Abstract
Seagrasses are among the Earth's most efficient and long-term carbon sinks, but coastal development threatens this capacity. We report new evidence that disturbance to seagrass ecosystems causes release of ancient carbon. In a seagrass ecosystem that had been disturbed 50 years ago, we found that soil carbon stocks declined by 72%, which, according to radiocarbon dating, had taken hundreds to thousands of years to accumulate. Disturbed soils harboured different benthic bacterial communities (according to 16S rRNA sequence analysis), with higher proportions of aerobic heterotrophs compared with undisturbed. Fingerprinting of the carbon (via stable isotopes) suggested that the contribution of autochthonous carbon (carbon produced through plant primary production) to the soil carbon pool was less in disturbed areas compared with seagrass and recovered areas. Seagrass areas that had recovered from disturbance had slightly lower (35%) carbon levels than undisturbed, but more than twice as much as the disturbed areas, which is encouraging for restoration efforts. Slow rates of seagrass recovery imply the need to transplant seagrass, rather than waiting for recovery via natural processes. This study empirically demonstrates that disturbance to seagrass ecosystems can cause release of ancient carbon, with potentially major global warming consequences.
Keywords: seagrass, blue carbon, disturbance, bacteria, carbon sink, restoration
1. Background
Seagrass ecosystems are among the most effective carbon sinks on the Earth; they bury organic carbon (often referred to as ‘blue carbon’) into the seabed at a rate 35 times faster than tropical rainforests [1], and where rainforests bury carbon for decades, seagrasses are capable of storing carbon for millennia [2–4]. However, there is concern that if seagrass ecosystems are disturbed they could leak vast amounts of stored carbon back into the atmosphere, thereby shifting them from carbon sinks into carbon sources [5,6], as has been shown for high-profile terrestrial carbon sinks such as forests, peatlands and permafrost [7–9]. Importantly, the latter studies show that the rate of carbon loss is much greater than the rate of accumulation.
While loss of stored carbon has been demonstrated for other coastal vegetated habitats, such as saltmarshes and mangroves [10–12], there is still major uncertainty regarding whether seagrass ecosystems release soil carbon following disturbance [1]. Fourqurean et al. [13], who provided the first comprehensive survey of seagrass carbon stocks, estimated that present rates of decline in the aerial extent of seagrass ecosystems could result in the release of up to 299 Tg carbon per year, assuming all of the organic carbon in seagrass biomass and the top metre of seagrass soils is remineralized (broken down and released as CO2). However, this assumption—that the entire top metre of organic soil will be remineralized following seagrass loss—has lacked empirical support, creating uncertainty in the influence of seagrasses in global carbon budgets.
One study that recently investigated the impacts of small-scale disturbances (mimicking anchor damage) on seagrass carbon stocks reported no effect [14], and suggested that larger-scale disturbances and longer-time periods (greater than 2 years) might be needed to detect significance losses of seagrass carbon stocks. Larger-scale disturbances and longer time periods would increase the potential for physical removal of carbon (via scouring and erosion) and would reduce the possibility of carbon losses being masked by carbon supply from the surrounding meadow [14]. As for carbon losses via microbial remineralization, it would be expected that large-scale disturbances would increase vertical mixing at the soil–water interface, thereby increasing the input and spread of oxygen into the soil surface, increasing the potential for aerobic heterotrophs—which break down carbon much more efficiently than anaerobes while using O2 as the electron acceptor [15,16]. Defining how bacteria change in response to disturbance can therefore provide important insight into the factors that underpin carbon release via the process of remineralization.
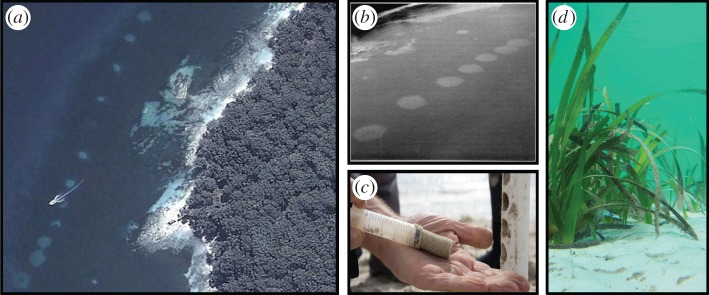
Finding study sites that have sustained long-term and well-defined losses of seagrass is a challenge. This study focuses on seagrass (Posidonia australis) losses that occurred in Jervis Bay on the east-coast of NSW, Australia (35°07′52.96″ S; 150°44′50.22″ E), a site containing one of the oldest documented records of seagrass disturbance [17], which occurred as a result of seismic testing within the seagrass beds during the 1960s to test the suitability of the site for a nuclear facility. The seismic testing resulted in 11 large (10–30 m diameter, water depth 4–6 m) circular holes that remain to this day as bare sand (figure 1a–d). Partial recovery has occurred via rhizome encroachment from the surrounding meadow, but the process is very slow. Mean spreading rate was estimated to be 21(±2) cm per year over a 25 year period, with recovery not expected until 2034 at the earliest and 2071 at the latest [17].
Figure 1.
Sampling the effects of disturbance on soil organic carbon stocks in a seagrass ecosystem. Aerial photograph of the southeastern shoreline of Jervis Bay (NSW, Australia) in 2014 showing ‘halos’ of bare sand (disturbed) within an otherwise continuous, undisturbed meadow of Posidonia australis seagrass in (a) 2014 and (b) 1970; (c) subcoring of PVC soil cores using an open-faced syringe; and (d) underwater image of the seagrass edge. Image credits in order (a–d): Google Earth; NSW Fisheries; Sarah Hoyt and Peter Macreadie, respectively. (Online version in colour.)
The goal of this study was to investigate the impact of disturbance on the organic carbon stocks of P. australis seagrass at Jervis Bay. Specifically, we tested the following main hypotheses: (i) soil organic carbon (SOC) stocks will be lower in seagrass areas that have undergone disturbance; (ii) the rate of SOC loss will be much greater than the rate of SOC accumulation; and (iii) disturbance will cause shifts in bacterial abundance and community composition within seagrass meadows.
2. Material and methods
During February 2013, soil samples were taken from three habitats in four different seismic testing holes: ‘disturbed’ soils within the middle of the circular holes that were barren of any seagrass; ‘recovered’ soils where seagrass had recently (during the past approx. 5–10 years as determined from Google Earth historical imagery—see below) recovered from disturbance via rhizome encroachment; and ‘undisturbed’ seagrass soils were treated as controls. The location of the holes and rates of recovery had been determined and mapped using GIS by Meehan & West [17] from aerial photographs for the years 1972–1997. We used this data to determine the original size and location of the disturbed areas, and then we used Google Earth historical images (a total of eight images were available spanning 2004–2013) to locate recovered areas. The latter was cross-referenced against recovery rate estimates (21 ± 2 cm per year) by Meehan & West [17]. Holes were easily visualized from the water surface due to the clear water and divers checked the exact positioning of the core entry point at the time of core insertion.
For radiocarbon dating, deep cores (up to 1 m) were collected using a vibracorer as described in Macreadie et al. [2]. For geochemical analyses, soils were collected using push cores, which involved hammering open-barrel PVC pipes (200 cm length, 5 cm internal diameter) into soils with a fence post driver until refusal was reached. Expanding rubber plugs were then inserted into the top end of the PVC pipes to achieve internal suction while the cores were pulled out of the soil and returned to the boat. Next, throughout the length of the core, 15.7 cm3 subsamples at 2 cm intervals were taken by inserting subcorers (fashioned by cutting the end off of a 20 cc, 2 cm internal diameter syringe) into sampling ports along the PVC pipe that had been pre-drilled and taped over (figure 1c).
In the laboratory, soils were dried at 50°C and weighed to determine dry bulk density (DBD; Mg m−3), calculated by dividing the mass of the dried soil by sample volume. SOC concentration and the stable isotope composition of the SOC (reported as δ13C) were measured on a Europa Scientific 20–20 IRMS linked to ANCA SL Prep Unit, with 29 internal standards and five certified protein standards (IsoEnvironmental cc. South Africa). Owing to the presence of carbonates, samples destined for carbon analysis were decalcified (acidified) prior to analysis. The decalcification procedure, which was repeated twice for each sample, involved addition of 2N HCl to soils, followed by repeated washing in deionized water. Inorganic carbon concentration was estimated as 0.12 × mass loss (%) during acidification. 14C concentration and/or radiocarbon age was determined via accelerator mass spectroscopy (AMS; electronic supplementary material). 14C concentration is reported as per cent modern carbon (pMC) and ages as conventional radiocarbon age (yr BP).
Bacterial samples were taken adjacent to where cores had been extracted by inserting 3 ml cut-off syringes into the soil to a depth of 2 cm. Soil was extruded from syringes and transferred into 2.0 ml cryotubes (Sarstedt AG & Co.), refrigerated while being transferred to the laboratory and then flash frozen in liquid nitrogen and stored at −80°C. For flow cytometry samples, 1 ml of 0.22 µm-filtered seawater containing glutaraldehyde (1% final conc.) was added before freezing.
Determination of bacterial abundance was performed via flow cytometry following the methodology of Trevathan-Tackett et al. [18]. Cell counts were quantified using an LSRII flow cytometer (BD Biosciences). Bacteria were discriminated according to SYBR Green fluorescence (nucleic acid content) and light side scatter [19,20]. Cell concentrations were calculated using Flowing Software v. 2.5 (www.flowingsoftware.com). Nucleic acid contents of individual bacteria were used to discriminate the fraction of putative active cells from the fraction of inactive (dormant) cells according to Lebaron et al. [21]. In brief, this was done by separating bacteria with high apparent nucleic acid content from those with low nucleic acid contents.
DNA sequencing was performed on the Illumina MiSeq platform and processed via the QIIME package (electronic supplementary material). Operational taxonomic units (OTUs) were compared against the Greengenes database using BLAST to assign taxonomy (electronic supplementary material). Metagenomes were also predicted for the bacterial samples using the PICRUSt package (electronic supplementary material).
In order to calculate unbiased estimates of SOC stocks across cores of differing depths, we adopted an equivalent mass approach often used in terrestrial soil carbon studies [22]. Stocks are reported to the median cumulative soil mass (250 kg m−2). For cores with mass >250 kg m−2, SOC stocks per depth interval were summed up to and including the depth that equalled this value. For cores with mass <250 kg m−2, additional SOC was ‘added’ to the bottom of the core using the mean SOC of the previous three depth intervals until a mass of 250 kg m−2 was reached. For statistical comparisons, δ13C values are reported as SOC weighted mean values for each core.
Geochemical and flow cytometry data were analysed using a factorial one-way blocked analysis of variance (ANOVA), which accounts for the spatial variation in response variables between holes, to compare the effects of habitat type (disturbed, recovered and undisturbed) on each response variable. Each ‘block’ consisted of a hole (disturbed), edge (recovered) and neighbouring pristine seagrass (undisturbed). Prior to analysis, assumptions of homogeneity of variance and normality were checked using Levene's test and graphical inspection of data, with natural log transformations made where necessary to improve data fitness. Tukey's post hoc analysis was used to compare means when significant differences were detected by ANOVA.
Bacterial rarefied OTU abundance data were square-root transformed and Bray–Curtis similarity between profiles was ordinated using multidimensional scaling (MDS) using Primer 6 [23]. Analysis of similarities (ANOSIM) was applied to determine if differences in treatments were significantly different from a randomly permutated distribution. Similarity percentage analysis (SIMPER), with 90% cut-off for low contributions, was used to identify the OTUs driving the shift between the clusters observed in the MDS plot. The predicted PICRUSt metagenome functions were analysed using the statistical analysis of metagenomic profiles (STAMP) program [24]. All STAMP analyses were performed with a two-sided Welch's t-test and Bonferroni correction to minimize Type I errors.
3. Results
We found no significant differences in mean core depth, DBD, soil mass or carbonate content among the disturbed, recovered and undisturbed habitats (electronic supplementary material, figure S1). However, stocks of SOC to an equivalent mass varied nearly threefold between disturbed and undisturbed habitats with the recovered habitats having an intermediate value (figure 2a). The difference in SOC stocks was primarily a result of significant differences in the concentration of SOC between habitats (F = 7.57, p = 0.02). The stable carbon isotope composition also varied significantly across habitats (F = 5.44; p = 0.044), with the disturbed sites having significantly more negative δ13C values than the undisturbed habitats (figure 2b). For each core, there is a sequential progression in age down the core (electronic supplementary material, table S1). Owing to limited 14C data, it is not possible to confidently determine the depth of disturbance; however, based on calculated soil accumulation rates (electronic supplementary material, Methods/Results) and a palaeoreconstruction (incl. lithological and geophysical analyses) of long cores taken from the site [25], it was estimated that disturbance probably affected the top 90–100 cm of soil—a period covering approximately 6000 calendar years before present (cal. yr BP). Our SOC analyses were restricted to the top 30 cm, which represented the past approximately 1300–3000 cal. yr BP (electronic supplementary material, table S1; [25]).
Figure 2.
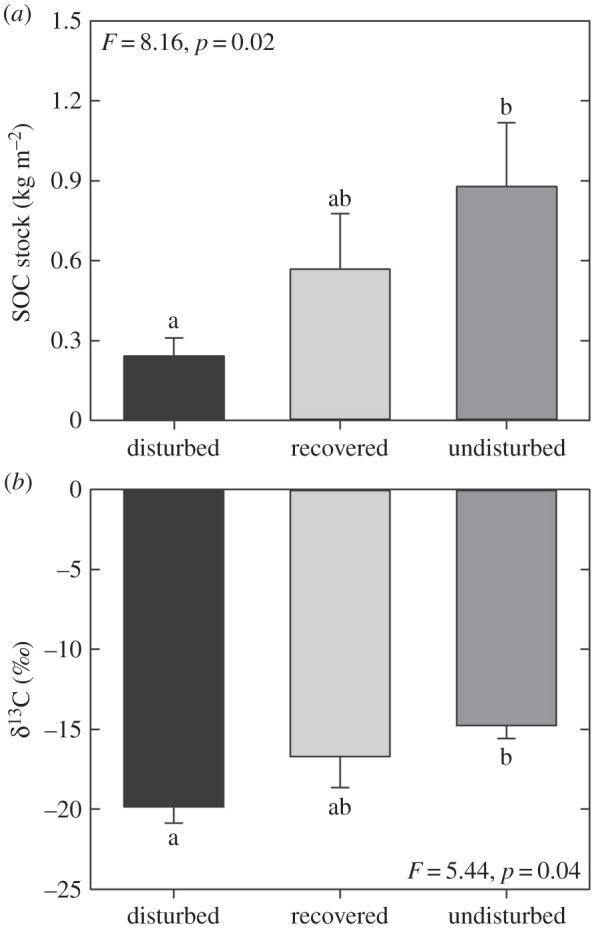
Impacts of disturbance on soil carbon. (a) Soil organic carbon stock (SOC; mean ± s.e.) and (b) carbon isotope (δ13C) values in taken from disturbed, recovered and undisturbed soils (top approx. 0–30 cm). Means with the same letters are not significantly different.
According to flow cytometry analysis, there was no difference in bacterial abundance among sites for total (F2,14 = 0.229; p = 0.801), or high- (F2,14 = 0.509; p = 0.619) or low-DNA content bacterial populations (F2,14 = 0.232; p = 0.798). ANOSIM analysis of the 16S rRNA sequence data revealed significantly different bacterial community compositions among the three site (R = 0.728, p = 0.001). The MDS (figure 3) further illustrates clustering of microbial communities among sites at the OTU level, from bare to edge to seagrass. SIMPER analyses revealed that at both the class and genus level there were multiple groups causing separation in the data, but some clear ecological trends were apparent (figure 4; electronic supplementary material, table S2).
Figure 3.
Shifts in seagrass microbial community composition in response to disturbance. MDS plot showing clustering of bacterial operational taxonomic units (OTUs) across disturbed, recovered and undisturbed soils.
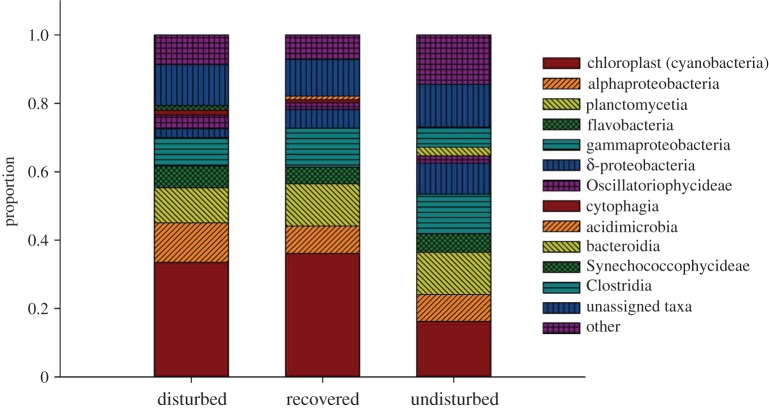
Figure 4.
Identities of bacterial communities across habitat types. Average relative abundance of dominant bacterial classes in disturbed, recovered and undisturbed soils. Top 10 most abundant classes shown. The ‘other’ category cumulatively represents less abundant taxa (less than 2%). (Online version in colour.)
At the bacterial class level, there was a trend of increasing importance of δ-proteobacteria and Clostridium from the bare to edge to seagrass meadow samples. There was an opposing trend of increasing Oscillatoriophycideae (cyanobacteria) and chloroplasts from the seagrass to the bare soil. These patterns were also adhered to at the genus level and it became apparent that the patterns in δ-proteobacteria were driven by an increase in the sulfate reducing genus Desulfococcus, while the patterns in Clostridia were driven by Clostridium and Fusibacter genera. The chloroplasts matched stramenopile chloroplasts, indicating an increase in eukaryotic algae, such as diatoms, in the non-seagrass soil. These data were supported by the greater abundances of energy metabolism genes involved in photosynthesis in the disturbed soils compared with undisturbed soils (level 3 KEGG pathways; electronic supplementary material, table S2). As predicted, the edge samples form an intermediate between bare and seagrass states, suggesting that seagrass recovery from disturbance causes a shift in the microbial community composition making them more similar to the seagrass state, but this transition is not complete.
4. Discussion
This study provides empirical evidence of the impact of disturbance on SOC stocks bound by seagrass meadows, as demonstrated by disturbed areas of seagrass having significantly lower SOC levels (72% less) than undisturbed controls, and an apparent decline in the proportion of autochthonous (plant-derived) carbon within their SOC pool (according to stable isotopes). Disturbance affected soils in the top 1 m, which, according to 14C analyses, had taken up to 6000 years to accumulate. Disturbed soils had high proportions of aerobic heterotrophs relative to undisturbed seagrass, indicative of a fundamental shift in the biogeochemical nature of the two soil types.
The recovered treatment, which represents seagrass regrowth, is promising for seagrass restoration prospects because it indicates that natural recovery of seagrass can lead to recovery of SOC stocks. Greiner et al. [26] provided evidence for the potential for seagrass habitat restoration to enhance SOC sequestration, but this was for manually transplanted seagrass, whereas little is known about natural recovery. The clonal nature of seagrasses [27] means that if the original meadow remains viable, and the system has not shifted towards an alternative stable state [28,29], then there is exponential potential for recovery of SOC stocks through recovery of autochthonous (carbon produced through photosynthesis; plant-derived) and allochthonous (carbon captured from external sources—e.g. terrestrial run-off, seston) SOC accumulation capacity [5,30], and through capping emissions and restoring anoxic conditions in disturbed areas [31].
Our findings suggest that recovery through clonal growth during the past approximately 5–10 years has led to partial recovery of SOC stocks; SOC stocks in recovered were lower (35%) than undisturbed, but this difference was not significant, whereas disturbed were significantly lower (72%) than undisturbed. Thus, approximately half of the carbon lost was recovered within 5–10 years, which is promising for restoration efforts as it implies that revegetation projects can restore seagrass SOC stocks in relatively short time frames. Supporting this conclusion is a recent study by Marba et al. [32] who reported that carbon burial rates of restored P. australis meadows within Oyster Harbour (Western Australia) were similar to continuously vegetated meadows 18 years after planting. Clonal growth for P. australis in Jervis Bay is very slow (2.5 cm per annum) [33], as it is for other members of the Posidonia genus (e.g. P. oceanica, 2.3 cm per annum) [34], so we would expect that the capacity for recovery of Posidonia SOC stocks to represent among the ‘worst case’ scenarios for seagrass ecosystems.
While no significant differences in the total abundance of soil bacteria were observed between the three treatments, 16S rRNA sequencing and predicted metagenomes revealed a change in the composition of the community between the habitat types. The bacterial community in the disturbed soil was significantly different to the undisturbed soil, with the recovered soil representing an intermediate environment. These community differences were driven by shifts among several organisms, but there were trends in the relative abundance of bacterial groups that may be reflective of changes in the biogeochemical nature and oxic conditions within the habitats. Undisturbed had a higher relative proportion of the sulfate reducing δ-proteobacteria Desulfococcus and obligate anaerobic bacteria belonging to Clostridia. This, in addition to the increase in some aerobic heterotrophic groups of marine bacteria (e.g. Pseudoalteromonas) in the disturbed and recovered environments, is potentially indicative of higher levels of available oxygen within the disturbed soils than within the undisturbed soils. The increase in the δ-proteobacteria, in particular Desulfococcus, in the undisturbed soils is also consistent with previous observations of the bacterial communities inhabiting Posidonia soils, where Desulfococcus has been shown to be highly abundant, with the δ-proteobacteria representing the dominant class of identified bacteria [35]. A higher proportion of sequences matching cyanobacteria and chloroplasts (stramenopiles) and of genes involved in photosynthetic energy metabolism in the disturbed, relative to the undisturbed, is indicative of higher levels of microbial photoautotrophy in the non-seagrass environments, which is probably related to the differences in shading effects between the seagrass and non-seagrass soils. These shifts suggest an overall change in the composition of the microbial community between the undisturbed and disturbed habitats, and may underpin significant differences in the metabolic capacity of these communities and their biogeochemical roles.
In conclusion, we find that the legacy of disturbance caused to P. australis seagrass in Jervis Bay is still evident after 50 years. Areas that remain bare due to disturbance have 72% less SOC than the surrounding undisturbed seagrass areas, which is remarkably close to Pendleton's [36] central estimate of a 63% loss. Shifts in bacterial consortia from undisturbed, recovered, and disturbed are perhaps indicative of significant differences in the biogeochemical state and/or oxic conditions in each habitat type, with a higher relative abundance of aerobic heterotrophs in disturbed areas, and more sulfate reducing bacteria and obligate anaerobic bacteria in undisturbed. This study provides among the first empirical evidence that loss of seagrass habitat through human activities can have substantial impacts on SOC stocks, providing further evidence for the need to protect globally declining seagrass populations. This study was made possible because of a peculiar event; seismic testing. Because the event was well documented, and recovery is still incomplete, it was an ideal case study for testing the effects of long-term disturbance on seagrass SOC stocks. However, we acknowledge that there are many causes of seagrass disturbance, and seismic testing—although it occurs in seagrass meadows around the world—is considered rare. Hence, the generality of our findings needs to be considered within this specific disturbance context. As more studies emerge on the impacts of seagrass disturbance on SOC stocks and associated biogeochemical processes, we will better understand if the findings here are typical of other types of disturbances. Overall, the findings of this study, as well as other recent work on the carbon-sink capacity of restored seagrass meadows [30,32,37], provide promising evidence that restoration of seagrass meadows represents an important strategy for offsetting carbon emissions and thereby mitigating climate change.
Supplementary Material
Acknowledgements
We thank Booderee National Park Managers and the people of the Wreck Bay Aboriginal Community for access to the field site. Seagrass removal during coring was carried out under Department of Primary Industries permit P12/0020 and OUT12/12537.
Authors' contributions
All authors contributed to the work presented in this paper.
Competing interests
The authors declare no competing financial interests.
Funding
This study was financially supported by an Australian Research Council DECRA Fellowship (DE130101084) to P.I.M. and an Australian Institute for Nuclear Science and Engineering (AINSE) grant (ALNGRA14031) to P.I.M. J.S. was supported by Funding from CSIRO Oceans and Atmosphere.
References
- 1.McLeod E, Chmura GL, Bouillon S, Salm R, Bjork M, Duarte CM, Lovelock CE, Schlesinger WH, Silliman BR. 2011. A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 9, 552–560. ( 10.1890/110004) [DOI] [Google Scholar]
- 2.Macreadie PI, Allen K, Kelaher BP, Ralph PJ, Skilbeck CG. 2012. Paleoreconstruction of estuarine sediments reveal human-induced weakening of coastal carbon sinks. Glob. Change Biol. 18, 891–901. ( 10.1111/j.1365-2486.2011.02582.x) [DOI] [Google Scholar]
- 3.Mateo MA, Romero J, Perez M, Littler MM, Littler DS. 1997. Dynamics of millenary organic deposits resulting from the growth of the Mediterranean seagrass Posidonia oceanica. Estuar. Coast. Shelf Sci. 44, 103–110. ( 10.1006/ecss.1996.0116) [DOI] [Google Scholar]
- 4.Serrano O, Mateo MA, Renom P, Julia R. 2012. Characterization of soils beneath a Posidonia oceanica meadow. Geoderma 185, 26–36. ( 10.1016/j.geoderma.2012.03.020) [DOI] [Google Scholar]
- 5.Macreadie PI, Baird ME, Trevathan-Tackett SM, Larkum AWD, Ralph PJ. 2014. Quantifying and modelling the carbon sequestration capacity of seagrass meadows: a critical assessment. Mar. Pollut. Bull. 83, 430–439. ( 10.1016/j.marpolbul.2013.07.038) [DOI] [PubMed] [Google Scholar]
- 6.Duarte CM, Losada IJ, Hendriks IE, Mazarrasa I, Marba N. 2013. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Change 3, 961–968. ( 10.1038/nclimate1970) [DOI] [Google Scholar]
- 7.Wei XR, Shao MG, Gale W, Li LH. 2014. Global pattern of soil carbon losses due to the conversion of forests to agricultural land. Sci. Rep. 4, 4062 ( 10.1038/srep04062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rooney RC, Bayley SE, Schindler DW. 2012. Oil sands mining and reclamation cause massive loss of peatland and stored carbon. Proc. Natl Acad. Sci. USA 109, 4933–4937. ( 10.1073/pnas.1117693108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang GX, Li YS, Wang YB, Wu QB. 2008. Effects of permafrost thawing on vegetation and soil carbon pool losses on the Qinghai-Tibet Plateau, China. Geoderma 143, 143–152. ( 10.1016/j.geoderma.2007.10.023) [DOI] [Google Scholar]
- 10.Macreadie PI, Hughes AR, Kimbro DL. 2013. Loss of ‘blue carbon’ from coastal salt marshes following habitat disturbance. PLoS ONE 8, 1–8. ( 10.1371/journal.pone.0069244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Couwenberg J, Dommain R, Joosten H. 2010. Greenhouse gas fluxes from tropical peatlands in south-east Asia. Glob. Change Biol. 16, 1715–1732. ( 10.1111/j.1365-2486.2009.02016.x) [DOI] [Google Scholar]
- 12.Donato DC, Kauffman JB, Murdiyarso D, Kurnianto S, Stidham M, Kanninen M. 2011. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 4, 293–297. ( 10.1038/ngeo1123) [DOI] [Google Scholar]
- 13.Fourqurean JW, et al. 2012. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 5, 505–509. ( 10.1038/ngeo1477) [DOI] [Google Scholar]
- 14.Macreadie PI, York PH, Sherman CDH, Keough MJ, Ross DJ, Ricart AM, Smith TM. 2014. No detectable impact of small-scale disturbances on ‘blue carbon’ within seagrass beds. Mar. Biol. 161, 2939–2944. ( 10.1007/s00227-014-2558-8) [DOI] [Google Scholar]
- 15.Jørgensen BB, Revsbech NP. 1985. Diffusive boundary-layers and the oxygen-uptake of sediments and detritus. Limnol. Oceanogr. 30, 111–122. ( 10.4319/lo.1985.30.1.0111) [DOI] [Google Scholar]
- 16.Fenchel T, King GM, Blackburn TH. 1998. Bacterial biogeochemistry: the ecophysiology of mineral cycling. San Diego, CA: Academic Press. [Google Scholar]
- 17.Meehan AJ, West RJ. 2000. Recovery times for a damaged Posidonia australis bed in south eastern Australia. Aquat. Bot. 67, 161–167. ( 10.1016/S0304-3770(99)00097-2) [DOI] [Google Scholar]
- 18.Trevathan-Tackett S, Macreadie P, Ralph P, Seymour J. 2014. Detachment and flow cytometric quantification of seagrass-associated bacteria. J. Microbial Methods 102, 23–25. ( 10.1016/j.mimet.2014.04.008) [DOI] [PubMed] [Google Scholar]
- 19.Marie D, Partensky F, Jacquet S, Vaulot D. 1997. Enumeration and cell cycle analysis of natural populations of marine picoplankton by flow cytometry using the nucleic acid stain SYBR Green I. Appl. Environ. Microbiol. 63, 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seymour JR, Seuront L, Mitchell JG. 2007. Microscale gradients of planktonic microbial communities above the sediment surface in a mangrove estuary. Estuar. Coast. Shelf Sci. 73, 651–666. ( 10.1016/j.ecss.2007.03.004) [DOI] [Google Scholar]
- 21.Lebaron P, Servais P, Agogue H, Courties C, Joux F. 2001. Does the high nucleic acid content of individual bacterial cells allow us to discriminate between active cells and inactive cells in aquatic systems? Appl. Environ. Microbiol. 67, 1775–1782. ( 10.1128/aem.67.4.1775-1782.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gifford RM, Roderick ML. 2003. Soil carbon stocks and bulk density: spatial or cumulative mass coordinates as a basis of expression? Glob. Change Biol. 9, 1507–1514. ( 10.1046/j.1365-2486.2003.00677.x) [DOI] [Google Scholar]
- 23.Clarke KR, Gorley RN. 2006. PRIMER, 6th edn Plymouth, UK: PRIMER-E. [Google Scholar]
- 24.Parks DH, Tyson GW, Hugenholtz P, Beiko RG. 2014. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30, 3123–3124. ( 10.1093/bioinformatics/btu494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Keefe B. 2014. Stability of Holocene ‘blue’ carbon in seagrass meadows in Jervis Bay, NSW. Sydney, Australia: University of Technology Sydney. [Google Scholar]
- 26.Greiner JT, McGlathery KJ, Gunnell J, McKee BA. 2013. Seagrass restoration enhances ‘blue carbon’ sequestration in coastal waters. PLoS ONE 8, e72469 ( 10.1371/journal.pone.0072469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sintes T, Marba N, Duarte CM. 2006. Modeling nonlinear seagrass clonal growth: assessing the efficiency of space occupation across the seagrass flora. Estuar. Coasts 29, 72–80. ( 10.1007/BF02784700) [DOI] [Google Scholar]
- 28.Macreadie PI, York PH, Sherman CDH. 2014. Resilience of Zostera muelleri seagrass to small-scale disturbances: the relative importance of asexual versus sexual recovery. Ecol. Evol. 4, 450–461. ( 10.1002/ece3.933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unsworth RKF, Collier CJ, Waycott M, Mckenzie LJ, Cullen-Unsworth LC In press. A framework for the resilience of seagrass ecosystems. Mar. Pollut. Bull. ( 10.1016/j.marpolbul.2015.08.016) [DOI] [PubMed] [Google Scholar]
- 30.Duarte CM, Sintes T, Marba N. 2013. Assessing the CO2 capture potential of seagrass restoration projects. J. Appl. Ecol. 50, 1341–1349. ( 10.1111/1365-2664.12155) [DOI] [Google Scholar]
- 31.Eldridge PM, Morse JW. 2000. A diagenetic model for sediment-seagrass interactions. Mar. Chem. 70, 89–103. ( 10.1016/s0304-4203(00)00018-9) [DOI] [Google Scholar]
- 32.Marba N, Arias-Ortiz A, Masque P, Kendrick GA, Mazarrasa I, Bastyan GR, Garcia-Orellana J, Duarte CM. 2015. Impact of seagrass loss and subsequent revegetation on carbon sequestration and stocks. J. Ecol. 103, 296–302. ( 10.1111/1365-2745.12370) [DOI] [Google Scholar]
- 33.West RJ. 1990. Depth related structural and morphological variations in an Australian Posidonia seagrass bed. Aquat. Bot. 36, 153–166. ( 10.1016/0304-3770(90)90079-Z) [DOI] [Google Scholar]
- 34.Marba N, Duarte CM, Cebrian J, Gallegos ME, Olesen B, SandJensen K. 1996. Growth and population dynamics of Posidonia oceanica on the Spanish Mediterranean coast: elucidating seagrass decline. Mar. Ecol. Prog. Ser. 137, 203–213. ( 10.3354/meps137203) [DOI] [Google Scholar]
- 35.Garcia-Martinez M, Lopez-Lopez A, Calleja ML, Marba N, Duarte CM. 2009. Bacterial community dynamics in a seagrass (Posidonia oceanica) meadow sediment. Estuar. Coasts 32, 276–286. ( 10.1007/s12237-008-9115-y) [DOI] [Google Scholar]
- 36.Pendleton L, et al. 2012. Estimating global ‘blue carbon’ emissions from conversion and degradation of vegetated coastal ecosystems. PLoS ONE 7, e43542 ( 10.1371/journal.pone.0043542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hejnowicz AP, Kennedy H, Rudd MA, Huxham MR. 2015. Harnessing the climate mitigation, conservation and poverty alleviation potential of seagrasses: prospects for developing blue carbon initiatives and payment for ecosystem service programmes. Front. Mar. Sci. 2, 1–22. ( 10.3389/fmars.2015.00032) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.