Abstract
Pancreatic cancer is a deadly malignancy associated with rapid progression and poor prognosis. Perineural invasion (PNI) in pancreatic cancer is one of the most common characteristics of this disease, with incidence of nerve invasion between 53.3% and 90%. PNI is also associated with disease recurrence and pain. Despite research efforts, the detailed mechanisms underlying PNI in pancreatic cancer remains unknown. The main factors of PNI in pancreatic cancer will be introduced in the following: 1. The anatomy of the pancreas nerve: The cancer cells along the blood vessels, lymphatic vessels, peripheral nerve gap and perineurium to Invasion. 2. Adhesion molecules in PNI: Neural cell adhesion molecules. 3. Growth factor: For example, nerve growth factor and tyrosine kinase receptor A; Neurotrophic factor and its receptor, etc. 4. The others: Such as matrix metalloproteinases, integrin. A neurite growth promoting factor and neuritrophic factor known to have a role in the pathophysiology of pancreatic cancer by inducing neuritis growth is midkine. In this review, we discuss the role of midkine and other growth and neurotrophic factors on the pathophysiology of PNI in pancreatic cancer.
Keywords: Midkine (MK), MtK, peripheral invasion (PNI) in pancreatic cancer
The discovery of midkine
Pancreatic cancer is one of the most aggressive and intractable human malignant cancers. PNI extending into the pancreatic nerve plexus is a histopathologic characteristic of pancreatic cancer. Multiple factors involved in PNI of pancreatic cancer (Figure 1). Midkine (MK) is a neurite growth promoting factor and heparin-binding growth factor discovered in 1988. Midkine is highly expressed in mice during the mid-gestational period [1]. In humans, it was found that pleiotrophin (PTN, a heparin-binding growth factor also known as neurite growth-promoting factor) is another growth factor that is structurally similar to midkine. Midkine is a secretory alkaline heparin binding protein consisting of 10 cysteine residues that are conserved in vertebrates. It contains 2 domains, linked by two or three disulfide bonds. The N-terminal is essential for cell exocrine signal peptide, while protects the C-terminus from proteolytic enzyme degradation. The C-terminus can interact with retinoic acid (RA), promoting neurite outgrowth and strengthening artery endothelial fibrinolysis [2]. Midkine is found primarily expressed on the surface of cells and extracellular matrix, and has high affinity for glucose glycan heparin and heparin sulfate.
Figure 1.
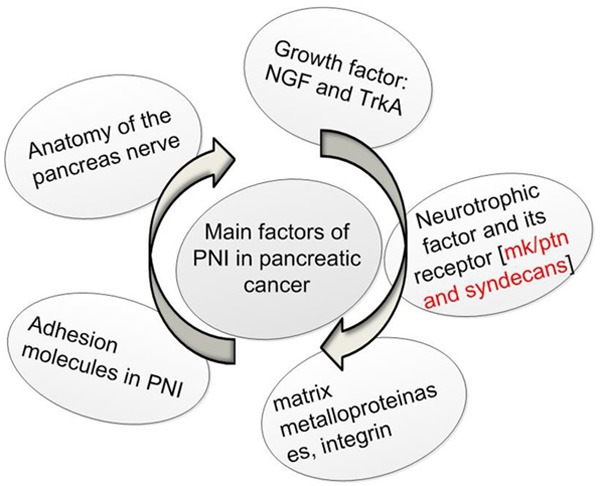
The main factors of PNI in pancreatic cancer.
The human gene encoding for MK is located at 11p11.2, and is flanked by genes encoding diacyglycerokinase Z and muscarnic choline receptor 4. MK genes are highly conservative in different species [3]. Known as MdK in rats, this gene consists of 7 exons and 4 introns. MDK is used to represent the genes for human and consists of 5 exons and 4 introns. Exon I of MDK in humans contain non-coding regions, while exons II encode for a signaling peptide and several amino acid residues in the N-terminus. Exons III and IV consists of amino acid residues in the core domain, while exon 5 contain amino acid residues in the C-terminus. The promoter of the MK gene also contains retinoic acid-responsive element sites, IgG enhancer elements, AP-1 and NF-KB binding sites, WTl (a suppressor gene product)-responsive elements, and sterols/thyroid hormone receptor binding sites.
Studies have shown that the following are structurally related to MK (Figure 2): protein tyrosine phosphataseζ (PTPζ), LDL receptor-related protein (LRP), ALK tyrosine kinase, syndecans compounds [4] and integrin [5], anaplastic lymphoma kinase (ALK) [6]. As described above, MK binds to syndecan-1, syndecan-3, syndecan-4 [7,8] and PTN binds to syndecan-3 [9]. MK has been reported to modulate PTPζ, PI3 kinase, PKC, and MAP kinase activity, but its specific transduction mechanism remains to be further elucidated by future research.
Figure 2.
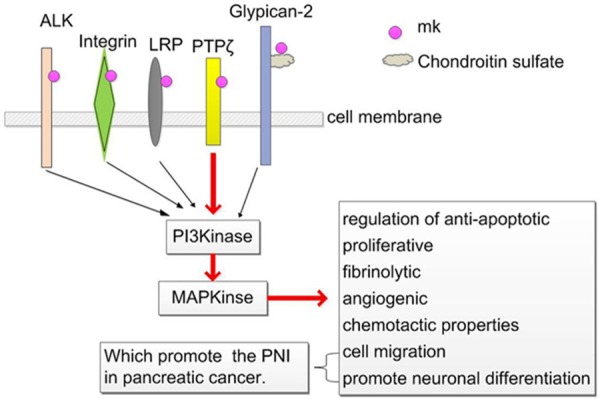
Glypican-2: syndecans compounds syndecan-1, -2, -3, -4. PTPζ: protein tyrosine phosphataseζ; LRP: LDL receptor-related protein; ALK: anaplastic lymphoma kinase; PI3K: phosphatidylinositol 3-kinase; MAPK: mitogen-activated protein kinase.
Biological activities of MK
MK and PTN have several cancer-related properties (Figure 2). These include regulation of anti-apoptotic, proliferative, fibrinolytic, angiogenic, and chemotactic properties. MK and PTN also have neurotrophic activities, which promote neuronal differentiation and cell migration in peripheral invasion of pancreatic cancer. In addition, both of these proteins promote the survival of dorsal root ganglion neurons and embryonic midbrain tyrosine carboxylase positive neurons in vitro [10,11]. MK also has been described to promote the differentiation of p19 cells to neurons. Thus, these observations support the activating role of MK in the differentiation of neural stem cells [12]. MK has also been demonstrated to increase cell migration of neutrophils [13], osteoblastic osteosarcoma cells [14], neural cells [15], macrophages, and smooth muscle cells [13,16], mediated by chondroitin sulfate chain [17], syndecan-3 and PTPζ [15]. Given the above, we assume that whether there is such a mechanism and what the PNI in pancreatic cancer nature is; First of all, pancreatic cancer cells attached to the weak place of the nerve gap and perineurium; Second, MK binded its receptor syndecan-3 and PTPζ through the signal transduction pathway mediated by chondroitin sulfate chain, which promote cancer infiltration or migration and rapid development of peripheral invasion. While the occurrence of peripheral invasion attract more MK to repair by its neurotrophic and chemotaxis function. Such a vicious circle, which make the further occurrence of PNI.
MK and cancer
In adults, MK is found at low levels in normal tissues, including the kidneys [18,26]. High expression of MK have been confirmed in a variety of solid tumors, including esophageal cancer, stomach cancer, colon cancer, pancreatic cancer, hepatocellular carcinoma, and lung cancer [19]. MK is found highly expressed during early stage tumor development and precancerous lesions [20]. Nakanishi [21] and Miyashiro [22] showed that MK is overexpressed in ovarian cancer and breast cancer, respectively. It is reported that MK is also highly expressed in Wilms’ tumor [23]. In a number of neurodegenerative diseases, MK is detected in patients with Alzheimer’s [24] and in precipitated glial cells of patients with multiple system atrophy [25]. Elevated MK levels have also been detected in urine of cancer patients. A number of studies have also reported that MK expression is a viable prognostic biomarker in a variety of tumors. Whether MK could also be used as a biomarker for the diagnosis and screening of other cancers remains to be determined.
MK and PNI
Pancreatic cancer is an aggressive tumor that is resistant to many types of therapies, with a five-year survival rate of only 1-4℅ [27]. Surgery is a treatment option for patients with pancreatic cancer. However, many patients initially present with local invasion and distant metastasis which hinder surgical success [28-30]. Peripheral nerve infiltration is associated with tumor invasion and local recurrence, and correlates with poor prognosis [31]. MK is a secretory alkaline heparin-binding protein and a neural growth factor. The MK family is comprised of two members, MK and PTN [32]. MK and PTN share a number of common receptors and biological characteristics, including interaction with heparin [33] and development of the nervous system [32]. Studies have showed that MK is overexpressed in pancreatic cancer [33] and levels of MK and PTN are also increased in pancreatic head carcinoma [34,35]. Levels of MK and syndecan-3 protein in tumor samples of patients with pancreatic cancer are significantly higher compared to control samples. Additionally, the expression of MK is being closely related to PNI, while levels of MK and syndecan-3 are significantly higher in patients with PNI than in those without PNI [36]. Thus, these observations provide evidence that MK may promote tumorigenesis and peripheral nerve infiltration of pancreatic cancer. Additional studies are needed to further understand the role of MK in pancreatic cancer development and whether it is a viable therapeutic target. These include determining the relationship between MK and nerve infiltration using gene silencing and siRNA technology.
Significance of MK in pancreatic cancer
MK has tumorigenic potential and is highly expressed in several malignant tumors. This leads to the notion of whether MK could serve as a prognostic serum biomarker. MK is a type of secretory heparin binding protein, when its level reaches certain in the organization, circulation system of MK level began to rise. Therefore, the serological detection of MK in the serum is becoming a hot spot of research at present. Studies have demonstrated that up to 60% of patients with malignant tumors have highly detectable levels of serum MK [37], and its levels are reduced upon tumor removal [38]. Further studies have also showed that patients with neuroblastoma have increasing MK levels that correlate with worsening clinical stage. In neuroblastoma, MK levels is also closely associated with MYCN amplification, TrkA expression, and proliferation [39]. In addition to MK, whether there are other biomarkers, such as MtK, that have potential prognostic value remains to be determined.
MtK
MtK is a truncated form of MK and founded by Mitashir in 1996. Compare to MK, MtK has a shorter mRNA, and lacks exon 3, N-terminus and neighboring amino acids (Asp26~Gly81). MtK contains a functional C-terminal end which performs a number biological activity. MtK is expressed in tumor tissue and metastastic lymph node, while it is undetectable in healthy human. Thus, like MK, MtK could be a potential biomarker for the diagnosis of precancerous lesions and the monitoring of tumor metastasis. A study by Miyashiro showed that MtK is detectable in approximately 25% (6/26) of breast cancer tissues that have MK expression. Additionally, high levels of MtK are also found in colorectal cancer [40], Wilms’ tumor, and various other tumors [41]. While MK is detectable at early stages of cancer, MtK is found expressed at all stages. These observations potentially demonstrate that MtK may be a more accurate biomarker than MK. However, levels of MtK are undetectable in serum of cancer patients that may be attributed to the lack of a N-terminal end. As a result, this may effect its stability, resistant to protease degradation, and ability to dimerize with other proteins. Specific mutations in MK can lead to the formation of MtK. Further studies are needed to assess the prognostic value of MtK in cancer, such as pancreatic cancer, and its biological mechanism underlying tumorigenesis and metastasis.
Gene therapy as treatment for pancreatic cancer
MK confers antiapoptotic and protect the cell, its characteristics of low expression in normal tissue even noexpression, which make it a promising therapeutic target. Interaction of both MK and N-syndecan induced expression of axons and neuronal movement, which contributes to nerve infiltration observed in pancreatic cancer. Based on these observations, several therapeutic strategies could potentially be implemented for treatment of pancreatic cancer. These may include using neutralizing antibody which block MK binding to, but not secretion by, cancer cells. Additionally, decreasing MK levels by antisense oligonucleotides (antisense oligonucleotides, ASODN) could be potentially treatment strategy for cancer. Studies have showed that the knockdown of MK in rat colon cancer cells and nerve fibroma inhibited cell proliferation and AGAR colony formation, and significantly decreased tumor growth in nude mice [42,43]. Furthermore, Latest studies confirm that application for MK genes of siRNA can inhibit the liver metastasis of human pancreatic cancer cells in a concentration and time dependent manner [44]. The application of suicide gene fragment could also be implemented to block MK activity. Because MK is primarily expressed in tumors, its gene promoter could be used to selectively expressed regulators that inhibit tumor growth. Studies have reported that the 5’ control region of certain genes associated with pancreatic cancer is transduced by thymidine kinase of herpes simplex virus, which inhibited tumor growth [45]. This strategy could also be intergrated by using adenovirus to enhance specificity for killing of caner cells.
Conclusion
MK is highly expressed in a various tumors and is detectable in serum and urine. This provides support that MK could be a potential prognostic or diagnostic biomarker that will aid in treatment decisions in patients with cancer. As a biomarker, high levels of MK may assist in early detection of nerve infiltration in patients with pancreatic cancer. Studies have demonstrated that MtK, a truncated form of MK, might also serve as a more accurate biomarker than MK for the diagnosis and prognosis of a number of cancers. However, future studies are needed to further understand the biological mechanisms underlying the role of MK and MtK in cancer and to assess the feasibility of using these as diagnostic and prognostic biomarkers.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. U1204819) and by Health Science and Technology Innovation Talents Program of Henan Province (No. 4203).
Disclosure of conflict of interest
None.
References
- 1.Muramatsu H, Inui T, Kimura T, Sakakibara S, Song XJ, Maruta H, Muramatsu T. Localization of heparin-binding, neurite outgrowth and antigenic regions in midkine molecule. Biochem Biophys Res Commun. 1994;203:1131–1139. doi: 10.1006/bbrc.1994.2300. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda Y, Talukder AH, Ishihara M, Hara S, Yoshida K, Muramatsu T, Kaneda N. Limited proteolysis by chymotrypsin of midkine and inhibition by heparin binding. Biochem Biophys Res Commun. 1996;228:176–181. doi: 10.1006/bbrc.1996.1635. [DOI] [PubMed] [Google Scholar]
- 3.Unoki K, Ohba N, Arimura H, Muramatsu H, Muramatsu T. Rescue of photoreceptors from the damaging effects of constant light by midkine, a retinoic acid-responsive gene product. Invest Ophthalmol Vis Sci. 1994;35:4063–4068. [PubMed] [Google Scholar]
- 4.Muramatsu T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem. 2002;132:359–371. doi: 10.1093/oxfordjournals.jbchem.a003231. [DOI] [PubMed] [Google Scholar]
- 5.Muramatsu H, Zou P, Suzuki H, Oda Y, Chen GY, Sakaguchi N, Sakuma S, Maeda N, Noda M, Takada Y, Muramatsu T. α4β1- and α6β1-integrins are functional receptors for midkine, a heparin-binding growth factor. J Cell Sci. 2004;117:5405–5415. doi: 10.1242/jcs.01423. [DOI] [PubMed] [Google Scholar]
- 6.Stoica GE, Kuo A, Powers C, Bowden ET, Sale EB, Riegel AT, Wellstein A. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J Biol Chem. 2002;277:35990–35998. doi: 10.1074/jbc.M205749200. [DOI] [PubMed] [Google Scholar]
- 7.Kojima T, Katsumi A, Yamazaki T, Muramatsu T, Nagasaka T, Ohsumi K, Saito H. Human ryudocanfrom endothelium-like cells binds basic fibroblast growth factor, midkine, and tissue factor pathway inhibitor. J Biol Chem. 1996;271:5914–5920. doi: 10.1074/jbc.271.10.5914. [DOI] [PubMed] [Google Scholar]
- 8.Nakanishi T, Kadomatsu K, Okamoto T, Ichihara-Tanaka K, Kojima T, Saito H, Tomoda Y, Muramatsu T. Expression of syndecan-1 and -3 during embryogenesis of the central nervous system in relation to binding with midkine. J Biochem. 1997;121:197–205. [PubMed] [Google Scholar]
- 9.Landgraf P, Mikhaylova M, Macharadze T, Borutzki C, Zenclussen AC, Wahle P, Kreutz MR. Binding of Y-P30 to syndecan 2/3 regulates the nuclear localization of CASK. PLoS One. 2014;9:e85924. doi: 10.1371/journal.pone.0085924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao J, Li WY, Li SG, Feng XS, Gao SG. Recombinant lentivirus targeting the pleotrophin gene reduces pleotrophin protein expression in pancreatic cancer cells and inhibits neurite outgrowth of dorsal root ganglion neurons. Mol Med Rep. 2014;9:999–1004. doi: 10.3892/mmr.2014.1918. [DOI] [PubMed] [Google Scholar]
- 11.Yao J, Zhang M, Ma QY, Wang Z, Wang LC, Zhang D. PAd-shRNA-PTN reduces pleiotrophin of pancreatic cancer cells and inhibits neurite outgrowth of DRG. World J Gastroenterol. 2011;17:2667–2673. doi: 10.3748/wjg.v17.i21.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sotogaku N, Tully SE, Gama CI, Higashi H, Tanaka M, Hsieh-Wilson LC, Nishi A. Activation of phospholipase C pathways by a synthetic chondroitin sulfate-E tetrasaccharide promotes neurite outgrowth of dopaminergic neurons. J Neurochem. 2007;103:749–760. doi: 10.1111/j.1471-4159.2007.04849.x. [DOI] [PubMed] [Google Scholar]
- 13.Sato W, Kadomatsu K, Yuzawa Y, Muramatsu H, Hotta N, Matsuo S, Muramatsu T. Midkine is involved in neutrophil infiltration into the tubulointerstitium in ischemic renal injury. J Immunol. 2001;167:3463–3469. doi: 10.4049/jimmunol.167.6.3463. [DOI] [PubMed] [Google Scholar]
- 14.Qi M, Ikematsu S, Maeda N, Ichihara-Tanaka K, Sakuma S, Noda M, Muramatsu T, Kadomatsu K. Haptotactic migration induced by midkine. Involvement of protein-tyrosine phosphataseζ. Mitogen-activated protein kinase, and phosphatidylinositol 3-kinase. J Biol Chem. 2001;276:15868–15875. doi: 10.1074/jbc.m005911200. [DOI] [PubMed] [Google Scholar]
- 15.Maeda N, Ichihara-Tanaka K, Kimura T, Kadomatsu K, Muramatsu T, Noda M. A receptorlike proteintyrosine phosphatase PTPζ/RPTPβ binds a heparin-binding growth factor midkine. Involvement of arginine 78 of midkine in the high affinity binding to PTPζ. J Biol Chem. 1999;274:12474–12479. doi: 10.1074/jbc.274.18.12474. [DOI] [PubMed] [Google Scholar]
- 16.Horiba M, Kadomatsu K, Nakamura E, Muramatsu H, Ikematsu S, Sakuma S, Hayashi K, Yuzawa Y, Matsuo S, Kuzuya M, Kaname T, Hirai M, Saito H, Muramatsu T. Neointima formation in a restenosis model is suppressed in midkine-deficient mice. J Clin Invest. 2000;105:489–495. doi: 10.1172/JCI7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi M, Ikematsu S, Maeda N, Ichihara-Tanaka K, Sakuma S, Noda M, Muramatsu T, Kadomatsu K. Haptotactic migration induced by midkine. Involvement of protein-tyrosine phosphatase zeta. Mitogen-activated protein kinase, and phosphatidylinositol 3-kinase. J Biol Chem. 2001;276:15868–15875. doi: 10.1074/jbc.m005911200. [DOI] [PubMed] [Google Scholar]
- 18.Sakamoto K, Kadomatsu K. Midkine in the pathology of cancer, neural disease, and inflammation. Pathol Int. 2012;62:445–455. doi: 10.1111/j.1440-1827.2012.02815.x. [DOI] [PubMed] [Google Scholar]
- 19.Takada T, Toriyama K, Muramatsu H, Song XJ, Torii S, Muramatsu T. Midkine, a retinoic acid-inducible heparin-bingding cytokine in inflammatory responses: chemotactic activity to neutrophils and association with inflammatory synovitis. J Biochem. 1997;122:453–458. doi: 10.1093/oxfordjournals.jbchem.a021773. [DOI] [PubMed] [Google Scholar]
- 20.Konishi N, Nakamura M, Nakaoka S, Hiasa Y, Cho M, Uemura H, Hirao Y, Muramatsu T, Kadomatsu K. ImmunohistochemicaI analysis of midkine expression in human prostate carcinoma. Oncology. 1999;57:253–257. doi: 10.1159/000012039. [DOI] [PubMed] [Google Scholar]
- 21.Nakanishi T, Kadomatsu K, Okamoto T, Tomoda Y, Muramatsu T. Expression of midkine and pleiotropin in ovarian tumors. Obstet Gynecol. 1997;90:285–290. doi: 10.1016/S0029-7844(97)00237-8. [DOI] [PubMed] [Google Scholar]
- 22.Miyashiro I, Kaname T, Shin E, Wakasugi E, Monden T, Takatsuka Y, Kikkawa N, Muramatsu T, Monden M, Akiyama T. Midkine expression in human breast cancers: Expression of truncated form. Breast Cancer Res Treat. 1997;43:1–6. doi: 10.1023/a:1005748728351. [DOI] [PubMed] [Google Scholar]
- 23.Tsutsui J, Kadomatsu K, Matsubara S, Nakagawara A, Hamanoue M, Takao S, Shimazu H, Ohi Y, Muramatsu T. A new family of heparin-binding growth/differentiation factors: Increased midkine expression in Wilms’ tumor and other human carcinomas. Cancer Res. 1993;53:1281–1285. [PubMed] [Google Scholar]
- 24.Yasuhara O, Muramatsu H, Kim SU, Muramatsu T, Maruta H, McGeer PL. Midkine, a novel neurotrophic factor, is present in senile plaques of Alzheimer disease. Biochem Biophys Res Commun. 1993;192:246–251. doi: 10.1006/bbrc.1993.1406. [DOI] [PubMed] [Google Scholar]
- 25.Kato S, Shinozawa T, Takikawa M, Kato M, Hirano A, Awaya A, Ohama E. Midkine, a new neurotrophic factor, is present in glial cytoplasmic inclusions of multiple system atrophy brains. Acta Neuropathol. 2000;100:481–489. doi: 10.1007/s004010000214. [DOI] [PubMed] [Google Scholar]
- 26.Ikematsu S, Okamoto K, Yoshida Y, Oda M, Sugano-Nagano H, Ashida K, Kumai H, Kadomatsu K, Muramatsu H, Takashi Muramatsu, Sakuma S. High levels of urinary midkine in various cancer patients. Biochem Biophys Res Commun. 2006;306:329. doi: 10.1016/s0006-291x(03)00984-7. [DOI] [PubMed] [Google Scholar]
- 27.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 28.Mossner J. What’s new in therapy of pancreatic cancer? Dig Dis. 2010;28:679–683. doi: 10.1159/000320096. [DOI] [PubMed] [Google Scholar]
- 29.Han SL, Zhang WJ, Zheng XF, Shen X, Zeng QQ, Ke QH. Radical resection and outcome for malignant tumors of the pancreatic body and tail. World J Gastroenterol. 2009;15:5346–5351. doi: 10.3748/wjg.15.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiang KC, Yeh CN, Lee WC, Jan YY, Hwang TL. Prognostic analysis of patients with pancreatic head adenocarcinoma less than 2 cm undergoing resection. World J Gastroenterol. 2009;15:4305–10. doi: 10.3748/wjg.15.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bapat AA, Hostetter G, Von Hoff DD, Han H. Peripheral invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695–707. doi: 10.1038/nrc3131. [DOI] [PubMed] [Google Scholar]
- 32.Kadomatsu K, Muramatsu T. Midkine and pleiotrophin in neural development and cancer. Cancer Lett. 2004;204:127–143. doi: 10.1016/S0304-3835(03)00450-6. [DOI] [PubMed] [Google Scholar]
- 33.Ohhashi S, Ohuchida K, Mizumoto K, Egami T, Yu J, Cui L, Toma H, Takahata S, Nabae T, Tanaka M. Midkine mRNA Is Overexpressed in Pancreatic Cancer. Dig Dis Sci. 2009;54:811–815. doi: 10.1007/s10620-008-0434-4. [DOI] [PubMed] [Google Scholar]
- 34.Maeda S, Shinchi H, Kurahara H, Mataki Y, Noma H, Maemura K, Aridome K, Yokomine T, Natsugoe S, Aikou T, Takao S. Clinical significance of midkine expression in pancreatic head Carcinoma. Brit J Cancer. 2007;97:405–411. doi: 10.1038/sj.bjc.6603879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao J, Ma Q, Wang L, Zhang M. Pleiotrophin expression in human pancreatic cancer and its correlation with clinicopathological features, perineural invasion, and prognosis. Digest Dis Sci. 2009;54:895–901. doi: 10.1007/s10620-008-0433-5. [DOI] [PubMed] [Google Scholar]
- 36.Yao J, Li WY, Li SG, Feng XS, Gao SG. Midkine promotes perineural invasion in human pancreatic cancer. World J Gastroenterol. 2014;20:3018–3024. doi: 10.3748/wjg.v20.i11.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muramatsu H, Song XJ, Koide N, Hada H, Tsuji T, Kadomatsu K, Inui T, Kimura T, Sakakibara S, Muramatsu T. Enzyme-linked immunoassay for midkine, and its application to evaluation of midkine levels in developing mouse brain and sera from patients with hepatocellular carcinomas. J Biochem (Tokyo) 1996;119:1171–1175. doi: 10.1093/oxfordjournals.jbchem.a021364. [DOI] [PubMed] [Google Scholar]
- 38.Ikematsu S, Yano A, Aridome K, Kikuchi M, Kumai H, Nagano H, Okamoto K, Oda M, Sakuma S, Aikou T, Muramatsu H, Kadomatsu K, Muramatsu T. Serum midkine levels are increased in patients with various types of carcinomas. Brit J Cancer. 2000;83:701–706. doi: 10.1054/bjoc.2000.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ikematsu S, Nakagawara A, Nakamura Y, Sakuma S, Wakai K, Muramatsu T, Kadomatsu K. Correlation of elevated level of blood midkine with poor prognostic factors of human neuroblastomas. Brit J Cancer. 2003;88:1522–1526. doi: 10.1038/sj.bjc.6600938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyashiro I, Kaname T, Nakayama T, Nakamori S, Yagyu T, Monden T, Kikkawa N, Nishisho I, Muramatsu T, Monden M, Akiyama T. Expression of truncated midkine in human colorectal cancers. Cancer Lett. 1996;106:287–291. doi: 10.1016/0304-3835(96)04333-9. [DOI] [PubMed] [Google Scholar]
- 41.Paul S, Mitsumoto T, Asano Y, Kato S, Kato M, Shinozawa T. Detection of truncated midkine in Wilms’ tumor by a monoclonal antibody against human recombinant truncated midkine. Cancer Lett. 2001;163:245–251. doi: 10.1016/s0304-3835(00)00696-0. [DOI] [PubMed] [Google Scholar]
- 42.Takei Y, Kadomatsu K, Matsuo S, Itoh H, Nakazawa K, Kubota S, Muramatsu T. Antisense oligodeoxynucleotide targeted to Midkine, a heparin-binding growth factor, suppresses tumorigenicity of mouse rectal carcinoma cells. Cancer Res. 2001;61:8486–8491. [PubMed] [Google Scholar]
- 43.Takei Y, Kadomatsu K, Yuasa K, Sato W, Muramatsu T. Morpholino antisense oligomer targeting human midkine: its application for cancer therapy. Int J Cancer. 2005;114:490–497. doi: 10.1002/ijc.20781. [DOI] [PubMed] [Google Scholar]
- 44.Yu L, Fan Y, Chen BD, Hu Y, Gao Y, Wei D. Suppression of metastasis of human pancreatic cancer cells to the liver by small interfering RNA-mediated targeting of the midkine gene. Oncol Lett. 2013;6:1338–1342. doi: 10.3892/ol.2013.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshida Y, Tomizawa M, Bahar R, Miyauchi M, Yamaguchi T, Saisho H, Kadomatsu K, Muramatsu T, Matsubara S, Sakiyama S, Tagawa M. A promoter region of midkine gene can activate transcription of an exogenous suicide gene in human pancreatic cancer. Antieancer Res. 2002;22:117–120. [PubMed] [Google Scholar]


