Abstract
Initial mate choice and re-mating strategies (infidelity and divorce) influence individual fitness. Both of these should be influenced by the social environment, which determines the number and availability of potential partners. While most studies looking at this relationship take a population-level approach, individual-level responses to variation in the social environment remain largely unstudied. Here, we explore carry-over effects on future mating decisions of the social environment in which the initial mating decision occurred. Using detailed data on the winter social networks of great tits, we tested whether the probability of subsequent divorce, a year later, could be predicted by measures of the social environment at the time of pairing. We found that males that had a lower proportion of female associates, and whose partner ranked lower among these, as well as inexperienced breeders, were more likely to divorce after breeding. We found no evidence that a female's social environment influenced the probability of divorce. Our findings highlight the importance of the social environment that individuals experience during initial pair formation on later pairing outcomes, and demonstrate that such effects can be delayed. Exploring these extended effects of the social environment can yield valuable insights into processes and selective pressures acting upon the mating strategies that individuals adopt.
Keywords: social environment, divorce, pair formation, great tit, mate choice
1. Introduction
The social environment has repeatedly been shown to shape individual fitness, population dynamics and selection acting on behavioural and morphological traits, not only in humans [1–3], but also in other animals [4–7]. One of the main pathways for these effects is via the influence of the social environment on reproductive success. For example, Oh & Badyaev [5] found that male house finches (Carpodacus mexicanus) with less elaborate plumage (which are less preferred by females) can increase their mating success if they change winter flocks often (thus changing selection on ornamentation) [5]. Social effects need not be immediate, but may have extended fitness consequences, even several years after the interactions have happened. For example, the connectivity of young long-tailed manakin (Chiroxiphia linearis) males with other males in their social network predicted their social rise and mating success up to 8 years later [4]. The social environment should be especially important for primary mating strategies (initial mate choice) and secondary mating strategies (divorce and infidelity) in socially monogamous species where mate sampling might be limited and hence constrained by the social environment; such constraints might lead to the formation of a suboptimal partnership [8].
Suboptimal partnerships in socially monogamous species can be adjusted either by mating outside of the social pair (i.e. infidelity) and/or by divorcing a partner after initial breeding [9–12]. Consequently, when the social environment influences initial mate choice by determining the number of opposite sex associates [13,14], it should also influence the emergence of secondary mating strategies. In this way, the social environment may not only impact breeding success through the immediate effects of the quality of the social partnership, but also through later fitness effects of secondary mating strategies. For example, across socially monogamous birds, divorce has been shown to lead to an increase in reproductive success with a new partner [15].
However, the social environments of individuals, and their subsequent effects, are challenging to study because they require detailed knowledge of the behaviour of large numbers of individuals. In free-ranging animal populations, data on the social environment are difficult and time-consuming to obtain. Consequently, studies often use a range of demographic proxies, such as sex ratio, coloniality or mortality rates as an estimate of the social environment to which individuals are exposed [16–18]. For example, in their recent phylogenetic comparative analysis, Liker et al. [18] showed that divorce rates are higher in species with a female-biased adult sex ratio [18]. While these studies have given valuable insights into the ways demographic factors influence divorce rates at the level of a species and populations, responses to the social environment at the individual level have received less attention. Moreover, studies using large-scale demographic factors may not easily quantify spatial and temporal variability in the social environment, and do not distinguish between the demography of the environment in which a pair had formed (i.e. before the first breeding), and of the environment in which divorce has happened (i.e. after the first breeding). Advances in the application of social network theory to study heterogeneous associations within animal groups, together with the development of new tracking technologies, have enabled easier quantification, and statistically more robust description, of the social environment that individuals experience (reviewed in references [19,20]).
In this study, we used novel tracking techniques to construct social networks in winter flocks of great tits (Parus major) and to quantify how the social environment at the time a pair forms predicts its future divorce probability (i.e. after its first breeding season). The main hypotheses we tested were as follows (i) we predicted that birds that had had fewer associates of the opposite sex (we term this ‘between-sex degree’), or a lower proportion of the opposite sex as associates in their winter social network prior to breeding season t, would be more likely to divorce between breeding seasons t and t + 1. We reasoned that a smaller number of opposite sex associates would restrict the pool of potential partners from which to sample and choose from. Similarly, it might be that the proportion of opposite sex associates, rather than, or in addition to, their absolute number determines their availability owing to competition with other males. Thus, lower numbers, as well as a lower proportion, of opposite sex associates should increase the incidence of suboptimal partnerships, and consequently, lead to higher divorce probability later. (ii) We predicted that birds with a weaker association strength with their future partner, and/or lower rank of their future partner (in terms of the association strength) within their opposite sex associates in the winter in which they paired would divorce more often between breeding seasons t and t + 1. We reasoned that a lower association strength and, even more importantly, lower partner association–strength rank (the position, in terms of the association strength, a partner had on the list of all opposite sex associates) should indicate a lower level of preference for that partner (in comparison with other birds of the opposite sex).
2. Material and methods
(a). Great tits
Great tits breed in socially monogamous, territorial pairs and spend the winter in mixed species flocks composed of variable numbers of individuals [21]. Although the great tit is one of the most extensively studied bird species, knowledge about the process of pair formation, winter social behaviour and its influence on pairing outcome is still comparatively sparse. Previous studies have indicated that breeding pairs generally form between birds belonging to the same core winter flock [22], mostly after the winter flocks dissolve; however, some birds pair while still within the flock [22,23]. More recent work has demonstrated that pairs do form throughout the winter, with possibly several peak periods of pair formation, depending on the winter [24]. Reported divorce rates (i.e. the rate at which pairs that formerly bred together separate and pair with other birds, given that both survive) in great tits vary between 0% and 51% [25], but have been estimated at 31–50% in this population [26]. Divorce in great tits sometimes follows breeding attempts with low breeding success [27,28], and happens more often in pairs containing first year breeders (i.e. birds between their first and second breeding season [23,29]). Two studies have suggested that social organization in winter and winter residency might influence divorce probability in great tits [23,25]. Both these studies concentrated on divorce in relation to whether pair members spent winter in the same flock or not, after they had already bred. Dhondt et al. [25] found that divorce occurred more often in populations with lower winter residency. On the other hand, Saitou [23] carried out more detailed observations of flocks of colour-ringed birds. He found that divorce happened more often in pairs that, after breeding together, belonged to different winter flocks, but also in pairs that belonged to different flocks before breeding.
(b). Data collection
We collected data on winter social behaviour (2007–2008, 2008–2009, 2009–2010, 2011–2012 and 2012–2013 winters) and breeding pairs (2008–2014 breeding seasons) from a free-ranging great tit population in Wytham Woods (51°46′ N, 1°19′ W). The breeding population of great tits at this site has been monitored since the 1960s, whereas the collection of the winter social behaviour started, as a part of a larger project, in the 2007 winter. As a part of this long-term project, all birds receive a standard British Trust for Ornithology ring as well as a passive integrated transponder (PIT-tag) when first captured, either as breeders or nestlings during the standardized monitoring of the breeding population [21,30,31], or during constant winter catching (mostly immigrants). This ensured that the majority of birds wintering in the woods were tagged (more than 80% of birds, [32]). To study the influences of the social environment in the winter of pair formation on the probability of a pair to divorce between breeding seasons t and t + 1, we needed data on winter flocks in the winter prior to the breeding season t (e.g. data from the 2011 to 2012 winter were used to explore influences on divorce between 2012 and 2013 breeding seasons). Data on winter flocks were collected using radiofrequency identification technology where birds marked with PIT-tags are detected at feeders equipped with PIT-detecting antennae. Tagged birds were registered at an array of logger-equipped feeders placed at different feeding locations. During winters 2007–2008 to 2009–2010, 16 (out of overall 67) feeders were open (and thus available for birds) at any time between August and March (2007–2008 winter: from 3 August 2007 to 9 March 2008; 2008–2009 winter: from 22 August 2008 to 12 March 2009; 2009–2010 winter: from 24 August 2009 to 8 March 2010). These feeders were rotated every 4 days around 67 feeding locations following a structured randomized design, so that each of eight approximately equally sized sections of the woods always had two active loggers in it. In the 2011–2012 and 2012–2013 winters, there were 65 feeders (same locations as previous data), all of which were simultaneously opened once a week, for 2 subsequent days (weekends), between September and March (in 2011–2012 winter: from 8 September 2011 to 3 March 2012; in 2012–2013 winter: from 1 September 2012 to 3 March 2013). Over the five studied winters, feeders recorded 6 743 553 visits by 3198 tagged individual great tits. The data-collection set-up reduced the possibility that flocks would get attracted to a constant food source, and we assume that data gathered at feeders represent a ‘snapshot’ of the social composition at the time of feeding. Data on breeding pairs (i.e. identities of pair members and details of their breeding parameters) were collected using standardized protocols during the breeding season [21,30,31]. We used this information to determine the incidence of pair fidelity (i.e. pairs that breed together in the year t and again in the year t + 1), and divorce (i.e. pairs that bred in the year t, where both members survive to the next season, but breed with different partners in the year t + 1).
(c). Methods to describe the winter social environment
We based the description of the winter social environment on the spatio-temporal detection of co-occurrence of individuals at feeders (i.e. gambit of the group approach [33]). We used networks constructed using the Gaussian mixture model for event streams (GMMEvent) method [34] to identify gathering events (representing feeding flocks) from the data collected on loggers. The method has been shown to be a robust way to derive social networks from spatio-temporal data streams of co-occurrences of individuals [35]. There were 41 226 (2007–2008), 39 046 (2008–2009), 12 740 (2009–2010), 83 152 (2011–2012) and 132 143 (2012–2013) such gathering events in each winter. Gathering events were used to identify all associates of a focal individual (i.e. all other individuals seen at least once in the same gathering event as a focal individual). For each winter, we calculated the degree (the number of individuals the focal bird was associated with) and between-sex degree (the number of opposite sex individuals the focal bird was associated with). Degree and between-sex degree were strongly correlated in both datasets and in both sexes (males: Kendall's tau(2007–2010) = 0.91, p < 0.001; tau(2011–2013) = 0.90, p < 0.001; females: tau(2007–2010) = 0.93, p < 0.001, tau(2011–2013) = 0.92, p < 0.001). We used between-sex degree for our analysis as it better reflects the number of the opposite sex associates. We calculated the strength of the association between the two individuals (i.e. between males and females) using the half weight index (HWI) [36,37]:
where x is number of gathering events where both individuals a and b were part of the same gathering event; Ya (or Yb) number of gathering events in which only individual a (or b) was seen, and Yab number of times individuals were simultaneously detected in different gathering events.
In the first three winters, we calculated the association strength for each month, and for the 2011–2012 and 2012–2013 winters for each weekend of data collection. We scored partner association–strength rank using the following procedure: first, we calculated the association strength between the focal bird and each bird of the opposite sex (including its future breeding partner) that the focal bird associated with; second, we ordered these from the highest to the lowest; third, we determined the placement (i.e. the rank) of the focal bird's breeding partner on the list. For example, if the future partner association–strength rank is 1, this means that, considering all opposite sex associations, the focal bird associated most strongly with its future partner. We provide the frequency distribution of partner strength, female's and male's partner rank, female and male between-sex degree, for winters 2007–2008 to 2009–2010, for the 2011–2012 winter and for the 2012–2013 winter in the electronic supplementary material, figures S1 and S2.
(d). Data analysis
To test for the influence of social network traits in the winter of pair formation (i.e. winter prior to the breeding season t) on the probability of a pair to divorce between breeding seasons t and t + 1, we considered only newly formed pairs (i.e. pairs that formed in the winter prior to the breeding season t). Calculating association strength (and partner association–strength rank) and determining whether a pair divorced before the next season, required that both pair members were tagged (i.e. observable) in the winter prior to the breeding season t, and survived (and were seen breeding) between t and t + 1 breeding seasons. We excluded those pairs where one or both the pair members was first detected in February (2007–2008 to 2009–2010 winters) or in just the last two weekends of data collection (2011–2012 and 2012–2013 winters), and pairs whose members were never seen associated (in all but two cases, these were pairs where one member was not detected at all in winter). Consequently, sample sizes (especially for the first three winters of data collection) were reduced despite the large number of birds tagged in the entire population (electronic supplementary material, table S1). Because of the small sample size, and because we were interested in the absolute number of associates of the opposite sex (i.e. the pool of the potential breeding partners), the effect of which should be independent of winter, we combined the first three winters into one dataset. However, we kept the 2011–2012 and 2012–2013 winters separate from the previous three winters, because the data-collection methods were different in these two sets of winters. Similarly, partner association–strength rank (that we assume relates to the level of preference of the focal bird to associate with its partner compared with other birds of the opposite sex), should be independent of winter too (even if winters differ in the distribution of association strengths among all birds). On the other hand, the association strength between the future pair members is more likely to relate to winter-specific effects (i.e. if the distribution of association strength differs between different winters). Thus, we checked whether winter had a strong influence on the association strength between pair members (including all pairs in the network, and not only the newly formed pairs). We compared Akaike information criterion (AIC) values [38] of the two simple linear models with the association strength as the response, and with or without (i.e. the intercept model) winter as the explanatory variable. We did this for each set of winters separately (i.e. 2007–2008 to 2009–2010; and 2011–2012 and 2012–2013 winters). The two sets of models (i.e. winter versus no winter effect on association strength) gained similar support for 2007–2008 to 2009–2010 winters (AICno winter = −340.76, AICwinter = −339.66, LR test p = 0.23), further justifying the treatment of the first three winters as one dataset. However, for the 2011–2012 and 2012–2013 winters, there was a winter-specific effect on the association strength (AICno winter = −358.01, AICwinter = −363.50, LR test p = 0.006). We thus coded for three sets of winters in our analysis: 2007–2008 to 2009–2010, 2011–2012 and 2012–2013.
We ran binomial generalized linear models, with pair status in year t + 1 (divorced or faithful) as a binomial response variable to test for the variables influencing divorce probability, for each sex separately. Because members of 62 future pairs were never detected in the same winter flock (i.e. not associated, in all but two cases, this was due to one of the partners not being detected in winter), we restricted the main analysis to only those pairs that were detected in the same flock at least once. This was necessary, as partner strength and partner association–strength rank can only be estimated for those pairs that were seen associated; pairs never seen associated in winter were not more likely to divorce (AIC of model, controlled for clutch size and winter, was similar in model with: AIC = 139.35 and without: AIC = 137.70 the variable coding for whether a pair was seen to be associated). Thus, we assumed that these pairs represent an unbiased sample of all pairs, where one or both pair members failed to be detected at feeders (in 36 of these pairs one of the partners was not tagged in the winter of interest). We considered five main explanatory variables, and two control variables in our models:
(i) main explanatory variables: partner HWI strength, between-sex degree (i.e. number of opposite sex associates), proportion of opposite sex associates in the social network of all associates, partner association–strength rank among opposite sex associates (all of these calculated in the winter before the breeding season t), experience of the breeder (two possible values: bred previously, or not);
(ii) control variables: winter (three possible values: winters 2007–2008 to 2009–2010, 2011–2012 or 2012–2013), and clutch size (in breeding season t).
We first compared the performance of competing models, for each sex separately (males: electronic supplementary material, table S2, females: electronic supplementary material, table S3) including three main explanatory variables (partner strength, between-sex degree and partner association–strength rank; and two-way interactions between partner strength and partner rank, and partner strength and between-sex degree). We also compared the performance of the same set of models, but with breeding experience added as a covariate. Then, we added the proportion of opposite sex associates (as a measure of the intensity of the competition for partners) as an interactive effect with the between-sex degree to the main model structure, or replaced between-sex degree with the proportion of same-sex associates (males: electronic supplementary material table S2, females: electronic supplementary material, table S3). We controlled for the winter block of data. We also controlled for clutch size in each model because we have evidence [14,24] that clutch size is an important predictor of divorce in great tits and closely related tit species.
To additionally test for the influence of between-sex degree and proportion of the opposite sex associates on divorce probability, we used the full dataset, including all new pairs (i.e. irrespective of whether both partners were seen in winter or not). Here, we also included a variable coding for whether or not a pair was ever seen associating (list of models in electronic supplementary material, tables S4 and S5). We ran additional models to control for the number of weeks a bird was seen in the network (additive or interactive effect with the between-sex degree) and models where only birds seen in more than four weekends were considered. These models gave results similar to those of the main models.
We considered the model with the lowest AIC value as the best supported one, if this value was 3 or more units lower than the AIC of the next lowest AIC model. All statistical analysis were done in the R package lme4 [39], and predicted values of the response variables (with corresponding 95% CI) calculated using the predict() function.
3. Results
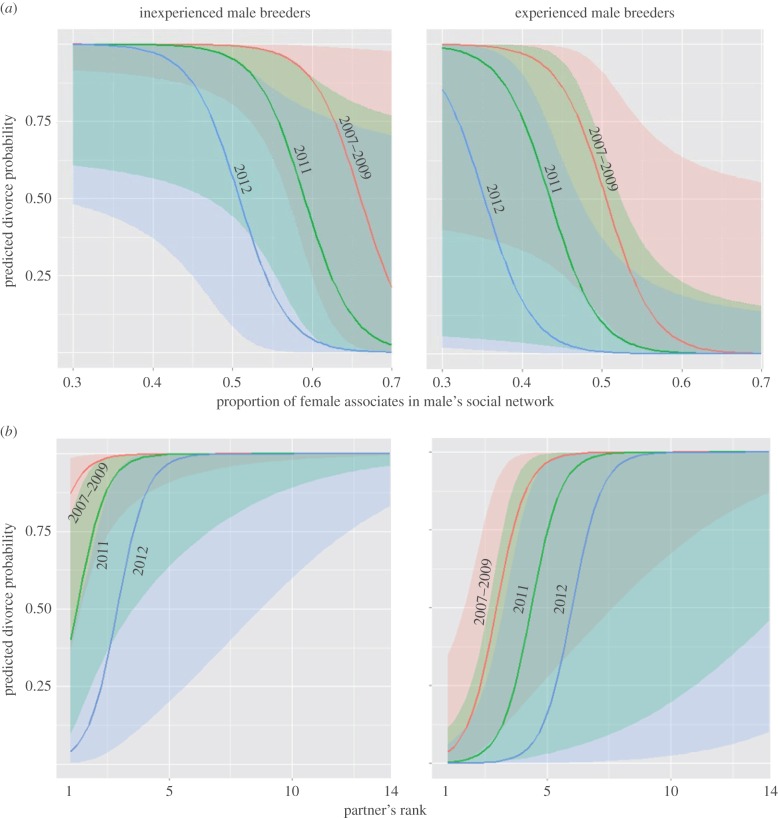
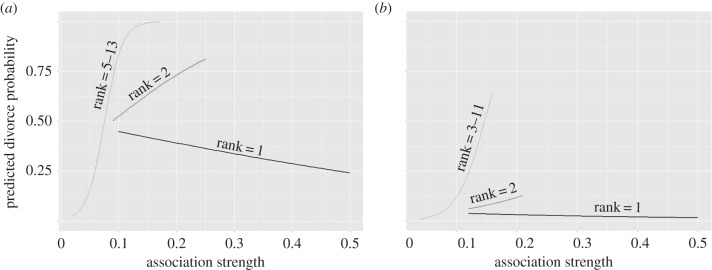
The likelihood of divorce for male great tits belonging to those pairs where pair members were seen associated in winter was best explained by male's breeding experience, proportion of female associates, partner association–strength rank, partner strength and the interaction between the last two (electronic supplementary material, tables S1 and S2; estimate for the term = −13.85, z-value = −2.47, p = 0.013). The absolute number of female associates (i.e. between-sex degree) did not influence the divorce probability of a male. Divorce probability decreased with an increase in the proportion of female associates a male had in the winter of pair formation (figure 1a). Divorce probability also decreased as the association–strength rank of a female partner increased (figure 1b). For example, the probability that an inexperienced male with the lowest proportion of female associates (which was 0.38) in the 2012–2013 winter would divorce before the next breeding season (i.e. between 2013 and 2014) was 0.98 (95% CI = 0.39–0.99). This probability decreased to 0.22 (95% CI = 0.11–0.86) for a male with the highest proportion of female associates (0.54). In the same winter, the divorce probability ranged between 0.04 (95% CI = 0.002–0.42) for an inexperienced male whose breeding partner ranked first among all of his female associates, and 0.98 (95% CI = 0.82–1) for an inexperienced male whose female ranked as 13th. A similar pattern of increased divorce probability with a decrease in the proportion of female associates, and a decrease in partner's association–strength rank, was evident in experienced males, and in other sets of winters (i.e. 2007–2009, and 2011–2012; figure 1a,b). Finally, there was an interaction between partner association–strength rank and partner strength. While males whose female was ranked as their first female associate had a low probability of divorcing (and this probability decreased with the increase in partner strength), for males whose female was ranked lower, the divorce probability increased with the increase in partner strength (figure 2a,b). The divorce probability was lower in newly formed pairs where the male was an experienced breeder. Divorce probability of an experienced male in a newly formed pair was 0.27 (95% CI = 0.05–0.71), and that of an inexperienced male 0.88 (0.49–0.98). To additionally test for the influence of between-sex degree and proportion of female associates on male's divorce probability, we considered males belonging to all newly formed pairs (not just those that were seen associated with their future females, as we did in the analysis above). The results of model selection (electronic supplementary material, table S4) gave weak support to the models where the between-sex degree influenced males divorce probability.
Figure 1.
The predicted probability of a newly formed great tit pair (breeding in season t) divorcing (as opposed to staying faithful) between breeding seasons t and t + 1 according to (a) proportion of female associates in male's social network, and (b) rank of male's partner (in terms of association strength) among all of his opposite sex associates (rank 1 is the female bird with whom the male has the strongest association), in the winter prior to breeding season t. Results are given for inexperienced breeders (i.e. males who were recorded breeding for the first time in breeding season t) and experienced breeders, and for three sets of winters (2007–2009 winters, winter 2011, winter 2012). Shaded areas on the graph represent the 95% CI of the estimate. Please note that the 95% CI of the adjacent years overlap, leading to the darker shade of the area. The estimates were obtained from the best binomial generalized linear model, controlling for the effect of the clutch size of a pair. (Online version in colour.)
Figure 2.
The predicted probability of divorce for a newly formed great tit pair (breeding in season t) between breeding seasons t and t + 1 according to association strength between pair members and association–strength rank of male's partner, among all his opposite sex associates (rank 1 is the female bird with whom the male has the strongest association), in the winter prior to breeding season t for (a) inexperienced males; (b) experienced males. The probability is plotted for all years (2007–2013) combined. The estimates were obtained by the best binomial glm, controlling for the effect of the clutch size and winter (winters 2007–2009, winter 2011 and winter 2012).
In contrast to males, we found no evidence that female social network traits, partner association–strength rank, or her breeding experience predicted divorce probability. Neither strength of the association with the partner, partner association–strength rank, between-sex degree, proportion of male associates in female's social network, nor breeding experience of females that were observed associating with their future breeding partners influenced divorce probability (see electronic supplementary material, table S3 for model selection). Similarly, neither between-sex degree nor proportion of male associates predicted divorce probability of females in new partnerships (all females of newly formed pairs, and not just those that were seen associated with their future breeding partners; electronic supplementary material, table S5).
4. Discussion
We used a detailed dataset of associations between males and females in winter flocks of wild great tits to describe how the social environment, including pair bond, affects future divorce probability. Our results showed that the male (but not the female) social environment and the association–strength rank of a male's future breeding female among his associates influenced the divorce probability of newly formed pairs. Males with a lower proportion of female associates, and males whose future breeding partner ranked relatively low among these, had a higher divorce probability after the first breeding of a pair. An increase in association strength between future breeding pair members decreased divorce probability only if a female was the first-ranked female associate of her male, but increased divorce probability otherwise. Previous studies on the influence of the social environment (approximated through demographic factors) on divorce probability have not considered the social environment in which a pair formed, but only the environment in which divorce happens. Our findings should be considered from the three main perspectives: (i) the importance of the social environment (in terms of the possible mating options) in mate choice; (ii) males as a sex that exercise partner preferences; and (iii) the extended influence of the social environment in which mate choice takes place on future reproductive decisions.
The influence of the social environment on mate choice decisions has been studied in the context of non-independent mate choice (commonly mate choice copying, [40]), and the relative attractiveness of a male compared with his associates [5,8,41]. Both empirical and theoretical work on these two aspects has emphasized that social influences on mate choice can substantially affect sexual selection [8,40]. A third aspect of the social influence on mate choice is the number (and quality) of possible partners [42]. For example, the frequency of extra-pair fertilizations in a house finch population was found to be largely determined by the availability of suitable extra-pair mates [43], and is thus specific for each individual in the population. Our study demonstrates that divorce, which can be considered as a form of mate choice [10], and a way to correct for a suboptimal mating situation [15], may also depend on aspects of the social environment in which initial mate choice happens. Our finding that males with a lower proportion of female associates during the period of pair formation divorced more often after breeding indicates that the intensity of competition (which we expressed as the proportion of the opposite sex associates) may limit the possibilities available for preferred pairing, regardless of the absolute number of female associates. However, there are other explanations, such as the possibility of a correlation between personality, social behaviour [44] and divorce probability. More detailed analysis of the composition of flocks and the way the composition changes over winter could help to reveal the mechanism behind our findings. Finally, when we looked at males of all new pairs, which also included those males for which we did not have information on the association strength with their females (mainly because she was not recorded in the network), the effect was no longer significant. One possible explanation is that the effect of the proportion of the opposite sex associates can be detected only when the data on the association between pair members are fully known. The support for this claim also comes from the results of the main model selection; if the information on partner strength and association–strength rank were excluded, the proportion of female associates was not selected as a covariate influencing divorce probability. However, why this might be so is not fully clear. A likely explanation is that the effect of the proportion of female associates explains the significant amount of the variance that remains after the effect of partner strength and association–strength rank are accounted for.
Although mate preferences have traditionally been studied in females [40,45], there is increasing evidence that males might be choosy [46], especially in social monogamous species where both parents care for offspring [47]. Results of some studies indicate that divorce, as a type of secondary mate choice, could also to be driven by both sexes [48]. For example, while divorce seems to be female driven in black-capped chickadees (Poecile atricapillus), where females abandon their partner for a male of higher quality [49], it is thought to be driven by males in house wrens, Troglodytes aedon [50]. Our result, that the male's but not the female's level of preference for the future breeding partner (as inferred from association–strength rank) influenced the probability of divorce after their first breeding, indicates that males likely use divorce to correct for suboptimal partnerships. Low partner association–strength rank of a female among other female associates most likely reflects the situation where a male was not able (because of competition or/and female choice) to breed with his preferred female, and ended up breeding with a less preferred one. Interestingly, while low partner association–strength rank was related to the high divorce probability in all three sets of winters, males that had their partners ranked high were more likely to divorce in the first 3 years (i.e. 2007–2009) than in the later years of data collection. This difference in the magnitude of the effect of partner association–strength rank on the divorce probability could likely be connected to the difference in the time periods when most future pairs start associating, and thus expressing partner preferences (see reference [23]). Exploring finer temporal changes in partner association–strength rank, and keeping track of the most preferred female over time would be a good way to study the observed pattern in more detail.
The lack of any influence of the components of the social environment on female divorce probability is difficult to explain, but we suggest several possible reasons for this. First, in great tits, males hold territories, and because of this, females that divorce their males, in general, also change the territory [51]. Thus, it could be that females base their decision on the territory rather than on preference for the previous male. Second, it has been shown that larger flocks have more juvenile females and fewer adult males than expected by chance [52]. Thus, one explanation could be that juvenile females have a relatively strong association with these males, which does not lead to formation of a pair. As previously stated, further more detailed exploration of the flock composition and its changes through time might help to explain the lack of any influence of female social traits and level of a preference for a male on future mating decisions.
Finally, a growing number of studies show that social experiences might have an extended influence on individual fitness [4,53–55]. For example, juvenile male zebra finches (Taeniopygia guttata) that matured with a single female showed more intense courtship, aggressiveness, and were more attractive to females later in life than were males reared in mixed-sex groups [56]. Because breeding success [10,15], and even survival [26,56], can be affected by fidelity to a partner and partner change in monogamous birds, the social environment has the potential to influence overall fitness, not just through initial mate choice, but also through later mating outcomes. Moreover, divorce and fidelity have the potential to influence overall population dynamics and productivity [14,57], as well as the intensity of sexual selection operating on individual traits [58].
5. Conclusion
Our study demonstrates that divorce in monogamous species might not only be affected by the social environment to which existing pairs are exposed, but also by the social environment in which pairs have formed. This environment is likely to constrain initial mate choice, subsequently shaping the secondary mating strategies. At the same time, our results also indicate that there may be sex differences in the links between the social environment and partner choice. Further exploration of these carry-over effects of the social environment, and how they differ between males and females, might give valuable new insights into processes of mate choice, population dynamics and sexual selection.
Supplementary Material
Acknowledgements
We thank Reinder Radersma for the advice on the data analysis. We thank Joshua Firth and Damien Farine for constructing the social networks and generating the association matrix that formed the basis of our subsequent analyses. We are grateful to all the people that helped to collect data on social winter behaviour and breeding data of great tits in Wytham woods.
Data accessibility
Data are available on Dryad, doi:10.5061/dryad.1s5rh.
Authors' contributions
A.C. participated in data collection, performed the main data analysis and wrote the main manuscript. C.A.H. and B.C.S. participated in data collection, and interpretation of the data. All three authors contributed substantially to revisions.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by an ERC Advanced grant no. (AdG 250164) to B.C.S.
References
- 1.Smith EA, Mulder MB, Hill K. 2001. Controversies in the evolutionary social sciences: a guide for the perplexed. Trends Ecol. Evol. 16, 128–135. ( 10.1016/S0169-5347(00)02077-2) [DOI] [PubMed] [Google Scholar]
- 2.Kameda T, Nakanishi D. 2003. Does social/cultural learning increase human adaptability? Rogers's question revisited. Evol. Hum. Behav. 24, 242–260. ( 10.1016/S1090-5138(03)00015-1) [DOI] [Google Scholar]
- 3.Apicella CL, Marlowe FW, Fowler JH, Christakis NA. 2012. Social networks and cooperation in hunter–gatherers. Nature 481, 497–501. ( 10.1038/nature10736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald DB. 2007. Predicting fate from early connectivity in a social network. Proc. Natl Acad. Sci. USA 104, 10 910–10 914. ( 10.1073/pnas.0701159104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh KP, Badyaev AV. 2010. Structure of social networks in a passerine bird: consequences for sexual selection and the evolution of mating strategies. Am. Nat. 176, E80–E89. ( 10.1086/655216) [DOI] [PubMed] [Google Scholar]
- 6.Kurvers R, Krause J, Croft DP, Wilson ADM, Wolf M. 2014. The evolutionary and ecological consequences of animal social networks: emerging issues. Trends Ecol. Evol. 29, 326–335. ( 10.1016/j.tree.2014.04.002) [DOI] [PubMed] [Google Scholar]
- 7.Farine DR, Sheldon BC. 2015. Selection for territory acquisition is modulated by social network structure in wild songbird. J. Evol. Biol. 28, 547–556. ( 10.1111/jeb.12587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bateson M, Healy SD. 2005. Comparative evaluation and its implications for mate choice. Trends Ecol. Evol. 20, 659–664. ( 10.1016/j.tree.2005.08.013) [DOI] [PubMed] [Google Scholar]
- 9.Choudhury S. 1995. Divorce in birds: a review of the hypotheses. Anim. Behav. 50, 413–429. ( 10.1006/anbe.1995.0256) [DOI] [Google Scholar]
- 10.Black JM. 1996. Partnerships in birds: the study of monogamy. Oxford, UK: Oxford University Press. [Google Scholar]
- 11.Petrie M, Kempenaers B. 1998. Extra-pair paternity in birds: explaining variation between species and populations. Trends Ecol. Evol. 13, 52–58. ( 10.1016/S0169-5347(97)01232-9) [DOI] [PubMed] [Google Scholar]
- 12.Griffith SC, Owens IPF, Thuman KA. 2002. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 11, 2195–2212. ( 10.1046/j.1365-294X.2002.01613.x) [DOI] [PubMed] [Google Scholar]
- 13.Ens BJ, Safriel UN, Harris MP. 1993. Divorce in the long-lived and monogamous oystercatcher, Haematopus ostralegus: incompatibility or choosing the better option. Anim. Behav. 45, 1199–1217. ( 10.1006/anbe.1993.1142) [DOI] [Google Scholar]
- 14.Maxin D, Berec L. 2010. A two-sex demographic model with single-dependent divorce rate. J. Theor. Biol. 265, 647–656. ( 10.1016/j.jtbi.2010.06.013) [DOI] [PubMed] [Google Scholar]
- 15.Culina A, Radersma R, Sheldon BC. 2014. Trading up: fitness consequences of divorce in monogamous birds. Biol. Rev. ( 10.1111/brv.12143) [DOI] [PubMed] [Google Scholar]
- 16.Dubois F, Cezilly F, Pagel M. 1998. Mate fidelity and coloniality in waterbirds: a comparative analysis. Oecologia 116, 433–440. ( 10.1007/s004420050607) [DOI] [PubMed] [Google Scholar]
- 17.Jeschke JM, Kokko H. 2008. Mortality and other determinants of bird divorce rate. Behav. Ecol. Sociobiol. 63, 1–9. ( 10.1007/s00265-008-0646-9) [DOI] [Google Scholar]
- 18.Liker A, Freckleton RP, Szekely T. 2014. Divorce and infidelity are associated with skewed adult sex ratios in birds. Curr. Biol. 24, 880–884. ( 10.1016/j.cub.2014.02.059) [DOI] [PubMed] [Google Scholar]
- 19.Sih A, Hanser SF, McHugh KA. 2009. Social network theory: new insights and issues for behavioral ecologists. Behav. Ecol. Sociobiol. 63, 975–988. ( 10.1007/s00265-009-0725-6) [DOI] [Google Scholar]
- 20.Garroway CJ, Radersma R, Hinde CA. 2014. Perspectives on social network analyses of bird populations. In Animal social networks (eds Krause J, Croft DP, James R), pp. 171–184. Oxford, UK: Oxford University Press. [Google Scholar]
- 21.Gosler AG. 1993. The great tit. London, UK: Paul Hamlyn. [Google Scholar]
- 22.Hinde RA. 1952. The behavior of the great tit (Parus major) and some other related species. Behaviour Suppl. 2, 1–201 [Google Scholar]
- 23.Saitou T. 2002. Factors affecting divorce in the great tit Parus major. Ibis 144, 311–316. ( 10.1046/j.1474-919X.2002.00016.x) [DOI] [Google Scholar]
- 24.Culina A. 2014. With or without you: divorce and pair fidelity in monogamous birds. DPhil thesis. Oxford University.
- 25.Dhondt AA, Adriaensen F, Plompen W. 1996. Between and within-population variation in mate fidelity in the great tit. In Partnerships in birds: the study of monogamy (ed. Black JM.), pp. 235–248. Oxford, UK: Oxford University Press. [Google Scholar]
- 26.Culina A, Lachish S, Pradel R, Choquet R, Sheldon BC. 2013. A multievent approach to estimating pair fidelity and heterogeneity in state transitions. Ecol. Evol. 3, 4326–4338. ( 10.1002/ece3.729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perrins CM, McCleery RH. 1985. The effect of age and pair bond on the breeding success of great tits Parus major. Ibis 127, 306–315. ( 10.1111/j.1474-919X.1985.tb05072.x) [DOI] [Google Scholar]
- 28.Szulkin M, Sheldon BC. 2008. Correlates of the occurrence of inbreeding in a wild bird population. Behav. Ecol. 19, 1200–1207. ( 10.1093/beheco/arn086) [DOI] [Google Scholar]
- 29.Pampus M, Schmidt KH, Wiltschko W. 2005. Pair bond and breeding success in blue tits Parus caeruleus and great tits Parus major. Ibis 147, 92–108. ( 10.1111/j.1474-919x.2004.00376.x) [DOI] [Google Scholar]
- 30.Perrins CM. 1965. Population fluctuations and clutch size in the great tit, Parus major L. J. Anim. Ecol. 34, 601–647. ( 10.2307/2453) [DOI] [Google Scholar]
- 31.Perrins CM. 1979. British tits. London, UK: Collins. [Google Scholar]
- 32.Matechou E, Cheng SC, Kidd LR, Garroway CJ. 2015. Reproductive consequences of the timing of seasonal movements in a non-migratory wild bird population. Ecology 96, 1641–1649. ( 10.1890/14-0886.1) [DOI] [Google Scholar]
- 33.Franks DW, Ruxton GD, James R. 2010. Sampling animal association networks with the gambit of the group. Behav. Ecol. Sociobiol. 64, 493–503. ( 10.1007/s00265-009-0865-8) [DOI] [Google Scholar]
- 34.Psorakis I, Roberts SJ, Rezek I, Sheldon BC. 2012. Inferring social network structure in ecological systems from spatio-temporal data streams. J. R. Soc. Interface 9, 3055–3066. ( 10.1098/rsif.2012.0223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Psorakis I, et al. 2015. Inferring social structure from temporal data. Behav. Ecol. Sociobiol. 69, 857–866. ( 10.1007/s00265-015-1906-0) [DOI] [Google Scholar]
- 36.Cairns SJ, Schwager SJ. 1987. A comparison of association indexes. Anim. Behav. 35, 1454–1469. ( 10.1016/S0003-3472(87)80018-0) [DOI] [Google Scholar]
- 37.Whitehead H. 2008. Analyzing animal societies: quantitative methods for vertebrate social analysis. Chicago, IL: University of Chicago Press. [Google Scholar]
- 38.Anderson DR, Burnham KP. 2002. Avoiding pitfalls when using information-theoretic methods. J. Wild. Manag. 6, 912–918. ( 10.2307/3803155) [DOI] [Google Scholar]
- 39.R Core Team. 2012. R: a language and environment for statistical computing. Vienna, Austria: R Core Team. [Google Scholar]
- 40.Westneat DF, Walters A, McCarthy TM, Hatch MI, Hein WK. 2000. Alternative mechanisms of nonindependent mate choice. Anim. Behav. 59, 467–476. ( 10.1006/anbe.1999.1341) [DOI] [PubMed] [Google Scholar]
- 41.Callander S, Jennions MD, Backwell PRY. 2011. Female choice over short and long distances: neighbour effects. Behav. Ecol. Sociobiol. 65, 2071–2078. ( 10.1007/s00265-011-1216-0) [DOI] [Google Scholar]
- 42.Shuster SM, Wade MJ. 2003. Mating systems and strategies. Princeton, NJ: Princeton University Press. [Google Scholar]
- 43.Lindstedt ER, Oh KP, Badyaev AV. 2007. Ecological, social, and genetic contingency of extrapair behavior in a socially monogamous bird. J. Avian Biol. 2, 214–223. ( 10.1098/rspb.2006.3528) [DOI] [Google Scholar]
- 44.Aplin LM, Farine DR, Morand-Ferron J, Cole EF, Cockburn A, Sheldon BC. 2013. Individual personalities predict social behaviour in wild networks of great tits (Parus major). Ecol. Lett. 16, 1365–1372. ( 10.1111/ele.12181) [DOI] [PubMed] [Google Scholar]
- 45.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 46.Clutton-Brock T. 2009. Sexual selection in females. Anim. Behav. 77, 3–11. ( 10.1016/j.anbehav.2008.08.026) [DOI] [Google Scholar]
- 47.Johnstone RA, Reynolds JD, Deutsch JC. 1996. Mutual mate choice and sex differences in choosiness. Evolution 50, 1382–1391. ( 10.2307/2410876) [DOI] [PubMed] [Google Scholar]
- 48.Cézilly F, Preault M, Dubois F, Faivre B, Patris B. 2000. Pair-bonding in birds and the active role of females: a critical review of the empirical evidence. Behav. Process. 51, 83–92. ( 10.1016/S0376-6357(00)00120-0) [DOI] [PubMed] [Google Scholar]
- 49.Ramsay SM, Otter KA, Mennill DJ, Ratcliffe LM, Boag PT. 2000. Divorce and extrapair mating in female black-capped chickadees (Parus atricapillus): separate strategies with a common target. Behav. Ecol. Sociobiol. 49, 18–23. ( 10.1007/s002650000270) [DOI] [Google Scholar]
- 50.Poirier NE, Whittingham LA, Dunn PO. 2003. Effects of paternity and mate availability on mate switching in house wrens. Condor 105, 816–821. ( 10.1650/7201) [DOI] [Google Scholar]
- 51.Andreu J, Barba E. 2006. Breeding dispersal of great tits Parus major in a homogeneous habitat: effects of sex, age, and mating status. Ardea 94, 45–58. [Google Scholar]
- 52.Farine DR, et al. 2015. The role of social and ecological processes in structuring animal populations: a case study from automated tracking of wild birds. R. Soc. open sci. 2, 150057 ( 10.1098/rsos.150057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gersick AS, Snyder-Mackler N, White DJ. 2012. Ontogeny of social skills: social complexity improves mating and competitive strategies in male brown-headed cowbirds. Anim. Behav. 83, 1171–1177. ( 10.1098/rstb.2011.0223) [DOI] [Google Scholar]
- 54.Gilby IC, Brent LJN, Wroblewski EE, Rudicell RS, Hahn BH, Goodall J, Pusey AE. 2013. Fitness benefits of coalitionary aggression in male chimpanzees. Behav. Ecol. Sociobiol 67, 373–381. ( 10.1007/s00265-012-1457-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stanton MA, Mann J. 2012. Early social networks predict survival in wild bottlenose dolphins. PLoS ONE 7, e47508 ( 10.1371/journal.pone.0047508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicolai CA, Sedinger JS, Ward DH, Boyd WS. 2012. Mate loss affects survival but not breeding in black brant geese. Behav. Ecol. 23, 643–648. ( 10.1093/beheco/ars009) [DOI] [Google Scholar]
- 57.Berec L, Boukal DS. 2004. Implications of mate search, mate choice and divorce rate for population dynamics of sexually reproducing species. Oikos 104, 122–132. ( 10.1111/j.0030-1299.2004.12753.x) [DOI] [Google Scholar]
- 58.DuVal EH. 2013. Female mate fidelity in a lek mating system and its implications for the evolution of cooperative lekking behavior. Am. Nat. 181, 213–222. ( 10.1086/668830) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on Dryad, doi:10.5061/dryad.1s5rh.