Abstract
Antarctic penguins survive some of the harshest conditions on the planet. Emperor penguins breed on the sea ice where temperatures drop below −40°C and forage in −1.8°C waters. Their ability to maintain 38°C body temperature in these conditions is due in large part to their feathered coat. Penguins have been reported to have the highest contour feather density of any bird, and both filoplumes and plumules (downy feathers) are reported absent in penguins. In studies modelling the heat transfer properties and the potential biomimetic applications of penguin plumage design, the insulative properties of penguin plumage have been attributed to the single afterfeather attached to contour feathers. This attribution of the afterfeather as the sole insulation component has been repeated in subsequent studies. Our results demonstrate the presence of both plumules and filoplumes in the penguin body plumage. The downy plumules are four times denser than afterfeathers and play a key, previously overlooked role in penguin survival. Our study also does not support the report that emperor penguins have the highest contour feather density.
Keywords: feathers, penguin, insulation, feather density, emperor penguin
1. Introduction
Antarctic penguins must endure some of the greatest environmental challenges on the Earth. Emperor penguins (Aptenodytes forsteri) spend six months a year in one of the coldest habitats on the planet, breeding during the Antarctic winter where air temperatures fall below −40°C and winds sometimes reach 26 m s−1 (50 knots). To feed their offspring, they dive in −1.8°C waters to depths in excess of 500 m, deeper than any other diving animal that relies on an exterior coat of feathers or fur. Individuals survive in large part owing to their thick and morphologically specialized plumage [1], which can provide 80–90% of insulation requirements [2,3] and enables emperor penguins to maintain a core body temperature of 38°C [4]. The insulative integrity of the feathers probably persists even at their maximum dive depth of 560 m [5] because their deep dives continue serially for several hours with short surface intervals [6].
The penguin's plumage is one of the keys to survival in Antarctica. Yet there is a dearth of information about penguin body feathers. In addition, much of what is reported about penguin feathers is either unsubstantiated or conflicting. The objectives of this study were to examine the types, density and distribution patterns of body feathers on the emperor penguin, including (i) contour feathers, (ii) afterfeathers, (iii) plumules and (iv) filoplumes.
The contour feathers are the stiff outer layer of body feathers that protect the bird's skin. In the emperor penguin, contour feathers provide an impenetrable and rigid waterproof cover over a thick, insulative layer of down [1]. There are numerous reports stating that emperor penguins or penguins as a taxonomic order have the highest contour feather density of any bird [7,8]. Despite these reports from popular media, we could find no published studies that have examined this question. In addition, there is no consensus of penguin feather density values and published reports vary fourfold, from 11 to 46 feathers cm−2 [9–12]. Surprisingly, none of these studies discusses methods or cites sources for determining feather density.
Afterfeathers and plumules are both soft downy feathers that make up the insulation component of many avian species plumage. Afterfeathers are attached to contour feathers, while plumules are separate feathers that attach directly to the skin. Although an early study of penguin feathers reported uniform distribution of both plumules and contour feathers in penguins as a taxonomic order [13], more recent studies omit any reference to plumules and their role in insulation [9,11,14–16]. The impressive thermal qualities of penguin body feathers have prompted modelling studies and inspired potential future applications [9,14,17–20]. However, the models in these studies attribute insulative properties of the plumage solely to the afterfeather, with no mention of plumules [9,14].
Filoplumes are very small feather structures consisting of a long, bare rachis (shaft) with short barbs and barbules only at the very tip of the rachis. They are believed to play a sensory role in alerting flying birds to displaced wing feathers [21]. Past studies have not found filoplumes present in penguin plumage [13]. Other reports also suggest filoplumes are absent in penguins and other non-flying birds, but methods for determining that penguins lack filoplumes were not discussed [22,23]. No studies have specifically examined the penguin plumage for filoplumes.
Despite the critically important role that body plumage plays in penguin survival, most of the aforementioned reports on penguin feathers are either conflicting or not supported by data. Yet this information has been perpetuated in numerous studies on heat transfer properties [9,14,24], mechanical properties [15] and feather evolution [25], and has inspired surveys and models of the potential biomimetic applications using the penguin plumage design for more effective thermal insulating materials in clothing or buildings [17–20]. However, the basic information on penguin feathers used in these studies may be inaccurate. The objectives of this study were to (i) identify the types of emperor penguin body feathers, including plumules, afterfeathers and filoplumes; (ii) determine feather density for each type of body feathers; (iii) model feather distribution patterns; and (iv) comment on the functional roles of these feather types.
2. Material and methods
Emperor penguin carcasses (n = 4) were collected opportunistically at Cape Washington, Antarctica during the spring of 2005 or at Cape Crozier, Antarctica in 2001 and kept in a −20°C freezer until examination in 2013–2014. Mass and girth of penguin carcasses are shown in table 1. Only feather samples were obtained from one penguin (EP1), as the carcass had been dissected in certain areas so an accurate mass and girth could not be obtained. EP4, a female, was found in an ice rubble field created by a large iceberg (B15A) colliding with, and crumpling, the sea ice around the Cape Crozier colony adjacent to the Ross Ice shelf [26]. EP4 was emaciated and probably starved as a result of the disruption of the sea ice.
Table 1.
Penguin characteristics and model inputs.
| mass (m) | height (h) | upper girth (g1) | widest girth (g2) | lower girth (g3) | |
|---|---|---|---|---|---|
| penguin | kg | cm | cm | cm | cm |
| EP2 | 22.2 | 42 | 71.9 | 85.4 | 74.2 |
| EP3 | 24.7 | 48 | 72 | 91.5 | 70.1 |
| EP4 | 11.2 | 36.5 | 59.2 | 73.2 | 62.6 |
(a). Feather types
The presence or absence of different feather types was assessed first. Contour feathers were removed from excised skin sections on the ventral and dorsal aspects of the bird body, and attached afterfeathers were visually identified. Plumules were identified visually on the excised skin sections after contour feathers and afterfeathers were removed. Details of the plumules were determined microscopically. Microscopy was also used to confirm filoplume presence on the excised skin sections. While the primary focus of this study was on the body plumage, for the purpose of a more complete and broader orientation, a descriptive feathergram showing the main feather types was made by removing a feather from each area of the penguin, including head, body, wings, tail and legs (figure 1).
Figure 1.
Feather types of emperor penguins: tail feather, or rectrice (1); dorsal, base of the tail (2); dorsal, base of the leg, or leg fringe (3); ventral, base of the leg, or leg fringe (4); dorsal contour (5); ventral contour with afterfeather attached (6); fringe of brood patch (7); distal, dorsal primary—double-layered with a dorsal and ventral primary (8); dorsal primary (9); ventral primary (10); dorsal primary (11); dorsal secondary (12); ventral secondary (13); dorsal covert (14); ventral covert (15); leading edge covert (16); skull (17); auricular patch contour (18); dorsal neck contour (19); ventral neck contour (20); dorsal plumulaceous (plumules) (21); ventral plumulaceous (22). See Rutschke [1] for further details on specialized feather morphology. (Online version in colour.)
(b). Feather density
Feather density on the ventral and dorsal aspects of the bird was determined for four types of body feathers: contour feathers, afterfeathers, plumules and filoplumes. As this part of the study focused on the penguin body, we did not examine feather density of other areas (e.g. wings, legs, head, neck) of the penguin.
Contour feathers were counted by marking 5 cm2 sections on the dorsal and ventral sides of each carcass. All contour and down feathers within the sections were cut with fine-tipped clippers as close to the skin as possible. Marked sections were measured and photographed and then, using a graphic software program (Photoshop v. 5, Adobe Systems Inc., San Jose, CA), the remnant of each shaft of cut contour feathers was marked and automatically summed in the program. Distribution of contour feathers was confirmed from the photographed and marked sections. This method was deemed the most suitable for quantifying contour feather density because (i) the attachment point of each contour feather was easily visible, (ii) the photos created an archival record allowing counts to be confirmed by different researchers and (iii) the skin could not stretch or shrink as possible in an excised sample.
To examine afterfeather density, all contour feathers from a 2.5 cm2 excised skin section were removed. Afterfeathers still attached to contour feathers removed from excised sections were counted. However, despite careful removal of contour feathers from the excised sections, it was difficult to preserve the connection between every contour feather and its afterfeather (figure 2a,b). Thus, any unattached afterfeathers were also collected and compared with the number of contour feathers without afterfeathers. Because the number of contour feathers without afterfeathers matched the number of unattached afterfeathers, we determined afterfeather density by counting contour feathers. In addition, during our examination of numerous individual contour feathers we did not find any contour feathers without an attached afterfeather or an attachment remnant.
Figure 2.

(a) Ventral contour feather with plumulaceous afterfeather connected by a long, thin hyporachis at the top of calamus, very close to the base of the rachis. The contour feathers have a broad, flattened rachis. The calamus of the ventral and dorsal contour feathers is curved at an almost 90° angle. (b) Close-up of hyporachis (diameter = 0.15 mm). (Online version in colour.)
The density of filoplumes and plumules was determined by establishing the number of filoplumes and plumules associated with each contour feather using microscopy. Once these patterns were established, plumule and filoplume densities were calculated based on contour feather density. As an additional confirmation of density, plumules were manually counted from one 2.4 × 2.8 cm2 section after contour feathers were plucked.
(c). Feather distribution pattern and a model of total body feather counts
The distribution pattern of all body feathers was determined by examining excised skin sections microscopically to establish the location of plumules and filoplumes in association with contour feathers (and attached afterfeathers). In addition to location, filoplume height was also examined microscopically.
To determine a gross estimate of the total number of feathers on the body (excluding the head, tail, legs and wings), a model was developed using feather density and distribution patterns as well as morphological measurements of emperor penguins. Model estimates were made based on the lateral surface area (LSA) equations for two conical frustums (figure 3).
Height, mass and three girth (g) measurements were made on each penguin as shown in table 1. Radius (r) was taken as g÷2π. All girth, height and surface area measurements were in centimetres. To determine body feather counts, LSA was multiplied by feather density. Feathers per mass calculations were made by dividing the total feather count by penguin mass.
Figure 3.
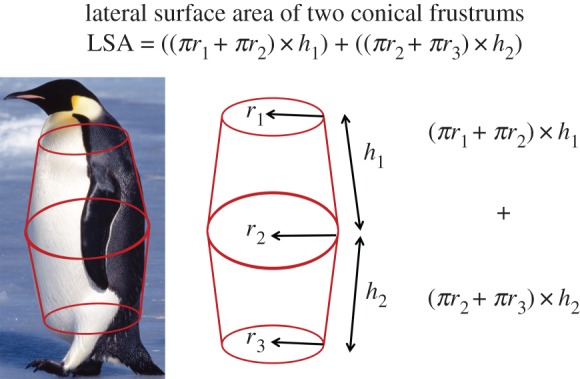
Feather count model illustration. (Online version in colour.)
(d). Statistics
Paired t-tests were conducted to compare the density of contour feathers on the dorsal and ventral sides, with significance level set at p < 0.05.
3. Results
(a). Feather types
The wide array of different feather types found on the emperor penguin from the head to the tail is illustrated in a feathergram (figure 1). This feathergram shows 12 main feather types. Note the exceptional length of the calamus (quill) of the wing and tail feathers. Filoplumes are excluded as they are too small to visualize in a feathergram, but are shown separately in figure 4a.
Figure 4.
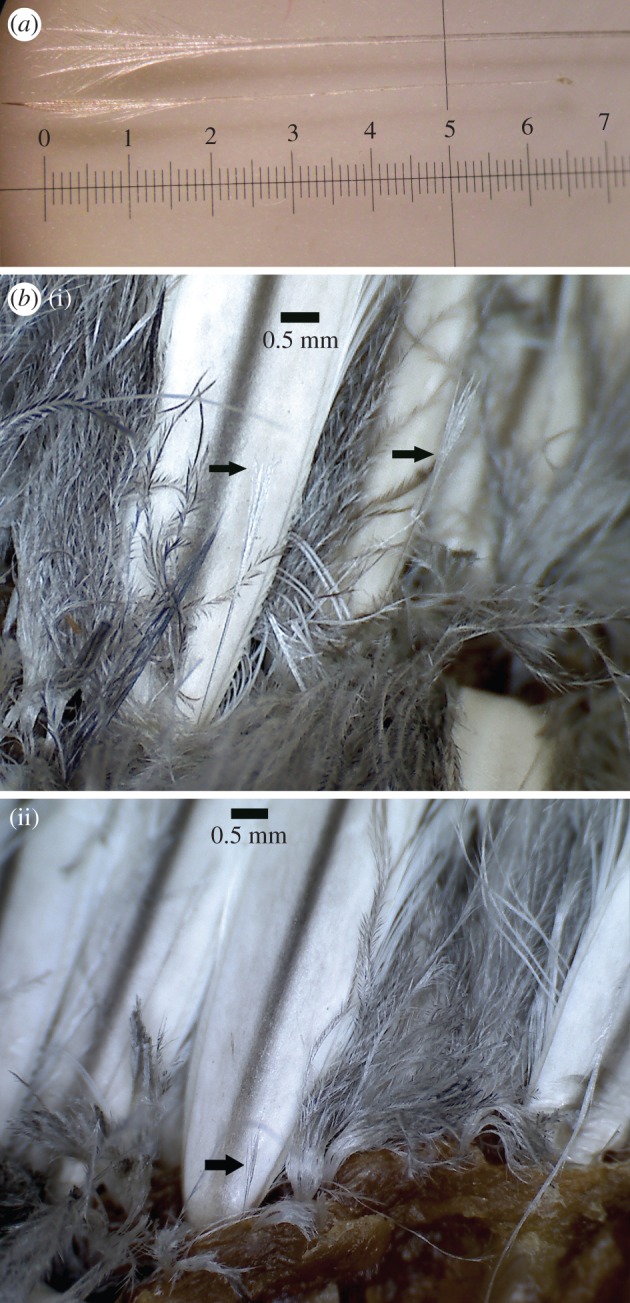
Filoplumes. (a) Ventral (top) and dorsal (bottom) filoplumes. The ventral filoplume has three barbs, whereas the dorsal filoplume has two barbs at the tip. Ventral filoplume is 14.6 mm in length and dorsal filoplume is 7.1 mm. (b(i,ii)) Filoplumes, varying in length and size, at the base of rachis of contour feathers. (Online version in colour.)
The presence of afterfeathers, filoplumes and plumules on each of the four emperor penguins carcasses was confirmed. Contour feathers, with attached afterfeathers, were as previously described [16]. The hyporachis, attaching the afterfeather to the contour feather near the base of the rachis, was approximately 0.15 mm in diameter (figure 2a,b). Plumules have a short calamus after which a hyporachis is attached to the rachis, dividing the seemingly single downy feather into a plumule with an attached downy afterfeather (figure 5). The plumule and afterfeather are similar in size. Filoplumes were mature as defined by Lucas & Stettenheim [27] and consist of a bare rachis with a thin, curved calamus (figure 4a). The rachis is transparent, with a diameter that varies considerably, from less than 0.05 to 0.1 mm. The tip of the filoplume has two to four thin barbs that fan out for the last 2–3 µm of the filoplume (figure 4a). Filoplumes varied in size and height (figure 4a,b).
Figure 5.
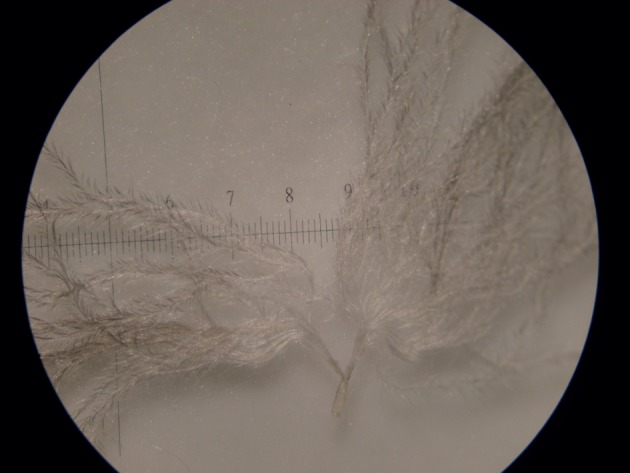
Close-up of plumule (scale division = 1 mm). (Online version in colour.)
(b). Feather density
The density of contour feathers varied among the three emperor penguin carcasses (table 2). Contour feather density on the chest was 29–50% greater than dorsal feather density (p = 0.003, n = 3; table 2). An afterfeather was attached to each contour feather counted, thus afterfeather density was equal to contour feather density. Density counts for specimen EP4, the emaciated bird, were approximately 60% higher than the mean density for the other two specimens (EP2 and EP3; table 2). The density of plumules was four times the contour feather density. There was a 1 : 1 ratio of filoplumes to contour feathers.
Table 2.
Calculated feather density and counts from model. LSA, lateral surface area.
| penguin | ventral contour (feathers cm−2) | dorsal contour (feathers cm−2) | LSA cm2 | contour feather estimate | insulative feather estimate |
|---|---|---|---|---|---|
| EP2 | 8.7 | 5.8 | 3327 | 24 124 | 120 620 |
| EP3 | 9 | 6.5 | 3901 | 30 234 | 151 172 |
| EP4 | 13.5 | 10.5 | 2447 | 29 368 | 146 840 |
(c). Feather distribution pattern and total body feather counts
A drawing was created to illustrate the distribution pattern of body plumage (contour feathers, afterfeathers, plumules and filoplumes) on emperor penguin skin (figure 6). Although contour feathers are uniformly distributed across the penguin body, the pattern of all insulative feather types is complex and non-uniform (figure 6). Plumules are not evenly spaced between each other or contour feathers. Each contour feather is surrounded by nine plumules (figure 6). Three of the plumules are shared by three contour feathers, resulting in one ‘plumule equivalent’ for each contour feather. The remaining six plumules are shared between only two contour feathers, resulting in three ‘plumule equivalents’ for each contour feather. Thus, there are four plumules per contour feather. One filoplume is found at the base of each contour feather (figure 4b) on both the ventral and dorsal sides of the penguins. Filoplumes varied in height and were always located on the right side in front of each contour feather rachis (figure 4b).
Figure 6.
Drawing of feather distribution pattern. Filoplumes are very small, difficult to see and located right along the rachis. Plumules are located between contour feathers. Nine plumules (numbered 1–9) are adjacent to each contour feather. Three of the plumules (1, 4 and 7) are shared with two other contour feathers (e.g. plumule 1 is shared by contour feathers A, B and C). The six other plumules (2–3, 5–6, 8–9) are shared by one other contour feather (e.g. plumule 2 is shared by feathers A and B and plumule 9 is shared by feathers A and C). In order to show the pattern, feathers are not drawn to scale. Illustration by C. N. Cruz.
The model (figure 3) resulted in gross estimates of feathers on the body of each penguin ranging from 24 000 to 30 000 for contour feathers and 120 000 to 150 000 for insulative feathers (afterfeathers and plumules; table 2).
4. Discussion
Our results contradict recent studies in three important ways. First, and perhaps most importantly, plumules were clearly identified on the penguin plumage and had a density four times that of afterfeathers. Second, filoplumes were present and associated with each contour feather. Third, contour feather density was much lower than all previous reports despite the general perception that density should be greatest of all birds given the cold extremes emperor penguins face. In short, previously reported information on penguin plumage, and emperor penguin plumage in particular, has been erroneous. The prevailing idea that emperor penguin contour feather density is the highest of any bird is not supported by the results in this study.
We found that emperor penguin plumage is arranged in a distinctive pattern (figure 6). Each contour feather has an attached afterfeather and an adjacent filoplume, and is surrounded by plumules. Plumules are the main source of insulation, as these feathers form a dense mat beneath the contour feathers and are four times as numerous as other body feathers (table 2). Although plumules were mentioned by Chandler [13] and Lucas & Stettenheim [27], no densities or distribution patterns were discussed in these studies. It is puzzling that later studies do not mention plumules, and the afterfeather is assumed to be the only insulating feather [9,11,14–16]. This omission has been repeated in recent research [25,28] and undoubtedly leads to errors in models that assume the afterfeather is entirely responsible for the insulation provided by the plumage [9,29].
This downy layer of plumules and afterfeathers may also play a role in penguins' rapid underwater ascent, allowing them to fly out of the water on to the sea ice. The air lubrication hypothesis suggests that the release of air trapped in the downy layer into the boundary layer reduces drag, allowing penguins to reach high underwater speeds before exiting the water [28]. However, the hypothesis developed by Davenport et al. assumed that the afterfeather was the only downy feather in the penguin plumage [28]. The presence and high density of plumules also support the air lubrication hypothesis, as the plumules and the accompanying barbule structure should contribute to even finer bubble formation. The resulting bubbles are so small that it appears as if a trail of smoke is coming from the feathers. The volume of air and feathers for emperor penguins determined by whole-body CT scans under anaesthesia and at room air is as much as 5 l (P. Ponganis 2014, personal observation).
Contour feather density determined in this study is lower than all previous reports. Feather density typically refers to the number of contour feathers in a given area [11]. Although contour feather density varied between specimens, it was not as high as past studies have suggested (except for the emaciated penguin discussed below). In previous studies, penguin feather densities have ranged from 11 to 46 contour feathers cm−2 [9–12,14,16]. None of these studies have described the method of determining density or, in some cases, the penguin species used [11,16]. Our results (less than or equal to 9 cm−2) come closest to Stonehouse, although these values are still 20–50% lower than his values of approximately 11–12 cm−2 for all penguins [11,16]. Our results are the first detailed analysis of feather density in any penguin species. Whether feather density is similar among penguin species, as Stonehouse suggested [11,16], is unknown. The different thermal environments in which penguin species live suggest density might differ among species.
Although there have been only a few studies on the density of feathers in other birds, it is clear emperor penguins do not have the highest contour feather density of any bird. White-throated dippers (Cinclus cinclus), the smallest adult diving bird, are found in cold temperate areas, where air temperatures drop to −40°C and water temperatures in rivers and streams are often near 0°C [30]. The white-throated dipper has over six times higher contour feather density than the emperor penguin [30]. However, emperor penguins have about four times the number of downy plumes per contour feather (4 : 1 in emperor penguins versus 1 : 3.55 in dippers) [30].
Our finding that there is a higher density of contour feathers on the ventral side compared with the dorsal of emperor penguins may be important for tobogganing, exiting the water and resting on ice. The higher density on the chest provides more cushion for tobogganing over rough edges on the snow and for landing on their chest after leaping exits from the water. In addition, the higher ventral feather density provides increased insulation while resting prone on the ice. Given that most penguins do not travel by tobogganing (except the emperor and Adélie penguin, Pygoscelis adeliae) or land on their chest when exiting the water, we hypothesize there may be no difference in ventral and dorsal feather density in other species.
Body feather density is not static in emperor penguins, and will increase and decrease through the annual cycle as body girth changes. Emperor penguins' foraging cycle is almost continuous after moulting in January to prepare for the breeding season [31]. In April, at the start of the breeding season, penguins weigh 30–40 kg, with lipid mass accounting for up to 25% of body mass [32,33]. At this time, feather density will be lowest, and increased subcutaneous fat will provide more insulation. Over the next three months, body mass can drop 35–50% in the fasting male [32–34], with 80–90% of the loss owing to subcutaneous fat reduction [32]. The resulting reduction in girth and surface area will increase feather density without changing the number of feathers. At the end of the males' fast, when temperatures are near the coldest of the year and males have lost most of their lipid mass, feather density will be the highest. Although only a function of geometry, the increased feather density with decreased girth is advantageous and counteracts the loss of the insulative subdermal fat layer. An example of the extreme difference can be seen in comparing feather densities and girths in birds EP4 and EP3. The carcass of EP4 was found at Cape Crozier after a large iceberg blocked easy access to the sea and EP4 most likely starved (G. Kooyman 2001, personal observation). The resulting drop in body surface area increased feather density by about 50% on both the ventral and dorsal sides, although the total number of feathers was similar to other penguins (table 2).
Our findings also contradict the reported absence of filoplumes on emperor penguin plumage. No prior studies have specifically identified filoplumes in emperor penguins, and other sources state they are absent in penguins and other non-flying birds such as ostriches [13,22,23]. In flying birds, filoplumes are present on the wing plumage and appear to play a role in avian flight [21]. Necker proposed that filoplumes associated with flight feathers serve a sensory function, firing when feathers are displaced [21]. A slow-acting receptor at the base of filoplumes in pigeons is excited by lateral displacement of the nearby contour feather or movement of the associated filoplume, suggesting filoplumes act as part of a signal mechanism to alert the bird of the location of displaced feathers [21]. The highly modified, fish-scale-like wing feathers of penguins [1] are immobile and not likely to have filoplumes, but we suggest filoplumes may play a role in management of contour feather arrangement elsewhere on the body. Feathers disrupted by air or water will affect the insulative properties of the uniform, overlapping feather plumage and may increase drag during diving.
The ability of emperor penguins to maintain a body temperature of 38°C while foraging in freezing water or in adverse weather conditions is a result of specialized feather morphology [1] and the insulative properties of a well-preened plumage [3,34]. Filoplumes may assist in the maintenance of the smooth plumage of contour feathers, similar to the role they play in flying birds on displaced wing feathers. If there is a mechanoreceptor at the contour/afterfeather complex, displacement of a contour feather would cause an alteration of air flow or water flow, causing differential pressure on the tip of the filoplume, eliciting a signal from the mechanoreceptor and ultimately signalling the bird to preen at that location. The varying heights of filoplumes support this role, allowing for detection of displaced feathers from the skin to the edge of the contour feather layer.
5. Conclusion
The findings in this study demonstrate that emperor penguins have a much more complex feather distribution than was previously appreciated. Different penguin species inhabit polar to tropical environments, suggesting there must be considerable variation in feather pelage. It has yet to be determined, however, whether other penguins have plumage structures as complex as emperor penguins. While emperor penguin contour feather density is not the highest of any bird, a much greater concentration of plumules provides an additional fourfold layer of insulation, vital for survival during the harsh Antarctic winter. The filoplumes we discovered adjacent to contour feathers may play a similarly important survival role. By signalling the occurrence and location of a displaced feather, filoplumes may be key to maintaining an impermeable exterior, as well as the smooth hydrodynamic shape that probably contributes to a low cost of diving in emperor penguins [35]. Finally, perhaps explaining earlier omissions, we find it a special irony that the most abundant feathers on the penguin, the plumules, are never seen under normal circumstances.
Supplementary Material
Acknowledgements
We thank Paul Ponganis for use of collected specimens. We also thank Paul Ponganis, Allyson Hindle and two anonymous referees for helpful comments on the manuscript.
Ethics
Emperor penguin carcasses were collected at Cape Washington in 2001 and 2005 under an US Antarctic Treaty Permit and NSF OPP grants 9814794 and 0229638 (to Paul Ponganis).
Data accessibility
Feather density data are provided in the electronic supplementary material.
Authors' contributions
C.L.W. and G.L.K. designed and performed research. All authors analysed the data, discussed the results and wrote the paper. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
Research was supported in part by NSF OPP grants 9814794, 0229638 (to Paul Ponganis) and 1043454 (to G.L.K.). C.L.W. was supported by a National Institute of Health grant (NIH-NIAMS T32AR047752 to V. Caiozzo).
References
- 1.Rutschke E. 1965. Beitrage zur morphologie der pinguinfeder. Z. Morph. u. Okol. Tiere 55, 835–858. ( 10.1007/BF00391799) [DOI] [Google Scholar]
- 2.Le Maho Y. 1977. The emperor penguin: a strategy to live and breed in the cold. Am. Sci. 65, 680–693. [Google Scholar]
- 3.Le Maho Y, Delclitte P, Chatonnet J. 1976. Thermoregulation in fasting emperor penguins under natural conditions. Am. J. Physiol. 231, 913–922. [DOI] [PubMed] [Google Scholar]
- 4.Ponganis PJ, Van Dam RP, Levenson DH, Knower T, Ponganis KV, Marshall G. 2003. Regional heterothermy and conservation of core temperature in emperor penguins diving under sea ice. Comp. Biochem. Physiol. A, Physiol. 135, 477–487. ( 10.1016/S1095-6433(03)00133-8) [DOI] [PubMed] [Google Scholar]
- 5.Wienecke B, Robertson G, Kirkwood R, Lawton K. 2007. Extreme dives by free-ranging emperor penguins. Polar Biol. 30, 133–142. ( 10.1007/s00300-006-0168-8) [DOI] [Google Scholar]
- 6.Kooyman GL, Kooyman TG. 1995. Diving behavior of emperor penguins nurturing chicks at Coulman Island, Antarctica. The Condor 97, 536–549. ( 10.2307/1369039) [DOI] [Google Scholar]
- 7.Gavin F. 2013. Empire Antarctica: ice, silence, and emperor penguins. Berkeley, CA: Counterpoint. [Google Scholar]
- 8.Waters H.2013. 14 fun facts about penguins. See www.smithsonianmag.com/science-nature/14-fun-facts-about-penguins-41774295 .
- 9.Dawson C, Vincent JFV, Jeronimidis G, Rice G, Forshaw P. 1999. Heat transfer through penguin feathers. J. Theor. Biol. 199, 291–295. ( 10.1006/jtbi.1999.0959) [DOI] [PubMed] [Google Scholar]
- 10.Lowe PR. 1933. On the primitive characters of the penguins, and their bearing on the phylogeny of birds. Proc. Zool. Soc. Lond. 103, 483–538. [Google Scholar]
- 11.Stonehouse B. 1970. Adaptation in polar and subpolar penguins (Speniscidae). In Antarctic ecology (ed. Holdgate MW.), pp. 526–541. London, UK: Academic Press. [Google Scholar]
- 12.Stahel CD, Gales R. 1987. Little penguin: fairy penguins in Australia. Kensington, Australia: New South Wales University Press. [Google Scholar]
- 13.Chandler AC. 1916. A study of the structure of feathers: with reference to their taxonomic significance. Univ. Calif. Publ. Zool. 13, 244–447. [Google Scholar]
- 14.Du N, Fan J, Wu H, Chen S, Liu Y. 2007. An improved model of heat transfer through penguin feathers and down. J. Theor. Biol. 248, 727–735. ( 10.1016/j.jtbi.2007.06.020) [DOI] [PubMed] [Google Scholar]
- 15.Bonser RHC, Dawson C. 2000. The mechanical properties of down feathers from gentoo penguins (Pygoscelis papua). J. Zool. (Lond.) 251, 545–547. ( 10.1111/j.1469-7998.2000.tb00812.x) [DOI] [Google Scholar]
- 16.Stonehouse B. 1967. The general biology and thermal balance of penguins. In Advances in ecological research (ed. Cragg JB.), pp. 183–184. London, UK: Academic Press. [Google Scholar]
- 17.John G, Clements-Croome D, Jeronimidis G. 2005. Sustainable building solutions: a review of lessons from the natural world. Build. Environ. 40, 319–328. ( 10.1016/j.buildenv.2004.05.011) [DOI] [Google Scholar]
- 18.Eadie L, Ghosh TK. 2011. Biomimicry in textiles: past, present and potential: an overview. J. R. Soc. Interface 8, 761–775. ( 10.1098/rsif.2010.0487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao P, Shang W, Song C, Shen Q, Zhang F, Luo Z, Yi N, Zhang D, Deng T. 2015. Bioinspired engineering of thermal materials. Adv. Mater. 27, 428–463. ( 10.1002/adma.201401449) [DOI] [PubMed] [Google Scholar]
- 20.Wan XF, Fan JT, Wu HJ. 2009. Measurement of thermal radiative properties of penguin down and other fibrous materials using FTIR. Polym. Test. 28, 673–679. ( 10.1016/j.polymertesting.2009.04.007) [DOI] [Google Scholar]
- 21.Necker R. 1985. Observations on the function of a slowly-adapting mechanoreceptor associated with filoplumes in the feathered skin of pigeons. J. Comp. Physiol. A, Sens. Behav. Physiol. 156, 391–394. ( 10.1007/BF00610731) [DOI] [Google Scholar]
- 22.Young JR. 2012. Animal behavior. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 23.Gill FB. 2007. Ornithology. New York, NY: WH. Freeman and Company. [Google Scholar]
- 24.Taylor JR. 1986. Thermal insulation of the down and feathers of pygoscelid penguin chicks and the unique properties of penguin feathers. The Auk 103, 160–168. [Google Scholar]
- 25.Clarke JA, Ksepka DT, Salas-Gismondi R, Altamirano AJ, Shawkey MD, D'Alba L, Vinther J, DeVries TJ, Baby P. 2010. Fossil evidence for evolution of the shape and color of penguin feathers. Science 330, 954–957. ( 10.1126/science.1193604) [DOI] [PubMed] [Google Scholar]
- 26.Kooyman GL, Ainley DG, Ballard G, Ponganis PJ. 2007. Effects of giant icebergs on two emperor penguin colonies in the Ross Sea, Antarctica. Antarct. Sci. 19, 31–38. ( 10.1017/S0954102007000065) [DOI] [Google Scholar]
- 27.Lucas AM, Stettenheim PR. 1972. Avian anatomy: integument, pp. 266–276. Washington, DC: Agricultural Research Service. [Google Scholar]
- 28.Davenport J, Hughes RN, Shorten M, Larsen PS. 2011. Drag reduction by air release promotes fast ascent in jumping emperor penguins—a novel hypothesis. Mar. Ecol. Prog. Ser 430, 171–182. ( 10.3354/meps08868) [DOI] [Google Scholar]
- 29.Bonser RHC, Dawson C. 1999. The structural mechanical properties of down feathers and biomimicking natural insulation materials. J. Mater. Sci. Lett. 18, 1769–1770. ( 10.1023/A:1006631328233) [DOI] [Google Scholar]
- 30.Davenport J, O'Halloran J, Hannah F, McLaughlin O, Smiddy P. 2009. Comparison of plumages of white-throated dipper Cinclus cinclus and blackbird Turdus merula. Waterbirds 32, 169–178. ( 10.1675/063.032.0121) [DOI] [Google Scholar]
- 31.Kooyman GL, Siniff DB, Stirling I, Bengtson JL. 2004. Moult habitat, pre- and post-moult diet and post-moult travel of Ross Sea emperor penguins. Mar. Ecol. Prog. Ser. 267, 281–290. ( 10.3354/meps267281) [DOI] [Google Scholar]
- 32.Robin J-P, Groscolas R, Le Maho Y. 1988. Protein and lipid utilization during long term fasting in emperor penguins. Am. J. Physiol. 254, R61–R68. [DOI] [PubMed] [Google Scholar]
- 33.Ancel A, et al. 1992. Foraging behavior of emperor penguins as a resource detector in winter and summer. Nature 360, 336–339. ( 10.1038/360336a0) [DOI] [Google Scholar]
- 34.Jarman M. 1973. Experiments on the emperor penguin, Aptenodytes forsteri in various thermal environments. Brit. Antarct. Surv. B 33, 57–63. [Google Scholar]
- 35.Williams CL, Meir JU, Ponganis PJ. 2011. What triggers the aerobic dive limit? Patterns of muscle oxygen depletion during dives of emperor penguins. J. Exp. Biol. 214, 1802–1812. ( 10.1242/jeb.052233) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Feather density data are provided in the electronic supplementary material.




