Abstract
Sex ratio and sexual dimorphism have long been of interest in population and evolutionary ecology, but consequences for communities and ecosystems remain untested. Sex ratio could influence ecological conditions whenever sexual dimorphism is associated with ecological dimorphism in species with strong ecological interactions. We tested for ecological implications of sex ratio variation in the sexually dimorphic western mosquitofish, Gambusia affinis. This species causes strong pelagic trophic cascades and exhibits substantial variation in adult sex ratios. We found that female-biased populations induced stronger pelagic trophic cascades compared with male-biased populations, causing larger changes to key community and ecosystem responses, including zooplankton abundance, phytoplankton abundance, productivity, pH and temperature. The magnitude of such effects indicates that sex ratio is important for mediating the ecological role of mosquitofish. Because both sex ratio variation and sexual dimorphism are common features of natural populations, our findings should encourage broader consideration of the ecological significance of sex ratio variation in nature, including the relative contributions of various sexually dimorphic traits to these effects.
Keywords: sexual dimorphism, trophic cascade, mosquitofish, Gambusia, freshwater ecology
1. Introduction
Sex ratio variation is a longstanding theme in evolutionary biology. Fisher [1] famously theorized that natural selection should maintain 1 : 1 sex ratios by continuously favouring the rare sex, thereby always returning skewed sex ratios to equality. In nature, skewed sex ratios are a common observation across the tree of life, and explanations include differential mortality rates for males and females [2], inbreeding and local competition for mates [3], endocrine-disrupting environmental pollutants [4,5] and adaptive maternal effects that allow differential investment in male or female offspring [6–8]. Despite the attention paid to the causes of sex ratio variation in nature, and in some cases its consequences for population growth [9], theory and tests of its effects on communities and ecosystems are lacking. This lack of attention may be in part due to a presumption that the sexes of most species are ecologically equivalent in their effects on communities and ecosystems.
However, many species show marked sexual dimorphism in body size and other traits related to ecological function. Sexual size dimorphism has the potential to influence resource use because prey capture is size-dependent [10] and body size influences overall feeding rates [11]. Body size and physiology also influence rates of nutrient excretion [12], which have important effects for ecosystem processes [13]. Males and females can also be dimorphic in behavioural or morphological traits, affecting resource use [14]. The widespread observations that sex ratios vary in nature, and that males and females often differ in key ecological traits, suggest that sex ratio variation may have effects on communities and ecosystems. These effects may be particularly prevalent when sex ratio variation is present in ecologically important species, such as keystone, foundation, dominant or invasive species.
We examined the ecological consequences of sex ratio variation in the western mosquitofish (Gambusia affinis). Mosquitofish (G. affinis and its congener G. holbrooki) are small (<6 cm) livebearing fishes (Poeciliidae), which lack sexual dimorphism in age and size at maturity [15], but show pronounced sexual size dimorphism (figure 1) due to differences in post-maturation growth rates. Empirical tests show female mosquitofish display higher feeding rates per unit body size [20,21] and typically show greater niche breadths, with a notable preference for prey of larger body sizes [22,23]. Females also spend relatively more time foraging when in the presence of other females compared with when in the presence of males [24,25]. In addition to these dimorphic foraging characteristics, the relatively large size of females could increase overall nutrient excretion rates for the same density of fish in female-biased populations compared with male-biased populations, which could in turn affect primary production [13]. As a result of these sex-specific tendencies, we predict that female-biased populations induce stronger pelagic trophic cascades compared to male-biased populations. We expect these effects to be strong because mosquitofish play a major role in aquatic ecosystems by altering invertebrate communities and driving strong trophic cascades that can even change abiotic conditions, including light penetration and nutrient dynamics [26,27].
Figure 1.
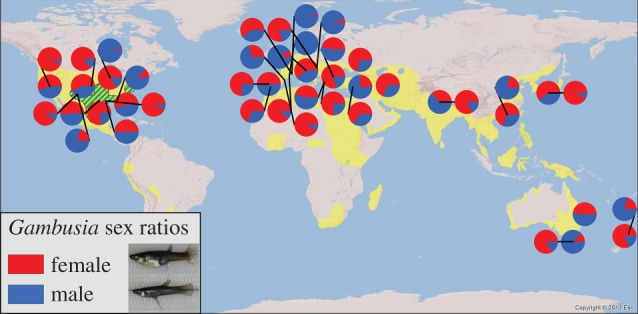
Sex ratio variation over part of the present-day range of mosquitofish (Gambusia affinis and G. holbrooki). These species are both widely introduced for mosquito control and were grouped together into a single species until 1988 [16]. Therefore, data for both species are presented. Yellow represents the approximate present-day range of mosquitofish, while green hatching represents their native range (adapted from [17], with supplemental data from [18,19]). Their range is likely to be larger than presented due to the unreported nature of historical transplantations. For studies reporting more than two sex ratios (within a region or in a single location through time), two pie charts were plotted representing the most female-biased and most male-biased sample taken. Data and sources are reported in electronic supplementary material, table S1.
We performed a pond mesocosm experiment to test this prediction. The experiment was conducted in California (USA), where the western mosquitofish was introduced in 1922 and has since been spread by Mosquito & Vector Control Districts (MVCDs) as a means of disease control [28], most notably West Nile virus [29]. We obtained fish for our experiments from the Sacramento–Yolo MVCD (Elk Grove, CA, USA). This district alone stocks about one million mosquitofish annually over an area of about 5000 hectares of agricultural fields, wildlife refuges and private lands in Sacramento and Yolo Counties, California [30]. Mosquitofish were historically introduced for the same purposes elsewhere and are today one of the most widespread and abundant freshwater fishes in the world [31] (figure 1). Their strong negative consequences for native fauna across their global range have resulted in their being listed as one of the world's 100 worst invasive species by the IUCN [18].
Mosquitofish sex ratios vary substantially across their present-day range (figure 1). The mechanism of sex determinism is chromosomal, and primary and secondary sex ratios have rarely been found to differ from 1 : 1 (but see [8]). Therefore, sex ratio variation has primarily been attributed to differential postpartum mortality induced by a variety of physiological and ecological mechanisms, including temperature, salinity, seasonality and interactions with other species. Predation may be a particularly important driver. For example, avian predators prefer females over males, leading to sex ratio estimates as high as 97% males in some locations [32]. Female-biased populations are more common, and are often attributed to the greater longevity of females (often >6 months) relative to males (often <6 months) [33]. Therefore, mosquitofish are a relevant system to understand whether sex ratio variation shapes ecological effects because they are dimorphic, ecologically important and show substantial sex ratio variation across their global range.
2. Material and methods
(a). Design
We created a 6 × 6 array of experimental pond mesocosms (1136-litre Rubbermaid stock tanks) on a level field on the grounds of Long Marine Laboratory at the University of California, Santa Cruz. Each mesocosm was layered with 19 litres of sand and filled with dechlorinated municipal water. We inoculated each mesocosm with 4 litres of thoroughly homogenized sediment from Westlake Pond (Santa Cruz, CA, USA) to introduce nutrients and benthic communities. After the sediment settled (approx. 5 days) a cinderblock (9.3 × 19.0 × 39.3 cm3) was placed in the centre of each mesocosm for habitat cover, and the water was inoculated with a homogenized plankton community comprising multiple horizontal plankton tows (80 µm mesh) at Westlake Pond.
Experimental fish were obtained from the husbandry facility operated by the Sacramento–Yolo MVCD. Fish at or above the approximate size at maturity were sex sorted before being assigned to treatments. Fish were identified as adult males if they had evidence of a gonopodium, and as adult females if they were larger than the smallest adult male and lacked evidence of a gonopodium [34]. Our experiment thus incorporated the normal range of sexual size dimorphism, as well as other aspects of dimorphism, inherent to the species. We then counted out sex ratio treatments of 0, 25, 50, 75 and 100% males at a density of 12 individual fish per mesocosm. We chose this density because it is consistent with stocking densities used by MVCDs and is within the range of densities used in similar experiments [26,27]. Our design included a fishless reference treatment, yielding a grand total of six treatments, each with six replicates. Treatments were assigned to the mesocosm array using a Latin-squares random number generator. Fish were introduced to the mesocosms on 31 March 2014, one week after adding plankton to the pond communities. On the same day, we placed two unglazed ceramic tiles (2.8 × 4.3 cm2) on the cinderblock in each mesocosm to measure periphyton accrual. A 1.27 cm mesh bird-netting was used over all mesocosms throughout the experiment to prevent catastrophic avian and mammalian disturbance, meanwhile allowing for exposure to all other natural physical, chemical and biological elements, including oviposition by insects and amphibians.
(b). Sampling
Mesocosms were sampled 2 and 4 weeks after fish introduction for various pelagic responses including zooplankton abundance, phytoplankton abundance, primary productivity and respiration, pH, temperature at dusk and water nutrient concentrations. Zooplankton samples were taken from a 1-litre depth-integrated water sample filtered through 80 µm mesh and preserved in 80% ethanol. All zooplankters were thereafter counted and identified to the lowest taxonomical distinction possible at 100× magnification. Phytoplankton abundance was estimated as the pelagic chlorophyll a concentration in 1 litre of water. Water was filtered through a 0.7 µm glass microfibre filter (Whatman GF/F) and the filters frozen for less than 4 weeks when we extracted the chlorophyll a with 90% acetone for 24 h at 2°C [35]. The concentration of chlorophyll a in acetone was then measured fluorometrically on a Trilogy Laboratory Fluorometer (Turner Designs) using the Turner Designs non-acid module. Ecosystem respiration (ER), net primary productivity (NPP) and gross primary productivity (GPP) were estimated using diel change in dissolved oxygen measured at dawn, dusk and the following dawn, using a handheld sonde (YSI Pro 2030) as in [36]. Responses are reported in units of dissolved oxygen concentration (mg l−1) change over time. ER is, by convention, reported with negative values, which represent the decrease in dissolved oxygen with increasing respiration. Pond temperature and pH were measured with a handheld sonde (Oakton PTTestr 35). To determine water nutrient concentrations, 50 ml water samples were taken from 5 cm below the surface of each mesocosm, filtered through 0.7 µm glass microfibre filters (Whatman GF/F) and frozen. A week later the water samples were thawed and analysed for soluble reactive phosphorus (PO4) and nitrate + nitrite (NOx) concentrations on a flow injection analyser (Lachat QuickChem) following standard methods [35].
At the end of the experiment (28 April, week 4), we collected samples for benthic community composition, snail abundance, periphyton accrual and larval amphibian abundance. While mosquitofish primarily feed on pelagic food resources [15], their use of benthic resources has also been observed to cause changes to benthic communities [26]. Benthic community composition was determined from invertebrate counts taken from an 18 cm diameter benthic core in a common central location of each mesocosm. The dry mass of the dominant benthic invertebrate group, Chironomidae (non-biting midges), was then determined after drying at 60°C for 48 h. All snails (Planorbidae and Physidae) were then picked from each mesocosm, counted, and then dried and weighed in the same manner as the chironomids above. Periphyton tiles were scrubbed and rinsed into a filter apparatus and then filtered through a 0.7 µm glass microfibre filter (Whatman GF/F). The filters were frozen and then analysed for chlorophyll a as in the phytoplankton abundance estimation above. Larval Pacific tree frogs (Pseudacris regilla) were counted in mesocosms where they were present, euthanized using an overdose of tricaine methanesulfonate (MS-222) and dry mass obtained using methods above.
On 29 April, we captured all fish from each mesocosm and ran excretion trials to estimate mesocosm-level fish N and P excretion. Fish within a mesocosm were temporarily held in a 10-litre floating tub until all fish were captured. Thereafter, all fish from a given mesocosm were introduced to a 2-litre Nalgene bottle filled with 1.5 litres of dechlorinated city water and floated in their respective mesocosm for 30–60 min. In fishless treatments, a Nalgene bottle was floated in the same manner with the same water, but without any fish added. A water sample was then taken from each bottle as in the NOx and PO4 measurements above, and its ammonium (NH4) and PO4 concentrations were determined using the same instrumentation and standard practice [35]. Excretion rate was calculated for all fish treatments as the concentration measured in each tank minus the mean fishless concentration, all divided by the excretion trial time length. After the excretion assay, we euthanized experimental fish using an overdose of MS-222. Fish were then dried and weighed as above.
(c). Analysis
At the end of the experiment we discovered that four fish had been initially misidentified to sex and three individuals had died (0.8% overall mortality). No fish in the even sex ratio treatment died, nor were any misidentified to sex, so to avoid dropping replicates, we combined the 0 and 25% male treatments into a single ‘female-biased’ treatment (n = 12 replicates) and the 75 and 100% male treatments into a single ‘male-biased’ treatment (n = 12 replicates). We then ran two separate but identical analyses to determine the differences between (1) the male-biased treatment versus the female-biased treatment, representing the ecological effects of sex ratio variation, and (2) the fishless treatment versus the ‘even’ (50 : 50 female : male) treatment, representing the ecological effects of addition of mosquitofish as occurs in the context of an introduction or invasion. Mosquitofish introduction is known to have very strong impacts on most of the ecological variables measured, and the main purpose of this latter analysis was to provide a frame of reference effect size to better interpret the relative importance of sex ratio effects.
We employed MANOVA of repeated measures for pelagic (time series) responses. Treatment and time × treatment interaction effects were of primary interest. Treatment effects were also tested independently for each time point using Student's t-tests, after checking for equality of variances using Levene's test (α = 0.05) [37]. If Levene's test was significant for a given response, then Welch's t-test for unequal variances was used [38]. Bonferroni corrections were implemented for interpretation of significance for the two non-independent t-tests performed on time-series data (i.e. those performed at both times separately). Benthic responses, excretion rates and fish biomass were measured once at the end of the experiment, and thus were analysed using t-tests as above. Analyses were performed in JMP Pro v. 11.2.0 and R v. 3.1.2 [39]. Zooplankton abundance data were log 10(x + 1)-transformed in order to meet assumptions of normality of residuals. A summary of all the above statistical tests is provided in electronic supplementary material, table S2.
Calculations of effect size (Cohen's d, where 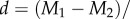
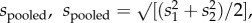 M = mean, and s = standard deviation) [40] were used to compare sex ratio effects with the effect of mosquitofish introduction. For pelagic responses, which were measured twice, we used data from the sampling time point showing the greatest sex ratio effect. Post hoc path analyses were used to explore the strength of potential causal linkages among response variables. These analyses were performed on standardized data in R with the package lavaan [41]. Lastly, we examined whether total fish biomass explained variation in important pelagic responses within grouped sex ratio treatments using simple linear regression.
M = mean, and s = standard deviation) [40] were used to compare sex ratio effects with the effect of mosquitofish introduction. For pelagic responses, which were measured twice, we used data from the sampling time point showing the greatest sex ratio effect. Post hoc path analyses were used to explore the strength of potential causal linkages among response variables. These analyses were performed on standardized data in R with the package lavaan [41]. Lastly, we examined whether total fish biomass explained variation in important pelagic responses within grouped sex ratio treatments using simple linear regression.
3. Results
Female-biased treatments had lower abundances of crustacean zooplankton (dominated by Daphnia, Bosmina, Sida and calanoid copepods) than male-biased treatments (F1,22 = 11.620, p = 0.003). Rotifer abundance (dominated by the family Brachionidae) did not differ between sex ratio treatments (F1,22 = 1.069, p = 0.312). Female-biased treatments had higher phytoplankton abundance (F1,22 = 3.953, p = 0.059) and higher rates of NPP (F1,22 = 4.819, p = 0.039), ER (F1,22 = 12.315, p = 0.002) and GPP (F1,22 = 9.441, p = 0.006). Although the sex ratio effect was non-significant for pH (F1,22 = 2.084, p = 0.163) and temperature (F1,22 = 2.698, p = 0.115), the time × sex ratio interaction effects were significant (pH: F1,22 = 4.400, p = 0.048; temperature: F1,22 = 9.333, p = 0.006). Overall, these results suggest that female-biased populations induced stronger pelagic trophic cascades when compared with male-biased populations by driving a relative decrease in crustacean zooplankton abundance, an increase in phytoplankton abundance and an increase in ecosystem production and respiration (figure 2). Concentrations of NOx and PO4 were not influenced by sex ratio (NOx: F1,22 = 0.021, p = 0.887; PO4: F1,22 = 1.742, p = 0.201) or the time × sex ratio interaction (NOx: F1,22 = 0.078, p = 0.783; PO4: F1,22 = 2.046, p = 0.167). The t-tests performed to analyse pelagic response trends at each time separately were in general support of results from the MANOVA of repeated measures. Pelagic responses, other than crustacean zooplankton abundance, were significant at week 2 but not at week 4 (electronic supplementary material, table S2).
Figure 2.
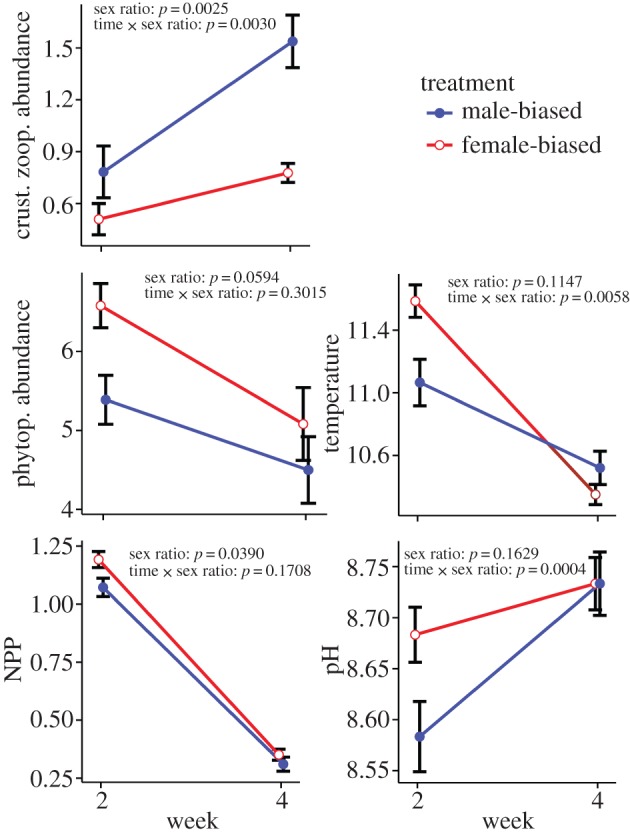
Impacts of mosquitofish sex ratio on the strength of various components of the pelagic trophic cascade (mean ± s.e.). Ponds with female-biased sex ratios had relatively lower densities of crustacean zooplankton, higher densities of phytoplankton, higher NPP, higher pH and higher temperature. p-values are reported for the sex ratio and time × sex ratio effects from the MANOVA of repeated measures.
Benthic invertebrate samples were dominated by chironomid larvae (98% of all organisms counted). Sex ratio had no effect on their abundance (t22 = 0.164, p = 0.871), total mass (t22 = 0.499, p = 0.622) or mean individual mass (t22 = 0.154, p = 0.879). Mean individual snail mass was larger in female-biased treatments (mean = 0.061 g, s.d. = 0.025) than male-biased treatments (mean = 0.045 g, s.d. = 0.013) (Welch's t14.83 = 1.883, p = 0.040), but there were no differences in overall snail abundance (t22 = 1.189, p = 0.247) or biomass (t22 = 0.242, p = 0.811). Periphyton accrual was not affected by sex ratio (t22 = 1.243, p = 0.227).
Female-biased treatments had a mean fish biomass of 1.877 g (s.d. = 0.1707) and excretion rates of 4.470 µg N min−1 (s.d. = 1.0197) and 0.485 µg P min−1 (s.d. = 0.4430), while male-biased treatments had a mean fish biomass of 0.962 g (s.d. = 0.2454) and excretion rates of 1.807 µg N min−1 (s.d. = 0.4667) and 0.132 µg P min−1 (s.d. = 0.3445). These differences were significant for biomass of mosquitofish (t22 = 10.602, p < 0.001) and excretion rates for both N (t22 = 8.225, p < 0.001) and P (t22 = 2.178, p = 0.040).
Amphibian eggs were deposited in at least one replicate of each treatment within the first week of fish introduction. However, larval amphibians were only recovered in three replicates of the fishless treatment at week 4. The biomass of tadpoles in those replicates ranged from 0.051 to 0.289 g.
Mosquitofish addition (relative to fishless conditions) affected all pelagic responses except for NOx concentration (electronic supplementary material, table S2 and figure S1). Mosquitofish addition also had no effect on the number of snails (t10 = 0.282, p = 0.784) and chironomids (t10 = 0.772, p = 0.458), the mean individual snail mass (t10 = 0.444, p = 0.667) and the mean individual chironomid mass (t10 = 1.031, p = 0.327), or the overall snail mass (t10 = 0.005, p = 0.996) and overall chironomid mass (t10 = 1.367, p = 0.202). Periphyton accrual was higher in the mosquitofish addition treatment (mean = 0.172 µg cm−2, s.d. = 0.1229) than in the fishless treatment (mean = 0.049 µg cm−2, s.d. = 0.0158) (t10 = 2.433, p = 0.035).
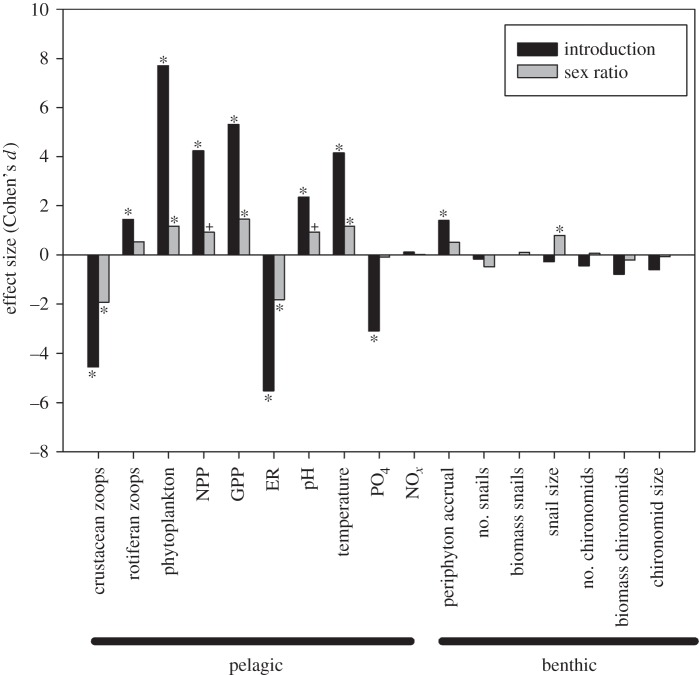
Upper effect sizes were calculated on week 2 data for all pelagic responses except for zooplankton abundance, which was performed on week 4 data. Sex ratio effect sizes for all significant responses were ‘large’ (i.e. greater than 0.8 [40]) (figure 3). Interestingly, although sex ratio effects were mostly less than half those of mosquitofish introduction (presence versus absence), female-biased sex ratio effects nearly always reinforced introduction effects (figure 3). Path analyses were performed to investigate the drivers of phytoplankton abundance, NPP and snail size across all treatments. We found that phytoplankton abundance at week 2 across all treatments with fish was affected by fish excretion rather than by crustacean zooplankton abundance (electronic supplementary material, figure S2). NPP was found to be driven by direct effects of both temperature and phytoplankton abundance (electronic supplementary material, figure S3). Snail size was related to the number of females present in a mesocosm, but not significantly related to temperature or the amount of primary production (electronic supplementary material, figure S4). Linear regressions of significant pelagic responses on biomass were not significant (α = 0.10) in any case (electronic supplementary material, table S3).
Figure 3.
Comparisons of upper sex ratio effect sizes with effect sizes of mosquitofish introduction at an even sex ratio. Significance symbols from t-tests (p < 0.10+, p < 0.05*; electronic supplementary material, table S2) are based on unadjusted p-values for benthic responses and Bonferroni-adjusted p-values (i.e. 2 × p) for pelagic responses since they were measured twice. Effects going in the same direction (positive or negative) indicate that female-biased treatments exacerbated the effects of mosquitofish introduction relative to male-biased treatments.
4. Discussion
Mosquitofish are a globally introduced freshwater fish showing pronounced sexual and ecological dimorphism and widespread variation in adult sex ratios (figure 1) [15,34]. Female mosquitofish are larger than males, prefer larger food items [22,23], exhibit higher feeding rates [20,21] and spend more time foraging in the presence of other females [24,25]. We therefore predicted that female-biased populations would induce stronger trophic cascades than male-biased populations. Our results show that female-biased populations also exhibit higher nitrogen and phosphorus excretion rates, as expected given the sexual size dimorphism, which could also lead to an increase in trophic cascade strength.
Consistent with this prediction, we found that experimental ponds with female-biased populations had reduced crustacean zooplankton abundances and increased phytoplankton abundances relative to ponds with male-biased populations (figure 2). Female-biased ponds had higher water temperatures, probably caused by the increase in turbidity associated with increased phytoplankton abundance, which can increase the absorption of solar heat energy [42]. Female-biased ponds also had higher NPP and pH compared with male-biased ponds. Increases in NPP can be attributed to both the increase in pond temperature and the increase in phytoplankton abundance (electronic supplementary material, figure S3). Pond pH increases when primary production reduces the amount of dissolved carbon dioxide, shifting the equilibrium in the aqueous carbonate system [43]. While the large effect of mosquitofish introduction on pond temperature and pH has been noted in the past [26,44], our results suggest that sex ratio has a surprisingly large role mediating the magnitude of these effects (figure 3).
Early on in our experiment (week 2), the effect of sex ratio for trophic cascades was clear, despite relatively modest effects of sex ratio on crustacean zooplankton abundance. Excretion is known to play a large role in contributing to trophic cascades [45], and thus treatment differences in excretion could have driven this early cascade (electronic supplementary material, figure S2). This trend may additionally highlight the importance of behavioural changes induced by perceived threats of predation [46]. In this case, zooplankton could have reduced their foraging rates to a greater extent in female-biased treatments relative to male-biased treatments. Later in our experiment (week 4) the cascading effects of sex ratio diminished, while zooplankton effects greatly strengthened. It is possible that proliferation of grazing-resistant forms of phytoplankton led to the observed reduction in trophic cascade strength through time (figure 2; and electronic supplementary material, figure S1) [45] or that these patterns represent natural cycling of zooplankton and phytoplankton abundances.
Mosquitofish are typically consumers of pelagic and epipelagic resources [15]. However, mosquitofish may also consume benthic food resources, especially in the littoral zone. In our experiment, mosquitofish introduction did not decrease chironomid density, which may be due to greater resources available in the pelagic zone of our artificial pond mesocosms. Indeed, the only significant effect of mosquitofish on benthic resources (p = 0.04) was for snails. Snails are not a major diet item, yet there is evidence that they are occasionally consumed, especially by large females [47]. Surprisingly, sex ratio influenced the average individual size of snails while fish introduction had no effect on snail size. Predation is known to plastically induce higher growth rates in snails [48]. Female-biased mesocosms had larger snails than male-biased mesocosms, which could be the result of faster growth rates of snails in response to the presence of snail-consuming females. Alternatively, both the higher temperatures and the relatively high rate of primary production in ponds with female-biased sex ratios could have led to faster snail growth. Path analysis suggests that the number of females present had a larger effect on snail size than did temperature or NPP (electronic supplementary material, figure S4); however, the mechanism driving this effect remains unclear.
Mosquitofish have been introduced worldwide throughout the past century for mosquito biocontrol purposes, resulting in unintended consequences for native biodiversity. Their negative impacts on amphibians and native fishes have been the subject of extensive study (reviewed in [15]). In our experiment, amphibians deposited eggs in all treatments, but larval amphibians were found only in mesocosms lacking mosquitofish. The introduction of mosquitofish into historically fishless habitats (e.g. isolated springs) has led to declines in native invertebrates as well [49]. Because of these global impacts, mosquitofish have been nicknamed the ‘plague minnow’ [50] and identified as one of the world's 100 worst invasive species [18]. Where mosquitofish have invaded, population control efforts are commonly employed to mitigate such negative consequences. Traditional methods of mosquitofish removal such as the use of minnow traps may selectively remove females due to their larger body size [24]. A recently described control method proposes to use ‘Trojan sex chromosomes’ to control mosquitofish abundance by generating females that can only produce male offspring, with the goal of creating male-biased populations in order to lower reproductive output and increase the probability of local extinction [49]. Our results suggest that control measures that reduce the relative abundance of females may have added benefits for aquatic communities and ecosystems since it is the females that cause the strongest ecological effects.
Our experimental design sought to control fish density while allowing for natural variation in biomass associated with sexual size dimorphism. Controlling density was necessary because density strongly influences behaviour of these social fish [51,52]. At the same time, including the effects of sexual size dimorphism was important because it is a principal expression of sexual dimorphism in mosquitofish, a widespread form of dimorphism in general, and theoretically important due to its effects on consumption and excretion. Biomass and sex ratio were strongly collinear in our experiment (electronic supplementary material, figure S5), suggesting sexual size dimorphism is indeed an important component of our findings, but it is important to recognize that our study design does not preclude effects of other forms of ecological dimorphism. Indeed biomass variation from size dimorphism did not explain a significant amount of variation in pelagic responses within treatments (electronic supplementary material, table S3). Moreover, one might predict some amount of sex ratio effects tied to other ecological aspects of sexual dimorphism. Empirical evidence suggests females have higher feeding rates than males per unit body size [20,21] and spend more time feeding when in the presence of other females [24,25]. In addition, mosquitofish size dimorphism is largely related to differences in post-maturation growth rates. Since excretion rates are determined partly by instantaneous growth rate [53], it is likely that there exists some size-specific sexual dimorphism in excretion rates. Future investigation of sex ratio effects might thus profitably employ study designs to isolate and estimate the ecological effects of size- and non-size components of sexual dimorphism.
5. Conclusion
Our work demonstrates that sex ratio variation in ecologically important species showing sexual dimorphism can lead to marked ecological effects. This study adds to a growing literature suggesting that intraspecific variation may be important for shaping ecology [54–56]. Indeed, sexual dimorphism is one of the most common and well-known forms of intraspecific trait variation in the wild and many populations in nature show marked demographic differences in sex ratios from the commonly assumed expectation of 1 : 1 [2–5,7]. As such, we suggest that sex ratios may be a common driver of community and ecosystem variation across a wide diversity of organisms and habitat types. We recommend future investigations into other study systems where there is known sexual dimorphism in functional traits, where the focal species is ecologically important (e.g. keystone species, invasive species, dominant species), and where there is substantial sex ratio variation in the wild. Subsequent work with size dimorphic species should aim to isolate biomass-dependent and biomass-independent sex ratio effects. Such work can further inform our understanding of the ecological importance of one of the most common forms of intraspecific trait variation in nature.
Supplementary Material
Supplementary Material
Acknowledgements
We thank S. Kelson, M. Michelson, S. Steiner, K. Roessler, W. Wright, L. Lambwotton and R. Franks for assistance in the lab and in the field. P. Raimondi and S. Munch provided invaluable analysis advice. We thank the Sacramento–Yolo MVCD for graciously providing the experimental fish. Two anonymous reviewers provided comments that considerably improved the manuscript.
Ethics
All handling of vertebrates was approved under UCSC IACUC protocol Palke1306-2.
Data accessibility
Data from the experimental pond study are available at Dryad http://dx.doi.org/10.5061/dryad.1f734.
Authors' contributions
D.C.F., H.A.A., M.T.K. and E.P.P. designed the experiment based on a pilot concept by H.A.A., E.P.P. and M.T.K. D.C.F., H.A.A. and T.M.A. carried out the experiment and analysed samples. D.C.F. performed statistical analyses. T.M.A. created the map. D.C.F. and E.P.P. led writing of the manuscript with contributions from all authors.
Competing interests
We have no competing interests.
Funding
This work was funded by the National Science Foundation (NSF-DEB #1457333, NSF-DEB #1457112), UC Santa Cruz, and the Maine Agricultural and Forest Experiment Station (#3442). D.C.F. was supported by a NSF EAPSI Fellowship (#1316649) and a NSF Graduate Research Fellowship.
References
- 1.Fisher RA. 1930. The genetical theory of natural selection. Oxford, UK: Clarendon Press. [Google Scholar]
- 2.Arendt JD, Reznick DN, López-Sepulcre A. 2014. Replicated origin of female-biased adult sex ratio in introduced populations of the Trinidadian Guppy (Poecilia reticulata). Evolution 68, 2343–2356. ( 10.1111/evo.12445) [DOI] [PubMed] [Google Scholar]
- 3.Hamilton WD. 1967. Extraordinary sex ratios. Science 156, 477–488. ( 10.1126/science.156.3774.477) [DOI] [PubMed] [Google Scholar]
- 4.Mills LJ, Chichester C. 2005. Review of evidence: are endocrine-disrupting chemicals in the aquatic environment impacting fish populations? Sci. Total Environ. 343, 1–34. ( 10.1016/j.scitotenv.2004.12.070) [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez EM, Medesani DA, Fingerman M. 2007. Endocrine disruption in crustaceans due to pollutants: a review. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 146, 661–671. ( 10.1016/j.cbpa.2006.04.030) [DOI] [PubMed] [Google Scholar]
- 6.Trivers RL, Willard DE. 1973. Natural selection of parental ability to vary the sex ratio of offspring. Science 179, 90–92. ( 10.1126/science.179.4068.90) [DOI] [PubMed] [Google Scholar]
- 7.Clutton-Brock TH, Iason GR. 1986. Sex ratio variation in mammals. Q. Rev. Biol. 61, 339–374. ( 10.1086/516403) [DOI] [PubMed] [Google Scholar]
- 8.Kahn AT, Kokko H, Jennions MD. 2013. Adaptive sex allocation in anticipation of changes in offspring mating opportunities. Nat. Commun. 4, 1603 ( 10.1038/ncomms2634) [DOI] [PubMed] [Google Scholar]
- 9.Thresher RE, Canning M, Bax NJ. 2013. Demographic effects on the use of genetic options for the control of mosquitofish, Gambusia holbrooki. Ecol. Appl. 23, 801–814. ( 10.1890/12-1324.1) [DOI] [PubMed] [Google Scholar]
- 10.Brose U, et al. 2006. Consumer-resource body-size relationships in natural food webs. Ecology 87, 2411–2417. ( 10.1890/0012-9658%282006%2987%5B2411%3ACBRINF%5D2.0.CO%3B2) [DOI] [PubMed] [Google Scholar]
- 11.Rall BC, Brose U, Hartvig M, Kalinkat G, Schwarzmuller F, Vucic-Pestic O, Petchey OL. 2012. Universal temperature and body-mass scaling of feeding rates. Phil. Trans. R. Soc. B 367, 2923–2934. ( 10.1098/rstb.2012.0242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall RO, Koch BJ, Marshall MC, Taylor BW, Tronstad LM. 2007. How body size mediates the role of animals in nutrient cycling in aquatic ecosystems. In Body size: the structure and function of aquatic ecosystems (eds Hildrew AG, Raffaelli DG, Edmonds-Brown R), pp. 286–305. New York, NY: Cambridge University Press. [Google Scholar]
- 13.Vanni MJ. 2002. Nutrient cycling by animals in freshwater ecosystems. Annu. Rev. Ecol. Syst. 33, 341–370. ( 10.1146/annurev.ecolsys.33.010802.150519) [DOI] [Google Scholar]
- 14.Shine R. 1989. Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Q. Rev. Biol. 64, 419–461. ( 10.1086/416458) [DOI] [PubMed] [Google Scholar]
- 15.Pyke GH. 2005. A review of the biology of Gambusia affinis and G. holbrooki. Rev. Fish Biol. Fish. 15, 339–365. ( 10.1007/s11160-006-6394-x) [DOI] [Google Scholar]
- 16.Wooten MC, Scribner KT, Smith MH. 1988. Genetic variability and systematics of Gambusia in the southeastern United States. Copeia 1988, 283–289. ( 10.2307/1445867) [DOI] [Google Scholar]
- 17.Lloyd L, Arthington AH, Milton DA. 1986. The mosquito fish—a valuable mosquito-control agent or a pest? In The ecology of exotic animals and plants: some Australian case histories (ed. Kitching RL.), pp. 5–25. New York, NY: John Wiley & Sons. [Google Scholar]
- 18.Lowe S, Browne M, Boudjelas S, De Poorter M. 100 of the world's worst invasive alien species. 2000. Auckland, New Zealand: Invasive Species Specialist Group. [Google Scholar]
- 19.Encyclopedia of Life. 2015. Gambusia affinis. See http://eol.org/pages/218297/data (accessed 25 March 2015).
- 20.Reddy SR. 1975. Effect of water temperature on the predatory efficiency of Gambusia affinis. Experentia 31, 801–802. ( 10.1007/BF01938473) [DOI] [Google Scholar]
- 21.Shakuntala K. 1977. Effect of feeding level on the rate and efficiency of food conversion in the cyprinodon fish Gambusia affinis. Ceylon J. Sci. 12, 177–184. [Google Scholar]
- 22.Bence JR, Murdoch WW. 1986. Prey size selection by the mosquitofish: relation to optimal diet theory. Ecology 67, 324–336. ( 10.2307/1938576) [DOI] [Google Scholar]
- 23.Homski D, Goren M, Gasith A. 1994. Comparative evaluation of the larvivorous fish Gambusia affinis and Aphanius dispar as mosquito control agents. Hydrobiologia 284, 137–146. ( 10.1007/BF00006885) [DOI] [Google Scholar]
- 24.Pilastro A, Benetton S, Bisazza A. 2003. Female aggregation and male competition reduce costs of sexual harassment in the mosquitofish Gambusia holbrooki. Anim. Behav. 65, 1161–1167. ( 10.1006/anbe.2003.2118) [DOI] [Google Scholar]
- 25.Arrington JJ, Thaman KRJ, Rettig JE, Smith GR. 2009. Foraging behavior of male and female mosquitofish (Gambusia affinis) in single- and mixed-sex groups. J. Freshw. Ecol. 24, 327–329. ( 10.1080/02705060.2009.9664299) [DOI] [Google Scholar]
- 26.Hurlbert SH, Zedler J, Fairbanks D. 1972. Ecosystem alteration by mosquitofish (Gambusia affinis) predation. Science 175, 639–641. ( 10.1126/science.175.4022.639) [DOI] [PubMed] [Google Scholar]
- 27.Lancaster HF, Drenner RW. 1990. Experimental mesocosm study of the separate and interaction effects of phosphorus and mosquitofish (Gambusia affinis) on plankton community structure. Can. J. Fish. Aquat. Sci. 47, 471–479. ( 10.1139/f90-051) [DOI] [Google Scholar]
- 28.Lenert LG. 1923. Gambusia affinis: its use in mosquito control. Calif. State Board Heal. Wkly. Bull. 2, 1–2. [Google Scholar]
- 29.Mosquito and Vector Control Association of California. 2015. About us. See http://www.mvcac.org/about-us.
- 30.Sacramento–Yolo Mosquito & Vector Control District. 2005. Annual Report 2005. See http://www.fightthebite.net/annual-report/AnnualReport2005.pdf.
- 31.Pyke GH. 2008. Plague minnow or mosquito fish? A review of the biology and impacts of introduced Gambusia species. Annu. Rev. Ecol. Evol. Syst. 39, 171–191. ( 10.1146/annurev.ecolsys.39.110707.173451) [DOI] [Google Scholar]
- 32.Britton RH, Moser ME. 1982. Size specific predation by herons and its effect on the sex-ratio of natural populations of the mosquito fish Gambusia affinis Baird and Girard. Oecologia 53, 146–151. ( 10.1007/BF00545657) [DOI] [PubMed] [Google Scholar]
- 33.Haynes JL, Cashner RC. 1995. Life history and population dynamics of the western mosquitofish: a comparison of natural and introduced populations. J. Fish Biol. 46, 1026–1041. ( 10.1111/j.1095-8649.1995.tb01407.x) [DOI] [Google Scholar]
- 34.Krumholz LA. 1948. Reproduction in the western mosquitofish, Gambusia affinis affinis (Baird & Girard), and its use in mosquito control. Ecol. Monogr. 18, 1–43. ( 10.2307/1948627) [DOI] [Google Scholar]
- 35.Rice EW, Baird RB, Eaton AD, Clesceri LS (eds). 2012. Standard methods for the examination of water and wastewater, 22nd edn Washington, DC: American Public Health Association. [Google Scholar]
- 36.Harmon LJ, Matthews B, Des Roches S, Chase JM, Shurin JB, Schluter D. 2009. Evolutionary diversification in stickleback affects ecosystem functioning. Nature 458, 1167–1170. ( 10.1038/nature07974) [DOI] [PubMed] [Google Scholar]
- 37.Levene H. 1960. Robust tests for equality of variances. In Contributions to probability and statistics: essays in honor of Harold Hotelling (eds Olkin I, Ghurge SG, Hoeffding W, Madow WG, Mann HB), pp. 278–292. Stanford, CA: Stanford University Press. [Google Scholar]
- 38.Welch BL. 1947. The generalisation of student's problems when several different population variances are involved. Biometrika 34, 28–35. ( 10.1093/biomet/34.1-2.28) [DOI] [PubMed] [Google Scholar]
- 39.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 40.Cohen J. 1992. A power primer. Psychol. Bull. 112, 155–159. ( 10.1038/141613a0) [DOI] [PubMed] [Google Scholar]
- 41.Rosseel Y. 2012. lavaan: an R package for structural equation modeling. J. Stat. Softw. 48, 1–36. ( 10.18637/jss.v048.i02) [DOI] [Google Scholar]
- 42.Mazumder A, Taylor WD, McQueen DJ, Lean DR. 1990. Effects of fish and plankton and lake temperature and mixing depth. Science 247, 312–315. ( 10.1126/science.247.4940.312) [DOI] [PubMed] [Google Scholar]
- 43.Dodson S. 2005. pH, inorganic carbon equilibrium, and photosynthesis. In Introduction to limnology (ed. Dodson S.), p. 235. New York: McGraw-Hill. [Google Scholar]
- 44.Hurlbert SH, Mulla MS. 1981. Impacts of mosquitofish (Gambusia affinis) predation on plankton communities. Hydrobiologia 83, 125–151. ( 10.1007/BF02187157) [DOI] [Google Scholar]
- 45.Persson L. 1999. Trophic cascades: abiding heterogeneity and the trophic level concept at the end of the road. Oikos 85, 385–397. ( 10.2307/3546688) [DOI] [Google Scholar]
- 46.Schmitz OJ, Beckerman AP, O'Brien KM. 1997. Behaviorally mediated trophic cascades: effects of predation risk on food web interactions. Ecology 78, 1388–1399. ( 10.1890/0012-9658%281997%29078%5B1388:BMTCEO%5D2.0.CO;2) [DOI] [Google Scholar]
- 47.Hubbs C. 1990. Snails as a food source for Gambusia. Texas J. Sci. 42, 245–256. [Google Scholar]
- 48.Crowl TA, Covich AP. 1990. Predator-induced life-history shifts in a freshwater snail. Science 247, 949–951. ( 10.1126/science.247.4945.949) [DOI] [PubMed] [Google Scholar]
- 49.Schaefer JF, Heulett ST, Farrell TM. 1994. Interactions between two poeciliid fishes (Gambusia holbrooki and Heterandria formosa) and their prey in a Florida marsh. Copeia 1994, 516–520. ( 10.2307/1447002) [DOI] [Google Scholar]
- 50.Pyke GH, White AW. 2000. Factors influencing predation on eggs and tadpoles of the endangered green and golden bell frog Litoria aurea by the introduced plague minnow Gambusia holbrooki. Aust. Zool. 31, 496–505. ( 10.7882/AZ.2000.011) [DOI] [Google Scholar]
- 51.Smith CC. 2007. Independent effects of male and female density on sexual harassment, female fitness, and male competition for mates in the western mosquitofish Gambusia affinis. Behav. Ecol. Sociobiol. 61, 1349–1358. ( 10.1007/s00265-007-0365-7) [DOI] [Google Scholar]
- 52.Cureton JC, Martin RE, Deaton R. 2010. Short term changes in sex ratio and density alter coercive male mating tactics. Behaviour 147, 1431–1442. ( 10.1163/000579510X519495) [DOI] [Google Scholar]
- 53.Kraft CE. 1992. Estimates of phosphorus and nitrogen cycling by fish using a bioenergetics approach. Can. J. Fish. Aquat. Sci. 49, 2596–2604. ( 10.1139/f92-287) [DOI] [Google Scholar]
- 54.Schoener TW. 2011. The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science 331, 426–429. ( 10.1126/science.1193954) [DOI] [PubMed] [Google Scholar]
- 55.Bolnick DI, et al. 2011. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 26, 183–192. ( 10.1016/j.tree.2011.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Post DM, Palkovacs EP. 2009. Eco-evolutionary feedbacks in community and ecosystem ecology: interactions between the ecological theatre and the evolutionary play. Phil. Trans. R. Soc. B 364, 1629–1640. ( 10.1098/rstb.2009.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the experimental pond study are available at Dryad http://dx.doi.org/10.5061/dryad.1f734.