Abstract
Background and objective
ABO blood types are determined by antigen modifications on glycoproteins and glycolipids and associated with altered plasma levels of inflammatory and endothelial injury markers implicated in AKI pathogenesis. We sought to determine the association of ABO blood types with AKI risk in critically ill patients with trauma or sepsis.
Design, setting, participants, & measurements
We conducted two prospective cohort studies at an urban, academic, level I trauma center and tertiary referral center; 497 patients with trauma admitted to the surgical intensive care unit between 2005 and 2010 with an injury severity score >15 and 759 patients with severe sepsis admitted to the medical intensive care unit between 2008 and 2013 were followed for 6 days for the development of incident AKI. AKI was defined by Acute Kidney Injury Network creatinine and dialysis criteria.
Results
Of 497 patients with trauma, 134 developed AKI (27%). In multivariable analysis, blood type A was associated with higher AKI risk relative to type O among patients of European descent (n=229; adjusted risk, 0.28 versus 0.14; risk difference, 0.14; 95% confidence interval, 0.03 to 0.24; P=0.02). Of 759 patients with sepsis, AKI developed in 326 (43%). Blood type A again conferred higher AKI risk relative to type O among patients of European descent (n=437; adjusted risk, 0.53 versus 0.40; risk difference, 0.14; 95% confidence interval, 0.04 to 0.23; P=0.01). Findings were similar when analysis was restricted to those patients who did not develop acute respiratory distress syndrome or were not transfused. We did not detect a significant association between blood type and AKI risk among individuals of African descent in either cohort.
Conclusions
Blood type A is independently associated with AKI risk in critically ill patients with trauma or severe sepsis of European descent, suggesting a role for ABO glycans in AKI susceptibility.
Keywords: ABO histoblood group system, AKI, sepsis, trauma, critical illness
Introduction
AKI is common among the critically ill and associated with significant mortality and morbidity (1–3). The mechanisms underlying the development of AKI remain incompletely understood, leading to a dearth of specific therapies beyond supportive care and renal replacement. Although evidence from human studies and animal models suggests some differences in AKI pathogenesis in the critically ill population, certain mechanisms are likely shared across at-risk groups (4,5). Factors that may influence the development of AKI, including renal ischemia, host immune response, and endothelial activation, are common in severe sepsis, major trauma, and other critical illness syndromes (5–10).
Recent studies have implicated the ABO histo-blood group system as a potential mediator of endothelial dysfunction, microcirculatory coagulation, and inflammation linked to risk of acute myocardial infarction (MI), venous thromboembolism (VTE), and acute respiratory distress syndrome (ARDS) (11–16). The ABO histo-blood group system, originally described in red blood cells, consists of terminal carbohydrate modifications on glycoproteins and glycolipids (17). The ABO antigens are major components of the glycocalyx coating the endothelium and epithelium of several tissues (11,18). In the kidneys, ABO antigens are located on the renal vascular endothelium of arteries, veins, and peritubular and glomerular capillaries in addition to the epithelial cells of the convoluted tubules and collecting ducts (19). Although the precise biologic significance of the ABO antigens is not fully known, they are hypothesized to be important mediators of cell-cell interactions, including the leukocyte-endothelial cell interactions that are important in AKI pathogenesis (5–7,11). Genetic polymorphisms in the ABO gene have been associated with circulating levels of glycoproteins important in endothelial function and inflammation, including soluble intercellular adhesion molecule-1 (ICAM-1), thrombomodulin, vWF, and the selectins (12,20–23). These same proteins have been implicated in the pathogenesis of AKI (24–28). Although non–O blood types are reported risk factors for MI, VTE, and ARDS, it is unknown if ABO blood type is associated with AKI risk (13–16).
The objective of this study was to determine if specific ABO blood types represent a common risk factor for AKI across two separate populations of critically ill patients: major trauma and severe sepsis. Because blood type A has shown the strongest association with higher ARDS, MI, and VTE risk, we hypothesized that blood type A would be associated with a higher risk of AKI relative to type O. Although many aspects of AKI pathogenesis likely differ between patients with trauma and patients with sepsis, we also hypothesized that shared mechanisms of AKI development would result in similar associations of ABO blood type and AKI risk in both populations. Some of the results described here have been presented in abstract form (Reilly J, et al., unpublished data).
Materials and Methods
Study Design
We conducted two independent cohort studies of critically ill patients admitted to the Hospital of the University of Pennsylvania, an urban tertiary referral center and level I trauma center. The first cohort consisted of severely injured patients with trauma, and the second cohort consisted of patients with severe sepsis.
Trauma Population
Severely injured patients with trauma admitted through the emergency department (ED) to the surgical intensive care unit (ICU) were screened prospectively for enrollment between 2005 and 2010. Detailed inclusion and exclusion criteria have been previously described (29,30). Patients were included if they had an injury severity score >15 (31). Patients were excluded for isolated severe head injury, death, or discharge from the ICU within 24 hours of presentation, age <14 years old, or need for chronic dialysis before presentation. The Institutional Review Board of the University of Pennsylvania approved the study with a waiver of informed consent.
Severe Sepsis Population
Patients admitted to the medical ICU from the ED or transferred from hospital wards or other institutions were screened for enrollment in the Molecular Epidemiology of Severe Sepsis in the ICU (MESSI) Cohort Study between 2008 and 2013. Patients were enrolled if the primary reason for ICU admission was severe sepsis as defined by the American College of Chest Physicians (ACCP) consensus definition (32). Patients were excluded for a lack of commitment to life-sustaining measures at the time of screening, admission from a long–term acute care facility, a primary reason for ICU admission unrelated to sepsis, or need for chronic dialysis before presentation. The Institutional Review Board of the University of Pennsylvania approved the study with a waiver of timely informed consent. Consent was obtained from patients or their surrogates as soon as feasible, and patients could withdraw consent at any time.
Data Collection
Trained research personnel collected clinical data for both cohorts, including demographics and chronic health information, and for patients with trauma, mechanism, severity, and location of injury. Blood transfusions, physiologic and laboratory variables, and microbiologic data were tracked over the first 6 days in the ICU. ABO blood type was determined using standard methods by the hospital blood bank for clinical purposes and extracted from the medical record. Patients missing ABO blood type were excluded from final analyses.
Outcome Definition
The primary outcome was the development of AKI within 6 days of ICU admission as defined by the Acute Kidney Injury Network (AKIN) creatinine and RRT consensus criteria (33). We chose to follow patients for 6 days to capture AKI reflective of the pathogenic mechanisms involved in acute trauma or severe sepsis and limit the confounding effects of later ICU interventions and complications. AKIN criteria define AKI as an increase in serum creatinine ≥0.3 mg/dl or ≥50% over a 48-hour period or the need for acute RRT. We determined whether such an increase occurred over any 2-day interval through the first 6 days of ICU admission starting at ED presentation or if transferred from a hospital ward, 8 hours before ICU admission. Staging was determined for each patient who met criteria for AKI using creatinine and RRT data collected for 7 additional days after the diagnosis of AKI. We collected creatinine values within the 6 months before enrollment when available. Because we would have had to estimate baseline creatinine for many patients, disproportionately so in the trauma cohort, we did not use prehospitalization values as part of the AKI definition (34). We did not include AKIN urine output criteria, because hourly urine output data were not available, and patients did not universally have urinary catheters during the study period.
Statistical Analyses
Analyses for the trauma and MESSI cohorts were conducted separately but with identical approaches. Clinical characteristics were compared between those patients with and without AKI as well as across ABO blood types using the Pearson chi–squared, Fisher exact, Wilcoxon rank sum, or Kruskal–Wallis test as appropriate. We used multivariable logistic regression to adjust the association of ABO blood type with AKI for potential confounders. Our primary analysis compared the two most common ABO blood types, A and O, and excluded patients with the less common blood types. Secondary analyses were conducted comparing each of four main blood types with each other. We considered all baseline variables listed in Table 1 that had an unadjusted association with AKI or ABO blood type at P<0.20 for inclusion in the multivariable models. Using backward elimination, we then removed variables that did not alter the odds ratio for the association of ABO blood type and AKI by ≥10% to reach our final models (35). Certain variables, including severity of illness, age, sex, and red blood cell transfusion, were included in all final models. To adjust for severity of illness, we used Acute Physiology, Age, Chronic Health Evaluation III (APACHE III) scores without renal components to avoid colinearity with the outcome of AKI. All analyses were a priori stratified by race given the substantial differences in blood type frequencies and genetic background between racial groups. Postestimation marginal analysis was used for each final logistic regression model to compute standardized, adjusted AKI risks as well as convert odds ratios to risk differences (RDs) by ABO blood type, including 95% confidence intervals (95% CIs) (36). On the basis of our fixed sample sizes and the relative frequencies of blood types A and O in each race, we estimated that we would have 80% power to detect 15% and 18% differences in AKI risk in patients with trauma of European and African descent, respectively, and 13% and 20% AKI RDs in patients with sepsis of European and African descent, respectively.
Table 1.
Patient characteristics by presence or absence of AKI
| Characteristics | Trauma Cohort | Severe Sepsis Cohort | ||||
|---|---|---|---|---|---|---|
| AKI (n=134; 27%) | No AKI (n=363; 73%) | P Valuei | AKI (n=335; 45%) | No AKI (n=417; 55%) | P Valuei | |
| Demographics | ||||||
| Race | <0.01 | 0.68 | ||||
| African descent | 85 (63%) | 168 (46%) | 106 (32%) | 136 (33%) | ||
| European descent | 48 (36%) | 181 (50%) | 201 (60%) | 236 (57%) | ||
| Asian descent | 1 (1%) | 10 (3%) | 7 (2%) | 12 (3%) | ||
| Other | 0 (0%) | 4 (1%) | 14 (4%) | 18 (4%) | ||
| Unknown | 0 (0%) | 0 (0%) | 7 (2%) | 15 (4%) | ||
| Age (yr) | 35 (23–69) | 36 (24–50) | 0.70 | 60 (52–68) | 63 (52–68) | 0.04 |
| Men | 102 (76%) | 268 (74%) | 0.60 | 207 (62%) | 221 (53%) | 0.02 |
| Body mass index (kg/m2) | 27 (24–31) | 26 (22–29) | <0.001 | 27 (23–32) | 26 (22–31) | <0.01 |
| Medical history | ||||||
| Hypertension | 24 (18%) | 56 (15%) | 0.50 | 161 (48%) | 207 (51%) | 0.52 |
| Diabetes mellitus | 13 (10%) | 13 (4%) | <0.01 | 97 (29%) | 119 (29%) | 0.92 |
| CKD | 0 (0%) | 2 (1%) | >0.99 | 41 (13%) | 39 (10%) | 0.22 |
| Congestive heart failurea | 0 (0%) | 0 (0%) | >0.99 | 56 (17%) | 47 (11%) | 0.03 |
| Malignancyb | 0 (0%) | 0 (0%) | >0.99 | 116 (35%) | 139 (34%) | 0.75 |
| Smoking historyc | 51 (40%) | 152 (43%) | 0.56 | 175 (60%) | 224 (60%) | 0.93 |
| Initial creatinine (mg/dl) | 1.2 (0.9–1.4) | 1.1 (0.9–1.3) | <0.01 | 1.6 (1.0–2.5) | 1.3 (0.9–2.0) | <0.001 |
| Severity and mechanism | ||||||
| APACHE III score | 46 (37–61) | 34 (26–41) | <0.001 | 82 (67–98) | 70 (56–85) | <0.001 |
| APACHE III without renald | 42 (30–51) | 31 (25–37) | <0.001 | 74 (61–89) | 64 (49–77) | <0.001 |
| Injury severity scoree | 25 (19–29) | 22 (19–29) | 0.17 | N/A | N/A | |
| Mechanism of traumae | <0.001 | N/A | N/A | |||
| Blunt | 76 (57%) | 272 (75%) | ||||
| Penetrating | 58 (43%) | 91 (25%) | ||||
| Surgery performede | 96 (72%) | 180 (50%) | <0.001 | N/A | N/A | |
| Blood transfusions (%)f | ||||||
| Red blood cells | 104 (78%) | 197 (55%) | <0.001 | 32 (10%) | 15 (4%) | <0.01 |
| Platelets | 51 (38%) | 68 (19%) | <0.001 | 34 (10%) | 23 (6%) | 0.02 |
| Fresh frozen plasma | 88 (66%) | 130 (36%) | <0.001 | 26 (8%) | 8 (2%) | <0.01 |
| Nephrotoxinsg | ||||||
| Intravenous contrast | 74 (55%) | 281 (77%) | <0.01 | 61 (18%) | 108 (26%) | 0.01 |
| Amioglycosides | 20 (15%) | 39 (11%) | 0.21 | 151 (45%) | 110 (26%) | <0.001 |
| NSAIDs | 4 (3%) | 8 (2%) | 0.62 | 17 (5%) | 23 (6%) | 0.79 |
| Calcineurin inhibitor | 0 (0%) | 0 (0%) | >0.99 | 48 (14%) | 43 (10%) | 0.09 |
| Source of infection | 0.07 | |||||
| Pulmonary | N/A | N/A | 148 (44%) | 172 (41%) | ||
| Genitourinary | 29 (9%) | 57 (14%) | ||||
| Abdominal/gastrointestinal | 50 (15%) | 52 (12%) | ||||
| Head/neck | 9 (3%) | 6 (1%) | ||||
| Blood | 25 (7%) | 38 (9%) | ||||
| Skin/soft tissue/bone | 12 (4%) | 27 (6%) | ||||
| Gynecologic | 0 (0%) | 2 (1%) | ||||
| Unknown | 62 (19%) | 63 (15%) | ||||
| ABO blood type | 0.01 | 0.08 | ||||
| A | 49 (37%) | 116 (32%) | 143 (43%) | 140 (34%) | ||
| B | 30 (22%) | 49 (14%) | 44 (13%) | 61 (15%) | ||
| AB | 3 (2%) | 24 (7%) | 14 (4%) | 22 (5%) | ||
| O | 52 (39%) | 174 (48%) | 134 (40%) | 194 (47%) | ||
| Outcome | ||||||
| 30-d Mortality | 27 (20%) | 15 (4%) | <0.001 | 198 (59%) | 131 (31%) | <0.001 |
| ICU length of stay (d)h | 20 (9–38) | 7 (4–17) | <0.001 | ∞ (7–∞) | 6 (2–∞) | <0.001 |
Data are shown as n (%) for categorical variables and median (interquartile range) for continuous variables. APACHE III, Acute Physiology, Age, Chronic Health Evaluation III; NSAID, nonsteroidal anti–inflammatory drug; ICU, intensive care unit; N/A, not applicable.
Patients with congestive heart failure were excluded from enrollment in the trauma cohort.
Metastatic solid malignancy or hematologic malignancy.
Smoking history includes current and former cigarette smokers.
APACHE III without renal refers to the APACHE III score with the renal components removed.
Injury severity score, mechanism of trauma, and surgery performed are only relevant to the trauma cohort. Source of infection is only relevant to the severe sepsis cohort. Surgery performed refers to patients undergoing emergent surgical intervention in an operating room between emergency department presentation and ICU admission.
Blood transfusion refers to the percentage of patients transfused during the first 24 hours of resuscitation (trauma cohort) or the first 24 hours of ICU admission (severe sepsis cohort).
Nephrotoxins refer to the exposure of intravenous contrast or aminoglycosides during the first 6 days of admission and a medical history of NSAID or calcineurin inhibitor use before admission. In our cohort, all patients with a medical history of calcineurin inhibitors had undergone a solid organ or bone marrow transplant.
Intensive care unit length of stay was assumed to be infinite in patients who died. Given that >50% of patients with AKI died in the severe sepsis cohort, the median ICU length of stay is infinity.
Continuous variables were compared using a Wilcoxon rank sum test, and categorical variables were compared using a Pearson chi–squared or Fisher’s exact test.
In secondary analyses, we determined the association of ABO blood type and AKI stage. Given the limited number of patients with stage 3 AKI, those with stages 2 and 3 AKI were combined. Patients with stage 2/3 AKI and those with stage 1 AKI were separately compared with patients without AKI using multivariable logistic regression. To determine independence from our previously reported associations between ABO blood types and ARDS (14), we also conducted analyses stratified by the development of ARDS. ARDS was phenotyped using the Berlin definition and our previously published methods over the first 6 days of ICU admission (37,38). We also conducted analyses within the subgroup of patients who did not receive blood product transfusions during the first 24 hours of enrollment. In the severe sepsis cohort, acute renal dysfunction at ICU admission was one measure of organ dysfunction that could qualify a patient’s sepsis as severe (32). To ensure that using renal dysfunction at enrollment as an inclusion criterion did not bias our results, we conducted additional analyses in this cohort restricted to patients who did not have acute renal dysfunction at ICU admission. All statistical analyses were performed using Stata/IC 12.0 (StataCorp LP, College Station, TX).
Results
Trauma Population
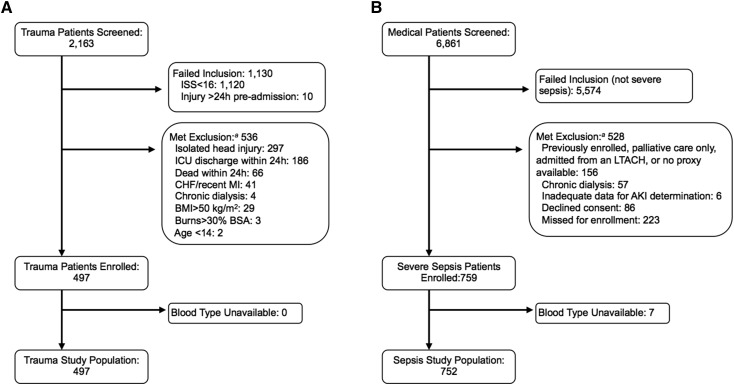
We enrolled 497 patients with trauma (Figure 1) with a median age of 35 years old and injury severity score of 22. The majority of patients were of African (51%) or European descent (45%). AKI developed in 134 (27%) patients. Comparisons of patient characteristics by AKI status are provided in Table 1. As we have reported previously, risk factors for AKI included African ancestry, increased body mass index, and transfusion of blood products (29). ABO blood type was available in all 497 (100%) patients with trauma. Frequencies of the ABO blood types by race were similar to reported distributions in the United States population (39). Distributions and unadjusted associations between clinical variables and ABO blood type are provided in Table 2.
Figure 1.
Screening and enrollment. (A) Trauma cohort. (B) Sepsis cohort. aSome patients met more than one exclusion criterion. BMI, body mass index; BSA, body surface area; CHF, congestive heart failure; ICU, intensive care unit; ISS, injury severity score; LTACH, long–term acute care hospital; MI, myocardial infarction.
Table 2.
Characteristics of patients with trauma by ABO blood type
| Characteristics | ABO Blood Type | P Valueg | |||
|---|---|---|---|---|---|
| A (n=165; 33%) | B (n=79; 16%) | AB (n=27; 6%) | O (n=226; 45%) | ||
| Demographics | |||||
| Race | <0.001 | ||||
| African descent | 64 (39%) | 50 (63%) | 12 (44%) | 127 (56%) | |
| European descent | 94 (57%) | 28 (35%) | 12 (44%) | 95 (42%) | |
| Asian descent | 6 (3%) | 1 (1%) | 2 (7%) | 2 (1%) | |
| Other | 1 (1%) | 0 (0%) | 1 (4%) | 2 (1%) | |
| Age (yr) | 39 (26–52) | 39 (22–54) | 47 (26–59) | 32 (23–46) | 0.08 |
| Men | 119 (72%) | 54 (68%) | 21 (78%) | 176 (78%) | 0.31 |
| Body mass index (kg/m2) | 26 (23–30) | 27 (24–31) | 27 (24–31) | 26 (23–30) | 0.90 |
| Medical history | |||||
| Hypertension | 27 (16%) | 18 (23%) | 9 (33%) | 26 (12%) | <0.01 |
| Diabetes mellitus | 8 (5%) | 6 (8%) | 3 (11%) | 9 (4%) | 0.25 |
| CKD | 1 (1%) | 1 (1%) | 0 (0%) | 0 (0%) | 0.24 |
| Smoking historya | 55 (34%) | 29 (39%) | 10 (37%) | 109 (49%) | 0.02 |
| Initial creatinine (mg/dl) | 1.1 (0.9–1.2) | 1.2 (0.9–1.4) | 1.1 (0.8–1.4) | 1.1 (0.9–1.3) | 0.10 |
| Severity and mechanism | |||||
| APACHE III score | 36 (28–46) | 39 (31–49) | 36 (25–41) | 36 (29–45) | 0.39 |
| APACHE III without renalb | 31 (26–42) | 36 (26–43) | 33 (25–37) | 32 (26–41) | 0.53 |
| Injury severity score | 22 (19–29) | 22 (19–29) | 22 (20–29) | 22 (18–29) | 0.89 |
| Mechanism of trauma | 0.17 | ||||
| Blunt | 125 (76%) | 50 (63%) | 20 (74%) | 153 (68%) | |
| Penetrating | 40 (24%) | 29 (37%) | 7 (26%) | 73 (32%) | |
| Surgery performedc | 87 (53%) | 45 (57%) | 13 (48%) | 131 (58%) | 0.63 |
| Blood transfusions (%)d | |||||
| Red blood cells | 99 (60%) | 53 (67%) | 12 (44%) | 137 (61%) | 0.22 |
| Platelets | 41 (25%) | 24 (30%) | 4 (15%) | 50 (22%) | 0.32 |
| Fresh frozen plasma | 73 (44%) | 39 (49%) | 4 (15%) | 50 (22%) | 0.71 |
| Nephrotoxinse | |||||
| Intravenous contrast | 119 (72%) | 54 (68%) | 18 (67%) | 164 (73%) | 0.84 |
| Aminoglycosides | 19 (12%) | 10 (13%) | 1 (4%) | 29 (13%) | 0.60 |
| NSAIDs | 4 (2%) | 1 (1%) | 1 (4%) | 6 (3%) | 0.88 |
| Outcomes | |||||
| 30-d Mortality | 21 (13%) | 5 (6%) | 1 (4%) | 15 (7%) | 0.11 |
| ICU length of stay (d)f | 10 (5–23) | 9 (4–26) | 10 (4–19) | 10 (4–19) | 0.46 |
| AKI (any stage) | 49 (30%) | 30 (38%) | 3 (11%) | 52 (23%) | 0.01 |
| AKI (stage 2 or 3) | 13 (8%) | 4 (5%) | 2 (7%) | 16 (7%) | 0.88 |
| Need for dialysis | 11 (7%) | 1 (1%) | 1 (4%) | 9 (4%) | 0.26 |
Data are shown as n (%) for categorical variables and median (interquartile range) for continuous variables. APACHE III, Acute Physiology, Age, Chronic Health Evaluation III; NSAID, nonsteroidal anti–inflammatory drug; ICU, intensive care unit.
Smoking history includes current and former cigarette smokers.
APACHE III without renal refers to the APACHE III score with the renal components removed.
Surgery performed refers to patient undergoing emergent surgical intervention in an operating room between emergency department presentation and ICU admission.
Blood transfusions are presented as the number (%) of patients who received a transfusion of the specified blood product in the first 24 hours after emergency department presentation.
Nephrotoxins refer to the exposure of intravenous contrast or aminoglycosides during the first 6 days of admission and a medical history of NSAID use just before admission.
ICU length of stay was assumed to be infinite in patients who died.
Continuous variables were compared using a Kruskal–Wallis test, and categorical variables were compared using a Pearson chi–squared or Fisher’s exact test.
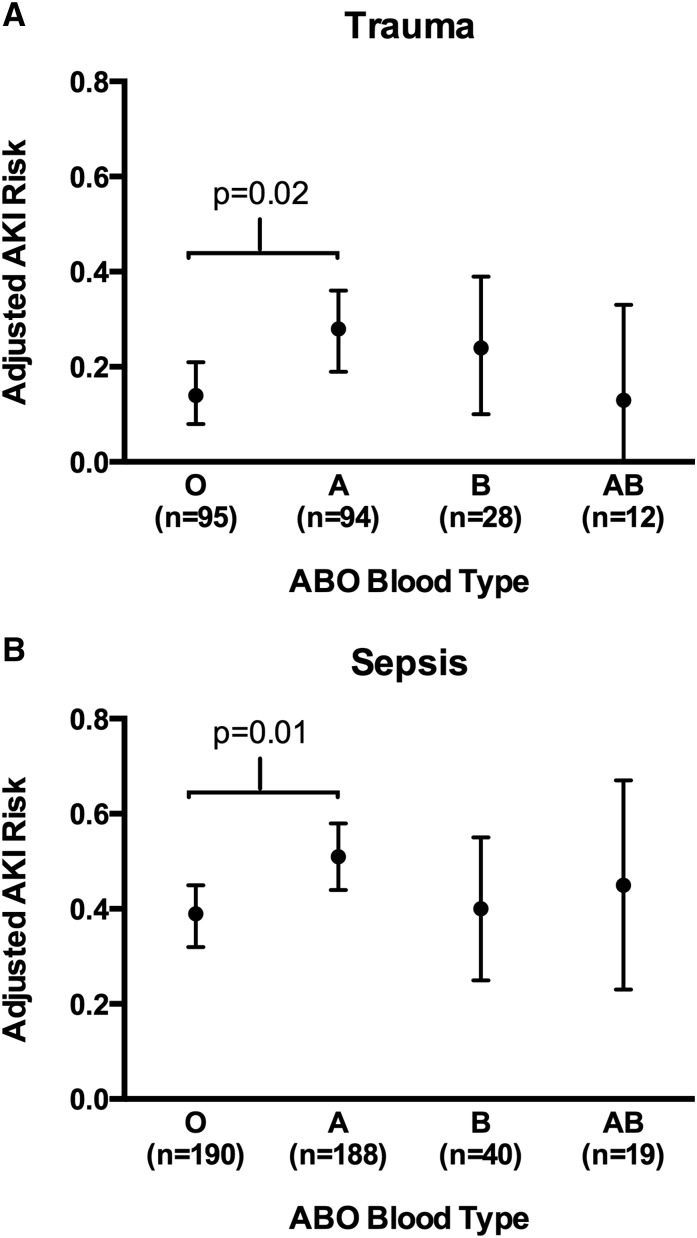
In multivariable analysis, blood type A was associated with higher AKI risk relative to blood type O among patients of European descent (adjusted RD, 0.14; 95% CI, 0.03 to 0.24; P=0.02) but not among patients of African descent (adjusted RD, 0.03; 95% CI, −0.09 to 0.15; P=0.61) (Figure 2A, Table 3). Comparisons of blood types B and AB with types O and A were limited by the smaller sample size of the B and AB types and did not result in any statistically significant associations (Supplemental Table 1). The association of blood type A relative to O with AKI was not substantially changed by excluding from the analysis patients who developed ARDS or were transfused during resuscitation (Table 3). Among patients of European descent, the RD between those who developed stage 2/3 AKI and those without AKI was similar to the RD observed between stage 1 AKI and no AKI (Supplemental Table 2), with no incremental increase in stage 2/3 AKI risk over stage 1 AKI risk. Initial creatinine at presentation and creatinine at discharge or death did not differ by ABO blood type in either racial group (Supplemental Table 3). A creatinine within the 6 months before trauma was only available in 86 (17%) patients and also, did not differ by ABO blood type (Supplemental Table 3); 22 (4%) patients required dialysis during their hospital stay (8 patients of European descent and 14 patients of African descent). Of eight patients of European descent, all eight were blood type A.
Figure 2.
Adjusted risk of AKI by ABO blood type among patients of European descent. (A) Patients with trauma. (B) Patients with severe sepsis. Points represent the adjusted AKI risk for each ABO blood type. Vertical error bars represent 95% confidence intervals. P values are for the comparison of blood type A with blood type O in adjusted logistic regression models. Tables 3 and 5 list the covariates included in the final multivariable models.
Table 3.
Associations of ABO blood types and AKI in the trauma cohort
| Population | No. | Adjusted AKI Risk (%)c | Adjusted RDd (95% Confidence Interval) for A Versus O | P Value | |||
|---|---|---|---|---|---|---|---|
| A | B | AB | O | ||||
| Overall trauma cohort | |||||||
| European descent | 229 | 28 | 24 | 13 | 14 | 0.14 (0.03 to 0.24) | 0.02 |
| African descent | 253 | 35 | 39 | 17 | 32 | 0.03 (−0.09 to 0.15) | 0.61 |
| Not transfuseda | |||||||
| European descent | 96 | 19 | 10 | 0 | 6 | 0.13 (0.00 to 0.27) | 0.05 |
| African descent | 71 | 17 | 18 | 0 | 14 | 0.03 (−0.18 to 0.25) | 0.74 |
| Not ARDSb | |||||||
| European descent | 156 | 26 | 18 | 14 | 10 | 0.16 (0.03 to 0.29) | 0.02 |
| African descent | 190 | 32 | 29 | 9 | 23 | 0.09 (−0.05 to 0.22) | 0.21 |
ARDS, acute respiratory distress syndrome; RD, risk difference.
Analyses limited to patients who were not transfused any blood products during the first 24 hours after emergency department presentation.
Analyses limited to patients who did not develop ARDS during the first 6 days of hospital admission.
AKI risk by ABO blood type on the basis of multivariable logistic regression models adjusted for age; Acute Physiology, Age, Chronic Health Evaluation III score without the renal components; body mass index; mechanism of injury; injury severity score; history of diabetes; and units of red blood cells transfused in the first 24 hours.
RDs are the adjusted differences in AKI risk between blood types A and O. All other comparisons between blood types A, B, AB, and O were not statistically significant and are displayed in Supplemental Table 1.
Severe Sepsis Population
We enrolled 759 critically ill patients with severe sepsis (Figure 1), with a median age of 61 years old and a median APACHE III score of 75. The source of sepsis was pulmonary in 43%. The majority of patients were of European (58%) or African descent (32%). Supplemental Table 4 provides the frequency of each organ dysfunction defining severe sepsis at ICU admission. Acute renal dysfunction according to ACCP sepsis criteria was present in 279 (37%) of patients at ICU admission. AKIN-defined AKI developed in 337 of 759 (44%) patients. Characteristics of those patients who did and did not develop AKI are provided in Table 1. ABO blood type was available in 752 of 759 (99%) enrolled patients. Seven patients without ABO blood type available, two of whom developed AKI, were excluded from additional analyses. Frequencies of the ABO blood types by race were similar to those in the trauma cohort and the reported distributions in the United States (39). Distributions and unadjusted associations between clinical variables and ABO blood type are shown in Table 4.
Table 4.
Characteristics of patients with severe sepsis by ABO blood type
| Characteristics | ABO Blood Typeg | P Valueh | |||
|---|---|---|---|---|---|
| A (n=283; 38%) | B (n=105; 14%) | AB (n=36; 5%) | O (n=328; 44%) | ||
| Demographics | |||||
| Race | <0.001 | ||||
| African descent | 76 (27%) | 50 (48%) | 13 (36%) | 103 (31%) | |
| European descent | 188 (66%) | 40 (38%) | 19 (53%) | 190 (58%) | |
| Asian descent | 3 (1%) | 5 (5%) | 2 (6%) | 9 (3%) | |
| Other | 7 (3%) | 6 (6%) | 1 (3%) | 18 (5%) | |
| Unknown | 9 (3%) | 4 (4%) | 1 (3%) | 8 (2%) | |
| Age (yr) | 62 (52–70) | 61 (52–72) | 62 (58–70) | 61 (51–70) | 0.57 |
| Men | 170 (60%) | 53 (51%) | 22 (61%) | 183 (56%) | 0.40 |
| Body mass index (kg/m2) | 26 (22–30) | 27 (23–32) | 26 (24–30) | 26 (22–31) | 0.11 |
| Medical history | |||||
| Hypertension | 134 (47%) | 59 (56%) | 23 (64%) | 152 (47%) | 0.07 |
| Diabetes mellitus | 77 (27%) | 35 (33%) | 12 (33%) | 92 (28%) | 0.48 |
| Congestive heart failure | 46 (16%) | 16 (15%) | 3 (8%) | 38 (12%) | 0.26 |
| CKD | 32 (11%) | 12 (11%) | 4 (11%) | 32 (10%) | 0.91 |
| Malignancya | 102 (36%) | 29 (28%) | 16 (44%) | 108 (33%) | 0.25 |
| Smoking historyb | 145 (51%) | 55 (52%) | 19 (53%) | 180 (55%) | 0.84 |
| Initial creatinine (mg/dl) | 1.3 (0.9–2.2) | 1.4 (1.0–2.1) | 1.2 (0.9–2.7) | 1.4 (0.9–2.3) | 0.99 |
| Severity of illness | |||||
| APACHE III score | 76 (59–93) | 73 (58–90) | 77 (64–96) | 75 (60–91) | 0.70 |
| APACHE III without renalc | 69 (54–84) | 66 (53–82) | 69 (59–90) | 69 (56–83) | 0.65 |
| Source of infection | |||||
| Pulmonary | 115 (41%) | 49 (47%) | 18 (50%) | 138 (42%) | 0.77 |
| Genitourinary | 27 (10%) | 14 (13%) | 4 (11%) | 41 (13%) | |
| Abdominal/gastrointestinal | 37 (13%) | 9 (9%) | 4 (11%) | 52 (16%) | |
| Head/neck | 6 (2%) | 4 (4%) | 0 (0%) | 5 (2%) | |
| Blood | 25 (9%) | 10 (10%) | 2 (6%) | 26 (8%) | |
| Skin/soft tissue/bone | 16 (6%) | 6 (6%) | 2 (6%) | 15 (5%) | |
| Gynecologic | 2 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Unknown | 55 (19%) | 13 (12%) | 6 (17%) | 51 (16%) | |
| Blood transfusions (%)d | |||||
| Red blood cells | 23 (8%) | 6 (6%) | 1 (3%) | 17 (5%) | 0.37 |
| Platelets | 24 (8%) | 9 (9%) | 3 (8%) | 21 (6%) | 0.76 |
| Fresh frozen plasma | 20 (7%) | 4 (4%) | 1 (3%) | 9 (3%) | 0.07 |
| Nephrotoxinse | |||||
| Intravenous contrast | 61 (22%) | 20 (19%) | 6 (17%) | 82 (25%) | 0.43 |
| Aminoglycosides | 113 (40%) | 29 (28%) | 11 (31%) | 108 (33%) | 0.09 |
| NSAIDs | 16 (6%) | 6 (6%) | 1 (3%) | 17 (5%) | 0.90 |
| Calcineurin inhibitor | 39 (14%) | 8 (8%) | 3 (8%) | 41 (13%) | 0.35 |
| Outcomes | |||||
| 30-d Mortality | 135 (48%) | 35 (33%) | 19 (53%) | 140 (43%) | 0.05 |
| ICU length of stay (d)f | 28 (4–∞) | 6 (3–∞) | ∞ (3–∞) | 12 (3–∞) | 0.05 |
| AKI (any stage) | 143 (51%) | 44 (42%) | 14 (40%) | 134 (41%) | 0.08 |
| AKI (stage 2 or 3) | 73 (26%) | 17 (16%) | 8 (22%) | 74 (23%) | 0.26 |
| Need for dialysis | 22 (8%) | 4 (4%) | 3 (8%) | 22 (7%) | 0.53 |
Data are shown as n (%) for categorical variables and median (interquartile range) for continuous variables. APACHE III, Acute Physiology, Age, Chronic Health Evaluation III; NSAID, nonsteroidal anti–inflammatory drug; ICU, intensive care unit.
Metastatic solid malignancy or hematologic malignancy.
Smoking history includes current and former cigarette smokers.
APACHE III without renal refers to the APACHE III score with the renal components removed.
Blood transfusions are presented as the number (%) of patients who received a transfusion of a specified blood product in the first 24 hours of ICU admission.
Nephrotoxins refer to the exposure of intravenous contrast or aminoglycosides during the first 6 days of admission and a medical history of NSAID use just before admission.
ICU length of stay was assumed to be infinite in patients who died. Given that >50% of patients with blood type AB died, the median is infinity.
Values do not add up to 100% because of rounding.
Continuous variables were compared using a Wilcoxon rank sum test, and categorical variables were compared using a Pearson chi–squared or Fisher’s exact test.
Similar to the trauma cohort, blood type A again conferred a higher AKI risk relative to type O among patients of European descent (adjusted RD, 0.14; 95% CI, 0.04 to 0.23; P=0.01) but not among patients of African descent (adjusted RD, 0.04; 95% CI, −0.10 to 0.18; P=0.55) (Figure 2B, Table 5). Comparisons of blood types B and AB with types O and A, again limited by the low frequency of the B and AB types, did not show any statistically significant differences in AKI risk (Supplemental Table 1). Compared with the overall sepsis cohort, the point estimates for the RD between blood types A and O were similar in patients who did not develop ARDS, were not transfused, and did not have acute renal dysfunction at ICU admission (Table 5). The AKI RD between blood types A and O in patients of European descent were similar comparing AKI stage 2/3 or 1 with no AKI (Supplemental Table 2), with no incremental increase in risk for stage 2/3 AKI over stage 1 AKI. Initial creatinine at enrollment and creatinine at death or discharge did not differ by ABO blood type (Supplemental Table 3). A lowest creatinine from the 6 months before ICU enrollment was available in 80% of patients, and it also did not differ by ABO blood type (Supplemental Table 3). We did not detect differences in the percentage requiring dialysis in patients of European descent (9% versus 5% for types A and O, respectively) and patients of African descent (7% versus 9% for types A and O, respectively).
Table 5.
Associations of ABO blood types and AKI in the severe sepsis cohort
| Population | No. | Adjusted AKI Risk (%)d | Adjusted RDe (95% Confidence Interval) for A Versus O | P Value | |||
|---|---|---|---|---|---|---|---|
| A | B | AB | O | ||||
| Overall sepsis cohort | |||||||
| European descent | 437 | 53 | 42 | 45 | 40 | 0.14 (0.04 to 0.23) | <0.01 |
| African descent | 242 | 47 | 43 | 39 | 43 | 0.04 (−0.10 to 0.18) | 0.55 |
| Not transfuseda | |||||||
| European descent | 374 | 50 | 40 | 48 | 37 | 0.13 (0.02 to 0.23) | 0.02 |
| African descent | 213 | 41 | 37 | 32 | 40 | 0.01 (−0.14 to 0.16) | 0.89 |
| Not ARDSb | |||||||
| European descent | 245 | 38 | 31 | 36 | 29 | 0.09 (−0.03 to 0.22) | 0.14 |
| African descent | 153 | 29 | 38 | 25 | 35 | −0.06 (−0.23 to 0.11) | 0.48 |
| No renal dysfunction at enrolmentc | |||||||
| European descent | 277 | 53 | 34 | 27 | 33 | 0.20 (0.08 to 0.32) | 0.001 |
| African descent | 131 | 42 | 28 | 39 | 43 | −0.01 (−0.20 to 0.18) | 0.93 |
ARDS, acute respiratory distress syndrome; RD, risk difference.
Analyses limited to patients who were not transfused any blood products during the first 24 hours after intensive care unit admission.
Analyses limited to patients who did not develop ARDS during the first 5 days of hospital admission.
Analyses limited to patients who did not have evidence of acute renal dysfunction by American College of Chest Physicians severe sepsis criteria at intensive care unit enrollment.
AKI risk by ABO blood type on the basis of multivariable logistic regression models adjusted for Acute Physiology, Age, Chronic Health Evaluation III score without renal components, age, sex, history of diabetes, history of congestive heart failure, malignancy, pulmonary source of infection, and units of red blood cells transfused in the first 24 hours of intensive care unit admission.
RDs are the adjusted differences in AKI risk between blood types A and O. All other comparisons between blood types A, B, AB, and O were not statistically significant and are displayed in Supplemental Table 1.
Discussion
In this study, we showed that ABO blood type A was associated with an estimated 14% higher risk of developing AKI in the ICU compared with type O among individuals of European descent in two critically ill populations: major trauma and severe sepsis. The ABO and AKI risk associations were also present within patients who were not transfused, showing the independence of the relationship from characteristics of blood transfusion (e.g., storage duration, volume, and compatible versus identical blood type). Additionally, our findings were similar in patients who did not develop ARDS, indicating an association between ABO and AKI independent of ARDS. Our findings are notable for several reasons. First, they point toward the possibility that ABO glycans may be important mediators of AKI. Second, the presence of a similar ABO-AKI association in both patients with sepsis and patients with trauma, despite differences in study population, comorbidities, and years of enrollment, indicates that the underlying mechanisms may be shared in these distinct populations. Placed in the context of prior demonstrations by us and others that blood type A is a risk factor for vascular diseases and ARDS, our findings also suggest that ABO blood types may have common effects on multiple organ dysfunctions in critical illness (13–16).
Prior literature on the ABO histo-blood group and AKI had focused on the now rare renal injury from ABO-incompatible transfusions and renal allograft dysfunction after ABO-incompatible transplantation (40,41). Our findings of an association between ABO blood type and AKI likely reflect a different underlying pathophysiology. We hypothesize that the ABO glycans increase AKI risk through their effects on inflammation, endothelial function, and microvascular coagulation (6,7). ABO blood types are major genetic determinants of blood concentrations of soluble ICAM-1, selectins, vWF, and thrombomodulin (12,20–23), and several of these proteins are known to be glycated with the ABO antigens in renal vascular endothelium (19). Animal models of renal ischemia-reperfusion injury show endothelial upregulation of integrins, selectins, and ICAM-1 (5,42), downregulation of thrombomodulin (43), and secretion of vWF (44). Although the exact biologic role of the ABO glycans is unknown, their effects on these endothelial mediators may underlie the relationship between ABO blood type and AKI. As treatments are developed targeting these mechanisms, blood type A may represent an easily identifiable population to test novel, tailored therapeutics aimed at glycoproteins possessing the ABO antigens.
We identified a significant association between blood type A and higher AKI risk only in patients of European descent, similar to the association between ABO blood type and ARDS. There are several potential explanations for this finding. African descent is an independent risk factor for AKI (29,45). It is possible that, in individuals of African descent, any protective effects of blood type O are overwhelmed by other mechanisms that increase their AKI risk. Alternatively, an unidentified genetic or environmental factor, with distinct frequencies between individuals of European and African descent, may modify the ABO-AKI association depending on race. For example, polymorphisms in the vWF gene are associated with expression of vWF in healthy Americans of European but not African descent, suggesting racial divergence in the genetic regulation of vWF expression (46,47) and possibly, contributing to the racial difference in ABO-AKI associations. The baseline characteristics of our cohorts also exhibit racial differences. Among patients with trauma, individuals of African descent were significantly more likely to suffer penetrating trauma; in sepsis, they were significantly less likely to have certain comorbidities, particularly hematologic malignancies. However, adjusting for these clinical characteristics did not explain the observed racial differences in our study. Lastly, because our study only had power to detect an 18%–20% difference in AKI risk by blood type among patients of African descent, it is possible that a smaller but clinically relevant association of ABO blood type and AKI went undetectable.
Our study has several important limitations. The two patient cohorts were enrolled at a single center, and generalizability to other centers may be limited. Additionally, enrollment procedures, including inclusion and exclusion criteria and era of enrollment, were different in the cohorts. However, we found a similar risk pattern by blood type in two distinct critically ill patient populations. Our study warrants replication at other centers and in additional at–risk populations. The AKI outcome definition did not include urine output criteria. These criteria are noted to be the least specific for AKI and therefore, may be suboptimal for studies of potential AKI pathophysiologic mechanisms (48). Additionally, we did not use premorbid baseline creatinine values given the high frequency of missingness that was disproportionately in patients with trauma. This approach sacrificed some sensitivity for admission creatinine elevations that did not subsequently worsen but avoided the lack of specificity that comes with using premorbid creatinine estimates. Sample size and cohort composition limited our ability to reach conclusions among the less common blood types (B and AB) and racial groups. Similarly, we did not have statistical power to determine the effects of genetic interactions on the ABO-AKI relationship. Severe sepsis is defined by sepsis in the setting of acute organ dysfunction, including renal dysfunction (32). Given that our inclusion criteria included renal dysfunction, we may have introduced selection bias. However, renal dysfunction at ICU admission was not associated with ABO blood type, but rather, ABO blood type was associated with the subsequent development of AKI in the ICU. Additionally, our analyses, which were restricted to patients without acute renal dysfunction at enrollment, show an association of ABO blood type and AKI. Finally, despite consideration of multiple potential confounders for adjusted analyses, unmeasured confounders or linked genetic traits may have affected the association between ABO blood type and AKI.
In conclusion, our study identified ABO blood type A as a risk factor for the development of AKI in critically ill patients of European descent with trauma or severe sepsis. This association was independent of blood transfusion and other clinical confounders, implicating the ABO glycans in AKI risk among the critically ill. Future research is warranted to identify and detail mechanisms underlying this relationship.
Disclosures
None.
Supplementary Material
Acknowledgments
We acknowledge the contributions of Esra Kalkan, Isabel Hiciano, Zachary Garcia, Nathaniel Oz, Ana Campbell, Sandra Kaplan, Arife Yildiz, and Dudley Charles to the collection of data for this study.
Financial support was provided by National Institutes of Health Grants P01HL079063, U01HL108636, K23DK097307, K23HL102254, F32HL122075, K24HL115354, T32HL007891, and K12HL109009.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12201214/-/DCSupplemental.
References
- 1.Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, Kellum JA: RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit Care 10: R73, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopes JA, Jorge S, Resina C, Santos C, Pereira A, Neves J, Antunes F, Prata MM: Acute kidney injury in patients with sepsis: A contemporary analysis. Int J Infect Dis 13: 176–181, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG, University of Toronto Acute Kidney Injury Research Group : Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302: 1179–1185, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Zarjou A, Agarwal A: Sepsis and acute kidney injury. J Am Soc Nephrol 22: 999–1006, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basile DP: The endothelial cell in ischemic acute kidney injury: Implications for acute and chronic function. Kidney Int 72: 151–156, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Kinsey GR, Li L, Okusa MD: Inflammation in acute kidney injury. Nephron, Exp Nephrol 109: e102–e107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doi K, Leelahavanichkul A, Yuen PS, Star RA: Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest 119: 2868–2878, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsukamoto T, Chanthaphavong RS, Pape HC: Current theories on the pathophysiology of multiple organ failure after trauma. Injury 41: 21–26, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, Kellum JA: A unified theory of sepsis-induced acute kidney injury: Inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 41: 3–11, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anstee DJ: The relationship between blood groups and disease. Blood 115: 4635–4643, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Franchini M, Capra F, Targher G, Montagnana M, Lippi G: Relationship between ABO blood group and von Willebrand factor levels: From biology to clinical implications. Thromb J 5: 14, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He M, Wolpin B, Rexrode K, Manson JE, Rimm E, Hu FB, Qi L: ABO blood group and risk of coronary heart disease in two prospective cohort studies. Arterioscler Thromb Vasc Biol 32: 2314–2320, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reilly JP, Meyer NJ, Shashaty MG, Feng R, Lanken PN, Gallop R, Kaplan S, Herlim M, Oz NL, Hiciano I, Campbell A, Holena DN, Reilly MP, Christie JD: ABO blood type A is associated with increased risk of ARDS in whites following both major trauma and severe sepsis. Chest 145: 753–761, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reilly MP, Li M, He J, Ferguson JF, Stylianou IM, Mehta NN, Burnett MS, Devaney JM, Knouff CW, Thompson JR, Horne BD, Stewart AF, Assimes TL, Wild PS, Allayee H, Nitschke PL, Patel RS, Martinelli N, Girelli D, Quyyumi AA, Anderson JL, Erdmann J, Hall AS, Schunkert H, Quertermous T, Blankenberg S, Hazen SL, Roberts R, Kathiresan S, Samani NJ, Epstein SE, Rader DJ, Myocardial Infarction Genetics Consortium. Wellcome Trust Case Control Consortium : Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: Two genome-wide association studies. Lancet 377: 383–392, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu O, Bayoumi N, Vickers MA, Clark P: ABO(H) blood groups and vascular disease: A systematic review and meta-analysis. J Thromb Haemost 6: 62–69, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Ravn V, Dabelsteen E: Tissue distribution of histo-blood group antigens. APMIS 108: 1–28, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Oriol R, Mollicone R, Coullin P, Dalix AM, Candelier JJ: Genetic regulation of the expression of ABH and Lewis antigens in tissues. APMIS Suppl 27: 28–38, 1992 [PubMed] [Google Scholar]
- 19.Tasaki M, Yoshida Y, Miyamoto M, Nameta M, Cuellar LM, Xu B, Zhang Y, Yaoita E, Nakagawa Y, Saito K, Yamamoto T, Takahashi K: Identification and characterization of major proteins carrying ABO blood group antigens in the human kidney. Transplantation 87: 1125–1133, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Kiechl S, Paré G, Barbalic M, Qi L, Dupuis J, Dehghan A, Bis JC, Laxton RC, Xiao Q, Bonora E, Willeit J, Xu Q, Witteman JC, Chasman D, Tracy RP, Ballantyne CM, Ridker PM, Benjamin EJ, Ye S: Association of variation at the ABO locus with circulating levels of soluble intercellular adhesion molecule-1, soluble P-selectin, and soluble E-selectin: A meta-analysis. Circ Cardiovasc Genet 4: 681–686, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paré G, Chasman DI, Kellogg M, Zee RY, Rifai N, Badola S, Miletich JP, Ridker PM: Novel association of ABO histo-blood group antigen with soluble ICAM-1: Results of a genome-wide association study of 6,578 women. PLoS Genet 4: e1000118, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbalic M, Dupuis J, Dehghan A, Bis JC, Hoogeveen RC, Schnabel RB, Nambi V, Bretler M, Smith NL, Peters A, Lu C, Tracy RP, Aleksic N, Heeriga J, Keaney JF, Jr., Rice K, Lip GY, Vasan RS, Glazer NL, Larson MG, Uitterlinden AG, Yamamoto J, Durda P, Haritunians T, Psaty BM, Boerwinkle E, Hofman A, Koenig W, Jenny NS, Witteman JC, Ballantyne C, Benjamin EJ: Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum Mol Genet 19: 1863–1872, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blann AD, Daly RJ, Amiral J: The influence of age, gender and ABO blood group on soluble endothelial cell markers and adhesion molecules. Br J Haematol 92: 498–500, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Sharfuddin AA, Sandoval RM, Berg DT, McDougal GE, Campos SB, Phillips CL, Jones BE, Gupta A, Grinnell BW, Molitoris BA: Soluble thrombomodulin protects ischemic kidneys. J Am Soc Nephrol 20: 524–534, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu KL, Lee KT, Chang CH, Chen YC, Lin SM, Chu PH: Elevated plasma thrombomodulin and angiopoietin-2 predict the development of acute kidney injury in patients with acute myocardial infarction. Crit Care 18: R100, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herter JM, Rossaint J, Spieker T, Zarbock A: Adhesion molecules involved in neutrophil recruitment during sepsis-induced acute kidney injury. J Innate Immun 6: 597–606, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Claus RA, Bockmeyer CL, Budde U, Kentouche K, Sossdorf M, Hilberg T, Schneppenheim R, Reinhart K, Bauer M, Brunkhorst FM, Lösche W: Variations in the ratio between von Willebrand factor and its cleaving protease during systemic inflammation and association with severity and prognosis of organ failure. Thromb Haemost 101: 239–247, 2009 [PubMed] [Google Scholar]
- 28.Claus RA, Bockmeyer CL, Sossdorf M, Lösche W: The balance between von-Willebrand factor and its cleaving protease ADAMTS13: Biomarker in systemic inflammation and development of organ failure? Curr Mol Med 10: 236–248, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Shashaty MG, Meyer NJ, Localio AR, Gallop R, Bellamy SL, Holena DN, Lanken PN, Kaplan S, Yarar D, Kawut SM, Feldman HI, Christie JD: African American race, obesity, and blood product transfusion are risk factors for acute kidney injury in critically ill trauma patients. J Crit Care 27: 496–504, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reilly JP, Bellamy S, Shashaty MG, Gallop R, Meyer NJ, Lanken PN, Kaplan S, Holena DN, May AK, Ware LB, Christie JD: Heterogeneous phenotypes of acute respiratory distress syndrome after major trauma. Ann Am Thorac Soc 11: 728–736, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Civil ID, Schwab CW: The Abbreviated Injury Scale, 1985 revision: A condensed chart for clinical use. J Trauma 28: 87–90, 1988 [DOI] [PubMed] [Google Scholar]
- 32.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, SCCM/ESICM/ACCP/ATS/SIS : 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31: 1250–1256, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network : Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pickering JW, Endre ZH: Back-calculating baseline creatinine with MDRD misclassifies acute kidney injury in the intensive care unit. Clin J Am Soc Nephrol 5: 1165–1173, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maldonado G, Greenland S: Simulation study of confounder-selection strategies. Am J Epidemiol 138: 923–936, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Graubard BI, Korn EL: Predictive margins with survey data. Biometrics 55: 652–659, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS, ARDS Definition Task Force : Acute respiratory distress syndrome: The Berlin Definition. JAMA 307: 2526–2533, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Shah CV, Lanken PN, Localio AR, Gallop R, Bellamy S, Ma SF, Flores C, Kahn JM, Finkel B, Fuchs BD, Garcia JG, Christie JD: An alternative method of acute lung injury classification for use in observational studies. Chest 138: 1054–1061, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garratty G, Glynn SA, McEntire R, Retrovirus Epidemiology Donor Study : ABO and Rh(D) phenotype frequencies of different racial/ethnic groups in the United States. Transfusion 44: 703–706, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Sazama K: Reports of 355 transfusion-associated deaths: 1976 through 1985. Transfusion 30: 583–590, 1990 [DOI] [PubMed] [Google Scholar]
- 41.Sekijima M, Shimizu A, Ishii Y, Kudo S, Horita S, Nakajima I, Fuchinoue S, Teraoka S: Early humoral-mediated graft injuries in ABO-incompatible kidney transplantation in human beings. Transplant Proc 42: 789–790, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Friedewald JJ, Rabb H: Inflammatory cells in ischemic acute renal failure. Kidney Int 66: 486–491, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Pinsky DJ, Yan SF, Lawson C, Naka Y, Chen JX, Connolly ES, Jr., Stern DM: Hypoxia and modification of the endothelium: Implications for regulation of vascular homeostatic properties. Semin Cell Biol 6: 283–294, 1995 [DOI] [PubMed] [Google Scholar]
- 44.Sutton TA, Mang HE, Campos SB, Sandoval RM, Yoder MC, Molitoris BA: Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol Renal Physiol 285: F191–F198, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Grams ME, Matsushita K, Sang Y, Estrella MM, Foster MC, Tin A, Kao WH, Coresh J: Explaining the racial difference in AKI incidence. J Am Soc Nephrol 25: 1834–1841, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou Z, Yu F, Buchanan A, Fu Y, Campos M, Wu KK, Chambless LE, Folsom AR, Boerwinkle E, Dong JF: Possible race and gender divergence in association of genetic variations with plasma von Willebrand factor: A study of ARIC and 1000 genome cohorts. PLoS One 9: e84810, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller CH, Haff E, Platt SJ, Rawlins P, Drews CD, Dilley AB, Evatt B: Measurement of von Willebrand factor activity: Relative effects of ABO blood type and race. J Thromb Haemost 1: 2191–2197, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Ricci Z, Cruz D, Ronco C: The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int 73: 538–546, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.