Abstract
Very preterm birth is associated with an increased prevalence of attention problems and may especially impair executive attention, i.e., top-down control of attentional selection in situations where distracting information interferes with the processing of task-relevant stimuli. While there are initial findings linking structural brain alterations in preterm-born individuals with attention problems, the functional basis of these problems are not well understood. The present study used an fMRI adaptation of the Attentional Network Test to examine the neural correlates of executive attention in a large sample of N = 86 adults born very preterm and/or with very low birth weight (VP/VLBW), and N = 100 term-born controls. Executive attention was measured by comparing task behavior and brain activations associated with the processing of incongruent vs. congruent arrow flanker stimuli. Consistent with subtle impairments of executive attention, the VP/VLBW group showed lower accuracy and a tendency for increased response times during the processing of incongruent stimuli. Both groups showed similar activation patters, especially within expected fronto-cingulo-parietal areas, but no significant between-group differences. Our results argue for a maintained attention-relevant network organization in high-functioning preterm born adults in spite of subtle deficits in executive attention. Gestational age and neonatal treatment variables showed associations with task behavior, and brain activation in the dorsal ACC and lateral occipital areas, suggesting that the degree of prematurity (and related neonatal complications) has subtle modulatory influences on executive attention processing.
Abbreviations: ACC, anterior cingulate cortex; ANT, Attentional Network Test; BLS, Bavarian Longitudinal Study; BW, birth weight; CSF, cerebrospinal fluid; DLPFC, dorsolateral prefrontal cortex; DNTI, duration of neonatal intensive treatment; EHI, Edinburgh Handedness Inventory; ELBW, extremely low birth weight; EP, extremely preterm; fMRI, functional magnetic resonance imaging; FWE, familywise error; GA, gestational age; GM, gray matter; ICV, intracranial volume; IVH, intraventricular hemorrhage; INTI, intensity of neonatal intensive treatment; PFC, prefrontal cortex; VLBW, very low birth weight; VP, very preterm; WM, white matter
Keywords: Preterm birth, Gestational age, Attentional Network Test, Executive attention, Anterior cingulate
Highlights
-
•
fMRI study examines neural correlates of executive attention in preterm-born adults.
-
•
Preterm-born adults show subtle behavioral deficits.
-
•
Preterm-born adults show maintained organization of attention-related networks.
-
•
Modulatory effects of gestational age and neonatal treatment variables are observed.
1. Introduction
Attentional problems are among the most consistently reported cognitive impairments related to prematurity (Aarnoudse-Moens et al., 2009; Anderson, 2014; Bhutta et al., 2002; Hack et al., 2009; Jaekel et al., 2013b; Mulder et al., 2009; Wilson-Ching et al., 2013), representing a hallmark feature of a “preterm behavioral phenotype” (Johnson and Marlow, 2011). This is supported by many behavioral studies that assess specific attentional functions in children (Anderson et al., 2011; Geldof et al., 2013), adolescents (Luu et al., 2011; Wilson-Ching et al., 2013) or adults (Eryigit-Madzwamuse et al., 2015; Nosarti et al., 2007; Solsnes et al., 2014) who were born very (VP, <32 weeks gestation) or extremely preterm (EP, <28 weeks gestation), or had a very (VLBW, <1500 g) or extremely low (ELBW, <1000 g) birth weight. They show significant impairments in task domains such as selective attention, sustained attention or shifting attention (Aarnoudse-Moens et al., 2009; Mulder et al., 2009), which afford increased “top-down” (=executive) control of attentional resources. Recent studies using the Attentional Network Test (ANT: Fan et al., 2002; Geldof et al., 2013; Pizzo et al., 2010) indicated that preterm children were selectively impaired in the executive attention component of the task (which assesses the ability to focus attention on a task-relevant central arrow stimulus despite interference by task-irrelevant incongruent vs. congruent flanker stimuli), while they showed intact alerting and orienting (Geldof et al., 2013; Pizzo et al., 2010), suggesting that the brain networks subserving executive attention are especially vulnerable to the detrimental effects of preterm birth.
Yet, although various studies show prematurity-related deficits in tasks measuring executive attention via similar interference processing tasks (e.g., Stroop and Flanker tasks: de Kieviet et al., 2014; Luu et al., 2011; Solsnes et al., 2014), other studies do not find significant differences (Aarnoudse-Moens et al., 2012; Elgen et al., 2004) or detect impairments only in younger children, suggesting the possibility of a developmental “catch-up” (e.g., Ritter et al., 2013). The latter observation points to the possibility that preterm-born individuals develop compensatory brain mechanisms to cope with existing functional deficits secondary to neonatal brain injury or aberrant brain development, e.g., by stronger recruitment of prototypical task related areas, or by recruiting alternative processing pathways (e.g., Nosarti et al., 2006; Peterson et al., 2002). While functional neuroimaging studies can help to test these assumptions, relevant evidence from preterm-born populations is limited, and shows either no differences (de Kieviet et al., 2014) or reduced activation of task-related brain networks (Griffiths et al., 2013) in preterm-born children. Complementary studies in preterm-born adults are not yet available.
1.1. Study aims
The present study investigated the neural correlates of executive attention (as measured by contrasting brain responses during the processing of incongruent versus congruent flanker stimuli of an ANT fMRI paradigm) in VP/VLBW and term-born controls at age 26, with the following aims. First, group comparisons examined whether the location and level of task-induced brain activations in VP/VLBW adults differed significantly from controls. In line with previous theoretical accounts (e.g., Just and Varma, 2007), it was expected that adults born preterm would compensate prematurity-related brain dysfunctions by an over-recruitment of task-relevant areas, which include the dorsal anterior cingulate cortex (ACC)/presupplementary motor area and lateral prefrontal cortex (PFC), as well as lateral parietal areas (de Kieviet et al., 2014; Fan et al., 2005; Neufang et al., 2011). Second, to explore whether VP/VLBW behavioral performance and activation patterns were influenced by the degree of immaturity at birth or neonatal risk factors (e.g., Kalpakidou et al., 2012; Narberhaus et al., 2009), we examined whether they were predicted by neonatal variables which are known risk factors for poor neurological outcome (e.g., Aanes et al., 2015): Gestational age (GA), birth weight (BW), duration of ventilation, and duration of neonatal intensive care.
2. Methods
This fMRI study was conducted as a part of the prospective Bavarian Longitudinal Study (BLS), a geographically defined whole-population sample of VP/VLBW and term-born individuals, who were followed from birth into early adulthood (Riegel et al., 1995; Wolke and Meyer, 1999). To examine their developmental status, they were repeatedly assessed with neurological and psychological test batteries, and parental interviews, during childhood, adolescence, and, most recently, at 26 years of age, by specially trained psychologists. Following the behavioral assessments in adulthood, eligible participants were invited for an additional MRI examination (including the ANT paradigm) on a separate occasion. For each participant, a careful screening for MR-related contraindications (e.g., severe claustrophobia, pregnancy, electrical or ferromagnetic implants) was conducted.
MRI examinations were conducted at two sites: The Department of Neuroradiology of the Klinikum Rechts der Isar, Technische Universität München, and the Department of Radiology of the University Hospital Bonn. All travel expenses and attendance were reimbursed. The study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki), and approved by the local Institutional Review Boards of both hospitals. All participants gave written informed consent.
2.1. Participants
2.1.1. VP/VLBW group
VP/VLBW infants were recruited from the whole population of at-risk infants born alive in Southern Bavaria between January 1985 and March 1986 who required admission to one of the 17 children's hospitals within the first 10 days after birth (N = 7505; 10.6% of all live births). Of this initial cohort, 682 children were born VP/VLBW (GA < 32 weeks, and/or BW < 1500 g). 172 died during initial hospitalization and 12 died between discharge and 26 year assessments. Seven parents did not give consent to participate, while 43 parents and their children were non-German speakers and excluded as cognitive assessments could not be administered. No contact information was available for 37 VP/VLBW adults. Of the eligible 411 VP/VLBW survivors, 260 (63.3%) participated in psychological assessments at 26 years, with 104 (25.3%) undergoing the additional MRI examination. For four of these participants, ANT imaging data were not available, one was excluded due to severe image artifacts, two due to either deviant Executive Network Scores (see Section 2.4) or high error/omission rates (both: >3SD from mean), and six because of excessive scan-to-scan movements (>2 mm in any direction). A further five cases where excluded due to unavailable IQ data at 26 years. In total, the present analysis included 86 VP/VLBW.
2.1.2. Term-born controls
A comparison sample of term-born born infants (GA > 36 weeks) was recruited from normal postnatal wards in the same obstetric hospitals. Of the initial 916 control children, 350 were randomly selected as term controls within the stratification variables sex and family socioeconomic status (SES) to be comparable with the VP/VLBW cohort at age 6 years 3 months. Of these, 308 were eligible for 26 year follow-up assessments, with 229 (74.4%) participating in the psychological assessments, and 110 (35.6%) undergoing the additional MRI examination. For two of these participants, ANT imaging data were not available, one was excluded because of severe image artifacts, two due to high error/omission rates (>3 SD above mean), and two because of excessive scan-to-scan movements (>2 mm in any direction). A further three cases were excluded due to unavailable IQ data at 26 years. In total, the final sample included 100 controls.
2.2. Background characteristics
Background information for neonatal parameters was drawn from earlier assessments (Gutbrod et al., 2000; Riegel et al., 1995): These included GA, BW, multiple births, maternal age, duration of neonatal intensive treatment (DNTI) and intensity of neonatal intensive treatment (INTI), duration of ventilation and duration of hospitalization. Moreover, standardized optimality scores (Prechtl, 1967) summarized the number of prepregnancy complications (0–8, e.g., prior disabled child, prior preterm birth), pregnancy complications (0–14, e.g., nicotine addiction, anemia), perinatal complications (0–15, e.g., no spontaneous labor, anesthesia), and neonatal complications (0–21, e.g., ventilation/intubation, neonatal seizures, sepsis; cf. Schmid et al., 2011). For VP/VLBW only, intraventricular hemorrhage (IVH) was assessed with ultrasound examination, graded 1–4. SES at birth was based on a weighted composite derived from the occupation of the highest educational qualification held by either parent and the occupation of the self-identified head of the family (Bauer, 1988). Developmental cognitive measurements included the German adaptations of the Griffiths Scales of Baby Abilities (Brandt, 1983) at 5 and 20 months, and the Kaufman-Assessment Battery for Children (Melchers and Preuss, 1991) at 6 and 8 years. At 26 years, a short version of the German Wechsler Adult Scale of Intelligence, Third edition (WAIS-III: von Aster et al., 2006) was administered to derive estimates for the Full Scale Intelligence Quotient (FSIQ). The battery included the subtests Vocabulary, Similarities, Digit Symbol Coding, Block Design, Matrix Reasoning, and Letter Number Sequencing (cf. Breeman et al., 2015).
In addition, adult handedness was examined with the Edinburgh Handedness Inventory (EHI: Oldfield, 1971). Participants were classified as left-handed (EHI: −100 to −61), ambidexter (EHI: −60 to +60), or right-handed (EHI: +61 to +100) (cf. Dragovic, 2004).
To examine dropout-related selection biases, neonatal and developmental scores for the presented VP/VLBW and term-born subsamples were compared with respective data from those participants in the original cohort who were not included in the following analyses.
2.3. Experimental task
The task was presented using Presentation® (Neurobehavioral Systems Inc., Albany, CA). Visual stimuli were projected onto a display positioned inside the scanner room, which was viewed through a mirror system mounted on the MR head coil. Responses were recorded with MR-compatible button boxes.
Participants completed an fMRI adaptation of the ANT paradigm (Fan et al., 2005; Fan et al., 2002), presented in a fast event-related design (Supplementary Fig. 1). Participants were instructed to indicate the left–right orientation of a centrally presented arrow which was flanked by four additional arrows of the same size that pointed either into the same (congruent condition) or opposite direction (incongruent condition). In contrast to the original behavioral paradigm (Fan et al., 2002; see also: Neufang et al., 2011), but similar to other fMRI adaptations of the task (Fan et al., 2005; Konrad et al., 2006; Thienel et al., 2009), no neutral target stimulus condition (e.g., dashes instead of arrow flankers) was included. The target stimuli were presented either above or below a fixation cross which was continuously visible in the center of the display. Each target stimulus was presented for a duration of 1050 ms, followed by a fixation cross baseline for 1950 ms. Participants were instructed to indicate the direction of the central arrow as fast and accurately as possible, by pressing a left or right button (which corresponded to the index or middle finger of the dominant hand). Approximately 600 ms before each target stimulus, a cue stimulus could appear for a duration of 150 ms to inform about the impending target stimulus. There were three cueing conditions: In the Double Cue condition, two asterisks appeared both above and below the fixation cross, indicating the timing, but not the location of the upcoming target. In the Spatial Cue condition, a single asterisk appeared at the same location as the upcoming target stimulus, indicating both the timing and the location of the upcoming target (i.e., no invalid cues were included; see also Fan et al., 2005). Finally, in the No Cue reference condition, only the default fixation cross was visible during the 600 ms phase before target presentation. In total, the task design included six task conditions (3 [No Cue/Double Cue/Spatial Cue] × 2 [Congruent/Incongruent]).
The ANT experiment comprised two separate runs. Each run included 96 active trial events (16 trials for each of the six task conditions), and additional 32 null events (fixation cross, presented for 2000 ms) randomly interspersed between the trials. The presentation order of the different trial types was pseudorandomized, using OptSeq2 (http://surfer.nmr.mgh.harvard.edu/optseq). Each run had a total duration of 7:10 min. They were separated by a short break (about 5 min).
2.4. Statistical analyses for behavioral data
Behavioral data were analyzed using SPSS 22 (IBM Corp., Armonk, NY, USA). Frequency distributions for categorical variables were analyzed using χ2 tests (or Fisher exact tests). Unless stated otherwise, mean differences for continuous variables were analyzed with Student's t-tests for independent samples (including Welch–Satterthwaite correction for unequal variances), using bootstrapping (Efron and Tibshirani, 1993) estimates of p values (based on 5000 samples, bias-corrected and accelerated method).
In line with earlier reports of ANT behavioral data (e.g., Fan et al., 2005; Pizzo et al., 2010), an Executive Network Score was computed that represented the difference between median reaction time (RT, in ms) for the incongruent minus congruent target stimuli (averaged across cue conditions). Therefore, higher Executive Network Score values indicate a stronger RT increase for the incongruent target stimuli, which is supposed to reflect stronger response interference, and, hence, weaker efficiency of the Executive Network. High accuracy rates in both groups (i.e., highly skewed data distributions) precluded complementary analyses for accuracy rate data. Instead, accuracy rates for incongruent and congruent task conditions, respectively, were collapsed to derive simple summary scores.
We used univariate and stepwise multiple regressions to examine whether Executive Network Scores in the VP/VLBW group were predicted by neonatal variables which are known risk factors for impaired neurological outcome: GA and BW, DNTI, and duration of ventilation (e.g., Aanes et al., 2015).
2.5. MRI data acquisition
At both sites, MR data were initially acquired on identical Philips Achieva 3 T TX systems (Philips, Best, Netherlands), using 8-channel SENSE head coils. Due to a scanner upgrade, Bonn had to switch to a complementary Philips Ingenia 3 T system after n = 15 participants, while Munich had to do the same switch after n = 105 participants (Supplementary Table 1). Yet, the identical sequence parameters were used on all scanners. To account for possible confounds introduced by the scanner-specific differences, all second-level functional data analyses included dummy regressors for scanner identity as covariates of no interest.
During each run, 215 T2*-weighted EPI volumes were acquired (TR = 2000 ms, TE = 35 ms, flip angle = 82°, parallel imaging with SENSE = 2 (A − P); 32 interleaved oblique axial slices with a slice thickness = 4 mm (no gap); field of view = 220 × 220 × 128 mm; reconstruction matrix = 96 × 96; reconstructed voxel size = 2.29 × 2.29 × 4 mm). Five additional dummy scans were acquired (to achieve longitudinal magnetization equilibrium), but were already discarded before image reconstruction. For image registration purposes, high-resolution T1-weighted 3D-MPRAGE volumes were acquired (TI = 1300 ms, TR = 7.7 ms, TE = 3.9 ms, flip angle = 15°; 180 sagittal slices, field of view: 256 × 256 mm, reconstructed voxel size = 13 mm3).
2.6. fMRI data analyses
Data were analyzed using SPM8 (Wellcome Trust Centre for Neuroimaging, University College London, UK: http://www.fil.ion.ucl.ac.uk/spm), under Matlab 8.2 (MathWorks, Natick, MA, USA).
2.6.1. Preprocessing
Preprocessing of the functional data included slice time correction, realignment and unwarping of the EPI series, co-registration of the T1-weighted image with the mean EPI volume, segmentation of the T1-weighted image using Unified Segmentation (Ashburner and Friston, 2005), application of segmentation-derived normalization parameters to the co-registered functional data (interpolated to an isotropic voxel size of 23 mm3), and spatial smoothing of the normalized EPI series with a Gaussian kernel of 6 mm FWHM.
2.6.2. First-level statistical modeling
Functional time series were modeled using General Linear modeling (GLM: Friston et al., 1994). The first-level design matrix included separate event regressors for each of the six task conditions, with each event onset corresponding to the onset of the cue (i.e., for the No Cue conditions, event onset sampled the fixation cross baseline stimulus presented 600 ms before target presentation: cf. (Fan et al., 2005). An additional error regressor that captured trial events with false responses (or omissions) was included as a covariate of no interest. To capture residual movement-related artifacts, six regressors for the individual realignment parameters were included (Friston et al., 1996). Additionally, we included the time course of the average signal from white matter for each participant as a nuisance covariate (Linzenbold and Himmelbach, 2012; Martin et al., 2015): Individual white matter masks from T1 segmentation were thresholded with a probability value of 0.99, and the mean signal time course within the white matter volume was read out from the realigned and normalized EPI series, using Marsbar (Brett et al., 2002). Task-related regressors were convolved with the SPM8 canonical hemodynamic response function. To remove slow frequency signal drifts, high-pass filtering with 128 s cut-off was applied. Parameter estimates were generated using Restricted Maximum-Likelihood estimation, modeling temporal autocorrelation with an AR(1) model.
For each participant, a first-level contrast for the critical incongruent > congruent comparison was computed (which measures brain activity evoked by stimulus conflict for the correct trials, and thus is supposed to reflect the executive attention component of the task). For this purpose, incongruent and congruent trials conditions were contrasted across cueing conditions and runs.
2.6.3. Group statistical analyses
The contrast maps were subsequently entered into second-level random effect analyses (e.g., Penny et al., 2003). To compare the spatial pattern of task-induced activations in both groups, initial one-sample t-test analyses were conducted for each group. Then, activation differences between groups were analyzed using two-sample t-tests. Each analysis included additional covariates for scanner identity (coded by three dummy variables), sex and age at examination (which varied slightly between groups). Moreover, an additional RT regressor (representing the average of the median RTs for the six individual task conditions) and a regressor for the total number of missing and errors were added to account for potential confounding influences of global RT (see Supplementary Fig. 2) and accuracy. Since cognitive testing showed significant group differences in global cognitive function (see Section 3.1), which may explain some variance in attentional processing (e.g., Eryigit-Madzwamuse et al., 2015), group comparisons also included FSIQ as an additional covariate (cf. de Kieviet et al., 2014).
Moreover, regression models for the VP/VLBW group with neonatal variables (GA, BW, DNTI and duration of ventilation) as covariates were set up to examine whether these parameters predicted activation changes within the VP/VLBW group.
Contrast maps were set at a cluster extent threshold of p < .05 FWE (family-wise error), based on a height threshold of p < .001 voxelwise. Anatomical labels for activation maxima were identified with Talairach Client 2.4.3 (http://www.talairach.org/daemon.html), after converting the original MNI (Montreal Neurological Institute) coordinates in SPM to Talairach space using icbm2tal (Lancaster et al., 2007).
2.7. Brain tissue volumes
In addition, segmentations as calculated during the normalization procedure were used to calculate the individual tissue class volumes for gray matter (GM), white matter (WM), cerebrospinal fluid (CSF) and intracranial volume (ICV), by summing each voxel value for each segmentation class (“spm_summarise.m”). Since ICV differed significantly between groups (M ± SD VP/VLBW = 1279 ± 136 ml, M ± SD Controls = 1348 ± 143, t (184) = −3.4, p = .001), we used GM/ICV, WM/ICV and CSF/ICV ratios, respectively, to examine group differences regarding the proportion of the tissue compartments.
3. Results
3.1. Background characteristics
Descriptive statistics and group comparisons for the background characteristics are provided in Table 1. In both groups, there was a similar overbalance of male participants. There were no differences in handedness. By definition, the VP/VLBW group had a significantly lower GA and BW, and also had higher neonatal risk scores and a longer duration of hospitalization at birth. There were no group differences regarding maternal age and socioeconomic status at birth. VP/VLBW participants showed a significantly lower level of global cognitive function, both in childhood and adult assessments.
Table 1.
Background characteristics — comparison of VP/VLBW and control samples, and groupwise dropouts.
| VP/VLBW |
Controls |
Between current samples: p | ||||||
|---|---|---|---|---|---|---|---|---|
| Current sample (n = 86) | Not included (n = 325) | Within group: p | Current sample (n = 100) | Not included (n = 208) | Within group: p | |||
| Sex: | Male | n = 54 (63%) | n = 158 (49%) | <.05 | n = 59 (59%) | n = 95 (46%) | p < .05 | n.s. |
| Female | n = 32 (37%) | n = 167 (51%) | n = 41 (41%) | n = 113 (54%) | ||||
| Age at examination (years) | 26.6 ± 0.6 | NA | NA | 26.8 ± 0.8 | NA | NA | <.1 | |
| Edinburgh Handedness Inventory | Right | n = 73 (84.9%) | NA | NA | n = 83 (83%) | NA | NA | >.9 |
| Ambidexter | n = 4 (4.7%) | NA | NA | n = 6 (6%) | NA | NA | ||
| Left | n = 8 (9.3%) | NA | NA | n = 9 (9%) | NA | NA | ||
| Missing | n = 1 (1.2%) | n = 2 (2%) | ||||||
| Gestational age (months) | 30.3 ± 2 | 30.6 ± 2.4 | n.s. | 39.8 ± 1 | 39.6 ± 1.2 | n.s. | <.001 | |
| Birth weight (g) | 1328 ± 321 | 1295 ± 304 | n.s. | 3419 ± 451 | 3366 ± 443 | n.s. | <.001 | |
| Multiple births | n = 23 (26.7%) | 80 (24.6%) | n.s. | n = 4 (4%) | 8 (3.8%) | n.s. | <.001 | |
| Maternal age (years) | 29.6 ± 4.7 | 28.2 ± 5.1 | <.05 | 29 ± 4.9 | 28.5 ± 4.8 | n.s. | n.s. | |
| Socioeconomic status at birth | Upper | n = 25 (29%) | n = 56 (17%) | <.05 | n = 32 (32%) | n = 60 (29%) | n.s. | n.s. |
| Middle | n = 37 (43%) | n = 136 (42%) | n = 44 (44%) | n = 78 (38%) | ||||
| Lower | n = 24 (28%) | n = 132 (41%) | n = 24 (24%) | n = 70 (34%) | ||||
| Complications | n = 86 | n = 323 | n = 100 | n = 208 | ||||
| Pre-pregnancy | 1.5 ± 0.8 | 1.3 ± 0.8 | n.s. | 1.1 ± 0.8 | 1.1 ± 0.8 | n.s. | .001 | |
| Pregnancy | 2.1 ± 1.2 | 2.5 ± 1.2 | <.05 | 0.7 ± 0.8 | 0.9 ± 1 | n.s. | <.001 | |
| Birth | 4.7 ± 1.4 | 4.5 ± 1.4 | n.s. | 2.3 ± 1.6 | 2 ± 1.5 | n.s. | <.001 | |
| Neonatal | 8.9 ± 2.6 | 9.5 ± 2.7 | <.05 | 0.3 ± 0.6 | 0.4 ± 0.7 | n.s. | <.001 | |
| Duration of neonatal treatment (DNTI) | 54.1 ± 30 (n = 85) | 57.5 ± 37.4 (n = 323) | n.s. | NA | NA | NA | NA | |
| Intensity of neonatal treatment (INTI) | 11.6 ± 3.8 (n = 85) | 11.8 ± 3.9 (n = 321) | n.s. | NA | NA | NA | NA | |
| Ventilation (days) | 12.5 ± 17.7 | 16.2 ± 21.8 | n.s. | NA | NA | NA | NA | |
| Duration of hospitalization (days) | 72.3 ± 26.4 | 79 ± 38.8 | n.s. | 7 ± 3.1 | 7.4 ± 3.9 | n.s. | <.001 | |
| Intraventricular hemorrhage | n = 86 | n = 323 | ||||||
| None | n = 71 (82.6%) | n = 251 (77.7%) | n.s. | NA | NA | NA | NA | |
| Stage 1 | n = 4 (4.7%) | n = 26 (8%) | NA | NA | NA | |||
| Stage 2 | n = 7 (8.1%) | n = 20 (6.2%) | NA | NA | NA | |||
| Stage 3 | n = 3 (3.5%) | n = 17 (5.3%) | NA | NA | NA | |||
| Stage 4 | n = 1 (1.2%) | n = 9 (2.8%) | NA | NA | NA | |||
| Cerebral palsy (56 months) | n = 86 | n = 310 | <.05 | n = 100 | n = 208 | NA | <.01 | |
| Severity # | Grade 1 and 2 | n = 6 (7%) | n = 24 (7.7%) | 0 (0%) | 0 (0%) | |||
| Grade 3 and 4 | n = 0 (0.0%) | n = 23 (7.4%) | 0 (0%) | 0 (0%) | ||||
| Neurosensory deficits (56 months), non-corrected | n = 82 | n = 269 | n = 100 | n = 207 | ||||
| Blindness | n = 1 (1.2%) | n = 2 (0.7%) | n.s. | 0 (0%) | 0 (0%) | NA | n.s. | |
| Deafness | n = 0 (0.0%) | n = 0 (0.0%) | NA | 0 (0%) | 2 (1%) | n.s. | NA | |
| Griffith Scales of Baby Abilities | 5 months | 102.1 ± 16.3 (n = 85) | 93.6 ± 21.2 (n = 299) | <.001 | 106.9 ± 10.6 | 106.2 ± 11 | n.s. | <.05 |
| 20 months | 100.2 ± 10.2 (n = 83) | 89.5 ± 22.8 (n = 287) | <.001 | 107.5 ± 6.3 | 105.8 ± 6.8 | <.05 | <.001 | |
| Kaufman assessment Battery for children | 6; 3 years | 92.6 ± 11.1 (n = 77) | 83.6 ± 17.1 (n = 254) | <.001 | 101.7 ± 10.1 | 99.9 ± 11.5 | n.s. | <.001 |
| 8; 5 years | 96.7 ± 10.5 (n = 79) | 85.6 ± 18.9 (n = 262) | <.001 | 102.7 ± 8.7 (n = 99) | 100 ± 10.6 (n = 206) | <.05 | <.001 | |
| WAIS-III: full-scale IQ | 26 years | 95.2 ± 12.2 | 84.4 ± 19.1 (n = 117) | p < .001 | 102.7 ± 12.2 | 102.6 ± 13 (n = 97) | n.s. | <.001 |
Within-group analyses compared present preterm-born (VP/VLBW) and term-born (Controls) samples with those preterm-born and term-born participants, respectively, not included in this study. Between-group analyses compared current preterm-born and term-born samples. For variables where data were not available for all participants, the actual group size is indicated separately. # Cerebral palsy: Grade 1 & 2 = can walk independently or with stick, Grade 3 & 4 = restricted mobility requiring wheel chair or no mobility. Abbreviations: WAIS-III — Wechsler Adult Intelligence Scale, Third edition. NA — not available. n.s. — not significant.
Drop-out analyses (comparing the present VP/VLBW and control samples with the excluded VP/VLBW and controls, respectively, of the original sample) showed that both groups similarly included a lower proportion of female participants. Neonatal characteristics of the included controls did not significantly differ from the remaining controls. Meanwhile, the included VP/VLBW resembled the remaining VP/VLBW cohort with regard to GA and BW, but showed significantly lower complications during pregnancy and in the neonatal period. Moreover, the included VP/VLBW sample had mothers with a higher age, higher proportion of individuals with high family SES, and a lower percentage of individuals with severe cerebral palsy than those lost to follow-up. While there was some evidence for a positive selection of controls with higher average IQ (only in comparison to the larger childhood sample), this effect was highly significant for the VP/VLBW group (both for the childhood and adult cohorts).
3.2. Behavioral results
In general, the average response accuracy in both groups was very high (Table 2), although VP/VLBW showed a slightly lower accuracy level, which was specific for the incongruent trials (Mann–Whitney-Test: U (187) = 5056, p = .047). The Executive Network Score indicated slightly higher RT differences between incongruent and congruent trials for the VP/VLBW group (Table 2), which approached significance (p = .051).
Table 2.
Attentional Network Test – Executive Network Score.
| VP/VLBW (n = 86) (M ± SD) |
Controls (n = 100) (M ± SD) |
p-Value | |
|---|---|---|---|
| Task accuracy | |||
| Congruent trials: % correct | 99.3 ± 1.5 | 99.4 ± 1.2 | n.s. |
| Incongruent trials: % correct | 97.8 ± 3.1 | 98.6 ± 1.7 | <.05 |
| Response speed | |||
| Executive Network Score (in ms) | 84 ± 45 | 73 ± 30 | <.1 |
Abbreviations: VP/VLBW — very preterm/very low birth weight. M — mean, SD — standard deviation. n.s. — not significant.
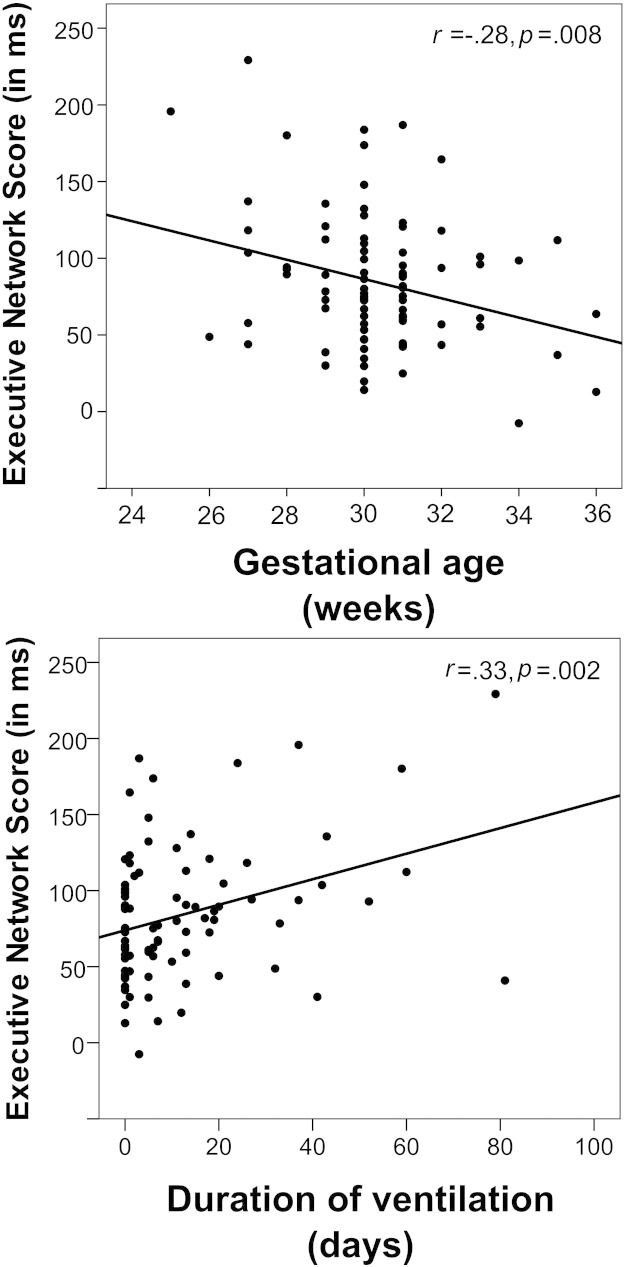
Within the VP/VLBW group, univariate regressions showed that the Executive Network Score was predicted by both GA (standardized β = −.28, p = .008), and duration of ventilation (standardized β = .33, p = .002) (Fig. 1), but not by BW (standardized β = −.07, p < .5), and only marginally by DNTI (standardized β = .19, p = .076). The predictors were moderately to strongly correlated (Supplementary Table 2), and may therefore explain overlapping variance. Actually, stepwise multiple regression showed that only the initial inclusion of duration of ventilation significantly improved model fit (ΔR2 = .109, p = .002), while adding GA or the other predictors did not explain additional variance. Meanwhile, when adding FSIQ into the regression model (which also predicted the Executive Network Score), FSIQ entered as the most important regressor (standardized β = −.28, p = .008), with only GA (but not duration of ventilation) explaining additional variance (standardized β = −.22, p = .036).
Fig. 1.
Associations between the Executive Network Score and neonatal risk variables in the preterm group. Note that higher Executive Network Scores indicate worse performance: therefore, preterm individuals with lower gestational age (negative association), and longer duration of ventilation (positive association) showed weaker executive attention performance. The complementary association with gestational age in the control group was not significant (r = .05, p = .643).
3.3. Brain tissue volumes
While there was no significant group difference for the GM/ICV ratio (t(184) = −0.7, p = .505), VP/VLBW adults showed a significantly lower WM/ICV ratio (t(184) = −2.5, p = .0.012), and significantly higher CSF/ICV ratio (t(184) = 2.4, p = .0.017; Table 3).
Table 3.
Brain tissues ratios.
| VP/VLBW (n = 86) (M ± SD) |
Controls (n = 100) (M ± SD) |
p-Value | |
|---|---|---|---|
| GM/ICV | 0.476 ± 0.023 | 0.479 ± 0.024 | 0.505 |
| WM/ICV | 0.326 ± 0.018 | 0.333 ± 0.019 | 0.012 |
| CSF/ICV | 0.197 ± 0.028 | 0.188 ± 0.024 | 0.017 |
Proportion of brain tissue volumes after controlling for total intracranial volume. Abbreviations: GM — gray matter, WM — white matter, CSF — cerebrospinal fluid, ICV — intracranial volume. VP/VBLW — very preterm/very low birth weight.
3.4. Functional neuroimaging results
3.4.1. Groupwise comparisons
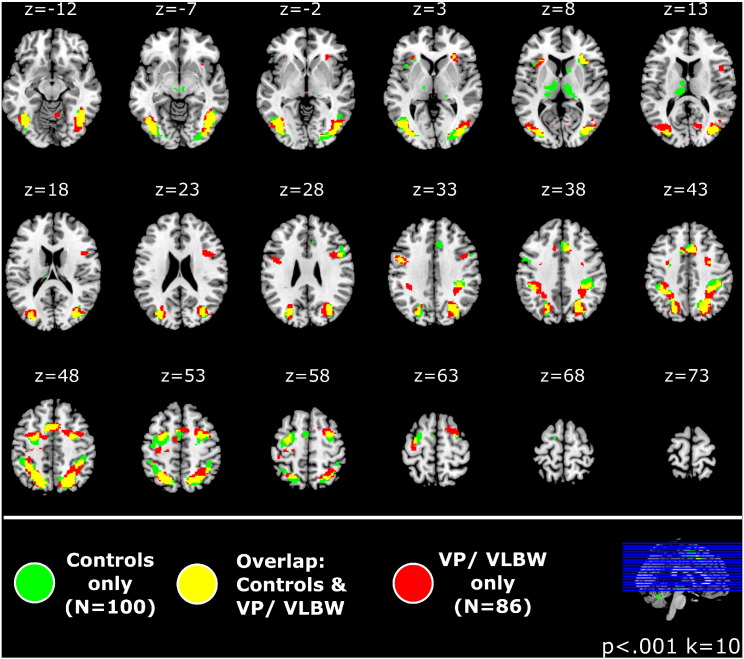
The anatomical distribution of suprathreshold activations for both the VP/VLBW and Control participants is depicted in Fig. 2, which shows the overlay of the statistical maps from the single group analyses (which already included the covariates). To give a better impression of the spatial extent of activations, statistical maps are presented at a liberal threshold of p < .001 uncorrected (see Supplementary Fig. 3, for a complementary display at p < .05 FWE, cluster-level corrected). Activation maxima are provided in Supplementary Tables 3 and 4.
Fig. 2.
Anatomical overlap of activation patterns for the incongruent > congruent contrast in the VP/VLBW and control group. The figure shows the statistical maps from the single group analyses (with all nuisance covariates included, e.g., Full Scale IQ) at a liberal voxelwise height threshold of p < .001 uncorrected, to visualize the extent of activations in both groups (for a complementary display at a p < .05 FWE, cluster-level corrected: Supplementary Figure 3; activation maxima: Supplementary Tables 3-4). Overlapping suprathreshold activations are shown in yellow, VP/VLBW-specific in red, and control-specific in green. Both groups show a distributed network of activations in broadly overlapping brain regions, suggesting a similar processing network, with no obvious reorganization in the VP/VLBW group. Although there are some additional clusters for the control group, especially in the thalamus, direct group comparisons showed no significant group differences. Abbreviation: VP/VLBW — very preterm/very low birth weight.
There was a broad anatomical overlap of activated regions across groups. Both groups showed bilateral activations in caudal aspects of the lateral PFC (precentral and middle frontal gyrus), dorsal ACC and medial frontal gyrus, the superior and inferior parietal lobule, postcentral gyri, and also in extrastriate occipital and fusiform areas. While visual display of the single group maps suggests that controls show more extensive activations than the VP/VLBW group in the bilateral thalamus, this was not confirmed by the direct statistical comparison which generally showed no significant differences between the two groups, i.e., neither for Controls > VP/VLBW, nor for VP/VLBW > Controls. Post hoc inspections of contrast estimates indicated that VP/VLBW the VP/VLBW group tended to show weaker thalamic activations than the controls (which therefore became visible only at more liberal voxelwise thresholds), but this was not sufficiently robust to produce significant group differences.
3.4.2. Associations between VP/VLBW activations and neonatal risk variables
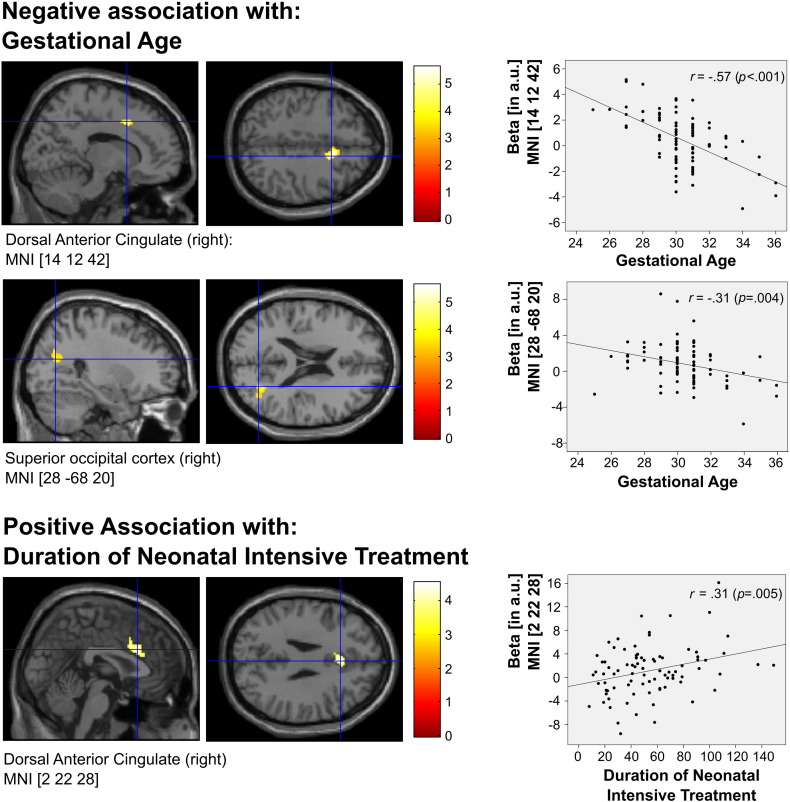
Regression analyses indicated that activation in the right dorsal ACC (MNImax [14, 12, 42]) showed a negative association with GA, indicating that those VP/VLBW born more prematurely showed stronger activations in this region (Table 4, Fig. 3). A similar pattern was found for a cluster in the right superior occipital lobe, extending from the superior and middle occipital gyrus to precuneus/cuneus (MNImax [28 −68 20]). Moreover, there was a positive association with DNTI in an adjacent region of the dorsal ACC (MNImax [2, 22, 28]), indicating that those VP/VLBW with longer intensive treatment also showed stronger activations in this region.
Table 4.
Associations between brain activations during incongruent > congruent trials and clinical variables.
| Cluster statistics |
Submaxima (MNI coordinates) |
Max. |
Anatomical region | ||||
|---|---|---|---|---|---|---|---|
| Size (k) | p(FWEc) | x | y | z | Z | ||
| Negative association with gestational age | 112 | 0.018 | 14 | 12 | 42 | 5.1 | R anterior cingulate (BA24) |
| 128 | 0.009 | 28 | −68 | 20 | 3.8 | R cuneus (BA18) | |
| 38 | −68 | 20 | 3.8 | R (white matter) | |||
| 28 | −70 | 28 | 3.7 | R precuneus (BA31) | |||
| Positive association with duration of neonatal intensive treatmenta | 201 | 0.001 | 2 | 22 | 28 | 4.3 | R cingulate gyrus (BA32) |
| 6 | 28 | 22 | 3.7 | R cingulate gyrus (BA32) | |||
| 4 | 14 | 42 | 3.2 | R medial frontal gyrus (BA32) | |||
Based on N = 85 participants, due to missing data for one participant.
Fig. 3.
Associations of task-induced activations in the VP/VLBW group with clinical variables. Brain activations for the incongruent > congruent contrast showed a negative association with gestational age in a region of the right dorsal ACC and in a right-lateralized superior occipital cluster, indicating stronger activations for VP/VLBW with a lower gestational age. Moreover, adjacent aspects of the right dorsal ACC showed a positive correlation with duration of neonatal intensive treatment, indicating stronger activations for VP/VLBW with longer treatment. Left: Cluster maxima shown in sagittal and axial view, indicated by the crosshairs. Right: scatterplots showing the association between beta weights (y-axis) and clinical variables (x-axis) in the corresponding cluster maxima.
While the areas showing a negative association with GA substantially overlapped with the group main effect (suggesting functional modulation of common task-relevant brain areas), the cluster showing a positive association with DNTI was located in a non-overlapping aspect of the dorsal ACC (Supplementary Fig. 4).
Additionally, a cluster in the right middle frontal gyrus showed a negative association with DNTI (MNImax [34 20 42]: Supplementary Fig. 5), indicating weaker activation for VP/VLBW with longer intensive treatment, but cluster extent only approached the statistical threshold (k = 86, p = .055 FWE, cluster-level corrected). For the other neonatal variables, no significant association was observed.
4. Discussion
To the best of our knowledge, this is the first functional neuroimaging study investigating the neural underpinnings of executive attention in adults born very preterm. While we found preliminary evidence for subtle performance deficits during the processing of incongruent arrow flanker stimuli in the context of an ANT fMRI paradigm, which converges with previous findings in VP children (de Kieviet et al., 2014; Geldof et al., 2013; Pizzo et al., 2010), there was no systematic compensatory recruitment, or even reorganization of the implicated fronto-cingulo-parietal processing networks. This could relate to the fact that we investigated a relatively high-functioning sample of VP/VLBW adults, which may have limited the need for compensatory functional reorganization: Yet, we also found that both behavioral performance and activations in some ACC and occipital areas were associated with lower gestational age and longer postnatal intensive treatment in the preterm-born group, suggesting at least subtle long-term influences of premature birth.
4.1. Brain activations: group comparisons
Contrary to our a priori assumptions, we found no imaging evidence for altered brain activation in the VP/VLBW adults on the group level. Compared with term-born controls, there were no significantly reduced activations that may indicate functional impairments in task-relevant networks (e.g., Griffiths et al., 2013), nor over-activations in the task-typical or supplementary brain areas that could be interpreted as compensatory activity (e.g., Peterson et al., 2002). Indeed, the activation main effects (incongruent > congruent arrow flanker trials) were strikingly similar in the two cohorts (Fig. 2), revealing similar activations in expected fronto-cingulo-parietal networks, which are consistent with observations from earlier ANT studies (Fan et al., 2005; Neufang et al., 2011), and meta-analyses for similar interference processing tasks (Cieslik et al., 2015). While meta-analyses for executive control tasks in general suggest a domain-general cognitive control network that may extend further into rostral DLPFC regions (Niendam et al., 2012), the caudal focus of frontal activations is in line with previous studies. Additionally, both groups showed activations in extrastriate occipital regions, which have also been observed in earlier studies (Backes et al., 2011; Kellermann et al., 2011; Korsch et al., 2014), and may reflect top-down modulation of stimulus processing in these sensory areas.
Although there were some regions where term control individuals showed more robust activations, e.g., in the thalamus (consistent with Fan et al., 2005), these differences were not stable enough to be confirmed by direct group comparisons. In sum, the observations provide no evidence for a systematic reorganization of the brain networks implicated in executive attention, at least not in this relatively high-functioning VP/VLBW cohort (see Section 4.5.1).
4.2. Brain activations: associations with clinical variables
Despite the absence of categorical group differences, we found that brain activations were partially predicted by neonatal risk variables (Fig. 3): We found a significant inverse association between GA and activation in the right dorsal ACC and lateral occipital cortex for the preterm born group, indicating a stronger activation of these task-relevant areas with increasing prematurity that would be compatible with a compensatory over-recruitment (see Nosarti et al., 2009 for a similar finding). This is further corroborated by the observation that VP/VLPBW adults with longer neonatal intensive care showed stronger dorsal ACC activations, yet in a non-overlapping region (Supplementary Fig. 4), which may reflect a compensatory recruitment of additional ACC regions. This would be consistent with a broader imaging literature implicating the dorsal ACC in monitoring aspects of cognitive control (e.g., Botvinick et al., 2001). Conversely, there was suggestive evidence that VP/VLBW with longer neonatal intensive care showed weaker activity in a right-sided middle frontal region. Yet, this area was located adjacent to task-typical processing networks for the ANT and similar interference processing tasks (Cieslik et al., 2015; Fan et al., 2005), making clear inferences about its functional relevance difficult. Yet, it seems to overlap with earlier meta-analytic findings of a domain-general cognitive control network (Niendam et al., 2012; Fig. 1), and could therefore indicate an impaired supplementary recruitment of this control-related region.
4.3. Relationship to other neuroimaging studies
Our findings do not clearly support the impaired recruitment of task-relevant brain networks that was observed by Griffiths et al. (2013) in 11-year-old EP/ELBW children who performed a combined Stroop/N-back task: These EP/ELBW children showed reduced activations in supplementary motor areas (SMA) and the dorsal ACC, the anterior insula and occipital brain regions. Although the single group statistical maps (Fig. 2) suggested reduced thalamic activations in our VP/VLBW group, which would converge with other studies finding thalamic alterations (Bäuml et al., 2014; Nosarti et al., 2006), this was not confirmed by the direct statistical comparison. Methodological differences between the studies may influence the divergent findings, including the varying developmental stage (11 vs. 26 years, with the possibility of function “catch-up” in the adult sample) and severity of prematurity (while the Griffith study focused on EP/ELBW children, we only had few EP/ELBW survivors in our cohort). Regarding task design, the present study used a simple Flanker paradigm, while the other study combined Stroop color-word interference stimuli with additional working memory demands: From a cognitive workload perspective (Jaekel et al., 2013a; Just and Varma, 2007), the simultaneous multi-item processing may have provoked an earlier breakdown of processing capacities.
On the other hand, our neuroimaging results are consistent with de Kieviet et al. (2014) who used a flanker task paradigm to examine 8-year old VP and term-born children, and did not find any groupwise activation differences despite significant impairments in task performance: This applied to both a whole brain analysis, and a subsequent ROI analysis for dorsal ACC, left parietal and right parietal regions, which showed significant activations in both groups. The latter aspect is similar to our observations, although we found more extended activations, including lateral prefrontal and anterior insular regions (which may result from our substantially larger sample size), and only subtle decrements in behavioral task performance. De Kieviet and colleagues speculate whether the potential for a compensatory over-recruitment of task-relevant brain networks may be limited in preterm-born individuals, and might therefore not translate into clear-cut group differences. Yet, our observation that preterm-born adults with lower gestational age (and longer duration of neonatal intensive treatment) showed stronger activations in dorsal ACC regions (Table 4, Fig. 3) suggests that there may be at least some compensatory potential in older preterm-born individuals.
In sum, the available fMRI literature provides inconclusive evidence for functional alterations of brain networks implicated in executive attention, and the present data extend these observations into early adulthood. Our data suggest that the basic functional organization of the relevant networks is largely preserved, although individuals with lower GA may be more likely to show at least subtle behavioral and functional alterations. Yet, our observations compare with fMRI studies that investigated other aspects of executive functioning (e.g., fluency, working memory, motor inhibition) in preterm-born individuals, and found variable evidence for activation differences within the dorsal fronto-cingulo-parietal networks linked with cognitive control (Niendam et al., 2012): Similar to the present experiment, some find no clear group differences in these brain regions (Daamen et al., 2015; Lawrence et al., 2009), while others show reduced (Griffiths et al., 2013), increased (Kalpakidou et al., 2014), or mixed patterns of both lower and higher activations (Nosarti et al., 2006; Nosarti et al., 2009). Whether these inconsistencies can be explained, e.g. by the varying degree of prematurity in the studied populations, and/or variations in task-specific processing demands (e.g., cognitive workload: Jaekel et al., 2013a) needs to be examined more systematically.
4.4. Behavioral results
Previous behavioral studies using the ANT found significant deficits for the Executive Network (Geldof et al., 2013; Pizzo et al., 2010). The fact that we could only detect subtle impairments of Executive Network function in the VP/VLBW participants, as indicated by marginally lower accuracy rates, and stronger RT increases for incongruent vs. congruent targets, could relate to specific requirements of the fMRI task adaptation, but, more likely, reflects an incidental positive selection of less impaired VP/VLBW individuals (see Section 4.5.1).
Meanwhile, there still was evidence that the efficiency of the Executive Network in the VP/VLBW group was linked to neonatal risk factors. Both lower gestational age and longer duration of ventilation predicted worse performance, which concurs with observations that showed a quadratic effect of gestational age on behavioral ratings of attention problems in childhood (Eryigit-Madzwamuse and Wolke, 2015). Stepwise regression suggests that both predictors explain overlapping behavioral variance, with duration of ventilation (as an indicator for neonatal intensive treatment) providing better predictive value than GA (as an indicator for biological maturity). After controlling for FSIQ, only GA was left as a significant predictor, which might suggest that the influence of ventilation duration is largely mediated by its effects on global cognitive function, while GA may explain some additional variance independent from FSIQ.
4.5. Methodological considerations
4.5.1. Sample size and selection
A major advantage of this study is its large sample size. This does not only reduce the probability of missing differences due to a lack of power, but may also be less sensitive to reporting bias (i.e., reporting of more activation clusters than could be expected due to sample size) than conventional small-scale studies (for further discussion: David et al., 2013; Ioannidis et al., 2014). Another distinctive feature is that VP/VLBW participants were not drawn from hospital-based cohorts (e.g., Gimenez et al., 2005), but came from a prospective epidemiological sample (Griffiths et al., 2013), which should promote generalizability (Kukull and Ganguli, 2012), and offered the opportunity to systematically evaluate the possible impact of selection biases.
Actually, drop-out analyses showed a methodological drawback of the study: There was a positive selection of VP/VLBW with relatively high levels of cognitive functioning, lower neonatal complications, and lesser neurological impairment, as indicated by a significantly lower rate of individuals with severe cerebral palsy. The fact that we did not observe an increased rate of non-right handers in the VP/VLBW group, which is frequently reported in the preterm literature (Domellöf et al., 2011), may also support the assertion of lesser neurological impairment, although the fact that WM and CSF proportion was decreased and increased, respectively, in VP/VLBW adults illustrates that deviant white matter development was still present in our sample.
While the average IQ in the VP/VLBW group was significantly lower than for controls, supporting previous studies (Bhutta et al., 2002), and findings for the whole BLS sample (Breeman et al., 2015), drop-out analyses confirmed that the VP/VLBW of the present MRI sample showed a higher IQ than those who were not scanned, or had to be excluded (e.g., due to excessive motion). Positive IQ attrition is also observed in other studies that followed up preterm-born populations into adulthood (Nosarti et al., 2007), and in some fMRI studies, the examined populations actually show average IQ performance (Lawrence et al., 2010; Narberhaus et al., 2009; White et al., 2014). While positive selection is not completely invalidating results, the group differences in our cognitively (and medically) “fitter” subsample can only provide a conservative estimate of possible differences, and are limited with regard to their generalizability (Kukull and Ganguli, 2012): Possibly, individuals with stronger cognitive deficits and neonatal adversities would have presented quantitatively or qualitatively different activation patterns that deviate more clearly from term-born controls. On the other hand, the inclusion of individuals who are potentially overcharged by the task (resulting in a substantial increase of error rates) would also introduce new analytical problems (Price and Friston, 1999).
4.5.2. Task design
The present study found only subtle attentional differences, which is most plausibly explained by a positive selection of “fitter” individuals. Meanwhile, some studies observe that attention problems in VP/VLBW children and adolescents may not impair task performance in a continuous manner, but mainly express as intermittent lapses of attention, i.e. higher rates of omissions, or outlier responses (e.g., Nosarti et al., 2007). Actually, brain activity coinciding or preceding errors and lapses may be quite informative. Yet, the brevity of the presented paradigm and the high accuracy rates in both groups precluded the collection of a sufficient number of trial events for systematic statistical analyses. To achieve this goal, a slow event-related design with irregular target presentation over an extended time course (similar to a Continuous Performance Task) may be more appropriate.
5. Conclusions
One may derive two main conclusions from the reported data: First, task-evoked brain activity mediating interference processing in VP/VLBW adults is not per se deviant at the macroscopic anatomical level, although there was evidence for subtle performance decrements, confirming recent observations in preterm-born children. Second, the observation of modest associations between brain activations and task performance during flanker task processing and gestational and neonatal adversities in VP/VLBW adults suggest that preterm birth has at least a subtle long-term effect on executive attention, which may become more salient in more severely affected preterm-born individuals. Due to the increasing survival rates of babies with extremely low gestational age and/or birth weight, this is a concern that deserves further examination.
Funding
Supported by German Federal Ministry of Education and Science (BMBF01ER0801: M.D., B.B., N.B., D.W.; BMBF01ER0803: C.S., J.B., A.W.) and the Kommission für Klinische Forschung, Technische Universität München (KKF 8765162: C.S.).
Acknowledgements
We thank Prof. Hans H. Schild (Head Department of Radiology, University Bonn) and Prof. Claus Zimmer (Head Department of Neuroradiology, Klinikum rechts der Isar München) for enabling this study, and the staff at the MR facilities in Bonn and Munich for their support during study execution. We kindly thank Dr. Jason A. Martin for providing additional support and input toward finalizing the manuscript. Moreover, we thank all current and former Bavarian Longitudinal Study Group members who contributed to study organization, recruitment, data collection, management and analyses, including (in alphabetical order): Stephan Czeschka, Claudia Grünzinger, Christian Koch, Diana Kurze, Sonja Perk, Andrea Schreier, Antje Strasser, Julia Trummer, and Eva van Rossum. Most importantly, we thank all our study participants for their efforts to take part in this study.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2015.09.002.
Contributor Information
Marcel Daamen, Email: mdaamen@posteo.de.
Josef G. Bäuml, Email: josef.baeuml@tum.de.
Lukas Scheef, Email: Lukas.Scheef@ukb.uni-bonn.de.
Chun Meng, Email: charlieofmeng@gmail.com.
Alina Jurcoane, Email: alinajurcoane@hotmail.com.
Julia Jaekel, Email: jjaekel@utk.edu.
Christian Sorg, Email: c.sorg@lrz.tum.de.
Barbara Busch, Email: Barbara.Busch@ukb.uni-bonn.de.
Nicole Baumann, Email: N.Baumann.1@warwick.ac.uk.
Peter Bartmann, Email: Peter.Bartmann@ukb.uni-bonn.de.
Dieter Wolke, Email: D.Wolke@warwick.ac.uk.
Afra Wohlschläger, Email: Afra.Wohlschlaeger@tum.de.
Henning Boecker, Email: Henning.Boecker@ukb.uni-bonn.de.
Appendix A. Supplementary data
Supplementary material.
References
- Aanes S., Bjuland K.J., Skranes J., Løhaugen G.C.C. Memory function and hippocampal volumes in preterm born very-low-birth-weight (VLBW) young adults. NeuroImage. 2015;105:76–83. doi: 10.1016/j.neuroimage.2014.10.023. 25451477 [DOI] [PubMed] [Google Scholar]
- Aarnoudse-Moens C.S., Duivenvoorden H.J., Weisglas-Kuperus N., Van Goudoever J.B., Oosterlaan J. The profile of executive function in very preterm children at 4 to 12 years. Dev. Med. Child Neurol. 2012;54(3):247–253. doi: 10.1111/j.1469-8749.2011.04150.x. 22126188 [DOI] [PubMed] [Google Scholar]
- Aarnoudse-Moens C.S.H., Smidts D.P., Oosterlaan J., Duivenvoorden H.J., Weisglas-Kuperus N. Executive function in very preterm children at early school age. J. Abnorm. Child Psychol. 2009;37(7):981–993. doi: 10.1007/s10802-009-9327-z. 19488851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P.J. Neuropsychological outcomes of children born very preterm. Semin. Fetal Neonat. 2014;19:90–96. doi: 10.1016/j.siny.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Anderson P.J., De Luca C.R., Hutchinson E., Spencer-Smith M.M., Roberts G., Doyle L.W., Victorian Infant Collaborative Study Group Attention problems in a representative sample of extremely preterm/extremely low birth weight children. Dev. Neuropsychol. 2011;36(1):57–73. doi: 10.1080/87565641.2011.540538. 21253991 [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. 15955494 [DOI] [PubMed] [Google Scholar]
- Backes V., Kellermann T., Voss B., Krämer J., Depner C., Schneider F., Habel U. Neural correlates of the attention network test in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2011;261:S155–S160. doi: 10.1007/s00406-011-0264-9. 21959916 [DOI] [PubMed] [Google Scholar]
- Bauer A. Deutsches Institut für Internationale Pädagogische Forschung; Frankfurt: 1988. Ein Verfahren zur Messung des für das Bildungsverhalten relevanten Sozial Status (BRSS) — überarbeitete Fassung. [Google Scholar]
- Bäuml J.G., Daamen M., Meng C., Neitzel J., Scheef L., Jaekel J., Busch B., Baumann N., Bartmann P., Wolke D., Boecker H., Wohlschläger A.M., Sorg C. Correspondence between aberrant intrinsic network connectivity and gray-matter volume in the ventral brain of preterm born adults. Cereb. Cortex. 2014 doi: 10.1093/cercor/bhu133. 24935776 [DOI] [PubMed] [Google Scholar]
- Bhutta A.T., Cleves M.A., Casey P.H., Cradock M.M., Anand K.J. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288(6):728–737. doi: 10.1001/jama.288.6.728. 12169077 [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. 11488380 [DOI] [PubMed] [Google Scholar]
- Brandt I. Beltz; Weinheim: 1983. Griffiths Entwicklungsskalen (GES) zur Beurteilung der Entwicklung in den Ersten Beiden Lebensjahren [Griffiths Developmental Scales for the First Two Years of Life] [Google Scholar]
- Breeman L.D., Jaekel J., Baumann N., Bartmann P., Wolke D. Preterm cognitive function into adulthood. Pediatrics. 2015;136(3):415–423. doi: 10.1542/peds.2015-0608. 26260714 [DOI] [PubMed] [Google Scholar]
- Brett M., Anton J.-L., Valabregue R., Poline J.-B. Region of interest analysis using an SPM toolbox. NeuroImage. 2002;16:497. [Google Scholar]
- Cieslik E.C., Mueller V.I., Eickhoff C.R., Langner R., Eickhoff S.B. Three key regions for supervisory attentional control: evidence from neuroimaging meta-analyses. Neurosci. Biobehav. Rev. 2015;48:22–34. doi: 10.1016/j.neubiorev.2014.11.003. 25446951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daamen M., Bäuml J.G., Scheef L., Sorg C., Busch B., Baumann N., Bartmann P., Wolke D., Wohlschläger A., Boecker H. Working memory in preterm-born adults: load-dependent compensatory activity of the posterior default mode network. Hum. Brain Mapp. 2015;36(3):1121–1137. doi: 10.1002/hbm.22691. 25413496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S.P., Ware J.J., Chu I.M., Loftus P.D., Fusar-Poli P., Radua J., Munafó M.R., Ioannidis J.P.A. Potential reporting bias in fMRI studies of the brain. PLOS One. 2013;8(7):e70104. doi: 10.1371/journal.pone.0070104. 23936149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kieviet J.F., Heslenfeld D.J., Pouwels P.J., Lafeber H.N., Vermeulen R.J., van Elburg R.M., Oosterlaan J. A crucial role for white matter alterations in interference control problems of very preterm children. Pediatr. Res. 2014;75(6):731–737. doi: 10.1038/pr.2014.31. 24695275 [DOI] [PubMed] [Google Scholar]
- Domellöf E., Johansson A.-M., Rönnqvist L. Handedness in preterm born children: a systematic review and a meta-analysis. Neuropsychologia. 2011;49(9):2299–2310. doi: 10.1016/j.neuropsychologia.2011.04.033. 21601584 [DOI] [PubMed] [Google Scholar]
- Dragovic M. Categorization and validation of handedness using latent class analysis. Acta Neuropsychiatrica. 2004;16(4):212–218. doi: 10.1111/j.0924-2708.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- Efron B., Tibshirani R. Chapman & Hall/CRC; Boca Raton, FL: 1993. An Introduction to the Bootstrap. [Google Scholar]
- Elgen I., Lundervold A.J., Sommerfelt K. Aspects of inattention in low birth weight children. Pediatr. Neurol. 2004;30(2):92–98. doi: 10.1016/S0887-8994(03)00402-8. 14984899 [DOI] [PubMed] [Google Scholar]
- Eryigit Madzwamuse S., Baumann N., Jaekel J., Bartmann P., Wolke D. Neuro-cognitive performance of very preterm or very low birth weight adults at 26 years. J Child Psychol Psychiatry. 2015;56(8):857–864. doi: 10.1111/jcpp.12358. 25382451 [DOI] [PubMed] [Google Scholar]
- Eryigit-Madzwamuse S., Wolke D. Attention problems in relation to gestational age at birth and smallness for gestational age. Early Hum. Dev. 2015;91(2):131–138. doi: 10.1016/j.earlhumdev.2015.01.004. 25617863 [DOI] [PubMed] [Google Scholar]
- Fan J., McCandliss B.D., Fossella J., Flombaum J.I., Posner M.I. The activation of attentional networks. NeuroImage. 2005;26(2):471–479. doi: 10.1016/j.neuroimage.2005.02.004. 15907304 [DOI] [PubMed] [Google Scholar]
- Fan J., McCandliss B.D., Sommer T., Raz A., Posner M.I. Testing the efficiency and independence of attentional networks. J. Cogn. Neurosci. 2002;14(3):340–347. doi: 10.1162/089892902317361886. 11970796 [DOI] [PubMed] [Google Scholar]
- Friston K.J., Holmes A.P., Worsley K.J., Poline J.-P., Frith C.D., Frackowiak R.S.J. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 1994;2(4):189–210. [Google Scholar]
- Friston K.J., Williams S., Howard R., Frackowiak R.S., Turner R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996;35(3):346–355. doi: 10.1002/mrm.1910350312. 8699946 [DOI] [PubMed] [Google Scholar]
- Geldof C.J., de Kieviet J.F., Dik M., Kok J.H., van Wassenaer-Leemhuis A.G., Oosterlaan J. Visual search and attention in five-year-old very preterm/very low birth weight children. Early Hum. Dev. 2013;89(12):983–988. doi: 10.1016/j.earlhumdev.2013.08.021. 24064408 [DOI] [PubMed] [Google Scholar]
- Giménez M., Junqué C., Vendrell P., Caldú X., Narberhaus A., Bargalló N., Falcón C., Botet F., Mercader J.M. Hippocampal functional magnetic resonance imaging during a face-name learning task in adolescents with antecedents of prematurity. NeuroImage. 2005;25(2):561–569. doi: 10.1016/j.neuroimage.2004.10.046. 15784435 [DOI] [PubMed] [Google Scholar]
- Griffiths S.T., Gundersen H., Neto E., Elgen I., Markestad T., Aukland S.M., Hugdahl K. fMRI: blood oxygen level-dependent activation during a working memory-selective attention task in children born extremely preterm. Pediatr. Res. 2013;74(2):196–205. doi: 10.1038/pr.2013.79. 23823155 [DOI] [PubMed] [Google Scholar]
- Gutbrod T., Wolke D., Soehne B., Ohrt B., Riegel K. Effects of gestation and birth weight on the growth and development of very low birthweight small for gestational age infants: a matched group comparison. Arch. Dis. Child. Fetal Neonatal. Ed. 2000;82(3):F208–F214. doi: 10.1136/fn.82.3.F208. 10794788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack M., Taylor H.G., Schluchter M., Andreias L., Drotar D., Klein N. Behavioral outcomes of extremely low birth weight children at age 8 years. J. Dev. Behav. Pediatr. 2009;30(2):122–130. doi: 10.1097/DBP.0b013e31819e6a16. 19322106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis J.P.A., Munafò M.R., Fusar-Poli P., Nosek B.A., David S.P. Publication and other reporting biases in cognitive sciences: detection, prevalence, and prevention. Trends Cogn. Sci. 2014;18(5):235–241. doi: 10.1016/j.tics.2014.02.010. 24656991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaekel J., Baumann N., Wolke D. Effects of gestational age at birth on cognitive performance: a function of cognitive workload demands. PLOS One. 2013;8(5):e65219. doi: 10.1371/journal.pone.0065219. 23717694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaekel J., Wolke D., Bartmann P. Poor attention rather than hyperactivity/impulsivity predicts academic achievement in very preterm and full-term adolescents. Psychol. Med. 2013;43(1):183–196. doi: 10.1017/S0033291712001031. 22608065 [DOI] [PubMed] [Google Scholar]
- Johnson S., Marlow N. Preterm birth and childhood psychiatric disorders. Pediatr. Res. 2011;69(5 Pt 2):11R–18R. doi: 10.1203/PDR.0b013e318212faa0. 21289534 [DOI] [PubMed] [Google Scholar]
- Just M.A., Varma S. The organization of thinking: what functional brain imaging reveals about the neuroarchitecture of complex cognition. Cogn. Affect. Behav. Neurosci. 2007;7(3):153–191. doi: 10.3758/cabn.7.3.153. 17993204 [DOI] [PubMed] [Google Scholar]
- Kalpakidou A.K., Allin M.P., Walshe M., Giampietro V., McGuire P.K., Rifkin L., Murray R.M., Nosarti C. Functional neuroanatomy of executive function after neonatal brain injury in adults who were born very preterm. PLOS One. 2014;9(12):e113975. doi: 10.1371/journal.pone.0113975. 25438043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpakidou A.K., Allin M.P., Walshe M., Giampietro V., Nam K.W., McGuire P., Rifkin L., Murray R.M., Nosarti C. Neonatal brain injury and neuroanatomy of memory processing following very preterm birth in adulthood: an fMRI study. PLOS One. 2012;7(4):e34858. doi: 10.1371/journal.pone.0034858. 22532832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermann T., Reske M., Jansen A., Satrapi P., Shah N.J., Schneider F., Habel U. Latencies in BOLD response during visual attention processes. Brain Res. 2011;1386:127–138. doi: 10.1016/j.brainres.2011.02.023. 21329677 [DOI] [PubMed] [Google Scholar]
- Konrad K., Neufang S., Hanisch C., Fink G.R., Herpertz-Dahlmann B. Dysfunctional attentional networks in children with attention deficit/hyperactivity disorder: evidence from an event-related functional magnetic resonance imaging study. Biol. Psychiatry. 2006;59(7):643–651. doi: 10.1016/j.biopsych.2005.08.013. 16197925 [DOI] [PubMed] [Google Scholar]
- Korsch M., Frühholz S., Herrmann M. Ageing differentially affects neural processing of different conflict types—an fMRI study. Front. Aging Neurosci. 2014;6:57. doi: 10.3389/fnagi.2014.00057. 24778615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukull W.A., Ganguli M. Generalizability: the trees, the forest, and the low-hanging fruit. Neurology. 2012;78(23):1886–1891. doi: 10.1212/WNL.0b013e318258f812. 22665145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Tordesillas-Gutiérrez D., Martinez M., Salinas F., Evans A., Zilles K., Mazziotta J.C., Fox P.T. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 2007;28(11):1194–1205. doi: 10.1002/hbm.20345. 17266101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence E.J., McGuire P.K., Allin M., Walshe M., Giampietro V., Murray R.M., Rifkin L., Nosarti C. The very preterm brain in young adulthood: the neural correlates of verbal paired associate learning. J. Pediatr. 2010;156(6):889–895. doi: 10.1016/j.jpeds.2010.01.017. 20346460 [DOI] [PubMed] [Google Scholar]
- Lawrence E.J., Rubia K., Murray R.M., McGuire P.K., Walshe M., Allin M., Giampietro V., Rifkin L., Williams S.C., Nosarti C. The neural basis of response inhibition and attention allocation as mediated by gestational age. Hum. Brain Mapp. 2009;30(3):1038–1050. doi: 10.1002/hbm.20564. 18412112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzenbold W., Himmelbach M. Signals from the deep: reach-related activity in the human superior colliculus. J. Neurosci. 2012;32(40):13881–13888. doi: 10.1523/JNEUROSCI.0619-12.2012. 23035097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu T.M., Ment L., Allan W., Schneider K., Vohr B.R. Executive and memory function in adolescents born very preterm. Pediatrics. 2011;127(3):e639–e646. doi: 10.1542/peds.2010-1421. 21300680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J.A., Karnath H.-O., Himmelbach M. Revisiting the cortical system for peripheral reaching at the parieto-occipital junction. Cortex. 2015;64:363–379. doi: 10.1016/j.cortex.2014.11.012. 25614234 [DOI] [PubMed] [Google Scholar]
- Melchers P., Preuss U. Swets & Zeitlinger; Frankfurt am Main: 1991. K−ABC: Kaufman Assessment Battery for Children − Deutschsprachige Fassung. [Google Scholar]
- Mulder H., Pitchford N.J., Hagger M.S., Marlow N. Development of executive function and attention in preterm children: a systematic review. Dev. Neuropsychol. 2009;34(4):393–421. doi: 10.1080/87565640902964524. 20183707 [DOI] [PubMed] [Google Scholar]
- Narberhaus A., Lawrence E., Allin M.P., Walshe M., McGuire P., Rifkin L., Murray R., Nosarti C. Neural substrates of visual paired associates in young adults with a history of very preterm birth: alterations in fronto-parieto-occipital networks and caudate nucleus. NeuroImage. 2009;47(4):1884–1893. doi: 10.1016/j.neuroimage.2009.04.036. 19376244 [DOI] [PubMed] [Google Scholar]
- Neufang S., Akhrif A., Riedl V., Förstl H., Kurz A., Zimmer C., Sorg C., Wohlschläger A.M. Disconnection of frontal and parietal areas contributes to impaired attention in very early Alzheimer's disease. J. Alzheimers Dis. 2011;25(2):309–321. doi: 10.3233/JAD-2011-102154. 21422523 [DOI] [PubMed] [Google Scholar]
- Niendam T.A., Laird A.R., Ray K.L., Dean Y.M., Glahn D.C., Carter C.S. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 2012;12(2):241–268. doi: 10.3758/s13415-011-0083-5. 22282036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C., Giouroukou E., Micali N., Rifkin L., Morris R.G., Murray R.M. Impaired executive functioning in young adults born very preterm. J. Int. Neuropsychol. Soc. 2007;13(4):571–581. doi: 10.1017/S1355617707070725. 17521479 [DOI] [PubMed] [Google Scholar]
- Nosarti C., Rubia K., Smith A.B., Frearson S., Williams S.C., Rifkin L., Murray R.M. Altered functional neuroanatomy of response inhibition in adolescent males who were born very preterm. Dev. Med. Child Neurol. 2006;48(4):265–271. doi: 10.1017/S0012162206000582. 16542513 [DOI] [PubMed] [Google Scholar]
- Nosarti C., Shergill S.S., Allin M.P., Walshe M., Rifkin L., Murray R.M., McGuire P.K. Neural substrates of letter fluency processing in young adults who were born very preterm: alterations in frontal and striatal regions. NeuroImage. 2009;47(4):1904–1913. doi: 10.1016/j.neuroimage.2009.04.041. 19376243 [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. 5146491 [DOI] [PubMed] [Google Scholar]
- Penny W.D., Holmes A.P., Friston K.J. Random effects analysis. In: Frackowiak R.S.J., Friston K.J., Frith C., Dolan R., Friston K.J., Price C.J., Zeki S., Ashburner J., Penny W.D., editors. Human Brain Function. second edition. Academic Press; San Diego, CA: 2003. pp. 843–850. [Google Scholar]
- Peterson B.S., Vohr B., Kane M.J., Whalen D.H., Schneider K.C., Katz K.H., Zhang H., Duncan C.C., Makuch R., Gore J.C., Ment L.R. A functional magnetic resonance imaging study of language processing and its cognitive correlates in prematurely born children. Pediatrics. 2002;110(6):1153–1162. doi: 10.1542/peds.110.6.1153. 12456913 [DOI] [PubMed] [Google Scholar]
- Pizzo R., Urben S., Van Der Linden M., Borradori-Tolsa C., Freschi M., Forcada-Guex M., Hüppi P., Barisnikov K. Attentional networks efficiency in preterm children. J. Int. Neuropsychol. Soc. 2010;16(1):130–137. doi: 10.1017/S1355617709991032. 19849881 [DOI] [PubMed] [Google Scholar]
- Prechtl H.F. Neurological sequelae of prenatal and perinatal complications. Br Med J. 1967;4(5582):763–767. doi: 10.1136/bmj.4.5582.763. 5625282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C.J., Friston K.J. Scanning patients with tasks they can perform. Hum. Brain Mapp. 1999;8(2–3):102–108. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<102::AID-HBM6>3.0.CO;2-J. 10524600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegel K., Ohrt B., Wolke D. Ferdinand Enke Verlag; Stuttgart, Germany: 1995. Die Entwicklung Gefährdet geborener Kinder bis zum fünften Lebensjahr [The Development of Children Born at Risk Until Their Fifth Year of Life] [Google Scholar]
- Ritter B.C., Nelle M., Perrig W., Steinlin M., Everts R. Executive functions of children born very preterm — deficit or delay? Eur. J. Pediatr. 2013;172(4):473–483. doi: 10.1007/s00431-012-1906-2. 23247616 [DOI] [PubMed] [Google Scholar]
- Schmid G., Schreier A., Meyer R., Wolke D. Predictors of crying, feeding and sleeping problems: a prospective study. Child Care Health Dev. 2011;37(4):493–502. doi: 10.1111/j.1365-2214.2010.01201.x. 21299592 [DOI] [PubMed] [Google Scholar]
- Sølsnes A.E., Skranes J., Brubakk A.M., Løhaugen G.C. Executive functions in very-low-birth-weight young adults: a comparison between self-report and neuropsychological test results. J. Int. Neuropsychol. Soc. 2014;20(5):506–515. doi: 10.1017/S1355617714000332. 24735984 [DOI] [PubMed] [Google Scholar]
- Thienel R., Kellermann T., Schall U., Voss B., Reske M., Halfter S., Sheldrick A.J., Radenbach K., Habel U., Shah N.J., Kircher T. Muscarinic antagonist effects on executive control of attention. Int. J. Neuropsychopharmacol. 2009;12(10):1307–1317. doi: 10.1017/S146114570999068X. 19793402 [DOI] [PubMed] [Google Scholar]
- von Aster M., Neubauer A., Horn R. [Wechsler Adult Intelligence Scale (WAIS III) German Adaption, Second Revised Edition. Pearson Assessment; Frankfurt: 2006. Wechsler Intelligenztest für Erwachsene WIE. Deutschsprachige Bearbeitung und Adaptation des WAIS-III von David Wechsler (2., korrigierte Auflage) [Google Scholar]
- White T.P., Symington I., Castellanos N.P., Brittain P.J., Froudist Walsh S.F., Nam K.-W., Sato J.R., Allin M.P.G., Shergill S.S., Murray R.M., Williams S.C.R., Nosarti C. Dysconnectivity of neurocognitive networks at rest in very-preterm born adults. Neuroimage Clin. 2014;4:352–365. doi: 10.1016/j.nicl.2014.01.005. 24567907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Ching M., Molloy C.S., Anderson V.A., Burnett A., Roberts G., Cheong J.L., Doyle L.W., Anderson P.J. Attention difficulties in a contemporary geographic cohort of adolescents born extremely preterm/extremely low birth weight. J. Int. Neuropsychol. Soc. 2013;19(10):1097–1108. doi: 10.1017/S1355617713001057. 24050646 [DOI] [PubMed] [Google Scholar]
- Wolke D., Meyer R. Cognitive status, language attainment, and prereading skills of 6-year-old very preterm children and their peers: the Bavarian Longitudinal Study. Dev. Med. Child Neurol. 1999;41(2):94–109. doi: 10.1017/s0012162299000201. 10075095 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.