Abstract
Amyloidosis derived from leukocyte cell–derived chemotaxin 2 is a recently recognized form of amyloidosis, and it has already been established as a frequent form of systemic amyloidosis in the United States, with predominant involvement of kidney and liver. The disease has a strong ethnic bias, affecting mainly Hispanics (particularly Mexicans). Additional ethnic groups prone to develop amyloidosis derived from leukocyte cell–derived chemotaxin 2 include Punjabis, First Nations people in British Columbia, and Native Americans. Most patients are elderly who present with chronic renal insufficiency and bland urinary sediment. Proteinuria is variable, being absent altogether in about one third of patients. Liver involvement is frequently an incidental finding. Amyloidosis derived from leukocyte cell–derived chemotaxin 2 deposits shows a characteristic distribution: in the kidney, there is consistent involvement of cortical interstitium, whereas in the liver, there is a preferential involvement of periportal and pericentral vein regions. Concurrent renal disease is frequent, with diabetic nephropathy and IgA nephropathy being the most common. Patient survival is excellent, likely because of the rarity of cardiac involvement, whereas renal survival is guarded, with a median renal survival of 62 months in those without concurrent renal disease. There is currently no efficacious therapy for amyloidosis derived from leukocyte cell–derived chemotaxin 2 amyloidosis. Renal transplantation seems to be a reasonable treatment for patients with advanced renal failure, although the disease may recur in the allograft. The pathogenesis of amyloidosis derived from leukocyte cell–derived chemotaxin 2 amyloidosis has not yet been elucidated. It could be a result of leukocyte cell–derived chemotaxin 2 overexpression by hepatocytes either constitutively (controlled by yet-uncharacterized genetic defects) or secondary to hepatocellular damage. It is critical not to misdiagnose amyloidosis derived from leukocyte cell–derived chemotaxin 2 amyloidosis as Ig light chain–derived amyloidosis to avoid harmful chemotherapy.
Keywords: leukocyte cell–derived chemotaxin 2, kidney biopsy, renal amyloidosis, liver amyloidosis, ALECT2 amyloidosis
Introduction
Amyloidosis is an uncommon group of diseases characterized by extracellular deposition of insoluble fibrils, which result from abnormal folding of proteins. Amyloid deposits characteristically stain with Congo red and show anomalous color change (so-called apple-green birefringence) when examined under polarized light. Thirty-one precursor proteins of amyloid have been identified so far (1). Amyloidosis can be systemic or localized. In the United States and Europe, Ig light chain–derived (AL) amyloidosis is, by far, the most frequent type, whereas in developing countries, AA amyloidosis is more common than AL amyloidosis (2). Kidney involvement is present in most patients with AL amyloidosis (3) and AA amyloidosis (4) and may also occur in several rare hereditary forms of amyloidosis, such as those derived from fibrinogen A, transthyretin (ATTR), gelsolin (particularly in homozygotes), lysozyme, apo AI (AApo AI), AApo AII, and AApo AIV (5–8). The overall renal biopsy incidence of amyloidosis ranges from 1.3% to 4% (5–7,9).
In 2008, Benson et al. (10) discovered a new form of amyloidosis derived from leukocyte cell–derived chemotaxin 2 (ALECT2) in a patient with nephrotic syndrome and renal insufficiency who underwent nephrectomy for resection of renal cell carcinoma. Three recent large studies (5,11,12) that included a total of 144 patients (112 patients with renal amyloidosis and 32 patients with liver amyloidosis) have established that ALECT2 amyloidosis is now a frequent type of renal and hepatic amyloidosis and highlighted its distinct clinical and pathologic characteristics, including clear ethnic predisposition, typical presentation with chronic renal insufficiency with or without proteinuria, and superior patient survival to AL amyloidosis and AA amyloidosis. This mini-review highlights the current status of ALECT2 amyloidosis, including incidence, organ distribution, clinical presentation, pathogenesis, histologic characteristics, renal and patient survival, poor prognostic indicators, and recurrence in the renal allograft.
Incidence and Ethnic Predisposition
ALECT2 amyloidosis is now as common as AA amyloidosis in the United States. Of 4139 patients with clinical amyloidosis diagnosed by proteomics (regardless of the specimen site) at the Mayo Clinic, the four most common types were AL (61.7%), ATTR (24.5%), AA (3.7%), and ALECT2 (3.6%) (13). It accounts for 2.7%–10% of patients with renal amyloidosis in the United States (5,6,14). In a recent series of 474 patients with biopsy-proven renal amyloidosis from the Mayo Clinic, 86% were Ig derived, 7% were AA derived, 3% were ALECT2 derived, and 1% were fibrinogen A derived (6). In another United States series from Nephropath of 414 patients, 83% were Ig derived, 10% were ALECT2 derived, and 5% were AA derived (5). In this series, ALECT2 amyloidosis accounted for 54% of patients with renal amyloidosis (2% of total kidney biopsies received during the study period) from the southwestern United States (New Mexico, Arizona, and far west Texas) (5), which has a high concentration of Hispanics. ALECT2 amyloidosis is also a frequent cause of hepatic amyloidosis (11,15). In a recent series of 130 patients with liver amyloidosis diagnosed by proteomics at the Mayo Clinic, 62% were AL, 25% were ALECT2, 7% were AApo AI, 4% were AA, 2% were ATTR, and 1% amyloidosis derived from lysozyme (11).
In sharp contrast to other types of systemic amyloidosis, ALECT2 amyloidosis exhibits a strong ethnic bias, with 88%–92% of reported patients being Hispanics (5,11,12). All reported Hispanic patients who disclosed their origin were of Mexican descent (12,16). Because Mexican Americans constitute the largest group of United States Hispanics (63%), it is still unknown if they are more likely to develop ALECT2 than Unites States Hispanics originating from other countries. Additional ethnic groups described to develop ALECT2 with an increased frequency included Punjabis, First Nations people in British Columbia, Arabs, Israelis, and Native Americans (5,12,17,18). It is worth noting that Native-American ancestry is shared by both First Nations people and Hispanics (higher level in Mexicans and Ecuadorians compared with Colombians, Puerto Ricans, and Dominicans) (19), which may explain the bias toward both of the these populations (17). As discussed later, the strong cosegregation of ALECT2 amyloidosis with Mexican-American ethnicity together with the reported familial occurrence in two siblings and the frequent G-allele homozygosity raise the possibility that this disease may represent another form of familial amyloidosis (5,12,20).
Affected Organs
ALECT2 amyloidosis is likely a systemic form of amyloidosis with predominant involvement of kidney and liver. Of 120 patients with ALECT2 amyloidosis diagnosed by liquid chromatography/mass spectrometry (LC/MS) at the Mayo Clinic between 2007 and 2012, 72 affected the kidney, 36 liver, five spleen, three prostate, and one each from gallbladder, pancreas, small bowel, and parathyroid gland (12). Interestingly, most patients with ALECT2 amyloidosis reported so far were diagnosed by kidney biopsy in patients without clinically evident extrarenal organ involvement (5,12,17) or liver biopsy in patients without clinically evident extrahepatic organ involvement (11). A patient with biopsy-proven pulmonary ALECT2 amyloidosis was also reported (21). No patient with cardiac ALECT2 amyloidosis has been confirmed histologically so far, although there was one reported patient with hepatic and cardiac amyloidosis in whom the hepatic (but not cardiac) amyloid was proven to be ALECT2 by immunohistochemistry (IHC) and amino acid sequencing (22). Patients with renal ALECT2 amyloidosis may have amyloid deposits in the bone marrow (12). Autopsy-based studies are underway in the southwest United States to confirm the systemic nature of ALECT2 amyloidosis and shed some light on its prevalence and pathogenesis.
Clinical Characteristics
Table 1 summarizes the clinical findings from the three largest series of ALECT2 amyloidosis reported to date. Similar to AL amyloidosis, ALECT2 amyloidosis mainly affects the older population. Patients with kidney involvement are slightly older at diagnosis (median age of 69 years old) than those with liver involvement (median age of 61 years old) (5,11,12). There have been only five reported patients who were younger than 50 years old at diagnosis, the youngest of whom was 33 years old with hepatic ALECT2 amyloidosis (11,12). Most patients with renal ALECT2 amyloidosis present with chronic renal insufficiency (5,12,16). The mean serum creatinine at diagnosis was 3 mg/dl in the series by Said et al. (12) and 2.8 mg/dl in the series by Larsen et al. (5) (Table 1). In contrast to other forms of renal amyloidosis, proteinuria is an inconsistent finding in renal ALECT2 amyloidosis and lacking altogether in 21%–67% of patients (5,12). Full nephrotic syndrome is uncommon in renal ALECT2 amyloidosis, which was present in only 10% of patients in the series by Said et al. (12) Rarely, renal ALECT2 amyloidosis is an incidental pathologic finding found in the non-neoplastic parenchyma of nephrectomy specimens performed for resection of renal cell carcinoma, which was the case in four (6%) patients in the series by Said et al. (12). Microhematuria is uncommon, encountered in 16% of patients in one study (12). Chronic hypertension and diabetes mellitus are frequent (Table 1) (5,12). A minority of reported patients with renal ALECT2 had carcinoma (10,12,21), which was present in 13% of patients in one study (12). A patient with kidney and lung ALECT2 amyloidosis presenting with acute renal failure and hemoptysis has been reported (21). This patient had positive myeloperoxidase-ANCA, and therefore, his pulmonary-renal syndrome was likely caused by small-vessel vasculitis and not amyloidosis, despite the lack of crescents in the limited renal biopsy sample (eight glomeruli only) (21).
Table 1.
Clinical characteristics of leukocyte cell–derived chemotaxin 2 amyloidosis
| Series | Said et al. (12; Renal ALECT2) | Larsen et al. (5; Renal ALECT2) | Mereuta et al. (11; Liver ALECT2) |
| No. of patients | 72 | 40 | 32 |
| Organs with histologically proven involvement | Kidney in all, liver in one, duodenum in one, and adrenal in one | Kidney in all, liver in one, and prostate in one | Liver in all |
| Median age (range), yr | 65.5 (43–88) | 70.5 (52–86) | 60.5 (33–79) |
| <50, % | 3 | 0 | 9 |
| Men/women | 37/35 | 25/15 | 12/20 |
| Hispanic ethnicity, % | 92 | 88 | 88 |
| Mean serum creatinine (range) at diagnosis, mg/dl | 3 | 2.8 | NA |
| Proteinuria, % | 79 | 33 | NA |
| Nephrotic-range proteinuria, % | 33 | 23 | NA |
| Median 24-h urine protein, g/d | 1 | 0.6 | NA |
| Other pathology, % | 26 | 32 | 66 |
| Diabetes mellitus, % | 26 | 38 | NA |
| Hypertension, % | 68 | 50 | NA |
| Monoclonal gammopathy, % | 10 | 8 | NA |
| Family history of amyloidosis, % | 0 | 5 | NA |
| Mean duration of follow-up, mo | 31 | 50 | NA |
| Progression to ESRD | 25/64 (39%) | 6/21 (29%) | NA |
ALECT2, amyloidosis derived from leukocyte cell–derived chemotaxin 2; NA, not applicable.
Hepatic ALECT2 amyloidosis is frequently discovered as an incidental finding in liver biopsy performed during surgery for nonhepatic conditions or specimens that show other pathology that explains the liver dysfunction, such as chronic viral hepatitis or steatohepatitis (11). The most frequent clinical abnormality, if any, in patients with liver ALECT2 amyloidosis is an elevation of alkaline phosphatase (11). In contrast, most patients with liver AL amyloidosis have evidence of liver abnormality, most commonly hepatomegaly and elevated liver function tests (11). Rarely, ALECT2 amyloidosis can cause portal hypertension and variceal bleeding (23).
Pathogenesis
Leukocyte cell–derived chemotaxin 2 (LECT2) is a 16-kD cytokine first isolated by Yamagoe et al. (24) in 1996. It is widely conserved in vertebrates and identical to bovine chondromodulin-II, a cartilage-derived protein involved in bone repair. The secreted protein consists of 133 amino acids and has three intermolecular disulfide bonds (25). The tertiary structure of this protein is still unknown. Its gene in humans is located on chromosome 5q31.1-q32 (26). The LECT2 gene also encodes an 18-amino acid secretory signal at the N terminus. A polymorphism in the LECT2 gene has been identified in which there is a DNA codon change from ATC (A allele) to GTC (G allele) at position 172 (SNP rs31517), resulting in replacement of isoleucine by valine at position 40 of mature protein (26). The G allele is common, with an overall frequency of 0.477; 51% of healthy Mexicans (27) and 47% of healthy Japanese individuals (28) are homozygous of the G allele. A Japanese study found that the A/A genotype is associated with higher severity of rheumatoid arthritis (28).
LECT2 is synthesized mainly by hepatocytes. IHC studies showed that LECT2 expression is restricted to pericentral vein hepatocytes in normal mouse liver, whereas it is diffuse throughout the lobule hepatocytes in normal human liver (29,30). One study found that LECT2 protein is also expressed in a variety of other cells in many organs, including adipocytes, smooth muscle cells, endothelial cells, and epithelial cells, although of much weaker and more variable expression than in hepatocytes (31). We, however, have not found any convincing positive intracellular LECT2 staining by IHC or in situ hybridization outside the liver (A. Dogan, unpublished data). LECT2 expression is upregulated by activation of the Wnt/β-catinin signaling pathway in the liver, a commonly dysregulated pathway in carcinogenesis (29). LECT2 receptor(s) and its downstream signaling pathways are yet to be characterized. A study on Ayu (plecoglossus altivelis) showed that LECT2 interacts with a C-type lectin receptor (32). Chen et al. (33) recently showed that the inhibitory function of LECT2 on hepatocellular carcinoma is mediated by binding and inactivation of MET receptor, leading to blockage of vascular invasion and metastasis of hepatocellular carcinoma. Other studies showed that LECT2 negatively regulates the homeostasis of NKT cells in the liver (34,35).
The turnover rate of LECT2 is unknown. Its half-life was predicted to be short because of the low molecular weight (36). Plasma LECT2 concentration in normal individuals is 19.7±3.4 ng/ml (37). Its serum level is increased in liver diseases, such as acute liver injury (38), hepatocellular carcinoma (39), fatty liver (40), obesity (40), and insulin resistance (36). In contrast, a recent study showed that plasma LECT2 concentrations in patients with sepsis were remarkably low and negatively correlated with the disease severity (37). LECT2 protein was initially purified from a culture fluid of phytohemagglutinin-activated human T cell leukemia SKW-3 cells as a potential chemotactic factor for human neutrophils (24). Subsequent studies indicated that it is a multifactorial cytokine involved in chemotaxis, cell proliferation, immunomodulation, damage/repair process, tumor suppression, and glucose metabolism (33,36,41–44).
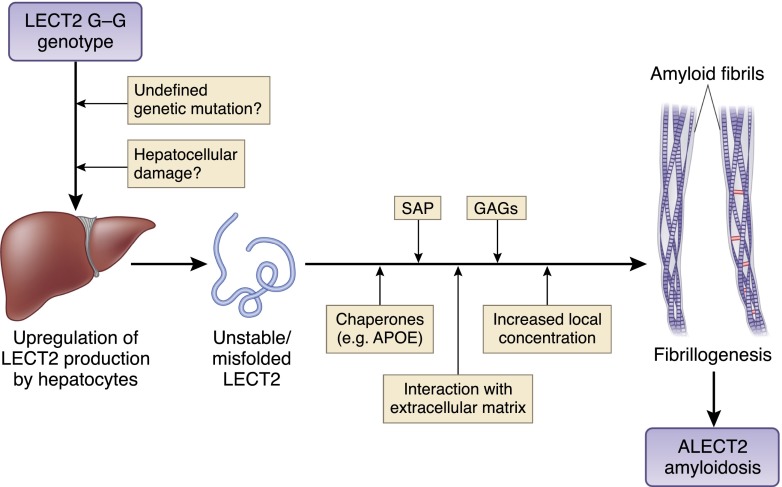
The pathogenesis of ALECT2 amyloidosis is still unknown. LECT2 is a folded protein with a β-sheet structure (45,46). The entire LECT2 protein was found in ALECT2 fibrils (10,27,47). It is unlikely that proteolytic degradation is necessary for fibril formation in ALECT2 amyloidosis in opposition to most other amyloid precursor proteins. Mereuta et al. (11) showed that hepatocytes in patients with liver ALECT2 amyloidosis strongly and uniformly expressed LECT2 mRNA, whereas hepatocytes of normal individuals and patients with AL amyloidosis did not, suggesting that constitutive or compensatory LECT2 overexpression could be responsible for ALECT2 amyloidosis (Figure 1). Because LECT2 is involved in hepatocarcinogenesis and severe liver injury, it is possible that hepatocellular damage causes upregulation of LECT2 expression and consequently, ALECT2 amyloidosis (11). However, LECT2 plasma levels tested in a few patients with ALECT2 amyloidosis were found to be normal (16). DNA sequencing performed in 26 patients with ALECT2 amyloidosis did not detect any mutation in the LECT2 gene (5,10,11,16). Interestingly, all patients tested were homozygous for the G allele (5,10,11,16). It is possible that the change from isoleucine to valine in the LECT2 protein decreases its stability and allows it to undergo amyloidogenic conformational alterations (Figure 1). Recent data indicated that LECT2 is capable of self-oligomerization in vitro (48). Oligomerization could be an intermediate step toward fibril formation, which has been shown recently in β2-microglobulin amyloidosis (49). Because of its strong ethnic bias and the reported familiar occurrence in two brothers, ALECT2 amyloidosis may represent a digenic or polygenic disorder that results from a combination of the common G/G genotype and other genetic mutations that have yet to be characterized (Figure 1) (5,20). Additional studies are needed to determine if ALECT2 is, in fact, a genetic disorder. It is also possible that ALECT2 amyloidosis is caused by interference, possibly due to a genetic defect, in the catabolic or transport pathways of LECT2, resulting in an increased local tissue concentration and amyloid fibril formation (12,16,47). Another possible etiology is an alteration in a binding partner of the LECT2 protein in the serum resulting in increased levels of free circulating LECT2. Tubular and glomerular cells in patients with renal ALECT2 amyloidosis do not overexpress LECT2 mRNA (S.H. Nasr, unpublished data). This finding and the documented disease recurrence in the renal allograft argue against the possibility that renal ALECT2 amyloidosis is caused by locally produced LECT2. Currently, we do not know whether ALECT2 deposition is a slow process that begins early in life and remains undetected until significant accumulation has occurred or whether it is a more rapid process that begins later in life (5).
Figure 1.
Proposed pathogenesis of amyloidosis derived from leukocyte cell–derived chemotaxin 2 (ALECT2) amyloidosis. The pathogenesis of ALECT2 amyloidosis is still unknown. It is likely caused by leukocyte cell–derived chemotaxin 2 (LECT2) overexpression by hepatocytes either constitutively resulting from a combination of the common G/G genotype and yet to be defined genetic mutations or compensatory to hepatocellular damage. Upregulation of LECT2 overexpression by hepatocytes potentially leads to production of misfolded LECT2 protein. The presence of unstable LECT2 protein combined with several other factors, such as increased local concentrations, interactions with components of extracellular matrix, such as laminin and type IV collagen, and bindings with serum amyloid P (SAP), apoE, and glycosaminoglycans (GAGs), particularly heparin sulfate, ultimately lead to amyloid fibril formation and stabilization. The accumulations of large amounts of ALECT2 amyloid fibrils in the interstitium can disrupt kidney or liver architecture and impede their physiologic functions.
Pathologic Characteristics
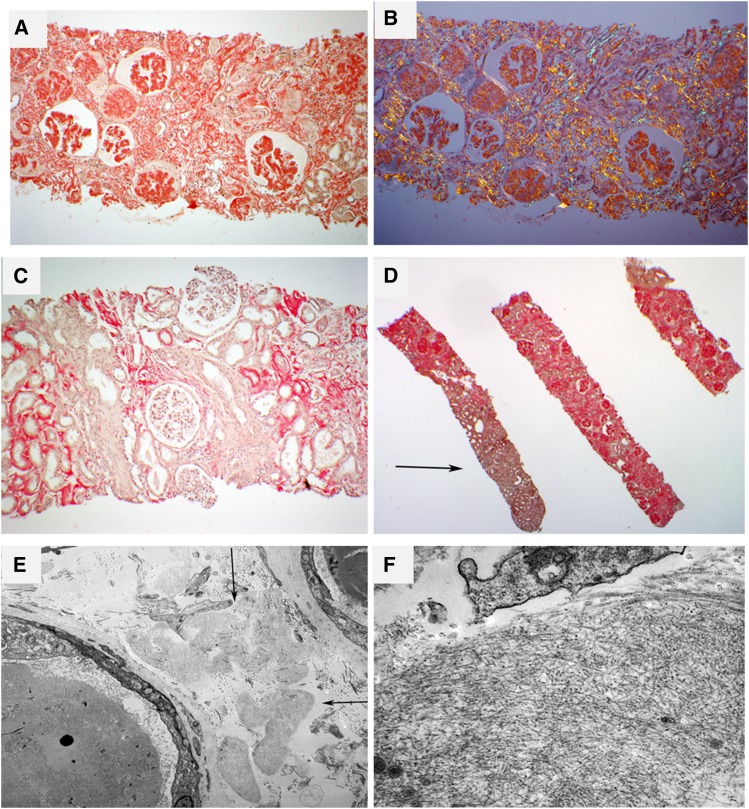
Histologically, renal ALECT2 amyloidosis shows preferential interstitial involvement, which is usually diffuse (Figure 2, A–D) (5,6,12,14,16). The glomerular and vascular amyloid deposits are variable, ranging from absent (Figure 2C) to mild to marked (Figure 2, A and B) (5,12). This is different from AL amyloidosis and AA amyloidosis, in which glomeruli and vessels are the prime sites of amyloid deposition (6). Contrary to AApo AI amyloidosis and AApo AIV amyloidosis, which mainly affect the medullary interstitium, ALECT2 amyloidosis shows a predominant involvement of cortical interstitium (Figure 2D) (5,12). ALECT2 deposits stain strongly positive with Congo red (5) and show anomalous colors, so called apple-green birefringence, under polarized light (Figure 2B). Similar to other forms of renal amyloidosis, the fibrils ultrastructurally appear randomly oriented with a mean diameter of 7–12 nm (Figure 2, E and F). Glomerular amyloid spicules, which result from parallel arrangement of amyloid fibrils perpendicular to the glomerular basement membranes, are far less common than in AL amyloidosis (6). Immunofluorescence is usually negative, although false-positive staining for IgG with/without staining for IgM, IgA, κ, and λ may rarely occur (12), albeit with a lesser frequency than in AA deposits (6). A minority (9%) of patients with ALECT2 amyloidosis show false-positive staining for serum amyloid A (SAA) (12). Importantly, concurrent renal disease was present in 32% and 26% of patients in the studies by Larsen et al. (5) and Said et al. (12), respectively, with diabetic nephropathy being the most common lesion followed by IgA nephropathy and membranous nephropathy (5,12). Compared with patients with ALECT2 amyloidosis without concurrent renal disease on biopsy, those with concurrent renal disease have higher proteinuria and lower renal amyloid load (consistent with ALECT2 amyloidosis detection at an earlier stage) (12).
Figure 2.
Renal pathology in amyloidosis derived from leukocyte cell–derived chemotaxin 2 (ALECT2) amyloidosis. (A) The case depicted shows extensive interstitial, glomerular, and vascular congophilic amyloid deposition. This patient had subnephrotic proteinuria (2.5 g/d) at diagnosis. Magnification, ×40. (B) Same field as in A. The congophilic amyloid deposits show anomalous colors (red, yellow, and green) under polarized light. Magnification, ×40. (C) The case depicted is from a patient who had a serum creatinine of 2.3 mg/dl at biopsy without proteinuria. There is diffuse cortical interstitial and focal arteriolar amyloid deposition with sparing of glomeruli (Congo red stain). Magnification, ×100. (D) This patient exhibits extensive involvement of cortical interstitium and glomeruli. The medullary interstitium (arrow) is spared (Congo red stain). Magnification, ×20. (E) A low-power electron microscopic image showing several interstitial collections of amyloid deposits (arrows). Magnification, ×5800. (F) A higher-magnification image reveals the fibrillar substructure of deposits. Magnification, ×49,000.
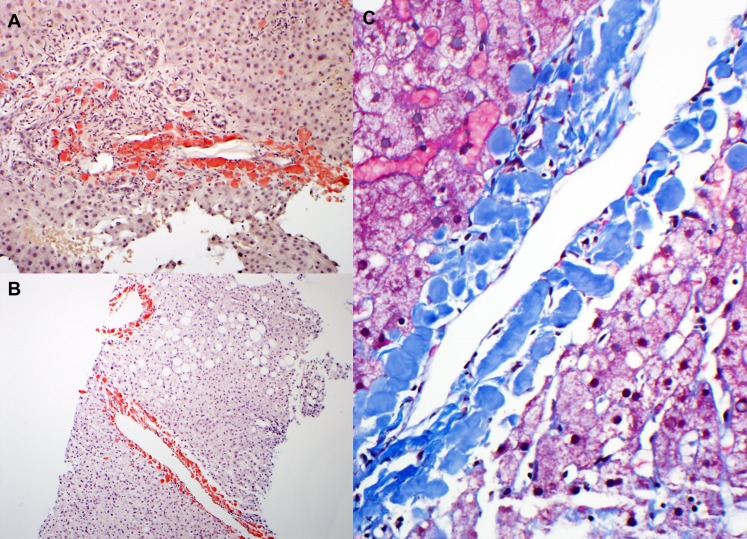
As in the kidney, ALECT2 deposits in the liver have a characteristic histologic morphology. In contrast to AL deposits, which typically exhibit a perisinusoidal distribution pattern, ALECT2 deposits are seen preferentially surrounding the central veins and in the periphery of portal tracts and exhibit a distinctive globular appearance (Figure 3) (11). As mentioned above, most specimens with liver ALECT2 deposits show other pathology that may account for liver dysfunction, most commonly chronic hepatitis C and steatohepatitis (11).
Figure 3.
Liver pathology in amyloidosis derived from leukocyte cell–derived chemotaxin 2 (ALECT2) amyloidosis. (A) There are periportal deposits of strongly congophilic amyloid deposits. Magnification, ×100. (B) In this low-power image, strongly congophilic amyloid deposits are seen surrounding the central veins. In contrast to Ig light chain–derived amyloidosis, the perisinusoidal spaces are spared. Magnification, ×40. (C) On high magnification, ALECT2 deposits form large acellular globules, which appear blue on trichrome stain. Magnification, ×200.
Diagnosis
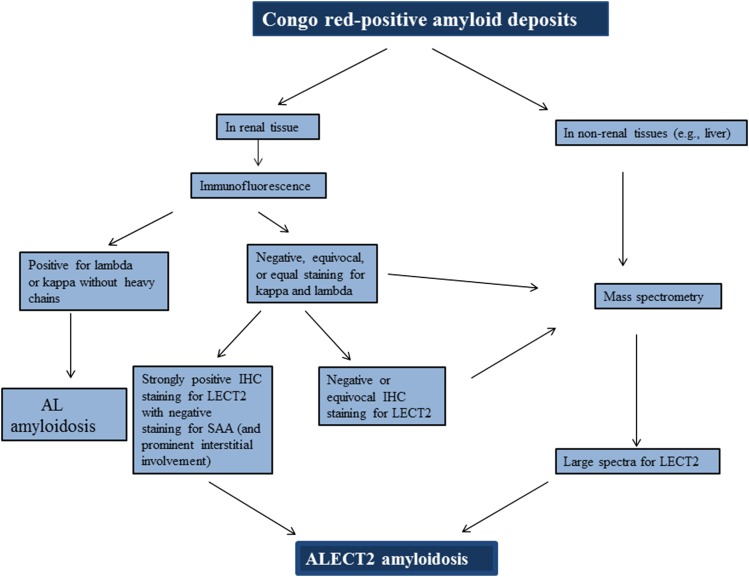
Typing amyloid deposits by LC/MS-based proteomics is currently the best tool to diagnose ALECT2 amyloidosis and other forms of amyloidosis because of its high sensitivity and specificity and because it is a single test that can detect the culprit protein in contrast to IHC typing of amyloidosis, which requires staining for multiple antibodies using several tissue sections (6,50,51). LECT2 protein has many lysine and arginine residues and therefore, can readily be digested by trypsin into peptides, and the amino acid sequence of the peptides can be determined by LC/MS. The diagnosis of ALECT2 amyloidosis by LC/MS is made according to clinically established criteria by identification of LECT2 peptide spectra and amyloid-associated proteins (serum amyloid P [SAP] and apoE) (Figure 4) (11,51). Because plasma concentration of LECT2 is trivial, contamination of amyloid deposits by serum LECT2 is not an issue, and the diagnosis is usually straightforward. Unfortunately, clinical amyloid typing by proteomics is available only in few selected centers, such as the Mayo Clinic. There are now several commercially available antibodies for IHC detection of LECT2 that seem to be highly sensitive (Figure 5); however, weak false-positive staining may occur (52), which may result from aggressive antigen retrieval (53). Therefore, it is crucial that LECT2 antibody is carefully validated and optimized for the diagnosis of ALECT2 (53). In cases that exhibit only weak staining for LECT2, false-positive staining for SAA, or staining for Ig light or heavy chains by immunofluorescence or immunoperoxidase, confirmation by LC/MS is strongly recommended. When amyloid typing is performed by IHC, it is critical that the testing is done with a panel of antibodies against several types of amyloidosis, so-called comparative IHC, instead of staining for a single precursor protein (54). In general, better results are obtained if amyloid typing by IHC is performed in specialized laboratories (54–56). Of note, monoclonal gammopathy of undetermined significance was present in 10% and 8% of patients reported by Said et al. (12) and Larsen et al. (5), respectively, highlighting the importance of accurate typing of amyloid deposits to avoid misdiagnosing ALECT2 amyloidosis as AL amyloidosis (see below). We propose the algorithm shown in Figure 6 for diagnosis of ALECT2 amyloidosis.
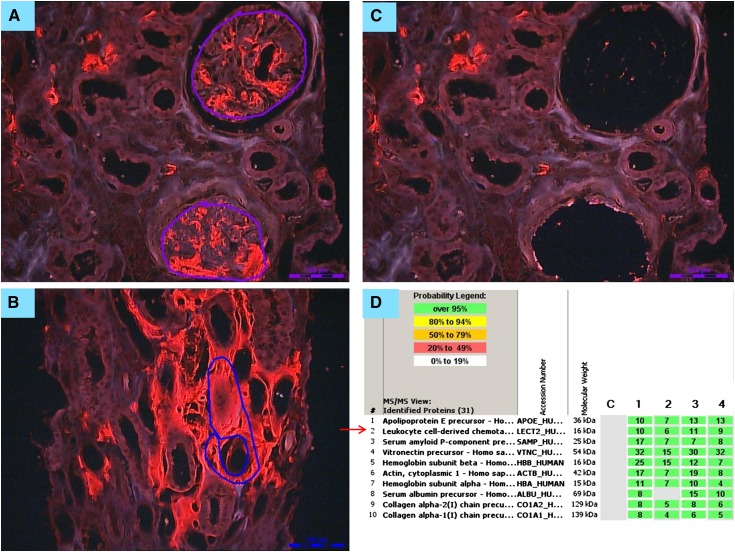
Figure 4.
Laser microdissection/mass spectrometry in a patient with renal amyloidosis derived from leukocyte cell–derived chemotaxin 2 (ALECT2) amyloidosis. (A) Glomerular and (B) interstitial congophilic amyloid deposits visualized under fluorescent light and marked for microdissection. Magnification, ×200. (C) Vacant spaces after microdissection of glomerular amyloid deposits. Magnification, ×200. (D) List of amyloid-associated proteins identified within the deposits by liquid chromatography/mass spectrometry (LC/MS) from a patient with ALECT2 displayed by Scaffold (proteome software). The four columns represent separate microdissected samples run in replicate. The spectra value indicates the total number of mass spectra collected on the mass spectrometer and matched to the protein using the proteomic software. A higher number of mass spectra is indicative of greater abundance of protein and greater amino acid sequence coverage. Our clinical amyloid testing requires a minimum number of four spectra with >95% probability in all samples before the protein identification will be deemed clinically valid. In this case, the presence of abundant spectra for apoE and serum amyloid P is indicative of amyloid, whereas the presence of abundant spectra for leukocyte cell–derived chemotaxin 2 (LECT2) establishes the type as ALECT2 amyloidosis.
Figure 5.
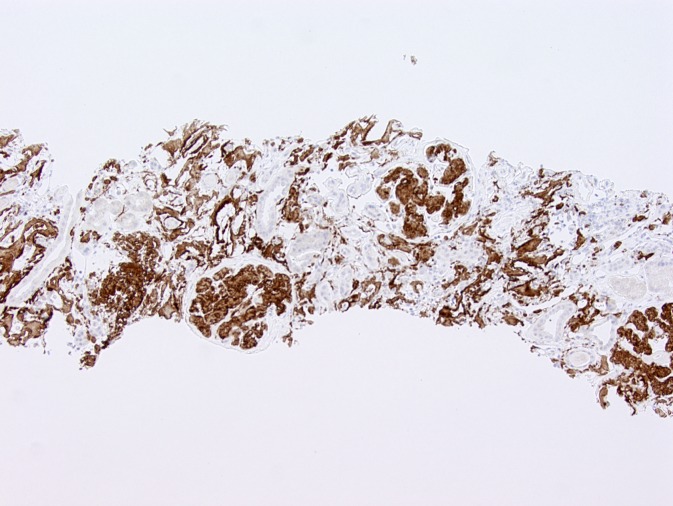
Immunohistochemical staining for leukocyte cell–derived chemotaxin 2 (LECT2). Glomerular and interstitial amyloid deposits are strongly positive for LECT2 by immunohistochemistry in this patient with renal amyloidosis derived from LECT2 amyloidosis. Magnification, ×100.
Figure 6.
Proposed algorithm for diagnosis of amyloidosis derived from leukocyte cell–derived chemotaxin 2 (ALECT2) amyloidosis. AL, Ig light chain–derived; IHC, immunohistochemistry; LECT2, leukocyte cell–derived chemotaxin 2; SAA, serum amyloid A.
Prognosis and Treatment
Patient survival in ALECT2 amyloidosis is significantly better than that of AL amyloidosis and AA amyloidosis, likely because of the rarity or absence of cardiac involvement in ALECT2 amyloidosis. In a series of 72 patients with renal ALECT2 amyloidosis, only 6% of 64 patients with available data died after a median follow-up of 22 months (12). The renal survival, however, is guarded; 39% of patients in the above-mentioned study progressed to ESRD (12). In the study by Larsen et al. (5), in which 32% of patients had concurrent renal disease, follow-up was available in 21 patients, and two of them had ESRD at diagnosis. The remaining 19 patients had an average deterioration in renal function of 0.5 ml/min per 1.73 m2 per month after a mean follow-up of 50 months (5). Outcome analysis was performed in the study by Said et al. (12). Independent predictors of renal survival in renal ALECT2 amyloidosis without concurrent kidney disease on biopsy were serum creatinine at diagnosis, with a value of 2.0 mg/dl being the best cutoff for predicting ESRD, degree of glomerulosclerosis, and presence of diabetes (12). Renal amyloid load and degree of proteinuria did not predict outcome (12).
There is currently no specific therapy for ALECT2 amyloidosis. Because the precursor protein is normal (nonmutant) LECT2, liver transplantation probably is not effective, although this approach has not been tested. Preliminary data suggest that renal transplantation is a reasonable therapeutic option for patients with advanced renal failure. In one recent series, the disease recurred in one of five patients transplanted, but the short-term outcome was good: after a mean duration of 20 months of post-transplant follow-up, no patient had graft loss, and two patients had normal final serum creatinine (12). Some potential future therapies for ALECT2 amyloidosis include reducing the supply of LECT2 (such as by Wnt/β-catinin signaling pathway inhibitors, etinoids, exisulind, and endostatin), inhibiting fibrillogenesis (such as by blocking the binding of glycosaminoglycans to amyloid fibrils), enhancing clearance of amyloid by immunotherapy, and promoting amyloid regression by targeting SAP. SAP is a major component of amyloid deposits of any type. Antibodies to human SAP were shown to eliminate visceral AA deposits in mice (57). It is critical not to misdiagnose ALECT2 amyloidosis as AL amyloidosis to avoid harmful therapy (20). This error may occur, because ALECT2 amyloidosis has only recently been recognized, most patients are elderly, and a minority have monoclonal gammopathy (comparable with the general elderly population) (5,12). In one series, four (6%) patients received chemotherapy with or without stem-cell transplant for an erroneous diagnosis of AL amyloidosis (12).
Summary
Nephrologists and pathologists should maintain a high level of suspicion for ALECT2 amyloidosis in older individuals of Mexican origin who present with renal impairment and bland urinary sediment, regardless of the degree of proteinuria, and a Congo red stain should be performed. Hepatic ALECT2 amyloidosis may not be the cause of clinically significant liver disease. Recent studies on ALECT2 amyloidosis remind us of the importance of accurate typing of the amyloid deposits and confirmation by LC/MS when indicated to establish that the culprit proteins causing amyloidosis really are Ig light chains and not other proteins, such as LECT2, TTR, or SAA, to avoid unnecessary and harmful chemotherapy.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, Saraiva MJ, Westermark P: Nomenclature 2014: Amyloid fibril proteins and clinical classification of the amyloidosis. Amyloid 21: 221–224, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Chugh KS, Datta BN, Singhal PC, Jain SK, Sakhuja V, Dash SC: Pattern of renal amyloidosis in Indian patients. Postgrad Med J 57: 31–35, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gertz MA, Leung N, Lacy MQ, Dispenzieri A, Zeldenrust SR, Hayman SR, Buadi FK, Dingli D, Greipp PR, Kumar SK, Lust JA, Rajkumar SV, Russell SJ, Witzig TE: Clinical outcome of immunoglobulin light chain amyloidosis affecting the kidney. Nephrol Dial Transplant 24: 3132–3137, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Lachmann HJ, Goodman HJ, Gilbertson JA, Gallimore JR, Sabin CA, Gillmore JD, Hawkins PN: Natural history and outcome in systemic AA amyloidosis. N Engl J Med 356: 2361–2371, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Larsen CP, Kossmann RJ, Beggs ML, Solomon A, Walker PD: Clinical, morphologic, and genetic features of renal leukocyte chemotactic factor 2 amyloidosis. Kidney Int 86: 378–382, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Said SM, Sethi S, Valeri AM, Leung N, Cornell LD, Fidler ME, Herrera Hernandez L, Vrana JA, Theis JD, Quint PS, Dogan A, Nasr SH: Renal amyloidosis: Origin and clinicopathologic correlations of 474 recent cases. Clin J Am Soc Nephrol 8: 1515–1523, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Hutten H, Mihatsch M, Lobeck H, Rudolph B, Eriksson M, Röcken C: Prevalence and origin of amyloid in kidney biopsies. Am J Surg Pathol 33: 1198–1205, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Maury CP, Kere J, Tolvanen R, de la Chapelle A: Homozygosity for the Asn187 gelsolin mutation in Finnish-type familial amyloidosis is associated with severe renal disease. Genomics 13: 902–903, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Rivera F, López-Gómez JM, Pérez-García R; Spsnish Registry of Glomerulonephritis: Frequency of renal pathology in Spain 1994-1999. Nephrol Dial Transplant 17: 1594–1602, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Benson MD, James S, Scott K, Liepnieks JJ, Kluve-Beckerman B: Leukocyte chemotactic factor 2: A novel renal amyloid protein. Kidney Int 74: 218–222, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Mereuta OM, Theis JD, Vrana JA, Law ME, Grogg KL, Dasari S, Chandan VS, Wu TT, Jimenez-Zepeda VH, Fonseca R, Dispenzieri A, Kurtin PJ, Dogan A: Leukocyte cell-derived chemotaxin 2 (LECT2)-associated amyloidosis is a frequent cause of hepatic amyloidosis in the United States. Blood 123: 1479–1482, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Said SM, Sethi S, Valeri AM, Chang A, Nast CC, Krahl L, Molloy P, Barry M, Fidler ME, Cornell LD, Leung N, Vrana JA, Theis JD, Dogan A, Nasr SH: Characterization and outcomes of renal leukocyte chemotactic factor 2-associated amyloidosis. Kidney Int 86: 370–377, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Theis JD, Dasari S, Vrana JA, Mereuta OM, Grogg KL, Gertz MA, Zeldenrust SR, Dispenzieri A, Kurtin PJ, Dogan A: Proteome of amyloidosis: Mayo Clinic experience in 4139 cases. Blood 122: 1900, 2013. 23896410 [Google Scholar]
- 14.Larsen CP, Walker PD, Weiss DT, Solomon A: Prevalence and morphology of leukocyte chemotactic factor 2-associated amyloid in renal biopsies. Kidney Int 77: 816–819, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vrana JA, Theis JD, Grogg KL, Wu TT, Chandan VS, Dogan A: Amyloidosis of the liver: Mass spectrometry-based proteomic analysis reveals diverse etiology associated with distinct histological features. Lab Invest 92: 425a, 2012 [Google Scholar]
- 16.Murphy CL, Wang S, Kestler D, Larsen C, Benson D, Weiss DT, Solomon A: Leukocyte chemotactic factor 2 (LECT2)-associated renal amyloidosis: a case series. Am J Kidney Dis 56: 1100–1107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutton HL, DeMarco ML, Magil AB, Taylor P: Renal leukocyte chemotactic factor 2 (LECT2) amyloidosis in First Nations people in Northern British Columbia, Canada: a report of 4 cases. Am J Kidney Dis 64: 790–792, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Dogan A, Theis JD, Vrana JA, Jimenez-Zepeda VH, Lacy MQ, Leung N, Dispenzieri A, Zeldenrust SR, Fonseca R, Gilbertson JA, Hunt T, Wechalekar AD, Lachmann HJ, Rowczenio D, Hawkins PN, Gillmore JD: Clinical and pathological phenotype of leukocyte cell-derived chemotaxin-2 (LECT2) amyloidosis (ALECT2). Amyloid 17: 69–70, 2010. 20462365 [Google Scholar]
- 19.Bryc K, Auton A, Nelson MR, Oksenberg JR, Hauser SL, Williams S, Froment A, Bodo JM, Wambebe C, Tishkoff SA, Bustamante CD: Genome-wide patterns of population structure and admixture in West Africans and African Americans. Proc Natl Acad Sci U S A 107: 786–791, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Picken MM: Alect2 amyloidosis: Primum non nocere (first, do no harm). Kidney Int 86: 229–232, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Khalighi MA, Yue A, Hwang M, Wallace WD: Leukocyte chemotactic factor 2 (LECT2) amyloidosis presenting as pulmonary-renal syndrome: A case report and review of the literature. Clin Kidney J 6: 618–621, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fix OK, Freise CE, Shores NJ, Damon LE, Lee BK, Brenner DA, McGlothlin DP, Ferrell LD, Olson JL, Liepnieks JJ, Benson MD: First liver and kidney transplant for leukocyte chemotactic factor 2-amyloidosis presenting with acute liver failure. ISN abstract OP53. Presented at the XIII International Symposium on Amyloidosis, Groningen, The Netherlands, May 6–10, 2012 [Google Scholar]
- 23.Damlaj M, Amre R, Wong P, How J: Hepatic ALECT-2 amyloidosis causing portal hypertension and recurrent variceal bleeding: A case report and review of the literature. Am J Clin Pathol 141: 288–291, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Yamagoe S, Yamakawa Y, Matsuo Y, Minowada J, Mizuno S, Suzuki K: Purification and primary amino acid sequence of a novel neutrophil chemotactic factor LECT2. Immunol Lett 52: 9–13, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Okumura A, Suzuki T, Dohmae N, Okabe T, Hashimoto Y, Nakazato K, Ohno H, Miyazaki Y, Yamagoe S: Identification and assignment of three disulfide bonds in mammalian leukocyte cell-derived chemotaxin 2 by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Biosci Trends 3: 139–143, 2009 [PubMed] [Google Scholar]
- 26.Yamagoe S, Kameoka Y, Hashimoto K, Mizuno S, Suzuki K: Molecular cloning, structural characterization, and chromosomal mapping of the human LECT2 gene. Genomics 48: 324–329, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Hardwick J, Murrell J, Kluve-Beckerman B, Liepnieks J, Benson MD: LECT2 amyloidosis in patients of Hispanic origin. Presented at the XIV International Symposium on Amyloidosis, Indianapolis, IN, April 27–May 1, 2014 [Google Scholar]
- 28.Kameoka Y, Yamagoe S, Hatano Y, Kasama T, Suzuki K: Val58Ile polymorphism of the neutrophil chemoattractant LECT2 and rheumatoid arthritis in the Japanese population. Arthritis Rheum 43: 1419–1420, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Ovejero C, Cavard C, Périanin A, Hakvoort T, Vermeulen J, Godard C, Fabre M, Chafey P, Suzuki K, Romagnolo B, Yamagoe S, Perret C: Identification of the leukocyte cell-derived chemotaxin 2 as a direct target gene of beta-catenin in the liver. Hepatology 40: 167–176, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Yamagoe S, Akasaka T, Uchida T, Hachiya T, Okabe T, Yamakawa Y, Arai T, Mizuno S, Suzuki K: Expression of a neutrophil chemotactic protein LECT2 in human hepatocytes revealed by immunochemical studies using polyclonal and monoclonal antibodies to a recombinant LECT2. Biochem Biophys Res Commun 237: 116–120, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Nagai H, Hamada T, Uchida T, Yamagoe S, Suzuki K: Systemic expression of a newly recognized protein, LECT2, in the human body. Pathol Int 48: 882–886, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Lu XJ, Yang HY, Shi YH: An interaction between a C-type lectin receptor and leukocyte cell-derived chemotaxin 2 of ayu, Plecoglossus altivelis. Fish Shellfish Immunol 28: 245–248, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Chen CK, Yang CY, Hua KT, Ho MC, Johansson G, Jeng YM, Chen CN, Chen MW, Lee WJ, Su JL, Lai TC, Chou CC, Ho BC, Chang CF, Lee PH, Chang KJ, Hsiao M, Lin MT, Kuo ML: Leukocyte cell-derived chemotaxin 2 antagonizes MET receptor activation to suppress hepatocellular carcinoma vascular invasion by protein tyrosine phosphatase 1B recruitment. Hepatology 59: 974–985, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Anson M, Crain-Denoyelle AM, Baud V, Chereau F, Gougelet A, Terris B, Yamagoe S, Colnot S, Viguier M, Perret C, Couty JP: Oncogenic β-catenin triggers an inflammatory response that determines the aggressiveness of hepatocellular carcinoma in mice. J Clin Invest 122: 586–599, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saito T, Okumura A, Watanabe H, Asano M, Ishida-Okawara A, Sakagami J, Sudo K, Hatano-Yokoe Y, Bezbradica JS, Joyce S, Abo T, Iwakura Y, Suzuki K, Yamagoe S: Increase in hepatic NKT cells in leukocyte cell-derived chemotaxin 2-deficient mice contributes to severe concanavalin A-induced hepatitis. J Immunol 173: 579–585, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Lan F, Misu H, Chikamoto K, Takayama H, Kikuchi A, Mohri K, Takata N, Hayashi H, Matsuzawa-Nagata N, Takeshita Y, Noda H, Matsumoto Y, Ota T, Nagano T, Nakagen M, Miyamoto K, Takatsuki K, Seo T, Iwayama K, Tokuyama K, Matsugo S, Tang H, Saito Y, Yamagoe S, Kaneko S, Takamura T: LECT2 functions as a hepatokine that links obesity to skeletal muscle insulin resistance. Diabetes 63: 1649–1664, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Ando K, Kato H, Kotani T, Ozaki M, Arimura Y, Yagi J: Plasma leukocyte cell-derived chemotaxin 2 is associated with the severity of systemic inflammation in patients with sepsis. Microbiol Immunol 56: 708–718, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Sato Y, Watanabe H, Kameyama H, Kobayashi T, Yamamoto S, Takeishi T, Hirano K, Oya H, Nakatsuka H, Watanabe T, Kokai H, Yamagoe S, Suzuki K, Oya K, Kojima K, Hatakeyama K: Serum LECT2 level as a prognostic indicator in acute liver failure. Transplant Proc 36: 2359–2361, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Okabe H, Delgado E, Lee JM, Yang J, Kinoshita H, Hayashi H, Tsung A, Behari J, Beppu T, Baba H, Monga SP: Role of leukocyte cell-derived chemotaxin 2 as a biomarker in hepatocellular carcinoma. PLoS ONE 9: e98817, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okumura A, Unoki-Kubota H, Matsushita Y, Shiga T, Moriyoshi Y, Yamagoe S, Kaburagi Y: Increased serum leukocyte cell-derived chemotaxin 2 (LECT2) levels in obesity and fatty liver. Biosci Trends 7: 276–283, 2013 [PubMed] [Google Scholar]
- 41.Okumura A, Saito T, Otani I, Kojima K, Yamada Y, Ishida-Okawara A, Nakazato K, Asano M, Kanayama K, Iwakura Y, Suzuki K, Yamagoe S: Suppressive role of leukocyte cell-derived chemotaxin 2 in mouse anti-type II collagen antibody-induced arthritis. Arthritis Rheum 58: 413–421, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Lu XJ, Chen J, Yu CH, Shi YH, He YQ, Zhang RC, Huang ZA, Lv JN, Zhang S, Xu L: LECT2 protects mice against bacterial sepsis by activating macrophages via the CD209a receptor. J Exp Med 210: 5–13, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ong HT, Tan PK, Wang SM, Hian Low DT, Ooi LL, Hui KM: The tumor suppressor function of LECT2 in human hepatocellular carcinoma makes it a potential therapeutic target. Cancer Gene Ther 18: 399–406, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Hiraki Y, Inoue H, Kondo J, Kamizono A, Yoshitake Y, Shukunami C, Suzuki F: A novel growth-promoting factor derived from fetal bovine cartilage, chondromodulin II. Purification and amino acid sequence. J Biol Chem 271: 22657–22662, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Ito M, Yamagoe S, Tomizawa K, Mizuno S, Tanokura M, Suzuki K: Preparation of recombinant six-histidine-tagged human LECT2, a chemotactic protein to neutrophils, in Escherichia coli. Cytotechnology 25: 235–238, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng H, Miyakawa T, Sawano Y, Yamagoe S, Tanokura M: Expression, high-pressure refolding and purification of human leukocyte cell-derived chemotaxin 2 (LECT2). Protein Expr Purif 88: 221–229, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Benson MD: LECT2 amyloidosis. Kidney Int 77: 757–759, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Okumura A, Suzuki T, Miyatake H, Okabe T, Hashimoto Y, Miyakawa T, Zheng H, Unoki-Kubota H, Ohno H, Dohmae N, Kaburagi Y, Miyazaki Y, Tanokura M, Yamagoe S: Leukocyte cell-derived chemotaxin 2 is a zinc-binding protein. FEBS Lett 587: 404–409, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Liu C, Sawaya MR, Eisenberg D: β₂-microglobulin forms three-dimensional domain-swapped amyloid fibrils with disulfide linkages. Nat Struct Mol Biol 18: 49–55, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sethi S, Vrana JA, Theis JD, Leung N, Sethi A, Nasr SH, Fervenza FC, Cornell LD, Fidler ME, Dogan A: Laser microdissection and mass spectrometry-based proteomics aids the diagnosis and typing of renal amyloidosis. Kidney Int 82: 226–234, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR, 3rd, Dogan A: Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood 114: 4957–4959, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Paueksakon P, Fogo AB, Sethi S: Leukocyte chemotactic factor 2 amyloidosis cannot be reliably diagnosed by immunohistochemical staining. Hum Pathol 45: 1445–1450, 2014 [DOI] [PubMed] [Google Scholar]
- 53.Larsen C: Leukocyte chemotactic factor 2 amyloidosis can be reliably diagnosed by immunohistochemical staining. Hum Pathol 45: 2179, 2014 [DOI] [PubMed] [Google Scholar]
- 54.Picken MM, Westermark P: Amyloid detection and typing: summary of current practice and recommendations of the consensus group. Amyloid 18[Suppl 1]: 48–50, 2011 [DOI] [PubMed] [Google Scholar]
- 55.Linke RP: On typing amyloidosis using immunohistochemistry. Detailled illustrations, review and a note on mass spectrometry. Prog Histochem Cytochem 47: 61–132, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Schönland SO, Hegenbart U, Bochtler T, Mangatter A, Hansberg M, Ho AD, Lohse P, Röcken C: Immunohistochemistry in the classification of systemic forms of amyloidosis: A systematic investigation of 117 patients. Blood 119: 488–493, 2012 [DOI] [PubMed] [Google Scholar]
- 57.Bodin K, Ellmerich S, Kahan MC, Tennent GA, Loesch A, Gilbertson JA, Hutchinson WL, Mangione PP, Gallimore JR, Millar DJ, Minogue S, Dhillon AP, Taylor GW, Bradwell AR, Petrie A, Gillmore JD, Bellotti V, Botto M, Hawkins PN, Pepys MB: Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature 468: 93–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]