Marek N, Myśliwska J, Hellmann A: First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol 133: 22–26, 2009 [DOI] [PubMed] [Google Scholar]
- 98.Geissler EK, Hutchinson JA: Cell therapy as a strategy to minimize maintenance immunosuppression in solid organ transplant recipients. Curr Opin Organ Transplant 18: 408–415, 2013 [DOI] [PubMed] [Google Scholar]
- 99.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA: In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med 199: 1455–1465, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Juvet SC, Whatcott AG, Bushell AR, Wood KJ: Harnessing regulatory T cells for clinical use in transplantation: the end of the beginning. Am J Transplant 14: 750–763, 2014 [DOI] [PubMed] [Google Scholar]
- 101.Hall BM, Tran GT, Verma ND, Plain KM, Robinson CM, Nomura M, Hodgkinson SJ: Do natural T regulatory cells become activated to antigen specific T regulatory cells in transplantation and in autoimmunity? Front Immunol 4: 208, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hall BM, Verma ND, Tran GT, Hodgkinson SJ: Distinct regulatory CD4+T cell subsets; differences between naïve and antigen specific T regulatory cells. Curr Opin Immunol 23: 641–647, 2011 [DOI] [PubMed] [Google Scholar]
- 103.Pearce NW, Spinelli A, Gurley KE, Hall BM: Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporine. V. Dependence of CD4+ suppressor cells on the presence of alloantigen and cytokines, including interleukin 2. Transplantation 55: 374–380, 1993 [DOI] [PubMed] [Google Scholar]
- 104.Daniel V, Sadeghi M, Wang H, Opelz G: CD4+CD25+Foxp3+IFN-γ+ human induced T regulatory cells are induced by interferon-γ and suppress alloresponses nonspecifically. Hum Immunol 72: 699–707, 2011 [DOI] [PubMed] [Google Scholar]
- 105.Hall AO, Beiting DP, Tato C, John B, Oldenhove G, Lombana CG, Pritchard GH, Silver JS, Bouladoux N, Stumhofer JS, Harris TH, Grainger J, Wojno ED, Wagage S, Roos DS, Scott P, Turka LA, Cherry S, Reiner SL, Cua D, Belkaid Y, Elloso MM, Hunter CA: The cytokines interleukin 27 and interferon-γ promote distinct Treg cell populations required to limit infection-induced pathology. Immunity 37: 511–523, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Feng T, Cao AT, Weaver CT, Elson CO, Cong Y: Interleukin-12 converts Foxp3+ regulatory T cells to interferon-γ-producing Foxp3+ T cells that inhibit colitis. Gastroenterology 140: 2031–2043, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Feng G, Wood KJ, Bushell A: Interferon-gamma conditioning ex vivo generates CD25+CD62L+Foxp3+ regulatory T cells that prevent allograft rejection: Potential avenues for cellular therapy. Transplantation 86: 578–589, 2008 [DOI] [PubMed] [Google Scholar]
- 108.Francis RS, Feng G, Tha-In T, Lyons IS, Wood KJ, Bushell A: Induction of transplantation tolerance converts potential effector T cells into graft-protective regulatory T cells. Eur J Immunol 41: 726–738, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maerten P, Shen C, Bullens DM, Van Assche G, Van Gool S, Geboes K, Rutgeerts P, Ceuppens JL: Effects of interleukin 4 on CD25+CD4+ regulatory T cell function. J Autoimmun 25: 112–120, 2005 [DOI] [PubMed] [Google Scholar]
- 110.Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY: Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature 458: 351–356, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F: A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204: 1757–1764, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weiner HL, da Cunha AP, Quintana F, Wu H: Oral tolerance. Immunol Rev 241: 241–259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huter EN, Punkosdy GA, Glass DD, Cheng LI, Ward JM, Shevach EM: TGF-beta-induced Foxp3+ regulatory T cells rescue scurfy mice. Eur J Immunol 38: 1814–1821, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huter EN, Stummvoll GH, DiPaolo RJ, Glass DD, Shevach EM: Cutting edge: Antigen-specific TGF beta-induced regulatory T cells suppress Th17-mediated autoimmune disease. J Immunol 181: 8209–8213, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR: TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 453: 236–240, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mantel PY, Schmidt-Weber CB: Transforming growth factor-beta: recent advances on its role in immune tolerance. Methods Mol Biol 677: 303–338, 2011 [DOI] [PubMed] [Google Scholar]
- 117.Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A, Kuchroo VK, Oukka M: IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A 105: 18460–18465, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, Khoury S, Oukka M, Kuchroo VK: IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol 9: 1347–1355, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Allan SE, Alstad AN, Merindol N, Crellin NK, Amendola M, Bacchetta R, Naldini L, Roncarolo MG, Soudeyns H, Levings MK: Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol Ther 16: 194–202, 2008 [DOI] [PubMed] [Google Scholar]
- 120.Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM: IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo. J Immunol 186: 6329–6337, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Koenecke C, Czeloth N, Bubke A, Schmitz S, Kissenpfennig A, Malissen B, Huehn J, Ganser A, Förster R, Prinz I: Alloantigen-specific de novo-induced Foxp3+ Treg revert in vivo and do not protect from experimental GVHD. Eur J Immunol 39: 3091–3096, 2009 [DOI] [PubMed] [Google Scholar]
- 122.Groux H: Type 1 T-regulatory cells: Their role in the control of immune responses. Transplantation 75[Suppl]: 8S–12S, 2003 [DOI] [PubMed] [Google Scholar]
- 123.Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, Hauben E, Roncarolo MG: Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood 116: 935–944, 2010 [DOI] [PubMed] [Google Scholar]
- 124.Gregori S, Goudy KS, Roncarolo MG: The cellular and molecular mechanisms of immuno-suppression by human type 1 regulatory T cells. Front Immunol 3: 30, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Starzl TE, Zinkernagel RM: Transplantation tolerance from a historical perspective. Nat Rev Immunol 1: 233–239, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zinkernagel RM, Planz O, Ehl S, Battegay M, Odermatt B, Klenerman P, Hengartner H: General and specific immunosuppression caused by antiviral T-cell responses. Immunol Rev 168: 305–315, 1999 [DOI] [PubMed] [Google Scholar]
- 127.Arakaki R, Yamada A, Kudo Y, Hayashi Y, Ishimaru N: Mechanism of activation-induced cell death of T cells and regulation of FasL expression. Crit Rev Immunol 34: 301–314, 2014 [DOI] [PubMed] [Google Scholar]
- 128.Weiss EM, Schmidt A, Vobis D, Garbi N, Lahl K, Mayer CT, Sparwasser T, Ludwig A, Suri-Payer E, Oberle N, Krammer PH: Foxp3-mediated suppression of CD95L expression confers resistance to activation-induced cell death in regulatory T cells. J Immunol 187: 1684–1691, 2011 [DOI] [PubMed] [Google Scholar]
- 129.Allione A, Bernabei P, Bosticardo M, Ariotti S, Forni G, Novelli F: Nitric oxide suppresses human T lymphocyte proliferation through IFN-gamma-dependent and IFN-gamma-independent induction of apoptosis. J Immunol 163: 4182–4191, 1999 [PubMed] [Google Scholar]
- 130.Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA: Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol 10: 1185–1192, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Saraiva M, O’Garra A: The regulation of IL-10 production by immune cells. Nat Rev Immunol 10: 170–181, 2010 [DOI] [PubMed] [Google Scholar]
- 132.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL: A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol 8: 1380–1389, 2007 [DOI] [PubMed] [Google Scholar]
- 133.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA: The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity 19: 645–655, 2003 [DOI] [PubMed] [Google Scholar]
- 134.Yoshida H, Nakaya M, Miyazaki Y: Interleukin 27: A double-edged sword for offense and defense. J Leukoc Biol 86: 1295–1303, 2009 [DOI] [PubMed] [Google Scholar]
- 135.Vignali DA, Kuchroo VK: IL-12 family cytokines: immunological playmakers. Nat Immunol 13: 722–728, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cobbold S, Waldmann H: Infectious tolerance. Curr Opin Immunol 10: 518–524, 1998 [DOI] [PubMed] [Google Scholar]
- 137.Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O’Shea JJ, Shevach EM: CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-β-dependent manner. J Exp Med 205: 1975–1981, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA: IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol 11: 1093–1101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr., Muller W, Rudensky AY: Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28: 546–558, 2008 [DOI] [PubMed] [Google Scholar]
- 140.Pillai MR, Collison LW, Wang X, Finkelstein D, Rehg JE, Boyd K, Szymczak-Workman AL, Doggett T, Griffith TS, Ferguson TA, Vignali DA: The plasticity of regulatory T cell function. J Immunol 187: 4987–4997, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC: Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 204: 1257–1265, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Beeston T, Smith TR, Maricic I, Tang X, Kumar V: Involvement of IFN-γ and perforin, but not Fas/FasL interactions in regulatory T cell-mediated suppression of experimental autoimmune encephalomyelitis. J Neuroimmunol 229: 91–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gondek DC, Devries V, Nowak EC, Lu LF, Bennett KA, Scott ZA, Noelle RJ: Transplantation survival is maintained by granzyme B+ regulatory cells and adaptive regulatory T cells. J Immunol 181: 4752–4760, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sakaguchi S, Vignali DAA, Rudensky AY, Niec RE, Waldmann H: The plasticity and stability of regulatory T cells. Nat Rev Immunol 13: 461–467, 2013 [DOI] [PubMed] [Google Scholar]
- 145.Hori S: Lineage stability and phenotypic plasticity of Foxp3⁺ regulatory T cells. Immunol Rev 259: 159–172, 2014 [DOI] [PubMed] [Google Scholar]
- 146.Sawant DV, Vignali DAA: Once a Treg, always a Treg? Immunol Rev 259: 173–191, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kleinewietfeld M, Hafler DA: The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol 25: 305–312, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ohkura N, Hamaguchi M, Morikawa H, Sugimura K, Tanaka A, Ito Y, Osaki M, Tanaka Y, Yamashita R, Nakano N, Huehn J, Fehling HJ, Sparwasser T, Nakai K, Sakaguchi S: T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 37: 785–799, 2012 [DOI] [PubMed] [Google Scholar]
- 149.Duarte JH, Zelenay S, Bergman M-L, Martins AC, Demengeot J: Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur J Immunol 39: 948–955, 2009 [DOI] [PubMed] [Google Scholar]
- 150.Xu L, Kitani A, Fuss I, Strober W: Cutting edge: Regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol 178: 6725–6729, 2007 [DOI] [PubMed] [Google Scholar]
- 151.Holdsworth SR, Tipping PG: Cell-mediated immunity in glumerulonephritis. In: Immunology of Renal Disease, edited by Pusey CD, London, Kluwer Academic Publishers, 1991, pp 97–122 [Google Scholar]
- 152.Tipping PG, Kitching AR, Huang XR, Mutch DA, Holdsworth SR: Immune modulation with interleukin-4 and interleukin-10 prevents crescent formation and glomerular injury in experimental glomerulonephritis. Eur J Immunol 27: 530–537, 1997 [DOI] [PubMed] [Google Scholar]
- 153.Holdsworth SR, Gan P-Y: Cytokines: Names and numbers you should care about [published online ahead of print May 4, 2015]. Clin J Am Soc Nephrol doi: 10.2215/CJN.07590714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cheng IKP, Dorsch SE, Hall BM: The regulation of autoantibody production in Heymann’s nephritis by T lymphocyte subsets. Lab Invest 59: 780–788, 1988 [PubMed] [Google Scholar]
- 155.Savransky V, Molls RR, Burne-Taney M, Chien CC, Racusen L, Rabb H: Role of the T-cell receptor in kidney ischemia-reperfusion injury. Kidney Int 69: 233–238, 2006 [DOI] [PubMed] [Google Scholar]
- 156.Kinsey GR, Okusa MD: Expanding role of T cells in acute kidney injury. Curr Opin Nephrol Hypertens 23: 9–16, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kinsey GR, Sharma R, Okusa MD: Regulatory T cells in AKI. J Am Soc Nephrol 24: 1720–1726, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Burne-Taney MJ, Yokota-Ikeda N, Rabb H: Effects of combined T- and B-cell deficiency on murine ischemia reperfusion injury. Am J Transplant 5: 1186–1193, 2005 [DOI] [PubMed] [Google Scholar]
- 159.Lai LW, Yong KC, Igarashi S, Lien YH: A sphingosine-1-phosphate type 1 receptor agonist inhibits the early T-cell transient following renal ischemia-reperfusion injury. Kidney Int 71: 1223–1231, 2007 [DOI] [PubMed] [Google Scholar]
- 160.Faubel S, Ljubanovic D, Poole B, Dursun B, He Z, Cushing S, Somerset H, Gill RG, Edelstein CL: Peripheral CD4 T-cell depletion is not sufficient to prevent ischemic acute renal failure. Transplantation 80: 643–649, 2005 [DOI] [PubMed] [Google Scholar]
- 161.Hall BM: Cells mediating allograft rejection. Transplantation 51: 1141–1151, 1991 [DOI] [PubMed] [Google Scholar]
- 162.Hall BM, Gurley KE, Dorsch SE: The possible role of cytotoxic T cells in the mediation of first-set allograft rejection. Transplantation 40: 336–339, 1985 [PubMed] [Google Scholar]
- 163.Plain KM, Fava L, Spinelli A, He XY, Chen J, Boyd R, Davidson CL, Hall BM: Induction of tolerance with nondepleting anti-CD4 monoclonal antibodies is associated with down-regulation of TH2 cytokines. Transplantation 64: 1559–1567, 1997 [DOI] [PubMed] [Google Scholar]
- 164.Hall BM, Jelbart ME, Gurley KE, Dorsch SE: Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporine. Mediation of specific suppression by T helper/inducer cells. J Exp Med 162: 1683–1694, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]