Abstract
Foraging herbivores face twin threats of predation and parasite infection, but the risk of predation has received much more attention. We evaluated, experimentally, the role of olfactory cues in predator and parasite risk assessment on the foraging behaviour of a population of marked, free-ranging, red-necked wallabies (Macropus rufogriseus). The wallabies adjusted their behaviour according to these olfactory cues. They foraged less, were more vigilant and spent less time at feeders placed in the vicinity of faeces from dogs that had consumed wallaby or kangaroo meat compared with that of dogs feeding on sheep, rabbit or possum meat. Wallabies also showed a species-specific faecal aversion by consuming less food from feeders contaminated with wallaby faeces compared with sympatric kangaroo faeces, whose gastrointestinal parasite fauna differs from that of the wallabies. Combining both parasite and predation cues in a single field experiment revealed that these risks had an additive effect, rather than the wallabies compromising their response to one risk at the expense of the other.
Keywords: foraging behaviour, macropodid marsupials, anti-predator behaviour, predator detection, parasite avoidance, faecal aversion
1. Introduction
It is widely understood that foraging animals trade-off the risk of predation against the risk of insufficient resources for maintenance and reproduction, typically by increasing their investment in predator vigilance and/or avoiding feeding in locations where they are vulnerable to attack [1,2]. Less well appreciated is whether animals also incorporate the risk of parasite infection into such foraging trade-offs [1], even though parasitism presents significant challenges to host fitness through negative impacts on growth, reproductive success or survival [3,4]. While several studies have investigated the combined effects of parasites and predators on fitness [5,6] and general behaviours, such as grooming [7,8], the combined effect on foraging behaviour is largely unknown. Yet foraging animals may often face these risks in combination, especially in times of peak parasite activity.
Accurate risk assessment of both predation and parasite infection requires a nuanced recognition of appropriate cues. The faeces of carnivorous vertebrates contain odour cues that are frequently used by herbivores to assess the risk of predation [9–13]. While the precise nature of these cues is probably complex, there is evidence that predator diet affects these volatile compounds, allowing prey to perceive differences between predator species, and even between individuals of the same species that have fed on different prey [14–16]. Similarly, many grazing herbivores avoid areas adjacent to faeces to reduce the risks of parasite transmission [1,17–20]. Herbivores infected with gastrointestinal parasites produce faeces containing parasite eggs that hatch into infective larvae on the sward, where the larvae may be ingested by other herbivores [4,21]. The parasite larvae are probably undetectable by the herbivore, so faeces are used as a cue for infection risk [20]. While a generalized aversion to all faeces is advantageous for herbivores foraging in the same areas as conspecifics, it may result in less efficient foraging if heterospecifics share foraging grounds but not parasites [22,23].
Here, we experimentally test the responses of free-ranging, red-necked wallabies (Macropus rufogriseus) to cues associated with the risks of predators, parasites and both in combination. Macropodid marsupials (kangaroos and wallabies) have been exposed to predation from past and present predators [24] and are typically wary of predator faeces [25,26]. Macropodids also have diverse and highly species-specific gastrointestinal parasite faunas [27] and often share foraging grounds (e.g. [28]). We asked the following questions: (i) do red-necked wallabies vary their response to predator olfactory cues according to variation in the predator's diet; (ii) is faecal aversion in red-necked wallabies host species-specific; and most importantly: (iii) how do the combined risks of predators and parasites impact on the foraging decisions of red-necked wallabies?
2. Material and methods
(a). Study site
We conducted this study at the Victoria Valley Airbase (Grampians National Park, Australia; 37°11′ S, 142°20′ E), which comprises two 80 × 800 m runways of mown native grasses and heath. Red-necked wallabies and grey kangaroos (Macropus giganteus and Macropus fuliginosus) are common, and they forage and defaecate throughout the runways. Dingoes (Canis lupus dingo) preyed on wallabies in the past [24], but have been extirpated from the Grampians region; the main extant predator is the red fox (Vulpes vulpes). In the Grampians region, red-necked wallabies have up to 22 parasitic helminth species in their gastrointestinal tracts, yet share very few species with sympatric grey kangaroos [29,30] (see the electronic supplementary material). Our experiments took place during autumn and winter in both 2012 and 2013, the peak time for parasite infection [31]. We observed the behaviour of 39 wallabies that were individually identified with unique colour combinations of Allflex® eartags.
(b). Predator faecal aversion
We assessed responses to predation risk by exposing wallabies to faeces from domestic dogs that had fed on five different herbivores: (i) conspecifics (Bennett's wallaby, Macropus rufogriseus rufogriseus); (ii) congenerics (a mix of kangaroos, Macropus rufus, M. giganteus and M. fuliginosus); (iii) larger non-native eutherians (sheep, Ovis aries); (iv) smaller, native marsupials (common brushtail possum, Trichosurus vulpecula); and (v) smaller, non-native eutherians (European rabbit, Oryctolagus cuniculus). The meat was obtained from Yarra Valley Game Meats (Healesville, Victoria, Australia). We fed four adult dogs, of varying breed, sex and size, with meat of one of the five prey types for 48 h to eliminate traces of previous diets, and then collected faeces produced while on that diet. We collected the faeces immediately on deposition and immediately froze them in airtight containers. Containers were later pooled into one set of experimental faeces for each diet.
We prepared feeders by placing 200 g of crushed maize and 100 g of an inedible matrix (eucalypt woodchips) in clear plastic freezer trays (36 × 25 cm) placed on the open runways. We placed four clear plastic lids, each with approximately 50 g of defrosted experimental predator faeces, 30 cm from the feeder in the four cardinal directions. Procedural controls had rocks on the plastic lids to imitate faeces. Each trial involved a randomly selected type of faeces or control; the observer was always blind to the type of faeces.
We observed wallabies using a Kowa spotting scope (20 × 60) from a distance of less than or equal to 40 m in a concealed position, first recording the distance between the feeder and the nearest cover, the weather conditions and the time of day. When a wallaby entered a 3 m radius around the feeder, we recorded its identity and time to subsequently arrive at the feeder. Once feeding, we noted the number of times it exhibited vigilance [32,33] and the time spent feeding until it either left the feeder or 1 h had elapsed. We then collected the feeders, weighed the remaining contents, removed any residual food and faeces and sealed them in a waste container. We discounted trials when animals knocked over trays or were scared away by vehicles, or when rain fell (affecting weights) during the trial. We analysed data only from individuals that participated in fewer than five trials on a specific predator cue type. In total, 21 wallabies contributed to 97 trials.
(c). Herbivore faecal aversion
We confirmed that the wallabies exhibited herbivore faecal aversion by presenting animals with paired feeders (as described below) containing 200 g of crushed maize contaminated with 100 g of mixed macropod faeces. Pilot studies (72 replicates from 14 individuals) indicated that wallabies always chose uncontaminated trays when presented with a choice, so we did not include uncontaminated trays in subsequent experiments.
We collected faeces, immediately after observing deposition, from animals foraging around the airbase, separating them by species. Each trial comprised a pair of adjacent feeders: one contaminated with 100 g of faeces of red-necked wallabies (conspecific feeder) and the other with 100 g of mixed faeces of eastern and western grey kangaroos (heterospecific feeder). As before, we observed wallabies from a concealed position. We recorded the identity of wallabies that approached and fed at the feeders, together with their choice of feeder and any switching between feeders. Observations lasted until the animal left the feeder or 1 h had elapsed, when we collected and weighed the feeders to determine the amount of food consumed. We discarded any remaining food and faeces as before. We only included in our analysis trials where the wallaby sniffed both feeders, the animal contributed to fewer than five trials, and rain did not fall before we collected the feeder. In total, 19 individuals contributed to 46 trials.
(d). Combined faecal aversion
We assessed the combined risk of predators and parasites by arranging feeders according to the previous predator faecal aversion experiment, but replaced the inedible woodchip matrix with 100 g of either wallaby or kangaroo faeces. We used dog faeces containing three prey cues: wallaby (conspecific), kangaroo (closely related) or possum (smaller prey) diet. We placed the faeces around the feeder and collected data on foraging choices and consumption as before.
(e). Statistical analysis
We analysed data using JMP v. 10 (SAS institute, USA). For the predator faecal aversion experiment, we used restricted maximum-likelihood (REML) models to determine the source of variation in foraging behaviours (approach time, number of vigilance bouts and amount of food eaten), with treatment (faecal source), distance to nearest cover, time of day (morning or evening) and their interactions as fixed effects, and animal identification (ID) as a random factor to account for multiple tests of the same individual. For the herbivore faecal aversion experiment, we used a paired t-test to compare the amount of food consumed from feeders contaminated with the faeces of either kangaroos or wallabies. In the combined faecal aversion experiment, we used REML to explore the sources of variation in foraging behaviours (approach time, number of vigilance bouts and amount of food eaten), with parasite type (kangaroo or wallaby), prey type (kangaroo, wallaby or possum), distance to nearest cover, time of day and the interaction terms as main effects, and animal ID as a random factor. The approach time and number of vigilance bouts were log transformed to improve the distribution. In all analyses we initially included the full model, but we report (see the electronic supplementary material) reduced models in which we sequentially removed interaction terms where they were not significant (p > 0.1).
3. Results
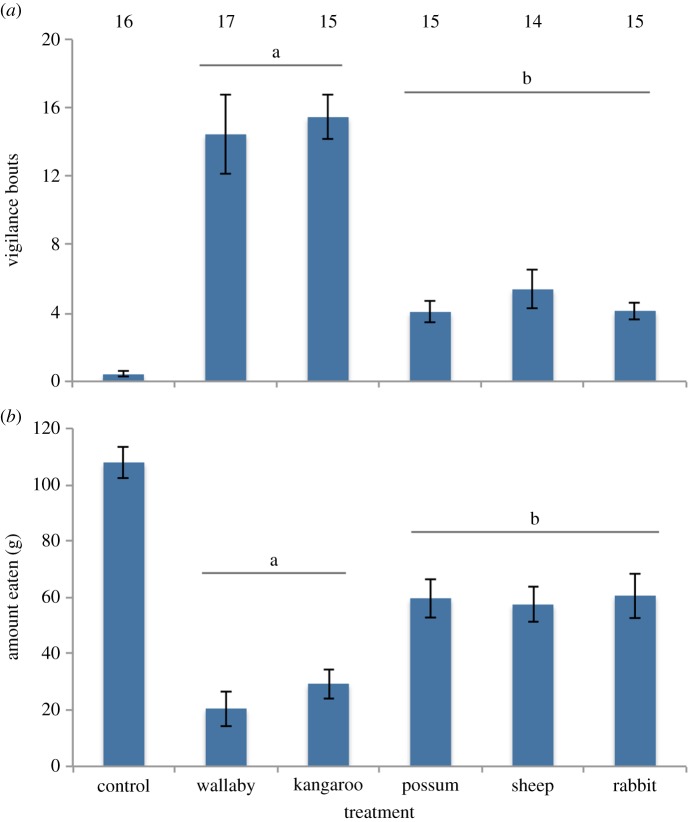
In the predator faecal aversion experiment, wallabies adjusted their foraging behaviour according to type of prey consumed. The wallabies took significantly longer (p < 0.05) to approach feeders surrounded with faeces of dogs fed on wallaby and kangaroo compared with the other prey types, were more vigilant (figure 1a), and consumed less food at these feeders (figure 1b and electronic supplementary material, table S3).
Figure 1.
The effects of the presence of the faeces of dogs, fed on different diets, on (a) the number of vigilance bouts and (b) the amount eaten (g) by red-necked wallabies. Values are means with standard error bars, with letters above indicating significant differences between treatments and numbers indicating sample size. (Online version in colour.)
In the herbivore faecal aversion experiment, wallabies typically approached the feeders, sniffed each, then began to feed from one of them, which we deemed their ‘choice’. Once the wallabies had made a choice, they switched feeders on only six (13%) occasions. The wallabies consumed five times more from heterospecific feeders (70.3 ± 7.1 g) than from conspecific feeders (13.8 ± 3.9 g; t45 = 6.4, p < 0.0001).
In the combined faecal aversion experiment, the time taken to approach the feeder was influenced by predator cues, but not parasite risk (figure 2a). The time spent vigilant was influenced by both predator and parasite cues (figure 2b), and an interaction term including predator cues, parasite cues and distance from cover (electronic supplementary material, table S3). The greater time spent vigilant in the wallaby faecal treatment may reflect a general unwillingness to feed, since there was no choice in this experiment. The amount of food consumed was similarly affected by cues indicating the risk of both predators and parasites (figure 2c), and the distance from cover (electronic supplementary material, table S3). The predator cue × parasite cue interaction term was not significant (F2,52.7 = 0.022, p = 0.98).
Figure 2.
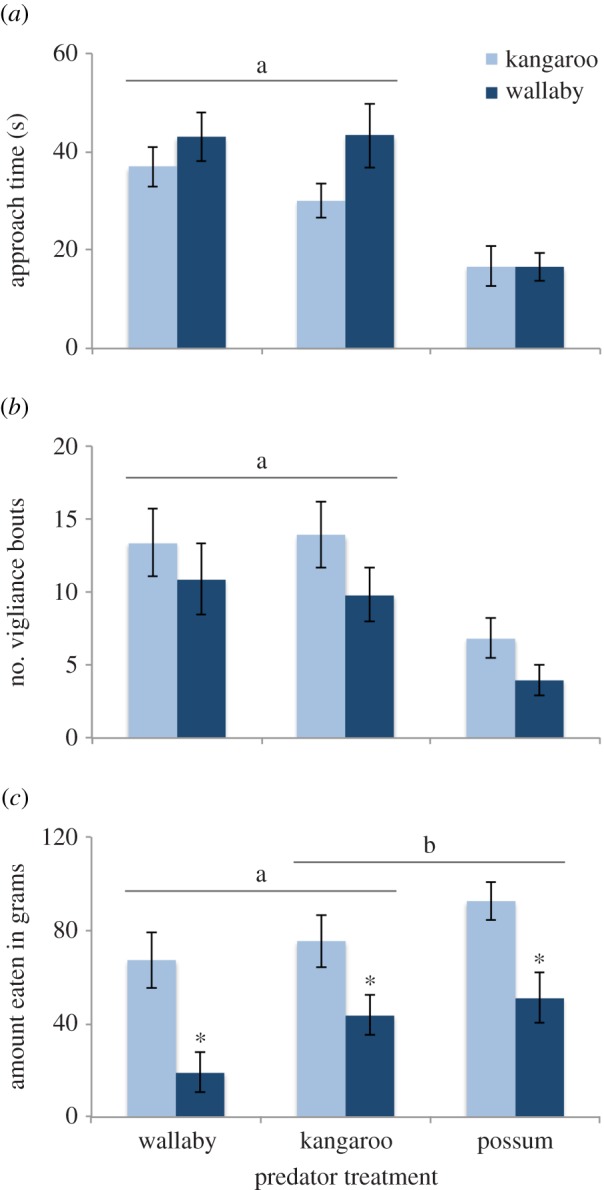
The combined effects of predator risk (the presence of dog faeces derived from different diets) and parasite risk (presence of conspecific and heterospecific macropod faeces) on: (a) the approach time (s); (b) the number of vigilance bouts; and (c) the amount eaten (g) by red-necked wallabies. Values are means with standard error bars. Connecting lines above the bars indicate non-significant (p > 0.05) differences between predator risk treatments, and an asterisk indicates significant differences between parasite risk treatments within predator risk treatments. (Online version in colour.)
4. Discussion
Red-necked wallabies make impressive use of olfactory cues to gauge the risk of predation and parasite infection. The wallabies approached feeders surrounded by the faeces of dogs that had fed on macropodids more warily than if the faeces came from dogs that had fed on other herbivores. Further, herbivore faecal aversion in red-necked wallabies is host species-specific; wallabies ate five times more from feeders contaminated by heterospecific than conspecific faeces. Finally, the wallabies' food consumption decreased additively when faced with the combined faecal cues for the risk of predation and parasite infection. While herbivore faeces may include social information (including identity, gender, dominance), this is unlikely to explain our results because the faeces included in each trial derive from numerous individuals, and therefore provide a non-specific cocktail of odours. We also think it unlikely that red-necked wallabies use conspecific faeces as a cue to prior foraging by conspecifics, thereby improving foraging efficiency by faecal avoidance. First, herbivores select foliage by direct visual and olfactory assessment, which provides far more precise cues of forage quality and quantity. Second, field experiments indicate that congeners will not trade-off their aversion to faecal contamination for a gain in foraging efficiency [20].
The precise chemical nature of the olfactory cues present in carnivore faeces is not known in this system, but they are clearly influenced by diet [34–36]. Such effects are also common in other chemical sensory systems [37]. The results of our field experiments caution against inferring that prey respond to different predator species per se, because the predators may also have different diets. Red-necked wallabies most strongly avoided faeces produced from diets that contained either conspecifics or closely related macropodids, rather than faeces from diets of larger rather than smaller prey, or from faeces containing other marsupial rather than eutherian material. Perhaps, the responses of red-necked wallabies are learnt, with the response reflecting the frequency of encounter [38,39].
Our results suggest that herbivore faecal aversion as a means of minimizing gastrointestinal parasite infection levels [18–20] may be more nuanced than previously thought. Although wallabies fed more from uncontaminated than contaminated food, their aversion was stronger if the food was contaminated by the faeces of conspecifics rather than heterospecifics. This finding suggests strong selection for parasite avoidance on the one hand, and efficient foraging on the other. Our results contrast with findings that reindeer (Rangifer tarandus) do not avoid sheep faeces less than conspecific faeces [40], perhaps reflecting relaxed selection either because these two species share a sufficiently broad gastrointestinal parasite fauna, or there has been little history of the two species foraging in the same location.
Studies report both additive and interactive effects of parasites and predators on fitness [5,6]. The wallabies might be expected to avoid feeders associated with predator cues, regardless of whether they had greater risk of parasite infection, if the more immediate threat of predation outweighed that of parasitism. This was not the case. Rather, avoidance behaviour was most evident at feeders that were associated with the greatest combined danger of predators and parasites. This suggests that while the fitness consequences of predation and parasites are often different, wallabies do not compromise their response to the risks of either predators or parasites in the face of the other. More complete analyses of foraging behaviour should consider these risks simultaneously: foraging herbivores are likely to trade-off foraging efficiency against avoiding both predators and parasites.
Supplementary Material
Acknowledgements
We thank Ian Beveridge for invaluable help identifying helminth parasites; Jemma Cripps for advice on parasite ecology; Daryl Panther and volunteers for field assistance; and Parks Victoria and the former Department of Sustainability and Environment for logistic support and access to the airbase.
Ethics
This research was approved by the University of Melbourne's Animal Ethics Committee (no. 1212475.1) and the former Department of Sustainability and Environment (no. 10004041).
Authors' contributions
S.G. and G.C. conceived the study with input from M.E. J.S. conducted the fieldwork with assistance from S.G. M.E. analysed the data. J.S. wrote the manuscript with editorial input from G.C., M.E. and S.G.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by the Holsworth Wildlife Research Endowment (to J.S.).
References
- 1.Hart BL. 1990. Behavioral adaptations to pathogens and parasites: five strategies. Neurosci. Biobehav. Rev. 14, 273–294. ( 10.1016/S0149-7634(05)80038-7) [DOI] [PubMed] [Google Scholar]
- 2.Lima SL, Dill LM. 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640. ( 10.1139/z90-092) [DOI] [Google Scholar]
- 3.Grenfell BT. 1992. Parasitism and the dynamics of ungulate grazing systems. Am. Nat. 139, 907–929. ( 10.1086/285366) [DOI] [Google Scholar]
- 4.Gunn A, Irvine RJ. 2003. Subclinical parasitism and ruminant foraging strategies: a review. Wildl. Soc. Bull. 31, 117–126. [Google Scholar]
- 5.Zanette L, Smith JNM, van Oort H, Clinchy M. 2003. Synergistic effects of food and predators on annual reproductive success in song sparrows. Proc. R. Soc. Lond. B 270, 799–803. ( 10.1098/rspb.2002.2311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belden LK, Wojdak JM. 2011. The combined influence of trematode parasites and predatory salamanders on wood frog (Rana sylvatica) tadpoles. Oecologia 166, 1077–1086. ( 10.1007/s00442-011-1946-8) [DOI] [PubMed] [Google Scholar]
- 7.Rutherford PL, Baker RL, Forbes MR. 2007. Do larval damselflies make adaptive choices when exposed to both parasites and predators? Ethology 113, 1073–1080. ( 10.1111/j.1439-0310.2007.01408.x) [DOI] [Google Scholar]
- 8.Szuroczki D, Richardson JML. 2012. The behavioral response of larval amphibians (Ranidae) to threats from predators and parasites. PLoS ONE 7, e49592 ( 10.1371/journal.pone.0049592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. 2005. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci. Biobehav. Rev. 29, 1123–1144. ( 10.1016/j.neubiorev.2005.05.005) [DOI] [PubMed] [Google Scholar]
- 10.Russell BG, Banks PB. 2007. Do Australian small mammals respond to native and introduced predator odours? Aust. Ecol. 32, 277–286. ( 10.1111/j.1442-9993.2007.01685.x) [DOI] [Google Scholar]
- 11.Belton LE, Ball N, Waterman JM, Bateman PW. 2007. Do Cape ground squirrels (Xerus inauris) discriminate between olfactory cues in the faeces of predators versus nonpredators? Afr. Zool. 42, 135–138. ( 10.3377/1562-7020%282007%2942%5B135%3ADCGSXI%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 12.Barrio IC, Bueno CG, Banks PB, Tortosa FS. 2010. Prey naivete in an introduced prey species: the wild rabbit in Australia. Behav. Ecol. 21, 986–991. ( 10.1093/beheco/arq103) [DOI] [Google Scholar]
- 13.Dickman CR, Doncaster CP. 1984. Responses of small mammals to red fox (Vulpes vulpes) odour. J. Zool. 204, 521–531. ( 10.1111/j.1469-7998.1984.tb02384.x) [DOI] [Google Scholar]
- 14.Jędrzejewski W, Rychlik L, Jękedrzejewska B. 1993. Responses of bank voles to odours of seven species of predators: experimental data and their relevance to natural predator–vole relationships. Oikos 68, 251–257. ( 10.2307/3544837) [DOI] [Google Scholar]
- 15.Barreto GR, Macdonald DW. 1999. The response of water voles, Arvicola terrestris, to the odours of predators. Anim. Behav. 57, 1107–1112. ( 10.1006/anbe.1998.1042) [DOI] [PubMed] [Google Scholar]
- 16.Schoeppner NM, Relyea RA. 2009. Interpreting the smells of predation: how alarm cues and kairomones induce different prey defences: unravelling the chemical cues of predation. Funct. Ecol. 23, 1114–1121. ( 10.1111/j.1365-2435.2009.01578.x) [DOI] [Google Scholar]
- 17.Hutchings MR, Kyriazakis I, Anderson DH, Gordon IJ, Coop RL. 1998. Behavioural strategies used by parasitized and nonparasitized sheep to avoid ingestion of gastrointestinal nematodes associated with faeces. Anim. Sci. 67, 97–106. ( 10.1017/S1357729800009838) [DOI] [Google Scholar]
- 18.Van der Wal R, Irvine J, Stien A, Shepherd N, Albon SD. 2000. Faecal avoidance and the risk of infection by nematodes in a natural population of reindeer. Oecologia 124, 19–25. ( 10.1007/s004420050020) [DOI] [PubMed] [Google Scholar]
- 19.Ezenwa VO. 2004. Selective defecation and selective foraging: antiparasite behavior in wild ungulates? Ethology 110, 851–862. ( 10.1111/j.1439-0310.2004.01013.x) [DOI] [Google Scholar]
- 20.Garnick SW, Elgar MA, Beveridge I, Coulson G. 2010. Foraging efficiency and parasite risk in eastern grey kangaroos (Macropus giganteus). Behav. Ecol. 21, 129–137. ( 10.1093/beheco/arp162) [DOI] [Google Scholar]
- 21.Hutchings MR, Kyriazakis I, Papachristou TG, Gordon IJ, Jackson F. 2000. The herbivores’ dilemma: tradeoffs between nutrition and parasitism in foraging decisions. Oecologia 124, 242–251. ( 10.1007/s004420000367) [DOI] [PubMed] [Google Scholar]
- 22.Smith LA, White PC, Marion G, Hutchings MR. 2009. Livestock grazing behavior and inter- versus intraspecific disease risk via the fecal–oral route. Behav. Ecol. 20, 426–432. ( 10.1093/beheco/arn143) [DOI] [Google Scholar]
- 23.Hoste H, Sotiraki S, Landau SY, Jackson F, Beveridge I. 2010. Goat–nematode interactions: think differently. Trends Parasitol. 26, 376–381. ( 10.1016/j.pt.2010.04.007) [DOI] [PubMed] [Google Scholar]
- 24.Jarman PJ, Coulson G. 1989. Dynamics and adaptiveness of grouping in macropods. In Kangaroos, wallabies and rat-kangaroos (eds Grigg G, Jarman PJ, Hume I), pp. 527–547. Chipping Norton, UK: Surrey Beatty. [Google Scholar]
- 25.Parsons MH, Blumstein DT. 2010. Familiarity breeds contempt: kangaroos persistently avoid areas with experimentally deployed dingo scents. PLoS ONE 5, e10403 ( 10.1371/journal.pone.0010403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox TE, Murray PJ, Bengsen AJ, Hall GP, Li X. 2015. Do fecal odors from native and non-native predators cause a habitat shift among macropods? Wildl. Soc. Bull. 39, 159–164. ( 10.1002/wsb.509) [DOI] [Google Scholar]
- 27.Beveridge I, Spratt DM, Johnson PM. 2010. Diversity and distribution of helminth parasites in macropodoid marsupials. In Macropods: the biology of kangaroos, wallabies, and rat-kangaroos (eds Coulson G, Eldridge MDB), pp. 231–242. Melbourne, Australia: CSRIO Publishing. [Google Scholar]
- 28.Coulson G. 1999. Monospecific and heterospecific grouping and feeding behavior in grey kangaroos and red-necked wallabies. J. Mammal. 80, 270–282. ( 10.2307/1383227) [DOI] [Google Scholar]
- 29.Aussavy M, Bernardin E, Corrigan A, Hufschmid J, Beveridge I. 2011. Helminth parasite communities in four species of sympatric macropodids in western Victoria. Aust. Mammal. 33, 13–20. ( 10.1071/AM10020) [DOI] [Google Scholar]
- 30.Beveridge I, Arundel JH. 1979. Helminth parasites of grey kangaroos, Macropus giganteus Shaw and M. fuliginosus (Desmarest), in Eastern Australia. Wildl. Res. 6, 69–77. ( 10.1071/WR9790069) [DOI] [Google Scholar]
- 31.Arundel JH, Dempster KJ, Harrigan KE, Black R. 1990. Epidemiological observations on the helminth parasites of Macropus giganteus Shaw in Victoria. Wildl. Res. 17, 39–51. ( 10.1071/WR9900039) [DOI] [Google Scholar]
- 32.Southwell C. 1987. Activity pattern of the eastern grey kangaroo, Macropus giganteus. Mammalia 51, 211–224. ( 10.1515/mamm.1987.51.2.211) [DOI] [Google Scholar]
- 33.Clarke JL, Jones ME, Jarman PJ. 1995. Diurnal and nocturnal grouping and foraging behaviors of free-ranging eastern grey kangaroos. Aust. J. Zool. 43, 519–529. ( 10.1071/ZO9950519) [DOI] [Google Scholar]
- 34.Nolte DL, Mason JR, Epple G, Aronov E, Campbell DL. 1994. Why are predator urines aversive to prey? J. Chem. Ecol. 20, 1505–1516. ( 10.1007/BF02059876) [DOI] [PubMed] [Google Scholar]
- 35.Banks P, Hughes KN, Rose T. 2003. Do native Australian small mammals avoid faeces of domestic dogs? Responses of Rattus fuscipes and Antechinus stuartii. Aust. Zool. 32, 406–409. ( 10.7882/AZ.2002.018) [DOI] [Google Scholar]
- 36.Mella VSA, Cooper CE, Davies SJJF. 2010. Predator odour does not influence trappability of southern brown bandicoots (Isoodon obesulus) and common brushtail possums (Trichosurus vulpecula). Aust. J. Zool. 58, 267–272. ( 10.1071/ZO10049) [DOI] [Google Scholar]
- 37.Henneken J, Jones TM, Goodger JQD, Dias DA, Walter A, Elgar MA. 2015. Diet influences female signal reliability for male mate choice. Anim. Behav. 108, 215–221. ( 10.1016/j.anbehav.2015.07.023) [DOI] [Google Scholar]
- 38.Mathis A, Smith RJF. 1993. Fathead minnows, Pimephales promelas, learn to recognize northern pike, Esox lucius, as predators on the basis of chemical stimuli from minnows in the pike's diet. Anim. Behav. 46, 645–656. ( 10.1006/anbe.1993.1241) [DOI] [Google Scholar]
- 39.Blumstein DT, Mari M, Daniel JC, Ardron JG, Griffin AS, Evans CS. 2002. Olfactory predator recognition: wallabies may have to learn to be wary. Anim. Conserv. 5, 87–93. ( 10.1017/S1367943002002123) [DOI] [Google Scholar]
- 40.Colman JE, Storlien S, Moe SR, Holand Ø, Reimers E. 2011. Reindeer avoidance of pasture contaminated with sheep and reindeer faeces. Rangifer 23, 313–320. ( 10.7557/2.23.5.1716) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.