Abstract
Background
Serum profiling using mass spectrometry-based proteomic techniques has great potential to detect biomarkers that might improve the management for advanced breast cancer patients. The albuminome has previously been investigated as a tool in biomarker discovery, however other high abundant blood proteins are also likely to sequester potentially interesting molecules.
Methods
Affinity resin purified and isolated Transferrin and associated bound proteins from normal control and breast cancer patient serum samples were analysed by label-free mass spectrometry during the discovery phase.
Results
21 significant proteins were identified with Fibrinogen and Fibronectin selected for further analysis in an independent sample set, with significant difference found when comparing the controls groups (normal healthy control, inflammatory bowel disease and benign breast disease) to stage IV breast cancer.
Conclusions
The area under the curve value for Fibrinogen compared favourably with cancer antigen 15-3, an established breast cancer tumour marker. A combination of all three biomarkers improved accuracy when comparing control/benign to stage IV breast cancer patient groups.
General significance
Mass spectrometry profiling of Transferrin-bound proteins has revealed serum proteins that can distinguish between serum from advanced breast cancer patients and healthy control subjects with high confidence.
Keywords: Biomarkers, Breast cancer, Mass spectrometry, Fibronectin, Fibrinogen, Transferrin
Highlights
-
•
Transferrin was found to interact with many candidate biomarkers.
-
•
21 significant proteins were identified bound to Transferrin.
-
•
Many of these proteins are associated with the complement and coagulations systems.
-
•
Fibrinogen and Fibronectin were found to have significant AUC values.
-
•
High abundant proteins harbour potential diagnostic molecules.
1. Introduction
Breast cancer is a very common disease that affects approximately 1 in 8 women at some point in their lives [1]. Breast cancer is commonly treated by various combinations of surgery, radiation therapy, chemotherapy, and hormone therapy with prognosis and selection of therapy influenced by clinical and pathology features [2]. In recent years, there has been a significant improvement in the understanding of molecular events and critical pathways involved in breast cancer. This has led to the identification of novel targets and development of anticancer therapies referred to as targeted therapy [3], [4].
In breast cancer care, three tumour markers, cancer antigen 15-3 (CA 15-3), cancer antigen 27.29 (CA 27.29), and carcinoembryonic antigen (CEA) have been used to help monitor advanced disease [5], [6], [7]. However, like most circulating protein biomarkers, they suffer from sub-optimum performance and more accurate molecules are needed, alone or in combination with existing biomarkers [8]. Therefore, the improved ability to monitor malignancy in the blood from breast cancer patients using biomarkers may be helpful for determining response to treatments in the future.
The discovery of clinically relevant cancer biomarkers using mass spectrometry (MS)-based proteomics has proven difficult, primarily because of the enormous dynamic range of blood-derived protein concentrations and the fact that the 22 most abundant blood-derived proteins constitute approximately 99% of the total serum protein mass [9]. While most strategies focus on removing or diluting such high abundant proteins, some studies have evaluated this protein group as a potential source of diagnostic material, with a particular emphasis on proteins, protein fragments or peptides that interact with this high abundance group [10].
Previous work performed on the analysis of high abundant protein cargo has focused on Albumin, as this protein accounts for more than 60% of the total serum proteins. Albumin has been found to bind many drugs, proteins, hormones and other chemicals, with many of these likely to be released directly from diseased tissue [11], [12], [13]. High abundant proteins sequestering other cargo proteins help to increase their abundance and at the same time protecting them from being eliminated by the body, therefore facilitating their detection in biomarker discovery analysis.
Transferrin, likely Albumin, is a high abundant serum protein and is the major iron-binding protein, having a vital role in iron transport. Additionally, Transferrin has many other uses including antimicrobial functions and growth factor effects on mammalian cell proliferation and differentiation [14]. This investigation centres on the analysis of Transferrin-bound proteins from control and breast cancer patients using resins to capture human serum Transferrin from blood samples. Transferrin-bound proteins/peptides isolated using this method were evaluated using label-free proteomic methods to identify differentially expressed between control and breast cancer samples. A number of candidate biomarkers were identified, with many of these found to be involved in the complement and coagulation cascades. Fibronectin and fibrinogen were selected for further analysis in a larger cohort of patient samples along with CA15-3, an established biomarker in breast cancer. All these molecules were found to be significantly altered in patient serum samples highlighting the usefulness of analysing the high-abundant fraction associated proteins.
2. Materials & methods
2.1. Patient information and sample collection
Samples were collected through a collaborative project involving St Vincent's University Hospital, Dublin 4, Ireland, coordinated through ICORG (the All Ireland Co-operative Oncology Research Group, www.icorg.ie). The samples were collected according to standard phlebotomy procedures from consented patients. Ethical consent was granted from each of the respective Hospital Ethics Committees. A total of 10 ml of blood was collected into additive free (serum) blood tubes and was allowed to clot for 30 min to 1 h at room temperature. The serum was denuded by pipette from the clot and poured into a clean tube. The tubes were centrifuged at 1000 ×g for 30 min at 4 °C. Serum was aliquoted in the cryovial tubes, labelled and stored at − 80 °C until time of analysis. The time from sample procurement to storage at − 80 °C was less than 3 h. Each serum sample underwent not more than three freeze/thaw cycles prior to analysis. Table 1 provides a summary of the total number of patients analysed and a breakdown of their clinicopathologic parameters.
Table 1.
Clinicopathologic features of the normal healthy control and breast cancer patient samples used for proteomic analysis of Transferrin-associated proteins (discovery set) with subsequent analysis of significant proteins in larger numbers (validation set).
| Discovery Set | n | Validation Set | n |
|---|---|---|---|
| Normal healthy control | Normal healthy control | ||
| No. cases | 10 | No. cases | 54 |
| Age (average ± standard deviation) | 51 ± 11 | Age (average ± standard deviation) | 52 ± 9 |
| Breast cancer | Inflammatory bowel disease | ||
| No. cases | 10 | No. cases | 19 |
| Age (average ± standard deviation) | 56 ± 10 | Age (average ± standard deviation) | 44 ± 8 |
| Tumour stage | |||
| IV | 10 | Benign breast disease | |
| Type | No. cases | 12 | |
| Invasive lobular carcinoma | 2 | Age (average ± standard deviation) | 43 ± 20 |
| Invasive ductal carcinoma | 8 | ||
| Breast cancer | |||
| No. cases | 155 | ||
| Age (average ± standard deviation) | 55 ± 12 | ||
| Tumour stage | |||
| I | 20 | ||
| II | 20 | ||
| III | 39 | ||
| IV | 76 | ||
| Type | |||
| Invasive lobular carcinoma | 16 | ||
| Invasive ductal carcinoma | 139 |
2.2. Transferrin isolation
CaptureSelect™ Transferrin Affinity Matrix (Life Technologies Corporation, USA) was used for the purification and isolation of Transferrin from normal control and breast cancer patient serum samples according to the manufacturer's protocol.
2.3. 1D gel electrophoresis
Protein quantification was performed using the Quick Start Bradford Protein Assay (Biorad) using bovine serum albumin as a protein standard. 20 μg of protein was mixed with reducing buffer 1:1 (50 mM Tris–HCl pH 6.8; 2% SDS; 10% glycerol; 1% β-mercaptoethanol; 12.5 mM EDTA; 0.02% bromophenol blue) and loaded onto NuPAGE® 12% Bis–Tris Gels (Invitrogen) and electrophoretically separated using a MOS/SDS buffer according to the manufacturer's instructions and stained with Coomassie Brilliant Blue R-250.
2.4. Protein digestion
Isolated Transferrin and bound proteins were precipitated using ice-cold acetone and resuspended in 8 M Urea with vortexing, sonication and centrifugation. Volumes and the concentration of protein suspensions were equalized and then reduced for 30 min with 10 mM DTT and alkylated for 20 min in the dark with 25 mM iodoacetamide in 50 mM ammonium bicarbonate. The proteolytic digestion of proteins was carried out in 2 steps. Firstly, digestion was performed with sequencing grade Lys-C at a ratio of 1:100 (protease/protein) for 4 h at 37 °C, followed by dilution with 4 times the initial sample volume in 50 mM ammonium bicarbonate. Secondly, further digestion was based on incubation with sequencing grade trypsin at a ratio of 1:25 (protease/protein) overnight at 37 °C. The protease-treated serum protein suspensions were diluted 3:1 (v/v) with 2% trifluoroacetic acid in 20% acetonitrile to stop the reaction and subject to C18 clean-up prior to mass spectrometry.
2.5. Label-free LC–MS/MS analysis
The nano LC–MS/MS analysis of normal control and breast cancer patient samples was carried out on an Ultimate 3000 nanoLC system (Dionex UK, Camberley, United Kingdom) coupled to an LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific, Dublin, Ireland) in the Proteomics Facility of the National Institute for Cellular Biotechnology, Dublin City University. The MS apparatus was operated in data-dependent mode and externally calibrated. Survey MS scans were acquired in the Orbitrap in the 300–2000 m/z range with the resolution set to a value of 30 000 at m/z 400 and lock mass set to 445.120025 u. Processing of the raw data generated from LC–MS/MS analysis was carried out with Progenesis label-free LC–MS software (version 3.1; Non-Linear Dynamics, Newcastle upon Tyne, UK). The retention times of all of the other runs were aligned to this reference run and peak intensities were then normalised. The following criteria were applied to assign a serum associated proteins as confidently identified: (i) an ANOVA score between experimental groups of ≤ 0.05, (ii) proteins with ≥ 1 peptides matched and (iii) a MASCOT score > 40.
2.6. ELISA analysis
ELISA kits for Fibrinogen (EF1040-1) and for Fibronectin (EF1045-1) from Assaypro were used as per manufacturer's instructions (AssayPro, Winfield, MO, USA). CA15-3 was assayed using an ELISA kit from Abcam (ab108633, Abcam UK). Standard curves were prepared for each ELISA kit and after completion of each assay, plates were read to determine the optical density (absorbance) of each well by reading the plate with the microplate reader (Bio-Tek instruments Inc., Vermont, USA) set to 450 nm with a wavelength correction set to 570 nm. Duplicate measurements were performed for each sample.
2.7. Statistical analysis
Student t-test was used to identify statistically significant changes in abundance levels for specific proteins between normal control and breast cancer patient serum samples. The area under the curve (AUC) was calculated to provide a summary of overall classifier effectiveness (MedCalc, version 13-0-0-0 64-bit, Medcalc Software, Mariakerke, Belgium). For multivariate analysis of biomarker combinations, logistic regression (LR) analysis of the serum biomarker levels in these patients groups was performed.
3. Results
3.1. Proteomic profiling
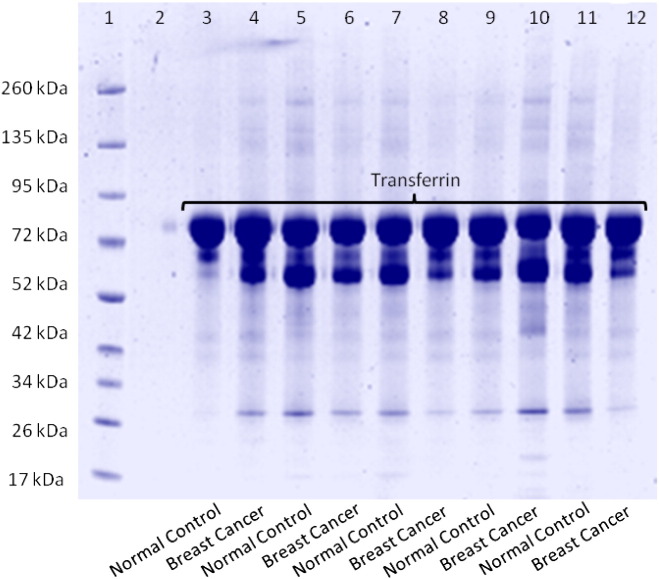
Prior to label-free MS analysis, serum samples from normal control and advanced breast cancer patients were used as biofluids to isolate Transferrin from using the CaptureSelect™ affinity resin specifically designed to capture Transferrin from complex samples such as serum (Table 1 — Discovery set). Analysis of this fraction was visualised by 1D-gel electrophoresis and Coomassie blue staining of lane 1 — molecular weight standards, lanes 3–7 Transferrin and Transferrin-bound proteins from normal control serum samples and lanes 8–12 Transferrin and Transferrin-bound proteins from advanced breast cancer patient serum samples (Fig. 1). The staining pattern reveals an abundant protein at approximately 80 kDa, subsequently identified as Transferrin by mass spectrometry (Mascot score: 341). Other noticeably weaker stained bands are also visible, most likely representing proteins found to be bound to Transferrin.
Fig. 1.
SDS-PAGE analysis of Transferrin-associated proteins. Samples were electrophoretically separated using a gradient (10–20%) polyacrylamide slab gel under reducing conditions with ß-mercaptoethanol. Aliquots of 20 μg were loaded and the gel was stained with Coomassie blue. Lane 1 — molecular weight standards; 3–12 Transferrin and Transferrin-associated proteins from normal healthy control and breast cancer patient serum samples. The most intense band at approximately 80 kDa was cut from the gel, digested and analysed by mass spectrometry. This band was identified as Transferrin (Mascot score 341).
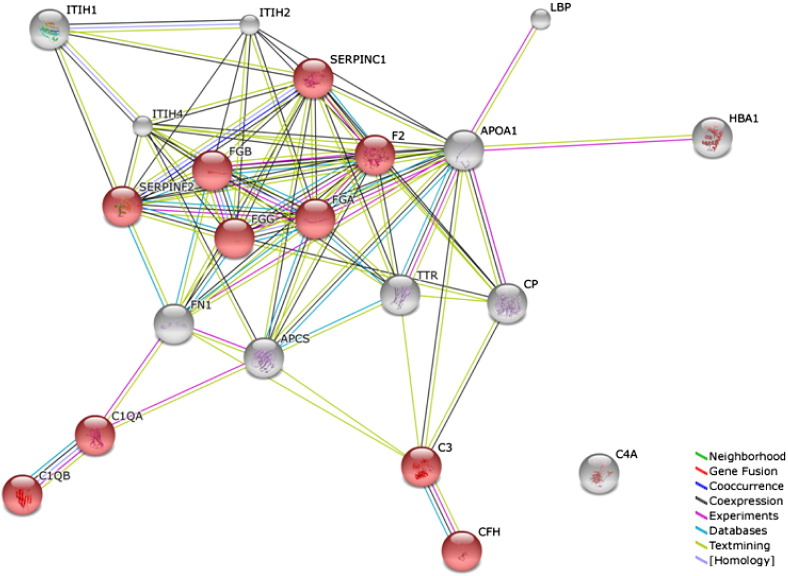
Affinity resin isolated Transferrin and associated bound proteins were analysed by label-free MS analysis, an established powerful method for peptide/protein quantitation. Progenesis LC–MS software was used to compare normal control serum samples to advanced breast cancer patient samples. Proteins that were only identified by a single peptide (the so-called “one-hit wonders”) were included in the protein list of Transferrin-bound proteins (Table 2). STRING analysis was performed to identify protein interactions and associations between the proteins of interest [15], [16], with KEGG analysis used to detect enrichment of functional systems for this network in STRING (Fig. 2). Of particular interest from this protein analysis was the number of protein involved in the complement and coagulation cascades, including FGG, FGA, FGB, SERPINC1, C1QA, C1QB, C3, CFH, F2 and SERPINF2. Of these only 2, F2 and SERPINF2 were found to have an elevated abundance level in normal control individual serum samples compared to advanced breast cancer patients.
Table 2.
List of proteins found to be differentially expressed at statistically significant levels (p ≤ 0.05, fold change ≥ 2) when comparing Transferrin-associated proteins in normal control (n = 10) to stage IV breast cancer (n = 10) patient serum samples (Discovery sample set). Included in the table is the gene symbol count, c, protein identification, peptide confidence score (Mascot), p-value, fold-change and highest/lowest mean change.
| Gene name | Protein identification | Peptide count | Confidence score | Anova (p) | Fold change | Highest mean condition | Lowest mean condition |
|---|---|---|---|---|---|---|---|
| FGG | Fibrinogen gamma chain | 4 | 212 | 0.0004 | 4.88 | Breast cancer | Normal control |
| FGA | Fibrinogen alpha chain | 3 | 177 | 0.0006 | 2.26 | Breast cancer | Normal control |
| FGB | Fibrinogen beta chain | 5 | 350 | 0.0007 | 2.75 | Breast cancer | Normal control |
| FN1 | Fibronectin | 7 | 379 | 0.0007 | 3.24 | Breast cancer | Normal control |
| APCS | Serum amyloid P-component | 1 | 63 | 0.0017 | 3.19 | Breast cancer | Normal control |
| SERPINC1 | Antithrombin-III | 1 | 44 | 0.0064 | 2.59 | Breast cancer | Normal control |
| C1QA | Complement C1q subcomponent subunit A | 1 | 67 | 0.0066 | 2.93 | Breast cancer | Normal control |
| C4A | Complement C4-A | 1 | 84 | 0.0102 | 3.91 | Breast cancer | Normal control |
| C1QB | Complement C1q subcomponent subunit B | 1 | 99 | 0.0111 | 3.40 | Breast cancer | Normal control |
| C3 | Complement C3 | 5 | 358 | 0.0127 | 3.17 | Breast cancer | Normal control |
| CFH | Complement factor H | 1 | 47 | 0.0169 | 4.03 | Breast cancer | Normal control |
| ITIH1 | Inter-alpha-trypsin inhibitor heavy chain H1 | 2 | 135 | 0.0170 | 3.07 | Breast cancer | Normal control |
| ITIH4 | Inter-alpha-trypsin inhibitor heavy chain H4 | 1 | 83 | 0.0201 | 2.03 | Normal control | Breast cancer |
| TTR | Transthyretin | 1 | 65 | 0.0254 | 3.10 | Normal control | Breast cancer |
| F2 | Prothrombin | 1 | 58 | 0.0317 | 4.07 | Normal control | Breast cancer |
| LBP | Lipopolysaccharide-binding protein | 1 | 47 | 0.0336 | 10.45 | Breast cancer | Normal control |
| SERPINF2 | Alpha-2-antiplasmin | 1 | 45 | 0.0350 | 2.95 | Normal control | Breast cancer |
| HBA1 | Hemoglobin subunit alpha | 1 | 52 | 0.0351 | 9.81 | Normal control | Breast cancer |
| ITIH2 | Inter-alpha-trypsin inhibitor heavy chain H2 | 1 | 58 | 0.0363 | 4.20 | Breast cancer | Normal control |
| CP | Ceruloplasmin | 1 | 43 | 0.0387 | 2.51 | Normal control | Breast cancer |
| APOA1 | Apolipoprotein A-I | 1 | 45 | 0.0413 | 2.27 | Normal control | Breast cancer |
Fig. 2.
STRING analysis of 21 differentially expressed Transferrin-associated proteins listed in Table 2. The STRING programme generates functional protein association networks. This evidence view uses different-coloured lines to depict the type of evidence that supports each interaction. Highlighted in red are proteins involved in the complement and coagulation cascades with the majority of these found to have higher levels in breast cancer patient samples.
3.2. ELISA analysis of Fibrinogen, Fibronectin and CA15-3
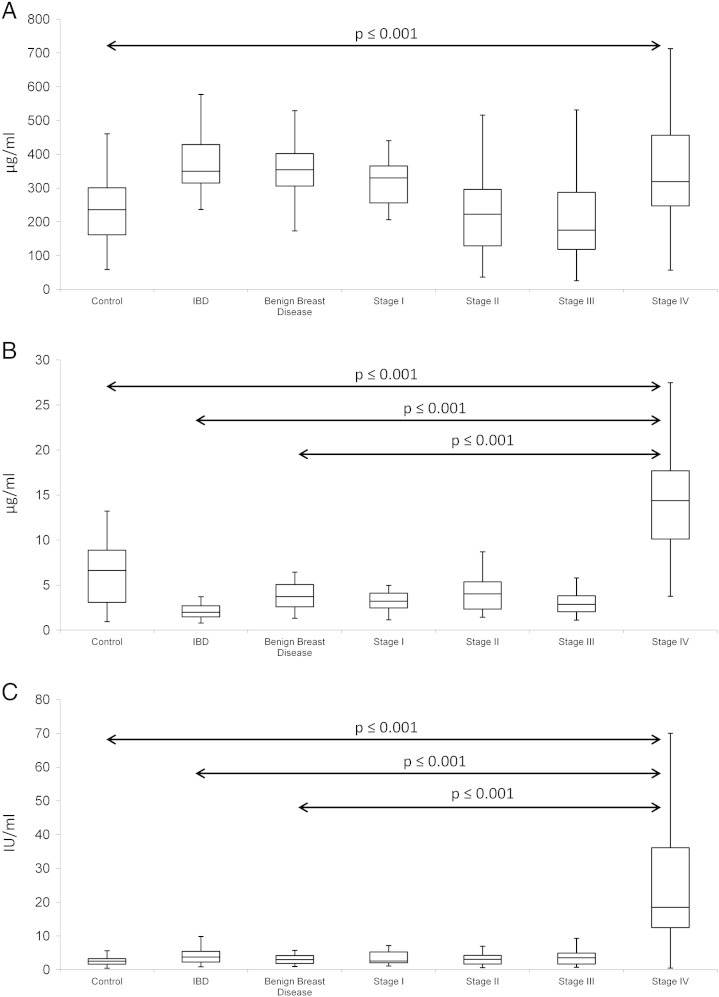
ELISA analysis of Fibrinogen, Fibronectin and CA15-3 in serum samples was performed in normal healthy control (n = 54), inflammatory bowel disease (n = 19), benign breast disease (n = 12), stage I breast cancer (n = 20), stage II breast cancer (n = 20), stage III breast cancer (n = 39) and stage IV breast cancer (n = 76) — (Table 1 — Validation set). Fibrinogen and Fibronectin were selected from the protein list for further analysis as these two molecules were determined to be the most statistically significant. CA15-3 was included at this validation stage as a reference because of the established utility this biomarker has in the management of advanced breast cancer patients. Box-and-whisker plot were constructed from this ELISA data showing the median and interquartile range (Fig. 3). Any outliers present in ELISA readings were determined using Tukey's method which considers values at a distance of 1.5 times the interquartile range (IQR) below Q1 (quartile one) or at 1.5 times the IQR above Q3 (quartile three) to be a possible outlier. Identified outliers were subsequently removed from the analysis.
Fig. 3.
Box and whisker plots (range, median, quartiles) of A. Fibronectin, B. Fibrinogen and C. CA15-3 in control, IBD (Inflammatory Bowel Disease), benign breast disease and stages I–IV breast cancer patient serum samples (validation sample set). Fibrinogen, Fibronectin and CA15-3 were all found to be statistically significant when comparing abundance levels between normal control and stage IV breast cancer patient serum samples. Both Fibrinogen and CA15-3 were also statistically significant comparing IBD or benign breast disease to stage IV breast cancer. Samples: normal healthy control (n = 54), inflammatory bowel disease (n = 19), benign breast disease (n = 12), stage I breast cancer (n = 20), stage II breast cancer (n = 20), stage III breast cancer (n = 39) and stage IV breast cancer (n = 76).
Fibrinogen was discovered to be statistically significant when comparing control (6.62 μg/ml), inflammatory bowel disease (1.98 μg/ml) and benign breast disease (3.71 μg/ml) to stage IV breast cancer (14.37 μg/ml). Fibronectin was significant when comparing abundance levels in control samples (235.95 μg/ml) to stage IV breast cancer (319.08 μg/ml). Similar to Fibrinogen, CA15-3 was statistically significant when comparing control (2.51 IU/ml), inflammatory bowel disease (3.76 IU/ml) and benign breast disease (3.00 IU/ml) to stage IV breast cancer (18.47 IU/ml) — (see Supplementary information).
Area Under the receiver operator Curve (AUC) value was also calculated for these three biomarkers and found to be 0.87, 0.72 and 0.93 for Fibrinogen, Fibronectin and CA15-3 respectively when comparing control to stage IV breast cancer; 1.0, 0.57 and 0.91 when comparing IBD to stage IV breast cancer; 0.95, 0.56 and 0.92 when comparing benign breast disease to stage IV breast cancer. Using logistic regression analysis to investigate the combination of all 3 biomarkers together, AUC-values of 0.98 (control v stage IV), 1.0 (IBD v stage IV) and 1.0 (benign breast disease v stage IV) were calculated.
4. Discussion
Albumin-bound peptides and proteins can yield important data for disease diagnosis and management, however, to our knowledge, this is the first investigation focused on identifying Transferrin-bound peptides and proteins as potential biomarkers. Transferrin consists of a polypeptide chain containing 679 amino acids, with alpha helices and beta sheets forming two domains. Transferrin is perfectly structured to perform its primary role of iron transport, although in this investigation, interactions with other peptides and proteins were investigated. Affinity resin isolated Transferrin and bound proteins were analysed by label-free mass spectrometry with 21 differentially expressed proteins identified during the discovery phase (n = 10 control v n = 10 advanced breast cancer). From this group, Fibrinogen and Fibronectin were selected for further analysis using ELISAs in larger numbers of clinical samples (normal healthy control (n = 54), inflammatory bowel disease (n = 19), benign breast disease (n = 12), stage I breast cancer (n = 20), stage II breast cancer (n = 20), stage III breast cancer (n = 39) and stage IV breast cancer (n = 76). Both these molecules were found to have significant alterations in their abundance levels in stage IV breast cancer patients' serum samples. CA15-3 was also included in the ELISA analysis, as this is a benchmark biomarker to compare AUC values to.
Fibrinogen and CA15-3 were found to have similar p-values and AUC-values for distinguishing stage IV breast cancer from the control, IBD and benign breast disease groups. IBD and benign breast disease groups (serum samples were from individuals diagnosed with fibroadenoma of the breast, fibrocystic mastopathy of the breast, fibrocystic change with fibroadenoma of the breast, duct papillomas of the breast or atypical ductal hyperplasia of the breast) were included as controls for inflammation/immunological reactions associated with cancer at all stages.
Fibronectin was found to be significant when comparing just the control group to stage IV breast cancer. None of these biomarkers were found to be significant when analysing the earlier stage breast cancer groups and it is evident that only the advanced breast cancer group was distinguishable from the other groups using these biomarkers. Using logistic regression analysis to look at the combination of the 3 biomarkers together, in all three comparisons (control v stage IV; IBD v stage IV and benign breast disease v stage IV), the panel was found to be more accurate in terms of AUC-values as opposed to the individual values.
Tumour biomarkers are sporadically used in the management of patients with breast cancer, for example for monitoring patients during therapy for metastatic disease, this molecule may serve as companion diagnostics to imaging and physical examination. CA15-3 has previously been found to increase in 75% of breast cancer patients with progressive disease, however with new treatment options available, the ability to monitor this cohort using reliable protein marker is important [17]. Treatment of metastatic breast cancer has changed with the availability of new cytotoxics and targeted therapies. This has contributed to a noticeable improvement in both survival and patients' quality of life [18], [19], [20]. Recent data by Hashim demonstrates that higher levels of CA15-3 could be considered a reliable prognostic biomarker for advanced stages and recurrent breast cancer as well as being associated to HER2/neu positivity in breast cancer patients [21].
Reproducible, safe and cost-effective protein-based predictors of response to these new treatment options are needed to avoid unnecessary and toxic therapies and identify suitable regimes. While circulating tumour cells (CTCs) have shown promise in this area, the ability to quickly and reliably detect the levels of specific proteins in patient blood samples makes this approach attractive over other strategies [22].
Fibrinogen is a 350-KDa glycoprotein synthesised mainly by liver epithelium, with an important role in the coagulation system. Fibrinogen is involved in cancer cell growth, progression and metastasis, with elevated levels found in a number of cancer types [23], [24], [25]. In this investigation, Fibrinogen alpha chain, beta chain and gamma chain were all found to be significantly elevated in advanced breast cancer, emphasising its role in cancer growth and development. Discovery phase analysis identified Fibrinogen chains as bound to Transferrin, indicating that many of these high abundant proteins may form complex with each other in the blood. Deposits of Fibrinogen in the stroma is considered a hallmark of breast cancer, and the ability of cancer cells to synthesise and secrete Fibrinogen may contribute to elevated levels found in the circulation, especially in patients with a large tumour burden [26].
Fibronectin is a multifunctional, extracellular matrix glycoprotein composed of two nearly identical disulfidebound polypeptides of molecular weight 220 kDa with functions including cell adhesion, cell migration and cell proliferation. Elevated levels of Fibronectin have been reported in patients diagnosed with gastrointestinal or head and neck cancer [27]. Saito and co-workers found that serum Fibronectin levels were significantly higher in the CRC patients than in the controls [28]. Williams and colleagues reported finding supporting a link between fibronectin expression and epithelial cell growth during development and oncogenesis in the mammary gland [29]. During tumorigenesis in the breast, primary tumours are characterized by increased deposition of fibronectin, similar to Fibrinogen, again potentially contributing to elevated circulatory levels [30]. The results presented in this study show a significant increase (~ 3-fold) in advanced breast cancer.
A significant proportion of the proteins identified as differentially expressed from the Transferrin-bound fraction are known to be involved in the complement and coagulation cascades. Cancer cells can directly secrete procoagulant and tissue factor, thereby activating the host haemostatic system and also indirectly through the production of tumour-associated cytokines [31]. Recent finding that the complement cascade contributes to tumour growth has generated renewed interest in this complex area with the possibility of anticomplement strategies to target cancer cells [32], [33]. The elevated level of many complement and coagulation cascade associated proteins reflects the advanced stage of the disease where most of the tumour specific processes are in over-drive.
Venous thromboembolism (VTE) is a major cause of morbidity and mortality among patients diagnosed with cancer. The risk of VTE is increased by a factor of approximately 6 in patients with cancer compared with non-cancer patients, with cancer patients contributing 20% of all newly diagnosed cases of VTE [34], [35], [36]. It would be beneficial to identify biomarkers that would allow for early identification of patients at high or low risk of VTE and consequently the selective use of suitable anticoagulants. Various established and novel biomarkers associated with VTE have been reported, with findings in this investigation signifying derangements in the coagulation cascade that can potentially result in VTE [37], [38].
5. Conclusions
In conclusion, the findings in this report suggest Transferrin harbours many bound proteins that are potentially useful especially in advanced patient management where more options are now available. Most of the proteins found and bound to Transferrin are high abundant molecules themselves, perhaps reflecting that such proteins form complexes with each other in the blood. Many of these proteins are associated with the complement and coagulation systems, areas that have been the subject of rejuvenated interest of late for potential diagnostic material and also try to manipulate these cascades from a therapeutic perspective.
The following are the supplementary data related to this article.
Information on Min, QI, Median, Q3, Max and IQR for Fibrinogen, Fibronectin and CA15-3 in normal healthy control (n = 54), inflammatory bowel disease (n = 19), benign breast disease (n = 12), stage I breast cancer (n = 20), stage II breast cancer (n = 20), stage III breast cancer (n = 39) and stage IV breast cancer (n = 76).
Acknowledgements
The All-Ireland Cooperative Oncology Research Group (ICORG) assisted in the provision of clinical samples. This work was supported by funding from Enterprise Ireland (EI) [grant number CF20122012].
References
- 1.DeSantis C., Ma J., Bryan L., Jemal A. Breast cancer statistics, 2013. CA Cancer J. Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 2.de la Mare J.A., Contu L., Hunter M.C., Moyo B., Sterrenberg J.N., Dhanani K.C., Mutsvunguma L.Z., Edkins A.L. Breast cancer: current developments in molecular approaches to diagnosis and treatment. Recent Pat. Anticancer Drug Discov. 2014;9:153–175. doi: 10.2174/15748928113086660046. [DOI] [PubMed] [Google Scholar]
- 3.Koka R., Ioffe O.B. Breast carcinoma: is molecular evaluation a necessary part of current pathological analysis? Semin. Diagn. Pathol. 2013;30:321–328. doi: 10.1053/j.semdp.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Rugo H.S., Perez E.A., Vahdat L.T. New developments in metastatic breast cancer: general discussion. Clin. Adv. Hematol. Oncol. 2013;11:16–17. [PubMed] [Google Scholar]
- 5.Mirabelli P., Incoronato M. Usefulness of traditional serum biomarkers for management of breast cancer patients. Biomed. Res. Int. 2013;2013:685641. doi: 10.1155/2013/685641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffy M.J. Serum tumor markers in breast cancer: are they of clinical value? Clin. Chem. 2006;52:345–351. doi: 10.1373/clinchem.2005.059832. [DOI] [PubMed] [Google Scholar]
- 7.Kurebayashi J., Nishimura R., Tanaka K., Kohno N., Kurosumi M., Moriya T., Ogawa Y., Taguchi T. Significance of serum tumor markers in monitoring advanced breast cancer patients treated with systemic therapy: a prospective study. Breast Cancer. 2004;11:389–395. doi: 10.1007/BF02968047. [DOI] [PubMed] [Google Scholar]
- 8.Duffy M.J. Tumor markers in clinical practice: a review focusing on common solid cancers. Med. Princ. Pract. 2013;22:4–11. doi: 10.1159/000338393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prieto D.A., Johann D.J., Wei B.R., Ye X., Chan K.C., Nissley D.V., Simpson R.M., Citrin D.E., Mackall C.L., Linehan W.M., Blonder J. Mass spectrometry in cancer biomarker research: a case for immunodepletion of abundant blood-derived proteins from clinical tissue specimens. Biomark. Med. 2014;8:269–286. doi: 10.2217/bmm.13.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koutroukides T.A., Guest P.C., Leweke F.M., Bailey D.M., Rahmoune H., Bahn S., Martins-de-Souza D. Characterization of the human serum depletome by label-free shotgun proteomics. J. Sep. Sci. 2011;34:1621–1626. doi: 10.1002/jssc.201100060. [DOI] [PubMed] [Google Scholar]
- 11.Gundry R.L., Fu Q., Jelinek C.A., Van Eyk J.E., Cotter R.J. Investigation of an albumin-enriched fraction of human serum and its albuminome. Proteomics Clin. Appl. 2007;1:73–88. doi: 10.1002/prca.200600276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gundry R.L., White M.Y., Nogee J., Tchernyshyov I., Van Eyk J.E. Assessment of albumin removal from an immunoaffinity spin column: critical implications for proteomic examination of the albuminome and albumin-depleted samples. Proteomics. 2009;9:2021–2028. doi: 10.1002/pmic.200800686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camaggi C.M., Zavatto E., Gramantieri L., Camaggi V., Strocchi E., Righini R., Merina L., Chieco P., Bolondi L. Serum albumin-bound proteomic signature for early detection and staging of hepatocarcinoma: sample variability and data classification. Clin. Chem. Lab. Med. 2010;48:1319–1326. doi: 10.1515/CCLM.2010.248. [DOI] [PubMed] [Google Scholar]
- 14.Andrés M.T., Fierro J.F. Antimicrobial mechanism of action of transferrins: selective inhibition of H+− ATPase. Antimicrob. Agents Chemother. 2010;54:4335–4342. doi: 10.1128/AAC.01620-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen L.J., Kuhn M., Stark M., Chaffron S., Creevey C., Muller J., Doerks T., Julien P., Roth A., Simonovic M., Bork P., von Mering C. STRING 8—a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 2009;37:D412–D416. doi: 10.1093/nar/gkn760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franceschini A., Szklarczyk D., Frankild S., Kuhn M., Simonovic M., Roth A., Lin J., Minguez P., Bork P., von Mering C., Jensen L.J. STRING v9.1: protein–protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–D815. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tondini C., Hayes D.F., Gelman R., Henderson I.C., Kufe D.W. Comparison of CA15-3 and carcinoembryonic antigen in monitoring the clinical course of patients with metastatic breast cancer. Cancer Res. 1988;48:4107–4112. [PubMed] [Google Scholar]
- 18.Senkus E., Cardoso F., Pagani O. Time for more optimism in metastatic breast cancer? Cancer Treat. Rev. 2014;40:220–228. doi: 10.1016/j.ctrv.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Cardoso F., Bischoff J., Brain E., Zotano Á., Lück H.J., Tjan-Heijnen V.C., Tanner M., Aapro M. A review of the treatment of endocrine responsive metastatic breast cancer in postmenopausal women. Cancer Treat. Rev. 2013;39:457–465. doi: 10.1016/j.ctrv.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Goel S., Chirgwin J., Francis P., Stuart-Harris R., Dewar J., Mileshkin L., Snyder R., Michael M., Koczwara B. Rational use of trastuzumab in metastatic and locally advanced breast cancer: implications of recent research. Breast. 2011;20:101–110. doi: 10.1016/j.breast.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Hashim Z.M. The significance of CA15-3 in breast cancer patients and its relationship to HER-2 receptor status. Int. J. Immunopathol. Pharmacol. 2014;27:45–51. doi: 10.1177/039463201402700107. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi N., Nakamura S., Tokuda Y., Shimoda Y., Yagata H., Yoshida A., Ota H., Hortobagyi G.N., Cristofanilli M., Ueno N.T. Prognostic value of HER2-positive circulating tumor cells in patients with metastatic breast cancer. Int. J. Clin. Oncol. 2012;17:96–104. doi: 10.1007/s10147-011-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamashita H., Kitayama J., Taguri M., Nagawa H. Effect of preoperative hyperfibrinogenemia on recurrence of colorectal cancer without a systemic inflammatory response. World J. Surg. 2009;33:1298–1305. doi: 10.1007/s00268-009-9992-7. [DOI] [PubMed] [Google Scholar]
- 24.Guo Q., Zhang B., Dong X., Xie Q., Guo E., Huang H., Wu Y. Elevated levels of plasma fibrinogen in patients with pancreatic cancer: possible role of a distant metastasis predictor. Pancreas. 2009;38:e75–e79. doi: 10.1097/MPA.0b013e3181987d86. [DOI] [PubMed] [Google Scholar]
- 25.Polterauer S., Grimm C., Seebacher V., Concin N., Marth C., Tomovski C., Husslein H., Leipold H., Hefler-Frischmuth K., Tempfer C., Reinthaller A., Hefler L. Plasma fibrinogen levels and prognosis in patients with ovarian cancer: a multicenter study. Oncologist. 2009;14:979–985. doi: 10.1634/theoncologist.2009-0079. [DOI] [PubMed] [Google Scholar]
- 26.Costantini V., Zacharski L.R., Memoli V.A., Kisiel W., Kudryk B.J., Rousseau S.M. Fibrinogen deposition without thrombin generation in primary human breast cancer tissue. Cancer Res. 1991;51:349–353. [PubMed] [Google Scholar]
- 27.Warawdekar U.M., Zingde S.M., Iyer K.S., Jagannath P., Mehta A.R., Mehta N.G. Elevated levels and fragmented nature of cellular fibronectin in the plasma of gastrointestinal and head and neck cancer patients. Clin. Chim. Acta. 2006;372:83–93. doi: 10.1016/j.cca.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Saito N., Nishimura H., Kameoka S. Clinical significance of fibronectin expression in colorectal cancer. Mol. Med. Rep. 2008;1:77–81. [PubMed] [Google Scholar]
- 29.Williams C.M., Engler A.J., Slone R.D., Galante L.L., Schwarzbauer J.E. Fibronectin expression modulates mammary epithelial cell proliferation during acinar differentiation. Cancer Res. 2008;68:3185–3192. doi: 10.1158/0008-5472.CAN-07-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koukoulis G.K., Howeedy A.A., Korhonen M., Virtanen I., Gould V.E. Distribution of tenascin, cellular fibronectins and integrins in the normal, hyperplastic and neoplastic breast. J. Submicrosc. Cytol. Pathol. 1993;25:285–295. [PubMed] [Google Scholar]
- 31.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutkowski M.J., Sughrue M.E., Kane A.J., Mills S.A., Parsa A.T. Cancer and the complement cascade. Mol. Cancer Res. 2010;8:1453–1465. doi: 10.1158/1541-7786.MCR-10-0225. [DOI] [PubMed] [Google Scholar]
- 33.Markiewski M.M., DeAngelis R.A., Benencia F., Ricklin-Lichtsteiner S.K., Koutoulaki A., Gerard C., Coukos G., Lambris J.D. Modulation of the antitumor immune response by complement. Nat. Immunol. 2008;9:1225–1235. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donnellan E., Kevane B., Bird B.R., Ainle F.N. Cancer and venous thromboembolic disease: from molecular mechanisms to clinical management. Curr. Oncol. 2014;21:134–143. doi: 10.3747/co.21.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacLean S., Mulla S., Akl E.A., Jankowski M., Vandvik P.O., Ebrahim S., McLeod S., Bhatnagar N., Guyatt G.H., A.C.o.C. Physicians Patient values and preferences in decision making for antithrombotic therapy: a systematic review: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e1S–e23S. doi: 10.1378/chest.11-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agnelli G., Verso M. Management of venous thromboembolism in patients with cancer. J. Thromb. Haemost. 2011;9(Suppl. 1):316–324. doi: 10.1111/j.1538-7836.2011.04346.x. [DOI] [PubMed] [Google Scholar]
- 37.Pabinger I., Thaler J., Ay C. Biomarkers for prediction of venous thromboembolism in cancer. Blood. 2013;122:2011–2018. doi: 10.1182/blood-2013-04-460147. [DOI] [PubMed] [Google Scholar]
- 38.Pabinger I., Ay C. Biomarkers and venous thromboembolism. Arterioscler. Thromb. Vasc. Biol. 2009;29:332–336. doi: 10.1161/ATVBAHA.108.182188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Information on Min, QI, Median, Q3, Max and IQR for Fibrinogen, Fibronectin and CA15-3 in normal healthy control (n = 54), inflammatory bowel disease (n = 19), benign breast disease (n = 12), stage I breast cancer (n = 20), stage II breast cancer (n = 20), stage III breast cancer (n = 39) and stage IV breast cancer (n = 76).