Abstract
CD47 is a membrane receptor that belongs to the immunoglobulin superfamily and plays an important role in the mechanisms of tumor immune escape. CD47 participates in tumor immune escape by combining with SIRPα to reduce the phagocytic activity of macrophages. There are six potential N-glycosylation sites on CD47, and glycosylation is known to be necessary for its membrane localization. However, it is still unknown to what extent glycosylation influences CD47 ligand binding properties and subsequent signaling. By using immunoprecipitation and confocal laser scanning microscopy, we showed that CD47 contains Lewis y antigen. Immunohistochemical analysis demonstrated that both the positive expression and the overexpression of CD47 and Lewis y antigen in cancer tissues and borderline tumors were significantly higher than those in benign ovarian tumors and normal ovarian tissues (P < 0.05). A linear correlation between the expression patterns of CD47 and Lewis y antigen was evident (r = 0.47, P < 0.01). The high expression of CD47 and Lewis y antigen showed significant correlations with the clinical pathological parameters of ovarian cancer [International Federation of Gynecology and Obstetrics (FIGO) standards, lymph node metastasis, and degree of differentiation] (P < 0.05). The Cox model and Kaplan-Meier tests showed that high expression of CD47 was an independent adverse risk factor for the prognosis of ovarian cancer. Cases with both high CD47 and Lewis y antigen expression had poor prognoses. Our study demonstrates that Lewis y antigens of CD47 may play a crucial role in the development of ovarian cancer, and could be new targets for immunotherapy for ovarian cancer.
Keywords: CD47, Lewis y, ovarian cancer, tumor immune escape, prognosis
Introduction
Ovarian carcinoma is the most common gynecological malignant tumor. The mortality rate of epithelial ovarian carcinoma (EOC) ranks first of all gynecological malignant tumors. EOC is a major type of tumor that threatens the health of women [1]. The early symptoms of EOC are not obvious, and approximately 70% of presenting patients are advanced-stage when they are diagnosed. Due to chemoresistance and recurrence, the five-year survival of ovarian cancer patients is less than 30% [2], and this situation has not changed in recent decades. Primary debulking surgery, followed by platinum and taxane-based chemotherapy, is still the main treatments for ovarian cancer, although immunotherapy in the treatment of ovarian cancer has been a recent focus.
Tumor cells are derived from normal cells. Immune function, particularly natural killer (NK) cells and cytotoxic T cells for tumor immune surveillance, can effectively prevent the development of cancer [3,4]. However, tumor-specific transplantation antigens and tumor-associated transplantation antigens expressed by tumor cells may mutate or not be expressed, which will affect the presentation and activation of T-cell antigens by dendritic cells. This would prevent cytotoxic T lymphocytes from effectively recognizing and killing the tumor cells, thus allowing the progeny cells to become tumor cells and escape immune surveillance [5-7]. Cancer stem cells (CSC) can have self-renewal, unlimited proliferation potential, lymphatic and distant metastases, exogenous cytotoxic drug resistance, and other characteristics [8,9], by expressing specific markers. CSCs shed from the primary tumors into the lymphatic vessels and peripheral circulation, and can occur very early during cancer development, providing the “seed” for tumor metastasis and recurrence. The cancer stem cells escape surveillance and clearance by the immune system, which plays an important role in the process of immune escape of tumor cells. Recently, there has been a significant breakthrough in the use of tumor-specific transplantation antigens as immunotherapy targets on the tumor, providing new possibilities for the treatment of this disorder.
Glycoprotein CD47 is also called integrin-associated protein (IAP), a member of the immunoglobulin superfamily. CD47 is involved in cell proliferation, apoptosis, and adhesion [10-12], playing an important role in the activation, proliferation and migration of T-cells [13-15], and inhibiting the phagocytosis of macrophages when combined with SIRP alpha 1 (SIRPα1) [16]. In addition, CD47 is highly expressed in a variety of hematologic malignancies, such as acute myeloid leukemia and non-Hodgkin’s lymphoma, with high expression significantly associated with poor prognosis [17]. CD47 is also abnormally expressed in a variety of solid tumors such as bladder cancer, breast cancer, colon cancer, and prostate cancer [18]. Recent studies suggest that CD47 plays an important role in tumor immune escape. In particular, the co-expression of CD47, CD44, and c-Met on the cell surface of breast cancer stem cells has an important role in breast cancer metastasis [19]. Anti-CD47 antibody could eliminate and suppress the metastasis and recurrence of many kinds of solid tumors. Without affecting normal cells, anti-CD47 antibody-mediated phagocytosis of targeted tumor cells can provide antitumor activity by promoting the phagocytosis of these cells by macrophages [20]. Furthermore, this antibody has anticancer effects on tumor stem cells (CSCs), and their differentiated subtypes can alter the tumorigenic tumor-associated macrophages (TAMs) into anti-neoplastic cells to enhance their phagocytic activity. Inhibition of CD47 expression in mice enhanced the radiosensitivity of tumor cells, and had a protective effect on normal tissues [21].
The Lewis y antigen is a blood type-related antigen that plays an important role in embryogenesis and the immune process of intercellular recognition. By expressing high levels of Lewis y antigen sperm acrosomes inhibit the innate and acquired immune responses of the female reproductive system, to improve the tolerance of the female reproductive system to maximize its own survival [22]. Lewis y antigen is also involved in metastasis, adhesion, resistance, and other malignant characteristics of tumor cells. It was reported that the expression of Lewis y antigen increased during tumorigenesis, such as during ovarian cancer, endometrial cancer, pancreatic cancer, prostate cancer, colon cancer, and non-small cell lung cancer [23-27]. By increasing expression of glycosylation of the surface antigen CA125 [28], tumor cells inhibit the cytotoxicity of NK cells, to induce immune tolerance and increase the chance of immune escape [29]. Our previous study showed that Lewis y antigen was a structural part of CD44, and enhanced the CD44-mediated proliferation, adhesion, and resistance ability of ovarian cancer cells [30,31]. There are six potential N-glycosylation sites for CD47, and it is currently thought that glycosylation influences the biological function and localization of CD47 on the cell membrane [32-34]. However, the specific forms of glycosylated CD47 on the membrane remain unknown. We speculate that CD47 also has Lewis y antigen. Therefore, the present study aimed to explore the correlations of Lewis y antigen and CD47, to analyze the possible correlations between CD47, clinical pathological features, and prognosis of ovarian cancer, and to investigate the effects of glycosylation on the biological functions of CD47. Furthermore, this study provides a theoretical basis for the use of CD47 as a target for immunotherapy of ovarian cancer.
Materials and methods
Tissue samples and follow-up
The study was approved by the Research Ethics Board at Shengjing Hospital affiliated to China Medical University (IACUC permit number 2015PS19K). The 192 chosen paraffin samples were obtained from operations performed from 2007 to 2012 in the Department of Gynecology and Obstetrics of our hospital. All the tissue sections had a final diagnosis provided by clinical specialists. There were 116 cases of primary malignant ovarian tumors (including 75 serous and 16 mucous cystadenocarcinomas, 17 ovarian clear cell carcinomas, OCCC, and 8 endometriosis), 20 borderline ovarian tumors (including 11 mucinous and 9 serous cystadenomas), 26 benign ovarian tumors, and 30 normal ovarian tissue samples (excised during the surgical removal of cervical cancers). According to pathological grading criteria, the ovarian cancer group contained 11 well differentiated, 34 moderately differentiated, and 71 poorly differentiated cases. The group included 50 stage I~II and 66 stage III~IV cases, based on the International Federation of Gynecology and Obstetrics (FIGO) standards. The clinical and pathological patient information was collected from their clinical records, and included their age, surgical stage, lymph node metastasis, pathological tumor grade and subtype, and residual tumor size. The age range was 16-77 years (52.7 years, median) in the malignant ovarian tumor group, 21-78 years (37 years, median) in the borderline ovarian tumor group, 25-61 years (44 years, median) in the benign ovarian tumor group, and 37-65 years (45.5 years, median) in the normal ovarian tissue group. There were no statistically significant differences in the ages of these groups (all P > 0.05). All cases were primary, and the information was complete. All patients received a gastroscopy or colposcopy to exclude other primary diagnoses. Patients were not subjected to chemotherapy prior to the operation. All the patients underwent lymphadenectomy during the operation. We collected information on the clinical chemotherapeutic treatments received and the follow-ups of 93 patients from a total 116 patients with malignant ovarian cancer. These 93 patients underwent treatment for ovarian cancer that included surgical debulking, followed by 6-8 postoperative cycles of conventional chemotherapy consisting of paclitaxel plus carboplatin, and were followed-up for a minimum of 3 years after the completion of chemotherapy. We defined the overall survival of the patients as extending from the date of surgery to the date of death or the last follow-up. After the operation, patients were observed at 6-month intervals. To determine the factors influencing survival after surgery and standard chemotherapy, conventional variables, together with CD47 and Lewis y antigen expression, were tested in the 93 ovarian carcinoma patients.
Coimmunoprecipitation and western blot
The CAOV3 and SKOV3 ovarian cancer cell lines were purchased from Cell Culture Collection of Shanghai (Shanghai, China) and coimmunoprecipitation and western blotting were performed as previously described [35]. Ten microliters of CD47 antibody (goat; Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used for coimmunoprecipitation studies. CD47 antibody (1:1000, rabbit; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and Lewis y antibody (1:1000, mouse; Abcam, Cambridge, MA, USA) were used for western blots.
Double-labeling immunofluorescence and immunohistochemistry
The methods of double-labeling immunofluorescence and immunohistochemistry were as previously described [36]. The working concentrations of primary antibodies against CD47 (Santa Cruz, rabbit) and Lewis y (Abcam, mouse) were 1:100 and 1:100 for double-labeling immunofluorescence, respectively, and 1:700 and 1:400 for immunohistochemistry, respectively. The procedures were performed according to the manufacturer’s instructions.
Assessment standards
Immunohistochemistry
A positive result was defined as buffy granules in the cell membrane and cytoplasm. According to the color intensity, no pigmentation, light yellow, buffy, and brown were scored as 0, 1, 2, and 3, respectively. We choose five high-power fields in a series from each slice, then scored them and calculated the mean percentage of the chromatic cells as follows: chromatic cells that accounted for less than 5% were scored 0; 5% to 25%, 1; 26% to 50%, 2; 51% to 75%, 3; and, greater than 75%, 4. After multiplying the two numbers, 0 to 2 was considered (2); 3 to 4, (+); 5 to 8, (++); and, 9 to 12, (+++). The completely negative (0) and weak (+) cases were considered as low expression; the moderate (++) and strong (+++) cases were considered as high expressers. Two observers read the sections to control for error.
Double-labeling immunofluorescence
The red fluorescent was CD47 antigen (tetramethylrhodamine, TRITC), the green fluorescent (fluorescein isothiocyanate, FITC) was Lewis y antigen, and the blue fluorescence was from nuclear staining with 4’,6-diamidino-2-phenylindole (DAPI). After images were obtained, picture analysis software was used for three images at different fluorescent wavelengths. The yellow fluorescence shows that the CD47 and Lewis y antigens were located in the same position.
Statistical analysis
SPSS version 19.0 software (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. The χ2 analysis, variance analysis, and t-test were employed. The Cox regression model was used for analysis of risk factors. The Kaplan-Meier and log-rank methods were used to analyze and compare survival curves. In addition, Spearman’s correlation analysis was used to analyze the correlation between the two proteins. A bilateral P < 0.05 was considered statistically significant.
Results
Coexpression of CD47 and Lewis y antigen in ovarian cell lines
Expression patterns of CD47 and Lewis y antigen in the ovarian cancer cell lines, CAOV3 and SKOV3, were examined using the coimmunoprecipitation methods. The molecular weight of CD47 is approximately 50 kDa, which includes Lewis y antigen (Figure 1).
Figure 1.
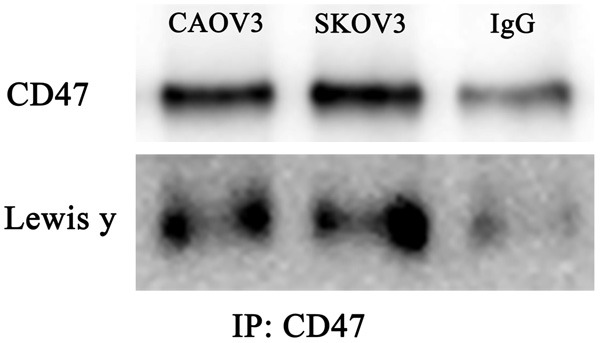
Coexpression of CD47 and Lewis y antigens in Ovarian cell lines (CAOV3, SKOV3), IgG is negative control (contained only 10 μl CD47 antibody).
Double-labeling immunofluorescence
In double-labeling immunofluorescence experiments, the red fluorescence-labeled CD47 was localized in the cell membrane and cytoplasm, while the green fluorescence-labeled Lewis y antigen also appeared at the cell membrane, but was observed only to a limited extent in the cytoplasm. The blue fluorescence represented nuclei after staining with DAPI. Images were obtained, and picture analysis software was used to accumulate three images at different fluorescent wavelengths. The yellow fluorescence appeared in the positions where red and green fluorescence overlapped simultaneously. Our findings clearly illustrate that CD47 and Lewis y colocalized at the same positions (Figure 2).
Figure 2.
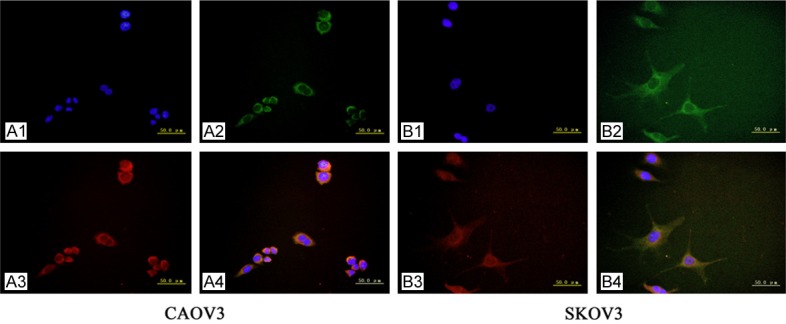
The double-labeling immunofluorescence shows the colocalization of CD47 and Lewis y antigen in ovarian cancer cell line CAOV3 and SKOV3 (original magnification ×400). Nucleus (A1, B1), Lewis y antigen (A2, B2), CD47 (A3, B3) and merged image (A4, B4).
Expression patterns of CD47 and Lewis y in ovarian tissues
CD47 is mainly expressed on the cell membrane, but is also expressed in the cytoplasm. The positive expression rates of CD47 in malignant ovarian tissues, borderline tumors, benign tumors, and normal ovarian tissues were 90.51%, 90%, 50%, and 0%, respectively. Further analysis of high expression found that the high expression of CD47 in cancer tissues and borderline tumors (60.34% and 60%, respectively) were significantly higher than in benign ovarian tumors and normal ovarian tissues (15.38% and 0%, respectively) (both P < 0.05). No significant difference was found between the malignant group and borderline tumor group (P > 0.05).
Similar with CD47, Lewis y antigen was mainly expressed in the cell membrane and cytoplasm, with positive expression percents in malignant, borderline, benign tumor, and normal ovarian tissues of 86.20%, 75%, 26.92%, and 0%, respectively. Percents of high expression in the ovarian cancer group and borderline tumor group (62.08% and 46%, respectively) were obviously higher than in the benign tissue group (15.38%) and normal group (0%) (P < 0.05). The expression of CD47 and Lewis y antigen was negative in normal ovarian tissues (Table 1; Figure 3).
Table 1.
The expression of CD47 and Lewis y in different Ovarian Tissue
| Groups | Cases | CD47 | Lewis y antigen | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Negative cases | Positive cases (%) | High cases (%) | Negative cases | Positive cases (%) | High cases (%) | ||
| Malignant | 116 | 11 | 105 (90.51) | 70 (60.34) | 16 | 100 (86.20) | 72 (62.08) |
| Borderline | 20 | 2 | 18 (90.00)* | 12 (60.00)* | 5 | 15 (75.00)* | 9 (45.00)* |
| Benign | 26 | 13 | 13 (50.00)Φ,Ψ | 4 (15.38)Φ,Ψ | 19 | 7 (26.92)Φ,Ψ | 4 (15.38)Φ,Ψ |
| Normal | 30 | 30 | 0 (0)Φ,Ψ | 0 (0)Φ,Ψ | 30 | 0 (0)Φ,Ψ | 0 (0)Φ,Ψ |
Compared with malignant group, P > 0.05;
Compared with malignant group, P < 0.01;
Compared with borderline group, P < 0.01.
Figure 3.
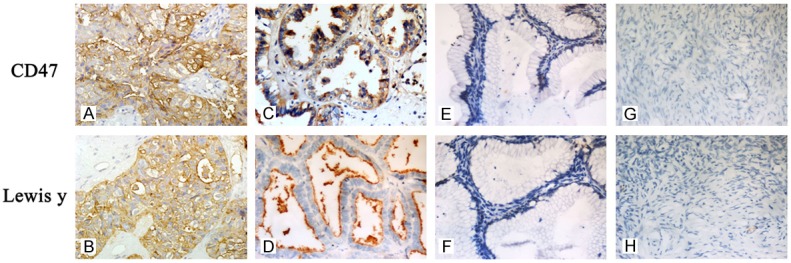
Expression of CD47 and Lewis y antigens in ovarian tissue groups. Immunohistochemical staining in ovarian malignant tumor (A, B), borderline tumor (C, D), benign tumor (E, F), and normal ovarian tissue (G, H). CD47 (A, C, E, G) and Lewis y (B, D, F, H; original magnification ×400).
Association between CD47 and Lewis y antigen expression and pathological features
No significant correlation was observed between the positive expression percents of CD47 and Lewis y antigen and the clinical pathological parameters of ovarian cancer (P > 0.05). We further analyzed the high expression of these two proteins in ovarian cancer tissue and found that the high expression of CD47 was 69.70% in Stage III-IV ovarian cancer, which was significantly higher than that of Stage I-II cancer (46%, P = 0.01).
With a decreasing degree of differentiation, the high expression of CD47 gradually increased, with the percent of high expression in the low differentiation group (67.60%) higher than that in the middle differentiation group (52.94%, P = 0.14) and the high differentiation group (27.27%, P = 0.01). However, a statistically significant difference was only observed between the low differentiation group and the high differentiation group. The percent high expression of CD47 in the lymph node metastasis group was 80%, significantly higher than that of the group without metastasis (55.2%, P = 0.04). The high expression of CD47 in different pathological types of ovarian cancer tissues were 65.33% (serous), 43.75% (mucinous), 75% (endometrial-like), and 58.82% (clear-cell carcinoma), with no statistically significant differences detected among the different cancer types.
The high expression of Lewis y antigen in Stage III-IV patients (69.70%) was significantly higher than that of Stage I-II patients (50%, P = 0.031). Similar to CD47, significant differences in high expression of Lewis y antigen was only detected between the low differentiation group (66.20%) and the high differentiation group (27.27%, P = 0.013). The high expression of Lewis y antigen in lymph node metastasis tissues was as high as 80%, significantly higher than those without lymph node metastasis, P = 0.048. The percent high expression of Lewis y antigen in different pathological types of ovarian cancer were consistent with that of CD47, with no significant differences observed (Table 2).
Table 2.
Correlation of CD47 and Lewis y antigen expression with clinical features of ovarian cancer
| Features | Cases | CD47 | Lewis y | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Positive cases (%) | P | High (%) | P | Positive cases (%) | P | High (%) | P | |||
| Pathological Type | ||||||||||
| Serous | 75 | 62 (82.67) | > 0.05 | 49 (65.33) | > 0.05 | 62 (82.67) | > 0.05 | 49 (65.33) | > 0.05 | |
| Mucinous | 16 | 14 (87.50) | 7 (43.75) | 13 (81.25) | 7 (43.75) | |||||
| Endometrioid | 8 | 7 (87.5) | 6 (75.00) | 8 (100) | 6 (75.00) | |||||
| OCCC | 17 | 17 (100) | 10 (58.82) | 17 (100) | 10 (58.82) | |||||
| Surgical Stage | ||||||||||
| I-II | 50 | 44 (88.00) | > 0.05 | 23 (46.00) | 0.01 | 40 (80.00) | > 0.05 | 25 (50.00) | 0.031 | |
| III-IV | 66 | 61 (92.42) | 46 (69.70) | 60 (90.00) | 46 (69.70) | |||||
| Differentiation | ||||||||||
| High | 11 | 8 (72.72) | > 0.05 | 3 (27.27) | 0.01* | 7 (63.63) | > 0.05 | 3 (27.27) | 0.013* | |
| Middle | 34 | 31 (90.32) | 18 (52.94) | 0.14* | 30 (88.24) | 21 (61.76) | 0.65* | |||
| Low | 71 | 63 (88.73) | 48 (67.61) | 63 (88.73) | 47 (66.20) | |||||
| Lymphatic Metastasis | ||||||||||
| No | 96 | 86 (89.58) | > 0.05 | 53 (55.21) | 0.04 | 81 (84.38) | > 0.05 | 52 (54.16) | 0.048 | |
| Yes | 20 | 19 (95.00) | 16 (80.00) | 19 (95.00) | 16 (80.00) | |||||
Compared with low differentiation group.
Relevance of CD47 and Lewis y expression in ovarian cancer
In total, 56 cases simultaneously had high expression of CD47 and Lewis y antigen, and 31 cases displayed simultaneously low expressions among all 116 cases of ovarian cancer. Linear regression and correlation analyses revealed that the expression of Lewis y antigen and CD47 showed a linear correlation (r = 0.477, P = 0.01) (Table 3).
Table 3.
Relevance of CD47 and Lewis y expression in ovarian cancer
| Lewis y expression | CD47 expression | Total | |
|---|---|---|---|
|
| |||
| +++, ++ | +, - | ||
| +++, ++ | 56 | 16 | 72 |
| +, - | 13 | 31 | 44 |
| Total | 69 | 47 | 116 |
Survival analysis
Follow-up was conducted for the 116 patients with ovarian cancer. Up to December of 2014, a total of 93 patients were contacted, with 23 cases lost, with follow-up times between 7~66 months. Among the 59 cases with high expression of CD47, 24 patients died, with an average survival time of 44.7 months. Among the 34 cases in the low expression group, 3 patients died, with an average survival time of 56.2 months. Kaplan-Meier tests were used to analyze the correlations between CD47 and prognosis of ovarian cancer patients. The results showed that the prognosis of the CD47 overexpression group was significantly poorer than the low expression group (P = 0.012). Multi-factor analyses of the prognosis of ovarian cancer were performed using Cox models that included as variables, the FIGO stage, lymph node metastasis level, and the expression levels of CD47 and Lewis y antigen for the 116 ovarian cancer patients. The stage and high expression of CD47 were found to be independent prognostic risk factors for ovarian cancer (Table 4; Figure 4).
Table 4.
Multivariate analysis of the prognosis of patients with ovarian carcinoma
| Variables | P-value | Exp. (B) | Hazard radio (95% CI) |
|---|---|---|---|
| FIGO (I+II vs. III+IV) | 0.028 | 3.165 | 1.133-8.844 |
| CD47 (low vs. high) | 0.049 | 3.577 | 1.008-12.55 |
Figure 4.
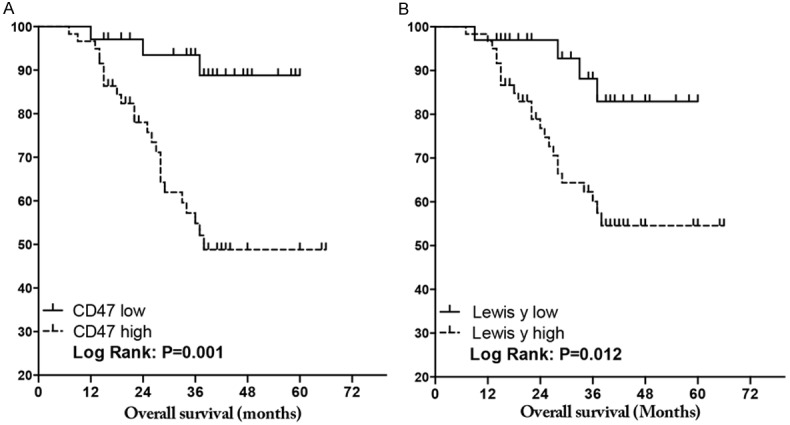
Kaplan-Meier survival analysis: (A) CD47 and (B) Lewis y antigen expression in ovarian cancer. P value, Log rank test.
Discussion
CD47 is a five transmembrane glycoprotein with molecular weight of 50 kD and an N-terminus at the extracellular domain, which could be glycosylated [37]. CD47 plays an important role in the physiological and pathological processes of the body, and is involved in apoptosis, proliferation, activation, and migration of many immune cells [10-12]. It is widely expressed in malignant tumor cells and is closely correlated with immune escape, apoptosis, proliferation, and metastasis of tumor cells [17,18,38]. In 2013, a study showed that the metastasis-initiating cells (MICs) had CD44, CD47, and receptor tyrosine kinase c-MET as biomarkers among circulating tumor cells (CTCs) of breast cancer [19], therefore CD47 became the subject of intense research efforts.
Carcinogenesis is a multi-gene process, during which surface antigens on the cells change in quantity and quality after a mutation. A few mutant cells may express some new antigen on their surface, which will mark the cells as “non-self.” Under normal circumstances, these cells are recognized, destroyed, and removed by the natural and acquired immunity, before tumor formation, via a process known as “immune surveillance”. However, tumor-specific transplantation antigens and tumor-associated transplantation antigens expressed by tumor genes may mutate or not be expressed. This process alters the presentation and activation of T-cell antigens by dendritic cells, preventing immune cells from effectively recognizing and killing the tumor cells, thus allowing the progeny cells to become tumor cells and escape from immune surveillance. By combining with SIRPα, CD47 can produce inhibitory signals to reduce the phagocytic activity of macrophages [39], which then allows hematopoietic stem cells to avoid phagocytosis. As a signal to suppress phagocytosis, CD47 could also alter the signal, which could then be used by tumor cells to escape immune surveillance. Therefore, during the early stages of tumorigenesis, cancer cells can survive by expressing CD47 to evade monitoring and removal by the immune system. Our results show that both the expression percentage and high expression of CD47 in ovarian cancer and borderline ovarian tumor tissues were significantly higher than those in benign ovarian tumors and normal ovarian tissues. In addition, a higher percentage of CD47 in the early stages of ovarian cancer (88%) correlates with a higher percentage positive in advanced stages (92.42%). High expression of CD47 significantly correlated with lymph node metastasis and FIGO stage (P < 0.05), indicating a close correlation between CD47 and the occurrence and development of ovarian cancer through cancer cell escape from immune surveillance.
It is currently suggested that glycosylation influences the biological function and localization of CD47 on the cell membrane. There are six potential N-glycosylation sites on CD47, all exposed on the surface of the cell membrane. However, the specific forms of glycosylation remain unknown. Lewis y antigen is one of the blood type antigens, playing an important role in embryo planting [40]. It is also a tumor-related antigen [27]. Our previous studies showed that Lewis y antigens expressed by cancer cells are involved in many cellular functions that influence the malignant behaviors of the cells, including adhesion, recognition, and signal transduction, by changing the phosphorylation levels of receptor signaling pathway proteins [41]. An increase of Lewis y antigens has been associated with cancer cell invasion and spread [42]. In this study, we confirmed that the expression of Lewis y antigen was similar to CD47. Both the expression percentage and overexpression ratio of CD47 in ovarian cancer and borderline ovarian tumor tissues were significantly higher than in benign ovarian tumors and normal ovarian tissues. Correlation analyses demonstrated a linear correlation between CD47 expression and Lewis y antigen in ovarian cancers. By using immunoprecipitation and confocal laser scanning microscopy, we showed colocalization of CD47 and Lewis y antigen, also suggesting that CD47 contains Lewis y antigen. Further analysis of possible correlations between CD47 and prognosis of ovarian cancer patients showed that prognosis of the CD47 overexpression group was significantly poorer than the low expression group. Cox model analyses also showed that overexpression of CD47 was an independent adverse risk factor for the prognosis of ovarian cancer. Studies have shown that overexpression of CD47 was an independent risk factor in patients with acute myeloid leukemia [38], with its expression level negatively correlated with prognosis of the disease, further supporting the results of our study. It is worth noting that the prognosis of the patients with both high expression of CD47 and Lewis y antigen was significantly poorer than the patients with low expression of both antigens. These data suggest that the Lewis y antigen plays an important role in the immune escape of CD47-mediated tumor cells, promoting the occurrence and development of ovarian cancer.
One study found that in melanoma, bladder cancer, and ovarian carcinoma, using specific antigens to inhibit CD47 could decrease its binding with type 4 collagen, to inhibit these cancer cells from metastasis in a process that depended on integrin αvβ3 [43]. It was reported that CD47 could be coexpressed with E-cadherin to mediate cell adhesion. The high expression of CD47 decreased intercellular adhesion, and promoted the metastasis of epithelial cells [44]. Therefore, CD47 combines with integrin αvβ3 and other receptors to promote the metastasis of ovarian cancer. Our previous results showed that the Lewis y antigen was an important component of integrin αvβ3 and CD44, influencing integrin αvβ3 or CD44-mediated malignant characteristics of ovarian cancer [30,31,45]. It was therefore hypothesized that the Lewis y antigen, as an important part of CD47, affected the interaction between CD47 and its ligands involved in mediation of the malignant characteristics of ovarian carcinoma.
It was formerly thought that CD47 was a specific tumor marker of ovarian cancer [37]. However, CD47 is also expressed in many tissues and organs. Although, CD47 is not a specific marker of ovarian cancer, its close relationship between high expression status and the development of ovarian cancer has been verified in recent years. Recent studies have also confirmed that CD47 plays an important role in immune escape of tumors. The development of anti-CD47 monoclonal antibodies for cancer immunotherapy has also made significant progress. However, characterization of the glycosylated forms of CD47 is not complete, and the role of glycosylation of CD47 in CD47-mediated physiological and pathological processes remains unknown. Future studies to relate glycosylation with biological functions of CD47 may help to provide a new target for anti-CD47 cancer immunotherapy.
Acknowledgements
Project supported by the National Natural Science Fund (project numbers 81172491, 81101527, 81472437, and 81402129), PhD Programs Foundation of Ministry of Education of China (Numbers 20112104110016 and 20112104120019), and the Shengjing Free Researchers Scheme (project number 201303).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Kipps E, Tan DS, Kaye SB. Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nat Rev Cancer. 2013;13:273–282. doi: 10.1038/nrc3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 4.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 5.Ugolini S, Vivier E. Immunology: Natural killer cells remember. Nature. 2009;457:544–545. doi: 10.1038/457544a. [DOI] [PubMed] [Google Scholar]
- 6.Liao KL, Bai XF, Friedman A. The role of CD200-CD200R in tumor immune evasion. J Theor Biol. 2013;328:65–76. doi: 10.1016/j.jtbi.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Bai XF, Liu J, Li O, Zheng P, Liu Y. Antigenic drift as a mechanism for tumor evasion of destruction by cytolytic T lymphocytes. J Clin Invest. 2003;111:1487–1496. doi: 10.1172/JCI17656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vermeulen L, de Sousa e Melo F, Richel DJ, Medema JP. The developing cancer stem-cell model: clinical challenges and opportunities. Lancet Oncol. 2012;13:e83–89. doi: 10.1016/S1470-2045(11)70257-1. [DOI] [PubMed] [Google Scholar]
- 9.Vermeulen L, Sprick MR, Kemper K, Stassi G, Medema JP. Cancer stem cells--old concepts, new insights. Cell Death Differ. 2008;15:947–958. doi: 10.1038/cdd.2008.20. [DOI] [PubMed] [Google Scholar]
- 10.Dorahy DJ, Thorne RF, Fecondo JV, Burns GF. Stimulation of platelet activation and aggregation by a carboxyl-terminal peptide from thrombospondin binding to the integrin-associated protein receptor. J Biol Chem. 1997;272:1323–1330. doi: 10.1074/jbc.272.2.1323. [DOI] [PubMed] [Google Scholar]
- 11.Manna PP, Frazier WA. CD47 mediates killing of breast tumor cells via Gi-dependent inhibition of protein kinase A. Cancer Res. 2004;64:1026–1036. doi: 10.1158/0008-5472.can-03-1708. [DOI] [PubMed] [Google Scholar]
- 12.Xing C, Lee S, Kim WJ, Wang H, Yang YG, Ning M, Wang X, Lo EH. Neurovascular effects of CD47 signaling: promotion of cell death, inflammation, and suppression of angiogenesis in brain endothelial cells in vitro. J Neurosci Res. 2009;87:2571–2577. doi: 10.1002/jnr.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stefanidakis M, Newton G, Lee WY, Parkos CA, Luscinskas FW. Endothelial CD47 interaction with SIRPgamma is required for human T-cell transendothelial migration under shear flow conditions in vitro. Blood. 2008;112:1280–1289. doi: 10.1182/blood-2008-01-134429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seiffert M, Brossart P, Cant C, Cella M, Colonna M, Brugger W, Kanz L, Ullrich A, Buhring HJ. Signal-regulatory protein alpha (SIRPalpha) but not SIRPbeta is involved in T-cell activation, binds to CD47 with high affinity, and is expressed on immature CD34(+)CD38(-) hematopoietic cells. Blood. 2001;97:2741–2749. doi: 10.1182/blood.v97.9.2741. [DOI] [PubMed] [Google Scholar]
- 15.Piccio L, Vermi W, Boles KS, Fuchs A, Strader CA, Facchetti F, Cella M, Colonna M. Adhesion of human T cells to antigen-presenting cells through SIRPbeta2-CD47 interaction costimulates T-cell proliferation. Blood. 2005;105:2421–2427. doi: 10.1182/blood-2004-07-2823. [DOI] [PubMed] [Google Scholar]
- 16.Han X, Sterling H, Chen Y, Saginario C, Brown EJ, Frazier WA, Lindberg FP, Vignery A. CD47, a ligand for the macrophage fusion receptor, participates in macrophage multinucleation. J Biol Chem. 2000;275:37984–37992. doi: 10.1074/jbc.M002334200. [DOI] [PubMed] [Google Scholar]
- 17.Jaiswal S, Chao MP, Majeti R, Weissman IL. Macrophages as mediators of tumor immunosurveillance. Trends Immunol. 2010;31:212–219. doi: 10.1016/j.it.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, Lovelace P, Scheeren FA, Chao MP, Weiskopf K, Tang C, Volkmer AK, Naik TJ, Storm TA, Mosley AR, Edris B, Schmid SM, Sun CK, Chua MS, Murillo O, Rajendran P, Cha AC, Chin RK, Kim D, Adorno M, Raveh T, Tseng D, Jaiswal S, Enger PO, Steinberg GK, Li G, So SK, Majeti R, Harsh GR, van de Rijn M, Teng NN, Sunwoo JB, Alizadeh AA, Clarke MF, Weissman IL. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, Klein C, Saini M, Bauerle T, Wallwiener M, Holland-Letz T, Hofner T, Sprick M, Scharpff M, Marme F, Sinn HP, Pantel K, Weichert W, Trumpp A. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31:539–544. doi: 10.1038/nbt.2576. [DOI] [PubMed] [Google Scholar]
- 20.Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, Weiskopf K, Willingham SB, Raveh T, Park CY, Majeti R, Weissman IL. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2:63ra94. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soto-Pantoja DR, Ridnour LA, Wink DA, Roberts DD. Blockade of CD47 increases survival of mice exposed to lethal total body irradiation. Sci Rep. 2013;3:1038. doi: 10.1038/srep01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pang PC, Tissot B, Drobnis EZ, Sutovsky P, Morris HR, Clark GF, Dell A. Expression of bisecting type and Lewisx/Lewisy terminated N-glycans on human sperm. J Biol Chem. 2007;282:36593–36602. doi: 10.1074/jbc.M705134200. [DOI] [PubMed] [Google Scholar]
- 23.Madjd Z, Parsons T, Watson NF, Spendlove I, Ellis I, Durrant LG. High expression of Lewis y/b antigens is associated with decreased survival in lymph node negative breast carcinomas. Breast Cancer Res. 2005;7:R780–787. doi: 10.1186/bcr1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arai Y, Nishida M. Differential diagnosis between normal endometrium and endometrial hyperplasia with immunostaining cytology using anti-LeY monoclonal antibody. Int J Gynecol Cancer. 2003;13:42–46. doi: 10.1046/j.1525-1438.2003.13009.x. [DOI] [PubMed] [Google Scholar]
- 25.Liu F, Qi HL, Chen HL. Regulation of differentiation- and proliferation-inducers on Lewis antigens, alpha-fucosyltransferase and metastatic potential in hepatocarcinoma cells. Br J Cancer. 2001;84:1556–1563. doi: 10.1054/bjoc.2001.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao K, Tan J, Ye P, Wang K, Xu W, ShenTu X, Tang X. Integrin beta1-mediated signaling is involved in transforming growth factor-beta2-promoted migration in human lens epithelial cells. Mol Vis. 2007;13:1769–1776. [PubMed] [Google Scholar]
- 27.Westwood JA, Murray WK, Trivett M, Haynes NM, Solomon B, Mileshkin L, Ball D, Michael M, Burman A, Mayura-Guru P, Trapani JA, Peinert S, Honemann D, Miles Prince H, Scott AM, Smyth MJ, Darcy PK, Kershaw MH. The Lewis-Y carbohydrate antigen is expressed by many human tumors and can serve as a target for genetically redirected T cells despite the presence of soluble antigen in serum. J Immunother. 2009;32:292–301. doi: 10.1097/CJI.0b013e31819b7c8e. [DOI] [PubMed] [Google Scholar]
- 28.Kui Wong N, Easton RL, Panico M, Sutton-Smith M, Morrison JC, Lattanzio FA, Morris HR, Clark GF, Dell A, Patankar MS. Characterization of the oligosaccharides associated with the human ovarian tumor marker CA125. J Biol Chem. 2003;278:28619–28634. doi: 10.1074/jbc.M302741200. [DOI] [PubMed] [Google Scholar]
- 29.Patankar MS, Jing Y, Morrison JC, Belisle JA, Lattanzio FA, Deng Y, Wong NK, Morris HR, Dell A, Clark GF. Potent suppression of natural killer cell response mediated by the ovarian tumor marker CA125. Gynecol Oncol. 2005;99:704–713. doi: 10.1016/j.ygyno.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 30.Gao L, Yan L, Lin B, Gao J, Liang X, Wang Y, Liu J, Zhang S, Iwamori M. Enhancive effects of Lewis y antigen on CD44-mediated adhesion and spreading of human ovarian cancer cell line RMG-I. J Exp Clin Cancer Res. 2011;30:15. doi: 10.1186/1756-9966-30-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Z, Gao J, Zhang D, Liu Q, Yan L, Gao L, Liu J, Liu D, Zhang S, Lin B. High expression of Lewis y antigen and CD44 is correlated with resistance to chemotherapy in epithelial ovarian cancers. PLoS One. 2013;8:e57250. doi: 10.1371/journal.pone.0057250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mawby WJ, Holmes CH, Anstee DJ, Spring FA, Tanner MJ. Isolation and characterization of CD47 glycoprotein: a multispanning membrane protein which is the same as integrin-associated protein (IAP) and the ovarian tumour marker OA3. Biochem J. 1994;304:525–530. doi: 10.1042/bj3040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parthasarathy R, Subramanian S, Boder ET, Discher DE. Post-translational regulation of expression and conformation of an immunoglobulin domain in yeast surface display. Biotechnol Bioeng. 2006;93:159–168. doi: 10.1002/bit.20684. [DOI] [PubMed] [Google Scholar]
- 34.Kaur S, Kuznetsova SA, Pendrak ML, Sipes JM, Romeo MJ, Li Z, Zhang L, Roberts DD. Heparan sulfate modification of the transmembrane receptor CD47 is necessary for inhibition of T cell receptor signaling by thrombospondin-1. J Biol Chem. 2011;286:14991–15002. doi: 10.1074/jbc.M110.179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhuang H, Tan M, Liu J, Hu Z, Liu D, Gao J, Zhu L, Lin B. Human epididymis protein 4 in association with Annexin II promotes invasion and metastasis of ovarian cancer cells. Mol Cancer. 2014;13:243. doi: 10.1186/1476-4598-13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhuang H, Gao J, Hu Z, Liu J, Liu D, Lin B. Co-expression of Lewis y antigen with human epididymis protein 4 in ovarian epithelial carcinoma. PLoS One. 2013;8:e68994. doi: 10.1371/journal.pone.0068994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell IG, Freemont PS, Foulkes W, Trowsdale J. An ovarian tumor marker with homology to vaccinia virus contains an IgV-like region and multiple transmembrane domains. Cancer Res. 1992;52:5416–5420. [PubMed] [Google Scholar]
- 38.Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barclay AN, Brown MH. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6:457–464. doi: 10.1038/nri1859. [DOI] [PubMed] [Google Scholar]
- 40.Dettke M, Palfi G, Loibner H. Activation-dependent expression of the blood group-related lewis Y antigen on peripheral blood granulocytes. J Leukoc Biol. 2000;68:511–514. [PubMed] [Google Scholar]
- 41.Liu JJ, Lin B, Hao YY, Li FF, Liu DW, Qi Y, Zhu LC, Zhang SL, Iwamori M. Lewis(y) antigen stimulates the growth of ovarian cancer cells via regulation of the epidermal growth factor receptor pathway. Oncol Rep. 2010;23:833–841. [PubMed] [Google Scholar]
- 42.Liu Q, Lin B, Wang PL, Yan LM, Hao YY, Li FF, Zhu LC, Zhang SL. [Effect of Lewis y antigen on regulating gene expression of partial drug resistance associated proteins in human ovarian cancer cell line RMG-I-H] . Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2009;31:481–487. [PubMed] [Google Scholar]
- 43.Shahan TA, Fawzi A, Bellon G, Monboisse JC, Kefalides NA. Regulation of tumor cell chemotaxis by type IV collagen is mediated by a Ca(2+)-dependent mechanism requiring CD47 and the integrin alpha(V)beta(3) J Biol Chem. 2000;275:4796–4802. doi: 10.1074/jbc.275.7.4796. [DOI] [PubMed] [Google Scholar]
- 44.Shinohara M, Ohyama N, Murata Y, Okazawa H, Ohnishi H, Ishikawa O, Matozaki T. CD47 regulation of epithelial cell spreading and migration, and its signal transduction. Cancer Sci. 2006;97:889–895. doi: 10.1111/j.1349-7006.2006.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao J, Hu Z, Liu D, Liu J, Liu C, Hou R, Gao S, Zhang D, Zhang S, Lin B. Expression of Lewis y antigen and integrin alphav, beta3 in ovarian cancer and their relationship with chemotherapeutic drug resistance. J Exp Clin Cancer Res. 2013;32:36. doi: 10.1186/1756-9966-32-36. [DOI] [PMC free article] [PubMed] [Google Scholar]


