Abstract
Classic socio-ecological theory holds that the occurrence of aggressive range defence is primarily driven by ecological incentives, most notably by the economic defendability of an area or the resources it contains. While this ecological cost–benefit framework has great explanatory power in solitary or pair-living species, comparative work on group-living primates has always found economic defendability to be a necessary, but not sufficient condition to account for the distribution of effective range defence across the taxon. This mismatch between theory and observation has recently been ascribed to a collective action problem among group members in, what is more informatively viewed as, a public goods dilemma: mounting effective defence of a communal range against intrusions by outgroup conspecifics. We here further develop this framework, and report on analyses at three levels of biological organization: across species, across populations within a single lineage and across groups and individuals within a single population. We find that communal range defence in primates very rarely involves collective action sensu stricto and that it is best interpreted as the outcome of opportunistic and strategic individual-level decisions. Whether the public good of a defended communal range is produced by solitary, joint or collective action is thus the outcome of the interplay between the unique characteristics of each individual, local and current socio-ecological conditions, and fundamental life-history traits of the species.
Keywords: between-group conflict, Chlorocebus, collective action problem, territoriality, Volunteer's Dilemma
1. Introduction
Although cooperation has long been recognized as a key puzzle in animal behaviour [1–3], by now awareness of its evolutionary significance has permeated the study of social interactions at all levels of biological organization. Widely seen as both a creative and organizing principle in the evolution of new levels of complexity throughout the history of life [4], cooperation has been implicated in many of the major evolutionary transitions [5] in which novel, composite units of selection (e.g. chromosomes, eukaryotic cells and multicellular organisms) evolved from the cooperative collectives of lower-level agents (e.g. nucleic acid molecules, prokaryotes and protists). Cooperation has also been a defining hallmark of hominin evolution, and our own species in particular is remarkably cooperative [6–9], with groups often acting collectively. Understanding why cooperation arises and how it is maintained is thus central to explaining many core features of the biological world, including human sociality.
In many animal groups, we see individuals working together in sometimes highly coordinated ways, for instance, in joint attacks on prey, defence against predators, rearing of offspring or defence of the group's territory. Arguably, some of the most dramatic examples of such joint action are found in the context of between-group conflict, in which multiple individuals cooperate to defend a communal range against intrusions by outgroup conspecifics, sometimes even with lethal consequences for those involved (e.g. ants [10], banded mongooses [11], wolves [12], monkeys [13] and chimpanzees [14]). This raises the question whether we can view the group rather than the individual as the primary unit of selection, as has often been suggested for eusocial insects [15,16] and humans [17,18]. For other taxa, we can ask to what extent groups of animals really ever act as true collectives (or ‘Darwinian individuals’ [19]) in the context of between-group conflict. The answer for non-human primates, moreover, may also affect how we think about human groups.
An excellent way to tackle such questions is to examine cooperative range defence from the perspective of the collective action problem (CAP [20,21]). To the extent that a communal range effectively represents a public good (i.e. a resource that is more or less equally shared among all group members), cooperation in defence is vulnerable to exploitation by free-riders, individuals who reap the benefits of access to the good without incurring the costs of producing it. This inherent instability of group-level cooperation is known as the CAP, and may prevent the production or defence of the public good, even in situations where it would be in the interest of all involved.
To explain how public goods are still procured in the presence of potential CAPs, a distinction can be made between altruistic and mutually beneficial cooperation in the production of the good. In the former, the actor incurs a direct fitness cost, whereas in the latter both actor and recipients increase their direct fitness component. Inclusive fitness theory [1] readily explains the evolutionary stability of altruistic behaviours through kin selection (the direct fitness cost to the actor is more than offset by indirect fitness gains from investing in relatives), whereas evolutionary game theory [22] has helped recognize three broad mechanisms to account for mutually beneficial cooperation [23]: (i) by-product mutualism [24], in which benefits to social partners arise as a by-product of an otherwise selfish behaviour of the actor; (ii) pseudo-reciprocity [25], which states that a costly behaviour evokes a selfish response from social partners that benefits the initial actor as a by-product; and (iii) reciprocity [2], in which a costly behaviour evokes a costly response that benefits the initial actor (but see [26]). These are by no means mutually exclusive mechanisms, but instead may often work in consort to stabilize cooperation.
From the above, it follows that although polyadic cooperation often results in the production of a public good, public good production does not necessarily require polyadic cooperation: it can, for example, also come about as a by-product of the self-serving actions of a single individual, as may be the case in species with a resource-defence polygyny mating system [27]. In the social units of other species, moreover, there will typically be a subset of ‘privileged individuals’ (e.g. dominants, with priority of access to resources [28]), whose selfish interests, based on a purely ecological cost–benefit analysis, should make them willing to contribute to the production of the good, regardless of any benefits this may generate for group members. In many situations then, social dilemmas do not involve all group members and public good production does not require collective action sensu stricto.
We should, therefore, expect to see the whole spectrum of public good production by means of solitary, joint and truly collective action (table 1). Here we examine the individual participation in public good production among group members in the context of between-group conflict in non-human primates. Primates are an interesting group, especially because there is a tendency in much of the anthropological literature to treat human groups as collective units of selection, to the point of positing that group selection in the context of warfare (i.e. escalated between-group conflict) has produced groups acting as corporate units [8,18,29,30]. A better understanding of the variation in the nature of between-group interactions among primates as a whole may thus help to delineate the conditions in which groups may act as Darwinian individuals.
Table 1.
Public good production does not necessarily require polyadic cooperation, and if it does, rarely encompasses all capable group members participating to the best of their abilities. Consequently, a collective action problem (CAP) can emerge amongst a subset (k), or all capable (N) individuals within a group. To distinguish between these two scenarios, throughout the text we speak of public good production by means of ‘joint’ versus ‘collective action sensu stricto’. Only if the latter occurs can a group be viewed as the primary unit of selection, or an emerging Darwinian individual [19]. N = all capable group members and 1 < k < N.
| public good production | |||
|---|---|---|---|
| action | nindividuals | social dilemma | potential mechanisms |
| solitary | 1 | no | — kin selection — by-product mutualism |
| joint | k | possible | — kin selection — by-product mutualism — (pseudo-) reciprocity |
| collective (sensu stricto) | N | effectively overcome | — kin selection — by-product mutualism |
In some species, groups may indeed operate as a collective unit, producing predictable monolithic, group-level decisions, which would allow us to consider the group, rather than the individual, as the primary unit of selection. At the other extreme, there is no group-level action at all, and between-group conflicts are mostly avoided, leaving the public good unproduced. In the majority of species, however, joint action involving only a subset of group members will be most common. We will argue that all modes of public good production can be observed in primates, but that the case of joint, yet non-collective, action is both widespread and perhaps most interesting. In such species, between-group encounters reflect the sum total of multiple opportunistic, individual-level decisions about participation, decisions that are subsequently affected by (and affect) the decisions of others in the group, as well as those of individuals in the opposing group. Disentangling the drivers of these individual decisions will reveal the benefits and costs for each individual in the groups, as well as the feedback effects of others' decisions to cooperate or to defect.
We will examine the problem of collective action in the context of communal range defence at three different levels of biological organization. First, we conduct broad-scale comparative analyses across the primate taxon to identify the macro-evolutionary correlates of communal range defence, and to quantify the proportion of species in which it may be affected by CAPs. Second, we investigate phenotypic flexibility among species and populations within a single taxon, the genus Chlorocebus, in which communal range defence typically requires joint action by multiple (but not all) group members. By focusing on a single lineage, the role of genetic canalization as a potential cause for the observed variation is strongly reduced, pointing more strongly at a role for behavioural flexibility (i.e. decisions based on assessment of the situation by potential participants). And third, we present results from a detailed study on group- and individual-level variability in participation in the production of the public good, as well as the complexity of the cooperative behaviours involved. Analyses at this level allow us to gain insights into individual-level decisions on whether and how to participate in each single between-group encounter.
2. Material and methods
(a). Species-level variation in communal range defence
A suite of socio-ecological variables was available from two previous studies on primate territoriality and between-group competition for a total of 138 group-living primates (for details see [31,32]). We censored this dataset to include only those taxa for which complete information was available on: (i) the rate of aggressive between-group encounters (n/daily activity period); (ii) the presence of territorial advertisement (yes/no); (iii) mobility, or economic defendability [the D-index, i.e. the ratio of average day journey length and the diameter of a circle with a surface area equal to the home range [33]); (iv) total home range overlap (expressed as a proportion of the home range area); and (v) group size (number of individuals).
The first two variables encapsulate key components of territorial behaviour [34,35], whereas the latter three are correlates of the probability of chance encounters under an ‘ideal gas' model of animal movement. This model postulates that groups are more likely to collide with increasing range use intensity, shared areas of use and group size [36,37], and thus represents the approximate need for effective range defence [31].
To extract the latent variables that best capture the variation in these traits across the primate taxon, we conducted a phylogenetic principal component analysis (PCA). Following Kaiser's criterion for samples with fewer than 30 variables [38], factors with eigenvalues greater than 1.0 were retained and used to define ‘axes of territoriality’. We next used Bonferroni-corrected phylogenetic generalized least squares analyses (PGLS) to test for associations between the extracted principal components and socio-ecological traits not used in their calculation. This helped identify the evolutionary correlates of communal range defence, and to estimate the proportion of species in which communal range defence may be affected by the CAP.
Analyses were conducted in R v. 3.2.0 [39] using the ‘phytools' [40] and ‘caper’ packages [41], with statistical significance set at α2-tailed < 0.05, corrected where needed for multiple testing.
(b). Population-level variation in communal range defence
Data were collected from the literature on free-ranging Chlorocebus populations to examine the naturally occurring variation in communal range defence within this species-complex. Chlorocebus makes an excellent taxon to investigate this for several reasons. First, animals typically live in large, multi-male multi-female groups, and both sexes are actively involved in between-group conflicts, providing ample opportunity for CAPs to emerge. Second, Chlorocebus is well studied throughout its range of occurrence, and highly opportunistic with broad socio-ecological reaction norms [42,43].
Because of the small sample size, no statistical inferences were attempted but descriptive statistics to illustrate the extent of variation in the efficacy of communal range defence within this species-complex could be calculated.
(c). Encounter-, group- and individual-level variation in communal range defence
Lastly, we used detailed behavioural observations on a long-term study population of vervet monkeys (Chlorocebus pygerythrus) at the Mawana Game Reserve (28° 00′ S, 31° 12′ E), KwaZulu-Natal, South Africa. Data were collected on three habituated groups ranging in size from 30 to 56 individuals, which were followed for a total of more than 11 000 observation hours between January 2012 and February 2014. All animals in the study groups were individually recognized, as were most adults in four frequently encountered neighbouring groups. We restricted our dataset to include only observations on communal range defence when the composition of the opposing group was fully known, and no experimental manipulations were undertaken on the day of the encounter on either of the groups involved. Individual participation and maximum complexity of cooperative behaviour (table 2) were scored for as many adults as feasible during each aggressive encounter, using all-occurrence sampling [46,47]. Definitions and the order of the levels of complexity of communal range defence behaviours were derived from a similar classification scheme devised by Boesch & Boesch [44] to characterize chimpanzee cooperative hunting (see also [45]). For more details on the data collection protocol, see [48].
Table 2.
Operational definitions of the increasing levels of complexity in cooperative range defence as observed in our study population. Definitions are based on those developed for cooperative hunting in chimpanzees [44] and carnivores [45], and number of observations stem from a targeted study on individual participation in communal range defence over the January 2012–February 2014 period.
| definition | description | nobservations (male/female) |
|---|---|---|
| defect | ||
| no participation | the individual does not direct any aggressive behaviour at any member/subgroup of the opposing group throughout the entire encounter | 988 (301/687) |
| cooperate | ||
| similarity | the individual directs aggressive behaviour(s) at any member/subgroup of the opposing group during the encounter. There is, however, no spatio-temporal relation between the actor's actions and those of its group members | 40 (29/11) |
| dyadic synchrony | two group members direct aggressive behaviours at any member/subgroup of the opposing group, and relate in time to each other's actions | 56 (25/31) |
| polyadic synchrony | more than two group members direct aggressive behaviours at any member/subgroup of the opposing group, and relate in time to each other's actions | 110 (28/82) |
| dyadic coordination | two group members concentrate aggressive behaviours at the same member/subgroup of the opposing group, and relate in space and time to each other's actions | 8 (2/6) |
| polyadic coordination | more than two group members concentrate aggressive behaviours at the same member/subgroup of the opposing group, and relate in space and time to each other's actions | 69 (18/51) |
To establish which characteristics influence an individual's likelihood to participate in communal range defence, we constructed a binomial Generalized Linear Mixed Model (GLMM), incorporating sex and dominance as fixed effects, and between-group encounter along with individual nested within group as random effects. Consistency in participation was subsequently assessed by a series of repeatability analyses [49,50], which allowed us to assess the fraction of behavioural variation owing to differences between encounters, groups and individuals within groups.
Next, we looked at whether the maximum level of complexity of a participant's cooperative behaviour during an encounter could be related to individual characteristics. Because cooperative complexity was measured on an ordinal scale, a Cumulative Link Mixed Model (CLMM) was fitted to the data and we investigated the effects of sex and dominance, while defining random effects to account for repeated observations on individuals within groups over multiple between-group encounters. Unfortunately, repeatability analyses are currently not yet implemented for ordinal response variables and could, therefore, not be performed.
All analyses were conducted in R v. 3.2.0 using the ‘lme4’ [51], ‘rptR’ [52] and ‘ordinal’ [53] packages, with statistical significance set at α2-tailed < 0.05.
3. Results
(a). Species-level variation in communal range defence
Our censored comparative dataset contained information on a total of 82 taxa encompassing all major radiations of social primate, including five representatives of the Lemuriformes, 24 Platyrrhini, 42 Cercopithecoidea and 11 Hominoidea (not including humans).
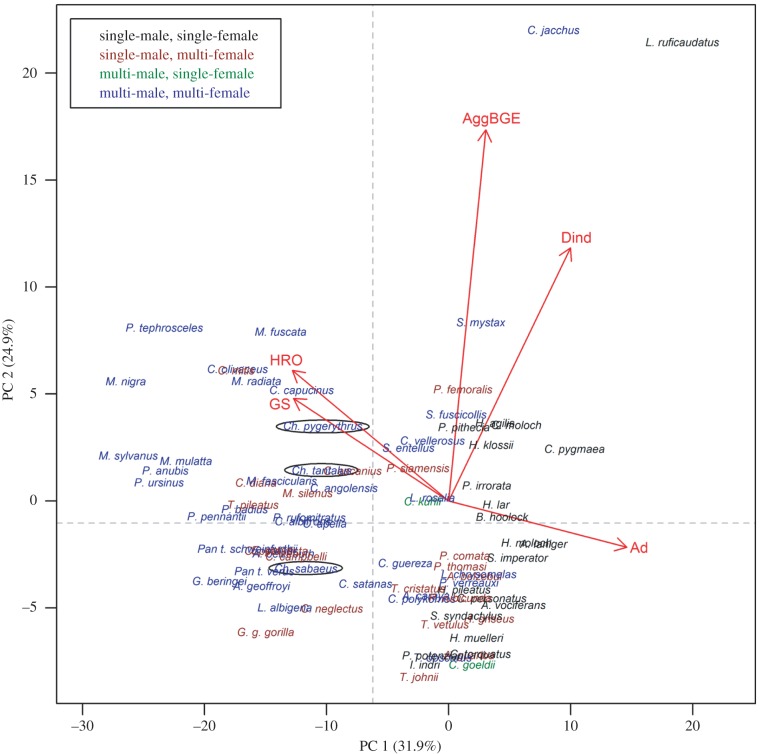
A phylogenetic PCA that controlled for the strong phylogenetic signal in the five range defence variables considered (λML = 0.849) extracted two significant, non-orthogonal ‘axes of territoriality’, which together accounted for 56.8% of the total observed variation (table 3). We focused on factor loadings greater than 0.5 (i.e. that explained more than 25% of the variance within a factor) to interpret these axes: species with high scores on PC 1 were advertisers in home ranges with low overlap that generally lived in small groups, while high scores on PC 2 were associated with high rates of aggressive between-group encounters and economically defendable ranges.
Table 3.
Factor loadings, eigenvalues and percentage of total variation explained by the two significant ‘axes of territoriality’ extracted by a phylogenetic PCA (λML = 0.849). Bold denotes factor loadings > 0.5, which were used to interpret the two principal components. BGE, between-group encounter.
| ‘axes of territoriality’ |
||
|---|---|---|
| PC 1 | PC 2 | |
| rate of aggressive BGEs | 0.151 | 0.860 |
| territorial advertisement | 0.724 | −0.107 |
| D-index | 0.495 | 0.587 |
| home range overlap | −0.635 | 0.303 |
| group size | −0.631 | 0.238 |
| eigenvalue | 1.59 | 1.24 |
| % of total variance | 31.9 | 24.9 |
We next related these two extracted axes to socio-ecological species traits not included in their calculation (electronic supplementary material), using Bonferroni-corrected PGLS analyses (Pcritical = 0.0036). This revealed that PC 1 was positively associated with a species' social system (λML = 0.942, 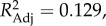 F3,78 = 5.00, p < 0.0036), with species living in single-male, single-female units having the highest scores, and species in multi-male, multi-female units the lowest. PC 1, furthermore, showed a negative association with the number of individuals of the larger sex within the modal group (λML = 0.930,
F3,78 = 5.00, p < 0.0036), with species living in single-male, single-female units having the highest scores, and species in multi-male, multi-female units the lowest. PC 1, furthermore, showed a negative association with the number of individuals of the larger sex within the modal group (λML = 0.930, 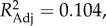 F1,80 = 10.43, p < 0.0036). PC 2 was also associated with a species' social system (λML = 0.898,
F1,80 = 10.43, p < 0.0036). PC 2 was also associated with a species' social system (λML = 0.898, 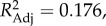 F3,78 = 6.76, p < 0.0036), such that species in single-male, single-female units lived in the most defendable ranges and experienced the highest rates of aggressive between-group encounters.
F3,78 = 6.76, p < 0.0036), such that species in single-male, single-female units lived in the most defendable ranges and experienced the highest rates of aggressive between-group encounters.
Figure 1 illustrates the overall pattern across the taxon. The combination of advertising and above average rates of aggressive between-group encounters (upper-right quadrant on the dashed-line axes) is found especially in species that live in pair-bonded or small polyandrous groups, and/or show cooperative breeding. These species engage in true collective action in that all adults of the social unit tend to engage in territorial defence [54], and often also advertising (when duetting, for instance [55]). Advertisers with below average rates of between-group conflicts (lower-right quadrant) tend to be species with single-male, multi-female groups and were often folivorous. Their diet limits their mobility, and the public good is often produced by the single male without any female participation [56].
Figure 1.
Principal component plot depicting the ‘axes of territoriality’ extracted by a phylogenetic PCA on 82 social species of primate. Species are colour-coded according to the social system of their modal social unit, and (scaled) factor loadings of the five range defence variables considered in the analysis are represented by red arrows. Dotted grey lines indicate the mean scores on PC 1 and PC 2 after omitting two extreme species (Callithrix jacchus and Lepilemur ruficaudatus), and were used to partition the plot into four quadrants. The three Chlorocebus species included in this analysis are encircled (note the very similar scores on PC 1, but divergence on PC 2). (Online version in colour.)
Among those that do not advertise, some species use their range with such low intensity that they can afford not to engage their neighbours in escalated encounters (lower-left quadrant). Other non-advertisers live in multi-male, multi-female groups but have a higher mobility that would in principle allow them to effectively defend their ranges, yet this is not observed (upper-left quadrant). Thus, a significant proportion of species expected to show territoriality (upper-left quadrant: approximately 30%, see also [32]) do not seem to do so in that they do not advertise and have high home range overlap. Groups in these species, therefore, do not act as collective units and may be subject to CAPs in the context of communal range defence. Obviously, many of these species do engage in between-group conflicts, which raises the question of how they organize range defence when they do so. One prominent, and well-studied taxon within this group is the cercopithecoid genus Chlorocebus, on which we focused our next analyses.
(b). Population-level variation in communal range defence
Within the Chlorocebus lineage, there are no records of territorial advertisement. We therefore assessed variation in communal range defence across populations on the basis of the remaining variables used in the species-level analyses (i.e. the rate of aggressive between-group encounters, mobility, home range overlap and group size). Data were collected on a total of 12 populations, representing four different species across sub-Saharan Africa: the green monkey (Ch. sabaeus) from west Africa, the tantalus monkey (Ch. tantalus) from western and central Africa, the Bale Mountains monkey (Ch. djamdjamensis) from the Ethiopian highlands and the vervet monkey from eastern and southern Africa. For one vervet monkey population (Amboseli, Kenya), data were included from two different time periods set apart by approximately 20 years, while one population of green monkey (St Kitts) was actually from outside of the species' natural geographical range (see the electronic supplementary material).
We found extensive variation in the investigated variables (figure 2). For instance, the rate of aggressive between-group encounters across populations varied from around once a day (Ch. pygerythrus; Samara, South Africa) to around once a month (Ch. pygerythrus; Loskop, South Africa). Even within the same population (Ch. pygerythrus; Amboseli, Kenya), the two time periods compared showed marked differences, with for instance average group size in 1985 being almost half of that in 1964 (electronic supplementary material). Inspections of the variance–covariance matrix revealed no pattern of association among these variables or any of the other socio-ecological variables measured. We interpret this as implying that the nature of between-group conflict depends on a variety of factors only partially captured in the ecological and demographical variables considered here. Range defence thus appears to be highly flexibly expressed within the Chlorocebus lineage. The most parsimonious explanation for this observation is that individuals show a large degree of behavioural plasticity, as would be expected given the highly flexible socio-ecology of the taxon. This, however, can only be confirmed by detailed behavioural observations on a focal population, and we next turned to our study on individual participation decisions in between-group conflict within a single population of vervet monkey at Mawana, South Africa.
Figure 2.
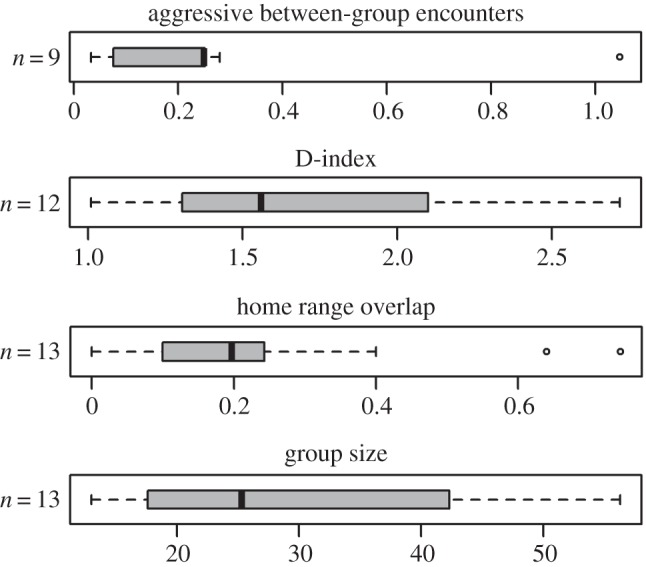
Boxplots of the four variables used to characterize communal range defence across the different Chlorocebus populations, revealing a considerable amount of variation.
(c). Encounter-, group- and individual-level variation in communal range defence
Information on participation in communal range defence and maximum complexity of individual cooperative behaviours was collected on 22 adult males and 36 adult females in three study groups in our study population. A total of 120 aggressive between-group encounters were used for analyses, comprising 1271 observations on individual participation (yes/no), and 283 observations on a participant's maximum cooperative involvement (table 2).
A binomial GLMM (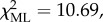 p < 0.05; table 4) revealed no difference in the probability of participation in communal range defence between males and females. In contrast, subordinate individuals were significantly less likely to participate than their top-dominant group members (B ± s.e. = −2.031 ± 0.68, z = −2.99, p < 0.005). The interaction between sex and rank just failed to reach statistical significance, but did marginally improve model fit (
p < 0.05; table 4) revealed no difference in the probability of participation in communal range defence between males and females. In contrast, subordinate individuals were significantly less likely to participate than their top-dominant group members (B ± s.e. = −2.031 ± 0.68, z = −2.99, p < 0.005). The interaction between sex and rank just failed to reach statistical significance, but did marginally improve model fit (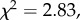 p = 0.092), and was therefore retained. Results from subsequent repeatability analyses (figure 3), moreover, indicated that the probability of individual participation varied significantly across encounters (RBGE ± s.e. = 0.638 ± 0.05, p < 0.001) and between group members (RInd ± s.e. = 0.115 ± 0.03, p < 0.001), but not between study groups (RGroup ± s.e. = 0.002 ± 0.003, p = 0.104). This implied that, although groups on average mobilised similar numbers of volunteers to participate in aggressive between-group encounters, the number of participants was highly variable across encounters, with some individuals within each group consistently more likely to participate than others.
p = 0.092), and was therefore retained. Results from subsequent repeatability analyses (figure 3), moreover, indicated that the probability of individual participation varied significantly across encounters (RBGE ± s.e. = 0.638 ± 0.05, p < 0.001) and between group members (RInd ± s.e. = 0.115 ± 0.03, p < 0.001), but not between study groups (RGroup ± s.e. = 0.002 ± 0.003, p = 0.104). This implied that, although groups on average mobilised similar numbers of volunteers to participate in aggressive between-group encounters, the number of participants was highly variable across encounters, with some individuals within each group consistently more likely to participate than others.
Table 4.
Individual characteristics accounting for differences in participation during aggressive between-group encounters in our study population of vervet monkeys. Parameter estimates (B) and significance values were obtained from a binomial GLMM. Italics denote p-values < 0.05. nobs. = 1271, on 58 individuals in three groups over 120 BGEs; 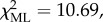 p < 0.05.
p < 0.05.
| variable | B | s.e. | z-value | p-value |
|---|---|---|---|---|
| intercept | −2.816 | 0.52 | ||
| sex | ||||
| male | — | — | — | — |
| female | −0.382 | 0.39 | 0.98 | 0.329 |
| rank | ||||
| dominant | — | — | — | — |
| subordinate | −2.031 | 0.68 | −2.99 | 0.003 |
| interaction | ||||
| sex × rank | 1.371 | 0.82 | 1.67 | 0.095 |
Figure 3.
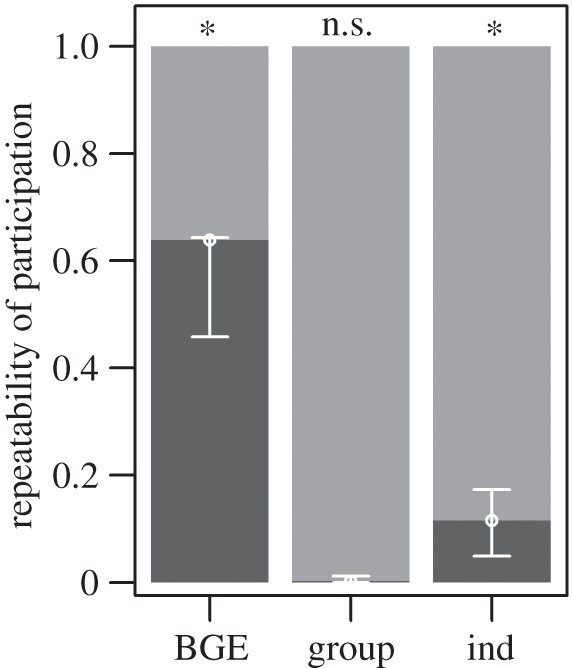
Repeatability (dark grey) and plasticity (light grey) in participation within different units of measurement (BGE, between-group encounter; group, study group; ind, individual within group). High values of repeatability indicate large variation between-units, low values large variation within-units. Permutation tests revealed significant (repeatability > 0) differences between encounters and group members, but not between the study groups. Error bars represent bootstrapped 95% confidence intervals.
To illustrate this individual variability, consider male participation. The average probability with which males participated (mean ± s.e. = 0.253 ± 0.022) was highly variable between individuals, with four males never participating in any encounter in which they were present, while the most active male participated in 60.0% of encounters in which he was present. Probability of participation for females (mean ± s.e. = 0.209 ± 0.014) showed a similar degree of between-individual variability: six females never participated when present, while the most active female was involved in 46.9% of encounters in which she was present. Note that no individual's participation approaches 100%, suggesting that decisions are made opportunistically, presumably based on instantaneous assessment of the situation.
Lastly, we looked at individual differences in the maximum complexity of cooperative behaviours exhibited during participation in communal range defence. Results from a CLMM (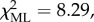 p < 0.05; table 5) revealed that females on average engaged in more complex cooperative behaviours during aggressive between-group encounters than males (B ± s.e. = 0.907 ± 0.31, z = 2.91, p < 0.005), while no differences between top-dominants and subordinates were detected.
p < 0.05; table 5) revealed that females on average engaged in more complex cooperative behaviours during aggressive between-group encounters than males (B ± s.e. = 0.907 ± 0.31, z = 2.91, p < 0.005), while no differences between top-dominants and subordinates were detected.
Table 5.
Individual characteristics accounting for differences in the maximum level of complexity of cooperative behaviours observed during aggressive between-group encounters in a study population of vervet monkeys. Parameter estimates (B) and significance values were obtained from a CLMM analysis. Italics denote p-values < 0.05. nobs
= 283, on 48 individuals in three groups over 72 BGEs; 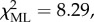 p < 0.05.
p < 0.05.
| variable | B | s.e. | z-value | p-value |
|---|---|---|---|---|
| sex | ||||
| male | — | — | — | — |
| female | 0.907 | 0.31 | 2.91 | 0.004 |
| rank | ||||
| dominant | — | — | — | — |
| subordinate | 0.017 | 0.31 | 0.06 | 0.956 |
4. Discussion
Our review of communal range defence across non-human primates has shown that primate groups hardly ever operate as true collectives, and that public good production in this context rarely involves collective action sensu stricto (table 1). The species coming closest to this, and whose groups therefore can possibly be viewed as (marginal) Darwinian individuals [19], are cooperative breeders or those that live in pairs (upper-right quadrant, figure 1). These small groups are able to operate as a collective unit because of a strong alignment of individual interests, through shared genes (e.g. callitrichids) and/or shared benefits (e.g. gibbons), arising from a special socio-ecology in which the requirements of food- and mate-defence coincide, and necessitate joint defence. In other species, the public good of the defended and advertised territory is produced by a single individual, typically a male (lower-right quadrant, figure 1), and is thus the product of solitary (and not collective) action.
Groups in most other species (left two quadrants, figure 1) operate as associations that, to varying degrees and in varying contexts, consist of more or less independent and interdependent individuals. Many primate species show between-group conflicts with multiple participants, yet do not advertise range ownership, which was associated with high home range overlap. They also tend to live in large groups with multiple members of either sex. These species live in ranges that are economically as defendable as those of species that actually do advertise range ownership, and individuals should therefore, in principle, stand to gain from such defence. Indeed, the more mobile species in this cluster do show joint action during conflicts between groups, but even then social units do not act as true collectives as some group members never participate and many participate only some of the time (e.g. [57–59]). These species thus suffer from some kind of CAP, as within-group competition inhibits group-level cooperation (see also [31,32]).
Focusing on a single taxon of this kind (the genus Chlorocebus), we subsequently could show that there is great variability in communal range defence between species in this genus, between populations of one species (Ch. pygerythrus) and, in a detailed study on a focal population, between encounters and individuals, though not between groups (figure 3). We take this pattern as observed in our focal population to be in line with predictions from the Volunteer's Dilemma [60–62].
The Volunteer's Dilemma is an n-player cooperation game in which a certain number of individuals is required to produce a public good, and cooperation is costly, but not as costly as a failure to produce the good [60]. Rather than being a linear function of the number of cooperators (as in an n-player Prisoner's Dilemma), however, the production curve is modelled by a step-function in which the public good is only secured if a minimum number of individuals, k, cooperate (figure 4). This nonlinearity allows cooperative and non-cooperative strategies to coexist within a social unit (as famously observed in lions [64]), even in the absence of any genetic or spatio-temporal assortment among individuals [65]. In the context of participation in communal range defence, the critical threshold, k, is intuitively understood as the number of individuals required to successfully repel an opposing group. A group's realized resource holding potential (i.e. the number of volunteers) thus reflects the context-specific and strategic decisions of individuals, which can differ markedly from one encounter to the next, depending on the particulars of the current and local socio-ecological circumstances. For groups to be able to coexist, however, the number of volunteers mobilized should on average not be too dissimilar, otherwise individuals in one of the groups would consistently lose all between-group conflicts and eventually no longer be able to secure sufficient resources to survive. This exactly matches the pattern observed in our study population (figure 3), and is also in line with many previous studies on the spatio-temporal contingency of individual participation in between-group conflicts (e.g. [48,66,67]).
Figure 4.
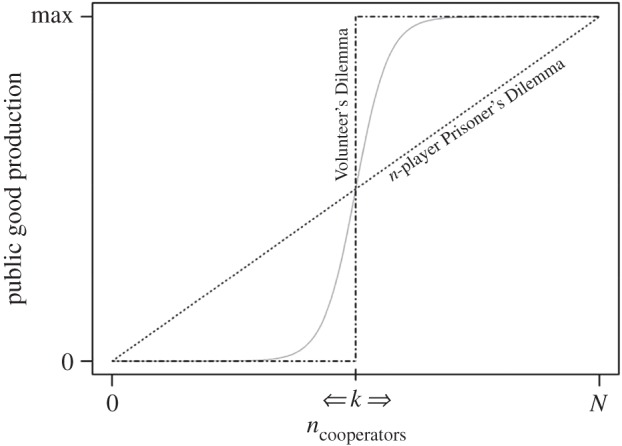
Schematic of the difference between the production functions of the n-player Prisoner's Dilemma (dotted line) and the Volunteer's Dilemma (dashed-dotted line). In the former, there is a linear relationship between the number of group members that cooperate and the likelihood (or amount) of the public good that is produced, whereas in the latter public good production is modelled by a step-function: the public good is only produced if the number of cooperators exceeds a critical threshold, k. The solid grey curve depicts a biologically more realistic, and intermediate scenario (such as communal range defence) between these two extreme scenarios. Theoretical work suggests that these intermediate production curves are often mathematically more accurately approximated by the Volunteer's Dilemma than by the n-player Prisoner's Dilemma [63], and our analyses on individual-level participation in aggressive between-group encounters in a focal population of vervet monkeys add empirical support to this.
We further found that the individuals most likely to volunteer were top-dominants of either sex (table 4), whose selfish cost–benefit analysis will be such that they have most to gain from securing the good. Public good production in our study population thus primarily comes about through joint action by ‘privileged individuals’ [28]: dominants with priority of access to the contested resources and whose behaviour is unaffected by that of group members, thereby effectively allowing subordinates to free-ride on their efforts (i.e. ‘the exploitation of the great by the small’ [20,68]). In as far as our study population can indeed be taken to be representative for a large proportion of primate species, inter-individual differences thus appear pivotal in stabilizing polyadic cooperation in the context of joint (i.e. not collective sensu stricto) communal range defence [68].
Interestingly, a difference between the sexes in how they mitigate the costs of participation was also apparent from our analyses, with males relying on less complex forms of cooperation than females (table 5). This almost certainly reflects the fact that in vervet monkeys, males run lower risks by participating in the production of a good as they are the larger sex, while females, being the philopatric sex, have more opportunities to cooperate with relatives. Moreover, the skew in resource distribution among individuals of the ecological sex tends to be less pronounced [69], while the stakes of losing territory are higher for the philopatric sex (e.g. in vervet monkeys [70]), further facilitating more complex forms of cooperation to evolve among females.
Lastly, turning to our closest living relatives, the chimpanzees, we observe that they do not clearly advertise territorial ownership. They are, nevertheless, resource defenders that obviously engage in between-group aggression, which sometimes may even turn lethal [14,71]. This action is joint and not collective, sensu stricto, and individual participation (almost exclusively by males) varies extensively for reasons that are only partly understood (e.g. [72]). Given some major similarities between the socio-ecology of human foragers and chimpanzees, it therefore seems most parsimonious to consider human groups, at least those in small-scale foraging or horticultural societies, as engaging in between-group conflicts based on individual-level assessments and decision-making [73–75] (much as in the vervet monkeys examined in more detail here), rather than as collective units of selection. Be that as it may, hominin social evolution may have seen two major transitions in cooperation [9]: first from a chimpanzee-like social organization to the societies of Pleistocene cooperative breeders/foragers, and second the Pleistocene–Holocene transition from cooperative breeders/foragers to the increasingly complex hierarchical societies of our species today. The hypothesis that group-level selection driven by escalated between-group conflict (i.e. warfare) has, uniquely among primates, shaped human cooperation (e.g. [8]), can therefore not yet fully be discarded.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Sergey Gavrilets, Luke Glowacki and Christopher von Rueden for taking the initiative on this theme issue, and for their invitation to contribute our work. We are furthermore grateful to two anonymous reviewers for thoughtful comments that improved the final version of this manuscript. This study would not have been possible without the efforts and hard work of numerous field primatologists, and we thank all authors of original studies who (unknowingly) contributed data to the comparative components of the paper. We also thank all collaborators, staff, students and volunteers at the Inkawu Vervet Project for their support and contributions to our own field data.
Ethics
All data collection protocols at the Inkawu Vervet Project were approved by local and national authorities, as well as the Ezemvelo KZN Wildlife Ethics Board in South Africa, and comply with EU-Directive 2010/63/EU (Art. 8, 10, 28, 31 and 32).
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
E.P.W. and C.P.v.S. designed and conceived the study. E.P.W., T.J.M.A. and X.S. collected data and E.P.W. performed statistical analyses. E.P.W., T.J.M.A., X.S. and C.P.v.S. wrote the paper, and all authors approved of the final version of this manuscript.
Competing interests
We declare we have no competing interests.
Funding
Financial support for this study was provided by the Swiss National Science Foundation (Sinergia grant CRSI33_133040 to C.P.v.S.) and the University of Zurich's Forschungskredit (postdoctoral grant to E.P.W.).
References
- 1.Hamilton WD. 1964. The genetical evolution of social behaviour I & II. J. Theor. Biol. 7, 1–52. ( 10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 2.Trivers RL. 1971. The evolution of reciprocal altruism. Q. Rev. Biol. 46, 35–57. ( 10.1086/406755) [DOI] [Google Scholar]
- 3.Wilson EO. 2000. Sociobiology: the new synthesis, 25th Aniversary edn Cambridge, MA: Belknap, Harvard University Press. [Google Scholar]
- 4.Michod RE, Herron MD. 2006. Cooperation and conflict during evolutionary transitions in individuality. J. Evol. Biol. 19, 1406–1409. ( 10.1111/j.1420-9101.2006.01142.x) [DOI] [PubMed] [Google Scholar]
- 5.Maynard Smith J, Szathmáry E. 1995. The major transitions in evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 6.Kaplan HS, Hooper PL, Gurven M. 2009. The evolutionary and ecological roots of human social organization. Phil. Trans. R. Soc. B 364, 3289–3299. ( 10.1098/rstb.2009.0115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvard M. 2012. Human sociality. In The evolution of primate societies (eds Mitani J, Call J, Kappeler PM, Palombit R, Silk JB), pp. 585–603. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 8.Bowles S, Gintis H. 2013. A cooperative species: human reciprocity and its evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 9.Sterelny K. 2014. Cooperation, culture, and conflict. Br. J. Philos. Sci. ( 10.1093/bjps/axu024) [DOI] [Google Scholar]
- 10.Adams ES. 1990. Boundary disputes in the territorial ant Azteca trigona: effects of asymmetries in colony size. Anim. Behav. 39, 321–328. ( 10.1016/s0003-3472(05)80877-2) [DOI] [Google Scholar]
- 11.Müller CA, Manser MB. 2007. ‘Nasty neighbours’ rather than ‘dear enemies’ in a social carnivore. Proc. R. Soc. B 274, 959–965. ( 10.1098/rspb.2006.0222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mech LD. 1994. Buffer zones of territories of gray wolves as regions of intraspecific strife. J. Mammal. 75, 199–202. ( 10.2307/1382251) [DOI] [Google Scholar]
- 13.Cheney DL. 1987. Interactions and relationships between groups. In Primate societies (eds Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT), pp. 267–281. Chicago, IL: University of Chicago Press. [Google Scholar]
- 14.Mitani JC, Watts DP, Amsler SJ. 2010. Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr. Biol. 20, R507–R508. ( 10.1016/j.cub.2010.04.021) [DOI] [PubMed] [Google Scholar]
- 15.Wilson EO. 1971. The insect societies. Cambridge, MA: Belknap Press. [Google Scholar]
- 16.Wilson DS, Edward O. 2007. Rethinking the theoretical foundation of sociobiology. Q. Rev. Biol. 82, 327–348. ( 10.1086/522809) [DOI] [PubMed] [Google Scholar]
- 17.Choi J-K, Bowles S. 2007. The coevolution of parochial altruism and war. Science 318, 636–640. ( 10.1126/science.1144237) [DOI] [PubMed] [Google Scholar]
- 18.Bowles S. 2009. Did warfare among ancestral hunter–gatherers affect the evolution of human social behaviors? Science 324, 1293–1298. ( 10.1126/science.1168112) [DOI] [PubMed] [Google Scholar]
- 19.Godfrey-Smith P. 2013. Darwinian individuals. In From groups to individuals: evolution and emerging individuality (eds Bouchard F, Huneman P), pp. 17–37. Cambridge, MA: MIT Press. [Google Scholar]
- 20.Olson M. 1965. The logic of collective action: public goods and the theory of groups. Cambridge, MA: Harvard University Press. [Google Scholar]
- 21.van Schaik CP. 1996. Social evolution in primates: the role of ecological factors and male behaviour. In Evolution of social behaviour patterns in primates and man (eds Runciman WG, Maynard Smith J, Dunbar RIM), pp. 9–32. Oxford, UK: Oxford University Press. [Google Scholar]
- 22.Maynard Smith J, Price GR. 1973. The logic of animal conflict. Nature 246, 15–18. ( 10.1038/246015a0) [DOI] [Google Scholar]
- 23.Bshary R. 2010. Cooperation between unrelated individuals—a game theoretic approach. In Animal behaviour: evolution and mechanisms (ed. Kappeler P.), pp. 213–240. Berlin, Germany: Springer. [Google Scholar]
- 24.West-Eberhard MJ. 1975. The evolution of social behavior by kin selection. Q. Rev. Biol. 50, 1–33. ( 10.2307/2821184) [DOI] [Google Scholar]
- 25.Connor RC. 1986. Pseudo-reciprocity: investing in mutualism. Anim. Behav. 34, 1562–1566. ( 10.1016/S0003-3472(86)80225-1) [DOI] [Google Scholar]
- 26.Clutton-Brock T. 2009. Cooperation between non-kin in animal societies. Nature 462, 51–57. ( 10.1038/nature08366) [DOI] [PubMed] [Google Scholar]
- 27.Emlen ST, Oring LW. 1977. Ecology, sexual selection, and evolution of mating systems. Science 197, 215–223. ( 10.1126/science.327542) [DOI] [PubMed] [Google Scholar]
- 28.Nunn CL. 2000. Collective benefits, free-riders, and male extra-group conflict. In Primate males (ed. Kappeler PM.), pp. 192–204. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 29.Boyd R, Gintis H, Bowles S, Richerson PJ. 2003. The evolution of altruistic punishment. Proc. Natl Acad. Sci. USA 100, 3531–3535. ( 10.1073/pnas.0630443100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puurtinen M, Mappes T. 2009. Between-group competition and human cooperation. Proc. R. Soc. B 276, 355–360. ( 10.1098/rspb.2008.1060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willems EP, van Schaik CP. 2015. Collective action and the intensity of between-group competition in nonhuman primates. Behav. Ecol. 26, 625–631. ( 10.1093/beheco/arv001) [DOI] [Google Scholar]
- 32.Willems EP, Hellriegel B, van Schaik CP. 2013. The collective action problem in primate territory economics. Proc. R. Soc. B 280, 20130081 ( 10.1098/rspb.2013.0081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitani JC, Rodman PS. 1979. Territoriality: the relation of ranging pattern and home range size to defendability, with an analysis of territoriality among primate species. Behav. Ecol. Sociobiol. 5, 241–251. ( 10.1007/BF00293673) [DOI] [Google Scholar]
- 34.Davies NB, Houston AI. 1984. Territory economics. In Behavioural ecology: an evolutionary approach (eds Krebs JR, Davies NB), pp. 148–169, 2nd edn. Oxford, UK: Blackwell Scientific Publications. [Google Scholar]
- 35.Kitchen DM, Cheney DL, Seyfarth RM. 2004. Factors mediating inter-group encounters in savannah baboons (Papio cynocephalus ursinus). Behaviour 141, 197–218. ( 10.1163/156853904322890816) [DOI] [Google Scholar]
- 36.Waser PM, Homewood K. 1979. Cost–benefit approaches to territoriality: a test with forest primates. Behav. Ecol. Sociobiol. 6, 115–119. ( 10.1007/BF00292557) [DOI] [Google Scholar]
- 37.Hutchinson JMC, Waser PM. 2007. Use, misuse and extensions of ‘ideal gas’ models of animal encounter. Biol. Rev. 82, 335–359. ( 10.1111/j.1469-185X.2007.00014.x) [DOI] [PubMed] [Google Scholar]
- 38.Field A, Miles J, Field Z. 2012. Discovering statistics using R, 1st edn London, UK: Sage. [Google Scholar]
- 39.R Core Team. 2015. R: a language and environment for statistical computing, 3.2.0 edn Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 40.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 41.Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W. 2013. caper: Comparative Analyses of Phylogenetics and Evolution in R. R package version 0.5.2. http://CRAN.R-project.org/package=caper . [Google Scholar]
- 42.Willems EP. 2008. From space to species: integrating remotely sensed information on primary productivity into investigations and systems models of vervet monkey (Cercopithecus aethiops) socio-ecology. PhD thesis, Durham University, Durham, UK.
- 43.Willems EP, Hill RA. 2009. A critical assessment of two species distribution models: a case study of the vervet monkey (Cercopithecus aethiops). J. Biogeogr. 36, 2300–2312. ( 10.1111/j.1365-2699.2009.02166.x) [DOI] [Google Scholar]
- 44.Boesch C, Boesch H. 1989. Hunting behavior of wild chimpanzees in the Taï National Park. Am. J. Phys. Anthropol. 78, 547–573. ( 10.1002/ajpa.1330780410) [DOI] [PubMed] [Google Scholar]
- 45.Bailey I, Myatt J, Wilson A. 2013. Group hunting within the Carnivora: physiological, cognitive and environmental influences on strategy and cooperation. Behav. Ecol. Sociobiol. 67, 1–17. ( 10.1007/s00265-012-1423-3). [DOI] [Google Scholar]
- 46.Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 49, 227–267. ( 10.1163/156853974X00534) [DOI] [PubMed] [Google Scholar]
- 47.Martin P, Bateson P. 1993. Measuring behaviour: an introductory guide, 2nd edn Cambridge, UK: Cambridge University Press. [Google Scholar]
- 48.Arseneau TJM, Taucher AL, Müller E, van Schaik CP, Willems EP. In press Male monkeys fight in between-group conflicts as protective parents or reluctant recruits. Anim. Behav. ( 10.1016/j.anbehav.2015.09.006) [DOI] [Google Scholar]
- 49.Bell AM, Hankison SJ, Laskowski KL. 2009. The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783. ( 10.1016/j.anbehav.2008.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakagawa S, Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956. ( 10.1111/j.1469-185X.2010.00141.x) [DOI] [PubMed] [Google Scholar]
- 51.Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-7. http://CRAN.R-project.org/package=lme4 . [Google Scholar]
- 52.Schielzeth H, Nakagawa S. 2013. rptR: Repeatability for Gaussian and non-Gaussian data. R package version 0.6.405/r52. http://R-Forge.R-project.org . [Google Scholar]
- 53.Christensen RHB. 2015. ordinal: Regression Models for Ordinal Data. R package version 2015.6-28. http://www.cran.r-project.org/package=ordinal/ . [Google Scholar]
- 54.Lazaro-Perea C. 2001. Intergroup interactions in wild common marmosets, Callithrix jacchus: territorial defence and assessment of neighbours. Anim. Behav. 62, 11–21. ( 10.1006/anbe.2000.1726) [DOI] [Google Scholar]
- 55.Bartlett TQ. 2011. The Hylobatidae: small apes of Asia. In Primates in perspective (eds Campbell CJ, Fuentes A, MacKinnon KC, Bearder SK, Stumpf RM), pp. 300–312, 2nd edn Oxford, UK: Oxford University Press. [Google Scholar]
- 56.van Schaik CP, Assink PR, Salafsky N. 1992. Territorial behavior in Southeast Asian langurs: resource defense or mate defense? Am. J. Primatol. 26, 233–242. ( 10.1002/ajp.1350260402) [DOI] [PubMed] [Google Scholar]
- 57.Nunn CL, Deaner RO. 2004. Patterns of participation and free riding in territorial conflicts among ringtailed lemurs (Lemur catta). Behav. Ecol. Sociobiol. 57, 50–61. ( 10.1007/s00265-004-0830-5) [DOI] [Google Scholar]
- 58.Kitchen DM, Beehner JC. 2007. Factors affecting individual participation in group-level aggression among non-human primates. Behaviour 144, 1551–1581. ( 10.1163/156853907782512074). [DOI] [Google Scholar]
- 59.Gilby I. 2012. Cooperation among non-kin: reciprocity, markets and mutualism. In The evolution of primate societies (eds Mitani J, Call J, Kappeler P, Palombit R, Silk J), pp. 514–530. London, UK: The University of Chicago Press. [Google Scholar]
- 60.Diekmann A. 1985. Volunteer's Dilemma. J. Conf. Resol. 29, 605–610. ( 10.2307/174243) [DOI] [Google Scholar]
- 61.Archetti M. 2009. Cooperation as a volunteer's dilemma and the strategy of conflict in public goods games. J. Evol. Biol. 22, 2192–2200. ( 10.1111/j.1420-9101.2009.01835.x) [DOI] [PubMed] [Google Scholar]
- 62.Archetti M. 2009. The volunteer's dilemma and the optimal size of a social group. J. Theor. Biol. 261, 475–480. ( 10.1016/j.jtbi.2009.08.018) [DOI] [PubMed] [Google Scholar]
- 63.Archetti M, Scheuring I, Hoffman M, Frederickson ME, Pierce NE, Yu DW. 2011. Economic game theory for mutualism and cooperation. Ecol. Lett. 14, 1300–1312. ( 10.1111/j.1461-0248.2011.01697.x) [DOI] [PubMed] [Google Scholar]
- 64.Heinsohn R, Packer C. 1995. Complex cooperative strategies in group-territorial African lions. Science 269, 1260–1262. ( 10.1126/science.7652573) [DOI] [PubMed] [Google Scholar]
- 65.Archetti M, Scheuring I. 2011. Coexistence of cooperation and defection in public goods games. Evolution 65, 1140–1148. ( 10.1111/j.1558-5646.2010.01185.x) [DOI] [PubMed] [Google Scholar]
- 66.Furrer RD, Kyabulima S, Willems EP, Cant MA, Manser MB. 2011. Location and group size influence decisions in simulated intergroup encounters in banded mongooses. Behav. Ecol. 22, 493–500. ( 10.1093/beheco/arr010) [DOI] [Google Scholar]
- 67.Crofoot MC, Gilby IC. 2012. Cheating monkeys undermine group strength in enemy territory. Proc. Natl Acad. Sci. USA 109, 501–505. ( 10.1073/pnas.1115937109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gavrilets S, Fortunato L. 2014. A solution to the collective action problem in between-group conflict with within-group inequality. Nat. Commun. 5, 3526 ( 10.1038/ncomms4526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Schaik CP, Kappeler PM. 2006. Cooperation in primates and human: closing the gap. In Cooperation in primates and humans: mechanisms and evolution (eds Kappeler PM, van Schaik CP), pp. 3–21. Berlin, Germany: Springer. [Google Scholar]
- 70.Wrangham RW. 1981. Drinking competition in vervet monkeys. Anim. Behav. 29, 904–910. ( 10.1016/S0003-3472(81)80027-9) [DOI] [Google Scholar]
- 71.Wilson ML, et al. 2014. Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature 513, 414–417. ( 10.1038/nature13727) [DOI] [PubMed] [Google Scholar]
- 72.Watts DP, Mitani JC. 2001. Boundary patrols and intergroup encounters in wild chimpanzees. Behaviour 138, 299–327. ( 10.1163/15685390152032488) [DOI] [Google Scholar]
- 73.Wrangham RW, Glowacki L. 2012. Intergroup aggression in chimpanzees and war in nomadic hunter–gatherers. Hum. Nat. 23, 5–29. ( 10.1007/s12110-012-9132-1) [DOI] [PubMed] [Google Scholar]
- 74.Glowacki L, Wrangham R. 2014. Warfare and reproductive success in a tribal population. Proc. Natl Acad. Sci. USA 112, 348–353. ( 10.1073/pnas.1412287112). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Glowacki L, Wrangham RW. 2013. The role of rewards in motivating participation in simple warfare. Hum. Nat. 24, 444–460. ( 10.1007/s12110-013-9178-8) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.