Abstract
The rhythm of life on earth is shaped by seasonal changes in the environment. Plants and animals show profound annual cycles in physiology, health, morphology, behaviour and demography in response to environmental cues. Seasonal biology impacts ecosystems and agriculture, with consequences for humans and biodiversity. Human populations show robust annual rhythms in health and well-being, and the birth month can have lasting effects that persist throughout life. This review emphasizes the need for a better understanding of seasonal biology against the backdrop of its rapidly progressing disruption through climate change, human lifestyles and other anthropogenic impact. Climate change is modifying annual rhythms to which numerous organisms have adapted, with potential consequences for industries relating to health, ecosystems and food security. Disconcertingly, human lifestyles under artificial conditions of eternal summer provide the most extreme example for disconnect from natural seasons, making humans vulnerable to increased morbidity and mortality. In this review, we introduce scenarios of seasonal disruption, highlight key aspects of seasonal biology and summarize from biomedical, anthropological, veterinary, agricultural and environmental perspectives the recent evidence for seasonal desynchronization between environmental factors and internal rhythms. Because annual rhythms are pervasive across biological systems, they provide a common framework for trans-disciplinary research.
Keywords: annual, fitness, desynchrony, one-health, biological rhythm, circannual
1. Introduction
Biological rhythms are ubiquitous in nature and occur on several temporal scales. Daily rhythms are important for the coordination of physiological, immunological and behavioural processes within organisms as well as for biotic interactions. The proper functioning of these rhythms is disrupted by modern human lifestyles—including sleep deprivation, light at night, jet lag and shift work [1,2], which induce temporal mismatches between the environment and circadian biology, and have detrimental effects on health and well-being. Problems of mismatch extend beyond daily rhythmicity. New research by Dopico et al. [3] has elegantly demonstrated massive seasonal changes in human immunity and physiology, adding to evidence for marked annual rhythms in the vast majority of organisms [4,5]. The disruption of annual rhythms under global climate change has potentially dramatic consequences for the health of animals, humans and ecosystems. Whether or not organisms can adapt to changing seasonality depends on the regulation of their annual rhythms. Principally, annual rhythms could be: (i) genetically programmed, i.e. genotype-dependent responses to the environment resulting from evolutionary adaptation to predictable annual change; (ii) direct environmental effects, e.g. accelerated growth owing to longer light hours in the day; or (iii) coincidental, e.g. human rhythms arising as a consequence from holidays.
This review will focus on genetically programmed, internal cell- and tissue-based mechanisms, which are predicted to track changes in environmental seasonality. In many species, tissue function is reprogrammed between subjective winter and summer states, generating endogenous rhythms that approximate a year (i.e. circannual rhythms) [3,6–9]. The existence of innate circannual rhythmicity has been demonstrated when organisms, from unicells to vertebrates, are maintained in constant environmental conditions for many years [6–11]. Species with genetically programmed annual rhythmicity occur globally, from high latitudes to the equator, and even in apparently ‘constant’ environments such as the deep sea [6]. Genetic programming is seen to be adaptive because it is pre-emptive and serves to predict and prepare organisms for alternations in seasonal environmental conditions [12–14].
In humans, the evidence in support of seasonal effects on disease risk, physiology and immune function is pervasive ([3,15–17]; electronic supplementary material, tables S1 and S2) and suggests present-day implications of evolutionarily inherited and refined mechanisms [5]. During the twentieth century, our species has developed technologies that allow precise photic and climate control over our living environments, and humans in developed societies now spend the vast majority of their lives in conditions that mimic ‘summer-like’ environments [15]. These so-called eternal summers are characterized by light and temperature conditions that lack seasonal rhythmicity. Presently, many of us no longer live in accordance with the naturally occurring variation in geophysical rhythms. The consequences of such modified environmental seasonality on human health are still being elucidated.
Additionally, human activities are also affecting seasonality in a wider ecological context, with implications for disease risk, ecosystem health and food security. In agricultural and natural ecosystems, there is growing evidence that seasonal patterns and ecological interactions are disrupted by global climate change [18]. Disruptions in annual rhythms are expected to become progressively more prevalent and detrimental consequences have already been documented [19,20]. Nevertheless, there is a surprising lack of data to determine if, and how seasonally generated and regulated functions can adapt [21,22] to altered environmental conditions. Thus, an overarching, integrated scientific understanding is needed of the mechanisms that underlie seasonality, including cyclical biology of humans and of the consequences for all organisms as they adapt to disrupted seasonality and a fast changing climate. Addressing the arising practical challenges requires an integrated, cross-disciplinary approach, as exemplified by the ‘one health’ initiative for advancing healthcare for humans, animals and the environment [23,24].
Here, we review current examples of disruption between seasonal environmental conditions and internal timing mechanisms. From this basis, we further emphasize the pervasiveness of seasonality (with a focus on humans). Finally, we outline potential threats of disruption for humans, industry, and managed and natural ecosystems, with a call for the development of a synergistic research agenda for seasonal biology.
2. Scenarios of seasonal disruption
Seasonal changes in environmental variables play a significant role in the regulation of many physiological and behavioural processes. Annual changes in day length, temperature and rainfall can all act as cues; they provide vital information used in the timing of seasonal behaviour and the synchronization of internal rhythms (electronic supplementary material, figure S1 and figure 1). In this way, organisms' behaviour and physiology is timed to optimize fitness in a given season [12,13,19]. However, the use of these cues to synchronize (entrain) biological rhythms is effective only to the extent that temporal relationships between the external and internal rhythms are predictable. Timing systems are therefore highly vulnerable to changes in the constellation of environmental factors under which they have evolved (figure 1). Changes in the seasonal timing of organismal functions provided some of the earliest evidence for biological effects of global climate change [25]. Such changes have now been documented across a wide range of habitat types and taxonomic groups (electronic supplementary material, figure S2 and [26]). Crucially, species and populations of wild plants and animals have demonstrated different rates of change in their overt seasonality [26].
Figure 1.
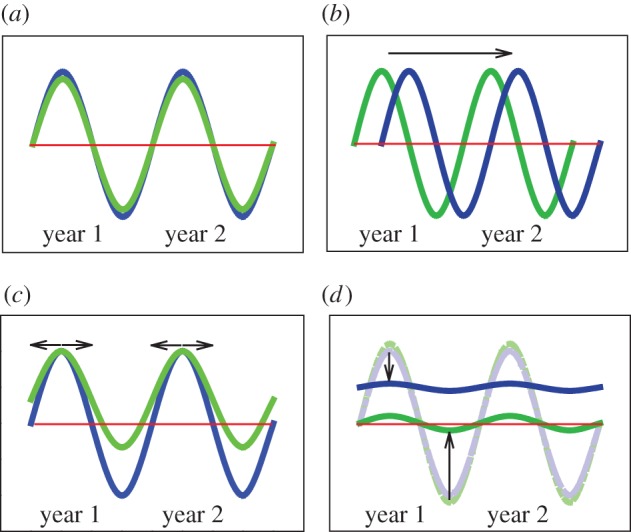
Schematic of annual rhythms. (a) All organisms on earth have evolved to time their physiology and behaviour (internal rhythm; blue) with seasonal changes in local climates/resources (external rhythm; green line). These internal rhythms can precede or follow those of resources, but for adaptive timing, the internal and external rhythms need to match. There are three theoretical scenarios that can account for disruptions of the match between seasonal timing to local climates: (b) phase shifts between internal and external rhythms; (c) increased (or decreased) duration of favourable environmental conditions (e.g. rising above the red line, which could indicate a rise in minimum temperature and day length); or (d) reduction of the amplitude (e.g. under ‘eternal summers’) and/or change in mean levels of seasonal rhythms. Red lines indicate the average seasonal mean of the rhythms in panel (a) for reference. Arrows indicate changes in rhythms. (Online version in colour.)
There are multiple, mutually non-exclusive and potentially interactive mechanisms by which altered external cues could disrupt the relationship between external and internal rhythmicity. For example, climate-change-induced shifts in the timing of the seasons can result in an abnormally delayed or advanced internal rhythm with respect to the environment (figure 1b). Such mismatches in the timing of critical life-history events can have a large impact on the reproductive output of plants and animals. Alternatively, disruptions might be induced if the durations of specific phases of an external rhythm become extended (e.g. longer growing season) or reduced (e.g. shorter time of snow cover). This could occur, for example, if local minimal winter temperatures increase, resulting in higher means in local annual temperatures with reduced amplitude of seasonal differences (figure 1c). A scenario with changes in the amplitude of seasonal differences is particularly relevant for humans (figure 1d). Cultural developments, including the ability to control local environmental conditions through the use of fire at night for heat and light, marked the beginning of human manipulation of natural day–night cycles. The seasonality experienced by humans in developed societies (and that of some closely associated species) is already largely damped by modern artificially induced photic and indoor climate conditions reminiscent of eternal summers [15].
3. Molecular, cellular and physiological basis of seasonal time-keeping
A better understanding of how species respond to seasonal changes in their environment and how they can adapt to their disrupted seasonality requires knowledge of the mechanisms of seasonal time-keeping. Endogenous annual rhythmicity is evident in a wide range of species (e.g. protists [6], insects [27], plants [28], fishes [29], birds [30] and mammals [4]), but is especially strong and widespread among vertebrates, making it highly likely that seasonal genes and/or intracellular pathways are also involved in long-term changes in human physiology and behaviour. Broadly, in multicellular organisms, the mechanisms for regulating annual rhythms involve cellular and molecular timers that interact closely with refined input pathways for transmitting day length (photoperiod) and other environmental cues (electronic supplementary material, figure S3). Highly photoperiodic species have been extremely valuable for the identification of key genetic, cellular and neuronal circuits that regulate annual rhythms [31]. Direct evidence for endogenous seasonal rhythms in humans is scarce owing to the challenges of collecting data over multiple annual oscillations and the near impossibility of isolating subjects from exogenous influences for extensive time periods. Historical and contemporary studies have demonstrated that humans exhibit seasonal reproduction, hypothesized to be driven by internal mechanisms [32,33]. Over the past few centuries, there has been a decrease in seasonal patterns in humans [32,33] that could have resulted from a reduction in the seasonal amplitude or desynchronization from environmental cues. Nevertheless, these data suggest that exogenous cues (i.e. photoperiod) can entrain seasonal human responses [15,33,34]. In most mammals, annual changes in day length affect seasonal rhythmicity via the suprachiasmatic nucleus in the hypothalamus, and consequently, altering the nocturnal secretion of the hormone melatonin from the pineal gland ([35]; electronic supplementary material, figure S3a). Melatonin receptors are localized in many of the brain regions implicated in cognitive, affective and homeostatic processes, and thus seasonal changes in melatonin secretion regulate key genes required for the neuroendocrine control of physiology, immune function and behaviour [36–40]. Although exact photoperiodic input pathways vary among vertebrate groups, similar day length-induced changes in neuroendocrine brain regions (electronic supplementary material, figure S3b) occur in fishes [41] and birds [42], which would suggest that seasonal changes in day length act to regulate a common, evolutionarily ancient internal timing system [43]. Recent work suggests that the fundamental nature of this timer may depend on cyclical histogenesis [44] and/or epigenetic mechanisms [45,46], potentially operating in multiple tissues to form a circannual clock-network.
In this framework, the hypothalamus of the brain acts as an important interface for many processes that are relevant for health and physiology, coordinating seasonal changes in autonomic and endocrine function. Of note, seasonal timing in immune function is vital for the survival of many small seasonal animals such as rodents and birds [39–41]. Seasonal- or light-induced changes in a range of innate humoral and cell-mediated systems are present in a wide range of species (electronic supplementary material, table S2). A general finding is that short days which mimic a winter environment enhance some immune functions [39]; for species where winter is a time of increased pathological risk, immune function is increased presumably in anticipation of increased need. It is important to note that different aspects of immunity may be differently regulated across the seasons. For example, rodent and bird models have shown that short days enhance many aspects of cell-mediated immunity while suppressing other specific immune defences ([39,41,47]; electronic supplementary material, table S2). The precise molecular and cellular mechanisms involved in the seasonal restructuring of immune function are not well described, but recent evidence suggests that a common molecular switch occurs in hypothalamic regions and immune tissues (e.g. leucocytes; [3,48]). Recent developments in next-generation sequencing platforms, transcriptomics and proteomic analyses, provide a powerful means to identify the precise molecular and cellular pathways that underlie annual rhythms [3,44,46]. Once the mechanisms that govern such rhythms are identified, experiments can be devised for field-based or clinical settings to examine the relationship between external environment and internal timing.
4. Seasonality and human health
In many tropical and equatorial areas, including the savannah–woodlands where humans are thought to have originated, seasonality is highly pronounced although there is little variation in day length. Indeed, striking seasonal patterns in behaviour are observed in the migration and breeding rhythms of many equatorial species, such as the flowering of trees [49] and the reproductive seasonality of some primates and traditional human societies [50]. Although at a population level many species appear to breed asynchronously with respect to the calendar year, individuals tend to be seasonally cyclical [51,52] when studied over a full life history. Environmental stimuli that provide seasonal cues in the tropics are often related to local non-photic cues, such as rainfall patterns that in turn determine food abundance (electronic supplementary material, figure S1c). In the Pleistocene, humans radiated out of the tropics to higher latitudes where they encountered novel seasonal environments, in particular marked annual rhythms in day length and ambient temperature (electronic supplementary material, figure S1a–c). However, because the underlying internal mechanisms that govern annual rhythms preceded the hominin lineage, humans probably possess much of the ancient molecular and cellular machinery characteristics of other seasonal species, potentially including genetic variation in seasonality [53]. Even today, human reproduction is not evenly spread across the year ([33,54]; electronic supplementary material, figure S4a–c). For example, a recent analysis indicated that human birth pulses across the USA fluctuate seasonally with an amplitude of about 10% ([33]; electronic supplementary material, figure S4). Despite the decline in birth seasonality (figure 1d; cf. [15,17,32,33]), it is still substantial for a species that effectively lives under conditions of eternal summer in its immediate habitat. Notably, the amplitude of these rhythms and the timing of their maxima vary with latitude, which strongly suggests an underlying physiological regulatory mechanism. These patterns of birth pulses have longer-term relevance because of the strong evidence [55] that birth month has lifelong impacts on health, including the likelihood of general, psychiatric or neurological illnesses ([17,54]; electronic supplementary material, table S1). That these time-of-birth effects may reflect day-length-dependent mechanisms is signified by the observation that some diagnoses, e.g. schizophrenia and multiple sclerosis, show an inverse pattern in the Northern and Southern Hemispheres (electronic supplementary material, table S1).
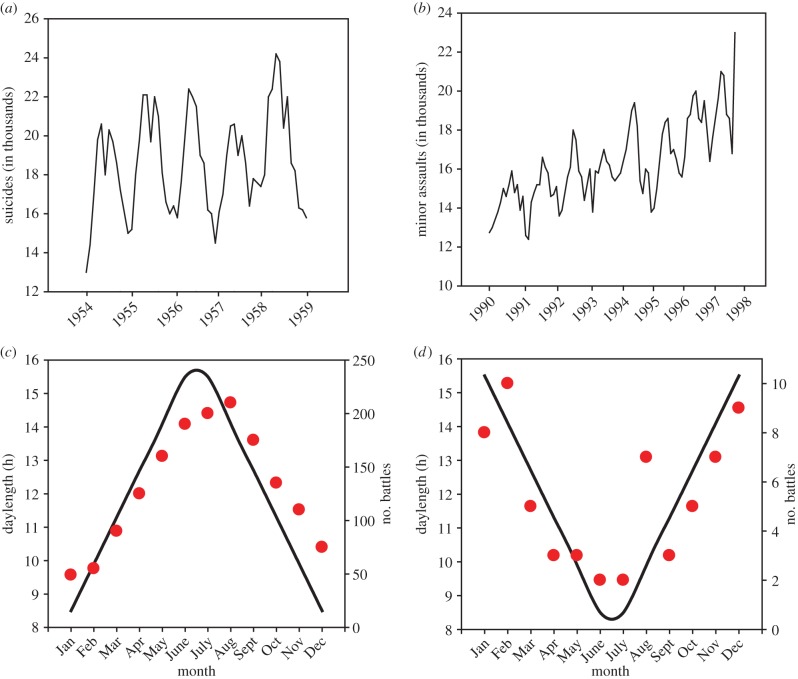
Seasonal human morbidity is observed in non-infectious diseases, including heart disease [56], cerebrovascular disease [57] and lung cancer [58]. Behaviour-driven mortality is also seasonal; cycles in the monthly numbers of suicides are one of the oldest and most replicated findings, with most, but not all, studies finding a peak in late spring/early summer (figure 2a). Interestingly, aggression and other violent acts such as homicide have a marked seasonal pattern of occurrence nearly coincident with that found for suicide (figure 2b). Seasonal patterns in aggression are not limited to the individual level. Historical records indicate a strong rhythm in population-level forms of aggression measured in onset of battles ([59]; figure 2c,d). Whether rhythms represent simple direct responses to seasonal environmental changes and/or the manifestation of endogenous seasonal timing mechanisms, these findings indicate that seasonality continues to be an important factor in human lives.
Figure 2.
Seasonal patterns in humans. (a) Number of suicides in Japan was significantly greater in the spring compared with autumn seasons. (b) The number of minor assaults in England and Wales significantly increased during the summer compared with winter seasons. (c) Number of battles in the Northern hemisphere peaked in August and were at a minimum in January; (d) the inverse pattern occurs in the Southern Hemisphere with peaks of battles occurring December–February; troughs in July. Data from (a,b) were kindly provided by Daniel Rock; (c,d) adapted from [59]. (Online version in colour.)
Humans also show seasonal changes in immunity and in the occurrence of infectious diseases [47]. Changes in immunity include seasonal variation in cytokine production [60], bacterial killing activity [61] and response to vaccination [62]. The most comprehensive characterization so far of annual cycles in human immunity comes from a recent study by Dopico et al. [3]. This study presented extensive data from geographically and ethnically diverse human populations, including mRNA expression in white blood cells and adipose tissue, inflammatory markers and blood count data. It reports seasonal differences in the expression of up to 23% of genes in peripheral blood mononuclear cells. Furthermore, the seasonal patterns of gene expression were inverse in Australia relative to the USA and the UK. The reverse pattern in Australia supports the idea that in humans, as well as in other animals, some immune responses may be modulated by day length [63]. Seasonal infections thus probably mirror internal rhythms of immunity as well as patterns of exposure. The relative importance of these mechanisms as drivers of any human infection remains to be established.
5. Disrupted seasonality and infectious disease dynamics
One important area for future research is the effect of disrupted seasonality on the dynamics of infectious disease. A recent review has highlighted the unprecedented rate at which vector-borne diseases have changed over the past decade, and has alerted a wide audience to the impact of changes in the climate [64]. The review details consequences of modified environmental seasonality, for example release from severe winters at higher latitudes and extended phases of seasonal activity. Environmental seasonal drivers of disease incidence include climate-sensitive pathogen dissemination and survival; seasonal variation in host recruitment, contact rates and susceptibility, and seasonal changes in vector abundance [65,66]. Directly transmitted and epidemic-prone diseases such as influenza, measles, polio, rotavirus and cholera exhibit pronounced seasonality and substantial heterogeneity in time and space [65,66]. The dominant seasonal drivers vary not only by disease, but also by geography. The best understood examples of climate-driven infections are influenza and cholera. Influenza is best transmitted when temperature and humidity are low, yielding winter epidemics in temperate regions [67], whereas cholera outbreaks are intensified by local increases in temperature during El Niño events [68].
Vector-borne pathogens—including those responsible for malaria, dengue, Lyme disease, Chagas, West Nile and sleeping sickness—present some of the most notable examples of seasonally driven infection [65,66]. Vector-borne diseases are particularly sensitive to phenological change, because numerous aspects of vector behaviour, demography and population dynamics are crucially dependent on environmental conditions [69]. For example, the growth rate of malaria vector populations in Africa explodes during the rainy seasons owing to the expansion of vector-larval habitat [70,71]. Temperature also has a strong impact on larval development rate, survival and the duration of the gonotrophic cycle in a wide range of Diptera [72–75]. Further complexity is introduced by seasonality in host populations. Examples include seasonal variation in the immune responsiveness and nutritional quality of plant hosts to their vectors [72,75]; herd immunity in cattle to tick-borne Babesia [76] and demonstration that the timing of peak human exposure to West Nile virus in North America is driven by seasonal patterns of avian migration [77]. The complexity of the seasonal interactions between vectors, pathogens and hosts is probably responsible for the lack of consistent evidence on how climate change will influence vector-borne disease [64,71,74]. While there is compelling evidence that some vector-borne diseases are being enhanced by climate change (e.g. Lyme disease; [78,79]; avian malaria; [80]), there are several other examples of diseases that have failed to expand as originally predicted (e.g. malaria; [69]). The least understood seasonal drivers of infection are those potentially governed by rhythms of host susceptibility and susceptible recruitment [47], although recent data suggest that annual cycles in human immune pathways may indeed affect susceptibility to specific diseases [3]. In summary, there is a need for a detailed understanding of how climate will affect all aspects of the pathogen life cycle before the consequences for transmission can be predicted.
6. Disrupted seasonality and ecosystem health
Ecological studies have identified potential problems associated with disruption of seasonality, owing to the desynchronization of key seasonal interactions among wild species (figure 1b). However, our existing understanding of the ecological implications of disrupted seasonality is mostly based upon studies of impacts upon single predator–prey relationships (such as the mismatch that can develop between the timing of seasonal coat colours and the annual duration of snow cover [81] and more recently also on plant–pollinator interactions [82–86]). Importantly, reproductive success in many species increases when reproductive timing and peak food availability are matched. Examples include egg hatching date in piscivorous seabirds, such as the Atlantic puffin (Fratercula arctica) [87], and in winter moths (Operophtera brumata) relative to tree budburst [88]; or calving date relative to vegetation growth in caribou (Rangifer tarandus) [89] or roe deer (Capreolus capreolus; [90]). An example that has been developed in some detail is the reducing match between the timing of breeding of forest songbirds, such as great tits (Parus major) and blue tits (Parus caeruleus), and the time that their prey, caterpillars feeding on oak leaves, are most abundant ([19,91–93]; electronic supplementary material, figure S5). Great tits that are most mismatched with the food peak have the fewest surviving offspring [94–96]. These ecological studies provide clear examples of how disrupted seasonality can affect the fitness of individuals living in an environment in which internal and external rhythms no longer match. The studies give numerous insights into how disrupted seasonality might also affect human and agricultural health.
Disrupted seasonality in natural systems can also be expected to affect ecosystem health. However, the effects at the ecosystem scale are more poorly understood than those at the individual level, because most studies have adopted the paradigm of the food chain (e.g. a single consumer population and a single resource species). While this has rendered the problem more tractable, patterns of species interaction in nature are in fact complex networks. Therefore, a major challenge is to move beyond relatively simple, mostly pairwise ecological interactions to consider the consequences of disrupted seasonality on population and community dynamics within broader, multispecies, interaction networks that include humans.
Despite the limitations of studies on dyadic interactions, there are already some clues that disrupted seasonality may affect ecosystem health. Under global change, the flowering phenology of plants and the seasonal activity phase of pollinators may shift to a different extent or even in opposing directions (electronic supplementary material, figure S2), thereby potentially leading to temporal mismatches and the disruption of existing interactions [97–99]. However, recent studies based on long-term phenology data indicate that bee emergence keeps pace with advanced plant-flowering, at least under current climatic conditions and for generalist plant–pollinator interactions [100]. Importantly, species-rich pollinator communities may also be able to buffer negative consequences of global warming [101], because plant pollinator networks exhibit plasticity with lost interactions being capable of replacement by new ones [102]. This underpins the importance of sustaining biodiversity for mitigating the impact of global climate change. Clearly, while there is ample scope for disrupted seasonality to strongly affect ecosystems, the few existing examples suggest that at present the sum impacts appear relatively small. In vertebrates, genetic variation in seasonality in wild populations of mammals and birds may not be sufficient to track changes in climate [103,104]. It remains to be seen if our collective perspective will hold once long-term research has been conducted as global climate change continues.
7. Disrupted seasonality and agricultural health
Ecological, physiological and epidemiological considerations suggest that seasonal disruption could also lead to substantial problems in agricultural industries, impacting both crop production and livestock viability, and hence, food security. The two most prevalent drivers of altered crop yields are changes in pollination and pest infestation. Notably, 70% of major crops and 35% of global crop production volume [82], with an estimated global economic value of $189 billion (€153 billion) per year [105], depend on seasonal pollination by bees and other insects. The spread of pest insects is a significant threat to human food security. For example, aphid outbreaks are expected to intensify owing to extended growing seasons (figure 1c) permitted by altered seasonal environmental conditions (reviewed by Bale & Hayward [106]). The peach–potato aphid and the grain aphid are examples of vectors of devastating plant virus diseases. In the past 20 years, increased occurrences of mild winters have resulted in earlier spring migrations of the winged form of these aphids into crops during their most vulnerable stages, resulting in epidemic outbreaks. The complex interactions between crops, pests and pathogens in the context of climate change urgently need more research [107].
Neglect of seasonal physiology and of the consequences of its disruption, negatively impact livestock health. Agricultural industries, notably the poultry industry, diminish seasonality by choosing light and temperature conditions to maximize reproduction and growth and thus profitability on an industrial scale (figure 1d). Some livestock, especially hens (Gallus gallus), may therefore be instructive models of longer-time effects of aseasonal conditions. Without the opportunity to seasonally pause laying and regenerate, notably by moulting [108], these hens become morbid and are typically slaughtered; only by allowing or forcing a moult can these effects be reversed. In other species used for human food production, breeding practices that select for animals which are capable of year round reproduction have also reduced seasonal rhythms. Farmed cattle (Bos primigenius taurus) have the ability to reproduce year round, but despite selection for decreased seasonal physiology, some ancient patterns remain, including the expression of poorly understood genes relating to hibernation and seasonal biology [109]. Many sheep breeds (Ovis aries) are excellent models for seasonal reproduction (electronic supplementary material, figure S6a) because they exhibit marked seasonal changes that are driven by internal rhythms synchronized by environmental factors [4]. Similar to the seasonal variation in humans discussed above, both sheep and cattle exhibit seasonal changes in disease diagnoses (electronic supplementary material, figure S6b).
To exemplify the possible implications of seasonal biology for morbidity and management of livestock, we highlight provisional data from passive surveillance in the UK on nutritional diseases (electronic supplementary material, figure S6b). Selenium (Se)-related deficiencies are common in farmed ruminants [110], so that sheep and cattle are commonly given supplementary Se. This perceived risk of deficiency and consequent timing of supplementation is driven by expected environmental supply and climatic- or management-driven challenge rather than by knowledge of underlying physiological processes that drive vulnerability. The contrasting peak months of diagnosis of Se deficiency syndromes in sheep versus cattle (electronic supplementary material, figure S6b) could be driven by different factors such as livestock management or disruptions in internal annual rhythms. Se deficiency is apparent in humans and is directly linked to viral pathology [111]. Seasonal variations in Se deficiency or supplementation in agriculture could, therefore, have significant implications for livestock health and human food systems.
8. Concluding remarks
Although our appreciation of the critical role of seasonal biology in the health and welfare of organisms (including humans), natural ecosystems and industries is growing, our ignorance of the effects of seasonal disruption on these systems is profound. We call for the development of an integrated, interdisciplinary, seasonality-focused research agenda (electronic supplementary material, table S3), inspired by a one health approach [24]. We propose three primary aims: understanding the internal seasonal clock, studying seasonality in human and veterinary clinical settings, and gaining ecological network perspectives.
(a). Understanding the internal seasonal clock
The mechanistic basis of the seasonal clock, including its conserved and variable elements across vertebrates, remains largely unknown. This is in contrast to remarkable advances in our understanding of the circadian clock where the expanding knowledge of individual genetic variation is paving the way to personalized medicine [112]. Obtaining a similar level of detail about genes and physiological processes involved in the seasonal timing mechanisms and functional variation in these genes, would aid in identifying people, livestock and crops susceptible to the impact of seasonal disruption. This research should include genomic and transcriptomic investigation of seasonal biology across seasonal gradients in nature and also long-term studies of human and animal health. The current research focus on short-lived animal models offers limited answers for long-term health management of humans, relative to the value of incorporating studies of long-lived seasonal species. Beyond animal systems, manipulating plant clocks might also enable the development of crops (by either artificial selection or transgenic approaches) that are more resistant to temporal mismatches owing to climate change, preserving and possibly improving crop production systems.
(b). Human and veterinary clinical settings
Seasonal patterns in health and physiology can be powerful indicators of possible underlying pathways, e.g. the neuroendocrine regulation of metabolism and obesity [113]. Data from human and veterinary pathology are often poorly integrated. Both are frequently only locally available, making it difficult to gain a broad understanding and to identify possible aetiologies. For example, revealing the seasonality of human diseases is now becoming possible as powerful epidemiological approaches are developed [33], but data often need to be tediously compiled from dispersed and poorly accessible sources. We encourage unified collection of seasonal health data, including possible use of big data analytics [114]. Analysis using improved empirical and epidemiological tools can help to identify drivers of seasonality (e.g. through trends in ambient temperature, latitude and day length) and also seasonal periods of particular vulnerability.
(c). Gaining ecological network perspectives
Because organisms are sensitive to changes in the rhythms of species with which they interact, consequential mismatches propagate across ecological networks. Most research focuses on pairwise predator–prey, plant–herbivore or plant–pollinator interactions, but pairwise interactions need to be scaled up to more complex food webs and host–vector–pathogen systems. We emphasize the value of longer-term monitoring in ecological studies, with a view of multiple components and ‘neighbours’ in the network of species of particular interest. There is a real need for large-scale experimental approaches to understand the ecosystem-level consequences of shifted or disrupted seasonal timing.
Overall, in our view: (i) understanding the internal seasonal clock, (ii) enhancing seasonal analyses in human and veterinary settings, and (iii) integrating data across ecological and agricultural networks, will enable an integrative platform for addressing seasonal disruptions in a rapidly changing world.
Supplementary Material
Acknowledgements
This paper first originated during the Symposium and Workshop ‘Seasons of Life’ hosted by the University of Glasgow in November 2013. Additional funding was kindly provided by BBSRC and the British Society for Neuroendocrinology (BSN).
Ethics
This review did not use human/animals.
Data accessibility
The review does not contain new data; all work was obtained from previously published material.
Authors' contributions
All authors contributed to the conception and design of the review. T.J.S., M.E.V. and B.H. drafted the review. All authors analysed, critically revised and approved the version published.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA. 2009. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl Acad. Sci. USA 106, 4453–4458. ( 10.1073/pnas.0808180106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. 2010. Light at night increases body mass by shifting the time of food intake. Proc. Natl Acad. Sci. USA 107, 18 664–18 669. ( 10.1073/pnas.1008734107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dopico XC, et al. 2015. Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat. Commun. 6, 7000 ( 10.1038/ncomms8000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lincoln GA, Clarke IJ, Hut RA, Hazlerigg DG. 2006. Characterizing a mammalian circannual pacemaker. Science 314, 1941–1944. ( 10.1126/science.1132009) [DOI] [PubMed] [Google Scholar]
- 5.Bradshaw WE, Holzapfel CM. 2007. Evolution of animal photoperiodism. Annu. Rev. Ecol. Evol. Syst. 38, 1–25. ( 10.1146/annurev.ecolsys.37.091305.110115) [DOI] [Google Scholar]
- 6.Andersen DM, Keafer BA. 1987. An endogenous annual clock in the toxic marine dinoflagellate (Gonyaulax tamarensis). Nature 325, 616–617. ( 10.1038/325616a0) [DOI] [PubMed] [Google Scholar]
- 7.Paul MJ, Zucker I, Schwartz WJ. 2008. Tracking the seasons: the internal calendars of vertebrates. Phil. Trans. R. Soc. B 363, 341–361. ( 10.1098/rstb.2007.2143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gwinner E. 1996. Circannual clocks in avian reproduction and migration. Ibis 138, 47–63. ( 10.1111/j.1474-919X.1996.tb04312.x) [DOI] [Google Scholar]
- 9.Gwinner E. 1986. Circannual rhythms. Heidelberg, Berlin, Germany: Springer. [Google Scholar]
- 10.Gwinner E, Dittami J. 1990. Endogenous reproductive rhythms in a tropical bird. Science 249, 906–908. ( 10.1126/science.249.4971.906) [DOI] [PubMed] [Google Scholar]
- 11.Kondo N, Sekijima T, Kondo J, Takamatsu N, Tohya K, Ohtsu T. 2006. Circannual control of hibernation by HP complex in the brain. Cell 125, 161–172. ( 10.1016/j.cell.2006.03.017) [DOI] [PubMed] [Google Scholar]
- 12.Visser ME, Caro SP, van Oers K, Schaper SV, Helm B. 2010. Phenology, seasonal timing and circannual rhythms: towards a unified framework. Phil. Trans. R. Soc. B 365, 3113–3127. ( 10.1098/rstb.2010.0111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helm B, Ben-Shlomo R, Sheriff MJ, Hut RA, Foster R, Barnes BM, Dominoni D. 2013. Annual rhythms that underlie phenology: biological time-keeping meets environmental change. Proc. R. Soc. B 280, 20130016 ( 10.1098/rspb.2013.0016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevenson TJ, Ball GF. 2011. Information theory and the neuropeptidergic regulation of seasonal reproduction in mammals and birds. Proc. R. Soc. B 278, 2477–2485. ( 10.1098/rspb.2010.2181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wehr TA. 2001. Photoperiodism in humans and other primates: evidence and implications. J. Biol. Rhythms 16, 348–364. ( 10.1177/074873001129002060) [DOI] [PubMed] [Google Scholar]
- 16.Wulff K, Gatti S, Wettstein JG, Foster RG. 2010. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat. Rev. Neurosci. 11, 589–599. ( 10.1038/nrn2868) [DOI] [PubMed] [Google Scholar]
- 17.Foster RG, Roenneberg T. 2008. Human responses to the geophysical daily, annual and lunar cycles. Curr. Biol. 18, R784–R794. ( 10.1016/j.cub.2008.07.003) [DOI] [PubMed] [Google Scholar]
- 18.IPCC. In Climate change 2014, Impacts, adaptation and vulnerability. Contribution of working group II to the fifth assessment report of the Intergovernmental panel on climate change.
- 19.Visser ME. 2008. Keeping up with a warming world: assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659. ( 10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vedder O, Bouwhuis S, Sheldon BC. 2013. Quantitative assessment of the importance of phenotypic plasticity in adaptation to climate change in wild bird populations. PLoS Biol. 11, 1–10. ( 10.1371/journal.pbio.1001605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gienapp P, Teplitsky C, Alho JS, Mills JA, Merila J. 2008. Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167–178. ( 10.1111/j.1365-294X.2007.03413.x) [DOI] [PubMed] [Google Scholar]
- 22.Visser ME, can Noordwijk AJ, Tinbergen JM. 1998. Warmer spring lead to mistimed reproduction in great tits (Parus major). Proc. R. Soc. Lond. B 265, 1867–1870. ( 10.1098/rspb.1998.0514) [DOI] [Google Scholar]
- 23.Zinsstag J, Schelling E, Waltner-Toews D, Tanner M. 2011. From ‘one medicine’ to ‘one health’ and systemic approaches to health and well-being. Prev. Vet. Med. 101, 148–156. ( 10.1016/j.prevetmed.2010.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coker R, Rushton J, Mounier-Jack S, Karimuribo E, Lutumba P, Kambarage D, Pfeiffer DU, Stärk K, Rweyemamu M. 2011. Towards a conceptual framework to support One Health research for policy on emerging zoonoses. Lancet Infect. Dis. 11, 326–331. ( 10.1016/S1473-3099(10)70312-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes L. 2000. Biological consequences of global warming: is the signal already apparent? Trends Ecol. Evol. 15, 56–61. ( 10.1016/S0169-5347(99)01764-4) [DOI] [PubMed] [Google Scholar]
- 26.Thackeray SJ. et al. 2010. Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Glob. Change Biol. 16, 3304–3313. ( 10.1111/j.1365-2486.2010.02165.x) [DOI] [Google Scholar]
- 27.Miyazaki Y, Nisimura T, Numata H. 2006. Phase responses in the circannual rhythm of the varied carpet beetle, Anthrenus verbasci, under naturally changing day lengths. Zool. Sci. 23, 1031–1037. ( 10.2108/zsj.23.1031) [DOI] [PubMed] [Google Scholar]
- 28.Song YH, Smith RW, To BJ, Millar AJ, Imaizumi T. 2012. FKF1 conveys timing information for CONSTANS stabilization in photoperiodic flowering. Science 336, 1045–1049. ( 10.1126/science.1219644) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eriksson LO, Lundqvist H. 1982. Circannual rhythms and photoperiod regulation of growth and smolting in Baltic salmon (Salmo salar L). Aquaculture 28, 113–121. ( 10.1016/0044-8486(82)90014-X) [DOI] [Google Scholar]
- 30.Helm B, Schwabl I, Gwinner E. 2009. Circannual basis of geographically distinct bird schedules. J. Exp. Biol. 212, 1259–1269. ( 10.1242/jeb.025411) [DOI] [PubMed] [Google Scholar]
- 31.Yasuo S, Yoshimura T. 2009. Comparative analysis of the molecular basis of photoperiodic signal transduction in vertebrates. Integr. Comp. Biol. 49, 507–518. ( 10.1093/icb/icp011) [DOI] [PubMed] [Google Scholar]
- 32.Roenneberg T, Aschoff J. 1990. Annual rhythm of human reproduction. I. Biology, sociology or both? J. Biol. Rhythms 5, 195–216. ( 10.1177/074873049000500303) [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Bakker M, Bakker KM, King AA, Rohani P. 2014. Human birth seasonality: latitudinal gradient and interplay with childhood disease dynamics. Proc. R. Soc. B 281, 20132438 ( 10.1098/rspb.2013.2438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roenneberg T, Aschoff J. 2014. Annual rhythm of human reproduction. II. Environmental correlations. J. Biol. Rhythms 5, 217–239. ( 10.1177/074873049000500304) [DOI] [PubMed] [Google Scholar]
- 35.Wood S, Loudon A. 2014. Clocks for all seasons: unwinding the roles and mechanisms of circadian and interval timers in the hypothalamus and pituitary. J. Endocrinol. 222, R39–R59. ( 10.1530/JOE-14-0141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dardente H, Wyse CA, Birnie MJ, Dupré SM, Loudon ASI, Lincoln GA, Hazlerigg DG. 2010. A molecular switch for photoperiod responsiveness in mammals. Curr. Biol. 20, 2193–2198. ( 10.1016/j.cub.2010.10.048) [DOI] [PubMed] [Google Scholar]
- 37.Ono H, Hoshino Y, Yasuo S, Watanabe M, Nakane Y, Murai A, Ebihara S, Korf H-W, Yoshimura T. 2008. Involvement of thyrotropin in photoperiodic signal transduction in mice. Proc. Natl Acad. Sci. USA 105, 18 238–18 242. ( 10.1073/pnas.0808952105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson RJ. 2004. Seasonal immune function and sickness responses. Trends Immunol. 25, 187–192. ( 10.1016/j.it.2004.02.001) [DOI] [PubMed] [Google Scholar]
- 39.Nelson RJ, Demas GE, Klein SL, Kriegsfeld LJ. 1995. The influence of season, photoperiod, and pineal melatonin on immune function. J. Pineal Res. 19, 149–165. ( 10.1111/j.1600-079X.1995.tb00184.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevenson TJ, Prendergast BJ. 2014. Photoperiodic time measurement and seasonal immunological plasticity. Front. Neuroendol. 37, 76–88. ( 10.1016/j.yfrne.2014.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakane Y, et al. 2013. The saccus vasculosus of fish is a sensor of seasonal changes in day length. Nat. Commun. 4, 2108 ( 10.1038/ncomms3108) [DOI] [PubMed] [Google Scholar]
- 42.Nakao N, et al. 2008. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature 452, 317–322. ( 10.1038/nature06738) [DOI] [PubMed] [Google Scholar]
- 43.O'Brien CS, Bourdo R, Bradshaw WE, Holzapfel CM, Cresko WA. 2012. Conservation of the photoperiodic neuroendocrine axis among vertebrates: evidence from the teleost fish, Gasterosteus aculeatus. Gen. Comp. Endocrinol. 178, 19–27. ( 10.1016/j.ygcen.2012.03.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hazlerigg DG, Lincoln GA. 2011. Hypothesis: cyclical histogenesis is the basis of circannual timing. J. Biol. Rhythms 26, 471–485. ( 10.1177/0748730411420812) [DOI] [PubMed] [Google Scholar]
- 45.Stevenson TJ, Prendergast BJ. 2013. Reversible DNA methylation regulates seasonal photoperiodic time measurement. Proc. Natl Acad. Sci. USA 110, 16 651–16 656. ( 10.1073/pnas.1310643110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Helm B, Stevenson T. 2015. Circannual rhythms: history, present challenges, future directions. In Annual, lunar and tidal clocks: patterns and mechanisms of nature's enigmatic rhythms (eds Numata H, Helm B), pp. 203–225. Tokyo, Japan: Springer. [Google Scholar]
- 47.Martinez-Bakker M, Helm B. 2015. The influence of biological rhythms on host–parasite interactions. Trends Ecol. Evol. 30, 314–326. ( 10.1016/j.tree.2015.03.012) [DOI] [PubMed] [Google Scholar]
- 48.Stevenson TJ, Onishi KG, Bradley SP, Prendergast BJ. 2014. Cell-autonomous iodothyronine deiodinase expression mediates seasonal plasticity in immune function. Brain Behav. Immunol. 36, 61–70. ( 10.1016/j.bbi.2013.10.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borchert R, Renner SS, Calle Z, Navarrete D, Tye A, Gautier L, Spichiger R, von Hildebrand P. 2005. Photoperiodic induction of synchronous flowering near the equator. Nature 433, 627–629. ( 10.1038/nature03259) [DOI] [PubMed] [Google Scholar]
- 50.Brockman DK, van Schaik CP. 2012. Seasonality and reproductive function. In Seasonality in primates: studies of living and extinct human and non-human primates (eds Brockman DK, van Schaik CP), pp. 269–306. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 51.Ashmole NP. 1963. The biology of the Wideawake or sooty tern Sterna fuscata on Ascension Island. Ibis 103, 297–364. ( 10.1111/j.1474-919x.1963.tb06757.x) [DOI] [Google Scholar]
- 52.Poole JH, Moss CJ. 1981. Musth in the African elephant, Loxodonta africana. Nature 292, 830–831. ( 10.1038/292830a0) [DOI] [PubMed] [Google Scholar]
- 53.Bronson FH. 2004. Are humans seasonally photoperiodic? J. Biol. Rhythms 19, 180–192. ( 10.1177/0748730404264658) [DOI] [PubMed] [Google Scholar]
- 54.Huber S, Fieder M, Wallner B, Moser G, Arnold W. 2004. Brief communication: birth month influences reproductive performance in contemporary women. Hum. Reprod. 19, 1081–1082. ( 10.1093/humrep/deh247) [DOI] [PubMed] [Google Scholar]
- 55.Boland MR, Shahn Z, Madigan D, Hripcsak G, Tatonetti NP. 2015. Birth month affects lifetime disease risk: a phenome-wide method. J. Am. Med. Inform. Assoc. 22, 1042–1053. ( 10.1093/jamia/ocv046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seretakis D, Lagiou P, Lipworth L, Signorello LB, Rothman KJ, Tirchopoulos D. 1997. Changing seasonality of mortality from coronary heart disease. J. Am. Med. Assoc. 278, 1012–1014. ( 10.1001/jama.1997.03550120072036) [DOI] [PubMed] [Google Scholar]
- 57.Haberman S, Capildeo R, Rose FC. 1981. The seasonal variation in mortality from cerebrovascular disease. J. Neurol. Sci. 52, 25–36. ( 10.1016/0022-510X(81)90131-3) [DOI] [PubMed] [Google Scholar]
- 58.Mackenbach JP, Kunst AE, Looman CW. 1992. Seasonal variation in mortality in the Netherlands. J. Epidemiol. Community Health 46, 261–265. ( 10.1136/jech.46.3.261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schreiber G, Avissar S, Tzahor Z, Grisaru N. 1991. Rhythms of war. Nature 352, 574–575. ( 10.1038/352574b0) [DOI] [Google Scholar]
- 60.Myrianthefs P, Karatzas S, Venetsanou K, Grouzi E, Evagelopoulou P, Boutzouka E, Fildissis G, Spiliotopoulou I, Baltopoulos G. 2003. Seasonal variation in whole blood cytokine production after LPS stimulation in normal individuals. Cytokine 24, 286–292. ( 10.1016/j.cyto.2003.08.005) [DOI] [PubMed] [Google Scholar]
- 61.Klink M, Bednarska K, Blus E, Kielbik M, Sulowska Z. 2012. Seasonal changes in activities of human neutrophils in vitro. Inflamm. Res. 61, 11–16. ( 10.1007/s00011-011-0382-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore SE, Collinson AC, Fulford AJC, Jalil F, Siegrist C-A, Goldblatt D, Hanson LÅ, Prentice AM. 2006. Effect of month of vaccine administration on antibody responses in Gambia and Pakistan. Trop. Med. Int. Health 11, 1529–1541. ( 10.1111/j.1365-3156.2006.01700.x) [DOI] [PubMed] [Google Scholar]
- 63.Haldar C, Ahmad R. 2010. Photoimmunomodulation and melatonin. J. Photochem. Photobiol. B Biol. 98, 107–117. ( 10.1016/j.jphotobiol.2009.11.014) [DOI] [PubMed] [Google Scholar]
- 64.Medlock JM, Leach SA. 2015. Effect of climate change on vector-borne disease risk in the UK. The Lancet Infect. Dis. 15, 721–730. ( 10.1016/S1473-3099(15)70091-5) [DOI] [PubMed] [Google Scholar]
- 65.Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P. 2006. Seasonality and the dynamics of infectious diseases. Ecol. Lett. 9, 467–484. ( 10.1111/j.1461-0248.2005.00879.x) [DOI] [PubMed] [Google Scholar]
- 66.Grassly NC, Fraser C. 2006. Seasonal infectious disease epidemiology. Proc. R. Soc. B 273, 2541–2550. ( 10.1098/rspb.2006.3604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shaman J, Pitzer VE, Viboud C, Grenfell BT, Lipsitch M. 2010. Absolute humidity and the seasonal onset of influenza in the continental United States. PLoS Biol. 8, e1000316 ( 10.1371/journal.pbio.1000316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pascual M, Rodo X, Ellner SP, Colwell R, Bouma MJ. 2000. Cholera dynamics and El Nino-Southern oscillation. Science 289, 1766–1769. ( 10.1126/science.289.5485.1766) [DOI] [PubMed] [Google Scholar]
- 69.Clements A. 1999. The biology of mosquitoes: sensory reception and behaviour. London, UK: Chapman and Hall. [Google Scholar]
- 70.Russell TL, Lwetoijera DW, Knols BGJ, Takken W, Killeen GF, Ferguson HM. 2011. Linking individual phenotype to density-dependent population growth: the influence of body size on the population dynamics of malaria vectors. Proc. R. Soc. B 278, 3142–3151. ( 10.1098/rspb.2011.0153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parham PE, Michael E. 2010. Modelling climate change and malaria transmission. Adv. Exp. Med. Biol. 673, 184–199. ( 10.1007/978-1-4419-6064-1_13) [DOI] [PubMed] [Google Scholar]
- 72.Morin CW, Comrie AC, Ernst K. 2013. Climate and dengue transmission: evidence and implications. Environ. Health Perspect. 121, 264–1272. ( 10.1289/ehp.1307303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Canto T, Aranda MA, Ferres A. 2009. Climate change effects on physiology and population processes of hosts and vectors that influence the spread of hemipteran-borne plant viruses. Glob. Change Biol. 15, 1884–1894. ( 10.1111/j.1365-2486.2008.01820.x) [DOI] [Google Scholar]
- 74.Kilpatrick AM, Randolph SE. 2012. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet 380, 1946–1955. ( 10.1016/S0140-6736(12)61151-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Searle KR, Blackwell A, Falconer D, Sullivan M, Butler A, Purse BV. 2013. Identifying environmental drivers of insect phenology across space and time: Culicoides in Scotland as a case study. Bull. Entomol. Res. 103, 155–170. ( 10.1017/S0007485312000466) [DOI] [PubMed] [Google Scholar]
- 76.Pérez de León AA, Teel PD, Auclair AN, Messenger MT, Guerrero FD, Schuster G, Miller RJ. 2012. Integrated strategy for sustainable cattle fever tick eradication in USA is required to mitigate the impact of global change. Front. Physiol. 3, 195 ( 10.3389/fphys.2012.00195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. 2006. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behaviour. PLoS Biol. 4, 606–610. ( 10.1371/journal.pbio.0040082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Estrada-Peña A, Ayllón N, de la Fuente J. 2012. Impact of climate trends on tick-borne pathogen transmission. Front. Physiol. 3, 1–12. ( 10.3389/fphys.2012.00064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ogden NH, Radojevic M, Wu X, Duvvuri VR, Leighton PA, Wu J. 2014. Estimated effects of projected climate change on the basic reproductive number of the Lyme disease vector Ixodes scapularis. Environ. Health Perspect. 122, 631–638. ( 10.1289/ehp.1307799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garamszegi LZ. 2011. Climate change increases the risk of malaria in birds. Glob. Change Biol. 17, 1751–1759. ( 10.1111/j.1365-2486.2010.02346.x) [DOI] [Google Scholar]
- 81.Mills LS, Zimova M, Oyler J, Running S, Abatzoglou JT, Lukacs PM. 2013. Camouflage mismatch in seasonal coat color due to decreased snow duration. Proc. Natl Acad. Sci. USA 110, 7360–7365. ( 10.1073/pnas.1222724110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klein AM, Vaissiere BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T. 2007. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 274, 303–313. ( 10.1098/rspb.2006.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Polce C, et al. 2014. Climate-driven spatial mismatches between British orchards and their pollinators: increased risks of pollination deficits. Glob. Change Biol. 20, 2815–2828. ( 10.1111/gcb.12577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brittain C, Kremen C, Klein AM. 2013. Biodiversity buffers pollination from changes in environmental conditions. Glob. Change Biol. 19, 540–547. ( 10.1111/gcb.12043) [DOI] [PubMed] [Google Scholar]
- 85.Garibaldi LA, et al. 2013. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339, 1608–1611. ( 10.1126/science.1230200) [DOI] [PubMed] [Google Scholar]
- 86.Rader R, Reilly J, Bartomeus I, Winfree R. 2013. Native bees buffer the negative impact of climate warming on honey bee pollination of watermelon crops. Glob. Change Biol. 19, 3103–3110. ( 10.1111/gcb.12264) [DOI] [PubMed] [Google Scholar]
- 87.Durant JM, Anker-Nilssen T, Stenseth NC. 2003. Trophic interactions under climate fluctuations: the Atlantic puffin as an example. Proc. R. Soc. Lond. B 270, 1461–1466. ( 10.1098/rspb.2003.2397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Visser ME, Holleman LJM. 2001. Warmer springs disrupt the synchrony of oak and winter moth phenology. Proc. R. Soc. Lond. B 268, 289–294. ( 10.1098/rspb.2000.1363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Post E, Forchhammer MC. 2008. Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Phil. Trans. R. Soc. B 363, 2369–2375. ( 10.1098/rstb.2007.2207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Plard F, Gaillard J-M, Coulson T, Hewison AJM, Delorme D, Warnant C, Bonenfant C. 2014. Mismatch between birth date and vegetation phenology slows the demography of roe deer. PLoS Biol. 12, e1001828 ( 10.1371/journal.pbio.1001828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reed TE, Grotan V, Jenouvrier S, Saether B-E, Visser ME. 2013. Population growth in a wild bird is buffered against phenological mismatch. Science 340, 488–491. ( 10.1126/science.1232870) [DOI] [PubMed] [Google Scholar]
- 92.Husby A, Visser ME, Kruuk LE. 2011. Speeding up microevolution: the effects of increasing temperature on selection and genetic variance in a wild bird population. PLoS Biol. 9, e1000585 ( 10.1371/journal.pbio.1000585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thomas DW. 2001. Energetic and fitness costs of mismatching resource supply and demand in seasonally breeding birds. Science 291, 2598–2600. ( 10.1126/science.1057487) [DOI] [PubMed] [Google Scholar]
- 94.Reed TE, Jenouvrier S, Visser ME. 2013. Phenological mismatch strongly affects individual fitness but not population demography in a woodland passerine. J. Anim. Ecol. 82, 131–144. ( 10.1111/j.1365-2656.2012.02020.x) [DOI] [PubMed] [Google Scholar]
- 95.Visser ME, Holleman LJM, Gienapp P. 2006. Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147, 164–172. ( 10.1007/s00442-005-0299-6) [DOI] [PubMed] [Google Scholar]
- 96.van Noordwijk AJ, McCleery RH, Perrins CM. 1995. Selection of timing of great tit (Parus major) breeding in relation to caterpillar growth and temperature. J. Anim. Ecol. 64, 451–458. ( 10.2307/5648) [DOI] [Google Scholar]
- 97.Visser ME, Both C. 2005. Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. B 272, 2561–2569. ( 10.1098/rspb.2005.3356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Memmott J, Craze PG, Waser NM, Price MV. 2007. Global warming and the disruption of plant–pollinator interactions. Ecol. Lett. 10, 710–717. ( 10.1111/j.1461-0248.2007.01061.x) [DOI] [PubMed] [Google Scholar]
- 99.Both C, van Asch M, Bijlsma RG, van den Burg AB, Visser ME. 2009. Climate change and unequal phenological changes across four trophic levels: constraints or adaptations? J. Anim. Ecol. 78, 73–83. ( 10.1111/j.1365-2656.2008.01458.x) [DOI] [PubMed] [Google Scholar]
- 100.Bartomeus I, Ascher JS, Wagner DL, Danforth BN, Colla S, Kornbluth S, Winfree R. 2011. Climate-associated phenology advances in bee pollinators and bee-pollinated plants. Proc. Natl Acad. Sci. USA 108, 20645–20649. ( 10.1073/pnas.1115559108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bartomeus I, Ascher JS, Gibbs J, Danforth BN, Wagner DL, Hedtke SM, Winfree R. 2013. Historical changes in northeastern US bee pollinators related to shared ecological traits. Proc. Natl Acad. Sci. USA 110, 4656–4660. ( 10.1073/pnas.1218503110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Burkle LA, Marlin JC, Knight TM. 2013. Plant–pollinator interactions over 120 years: loss of species, co-occurrence and function. Science 339, 1611–1615. ( 10.1126/science.1232728) [DOI] [PubMed] [Google Scholar]
- 103.Bronson FH. 2009. Climate change and seasonal reproduction in mammals. Phil. Trans. R. Soc. B 364, 3331–3340. ( 10.1098/rstb.2009.0140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gienapp P, Reed TE, Visser ME. 2014. Why climate change will invariably alter selection pressures on phenology. Proc. R. Soc. B 281, 1793 ( 10.1098/rspb.2014.1611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gallai N, Salles JM, Settele J, Vaissière BE. 2009. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 68, 810–821. ( 10.1016/j.ecolecon.2008.06.014) [DOI] [Google Scholar]
- 106.Bale JS, Hayward SAL. 2010. Insect overwintering in a changing climate. J. Exp. Biol. 213, 980–994. ( 10.1242/jeb.037911) [DOI] [PubMed] [Google Scholar]
- 107.Gregory PJ, Johnson SN, Newton AC, Ingram JSI. 2009. Integrating pests and pathogens into the climate change/food security debate. J. Exp. Bot. 60, 2827–2838. ( 10.1093/jxb/erp080) [DOI] [PubMed] [Google Scholar]
- 108.Berry WD. 2003. The physiology of induced molting. Poult. Sci. 82, 971–980. ( 10.1093/ps/82.6.971) [DOI] [PubMed] [Google Scholar]
- 109.Seldin MM, Byerly MS, Petersen PS, Swanson R. Balkema-Buschmann A, Groschup MH, Wong GW. 2014. Seasonal oscillation of liver-derived hibernation protein complex in the central nervous system of non-hibernating mammals. J. Exp. Biol. 217, 2667–2679. ( 10.1242/jeb.095976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kessler J. 2004. Selen-Vitamin-E-Versorgung überwachen. Forum Kleinwiederkäuer 11, 6–9. [Google Scholar]
- 111.Beck MA, Levander OA, Handy J. 2003. Selenium deficiency and viral infection. J. Nutr. 133, 1463S–1467S. [DOI] [PubMed] [Google Scholar]
- 112.Li XM, et al. 2013. A circadian clock transcription model for the personalization of cancer chronotherapy. Cancer Res. 73, 7176–7188. ( 10.1158/0008-5472.CAN-13-1528) [DOI] [PubMed] [Google Scholar]
- 113.Ebling FJ. 2014. On the value of seasonal mammals for identifying mechanisms underlying the control of food intake and body weight. Horm. Behav. 66, 56–65. ( 10.1016/j.yhbeh.2014.03.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sejnowski TJ, Churchland PS, Movshon JA. 2014. Putting big data to good use in neuroscience. Nat. Neurosci. 17, 1440–1441. ( 10.1038/nn.3839) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The review does not contain new data; all work was obtained from previously published material.