Abstract
Background
Elevated numbers of circulating fibrocytes are associated with inadequately controlled asthma, poor response to available therapies, and increased risk of adverse outcomes. The lack of reliable and clinically-applicable assays precludes a proper evaluation of blood fibrocyte count as a prognostic biomarker in asthma. This report concerns the use of a multiparameter flow cytometry assay for the enumeration of fibrocytes in the whole blood.
Methods
Consenting fibrocyte donors were 19 patients with asthma well controlled by current treatment, 16 patients with treatment-resistant asthma, 9 patients with transiently uncontrolled asthma and 14 age-matched normal individuals. Blood sampling was performed once in patients with transiently uncontrolled asthma and twice, at an interval of one week, in the other subjects. The assay was performed in 100 μl of whole blood and involved a sequential gating strategy and absolute fibrocyte counting with a single instrument (single-platform assay).
Results
The quantification of circulating fibrocytes by this assay was analytically and clinically valid. In individuals with stable clinical conditions, the repeatability of blood fibrocyte counts over one week was good. The intraclass correlation coefficient was 0.939 and 96.88% of the total variability reflected on-average differences among the tested subjects. Stabilized blood samples could be stored at 4 °C for up to 96 h before processing.
Conclusions
The novel assay for the enumeration of fibrocytes in the whole blood is reliable and clinically applicable.
General significance
This report demonstrates the validity and reliability of the first optimized assay for the enumeration of circulating fibrocytes in multicenter clinical trials.
Abbreviations: 7-AAD, 7-amino-actinomycin-D; AF, Alexa Fluor; α-SMA, α-smooth muscle actin; BMC, blood mononuclear cell; CCC, concordance correlation coefficient; CI, confidence interval; COL1, type I collagen; CT, threshold cycle; ET-1, endothelin-1; FSC, forward scatter; ICC, intraclass correlation coefficient; MFI, mean fluorescence intensity; PB, Pacific Blue; SD, standard deviation; SS, sum of squares; SSC, side scatter
Keywords: Asthma, Biomarker, Clinical outcome, Fibrocyte enumeration, Flow cytometry, Single-platform assay
Highlights
-
•
Elevated blood fibrocyte count is an emerging prognostic biomarker in asthma.
-
•
The lack of reliable, clinically-applicable assays precludes further evaluation.
-
•
An optimized whole-blood single-platform flow cytometry assay is described here.
-
•
The assay is analytically and clinically valid and provides reproducible measures.
-
•
Stabilized blood samples can be stored for 96 h at 4 °C before processing.
1. Introduction
Asthma is a common inflammatory disorder of the airway for which there is no cure [1]. Optimal asthma management consists in the achievement and maintenance of the best possible clinical control and in the prevention of disease exacerbations, which are most frequently triggered by allergen exposure and viral infections and increase the risk of future adverse outcomes [1], [2]. Clinical parameters of asthma control include the frequency of symptoms during the day and at night or on awakening, presence of activity limitation because of symptoms, and level of airflow obstruction [1]. The lack of clinical control of asthma reflects ongoing airway inflammation and indicates inadequate anti-inflammatory treatment [1], [2]. When patients with asthma responsive to the currently available anti-inflammatory therapies suffer from an acute exacerbation of their disease, asthma control can be achieved again by increasing the intensity of the anti-inflammatory treatment. In contrast, patients with treatment-resistant disease have frequent signs and symptoms of airflow obstruction, severe exacerbations requiring hospitalization, and persistent airway inflammation not responding to available therapies. Ongoing airway inflammation is associated with an increased risk of adverse outcomes because the chronic inflammatory process promotes the development of irreversible structural changes in the bronchial tubes that lead to fixed airway narrowing and progressive loss of lung function [1], [2]. Easily accessible biomarkers of airway inflammation and asthma control are therefore needed for monitoring the effects of available treatments in patients at increased risks of adverse outcomes and to assess their responsiveness to novel therapeutic candidates in clinical trials [2], [3], [4]. Blood fibrocyte count is emerging as one of these easily accessible biomarkers.
The fibrocytes are circulating CD45+CD34+HLA-DR+CD11b+CD13+CD16−CD115− cells that exhibit both hematopoietic and stromal-like features and possess antigen-presenting activity [5], [6], [7], [8], [9]. They produce several cytokines and growth factors and express type I collagen (COL1) genes and protein [5], [7], [10], [11]. Their proinflammatory and profibrotic properties [6], [9], [10], [11] are relevant to asthma [9], [12], [13], [14], [15], and there is mounting evidence [12], [13], [14], [15], [16], [17], [18] that these cells substantially contribute to the progression of the structural changes that ultimately lead to fixed airway narrowing in patients with frequent exacerbations and treatment-resistant disease. Elevated numbers of fibrocytes are present in the circulation and in the airways of these patients [10], [14], [17], [18] as a result of the release of ligands of the C—C chemokine receptor 5 and other cytokines that are known to mediate the output of fibrocytes from the bone marrow and their recruitment to the inflamed tissue [18], [19]. Moreover, the presence of an elevated number of fibrocytes in the peripheral blood of asthmatic individuals with inadequately controlled disease not only reflects ongoing airway inflammation [10], [14], [18] but is also associated with persistent airflow obstruction and accelerated reduction of lung function over time [17]. Thus, elevated blood fibrocyte counts in asthma are indicative of inflammatory and structural alterations that can otherwise be detected in a non-invasive way solely by combining cellular analysis of induced sputum with high-resolution computed tomography [20]. The use of blood fibrocyte count as an asthma outcome may also be advantageous in comparison with other prognostic biomarkers because it does not seem to be affected by treatment with corticosteroids. In fact, patients with treatment-refractory asthma by definition use the highest possible dose of inhaled corticosteroids, often in combination with oral corticosteroids, and yet they have substantially higher numbers of fibrocytes in their peripheral blood and airways than patients with treatment-responsive asthma of any severity [14], [18].
Unfortunately, the evaluation of the validity and utility of blood fibrocyte count as a novel cellular biomarker in asthma is currently precluded by the absence of reliable and clinically-applicable analytical assays. The experiments described in the present report were therefore conducted to evaluate the clinical applicability of an optimized multiparameter flow cytometry assay for the enumeration of fibrocytes in the whole blood, which involves a sequential gating strategy and direct absolute fibrocyte counting with a single instrument by using the single-platform technology [21], [22], [23].
2. Materials and methods
2.1. Patients
The experiments were conducted during the course of two recently published studies [16], [18], according to protocols approved by the appropriate review board, and the results are cumulatively reported here. Consenting fibrocyte donors were 19 patients with asthma well controlled by current treatment [18], 16 patients with treatment-resistant asthma [18], 9 patients with transiently uncontrolled asthma because of an allergen-induced exacerbation [16], and 14 age-matched normal individuals [16]. The demographic and clinical characteristics of these patients are described in every detail in the two published articles [16], [18]. Blood sampling was performed once in patients with transiently uncontrolled asthma and twice, at an interval of one week, in the other subjects.
2.2. Enumeration of circulating fibrocytes
Blood specimens were collected by venipuncture into evacuated tubes containing tripotassium ethylenediamine tetraacetate anticoagulant. Aliquots of the blood samples were either left untreated, and processed within 4 h, or mixed with the cellular surface antigen-stabilizing agent TransFix (200 μl per 1000 μl blood, Life Technologies, Carlsbad, CA, USA) [24], [25] and stored at 4 °C for up to 96 h before processing.
One hundred μl of treated or untreated blood were added to a 5-ml round-bottom tube containing 2 μl of the viability dye 7-amino-actinomycin-D (7-AAD, BD Biosciences, San Jose, CA, USA), which is detected on the PerCP-Cy5.5 channel, and appropriate dilutions in stain buffer (BD Biosciences) of the following fluorochrome-labeled monoclonal antibodies (all mouse IgG1κ): 2.5 μl CD3-PerCP-Cy5.5, 2.5 μl CD19-PerCP-Cy5.5, 2.5 μl CD20-PerCP-Cy5.5, 2.5 μl CRTH2/CD294-PerCP-Cy5.5, 5 μl CD45-AmCyan, 5 μl CD34-PE, 5 μl CD11b-Pacific Blue (PB), 10 μl CD16-FITC (BD Biosciences), and 5 μl CD115-APC (R&D Systems Europe, Abingdon, United Kingdom). After incubation for 20 min at room temperature in the dark, erythrocytes were lysed with 2 ml fixative-free High-Yield Lyse solution (Life Technologies). CountBright absolute counting beads (Life Technologies) were added to allow an absolute count of the target cell population according to the manufacturer's instruction, and samples were analyzed immediately without any wash steps.
The flow cytometer was a 4-laser BD SLRII (BD Biosciences) and the instrument was set up using BD Cytometer Setup and Tracking beads. Data were acquired and analyzed with the BD FACSDiva software (BD Biosciences). Compensation settings were performed with a set of compensation tubes using the BD CompBead antibody-capturing particles and the compensation setup tool in the BD FACSDiva software. Antibodies were titered for no-wash staining. Fluorescence-minus-one controls were used for accurate definition of cells that had fluorescence above the background levels [26].
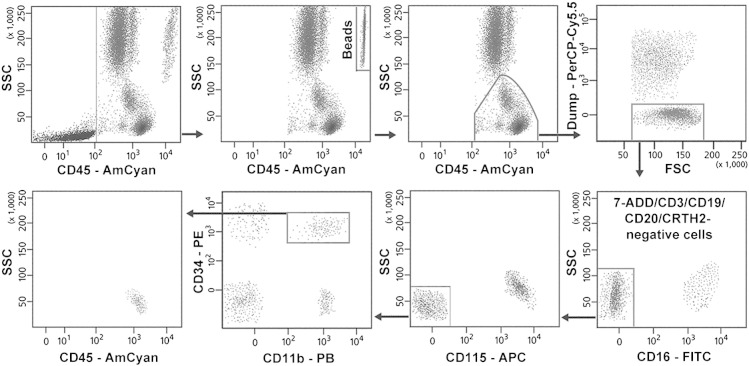
The gating strategy applied for the enumeration of fibrocytes is shown in Fig. 1. Because circulating fibrocytes are present in the CD45+ blood mononuclear cell (BMC) fraction [5], [6], [7], this population was initially gated on the basis of the side scatter (SSC) characteristics and CD45 expression, and debris and platelets were eliminated. The beads were gated for counting and excluded from cell analysis. A dump channel was then established on the PerCP-Cy5.5 channel to exclude 7-ADD+ non-viable cells and some of the unwanted cells that express CD11b, comprising CD3-positive T lymphocytes, CD19+CD20+ B lymphocytes and B blasts, CRTH2-positive immature and contaminating mature eosinophils, and CRTH2+ basophils [21], [27]. CD45+[7-ADD/CD3/CD19/CD20/CRTH2]− cells were gated in the plot PerCP-Cy5.5 versus forward scatter (FSC). Contaminating CD16+CD11b+ neutrophils, CD115+CD16+CD11b+ monocytes and CD115+ CD16−CD11b+ mature and immature monocytes [21], [28] were than sequentially excluded from this population by gating CD16- and CD115-negative cells in the plot CD16-FITC versus SSC and CD15-APC versus SSC, respectively. Fibrocytes were identified within the CD45+[7-ADD/CD3/CD19/CD20/CRTH2]−CD16−CD115− population on the basis of the coexpression of CD34 and CD11b, which distinguished these cells from the other remaining CD45+[7-ADD/CD3/CD19/CD20/CRTH2]−CD16−CD115− cells, including CD45dimCD34+CD11b− blasts/progenitors [29], circulating CD45+CD11b−CD34− myeloid and plasmacytoid dendritic cells [9], [30], and CD45+CD34− CD11b+CD16−CD56+ NK cells [21], [27]. In the CD45/SSC plot, the gated CD45+[CD3/CD19/CD20/CRTH2]−CD16−CD115−CD11b+CD34+ cells showed an SSC slightly higher than that of lymphocytes and similar to that of dendritic cells [27] and some myeloid progenitors [29]. At least 105 cell events were acquired for each assay before cell analysis and absolute cell counting. The intra-assay variability was assessed by analysis of the absolute fibrocyte counts in three separate 100-μl aliquots of each blood sample. The coefficient of variation ranged from 2.84 to 5.73% in fresh blood samples, from 4.15 to 8.28% in blood samples stored for 48 h and from 3.96 to 9.61% in blood samples stored for 96 h.
Fig. 1.
Gating strategy for the enumeration of viable CD45+[CD3/CD19/CD20/CRTH2]−CD16−CD115−CD11b+CD34+ fibrocytes in the whole blood. Absolute counting beads were successfully resolved from cells and gated for counting. The representative assay was performed with the fresh blood sample of a patient with treatment-resistant asthma.
7-AAD, 7-amino-actinomycin-D; FSC, forward scatter; PB, Pacific Blue; SSC, side scatter.
2.3. Phenotypic and functional analysis of sorted cells
Blood was collected into BD Vacutainer Glass Mononuclear Cell Preparation Tubes (Thermo Fisher Scientific, Waltham, MA, USA) and BMCs were isolated according to the manufacturer's instruction. Cells (1 × 107/ml stain buffer) were treated with 5% normal mouse serum (Sigma-Aldrich, St. Louis, MO, USA) for 10 min prior to staining with 7-AAD and the antibody cocktail mentioned above. Viable CD45+[CD3/CD19/CD20/CRTH2]−CD16−CD115−CD11b+CD34+ cells were aseptically sorted on a 4-laser BD FACS Aria II cytometer (BD Biosciences) using the BD FACSDiva software and the same setting procedures and gating strategy indicated above.
Sorted cells were cultured for 6 days in Dulbecco's modified Eagle's medium and nutrient mix Ham's F12 (1:1), containing glutamine (2 mmol/l), sodium pyruvate (1 mmol/l), nonessential amino acids (1% vol/vol), penicillin (50 U/ml), streptomycin (50 U/ml,) and 0.5% fetal calf serum (all from Life Technologies), in the presence or in the absence of 1 ng/ml endothelin-1 (ET-1, Merck Calbiochem, Darmstadt, Germany). Medium was replaced on the third day. Analysis of the expression of COL1 and α-smooth muscle actin (α-SMA) was performed before and at the end of the incubation period by flow cytometry. For intracellular staining, cells were fixed and permeabilized with the FIX & PERM kit (Life Technologies) and incubated with either an unconjugated anti-COL1 mouse IgG1 (Merck Millipore, Darmstadt, Germany) or mouse α-SMA-Alexa Fluor (AF) 700 IgG2A (R&D Systems). COL1 immunoreactivity was detected with an APC-conjugated goat anti-mouse IgG, F(ab′)2 (Santa Cruz Biotechnology, Dallas, TX, USA). Nonspecific staining was assessed by using the following recommended isotype controls for intracellular staining in place of the primary antibodies: unconjugated mouse IgG1 (BD Biosciences) and AF 700-conjugated mouse IgG2A (R&D Systems). By using aseptically sorted fibrocytes from 3 asthmatic patients with relatively high blood fibrocyte counts, the expression of COL1 was confirmed at the gene level. Gene expression analysis was performed by reverse-transcription and real time polymerase chain reaction with the use of a SYBR Green-based cells-to-threshold cycle (CT) kit (Life Technologies) for small cell samples, as previously described [10]. Specific primers for the target gene COL1A1, encoding the pro-α1 chain of COL1 (http://www.ncbi.nlm.nih.gov/gene/1277), were those used in a previous study [11]. The primer pairs for the reference gene ACTB, encoding β-actin (http://www.ncbi.nlm.nih.gov/gene/60), were included in the control kit from Life Technologies.
2.4. Statistical analysis
Differences across groups were analyzed by one-way analysis of variance followed by post-hoc Tukey comparison between groups. Repeatability of the fibrocyte counts over one week was assessed by Student's t test for paired data, correlation analysis and calculation of the intra-class correlation coefficient (ICC) as a reliability index (one-way random effect model) [31]. Agreement between fibrocyte counts determined in fresh and stored blood samples was assessed by linear regression analysis, Student's t test for paired data and calculation of the concordance correlation coefficient (CCC) [31]. The Bland–Altman plots were constructed to display repeatability of fibrocyte counts and the between-method comparison results [32]. The association between fibrocyte counts and clinical variables was assessed by calculating the Pearson correlation coefficient or the Spearman rank correlation coefficient, as appropriate. A p-value of less than 0.05 was considered to be statistically significant. Fibrocyte counts by group were reported as the means, standard deviations (SDs) and 95% confidence intervals (CIs).
3. Results
3.1. Ability of the single-platform assay to identify authentic fibrocytes
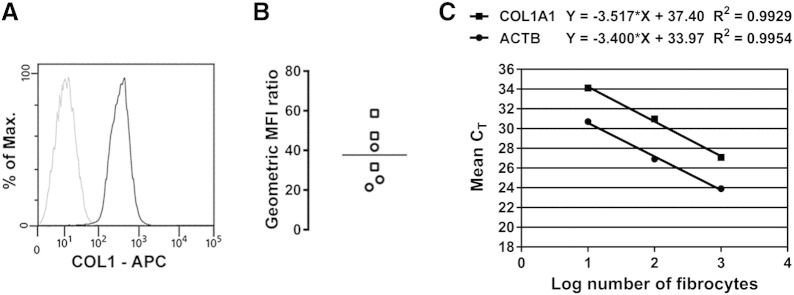
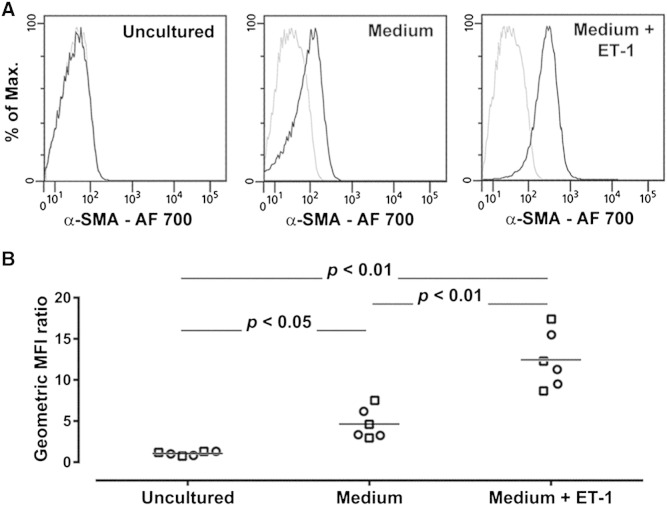
Proof-of-principle experiments were conducted in 3 patients with asthma well controlled by current treatment and 3 patients with treatment-resistant disease who were known to have appreciable numbers of authentic fibrocytes in their peripheral blood, on the basis of data collected at an earlier time point of the study and reported elsewhere [18]. Blood samples were analyzed by using the single-platform assay for the enumeration of fibrocytes as viable CD45+[CD3/CD19/CD20/CRTH2]−CD16−CD115−CD11b+CD34+ cells (Fig. 1). To evaluate the nature of the cells identified by this assay, circulating viable CD45+[CD3/CD19/CD20/CRTH2]−CD16−CD115−CD11b+CD34+ cells were sorted from the BMCs and analyzed for the expression of COL1 [5], [6], [7], [8], [11], [12], [13], [14] and response to stimulation with ET-1 [12], [33]. Specific immunoreactivity for COL1 was detected in the sorted cells from all 6 fibrocyte donors (Fig. 2A and B) and the percentage of COL1+ cells in individual samples ranged from 88.9% to 97.4%. COL1 expression was also confirmed at the gene level (Fig. 2C). Furthermore, sorted cells produced higher levels of α-SMA when cultured in the presence of ET-1 than in culture medium alone, while freshly sorted and uncultured cells did not express the contractile protein (Fig. 3A and B). These experiments confirmed that the circulating CD45+[CD3/CD19/CD20/CRTH2]−CD16−CD115−CD11b+CD34+ cells are authentic fibrocytes and demonstrated the analytical validity of the single-platform assay.
Fig. 2.
Analytical validity of the assay demonstrated by analysis of the expression of type I collagen protein and gene in sorted viable CD45+[CD3/CD19/CD20/CRTH2]−CD16−CD115−CD11b+CD34+ fibrocytes. (A) Representative flow cytometry analysis of the expression of intracellular type I collagen (COL1). The black line indicates staining with a specific anti-COL1 monoclonal antibody and the gray line shows nonspecific staining with the isotype control (B) Quantitative analysis of the expression of intracellular COL1. The geometric mean fluorescence intensity (MFI) for COL1 was divided by the corresponding value obtained with the isotype control. The graph shows individual geometric MFI ratio values obtained with the cells from 3 patients with controlled asthma (squares) and 3 patients with treatment-resistant asthma (circles). The horizontal lines indicate the means. (C) Analysis of the expression of the gene encoding the pro-α1 chain of COL1 (COL1A1) by reverse-transcription and real time polymerase chain reaction. The graph shows a representative example of quantification by using the relative standard curve method, where the mean threshold cycle (CT) values from duplicate reactions are plotted against the log of the number of cells used in the reaction. The endogenous control was ACTB, the gene encoding β-actin. The normalized levels of expression of COL1A1 in sorted fibrocytes from 3 patients with treatment-resistant asthma were 0.255, 0.334 and 0.469 (∆CT method with efficiency correction).
Fig. 3.
Analytical validity of the assay demonstrated by analysis of the response of sorted viable CD45+[CD3/CD19/CD20/CRTH2]−CD16−CD115−CD11b+CD34+ fibrocytes to stimulation with endothelin-1 (ET-1). (A) Representative flow cytometry analysis of the expression of intracellular α-smooth muscle actin (α-SMA) in freshly sorted and uncultured cells and in cells incubated for 6 days in culture medium alone or in culture medium supplemented with1 ng/ml ET-1. The black lines indicate staining with a specific anti α-SMA monoclonal antibody and the gray lines show nonspecific staining with the isotype control. (B) Quantitative analysis of the expression of intracellular α-SMA. The geometric mean fluorescence intensity (MFI) for α-SMA was divided by the corresponding value obtained with the isotype control. The graphs show individual geometric MFI ratio values obtained with the cells from 3 patients with controlled asthma (squares) and 3 patients with treatment-resistant asthma (circles) under each experimental condition. The horizontal lines indicate the means. AF, Alexa Fluor.
3.2. Clinical validity of the single-platform assay
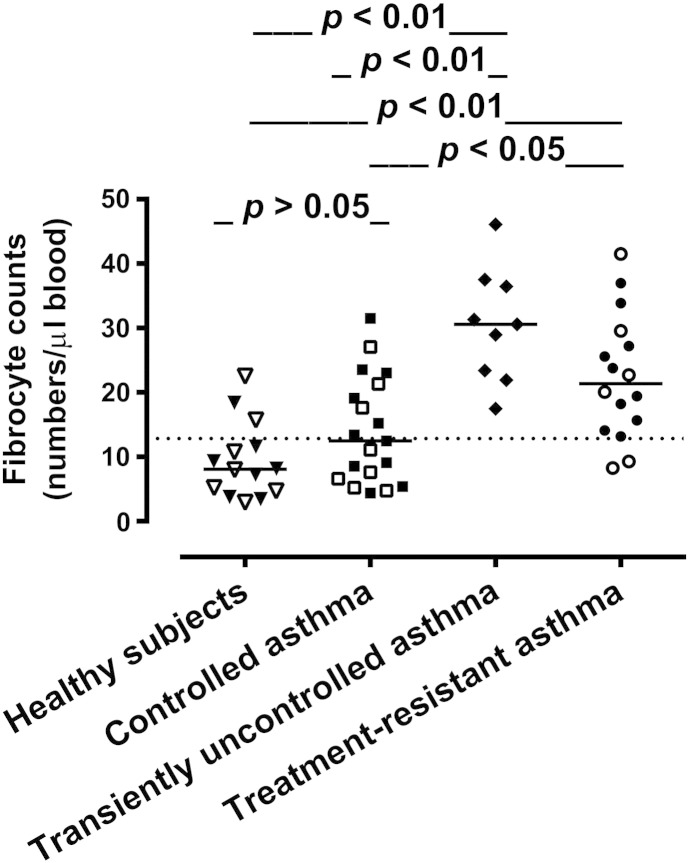
To investigate the ability of the single-platform assay to provide measures that correlate with the level of asthma control and therapeutic responsiveness, the blood fibrocyte counts obtained in patients with controlled asthma and patients with treatment-resistant disease by this method were compared with the counts similarly measured in patients with asthma transiently uncontrolled by current treatment, because of an exacerbation triggered by allergen exposure, and in healthy controls. Patients with transiently uncontrolled asthma and those with treatment-resistant disease had significantly elevated numbers of circulating fibrocytes in comparison with the other groups, while there were no statistically significant differences between the fibrocyte counts obtained in patients with controlled asthma and healthy individuals (Fig. 4). Although all patients with transiently uncontrolled asthma were suffering from a disease exacerbation triggered by allergen exposure, the atopic status alone did not appear to have an influence on the number of circulating fibrocytes because atopic subjects were present in all groups (Fig. 4). In asthmatic subjects, the blood fibrocyte counts did not correlate with the duration of asthma or measures of lung function alone (all p > 0.05).
Fig. 4.
Comparison of fibrocyte counts in the whole blood of healthy individuals and asthmatic patients. The graph shows individual values and the median value (horizontal line) for each group. Closed symbols represent atopic individuals. The dotted line indicates the upper limit of the 95% confidence interval (CI) in healthy subjects. The mean numbers of fibrocytes/μl blood, standard deviations and 95% CIs were 9.41, 5.92, 5.99–12.8 in healthy subjects; 14.05, 8.28, 10.06–18.04 in patients with controlled asthma; 30.42, 8.84, 23.62–37.21 in patients with transiently uncontrolled asthma; and 22.46, 9.65, 17.31–27.60 in patients with treatment-resistant asthma.
3.3. Repeatability of fibrocyte counts by the single-platform assay
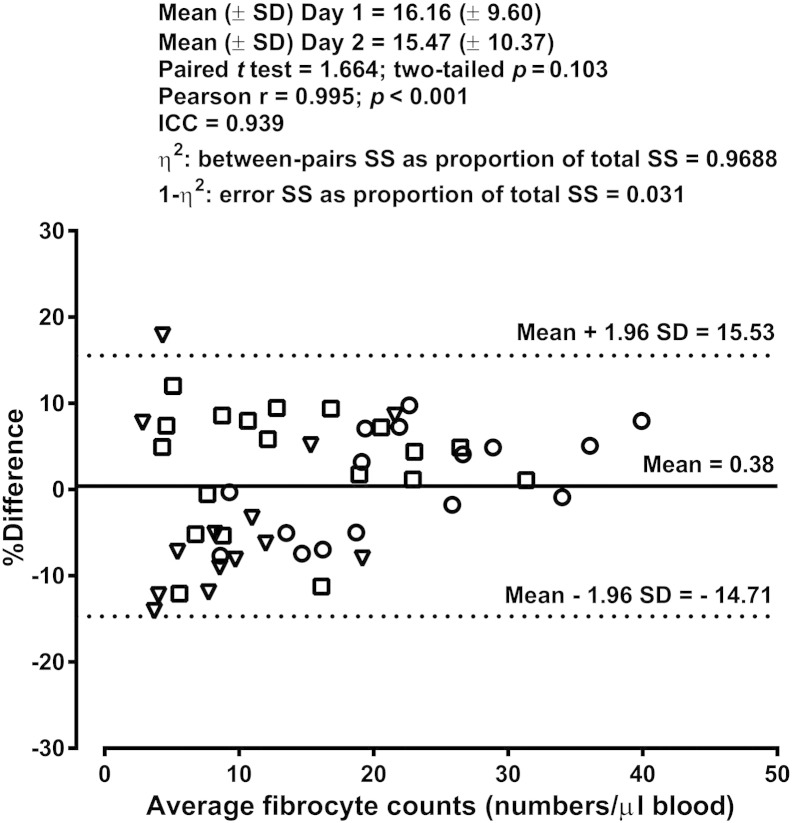
The enumeration of fibrocytes was performed again in consenting subjects at an interval of one week from the first blood sampling. Among the asthmatic patients, those with well-controlled asthma and treatment-resistant disease could be retested because they did not show notable changes in their clinical and functional characteristics. Comparative analysis of the blood fibrocyte counts obtained on the two occasions revealed low variability of the measures over one week (Fig. 5). The ICC represents the ratio of inter-subject variability to total variability and its high value denotes a good reproducibility of the measures. The variation attributable to the procedure was very low (1 − η2 = 0.031), with 96.88% of the total variability reflecting on-average differences among the 49 subjects.
Fig. 5.
Repeatability of the fibrocyte counts over one week in healthy subjects (triangles), patients with controlled asthma (squares) and patients with treatment-resistant asthma (circles). The Bland–Altman plot displays the differences in fibrocyte counts between day 1 and day 2 as a percentage of the average counts versus the average values, with individual % differences, the mean value and the limits of agreement. ICC, intraclass correlation coefficient; SD, standard deviation; SS, sum of squares (from analysis of variance, one way random model).
3.4. Comparison of fibrocyte counts in fresh and stored blood samples
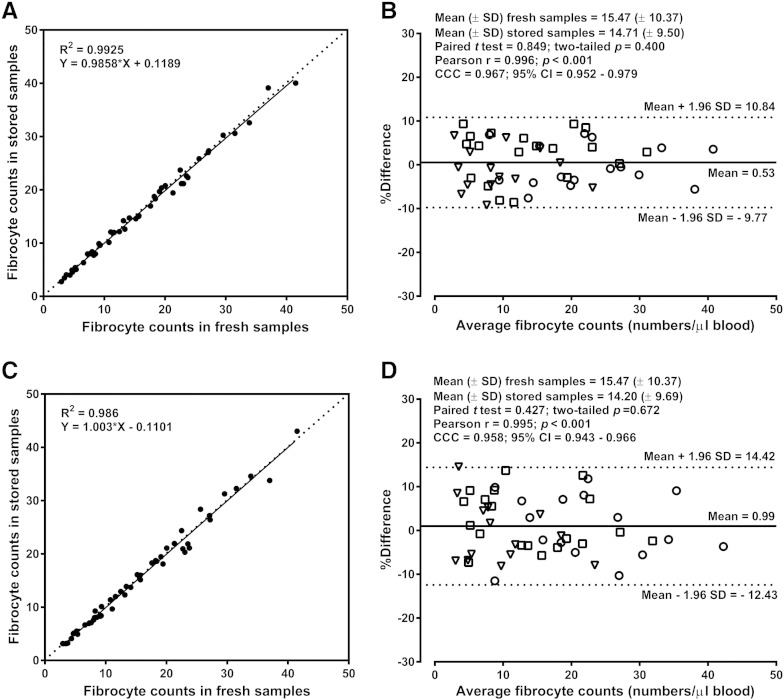
At the time of the second blood sampling in asthmatic patients and healthy controls, an aliquot of each blood sample was treated with the antigen-stabilizing agent and stored for 48 or 96 h at 4 °C before fibrocyte enumeration by using the single-platform assay. Robust between-method comparison by multiple statistical tests demonstrated a good agreement between the fibrocyte counts measured in fresh whole blood and the fibrocyte counts obtained in stored whole blood at 48 and 96 h (Fig. 6A–D).
Fig. 6.
Comparison of fibrocyte counts determined in fresh blood samples and in stabilized samples stored for 48 (A and B) or 96 (C and D) hours before processing. Panels A and C show the results of the linear regression analysis and the dotted line is the line of identity. Panels B and D show the Bland–Altman analysis by groups: healthy subjects (triangles), patients with controlled asthma (squares) and patients with treatment-resistant asthma (circles). The Bland–Altman plot displays the differences between the fibrocyte counts in fresh samples and those in stored samples as a percentage of the average counts versus the average values, with individual % differences, the mean value and the limits of agreement. CCC, concordance correlation coefficient; CI, confidence interval; SD, standard deviation.
4. Discussion
The methods currently used to identify authentic fibrocytes in the peripheral blood and to purify them for functional analyses [9], [10], [14], [16], [17], [18] are costly and time-consuming. Moreover, most of these methods involve the isolation of BMCs, which requires a large volume of venous blood because of the considerable cellular loss. The enumeration of CD34+COL1 mRNA+ fibrocytes on cytospins of blood leukocytes from normal and asthmatic subjects was previously performed to definitely ascertain if authentic fibrocytes are really present in the peripheral blood [10]. Because of the relatively low numbers of circulating fibrocytes, this method cannot be used on a large scale. Initial attempts to quantify fibrocytes in the leukocyte-enriched fraction of the whole blood by staining for intracellular COL1 have raised technical concerns [34]. More recently reported methods involve COL1 staining without previous cell permeabilization [35]. Thus, there is a lack of analytically valid and standardized assays for the enumeration of blood fibrocytes in a clinical setting and in multicenter clinical trials. Moreover, all previously described flow cytometry assays for the enumeration of fibrocytes in BMCs or in the leukocyte-enriched fraction of the whole blood involve the use of a dual-platform technology and therefore provide imprecise absolute cell counts [22].
The assay presented here is based on the single-platform counting technology, which is superior to the dual-platform approach in clinical trials because it greatly reduces the sources of variability, minimizes the risks of inaccuracy and improves inter-laboratory comparisons [22], particularly when the target cells represent a small percentage of the circulating leukocytes [36]. The assay eliminates three additional sources of variability specifically associated with the identification of circulating fibrocytes on the basis of the expression of intracellular COL1 by flow cytometry. Firstly, intracellular staining requires cells fixation and permeabilization and these procedures alter the light-scatter characteristics of the cells, increases autofluorescence and reduces the reactivity of the other fluorochrome-labeled antibodies included in the assay [37]. Secondly, cytoplasmic antigens require careful antibody titration because antibody binding is not saturable, and non-specific binding increases with each increase in antibody concentration [37], [38]. Thirdly, the percentage of circulating CD34+COL1 mRNA+ fibrocytes that express COL1 immunoreactivity is higher in asthmatic than in normal fibrocytes and may change over time [10]. Lastly, the frequency of circulating cells expressing receptors with a collagenous domain increases in some fibrotic conditions and in patients treated with systemic corticosteroids and such cells nonspecifically stain for COL1 [9]. Cell fixation and permeabilization also involve several washing steps that lead to an inevitable cell loss when decanting. Thus, staining for intracellular COL1 precludes a correct application of the single-platform counting method, which is only possible if any cell loss can be avoided before analysis with the use of the lyse-no-wash approach [22].
In the assay described here, the risk of non-specific staining are minimized as much as possible by adopting the following technical strategies [26], [29], [37]: non-viable cells are excluded; the effects of Fcγ receptor-mediated binding of antibodies are eliminated by excluding granulocytes and monocytes; fibrocytes are identified by sequential gating with the use of multiple surface markers; fluorescence-minus-one controls are employed for accurate definition of cells that have fluorescence above the background levels. Because of this strategic approach, isotype controls are not needed. Notably, identical results are obtained in fresh blood assays where the anti-CD16 antibody is omitted because CD16+ nonocytes are however excluded by gating out CD115+ cells and CD16+ neutrophils can be easily recognized on the basis of their high SSC. Nonetheless, the use of this antibody is important for excluding contaminating neutrophils in stabilized and stored whole blood, where slight changes in the SSC of granulocytes are commonly observed [25].
The enumeration of circulating fibrocytes by using the single-platform technology requires only 100 μl of venous blood. The assay is analytically valid because the enumerated cells are authentic fibrocytes, on the basis of their phenotype (CD45+CD34+CD11b+COL1+ cells) and functional characteristics (α-SMA expression in response to stimulation with ET-1). The assay is clinically valid because it is able to distinguish subjects with symptomatic asthma from healthy individuals and to detect significant differences among asthmatic patients on the basis of the level of asthma control and response to treatment. Moreover, the reproducibility of blood fibrocyte counts over one week is high in healthy individuals and asthmatic subjects with stable clinical conditions. The observation that stabilized blood samples can be stored at 4 °C for up to 96 h before analysis by using the single-platform assay is particularly important for the application of the assay in multicenter clinical trials, which require all analyses to be performed at a central laboratory.
In conclusion, this report describes the development and validation of a novel single-platform flow cytometry assay for the enumeration of circulating fibrocytes in the clinical setting and in multicenter clinical trials. The assay can be used to evaluate the utility and validity of blood fibrocyte count as an outcome measure in asthma. The description of a reliable and clinically applicable assay for the enumeration of fibrocytes in the whole blood is also of paramount importance for the conduction of translational research and clinical studies in other inflammatory and immunological disorders associated with tissue fibrosis, where fibrocytes may play a significant pathogenetic role [39].
Disclosures
S. Mattoli is founding shareholder and director of AVAIL GmbH. Her husband is a senior pharmaceutical executive and holds the shares of diverse pharmaceutical corporations and biotechnology industries. The other authors have no conflicts of interest or financial interests to disclose.
Author contributions
All authors made substantial contributions to study conception and design, or data acquisition, or data analysis and interpretation; drafting or critically revising the manuscript; final approval of the submitted version.
Funding sources
Funding sources were from DreiRosen Pharma (Research Fellowship to L.B.) and the international research projects FibroGENE (IRP 261009) and CellNet (IRP 210610).
Contributor Information
Lorenza Bianchetti, Email: lbianchetti@avail-research.com.
Mirko Isgrò, Email: misgro@avail-research.com.
Maurizio A. Marini, Email: mam.marini@yahoo.com.
Alberto Bellini, Email: abellini@avail-research.com.
Matthias Schmidt, Email: mschmidt@avail-research.com.
Sabrina Mattoli, Email: smattoli@avail-research.com.
References
- 1.Global Initiative for Asthma (GINA) Global strategy for asthma management and prevention. http://www.ginasthma.com Updated 2012, Available at: (last accessed on 06 March 2013)
- 2.Reddel H.K., Taylor D.R., Bateman E.D. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations. Standardizing endpoints for clinical asthma trials and clinical practice. Am. J. Respir. Crit. Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 3.Jia C.E., Zhang H.P., Lv Y. The Asthma Control Test and Asthma Control Questionnaire for assessing asthma control: systematic review and meta-analysis. J. Allergy Clin. Immunol. 2013;131:695–703. doi: 10.1016/j.jaci.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Hastie A.T., Moore W.C., Li H. Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J. Allergy Clin. Immunol. 2013;132:72–80. doi: 10.1016/j.jaci.2013.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bucala R., Spiegel L.A., Chesney J., Hogan M., Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol. Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 6.Chesney J., Metz C., Stavitsky A.B., Bacher M., Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J. Immunol. 1998;160:419–425. [PubMed] [Google Scholar]
- 7.Abe R., Donnelly S.C., Peng T., Bucala R., Metz C.N. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J. Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 8.Chesney J., Bacher M., Bender A., Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc. Natl. Acad. Sci. U. S. A. 1997;94:6307–6312. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isgrò M., Bianchetti L., Marini M.A., Mattoli S. Involvement of fibrocytes in allergen-induced T cell responses and rhinovirus infections in asthma. Biochem. Biophys. Res. Commun. 2013;437:446–451. doi: 10.1016/j.bbrc.2013.06.099. [DOI] [PubMed] [Google Scholar]
- 10.Bellini A., Marini M.A., Bianchetti L., Barczyk M., Schmidt M., Mattoli S. Interleukin (IL)-4, IL-13, and IL-17A differentially affect the profibrotic and proinflammatory functions of fibrocytes from asthmatic patients. Mucosal Immunol. 2012;5:140–149. doi: 10.1038/mi.2011.60. [DOI] [PubMed] [Google Scholar]
- 11.Bianchetti L., Barczyk M., Cardoso J., Schmidt M., Bellini A., Mattoli S. Extracellular matrix remodelling properties of human fibrocytes. J. Cell. Mol. Med. 2012;16:483–495. doi: 10.1111/j.1582-4934.2011.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt M., Sun G., Stacey M.A., Mori L., Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J. Immunol. 2003;170:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 13.Nihlberg K., Larsen K., Hultgårdh-Nilsson A., Malmström A., Bjermer L., Westergren-Thorsson G. Tissue fibrocytes in patients with mild asthma: a possible link to thickness of reticular basement membrane? Respir. Res. 2006;7:50. doi: 10.1186/1465-9921-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saunders R., Siddiqui S., Kaur D. Fibrocyte localization to the airway smooth muscle is a feature of asthma. J. Allergy Clin. Immunol. 2009;123:376–384. doi: 10.1016/j.jaci.2008.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellini A., Schmidt M., Mattoli S. Interactions between the bronchial epithelium and fibrocytes in the pathogenesis of airway remodeling in asthma. J. Epithelial Biol. Pharmacol. 2013;6:1–10. [Google Scholar]
- 16.Bianchetti L., Marini M.A., Isgrò M., Bellini A., Schmidt M., Mattoli S. IL-33 promotes the migration and proliferation of circulating fibrocytes from patients with allergen-exacerbated asthma. Biochem. Biophys. Res. Commun. 2012;426:116–121. doi: 10.1016/j.bbrc.2012.08.047. [DOI] [PubMed] [Google Scholar]
- 17.Wang C.H., Huang C.D., Lin H.C. Increased circulating fibrocytes in asthma with chronic airflow obstruction. Am. J. Respir. Crit. Care Med. 2008;178:583–591. doi: 10.1164/rccm.200710-1557OC. [DOI] [PubMed] [Google Scholar]
- 18.Isgrò M., Bianchetti L., Marini M.A., Bellini A., Schmidt M., Mattoli S. The C—C motif chemokine ligands CCL5, CCL11, and CCL24 induce the migration of circulating fibrocytes from patients with severe asthma. Mucosal Immunol. 2013;6:718–727. doi: 10.1038/mi.2012.109. [DOI] [PubMed] [Google Scholar]
- 19.Ishida Y., Kimura A., Kondo T. Essential roles of the CC chemokine ligand 3-CC chemokine receptor 5 axis in bleomycin-induced pulmonary fibrosis through regulation of macrophages and fibrocyte infiltration. Am. J. Pathol. 2007;170:843–854. doi: 10.2353/ajpath.2007.051213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szefler S.J., Wenzel S., Brown R. Asthma outcomes: biomarkers. J. Allergy Clin. Immunol. 2012;129:S9–S23. doi: 10.1016/j.jaci.2011.12.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faucher J.L., Lacronique-Gazaille C., Frébet E. ʺ6 markers/5 colorsʺ extended white blood cell differential by flow cytometry. Cytometry. 2007;A71:934–944. doi: 10.1002/cyto.a.20457. [DOI] [PubMed] [Google Scholar]
- 22.Brando B., Barnett D., Janossy G. Cytofluorometric methods for assessing absolute numbers of cell subsets in blood. European Working Group on clinical cell analysis. Cytometry. 2000;42:327–346. doi: 10.1002/1097-0320(20001215)42:6<327::aid-cyto1000>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 23.Mandy F.F., Nicholson J.K., McDougal J.S., CDC Guidelines for performing single-platform absolute CD4 + T-cell determinations with CD45 gating for persons infected with human immunodeficiency virus. Centers for disease control and prevention. MMWR Recomm. Rep. 2003;52(RR-2):1–13. [PubMed] [Google Scholar]
- 24.Bergeron M., Shafaie A., Ding T. Evaluation of stabilized blood cell products as candidate preparation for quality assessment programs for CD4 T-cell counting. Cytometry. 2002;50:86–91. doi: 10.1002/cyto.10090. [DOI] [PubMed] [Google Scholar]
- 25.Canonico B., Betti M., Luchetti F. Flow cytometry profiles, biomolecular and morphological aspects of transfixed leukocytes and red cells. Cytometry B Clin. Cytom. 2010;78:267–278. doi: 10.1002/cyto.b.20510. [DOI] [PubMed] [Google Scholar]
- 26.Perfetto S.P., Chattopadhyay P.K., Roederer M. Seventeen-colour flow cytometry: unravelling the immune system. Nat. Rev. Immunol. 2004;4:648–655. doi: 10.1038/nri1416. [DOI] [PubMed] [Google Scholar]
- 27.Autissier P., Soulas C., Burdo T.H., Williams K.C. Evaluation of a 12-color flow cytometry panel to study lymphocyte, monocyte and dendritic cell subsets in humans. Cytometry. 2010;77A:410–419. doi: 10.1002/cyto.a.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ingersoll M.A., Spanbroek R., Lottaz C. Comparison of gene expression profile between human and mouse monocyte subsets. Blood. 2010;115:e10–e19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bender J.G., Unverzagt K.L., Walker D.E. Identification and comparison of CD34-positive cells and their subpopulations from normal peripheral blood and bone marrow using multicolour flow cytometry. Blood. 1991;77:2591–2596. [PubMed] [Google Scholar]
- 30.Dzionek A., Fuchs A., Schmidt P. BDCA-2, BDCA-3, and BDC-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 31.Shoukri M.M. Second ed. Chapman & Hall/CRC Press; Boca Raton, FL: 2011. Measures of Interobserver Agreement and Reliability. [Google Scholar]
- 32.Bland J.M., Altman D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 33.Weng C.M., Chen B.C., Wang C.H. The endothelin A receptor mediates fibrocyte differentiation in chronic obstructive asthma. The involvement of connective tissue growth factor. Am. J. Respir. Crit. Care Med. 2013;188:298–308. doi: 10.1164/rccm.201301-0132OC. [DOI] [PubMed] [Google Scholar]
- 34.Bournazos S., Fahim A., Hart S.P. Identification of fibrocytes in peripheral blood. Am. J. Respir. Crit. Care Med. 2009;180:1279. doi: 10.1164/ajrccm.180.12.1279. [DOI] [PubMed] [Google Scholar]
- 35.Fang L., Moore X.L., Chan W., White D.A., Chin-Dusting J., Dart A.M. Decreased fibrocyte number is associated with atherosclerotic plaque instability in man. Cardiovasc. Res. 2012;95:124–133. doi: 10.1093/cvr/cvs156. [DOI] [PubMed] [Google Scholar]
- 36.Levering W.H.B.M., Preijers F.W.M.B., van Wieringen W.N. Flow cytometric CD34+ stem cell enumeration: lesson from nine years' external quality assessment within the Benelux countries. Cytometry. 2007;72B:178–188. doi: 10.1002/cyto.b.20351. [DOI] [PubMed] [Google Scholar]
- 37.Wood B. 9-Color and 10-color flow cytometry in the clinical laboratory. Arch. Pathol. Lab. Med. 2006;130:680–690. doi: 10.5858/2006-130-680-CACFCI. [DOI] [PubMed] [Google Scholar]
- 38.Lazarus A.H., Ellis J., Blanchette V., Freedman J., Sheng-Tanner X. Permeabilization and fixation conditions for intracellular flow cytometry detection of the T-cell receptor ζ chain and other intracellular proteins in lymphocyte subpopulations. Cytometry. 1998;32:206–213. doi: 10.1002/(sici)1097-0320(19980701)32:3<206::aid-cyto7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 39.Galligan C.L., Fish E.N. The role of circulating fibrocytes in inflammation and autoimmunity. J. Leukoc. Biol. 2013;93:45–50. doi: 10.1189/jlb.0712365. [DOI] [PubMed] [Google Scholar]