Abstract
The significant influence of tumor microenvironment on malignant cells has been investigated with enthusiasm in this era of targeted therapy. Transglutaminase 2 (TG2, EC 2.3.2.13), a multi-functional enzyme that catalyzes the formation of intermolecular isopeptide bonds between glutamine and lysine side-chains, has been reported to exert important pathophysiological functions. The aim of this review was to investigate the correlation between TG2 and malignant behaviors, which could provide the rationale for novel approaches in anti-cancer therapy. We performed a systematic and electronic search on Medline, Scopus, and Web of Science for relevant publications from inception to April 2015. The bibliographic references of retrieved articles were further reviewed for additional relevant studies. TG2 exerts important physiological functions and plays vital roles in inflammation mainly through its modulation on the structure and stability of extracellular matrix (ECM). It also regulates EMT of diverse malignant cells through various intracellular and extracellular pathways. TG2 also plays an important role in tumor progression and may serve as a novel prognostic biomarker and therapeutic target in various cancer types. TG2 promotes malignant cell mobility, invasion, and metastasis, and induces chemo-resistance of cancer cells, mainly through its pro-crosslink and signaling transduction mediation propensities. In conclusion, TG2 plays vital roles in malignancy progression, and may have important prognostic and therapeutic significances.
Keywords: Transglutaminase 2, inflammation, tumor progression, tumor microenvironment, epithelial-mesenchymal transition, chemo-resistance, prognosis, therapy
Introduction
Tumors are made up of heterogeneous cells, and are considered to be wounds that do not heal [1]. Tumor microenvironment, namely tumor stroma, is an important and active research field nowadays [2]. The components of the complex microenvironment, including various tumor stromal cells and cellular factors, modulate cancer cell growth and regulate their malignant biological behaviors via diverse intracellular signaling transduction pathways [3]. The features of tumor cells also determine their responses to tumor stroma: cancer cells with the same tissue origin and pathological classification are associated with diverse prognoses due to their different reactions to their surrounding microenvironment. Cancer cells are to tumor stroma what ‘seeds’ are to ‘soil’ [4]. Metastasis is a leading cause of poor prognosis and high mortality rate among many malignancies [5-8]. Epithelial-mesenchymal transition (EMT) is a major biological process promoting invasive and metastatic abilities of cancer cells [9]. Investigating possible mechanism modulating malignant cell EMT and discovering novel EMT regulators will further elucidate mechanism of metastasis, and provide novel biomarkers and efficient targets for preventative and curative anti-cancer approaches.
Transglutaminase 2 (TG2, Tgase 2, TGM 2, EC 2.3.2.13) is a trans-peptidase with a wide distribution in various tissues. It can mediate crosslink of proteins, and participate in signal transduction via activating and hydrolyzing guanidine triphosphate (GTP) enzyme. In normal tissues TG2-GTP binding is proportional to the TG2 expression level. Besides, TG2 actively exerts diverse biological functions, including regulation of extracellular matrix (ECM) stability and reconstruction of intracellular cytoskeleton. It’s also an active participant in promoting malignant cell mobility and invasion mainly through EMT induction, and in inducing chemo-resistance of cancer cells [10]. Aberrant regulation of TG2 gene has been documented in various cancer types, particularly those isolated from metastatic sites or selected for chemo-resistance. Its expression has been linked with activation of pathways that are known to play fundamental roles in cancer progression. This review systematically investigates the correlation between TG2 and malignant behaviors, especially EMT, which could be regulated by stromal components.
TG2 and cellular biology
TG2 is a member of the transglutaminase (TG) iso-enzyme family, which contains 8 kinds of discrepant enzyme subtypes consisting of the FXIII-A factor and other 7 enzymes (TG1 to TG7) which are able to catalyze transglutamine (band 4.2 is the only member of TG family that lacks catalytic function due to mutation in the active site), and to regulate calcium ion (Ca2+)- and thiol-dependent post-translational protein modification. All TG family members are basically made up of 4 different domains derived from a contiguous sequence [11]. The most well-known function of TGs is to crosslink proteins by catalyzing the amide group, and they can also modulate various post-translational modifications based on their esterification and hydrolysis activities, participating in various cellular biological processes, with diversities in function among different TG subtypes [10]. TG2 is an important subtype in the TG family with a molecular weight of around 77 kDa. As it is constitutively expressed in smooth muscle cells, fibroblasts, and various organ-specific cells, and is distributed in nearly all kinds of tissues, TG2 is also called tissue transglutaminase (tTGM, tTG). However, most epithelial cells in basal state lack TG2 expression. It could locate in ECM, on surface of cells adjacent to ECM, or inside cells (in cytoplasm, on nuclear membrane, or controversially, inside mitochondria) [12].
Like other TG family members, the diverse biological activities of TG2 are mainly modulated by Ca2+, which could cause reconstruction of TG2 subunits enabling exposure of the catalytic sites, and which could induce TG2 to catalyze the deamidation reaction and to promote crosslink of ECM proteins to form polyurethane conjugates, maintaining the stability of ECM and tissues [13,14]. Besides, TG2 along with fibronectin and collagen could form a stable ternary complex, which mediates cell-ECM adhesion via integrin. This process is vital in maintaining cell position locally and regulating cell migration which makes TG2 a key molecule in preserving cell polarity, and also in modulating damage repair, angiogenesis, bone remodeling, and apoptosis [15]. Without Ca2+, however, TG2 could function like GTPase and bind GTP/GDP, mediating intracellular G protein signaling transduction via adrenoceptor, thromboxane A2 receptor, and oxytocin receptor with the crosslink-catalytic function inhibited, and further modulating various cellular behaviors. Inside cells, due to the relatively high level of GTP/GDP and low level of Ca2+, TG2 mainly demonstrates weak pro-crosslink activity. Although the extracellular environment is “contrary” to the intracellular one, extracellular TG2 does not appear to be activated, which may be explained by the fact that the protein resting structure is maintained by the intra-molecular disulfide bonds between cysteine residues, the oxidation and degradation of which is another regulative method besides GTP/GDP and Ca2+. Thioredoxin could degrade the extracellular bisulfide bonds of TG2, and when the bonds are opened, TG2 could promote crosslink again after conformational change. Moreover, TG2 could modulate intracellular serine/threonine kinase activity via insulin-like growth factor binding protein [16].
Researches into cell signaling are focused on various pathways concerned with ras, Akt, Src, GSK3β, and nuclear factor-κB (NF-κB) [17-19]. Physiologically, TG2 has multiple functions including regulating cellular proliferation and receptor-mediated endocytosis. In mice with TG2 gene knockout, disorder occurs in serum insulin level as they grow older, suggesting that TG2 might be involved in insulin secretion by β cells [20]. Besides, TG2 could promote cellular adhesion and shows strong anti-pancreatin effect, suggesting that it might be associated with adhesive ability of fibroblasts [21]. Researches into TG2 are mainly concentrated on its relationship with inflammation and malignancy.
TG2 and inflammation
As there exists a close link between inflammation and tumor [4], herein we discuss initially the vital role of TG2 in inflammation, which could gradually develop into malignancies. TG2 is involved in many inflammatory processes, including damage limitation, tissue repair, fibrosis, celiac disease, atherosclerosis, and autoimmune disease [11]. It is necessary for in vivo allergic inflammation, such as triphasic cutaneous reaction, passive cutaneous anaphylaxis, and passive systemic anaphylaxis [22]. TG2, whose expression could be induced by antigen stimulation, shows an interaction and co-localization with FcRI. It plays a controversial role in modulating inflammation, and it’s debatable whether it is pro-inflammatory or tissue-protective. It is regulated by various inflammatory factors. Transforming growth factor (TGF)-β could activate the TG2 gene, causing high expression of TG2 on surfaces of keratinocytes and dermal fibroblasts [21,23]. Moreover, TNF-α could stimulate the NF-κB pathway via phosphorylating the inhibitory subunit α of NF-κB (IκBα), thus activating the TG2 gene, and promoting TG2 expression in hepatocytes; IL-6 could significantly enhance TG2 expression in hepatocellular carcinoma (HCC) cells. Besides cellular factors, vitamins A and D, and steroids could also stimulate TG2 expression in diverse tissues [10]. The wide distribution of TG2 and diversity of its expression regulation indicate that it might be involved in various cellular biological function modulations concerned with inflammation.
TG2 could regulate acute inflammatory exudation and inflammatory cell infiltration. Mice with TG2 gene knockout have significantly higher survival rates after undergoing endotoxic shock compared to wild-type ones, and this phenomenon is possibly associated with decreased NF-κB activation, which attenuates neutrophil deposition in peritoneum, kidney, and cardiac tissues, and weakens its tissue-damaging effect [24]. However, TG2 plays a hepatic protective role, and mice with TG2 gene knockout have lower survival rates after carbon tetrachloride-induced hepatic injury [25]. Besides, TG2 actively participates in modulating macrophage phagocytosis. It could bind with integrin promoting phagocytosis, and mediate cellular adhesion and movement [26]. In hepatic tissues of mice with TG2 gene knockout, the ability of macrophage phagocytosis of apoptotic debris and necrotic cells significantly weakens, which is possibly caused by the inhibition of TGF-β1 [27]. The binding capacity of TG2 with GDP or adenine nucleotide has been reported to play a key role in regulating the phagocytosis [28]. TG2 is also involved in scar formation and cellular apoptosis.
The key mechanism of these functions exerted by TG2 is its modulation of ECM structure and stability. After acute injury occurs, TG2 could crosslink and stabilize ECM components, preventing inflammation from expanding; meanwhile, TG2 along with integrin regulates signaling transduction which activates fibroblasts and endothelial cells, promoting angiogenesis. Contrarily and interestingly, TG2 largely exerts pro-inflammatory functions, which is also dependent on the specific signaling [28]. TG2 could regulate TGF-β binding protein-1 in ECM, and promote its activating effect of TGF-β, which is a potent inflammatory factor aggravating and protracting inflammation, and this indicates that TG2 plays an indirect regulative role in tissue TGF-β expression via its pro-crosslink activity. TG2 could also promote fibroblast proliferation, exacerbating fibrosis in chronic inflammation. Conversely, after pro-inflammatory factors are activated during inflammation, they could induce TG2 expression. Both TGF-β and TNF-α could up-regulate TG2 expression, further worsening the corresponding diseases. Chronic inflammation plays an important role in tumorigenesis and cancer progression, which are gradually evolved procedures, and where the abovementioned key roles of TG2 in inflammation might also be applicable.
TG2 and tumor progression
The correlation between TG2 and malignancies has long been a hot topic and an active research field. Normally, bladder, liver, and adrenal gland exhibit prominent expression and activity of TG2. In most other normal tissues, TG2 is less frequent and is usually confined either to the epithelium or in adjacent stroma. TG2, which is a ubiquitously-expressed Ca2+-dependent multifunctional protein-crosslinking enzyme and a dual function G-protein, which is encoded by a stress-responsive gene and is a transcriptional target of hypoxia-inducible factor (HIF)-1α, and which binds to fibronectin and exerts transamination function, plays key roles in regulating cell survival, differentiation, growth, migration, invasion, and apoptosis, and in impacting cellular interaction with the microenvironment (Figure 1). TG2 could enhance the survival of hypoxic cells. Hypoxia induces TG2 expression through an HIF-1-dependent pathway and concurrently activates intracellular TG2. The hypoxic cells overexpressing TG2 show resistance to apoptosis. Conversely, the hypoxic cells treated with either TG2 inhibitor or small interfering RNA (siRNA) become sensitive to apoptosis. Activation of TG2 in response to hypoxic stress inhibits caspase-3 activity by forming the cross-linked multimer, resulting in insoluble aggregates. TG2 also activates the NF-κB pathway under hypoxic stress, and thereby induces the expression of cellular inhibitor of apoptosis 2 [29]. TG2 is thus an anti-apoptotic mediator of HIF-1 through modulating both apoptosis and survival pathways and may confer a selective growth advantage to tumor cells. Additionally, TG2 forms a ternary complex with IκB/p65:p50 which causes constitutive activation of NF-κB. Tumor cells with TG2 overexpression exhibit an increased level of constitutively active NF-κB. Ectopic TG2 expression and activation of TG2 lead to NF-κB activation; conversely, inhibition of TG2 inhibits NF-κB activation. IκBα is physically associated with TG2. The enzymatic function of TG2 is required to induce phosphorylation of IκBα which leads to decreased inhibitory function and processing of the precursor protein p100 into the active p52 subunit, causing increases of pro-survival factors such as BCl-2. A specific target of TG2-activated p52/RelB complex is the hyaluronan receptor, CD44 [30]. Moreover, TG2 expression in cancer cells leads to constitutive activation of focal adhesion kinase (FAK) and its downstream phosphoinositide 3-kinase (PI3K)/Akt survival pathway. TG2 could also impede expansion of apoptosis, and participate in cellular proliferation. Generally, TG2 is down-regulated in primary tumors and during tumor progression, while it is up-regulated in secondary and chemo-resistant cancers [31]. The inhibition of TG2 may offer a novel strategy for anticancer therapy.
Figure 1.
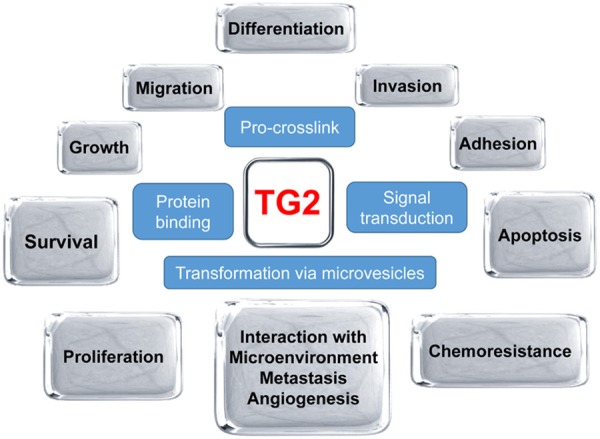
TG2 regulates various biological behaviors of tumor cells (shown in grey boxes) via four major mechanisms (shown in blue boxes). TG2, transglutaminase 2.
TG2 is the most diverse and ubiquitous member of the TG family. The multiple enzymatic activities of TG2 may be attributed to differential TG2 mRNA splicing. There exist significant differences in alternative splicing of TG2 in cancer cell lines, suggesting the alternative TG2 splicing as an active process [32]. In some malignancies, epigenetic mechanisms are involved in TG2 modulation [33]. Microvesicles (MVs) shed by cancer cells are capable of conferring onto normal fibroblasts and epithelial cells the transformed characteristics of cancer cells (e.g., anchorage-independent growth and enhanced survival capability), which requires the transfer of TG2. However, TG2 alone is not sufficient to transform fibroblasts and it must collaborate with the TG2-binding partner and cross-inking substrate fibronectin to mediate the transforming action of the cancer cell-derived MVs. TG2 crosslinks fibronectin in MVs from cancer cells, and the following MV-mediated transfer of cross-linked fibronectin and TG2 to recipient fibroblasts functions cooperatively to activate the mitogenic signaling activity and to induce their transformation. Thus, MVs play a vital role in the induction of cellular transformation with TG2 and fibronectin as essential participants [34]. Functions exerted by TG2 in tumors are mainly dependent on its pro-crosslink and signaling transduction mediation propensities. TG2 can associate with certain β members of the integrin family (β1, β3, β4, and β5) and promote stable interaction between cells and the ECM, resulting in increased cell survival, migration, and invasion. TG2 is involved in tumor progression through regulating malignancy mobility and invasion, and its expression is linked with activation of pathways that are known to play fundamental roles in cancer progression [35]. Inactivation of TG2 significantly attenuates cellular motility on fibronectin. TG2 which is fixed to fibronectin, regardless of its activity, could promote cellular adhesion, which is independent of integrin. TG2-containing cancer cells exhibit increased cell migration and invasion abilities in collagen- and Matrigel-coated Transwell plate assays [36].
ECM plays key regulative roles in tumor cell metastasis and angiogenesis. TG2 could affect intracellular adhesion and ECM via several ways. MMP1 and MMP2 could suppress fibronectin-mediated cancer cell adhesion and migration through hydrolyzing TG2 on cellular surface in the borderline between malignant and normal tissues, which however promotes collagen-mediated tumor cell migration [37]; moreover, exogenous TG2 could alleviate MMP-induced ECM degradation, and inhibit angiogenesis. TG2 promotes invasive ability of malignant cells via modulating the ECM. It could crosslink several proteins in the ECM, enhancing stability of fibronectin during initial matrix formation. Fibroblasts overexpressing TG2 have significantly more potent adhesive ability and better extensibility [38]. TG2 on cellular surface is involved in binding of various cellular surface receptors with relevant proteins in the ECM, mediating cell-ECM adhesion. TG2 could directly bind integrin β1/β3/β5 via G protein coupled cell adhesion receptor GPR56, a suppressor of tumor progression, facilitating adhesion of fibronectin onto fibroblasts [15,39,40]. TG2 is a ligand interacting with GPR56 in the ECM. TG2 affects cell adhesion function, and it has been implicated in suppression of tumor progression. It might serve as a host defense against the invading metastatic cells. The highly metastatic cells may escape from the inhibition by down-regulation of GPR56 [41]. Besides, TG2 could change ECM stability via regulating MMPs. TG2 could down-regulate PP2A-α and activate the response element binding protein (CREB), which could activate the expression of MMP2 gene. Expression and function of MMP2, a critical mediator of tissue invasiveness, are regulated by TG2 at the transcriptional level. TG2 and MMP2 expressions are significantly correlated. TG2 knockdown or inhibition of TG2 activity down-regulates MMP2 and decreases the gelatinase activity of MMP2 in cancer cells. Binding of CREB to the MMP2 promoter is diminished in cells with decreased TG2 expression, and TG2 knockdown decreases CREB phosphorylation. The effects of TG2 on CREB activity and MMP2 transcription are mediated by TG2-dependent degradation of the protein phosphatase 2 (PP2A-α) complex [42]. Contrarily, membrane MMPs could degrade ECM through hydrolyzing TG2 in ECM, while their combination with fibronectin in ECM could protect TG2 form being hydrolyzed by MMPs [37]. The interaction between various protein molecules in ECM forms a network, regulating cellular adhesion and detachment, and modulating tumor cell invasion and colonization. Clarification of relationship between TG2 and tumor invasion and metastasis in vivo will provide novel cancer prognostic markers and therapeutic targets.
TG2 might both mediate and inhibit tumor progression. TG2-dependent cross-link is important in ECM integrity and this TG2 activity establishes a barrier to tumor spreading. Active TG2 in vivo could result in the accumulation of a complex ECM leading to the suppression of endothelial tube formation without causing cell death. TG2 can make the ECM highly stable and resistant to proteolytic degradation. Tumors with negative lymph node show significantly higher expression of TG2 in the stroma, which is catalytically active. Pretreatment of Matrigel with catalytically active TG2 also results in strong inhibition of cancer cell invasion through the Matrigel Transwell filters. Thus, TG2-induced alteration in the ECM could effectively inhibit metastasis, and selective induction of catalytically active TG2 at the tumor site may offer a promising approach to limit metastasis [43]. TG2 modulation of cellular behavior via regulating the ECM may provide a novel approach for anti-tumor therapy. TG2 expression and activity in the tumor and surrounding matrix generally decrease with tumor progression, favoring matrix destabilization, but supporting angiogenesis and tumor invasion. In contrast, in the secondary metastasis TG2 is often highly expressed whereby its potential role in cell survival both at the intra- and extra-cellular levels becomes important [44]. Interestingly, TG2 either promotes or suppresses death-inducing signaling in tumors in a cell context-dependent manner. Depending on the cell type and its localization within the cell, TG2 can serve as either an anti-apoptotic or a pro-apoptotic protein. High level (> 1 mM) of Ca2+ activates TG2, which promotes inter- and intra-molecular crosslinking of proteins and results in cell death. The use of TG2-specific antisense RNA could protect cells against stress-induced cell death. In contrast, low level (< 1 mM) of Ca2+ and high concentration (> 9 mM) of GTP (the condition that generally prevails intra-cellularly) promote TG2-mediated cell survival signaling [45]. There exist N-epsilon γ-glutamyl lysine amino residues in tumor stroma, demonstrating the transamidation activity of TG2. TG2, a γ-glutamyl transferase which generates epsilon-lysine isopeptide bonds and which produces ammonia as a byproduct of its catalytic activity, is up-regulated by a decrease in cellular pH and helps protect cells from acid-induced cell death, which is similar to Kidney type glutaminase (GLS1) [46].
The apoptotic death of tumor cells occurs together with an increase in the expression and activity of TG2, which could be promptly antagonized by PKA inhibitor, suggesting the involvement of intracellular cAMP elevation and, consequently, PKA activation. Growth inhibition and TG2 expression and activity are potentiated by Retinoic acid (RA), a TG2 inducer [47]. Retinoid-induced TG2 mRNA up-regulation could be suppressed by 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) in human squamous carcinoma cell (SCC) line, which occurs without enhanced degradation of the mRNA and thus appears to result from altered transcription. The actions of all-trans-RA and synthetic retinoid, which resist metabolic degradation, are suppressed to the same extent without obvious changes in their EC50s. Sodium butyrate alone induces TG2 and augments retinoid induction. RA induction of TG2 displays a lag phase of > 24 h, indicating that the induction has an indirect component. Rather than depleting active retinoid in the culture medium or generally inactivating retinoid receptor function, TCDD may suppress the retinoid action by interfering with the late phase of induction [48]. A mechanistic model of theophylline action and anti-tumor strategies based on the concomitant use of theophylline and agents that potentiate TG2 activity can be hypothesized. The involvement of TG2 in specific cancer types are discussed as follows.
TG2 and breast cancer
Normal mammary tissues and those with benign hyperplasia rarely express TG2. TG2 is significantly expressed in breast cancer, and its expression is increased in both malignant breast epithelia and the surrounding stroma, although its selective spatial expression pattern in normal tissues also indicates a physiological role in the epithelium-stroma interaction. Stromal TG2 expression in breast cancer is significantly more frequent than that in normal breast tissues [49]. TG2-regulated pathways play a crucial role in promoting an aggressive phenotype in mammary epithelial cells [50]. Increased TG2 expression in breast cancer cells contributes to increased cell survival, invasion, and motility. Invasive ductal carcinoma and the adjacent normal tissue show a 10-fold higher TG2 activity compared to the normal tissues adjacent to fibro-adenoma and atypical ductal hyperplasia, and there exist interplays between membrane lipid peroxidation, TG2 activity, and COX-2 expression in the tissue adjoining to the neoplastic lesion during breast cancer progression [51]. In the highly aggressive breast cancer cell line where epithelial growth factor (EGF) stimulation is unnecessary for the migration and invasion activities, TG2 is already at the leading edge and activated. TG2 in metastatic breast cancer cells is present on the cell surface and is in close association with integrin β1, β4, and β5. Down-regulation of endogenous TG2 by siRNA inhibits fibronectin-mediated cell attachment, survival, and invasion. Conversely, ectopic expression of TG2 augments breast cancer cell invasion and attachment to fibronectin-coated surfaces. Thus, TG2 expression in breast cancer cells plays an important role in the development of metastasis [31]. Localization of TG2 to the leading edges of breast carcinoma cells, where it plays an essential role in their migration, has a strict requirement for heat shock protein 70 (Hsp70). TG2 inhibition by either TG2 inhibitors or transfection with TG2 shRNA, and treatment with a non-cell permeabilizing TG2 inhibitor in cancer cells with high TG2 expression levels could block S100A4-accelerated cell migration in the highly metastatic cells and in the non-metastatic cells treated with exogenous S100A4. There exists a direct interaction between TG2 and S100A4, which is a substrate of TG2. The syndecan-4 and α5β1 integrin co-signaling pathways linked by PKCα activation are involved in the TG2- and S100A4-mediated cell migration [52]. An increased TG2 level in breast cancer cells leads to decreased expression of IκBα, and enhancement of NF-κB activity. TG2 expression is inversely correlated with the IκBα level, implying that TG2 is responsible for the constitutive activation of NF-κB in breast tumor [53]. RA consistently induces TG2 expression and activation in breast cancer cells, and RA-induced TG2 expression is inhibited in fibroblasts co-stimulated with EGF. EGF alone is sufficient to potently promote TG2 expression and activation in cancer cells. Inhibiting PI3K activity severely diminishes the ability of EGF and RA to increase the TG2 level, whereas a constitutively active form of PI3K potentiates the EGF-induced TG2 expression in cancer cells. Moreover, expression of exogenous TG2 in cancer cells mimics the survival advantage of EGF, suggesting that TG2 activation is necessary and sufficient for the anti-apoptotic property of EGF [14]. IL-6 production in human breast cancer cells is dependent on the TG2 expression. Tumor-sphere formation in vitro, primary tumor growth in the mammary fat pads, and distant hematogenous metastasis into the lung are also dependent on both TG2 and downstream IL-6 expression. Thus, TG2 is an important link in IL-6-mediated tumor aggressiveness in breast cancer [54].
TG2 and cervical cancer
In cervical SCC cells, ectopic expression of TG2 increases cell motility and invasiveness [55]. Treatment of HeLa cells with EGF results in the activation of a plasma membrane-associated pool of TG2 and its redistribution to and accumulation at cell leading edges, which are essential for EGF-stimulated HeLa cell migration, whereas knock-down of TG2 or treating cells with TG2 inhibitors blocks EGF-stimulated cell migration and invasion. EGF signaling through Ras and c-Jun N-terminal kinase is responsible for targeting TG2 to the cell leading edges and activating it. The requirement of EGF to properly localize and activate TG2 can be circumvented by the expression of oncogenic Ras, whose ability to stimulate migration is also dependent on TG2. These demonstrate that TG2 plays a key role in cancer cell motility and invasiveness, and participates the EGF pathway that stimulates these processes in cancer cells [56]. The ability of TG2 localization to the leading edge is dependent on Hsp70, which could bind TG2, and which plays a vital role in cancer cell migration. Treatment of the cell lines with inhibitors against the ATP-hydrolytic activity of Hsp70 prevents TG2 from localizing to the leading edges and thereby blocks cancer cell migration. These highlight an unconventional and important role for the chaperonin activity of Hsp70 in the localization of TG2 at the leading edges of cancer cells and in the cellular migration ability [57]. TG2 is also an important mediator of the ligand-dependent phenotypic effect of oncostatin M receptor (OSMR) overexpression in cervical SCC cells. Its expression correlates with disease progression and with OSMR levels in clinical SCC samples. TG2 depletion in cervical SCC cells abrogates oncostatin M (OSM)-induced migration on fibronectin-coated surfaces and invasiveness through ECM. It interacts with integrin α5β1 in the presence of fibronectin in cervical SCC cells, with OSM treatment strengthening the interaction. Importantly, integrin α5β1 and fibronectin are also overexpressed in SCC, where their levels correlate with OSMR and TG2 expression. These demonstrate that stimulation of overexpressed OSMR in cervical SCC cells activates TG2-integrin α5β1 interaction and induces pro-malignancy changes, and suggest a biologically significant OSMR/TG2/integrin α5β1/fibronectin pathway in cervical SCC as a candidate for therapeutic targeting [55].
TG2 and ovarian cancer
In ovarian tumor, TG2 is upregulated and enhances intraperitoneal metastasis. All histological subtypes of ovarian cancer could express TG2 [58]. It is secreted abundantly in cancer ascites as an active enzyme. Extracellular TG2 also promotes the peritoneal metastasis, and the main pathway activated is the non-canonical NF-κB signaling. The TG2 gene is a direct transcriptional target of NF-κB, and there exists a self-reinforcing molecular feedback signaling loop where TG2 activates NF-κB and, in turn, NF-κB directly promotes TG2 transcription [59]. In epithelial ovarian cancer, TG2 enhances cancer cell adhesion to fibronectin and directional cell migration, and modulates β1 integrin subunit expression, and the tumor dissemination pattern in the peritoneal space is dependent on TG2. TG2 knockdown diminishes tumor dissemination on the peritoneal surface and mesentery [60]. TG2 could regulate MMP2 expression, which may facilitate tissue invasion and spread of cancer. TG2 induces degradation of PP2A-α which activates CREB, and thereby increases MMP2 transcription. These provide a novel mechanistic insight into the pro-metastatic function of TG2.
TG2 and pancreatic cancer
TG2 is overexpressed in pancreatic cancer [61], and TG2 gene is one of the most dramatically elevated genes in pancreatic cancer tissues [62]. Elevated expression of TG2 in pancreatic cancer cells has been implicated in chemo-resistance, metastasis and poor prognosis, and TG2 may serve as a promising therapeutic target [63]. Protein kinase C (PKC) δ constitutively suppresses autophagy, a type II programmed cell death, through TG2-induction in pancreatic cancer cells that are frequently insensitive to standard chemotherapies [64]. Rottlerin, a PKCδ-specific inhibitor, and PKCδ siRNA down-regulate TG2 expression and induce growth inhibition without inducing apoptosis in pancreatic cancer cells. Inhibition of PKCδ by rottlerin or knockdown of TG2 by a TG2-specific siRNA results in a marked increase in autophagy shown by presence of autophagic vacuoles in the cytoplasm, formation of the acidic vesicular organelles, membrane association of microtubule-associated protein 1 light chain 3 (LC3) with autophagosomes, and a marked induction of LC3-II protein, which are all important hallmarks of autophagy. Furthermore, inhibition of TG2 leads to the accumulation of green fluorescent protein (GFP)-LC3-II in autophagosomes in cancer cells transfected with a GFP-LC3-expressing vector. Inhibition of the PKCδ/TG2 signaling results in significant autophagic cell death mediated by Beclin 1, a specific autophagy-promoting protein and the product of Becn1 (ATG6). Knockdown of Beclin-1 inhibits rottlerin- and TG2 siRNA-induced autophagy. These suggest that PKCδ plays a critical role in TG2 expression, which regulates autophagy in pancreatic cancer cells [65]. In the invasive phenotype of pancreatic ductal adenocarcinoma (PDAC), down-regulation of eukaryotic elongation factor-2 kinase (eEF-2K) by siRNA or rottlerin displays an impairment of cancer cell invasion and migration, with a significant decrease in TG2 expression. Down-regulation of endogenous TG2 by siRNA could effectively block PDAC growth. The cancer cell motility and metastatic potential could be promoted through the TG2/β1 integrin/Src/uPAR/MMP2 signaling pathway [66]. However, activation of endogenous TG2 by calcium ionophore induces rapid and spontaneous apoptosis in PDAC cells in a caspase-independent manner. TG2-induced apoptosis is associated with release of apoptosis-inducing factor (AIF) [67]. TG2 expression in PDAC cells results in constitutive activation of the FAK/AKT cell survival signaling by modulating the expression of the tumor suppressor phosphatase PTEN, and TG2 expression is inversely correlated with PTEN. Ectopic TG2 expression inhibits PTEN phosphorylation and promotes its degradation by the ubiquitin-proteasomal pathway. Conversely, down-regulation of endogenous TG2 by siRNA up-regulates PTEN expression, and causes a significant retardation in PDAC xenograft growth. Clinically, TG2 overexpression is also associated with a loss of PTEN expression [68]. In PDAC, there exists a strong correlation between TG2 expression and NF-κB activation/overexpression, and TG2 induces constitutive NF-κB activation in tumor cells most likely independent of the IκBα kinase [69]. TG2 may be an attractive alternative target for inhibiting constitutive NF-κB activation and rendering pancreatic cancer cells sensitive to anticancer therapies.
TG2 and colorectal cancer
In colorectal cancer, TG2 expression increases, and is involved in tumor growth, suggesting it as a potentially important target for tumor chemoprevention and treatment [70,71]. Increased TG2 is detectable in the host tissue around the tumor. Intra-tumor injection of TG2 results in a reduction in tumor growth, and occasionally tumor regression. In tumors injected with TG2 there exists a fibrosis-like tissue containing increased collagen and TG2-mediated crosslinks, and reduced organized vasculature. TG2-knockout results in increased tumor progression and a reduced survival rate [72]. TG2-transfected cells show increased integrin β3 expression, and are more adherent to and less migratory on fibronectin. There exists a direct interaction between TG2 and β3 integrin, suggesting that TG2 acts as a co-receptor for fibronectin with β3 integrin. Cells transfected with active TG2 have increased levels of TGFβ1 and matrix-deposited fibronectin, which could be inhibited by TG2 site-directed inhibitors, and they are capable of inhibiting tumor growth. Thus in colonrectal carcinoma, an increased level of active TG2 is unfavorable to tumor growth due to its activating TGFβ1 and the increased matrix deposition, which in turn favor enhanced cell adhesion and a weakened migratory and invasive behavior [73].
TG2 and liver cancer
Serum TG2 level is significantly higher in HCC patients, and it correlates with the histological grade and tumor size. TG2 expression in liver tissues shows an inverse correlation with the level of serum α fetal protein (AFP) in the patients. TG2 is overexpressed in some AFP-deficient HCC cells and in about half of the tumor tissues with low levels of serum AFP, and trace amounts of TG2 are found to be expressed in the samples with high serum AFP levels. TG2 exists in the supernatant of the AFP-deficient cells, and it is induced in AFP-producing cells. These suggest that TG2 may serve as a novel histological/serologic candidate involved in HCC, especially for individuals with normal serum AFP [74].
TG2 and lung cancer
TG2 expression increases the invasive and migratory properties of non-small cell lung cancer (NSCLC) cells, which might be related to the induction of MMP9 [35]. The MMP9 expression is suppressed when TG2 is inhibited, suggesting that TG2 potentiates cell migration through up-regulating MMP9. Interferon (IFN) α could induce apoptosis, and cause an increase in the expression and activity of the TG2 protein in human lung cancer cells, which is more significant than the increase in TG2 mRNA level. This could be possibly explained by the fact that IFNα induces a time-dependent decrease in TG2 ubiquitination. Addition of the proteasome inhibitor lactacystin leads to the accumulation of ubiquitinated TG2 and to a consequent increase in its expression. Treatment of the cells with the combination agents antagonizes the accumulation of the ubiquitinated isoforms of TG2 induced by lactacystin and causes a potentiation of TG2 expression. The TG2 inducer RA is also able to cause increased expression and ubiquitination of TG2 in cancer cells. Addition of TG2 inhibitor to IFNα-treated cells completely antagonizes growth inhibition and apoptosis induced by the cytokine. Thus, in lung cancer TG2 is ubiquitinated and degraded by a proteasome-dependent pathway, through the modulation of which IFNα can at least partly induce apoptosis [75]. TG2 could suppress membrane resealing after mechanical damage in lung cancer cells. Additional expression of TG2 rescues the membrane resealing ability [76].
TG2 and melanoma
Tumor samples from melanoma patients show strong TG2 expression. TG2 is necessary for the enhanced metastatic potential of B16F1 melanoma cells by inducing passive systemic anaphylaxis and increasing the histamine release and the β-hexosaminidase activity [22]. Metastatic melanoma cell lines express a TG2 level up to 24-fold higher than the radial growth phase of primary melanoma cell lines, and TG2 expression is selectively up-regulated during advanced developmental stages of melanoma. The association of TG2 with cell surface integrin promotes strong attachment of cancer cells to fibronectin-coated surfaces, resulting in increased cell survival in the serum-free medium. Inhibition of TG2 by siRNA inhibits fibronectin-mediated cell attachment and survival. Activation of endogenous TG2 by calcium ionophore induces a rapid and strong apoptotic response in cancer cells and the induced apoptosis could be blocked by TG2-specific inhibitors [77]. TG2 could bind specifically with a novel G protein-coupled receptor GPR56, which is of significantly low expression in many highly malignant cells, and which potently inhibits melanoma cell growth, proliferation, and metastasis [15].
TG2 and prostate cancer
TG2 is a phenotypic biomarker in prostate cancer, and is down-regulated in primary cancer and in the surrounding premalignant field. There exist co-localization and a strong interaction between the TG moiety and protein kinase A anchor protein 13 [78]. TG2 could suppress apoptosis via the depletion of caspase 3 and cathepsin D. While in androgen-independent human prostate tumor, decreased progression into the S-phase particularly induced by γ-tocopherol is associated with increased expression and activity of TG2, suggesting that γ-tocopherol has an evident differentiative effect on tumor cells, leading to an increased TG2 expression, which affects cell cycle progression. Up-regulation and activation of TG2, which are associated with reduced proliferation, could be parts of a large-scale reprogramming that can attenuate the malignant phenotype of cancer cells [79].
TG2 and other cancers
In clinically-aggressive meningioma, TG2 expression is increased, and is associated with increased WHO malignancy grades and recurrences. Inhibition of TG2 by siRNA or cystamine induces meningioma cell death and reduces cell growth, which is associated with reduced AKT phosphorylation and caspase-3 activation [80]. In neuroblastoma, TG2 expression is inhibited by the transcription factor Sp1, which binds the corresponding promoter [81]. In primary renal cell carcinoma, TG2 is significantly higher in metastatic patients than in non-metastatic ones, indicating a correlation with metastasis [82].
TG2 and EMT
EMT, a key developmentally-regulated program, significantly influences cancer cell biological behaviors [2]. TG2 plays an important role in EMT via regulating diverse proteins. TG2 expression is also able to confer the cancer stem cell (CSC) traits. TG2 regulates diverse tumor biological behaviors including EMT through various intracellular and extracellular ways (Figure 2). Intra-cellularly, TG2 mediates deamination of various cytoskeleton-associated components, contributing to actin-actin and myosin-actin crosslinks, troponin polymerization, and β-tubulin reconstruction. Extra-cellularly, TG2 modulates formation, stability, and reconstruction of the ECM, via facilitating the collagen-collagen and fibronectin-collagen crosslinks. Vitronectin and osteonectin could also be catalyzed by TG2 to crosslink themselves with other ECM proteins. Inflammatory signals (TGFβ, TNFα, NF-κB, et al.) could induce EMT via inducing aberrant TG2 expression, which could also induce stem cell characteristics in epithelial cells, suggesting a possible link between TG2, inflammation, and cancer progression [83]. The regulation of tumor cell EMT, which plays important roles in inducing various malignant behaviors, especially enhanced chemo-resistance and a potent invasive ability, via TG2, is a complex biological procedure. TG2 is highly expressed in various tumor cells with inherent or acquired chemo-resistant and metastatic abilities, and the expression level is associated with tumor cell proliferative and invasive propensities. TG2 could activate FAK-, Akt-, and cAMP-binding proteins, and constitutively the NF-κB pathway, and down-regulate the expression of PTEN, a tumor-inhibitory protein; while suppression of TG2 by siRNA could remarkably attenuate the tumorigenic and cell invasive capacities [84].
Figure 2.

TG2 regulates EMT through both intracellular and extracellular pathways. TG2, transglutaminase 2; EMT, epithelial-mesenchymal transition; ECM, extracellular matrix.
TG2, which is highly expressed in ovarian cancer, promotes EMT, characterized by increased expressions of stromal markers and down-regulation of epithelial markers, contributing to increased cancer cell invasiveness and metastasis by activating oncogenic signaling. TG2 induces a mesenchymal phenotype, characterized by the E-to-M cadherin switch and invasive cell behavior in Matrigel. This is mediated at the transcriptional level by altering the expression levels and functions of several transcriptional repressors, especially Zeb1. One mechanism through which TG2 up-regulates Zeb1 is by activating the NF-κB complex. The effect of TG2 on ovarian cancer cell phenotype and invasiveness translates into increased tumor formation and metastasis in vivo [9,84]. Cells with a CSC phenotype isolated from human ovarian cancer express high levels of TG2, whose knock-down in cancer cells decreases the number of cells harboring a CSC phenotype [85]. In pancreatic cancer, inhibition of the eEF-2K/TG2 axis could suppress EMT, as demonstrated by the modulation of the zinc finger transcription factors, e.g. ZEB1/Snail, and the tight junction proteins, e.g. claudins [66]. The non-canonical NF-κB activation by extracellular TG2 induces CD44 up-regulation and EMT, contributing to increased cancer cell invasiveness and peritoneal dissemination [30]. In breast cancer cells, TG2 could also induce EMT, facilitating tumor cell Matrigel-penetration; in the non-adherent culture system, cancer cells highly expressing TG2 have more potent proliferative ability. The TG2-promoted EMT is mediated by activation of NF-κB and up-regulation of Snail, Zeb1, and Zeb2 [9]. TGF-β, a cytokine involved in tumor dissemination, is abundantly secreted in the cancer microenvironment, and could induce TG2 expression and its enzymatic activity, which regulate EMT and tumor metastasis. TGF-β is a potent EMT inducer in both physiological and pathological conditions, and TG2 could convert external stimulation from cellular factors into intracellular signals, and possibly function on the downstream of the molecules. Besides, TG2 also modulates IL-6-mediated cellular response to stimulation, suggesting that TG2 could be the signal transmission locus of various factors [9,86,87].
Our ongoing study along with one of our previous studies [88] shows that TG2 may be involved in mediating CAF-induced EMT in HCC cells via the IL-6/STAT3 signaling pathway, facilitating liver cancer progression and metastasis. In our ongoing study, TG2 expression is significantly elevated in HCC cells with an EMT phenotype. Overexpression of TG2 remarkably promotes CAF-induced EMT and metastasis of HCC cells both in vitro and in vivo, as well as their migratory and invasive abilities. TG2 gene knockout remarkably attenuates CAF-induced EMT in HCC cells, and TG2 expression is affected by the IL-6/STAT3 signaling pathway rather than HGF/Met. After phosphorylation and activation of STAT3, it could respond to and transduce signals transmitted by various factors, including integrin, EGF, IL-5, IL-6, HGF, and leukemia inhibitory factor (LIF), participating in many cellular reactions to stimulus including cell proliferation and apoptosis. Cellular factors of the IL-6 family could activate STAT3 via JAK2, after binding the GP130 receptor on cellular surface [89]. Consistently, our ongoing study also shows that high TG2 expression is significantly correlated with tumor size, tumor multiplicity, clinical stage, and vascular invasion, and that high TG2 expression associates with an unfavorable overall survival, suggesting it as an important independent negative prognosticator in HCC patients. As TG2 could promote tumor progression via various mechanisms, down-regulating TG2 expression with specific inhibitors or using gene interference could be novel approaches to battle malignancies.
TG2 and tumor chemo-resistance
TG2 is highly expressed in tumors with various tissue origins, and has emerged as a putative target involved in tumor chemo-resistance and evasion of apoptosis (Figure 3). Most studies indicate the pro-chemo-resistance, pro-survival, and anti-apoptosis roles for TG2 in malignancies, while few others suggest that TG2 can suppress tumor growth and enhance the growth inhibitory effects of anti-tumor agents. An in vivo experiment shows that compared to wild-type mice, those with TG2 gene knockout have larger tumor foci and a lower survival rate, and that TG2 could promote chemo-sensitivity of tumor cells [60]. TG2 mediates chemo-resistance not only through the activation of survival pathways and the inhibition of apoptosis, but also by regulating ECM formation, EMT, and autophagy. Chronic expression of TG2 in epithelial cancer cells initiates a complex series of signaling networks which contribute to the development of chemo-resistance and an invasive phenotype. For example, induced or basal high TG2 expression in mammary epithelial cells is associated with activation of NF-κB, Akt, FAK, and HIF [50].
Figure 3.
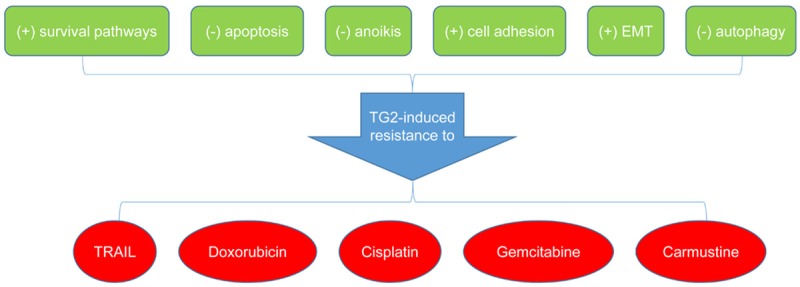
TG2-induced chemo-resistance and the underlined mechanisms. (+) indicates increasing, and (-) decreasing. TG2, transglutaminase 2; EMT, epithelial-mesenchymal transition; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
Cancer cells selected for resistance against chemotherapy or isolated from metastatic sites express an elevated level of TG2. TG2 could widely induce cell chemo-resistance, which contributes to the oncogenic potential, and which is mainly through promoting the integration of fibronectin in ECM and enabling ECM to protect malignant cells from exposure to chemotherapy. Besides, TG2 could impede anoikis of damaged tissue cells via recovering post-damage integrin-dependent adhesion, also mediating chemo-resistance [90]. Down-regulation of TG2 and the inhibition of endogenous TG2 by siRNA or by small molecule inhibitors could result in the reversal of chemo-resistance and the invasive phenotype, significantly enhancing the therapeutic efficacy of anticancer drugs and inhibiting the metastatic spread [91]. Conversely, ectopic TG2 expression promotes cancer cell survival, motility, and invasion [92]. When apoptosis occurs, abundant Ca2+ is released into the intracellular space, and TG2 is then activated with the pro-crosslink activity, and could localize fragments of cells undergoing apoptosis, thus avoiding recognition and phagocytosis of other cells by macrophages. This anti-apoptotic effect also enables TG2 to induce chemo-resistance of malignant cells. In lung cancer cells with acquired tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-resistance, TG2 is one of the most significantly increased genes. Suppressing TG2 dramatically alleviates TRAIL-resistance and cell migration, suggesting that TG2 contributes to these two phenotypes in TRAIL-resistant cells. TG2-mediated TRAIL-resistance is likely through c-FLIP since TG2 suppression significantly reduces c-FLIP. Suppression of EGF receptor (EGFR) dramatically reduces TG2 expression, which is mediated by JNK and ERK. These identify a novel pathway involving EGFR, MAPK (JNK and ERK), and TG2 for both acquired TRAIL-resistance and cell migration, and suggest TG2 as a potential molecular target for combating acquired chemo-resistance and metastasis in lung cancer [93]. Inhibition of TG2 provides an interesting strategy for sensitization to TRAIL-induced apoptosis in p53-deficient lung cancer cells through the up-regulation of death receptor 5 [94]. TG2 controls cell sensitivity to the chemotherapeutic drug doxorubicin. TG2 plays an important role in doxorubicin/cisplatin resistance in breast and ovarian cancers. The NSCLC cell lines with a methylated TG2 gene promoter demonstrate higher sensitivity to cisplatin than the TG2-expressing cell lines, suggesting a positive correlation between cisplatin sensitivity and TG2 inhibition. Thus, good responders of cisplatin in NSCLC could be identified by the promoter methylation of the TG2 gene, and TG2 inhibition might be an effective cisplatin-sensitizing modality in NSCLC [95].
Breast cancer cells express high basal TG2 levels. TG2-deficient cancer cells exhibit greater sensitivity to doxorubicin and are less invasive than the TG2-sufficient cells, and a higher proportion of tumors express TG2 in the chemoprevention group [96]. The sublines with increased TG2 expression will advance from a noninvasive to an invasive phenotype. Importantly, the metastatic lymph nodes from patients with breast cancer show significantly higher TG2 expression compared with the primary tumors from the same patients. TG2 is up-regulated in drug-resistant and metastatic breast cancer cells, and it might serve as a valuable prognosticator for these phenotypes [97]. When TG2 is inhibited, tumor cells undergo more frequently doxorubicin-induced cell death [49]. Down-regulation of TG2 by cystamine or synthetic peptide R2 promotes apoptosis in drug-resistant cancer cells by restoring the level of IκBα, leading to the inactivation of NF-κB. Blocked expression of TG2 renders the doxorubicin-resistant breast cancer cells highly susceptible to doxorubicin-induced apoptosis. TG2 leads to drug-resistance by up-regulating the levels of survival factors via NF-κB activation [98]. Exposure of cells to TG2 inhibitor or expression of a dominantly negative form of TG2 potently inhibits the EGF-mediated protection from doxorubicin-induced apoptosis. Expression of a dominantly negative form of TG2 reverses the EGF-mediated chemo-resistance in breast cancer cells. Doxorubicin-resistant cells have a higher level of TG2 compared with doxorubicin-sensitive cells independent of the EGFR expression. TG2 inhibition increases the chemo-sensitivity of drug-resistant cells, concomitant with a decrease in NF-κB activity. Increasing the TG2 level in drug-sensitive cells by transient transfection reduces the level of IκBα and increases the NF-κB activity in these cells. Inhibition of TG2 in drug-resistant cells by RNA interference increases the level of IκBα, and this correlates with a shift in the accumulation of NF-κB from the nucleus to the cytosol. TG2 activates NF-κB through polymerization and depletion of the free IκBα during inflammation. Therefore, increased expression of TG2 and subsequent activation of NF-κB may contribute to the drug-resistance in breast cancer cells independently of the EGF signaling [99]. TG2 closely associates with β1 and β5 integrins on the surface of the drug-resistant breast cancer cells. Incubation of the TG2-expressing chemo-resistant cancer cells on fibronectin-coated surfaces strongly activates FAK, which leads to the activation of several downstream signaling pathways and, in turn, can confer an apoptosis-resistant phenotype to the cancer cells. Inhibition of TG2 by siRNA inhibits the fibronectin-mediated cell attachment and survival in chemo-resistant cells [100]. Thus, TG2 expression in breast cancer cells contributes to the development of chemo-resistance by promoting the interaction between integrin and fibronectin.
TG2 regulates cisplatin-induced apoptosis in ovarian cancer cells. Interestingly, not only TG2 knockdown but also TG2 enzymatic inhibitors sensitize cancer cells to cisplatin. Over expression of the constitutively active p65 subunit of NF-κB, rather than of the constitutively active Akt, rescues cells with diminished TG2 expression from cisplatin-induced apoptosis. This implicates activation of NF-κB as the main cisplatin-resistance mechanism downstream of TG2. NF-κB activity is decreased and the level of the inhibitory subunit IκBα is increased in ovarian cancer cells engineered to express diminished levels of TG2 or treated with inhibitors. Thus, TG2 prevents cisplatin-induced apoptosis by activating the NF-κB survival pathway in ovarian cancer cells [101].
TG2 could induce gemcitabine-resistance in pancreatic cancer cells [102]. Down-regulation of TG2 significantly enhances the therapeutic efficacy of gemcitabine against PDAC and inhibits metastatic spread of the disease. Therefore, siRNA-mediated down-regulation of TG2 represents a promising therapeutic approach for improved treatment of PDAC [84]. TG2-inhibitors could also significantly attenuate carmustine-resistance of malignant glioma [103]. TG2 expression contributes to the development of chemo-resistance in malignant melanoma cells by exploiting integrin-mediated cell survival signaling pathways [77]. Nucleophosmin is a substrate of TG2 in cancer cells, and is polymerized by TG2. The polymerization could be inhibited by the TG2 inhibitor, cystamine. The nucleophosmin level in the cytosolic cell fraction is reduced when TG2 is expressed. Increased expression of TG2 is highly associated with drug-resistance, and polymerization of nucleophosmin by TG2 can also be correlated with the drug-resistance of cancer cells. Nucleophosmin cross-linked by TG2 is eradicated via the ubiquitin-proteasomal pathway. Depletion of cytosolic nucleophosmin by TG2 can decrease the Bax protein stability and is anti-apoptotic. Thus, TG2 inhibits the accumulation of cytosolic nucleophosmin through polymerization, which results in drug-resistance in cancer cells [104]. Since TG2 knockdown or TG2 enzymatic activity inhibition may reverse chemo-resistance and sensitize tumor cells to drug-induced apoptosis, many small molecules capable of blocking TG2 have recently been developed [105]. TG2 might also induce radiotherapy-resistance.
TG2 and prognosis
TG2 is considered majorly as a potential negative prognosticator, and is often associated with advanced disease stage, metastasis, and chemo-resistance. In renal cell carcinoma, TG2 expression is significantly reversely correlated with 5-year disease-free and cancer-specific survival rates [82]. In colorectal cancer, patients with high TG2 expression show a poorer overall survival (OS) rate than those with low TG2 expression, indicating increased TG2 expression as an independent unfavorable prognosticator [70]. TG2-mediated PTEN loss is a prognosticator for patients with stage II PDAC independent of the tumor stage/lymph node status and tumor differentiation grade [68]. Among laryngeal SCC patients receiving adjuvant radiotherapy, low TG2 expression is a significant independent prognosticator of an improved OS, and its expression correlates with another HIF-1α target, BNIP3 [106].
TG2 expression in NSCLC and in the non-adenocarcinoma subtypes including SCC is significantly correlated with recurrence. There also exists a significant correlation between strong TG2 expression and a shorter disease-free survival (DFS) in NSCLC (HR = 1.554) and in the non-adenocarcinoma subtype (HR = 2.184) [35]. Among NSCLC patients who receive the EGFR-tyrosine kinase inhibitor (TKI) therapy, the progression free survival (PFS) is significantly longer in the low TG2 expression group than that in the high TG2 expression group. Similarly, in wild-type EGFR patients treated with EGFR-TKI, PFS is also longer in patients with low TG2 expression. Thus, TG2 expression can predict PFS in NSCLC patients treated with EGFR-TKI [107].
In patients with advanced breast cancer, TG2 expression in the primary tumor is inversely correlated with recurrence-free survival and distant metastasis-free survival (DMFS). The combined high expression of TG2 and IL-6 is associated with a shorter DMFS, compared with the high expression of IL-6 only, suggesting that TG2 could be an important mediator of distant metastasis in breast cancer [54]. In invasive ductal carcinomas (IDCs) of the breast, patients showing stromal TG2 accumulation have significantly reduced DFS compared to those showing low TG2 expression, and stromal TG2 accumulation is an independent risk factor for recurrence, suggesting that TG2 overexpression in tumor stroma may serve as a poor prognosticator for breast IDC [108]. However, a research from Greece suggests that there is a beneficial effect of TG2 expression on OS, and that TG2 is an independent favorable prognosticator for survival possibly via enhancing the apoptotic effect of chemotherapy [109].
TG2-based anti-tumor strategies
Additional insights into the multifunctional nature of TG2 as well as translational studies concerning the correlation between expression, function, and location of TG2 and cancer behavior will shed light on novel anti-cancer therapies (Figure 4). A specific TG2 inhibitor may be a very useful treatment against cancer with a high TG2 level. Treatment of cancer cells with inhibitors of both TG2 and GLS1 results in synthetic cellular lethality. The combination of the inhibitors induces cell death, while individual treatment with either compound shows little or no cell-killing ability, suggesting that combination treatment simultaneously targeting TG2 and GLS1 might be a promising strategy to combat cancer [46].
Figure 4.
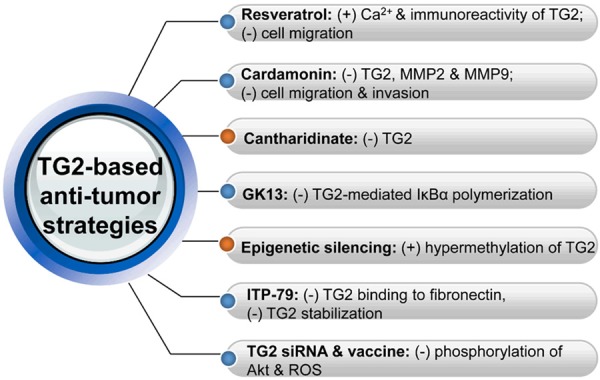
Current TG2-based anti-tumor strategies and the underlined mechanisms. (+) indicates increasing, and (-) decreasing. TG2, transglutaminase 2; MMP, matrix metalloproteinase; IκBα, the inhibitory subunit α of nuclear factor-κB; siRNA, small interfering RNA; ROS, reactive oxygen specie.
Resveratrol could prevent the migration of TG2-expressing cells. During migration, resveratrol increases the immunoreactivity of TG2 without affecting the total TG2 protein level in migrating cells, and with an increased Ca2+ level. Depletion of intracellular Ca2+ by a Ca2+ chelator attenuates the resveratrol-enhanced TG2 immunoreactivity. In the native-polyacrylamide gel, a non-phosphorylated TG2 protein form in resveratrol-treated cells correlates with a slower migration speed, and is exclusively present in the plasma membrane fractions and sensitive to the intracellular Ca2+ concentration, suggesting the Ca2+ requirement in TG2-regulated cell migration [36]. These suggest that resveratrol induces conformational changes of TG2, and that association of Ca2+-mediated TG2 with the plasma membrane is responsible for the inhibitory effect of resveratrol on cell migration.
Cardamonin suppresses TG2 expression in sarcoma cells, and directly inhibits TG2 activity. Silencing of TG2 gene suppresses the invasion and migration of sarcoma cells and inhibits the activities of MMP2 and MMP9. Migration of various cancer cells with high TG2 levels could also be inhibited by cardamonin [110]. This finding will evoke a novel idea of pharmacophore for the development of effective TG2-inhibitors. Cantharidinate could inhibit the TG2 expression in human colorectal cancer [71]. A competitive TG2-inhibiting quinoxaline compound GK13 inhibits TG2-mediated IκBα polymerization in a dose-dependent manner, and has greater anti-cancer efficacy than doxorubicin [111].
Both doxorubicin sensitivity and TG2 expression are highly variable in cultured human breast cancer cell lines and there exists a canonical CpG island within the 5’ flank of the human TG2 gene. These features, when combined with its potential tumor-suppressing activity, make TG2 an attractive candidate for epigenetic silencing, and this aberrant molecular event is a potential marker for chemotherapeutic drug sensitivity. Consistent with this, culturing breast tumor cells with DNA demethylating agents results in a robust increase in TG2 expression. TG2 gene often displays aberrant hyper-methylation, and there is a significant correlation between gene methylation and reduced expression. Doxorubicin-resistant cancer cells do not show TG2 silencing but doxorubicin-sensitive cells do. Culturing the sensitive cells with the DNA demethylating agent and subsequently restoring TG2 expression reduce cell sensitivity to doxorubicin [33].
In ovarian cancer, ITP-79 inhibits the binding of a TG2 peptide to a 42-kDa fragment of fibronectin in a dose-dependent manner, and reduces TG2 stabilization, which mimics the effect of GTP, a negative allosteric regulator of the TG2 enzymatic function, suggesting a potential allosteric mechanism for ITP-79 in light of its distal target site [112]. Down-regulation of TG2 by siRNA impairs adhesion and motility of HeLa cells by decreasing phosphorylation of the protein kinase Akt and reactive oxygen species (ROSs), and over-expression of TG2 shows opposite effects, suggesting the potential utility of TG2 for development of therapeutic anti-cancer vaccines [113].
Conclusions
Recent findings have produced great strides in developing an understanding of the molecular events involved in processes necessary for tumor cell invasion and subsequent metastasis formation. TG2 has important physiological functions and plays a vital role in inflammation mainly through its modulation of the ECM structure and stability with its influence on gene expression. It regulates tumor progression, especially EMT, via various intracellular and extracellular ways, mainly depending on its pro-crosslink and signaling transduction mediation propensities, which attenuates cell adhesion and promotes ECM proteolysis. It is controlled by a network of regulators, and provides an explanation for the association between inflammation and cancer progression. Progresses in investigating TG2 have become an exercise in coming to appreciate the level of complexity required for changing cellular identity. The underling mechanism highlights an integration of nuclear regulation and network signaling with alteration in the microenvironment. Here we have focused on the effects of various stroma-derived factors on epithelial cells. It is probable that the same factors also have vital effects on stromal cells, including fibroblasts, endothelial cells, and inflammatory cells. The malignant state is unleashed and exacerbated by defects in communication pathways. The cross-talk between tumor cells and stroma triggers pro-survival, proliferation, and invasion pathways in both the cancer cells and their hosts. However, it is unclear which signaling pathways should be inhibited in order to most effectively block tumor progression and to cause minimal toxicity in normal tissues simultaneously. TG2 is also involved in tumor chemo-resistance, highlighting the need to inhibit its activity during anti-cancer therapy.
It is notable that the models described in this review are not mutually exclusive, both across different patients and across different cancer cells in any specific tissue since malignant cells are quite heterogeneous. Most of the valuable insights have been derived from cell culture and tissue recombination xenograph experiments, and these results may not be applicable to the in vivo situation due to the fact that not all key micro-environmental factors are considered. The capability to overexpress specific factors or to conditionally knockout specific genes in vivo will further add greatly to our knowledge of the complex interaction involved in cancer progression. Future development will include a new class of therapies targeting the extra- and intra-cellular mediators, especially TG2, which acts at the tumor-host communication interface. Future efforts should also further focus on how TG2-mediated cell attachment, ECM proteolysis, and cell migration are controlled and integrated, which requires a better understanding of the involved transcriptional regulations and cell signaling mechanism.
An important issue for TG2 is its being regarded as having a high prognostic and therapeutic value. Further understanding the relevant genetics and epigenetics will be especially vital in order to differentiate TG2 as patient-specific therapeutic targets. Several molecular candidates have been proposed. Some of the TG2-associated signal transductions point to potential targets for therapy. A better understanding of the effects of targeting these pathways, however, is required. It is likely that further characterization of these interactions, and the molecular identification of the key mediators, will prove novel insights into oncology and indicate new therapeutic options. The inhibition of TG2 by small molecules may be useful in blocking metastasis. Pharmacological and biological agents that interfere with the signaling between malignant epithelial cells and the supporting stroma will likely continue to be tested. Much future work will be needed to test the hypothesis and to further our understanding of metastasis in general.
Acknowledgements
The authors would sincerely thank the reviewers and editors for critically reviewing this paper and for the constructive and thoughtful comments and suggestions.
Disclosure of conflict of interest
None.
References
- 1.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 2.Huang L, Xu AM, Liu S, Liu W, Li TJ. Cancerassociated fibroblasts in digestive tumors. World J Gastroenterol. 2014;20:17804–17818. doi: 10.3748/wjg.v20.i47.17804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brauer HA, Makowski L, Hoadley KA, Casbas-Hernandez P, Lang LJ, Roman-Perez E, D’Arcy M, Freemerman AJ, Perou CM, Troester MA. Impact of tumor microenvironment and epithelial phenotypes on metabolism in breast cancer. Clin Cancer Res. 2013;19:571–585. doi: 10.1158/1078-0432.CCR-12-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu AM, Huang L, Zhu L, Wei ZJ. Significance of peripheral neutrophil-lymphocyte ratio among gastric cancer patients and construction of a treatment-predictive model: a study based on 1131 cases. Am J Cancer Res. 2014;4:189–195. [PMC free article] [PubMed] [Google Scholar]
- 6.Huang L, Li TJ, Zhang JW, Liu S, Fu BS, Liu W. Neoadjuvant Chemotherapy Followed by Surgery Versus Surgery Alone for Colorectal Cancer: Meta-analysis of Randomized Controlled Trials. Medicine (Baltimore) 2014;93:e231. doi: 10.1097/MD.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu AM, Huang L, Han WX, Wei ZJ. Monitoring of peri-distal gastrectomy carbohydrate antigen 19-9 level in gastric juice and its significance. Int J Clin Exp Med. 2014;7:230–238. [PMC free article] [PubMed] [Google Scholar]
- 8.Xu AM, Huang L, Liu W, Gao S, Han WX, Wei ZJ. Neoadjuvant chemotherapy followed by surgery versus surgery alone for gastric carcinoma: systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9:e86941. doi: 10.1371/journal.pone.0086941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao M, Cao L, Shen C, Satpathy M, Chelladurai B, Bigsby RM, Nakshatri H, Matei D. Epithelial-to-mesenchymal transition and ovarian tumor progression induced by tissue transglutaminase. Cancer Res. 2009;69:9192–9201. doi: 10.1158/0008-5472.CAN-09-1257. [DOI] [PubMed] [Google Scholar]
- 10.Begg GE, Carrington L, Stokes PH, Matthews JM, Wouters MA, Husain A, Lorand L, Iismaa SE, Graham RM. Mechanism of allosteric regulation of transglutaminase 2 by GTP. Proc Natl Acad Sci U S A. 2006;103:19683–19688. doi: 10.1073/pnas.0609283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iismaa SE, Mearns BM, Lorand L, Graham RM. Transglutaminases and disease: lessons from genetically engineered mouse models and inherited disorders. Physiol Rev. 2009;89:991–1023. doi: 10.1152/physrev.00044.2008. [DOI] [PubMed] [Google Scholar]
- 12.Akimov SS, Krylov D, Fleischman LF, Belkin AM. Tissue transglutaminase is an integrinbinding adhesion coreceptor for fibronectin. J Cell Biol. 2000;148:825–838. doi: 10.1083/jcb.148.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin CY, Tsai PH, Kandaswami CC, Chang GD, Cheng CH, Huang CJ, Lee PP, Hwang JJ, Lee MT. Role of tissue transglutaminase 2 in the acquisition of a mesenchymal-like phenotype in highly invasive A431 tumor cells. Mol Cancer. 2011;10:87. doi: 10.1186/1476-4598-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonyak MA, Miller AM, Jansen JM, Boehm JE, Balkman CE, Wakshlag JJ, Page RL, Cerione RA. Augmentation of tissue transglutaminase expression and activation by epidermal growth factor inhibit doxorubicin-induced apoptosis in human breast cancer cells. J Biol Chem. 2004;279:41461–41467. doi: 10.1074/jbc.M404976200. [DOI] [PubMed] [Google Scholar]
- 15.Xu L, Begum S, Hearn JD, Hynes RO. GPR56, an atypical G protein-coupled receptor, binds tissue transglutaminase, TG2, and inhibits melanoma tumor growth and metastasis. Proc Natl Acad Sci U S A. 2006;103:9023–9028. doi: 10.1073/pnas.0602681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basso M, Berlin J, Xia L, Sleiman SF, Ko B, Haskew-Layton R, Kim E, Antonyak MA, Cerione RA, Iismaa SE, Willis D, Cho S, Ratan RR. Transglutaminase inhibition protects against oxidative stress-induced neuronal death downstream of pathological ERK activation. J Neurosci. 2012;32:6561–6569. doi: 10.1523/JNEUROSCI.3353-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN, Larue L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63:2172–2178. [PubMed] [Google Scholar]
- 18.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 19.Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26:711–724. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- 20.Bernassola F, Federici M, Corazzari M, Terrinoni A, Hribal ML, De Laurenzi V, Ranalli M, Massa O, Sesti G, McLean WH, Citro G, Barbetti F, Melino G. Role of transglutaminase 2 in glucose tolerance: knockout mice studies and a putative mutation in a MODY patient. FASEB J. 2002;16:1371–1378. doi: 10.1096/fj.01-0689com. [DOI] [PubMed] [Google Scholar]
- 21.Gross SR, Balklava Z, Griffin M. Importance of tissue transglutaminase in repair of extracellular matrices and cell death of dermal fibroblasts after exposure to a solarium ultraviolet A source. J Invest Dermatol. 2003;121:412–423. doi: 10.1046/j.1523-1747.2003.12353.x. [DOI] [PubMed] [Google Scholar]
- 22.Eom S, Kim Y, Kim M, Park D, Lee H, Lee YS, Choe J, Kim YM, Jeoung D. Transglutaminase II/microRNA-218/-181a loop regulates positive feedback relationship between allergic inflammation and tumor metastasis. J Biol Chem. 2014;289:29483–29505. doi: 10.1074/jbc.M114.603480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra S, Saleh A, Espino PS, Davie JR, Murphy LJ. Phosphorylation of histones by tissue transglutaminase. J Biol Chem. 2006;281:5532–5538. doi: 10.1074/jbc.M506864200. [DOI] [PubMed] [Google Scholar]
- 24.Falasca L, Farrace MG, Rinaldi A, Tuosto L, Melino G, Piacentini M. Transglutaminase type II is involved in the pathogenesis of endotoxic shock. J Immunol. 2008;180:2616–2624. doi: 10.4049/jimmunol.180.4.2616. [DOI] [PubMed] [Google Scholar]
- 25.Nardacci R, Lo Iacono O, Ciccosanti F, Falasca L, Addesso M, Amendola A, Antonucci G, Craxi A, Fimia GM, Iadevaia V, Melino G, Ruco L, Tocci G, Ippolito G, Piacentini M. Transglutaminase type II plays a protective role in hepatic injury. Am J Pathol. 2003;162:1293–1303. doi: 10.1016/S0002-9440(10)63925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shweke N, Boulos N, Jouanneau C, Vandermeersch S, Melino G, Dussaule JC, Chatziantoniou C, Ronco P, Boffa JJ. Tissue transglutaminase contributes to interstitial renal fibrosis by favoring accumulation of fibrillar collagen through TGF-beta activation and cell infiltration. Am J Pathol. 2008;173:631–642. doi: 10.2353/ajpath.2008.080025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toth B, Garabuczi E, Sarang Z, Vereb G, Vamosi G, Aeschlimann D, Blasko B, Becsi B, Erdodi F, Lacy-Hulbert A, Zhang A, Falasca L, Birge RB, Balajthy Z, Melino G, Fesus L, Szondy Z. Transglutaminase 2 is needed for the formation of an efficient phagocyte portal in macrophages engulfing apoptotic cells. J Immunol. 2009;182:2084–2092. doi: 10.4049/jimmunol.0803444. [DOI] [PubMed] [Google Scholar]
- 28.Akimov SS, Belkin AM. Cell surface tissue transglutaminase is involved in adhesion and migration of monocytic cells on fibronectin. Blood. 2001;98:1567–1576. doi: 10.1182/blood.v98.5.1567. [DOI] [PubMed] [Google Scholar]
- 29.Jang GY, Jeon JH, Cho SY, Shin DM, Kim CW, Jeong EM, Bae HC, Kim TW, Lee SH, Choi Y, Lee DS, Park SC, Kim IG. Transglutaminase 2 suppresses apoptosis by modulating caspase 3 and NF-kappaB activity in hypoxic tumor cells. Oncogene. 2010;29:356–367. doi: 10.1038/onc.2009.342. [DOI] [PubMed] [Google Scholar]
- 30.Yakubov B, Chelladurai B, Schmitt J, Emerson R, Turchi JJ, Matei D. Extracellular tissue transglutaminase activates noncanonical NFkappaB signaling and promotes metastasis in ovarian cancer. Neoplasia. 2013;15:609–619. doi: 10.1593/neo.121878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangala LS, Fok JY, Zorrilla-Calancha IR, Verma A, Mehta K. Tissue transglutaminase expression promotes cell attachment, invasion and survival in breast cancer cells. Oncogene. 2007;26:2459–2470. doi: 10.1038/sj.onc.1210035. [DOI] [PubMed] [Google Scholar]
- 32.Phatak VM, Croft SM, Rameshaiah Setty SG, Scarpellini A, Hughes DC, Rees R, McArdle S, Verderio EA. Expression of transglutaminase-2 isoforms in normal human tissues and cancer cell lines: dysregulation of alternative splicing in cancer. Amino Acids. 2013;44:33–44. doi: 10.1007/s00726-011-1127-4. [DOI] [PubMed] [Google Scholar]
- 33.Ai L, Kim WJ, Demircan B, Dyer LM, Bray KJ, Skehan RR, Massoll NA, Brown KD. The transglutaminase 2 gene (TGM2), a potential molecular marker for chemotherapeutic drug sensitivity, is epigenetically silenced in breast cancer. Carcinogenesis. 2008;29:510–518. doi: 10.1093/carcin/bgm280. [DOI] [PubMed] [Google Scholar]
- 34.Antonyak MA, Li B, Boroughs LK, Johnson JL, Druso JE, Bryant KL, Holowka DA, Cerione RA. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci U S A. 2011;108:4852–4857. doi: 10.1073/pnas.1017667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi CM, Jang SJ, Park SY, Choi YB, Jeong JH, Kim DS, Kim HK, Park KS, Nam BH, Kim HR Korean Thoracic Oncology Research Group (KTORG) Kim SY, Hong KM. Transglutaminase 2 as an independent prognostic marker for survival of patients with non-adenocarcinoma subtype of non-small cell lung cancer. Mol Cancer. 2011;10:119. doi: 10.1186/1476-4598-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar A, Hu J, LaVoie HA, Walsh KB, DiPette DJ, Singh US. Conformational changes and translocation of tissue-transglutaminase to the plasma membranes: role in cancer cell migration. BMC Cancer. 2014;14:256. doi: 10.1186/1471-2407-14-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belkin AM, Akimov SS, Zaritskaya LS, Ratnikov BI, Deryugina EI, Strongin AY. Matrixdependent proteolysis of surface transglutaminase by membrane-type metalloproteinase regulates cancer cell adhesion and locomotion. J Biol Chem. 2001;276:18415–18422. doi: 10.1074/jbc.M010135200. [DOI] [PubMed] [Google Scholar]
- 38.Stephens P, Grenard P, Aeschlimann P, Langley M, Blain E, Errington R, Kipling D, Thomas D, Aeschlimann D. Crosslinking and G-protein functions of transglutaminase 2 contribute differentially to fibroblast wound healing responses. J Cell Sci. 2004;117:3389–3403. doi: 10.1242/jcs.01188. [DOI] [PubMed] [Google Scholar]
- 39.Scarpellini A, Germack R, Lortat-Jacob H, Muramatsu T, Billett E, Johnson T, Verderio EA. Heparan sulfate proteoglycans are receptors for the cell-surface trafficking and biological activity of transglutaminase-2. J Biol Chem. 2009;284:18411–18423. doi: 10.1074/jbc.M109.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Collighan RJ, Gross SR, Danen EH, Orend G, Telci D, Griffin M. RGD-independent cell adhesion via a tissue transglutaminase-fibronectin matrix promotes fibronectin fibril deposition and requires syndecan-4/2 alpha5beta1 integrin co-signaling. J Biol Chem. 2010;285:40212–40229. doi: 10.1074/jbc.M110.123703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu L, Hynes RO. GPR56 and TG2: possible roles in suppression of tumor growth by the microenvironment. Cell Cycle. 2007;6:160–165. doi: 10.4161/cc.6.2.3760. [DOI] [PubMed] [Google Scholar]
- 42.Satpathy M, Shao M, Emerson R, Donner DB, Matei D. Tissue transglutaminase regulates matrix metalloproteinase-2 in ovarian cancer by modulating cAMP-response element-binding protein activity. J Biol Chem. 2009;284:15390–15399. doi: 10.1074/jbc.M808331200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mangala LS, Arun B, Sahin AA, Mehta K. Tissue transglutaminase-induced alterations in extracellular matrix inhibit tumor invasion. Mol Cancer. 2005;4:33. doi: 10.1186/1476-4598-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kotsakis P, Griffin M. Tissue transglutaminase in tumour progression: friend or foe? Amino Acids. 2007;33:373–384. doi: 10.1007/s00726-007-0516-1. [DOI] [PubMed] [Google Scholar]
- 45.Chhabra A, Verma A, Mehta K. Tissue transglutaminase promotes or suppresses tumors depending on cell context. Anticancer Res. 2009;29:1909–1919. [PubMed] [Google Scholar]
- 46.Katt WP, Antonyak MA, Cerione RA. Simultaneously targeting tissue transglutaminase and kidney type glutaminase sensitizes cancer cells to acid toxicity and offers new opportunities for therapeutic intervention. Mol Pharm. 2015;12:46–55. doi: 10.1021/mp500405h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caraglia M, Marra M, Giuberti G, D'Alessandro AM, Beninati S, Lentini A, Pepe S, Boccellino M, Abbruzzese A. Theophylline-induced apoptosis is paralleled by protein kinase A-dependent tissue transglutaminase activation in cancer cells. J Biochem. 2002;132:45–52. doi: 10.1093/oxfordjournals.jbchem.a003197. [DOI] [PubMed] [Google Scholar]
- 48.Krig SR, Rice RH. TCDD suppression of tissue transglutaminase stimulation by retinoids in malignant human keratinocytes. Toxicol Sci. 2000;56:357–364. doi: 10.1093/toxsci/56.2.357. [DOI] [PubMed] [Google Scholar]
- 49.Wakshlag JJ, McNeill CJ, Antonyak MA, Boehm JE, Fuji R, Balkman CE, Zgola M, Cerione RA, Page RL. Expression and activity of transglutaminase II in spontaneous tumours of dogs and cats. J Comp Pathol. 2006;134:202–210. doi: 10.1016/j.jcpa.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Agnihotri N, Kumar S, Mehta K. Tissue transglutaminase as a central mediator in inflammation-induced progression of breast cancer. Breast Cancer Res. 2013;15:202. doi: 10.1186/bcr3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balestrieri ML, Dicitore A, Benevento R, Di Maio M, Santoriello A, Canonico S, Giordano A, Stiuso P. Interplay between membrane lipid peroxidation, transglutaminase activity, and cyclooxygenase 2 expression in the tissue adjoining to breast cancer. J Cell Physiol. 2012;227:1577–1582. doi: 10.1002/jcp.22874. [DOI] [PubMed] [Google Scholar]
- 52.Wang Z, Griffin M. The role of TG2 in regulating S100A4-mediated mammary tumour cell migration. PLoS One. 2013;8:e57017. doi: 10.1371/journal.pone.0057017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park KS, Kim DS, Jeong KC, Kim SY. Increase in transglutaminase 2 expression is associated with NF-kappaB activation in breast cancer tissues. Front Biosci (Landmark Ed) 2009;14:1945–1951. doi: 10.2741/3354. [DOI] [PubMed] [Google Scholar]
- 54.Oh K, Ko E, Kim HS, Park AK, Moon HG, Noh DY, Lee DS. Transglutaminase 2 facilitates the distant hematogenous metastasis of breast cancer by modulating interleukin-6 in cancer cells. Breast Cancer Res. 2011;13:R96. doi: 10.1186/bcr3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caffarel MM, Chattopadhyay A, Araujo AM, Bauer J, Scarpini CG, Coleman N. Tissue transglutaminase mediates the pro-malignant effects of oncostatin M receptor over-expression in cervical squamous cell carcinoma. J Pathol. 2013;231:168–179. doi: 10.1002/path.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Antonyak MA, Li B, Regan AD, Feng Q, Dusaban SS, Cerione RA. Tissue transglutaminase is an essential participant in the epidermal growth factor-stimulated signaling pathway leading to cancer cell migration and invasion. J Biol Chem. 2009;284:17914–17925. doi: 10.1074/jbc.M109.013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boroughs LK, Antonyak MA, Johnson JL, Cerione RA. A unique role for heat shock protein 70 and its binding partner tissue transglutaminase in cancer cell migration. J Biol Chem. 2011;286:37094–37107. doi: 10.1074/jbc.M111.242438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singer CF, Hudelist G, Walter I, Rueckliniger E, Czerwenka K, Kubista E, Huber AV. Tissue array-based expression of transglutaminase-2 in human breast and ovarian cancer. Clin Exp Metastasis. 2006;23:33–39. doi: 10.1007/s10585-006-9015-0. [DOI] [PubMed] [Google Scholar]
- 59.Brown KD. Transglutaminase 2 and NFkappaB: an odd couple that shapes breast cancer phenotype. Breast Cancer Res Treat. 2013;137:329–336. doi: 10.1007/s10549-012-2351-7. [DOI] [PubMed] [Google Scholar]
- 60.Satpathy M, Cao L, Pincheira R, Emerson R, Bigsby R, Nakshatri H, Matei D. Enhanced peritoneal ovarian tumor dissemination by tissue transglutaminase. Cancer Res. 2007;67:7194–7202. doi: 10.1158/0008-5472.CAN-07-0307. [DOI] [PubMed] [Google Scholar]
- 61.Cheung W, Darfler MM, Alvarez H, Hood BL, Conrads TP, Habbe N, Krizman DB, Mollenhauer J, Feldmann G, Maitra A. Application of a global proteomic approach to archival precursor lesions: deleted in malignant brain tumors 1 and tissue transglutaminase 2 are upregulated in pancreatic cancer precursors. Pancreatology. 2008;8:608–616. doi: 10.1159/000161012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iacobuzio-Donahue CA, Ashfaq R, Maitra A, Adsay NV, Shen-Ong GL, Berg K, Hollingsworth MA, Cameron JL, Yeo CJ, Kern SE, Goggins M, Hruban RH. Highly expressed genes in pancreatic ductal adenocarcinomas: a comprehensive characterization and comparison of the transcription profiles obtained from three major technologies. Cancer Res. 2003;63:8614–8622. [PubMed] [Google Scholar]
- 63.Mehta K. Biological and therapeutic significance of tissue transglutaminase in pancreatic cancer. Amino Acids. 2009;36:709–716. doi: 10.1007/s00726-008-0128-4. [DOI] [PubMed] [Google Scholar]
- 64.Ozpolat B, Akar U, Mehta K, Lopez-Berestein G. PKC delta and tissue transglutaminase are novel inhibitors of autophagy in pancreatic cancer cells. Autophagy. 2007;3:480–483. doi: 10.4161/auto.4349. [DOI] [PubMed] [Google Scholar]
- 65.Akar U, Ozpolat B, Mehta K, Fok J, Kondo Y, Lopez-Berestein G. Tissue transglutaminase inhibits autophagy in pancreatic cancer cells. Mol Cancer Res. 2007;5:241–249. doi: 10.1158/1541-7786.MCR-06-0229. [DOI] [PubMed] [Google Scholar]
- 66.Ashour AA, Gurbuz N, Alpay SN, Abdel-Aziz AA, Mansour AM, Huo L, Ozpolat B. Elongation factor-2 kinase regulates TG2/beta1 integrin/Src/uPAR pathway and epithelial-mesenchymal transition mediating pancreatic cancer cells invasion. J Cell Mol Med. 2014;18:2235–2251. doi: 10.1111/jcmm.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fok JY, Mehta K. Tissue transglutaminase induces the release of apoptosis inducing factor and results in apoptotic death of pancreatic cancer cells. Apoptosis. 2007;12:1455–1463. doi: 10.1007/s10495-007-0079-3. [DOI] [PubMed] [Google Scholar]
- 68.Verma A, Guha S, Wang H, Fok JY, Koul D, Abbruzzese J, Mehta K. Tissue transglutaminase regulates focal adhesion kinase/AKT activation by modulating PTEN expression in pancreatic cancer cells. Clin Cancer Res. 2008;14:1997–2005. doi: 10.1158/1078-0432.CCR-07-1533. [DOI] [PubMed] [Google Scholar]
- 69.Mann AP, Verma A, Sethi G, Manavathi B, Wang H, Fok JY, Kunnumakkara AB, Kumar R, Aggarwal BB, Mehta K. Overexpression of tissue transglutaminase leads to constitutive activation of nuclear factor-kappaB in cancer cells: delineation of a novel pathway. Cancer Res. 2006;66:8788–8795. doi: 10.1158/0008-5472.CAN-06-1457. [DOI] [PubMed] [Google Scholar]
- 70.Miyoshi N, Ishii H, Mimori K, Tanaka F, Hitora T, Tei M, Sekimoto M, Doki Y, Mori M. TGM2 is a novel marker for prognosis and therapeutic target in colorectal cancer. Ann Surg Oncol. 2010;17:967–972. doi: 10.1245/s10434-009-0865-y. [DOI] [PubMed] [Google Scholar]
- 71.Yang LL, Liang CY, Lu TC, Zhi CY, Liu B, Zhou JH, Liu XM, Gao HC, Huang W. Role of tissue transglutaminase and effect of cantharidinate in human colorectal cancer. Mol Med Rep. 2013;8:1812–1816. doi: 10.3892/mmr.2013.1706. [DOI] [PubMed] [Google Scholar]
- 72.Jones RA, Kotsakis P, Johnson TS, Chau DY, Ali S, Melino G, Griffin M. Matrix changes induced by transglutaminase 2 lead to inhibition of angiogenesis and tumor growth. Cell Death Differ. 2006;13:1442–1453. doi: 10.1038/sj.cdd.4401816. [DOI] [PubMed] [Google Scholar]
- 73.Kotsakis P, Wang Z, Collighan RJ, Griffin M. The role of tissue transglutaminase (TG2) in regulating the tumour progression of the mouse colon carcinoma CT26. Amino Acids. 2011;41:909–921. doi: 10.1007/s00726-010-0790-1. [DOI] [PubMed] [Google Scholar]
- 74.Sun Y, Mi W, Cai J, Ying W, Liu F, Lu H, Qiao Y, Jia W, Bi X, Lu N, Liu S, Qian X, Zhao X. Quantitative proteomic signature of liver cancer cells: tissue transglutaminase 2 could be a novel protein candidate of human hepatocellular carcinoma. J Proteome Res. 2008;7:3847–3859. doi: 10.1021/pr800153s. [DOI] [PubMed] [Google Scholar]
- 75.Esposito C, Marra M, Giuberti G, D’Alessandro AM, Porta R, Cozzolino A, Caraglia M, Abbruzzese A. Ubiquitination of tissue transglutaminase is modulated by interferon alpha in human lung cancer cells. Biochem J. 2003;370:205–212. doi: 10.1042/BJ20021390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kawai Y, Wada F, Sugimura Y, Maki M, Hitomi K. Transglutaminase 2 activity promotes membrane resealing after mechanical damage in the lung cancer cell line A549. Cell Biol Int. 2008;32:928–934. doi: 10.1016/j.cellbi.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 77.Fok JY, Ekmekcioglu S, Mehta K. Implications of tissue transglutaminase expression in malignant melanoma. Mol Cancer Ther. 2006;5:1493–1503. doi: 10.1158/1535-7163.MCT-06-0083. [DOI] [PubMed] [Google Scholar]
- 78.Lewis TE, Milam TD, Klingler DW, Rao PS, Jaggi M, Smith DJ, Hemstreet GP, Balaji KC. Tissue transglutaminase interacts with protein kinase A anchor protein 13 in prostate cancer. Urol Oncol. 2005;23:407–412. doi: 10.1016/j.urolonc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 79.Torricelli P, Caraglia M, Abbruzzese A, Beninati S. gamma-Tocopherol inhibits human prostate cancer cell proliferation by up-regulation of transglutaminase 2 and down-regulation of cyclins. Amino Acids. 2013;44:45–51. doi: 10.1007/s00726-012-1278-y. [DOI] [PubMed] [Google Scholar]
- 80.Huang YC, Wei KC, Chang CN, Chen PY, Hsu PW, Chen CP, Lu CS, Wang HL, Gutmann DH, Yeh TH. Transglutaminase 2 expression is increased as a function of malignancy grade and negatively regulates cell growth in meningioma. PLoS One. 2014;9:e108228. doi: 10.1371/journal.pone.0108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu T, Tee AE, Porro A, Smith SA, Dwarte T, Liu PY, Iraci N, Sekyere E, Haber M, Norris MD, Diolaiti D, Della Valle G, Perini G, Marshall GM. Activation of tissue transglutaminase transcription by histone deacetylase inhibition as a therapeutic approach for Myc oncogenesis. Proc Natl Acad Sci U S A. 2007;104:18682–18687. doi: 10.1073/pnas.0705524104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Erdem S, Yegen G, Telci D, Yildiz I, Tefik T, Issever H, Kilicaslan I, Sanli O. The increased transglutaminase 2 expression levels during initial tumorigenesis predict increased risk of metastasis and decreased disease-free and cancer-specific survivals in renal cell carcinoma. World J Urol. 2015;33:1553–60. doi: 10.1007/s00345-014-1462-7. [DOI] [PubMed] [Google Scholar]
- 83.Kumar S, Mehta K. Tissue transglutaminase, inflammation, and cancer: how intimate is the relationship? Amino Acids. 2013;44:81–88. doi: 10.1007/s00726-011-1139-0. [DOI] [PubMed] [Google Scholar]
- 84.Verma A, Guha S, Diagaradjane P, Kunnumakkara AB, Sanguino AM, Lopez-Berestein G, Sood AK, Aggarwal BB, Krishnan S, Gelovani JG, Mehta K. Therapeutic significance of elevated tissue transglutaminase expression in pancreatic cancer. Clin Cancer Res. 2008;14:2476–2483. doi: 10.1158/1078-0432.CCR-07-4529. [DOI] [PubMed] [Google Scholar]
- 85.Cao L, Shao M, Schilder J, Guise T, Mohammad KS, Matei D. Tissue transglutaminase links TGF-beta, epithelial to mesenchymal transition and a stem cell phenotype in ovarian cancer. Oncogene. 2012;31:2521–2534. doi: 10.1038/onc.2011.429. [DOI] [PubMed] [Google Scholar]
- 86.Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796:75–90. doi: 10.1016/j.bbcan.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 87.Yang AD, Camp ER, Fan F, Shen L, Gray MJ, Liu W, Somcio R, Bauer TW, Wu Y, Hicklin DJ, Ellis LM. Vascular endothelial growth factor receptor-1 activation mediates epithelial to mesenchymal transition in human pancreatic carcinoma cells. Cancer Res. 2006;66:46–51. doi: 10.1158/0008-5472.CAN-05-3086. [DOI] [PubMed] [Google Scholar]
- 88.Jia CC, Wang TT, Liu W, Fu BS, Hua X, Wang GY, Li TJ, Li X, Wu XY, Tai Y, Zhou J, Chen GH, Zhang Q. Cancer-associated fibroblasts from hepatocellular carcinoma promote malignant cell proliferation by HGF secretion. PLoS One. 2013;8:e63243. doi: 10.1371/journal.pone.0063243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Berishaj M, Gao SP, Ahmed S, Leslie K, Al-Ahmadie H, Gerald WL, Bornmann W, Bromberg JF. Stat3 is tyrosine-phosphorylated through the interleukin-6/glycoprotein 130/Janus kinase pathway in breast cancer. Breast Cancer Res. 2007;9:R32. doi: 10.1186/bcr1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Verderio EA, Telci D, Okoye A, Melino G, Griffin M. A novel RGD-independent cel adhesion pathway mediated by fibronectin-bound tissue transglutaminase rescues cells from anoikis. J Biol Chem. 2003;278:42604–42614. doi: 10.1074/jbc.M303303200. [DOI] [PubMed] [Google Scholar]
- 91.Mehta K, Kumar A, Kim HI. Transglutaminase 2: a multi-tasking protein in the complex circuitry of inflammation and cancer. Biochem Pharmacol. 2010;80:1921–1929. doi: 10.1016/j.bcp.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 92.Verma A, Mehta K. Tissue transglutaminase-mediated chemoresistance in cancer cells. Drug Resist Updat. 2007;10:144–151. doi: 10.1016/j.drup.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 93.Li Z, Xu X, Bai L, Chen W, Lin Y. Epidermal growth factor receptor-mediated tissue transglutaminase overexpression couples acquired tumor necrosis factor-related apoptosis-inducing ligand resistance and migration through c-FLIP and MMP-9 proteins in lung cancer cells. J Biol Chem. 2011;286:21164–21172. doi: 10.1074/jbc.M110.207571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frese-Schaper M, Schardt JA, Sakai T, Carboni GL, Schmid RA, Frese S. Inhibition of tissue transglutaminase sensitizes TRAIL-resistant lung cancer cells through upregulation of death receptor 5. FEBS Lett. 2010;584:2867–2871. doi: 10.1016/j.febslet.2010.04.072. [DOI] [PubMed] [Google Scholar]
- 95.Park KS, Kim HK, Lee JH, Choi YB, Park SY, Yang SH, Kim SY, Hong KM. Transglutaminase 2 as a cisplatin resistance marker in non-small cell lung cancer. J Cancer Res Clin Oncol. 2010;136:493–502. doi: 10.1007/s00432-009-0681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martins FC, Teixeira F, Reis I, Geraldes N, Cabrita AM, Dias MF. Increased transglutaminase 2 and GLUT-1 expression in breast tumors not susceptible to chemoprevention with antioxidants. Tumori. 2009;95:227–232. doi: 10.1177/030089160909500215. [DOI] [PubMed] [Google Scholar]
- 97.Mehta K, Fok J, Miller FR, Koul D, Sahin AA. Prognostic significance of tissue transglutaminase in drug resistant and metastatic breast cancer. Clin Cancer Res. 2004;10:8068–8076. doi: 10.1158/1078-0432.CCR-04-1107. [DOI] [PubMed] [Google Scholar]
- 98.Kim DS, Park KS, Kim SY. Silencing of TGase 2 sensitizes breast cancer cells to apoptosis by regulation of survival factors. Front Biosci (Landmark Ed) 2009;14:2514–2521. doi: 10.2741/3394. [DOI] [PubMed] [Google Scholar]
- 99.Kim DS, Park SS, Nam BH, Kim IH, Kim SY. Reversal of drug resistance in breast cancer cells by transglutaminase 2 inhibition and nuclear factor-kappaB inactivation. Cancer Res. 2006;66:10936–10943. doi: 10.1158/0008-5472.CAN-06-1521. [DOI] [PubMed] [Google Scholar]
- 100.Herman JF, Mangala LS, Mehta K. Implications of increased tissue transglutaminase (TG2) expression in drug-resistant breast cancer (MCF-7) cells. Oncogene. 2006;25:3049–3058. doi: 10.1038/sj.onc.1209324. [DOI] [PubMed] [Google Scholar]
- 101.Cao L, Petrusca DN, Satpathy M, Nakshatri H, Petrache I, Matei D. Tissue transglutaminase protects epithelial ovarian cancer cells from cisplatin-induced apoptosis by promoting cell survival signaling. Carcinogenesis. 2008;29:1893–1900. doi: 10.1093/carcin/bgn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Verma A, Wang H, Manavathi B, Fok JY, Mann AP, Kumar R, Mehta K. Increased expression of tissue transglutaminase in pancreatic ductal adenocarcinoma and its implications in drug resistance and metastasis. Cancer Res. 2006;66:10525–10533. doi: 10.1158/0008-5472.CAN-06-2387. [DOI] [PubMed] [Google Scholar]
- 103.Yuan L, Siegel M, Choi K, Khosla C, Miller CR, Jackson EN, Piwnica-Worms D, Rich KM. Transglutaminase 2 inhibitor, KCC009, disrupts fibronectin assembly in the extracellular matrix and sensitizes orthotopic glioblastomas to chemotherapy. Oncogene. 2007;26:2563–2573. doi: 10.1038/sj.onc.1210048. [DOI] [PubMed] [Google Scholar]
- 104.Park KS, Han BG, Lee KH, Kim DS, Kim JM, Jeon H, Kim HS, Suh SW, Lee EH, Kim SY, Lee BI. Depletion of nucleophosmin via transglutaminase 2 cross-linking increases drug resistance in cancer cells. Cancer Lett. 2009;274:201–207. doi: 10.1016/j.canlet.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 105.Budillon A, Carbone C, Di Gennaro E. Tissue transglutaminase: a new target to reverse cancer drug resistance. Amino Acids. 2013;44:63–72. doi: 10.1007/s00726-011-1167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jin T, Lin HX, Lin H, Guo LB, Ge N, Cai XY, Sun R, Chen WK, Li QL, Hu WH. Expression TGM2 and BNIP3 have prognostic significance in laryngeal cancer patients receiving surgery and postoperative radiotherapy: a retrospective study. J Transl Med. 2012;10:64. doi: 10.1186/1479-5876-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jeong JH, Cho BC, Shim HS, Kim HR, Lim SM, Kim SK, Chung KY, Ul Islam SM, Song JJ, Kim SY, Kim JH. Transglutaminase 2 expression predicts progression free survival in non-small cell lung cancer patients treated with epidermal growth factor receptor tyrosine kinase inhibitor. J Korean Med Sci. 2013;28:1005–1014. doi: 10.3346/jkms.2013.28.7.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Assi J, Srivastava G, Matta A, Chang MC, Walfish PG, Ralhan R. Transglutaminase 2 overexpression in tumor stroma identifies invasive ductal carcinomas of breast at high risk of recurrence. PLoS One. 2013;8:e74437. doi: 10.1371/journal.pone.0074437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kyparidou E, Athanasiadis A, Xydakis E, Papadakou M, Panagos G. Correlation of tissue transglutaminase expression on breast cancer tissue with time to relapse, overall survival, and clinical and molecular prognostic factors: a preliminary report. J BUON. 2005;10:81–87. [PubMed] [Google Scholar]
- 110.Park MK, Jo SH, Lee HJ, Kang JH, Kim YR, Kim HJ, Lee EJ, Koh JY, Ahn KO, Jung KC, Oh SH, Kim SY, Lee CH. Novel suppressive effects of cardamonin on the activity and expression of transglutaminase-2 lead to blocking the migration and invasion of cancer cells. Life Sci. 2013;92:154–160. doi: 10.1016/j.lfs.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 111.Lee SH, Kim N, Kim SJ, Song J, Gong YD, Kim SY. Anti-cancer effect of a quinoxaline derivative GK13 as a transglutaminase 2 inhibitor. J Cancer Res Clin Oncol. 2013;139:1279–1294. doi: 10.1007/s00432-013-1433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khanna M, Chelladurai B, Gavini A, Li L, Shao M, Courtney D, Turchi JJ, Matei D, Meroueh S. Targeting ovarian tumor cell adhesion mediated by tissue transglutaminase. Mol Cancer Ther. 2011;10:626–636. doi: 10.1158/1535-7163.MCT-10-0912. [DOI] [PubMed] [Google Scholar]
- 113.Bae J, Lee YS, Jeoung D. Down-regulation of transglutaminase II leads to impaired motility of cancer cells by inactivation of the protein kinase, Akt, and decrease of reactive oxygen species. Biotechnol Lett. 2006;28:1151–1158. doi: 10.1007/s10529-006-9079-6. [DOI] [PubMed] [Google Scholar]


