Abstract
Background
The standard of care for newly diagnosed glioblastoma is maximal safe surgical resection, followed by chemoradiation therapy. We assessed carmustine wafer implantation efficacy and safety when used in combination with standard care.
Methods
Included were adult patients with (n = 354, implantation group) and without (n = 433, standard group) carmustine wafer implantation during first surgical resection followed by chemoradiation standard protocol. Multivariate and case-matched analyses (controlled propensity-matched cohort, 262 pairs of patients) were conducted.
Results
The median progression-free survival was 12.0 months (95% CI: 10.7–12.6) in the implantation group and 10.0 months (9.0–10.0) in the standard group and the median overall survival was 20.4 months (19.0–22.7) and 18.0 months (17.0–19.0), respectively. Carmustine wafer implantation was independently associated with longer progression-free survival in patients with subtotal/total surgical resection in the whole series (adjusted hazard ratio [HR], 0.76 [95% CI: 0.63–0.92], P = .005) and after propensity matching (HR, 0.74 [95% CI: 0.60–0.92], P = .008), whereas no significant difference was found for overall survival (HR, 0.95 [0.80–1.13], P = .574; HR, 1.06 [0.87–1.29], P = .561, respectively). Surgical resection at progression whether alone or combined with carmustine wafer implantation was independently associated with longer overall survival in the whole series (HR, 0.58 [0.44–0.76], P < .0001; HR, 0.54 [0.41–0.70], P < .0001, respectively) and after propensity matching (HR, 0.56 [95% CI: 0.40–0.78], P < .0001; HR, 0.46 [95% CI: 0.33–0.64], P < .0001, respectively). The higher postoperative infection rate in the implantation group did not affect survival.
Conclusions
Carmustine wafer implantation during surgical resection followed by the standard chemoradiation protocol for newly diagnosed glioblastoma in adults resulted in a significant progression-free survival benefit.
Keywords: carmustine wafers, chemoradiotherapy, glioblastoma, surgery, survival
Glioblastoma (World Health Organization [WHO] grade IV astrocytoma) is the most common malignant primary brain tumor in adults and among the most aggressive of all tumors.1,2 Maximal safe resection is recommended as the first treatment because it reduces symptoms, improves survival, and may increase the efficacy of adjuvant therapies.2–5 Following surgery, the current standard of care for newly diagnosed glioblastoma consists of combined chemoradiotherapy followed by adjuvant temozolomide (TMZ).2,4,5 This treatment regimen increases median overall survival (OS) by 2.5 months and the 2-year survival rate by 15% compared with radiotherapy alone in a randomized controlled trial.4,5 Recently, addition of bevacizumab to this standard treatment failed to improve overall survival but increased adverse effects.6,7 However, the effectiveness of this combined chemoradiation protocol is hampered by the recommended delay of 2 to 6 weeks between surgery and start of radiotherapy.8 Because carmustine wafer implantation in patients undergoing surgical resection provides a theoretical bridge during the nontherapeutic period between surgery and radiotherapy onset, it could offer a means to overcome this crucial “off treatment” period.9 Two randomized controlled trials have assessed the effectiveness of carmustine wafer and showed a significant increase in OS by 2 to 4 months in newly diagnosed glioblastoma.10–13 However, it remains inconclusive whether combining carmustine wafers with the chemoradiation standard protocol is safe and whether it improves survival in patients with newly diagnosed glioblastoma. These combined modalities have been reported in only a small number of studies, which suggested an extended survival and acceptable toxicity.9,14–19
Here we report a large multicenter study aiming at assessing the safety and efficacy of combined standard chemoradiation protocol, with or without prior carmustine wafer implantation at first surgery in patients with newly diagnosed glioblastoma. To address the methodological challenges due to the absence of a randomization design, we followed a comprehensive adjustment strategy based on both classical multivariate analyses and use of a propensity-matched cohort design.
Methods
Data Source
This study was conducted in 18 institutions in France. Inclusion criteria were (i) patients older than 18 at diagnosis, (ii) newly diagnosed glioblastoma,1 (iii) supratentorial hemispheric location, (iv) surgical resection with or without carmustine wafer implantation as first-line treatment, and (v) adjuvant treatment according to the combined standard chemoradiation protocol without bevacizumab as first-line treatment.4,5 The decision as to whether to implant carmustine wafers was not randomized but was decided according to: (i) the guidelines from the French Neurosurgical Society, (ii) the preoperative neurosurgical-based expectation of a gross total removal of the contrast-enhanced tumor mass, (iii) obtained informed consent, (iv) the feasibility of an intraoperative extemporaneous histopathological diagnosis, (v) the extemporaneous histopathological diagnosis of a malignant glioma, (vi) the intraoperative observation of an apparently gross total surgical removal, and (vii) the routine practice of each institution.
Between January 2005 and June 2011, a total of 820 patients were enrolled. We excluded 33 patients (4.0%) from the cohort (15 with carmustine wafer implantation, 18 without): 6 did not have the full radiotherapy dose, 3 did not receive concomitant chemoradiotherapy, and 24 were not available for follow-up. In the end, a total of 787 cases were available for survival analysis. The institutional review board of the Sainte-Anne Hospital Center–University Paris Descartes approved the study protocol (no. AC036).
Data Collection
Data were obtained from the medical records using a protocol designed for the study. The patient- and tumor-related characteristics collected at the time of histopathological diagnosis included the following: gender, age, Karnofsky performance status, the revised Radiation Therapy Oncology Group recursive partitioning analysis (RTOG-RPA) classification system for glioblastoma,20 and histopathological subtypes. Treatment-related characteristics included the following: carmustine wafer implantation, extent of surgical resection based on early postoperative MRI (within 48 h) on contrast-enhanced T1-weighted sequence (subtotal and total resections defined by removal of ≥90% of enhancing tumor21,22), adverse postoperative events (new neurological deficit, seizures, raised intracranial pressure, hematoma requiring surgical evacuation, wound-healing defect, cerebrospinal fluid leak, wound infection, bacterial and aseptic meningitis, bacterial abscess, systemic thromboembolic complications, postoperative KPS), postoperative first-line oncological treatment modalities (corticosteroid use, time interval between surgery and radiotherapy, radiotherapy dose, number of adjuvant TMZ therapy cycles, KPS at the end of first-line oncological treatment), oncological treatments at progression (none, surgical resection with or without carmustine wafer implantation, chemotherapy [TMZ, other chemotherapy], radiotherapy, bevacizumab therapy).
Endpoints
The aim of the study was to assess progression-free survival (PFS), OS, and treatment-related morbidity in patients receiving carmustine wafer implantation (implantation group) compared with those who did not (standard group), with both groups receiving standard chemoradiation. Overall survival was measured from the date of histopathological diagnosis to the date of death for any cause. Progression-free survival was measured from the date of histopathological diagnosis to the date of evidence of progression or to the date of death. Tumor progression was defined according to Macdonald criteria by any of the following: (i) 25% increase in total perpendicular diameters of an enhancing lesion, (ii) any new lesion, or (iii) clinical deterioration.23,24 According to the guidelines from the French Neurosurgical Society, a 3-month interval was systematically applied for the clinical and imaging follow-up and a new MRI was performed in case of clinical deterioration. Surviving patients were censored at the date of last follow-up.
Statistical Analyses
Univariate analyses were carried out using chi-square or Fisher's exact test for comparing categorical variables, and the unpaired t-test or Mann–Whitney rank sum test for continuous variables, as appropriate. Unadjusted survival curves for OS and PFS were plotted by the Kaplan–Meier method, using log-rank tests to assess significance for group comparison.
Because the baseline characteristics of subjects included in observational studies influence treatment choice, it is crucial to take into account such differences when estimating treatment effects to address bias arising from confounding. Among available methods, those based on the propensity score have recently attracted growing interest in clinical epidemiology.25 In particular, by pairing treated and control patients sharing similar values on the propensity score, the use of propensity-matched samples has the advantage of mimicking the analysis of a randomized controlled trial. At the cost of discarding all unmatched patients, it allows the direct comparison of the outcome of interest between treated and untreated subjects. In the present paper, we chose to use both classical regression adjustment on baseline characteristics and a propensity-matched cohort design so as to thoroughly test the robustness of our findings regarding the efficacy of carmustine wafer implantation. First, we constructed Cox proportional hazards regression models on the whole series using a backward stepwise approach, systematically adjusting for age, gender, and KPS, entering the predictors previously associated with mortality and progression in univariate analysis at the P < .2 level, and using the carmustine wafer implantation variable in the model as the main variable of interest. Based on Schoenfeld residuals, all the covariates were tested for the proportional hazards assumption, which was not found to be violated. Second, we performed a propensity-matched sample analysis by creating pairs of patients with and without carmustine wafer implantation at first surgery (1:1 matching), based on the closest logit of propensity scores within a predetermined range (caliper set to 0.2 standard deviation of the logit of the propensity score).26 The model to construct the propensity score included age, gender, KPS, RTOG-RPA class, histopathological diagnosis, and extent of resection as predictors of being treated by carmustine wafer implantation at first surgery. The matching procedure created 262 pairs of patients with and without carmustine wafer implantation. Post-matching imbalance between implantation and standard groups was assessed by computing standardized differences of proportions and means, a value inferior to 0.1 indicating a negligible difference in the mean/proportion of a covariate between groups27 (Supplementary Table 1). Because differences could be expected in the effect of carmustine wafer implantation on PFS and OS depending on the potential influence of the extent of resection, statistical interaction between extent of resection and carmustine wafer implantation was systematically evaluated. Likewise, propensity score calculations were performed separately among those with a total or subtotal resection and those with partial resection as a complementary analysis, yielding similar results for propensity score calculations and outcome comparison (data not shown). A 2-tailed P-value of <.05 was considered significant. Statistical analyses were performed with Stata software version 12.1, using the user-written psmatch2 program (http://ideas.repec.org/c/boc/bocode/s432001.html) to compute the propensity score and create pairs.
Results
Whole-Series Analysis
A total of 787 patients (288 women and 499 men) were included, with a median age of 58.0 years (range, 20–80); there were 354 patients in the implantation group and 433 in the standard group. Patients' main characteristics are summarized in Table 1.
Table 1.
Main characteristics and demographics of the patients
| Parameters | All Patients (n = 787) |
Carmustine Wafers at First Surgery Plus Standard Chemoradiation Protocol |
P | ||||
|---|---|---|---|---|---|---|---|
| Yes (n = 354) |
No (n = 433) |
||||||
| No. | % | No. | % | No. | % | ||
| Clinical parameters | |||||||
| Mean age, y (SD) | 57.4 (10.8) | 57.6 (10.5) | 57.3 (11.1) | .701 | |||
| Gender, n | .829 | ||||||
| Male | 499 | 63.4 | 223 | 63.0 | 276 | 63.7 | |
| Female | 288 | 36.6 | 131 | 37.0 | 157 | 36.3 | |
| KPS, n | |||||||
| 90–100 | 307 | 39.0 | 145 | 41.0 | 162 | 37.4 | .434 |
| 70–80 | 399 | 50.7 | 177 | 50.0 | 222 | 51.3 | |
| <70 | 81 | 10.3 | 32 | 9.0 | 49 | 11.3 | |
| RTOG-RPA classes, n | <.0001 | ||||||
| III + IV | 373 | 47.4 | 199 | 56.2 | 174 | 40.2 | |
| V + VI | 414 | 52.6 | 155 | 43.8 | 259 | 59.8 | |
| Histopathological parameters | |||||||
| Pathological diagnosis, n | <.0001 | ||||||
| Classical GBM | 608 | 77.3 | 273 | 77.1 | 335 | 77.4 | |
| GBM with oligodendroglial component | 136 | 17.3 | 73 | 20.6 | 63 | 14.5 | |
| Other subtype | 43 | 5.4 | 8 | 2.3 | 35 | 8.1 | |
| First-line oncological treatments, n | |||||||
| Mean, duration, days, of postoperative corticosteroids (SD) | 9.8 (12.9) | 12.9 (15.1) | 8.9 (12.1) | .050 | |||
| Mean time, mo, to radiotherapy (SD) | 2.2 (1.9) | 3.2 (2.3) | 1.7 (1.4) | <.0001 | |||
| Extent of resection, n | .001 | ||||||
| Partial | 242 | 30.7 | 90 | 25.4 | 152 | 35.1 | |
| Subtotal and total | 503 | 63.9 | 237 | 67.0 | 266 | 61.4 | |
| Missing | 42 | 5.3 | 27 | 7.6 | 15 | 3.5 | |
| Mean number of adjuvant TMZ cycles (SD) | 5.7 (3.8) | 6.1 (3.8) | 5.7 (3.7) | 0.217 | |||
| Treatments at progression (n = 684) | 684 | 100.0 | 280 | 40.9 | 404 | 59.1 | |
| Mean number of treatments at progression (SD) | 1.4 (1.2) | 1.0 (1.1) | 1.7 (1.2) | <.0001 | |||
| Mean number of surgical resections (SD) | 0.28 (0.5) | 0.19 (0.5) | 0.34 (0.5) | .0001 | |||
| Surgical resection(s) at progression | <.0001 | ||||||
| No | 512 | 75.0 | 236 | 84.3 | 277 | 68.5 | |
| Yes | 171 | 25.0 | 44 | 15.7 | 127 | 31.5 | |
| Carmustine wafer implantation at progression | <.0001 | ||||||
| No | 590 | 86.4 | 268 | 95.7 | 323 | 79.9 | |
| Yes | 93 | 13.6 | 12 | 4.3 | 81 | 20.1 | |
| Radiotherapy at progression | .131 | ||||||
| No | 652 | 95.3 | 271 | 96.8 | 381 | 94.5 | |
| Yes | 32 | 4.7 | 9 | 3.2 | 23 | 5.5 | |
| TMZ at progression | .023 | ||||||
| No | 527 | 77.0 | 228 | 81.4 | 299 | 74.2 | |
| Yes | 157 | 23.0 | 52 | 18.6 | 105 | 25.8 | |
| Bevacizumab at progression | .0004 | ||||||
| No | 350 | 51.2 | 166 | 59.3 | 184 | 45.7 | |
| Yes | 334 | 48.8 | 114 | 40.7 | 220 | 54.3 | |
| Other chemotherapy at progression | <.0001 | ||||||
| No | 438 | 64.0 | 227 | 81.1 | 211 | 52.4 | |
| Yes | 246 | 36.0 | 53 | 18.9 | 193 | 47.6 | |
Abbreviation: GBM, glioblastoma multiforme.
A subtotal or total resection was achieved in 72.5% of the implantation group and 63.6% of the standard group (P = .011, n = 745). The median duration of corticosteroid use in the immediate postoperative period was 5.5 days in the implantation group and 4 days in the standard group (P = .050). The median time from surgical resection to the start of radiotherapy was 8 weeks in the implantation group and 4 weeks in the standard group (P < .0001). After concomitant chemoradiotherapy, patients received a median of 6 cycles of adjuvant TMZ therapy in the implantation group and a median of 6 cycles in the standard group (P = .217); 65.5% and 56.8% of patients completed 6 cycles of TMZ therapy in the implantation group and in the standard group, respectively. Median duration of follow-up from histopathological diagnosis was 16.2 months (mean, 20.0; range, 0–95). Five hundred sixty-seven patients (72.0%) died over the follow-up period, of whom 9 (1.6%) died from an unrelated cause. Six hundred eighty-four patients (86.9%) re-presented with disease progression, which was subsequently histopathologically proven in 171 cases (25.0%) following a second surgical resection.
In the whole series, unadjusted hazard ratio (HR) for PFS in the implantation group compared with the standard group was 0.81 (95% CI: 0.69–0.94; P = .005) (Supplementary Table 2), indicating a 19% relative risk reduction for death or disease progression in patients treated with carmustine wafer implantation when combined with the standard chemoradiation protocol. The median PFS benefit was 2.0 months, with a median PFS of 12.0 months (95% CI: 10.7–12.6) in the implantation group against 10.0 months (95% CI: 9.0–10.0) in the standard group (P = .005). The rate of KPS under 70 at the end of first-line oncological treatment was 19% in the implantation group and 20% in the standard group (P = .423, n = 531). In the subgroup of patients with subtotal and total resection, unadjusted HR for PFS in the implantation group compared with the standard group was 0.79 (95% CI: 0.66–0.96; P = .018), indicating a 21% relative risk reduction for death or disease progression in patients treated with carmustine wafer implantation when combined with a subtotal or total resection and with the standard chemoradiation protocol. In this subgroup, the median PFS benefit was 2.1 months, with a median of 12.1 months (95% CI: 10.7–13.1) in the implantation group against 10.0 months (95% CI: 9.0–10.1) in the standard group (P = .017). Kaplan–Meier curves for PFS are shown in Fig. 1, stratified by the extent of surgical resection and whether carmustine wafers were implanted. In the whole series, 1-year and 2-year PFS rates were 47.1% (95% CI: 42.0–52.4) and 18.6% (95% CI: 14.5–23.5) in the implantation group compared with 33.0% (95% CI: 28.9–37.4) and 10.9% (95% CI: 8.3–14.1) in the standard group, respectively (Supplementary Table 3). In the subgroup of patients with subtotal and total resection, 1- and 2-year PFS rates were 50.2% (95% CI: 45.2–55.4) and 21.2% (95% CI: 17.3–25.1) in the implantation group compared with 34.8% (95% CI: 30.7–39.2) and 12.3% (95% CI: 9.6–15.4) in the standard group, respectively.
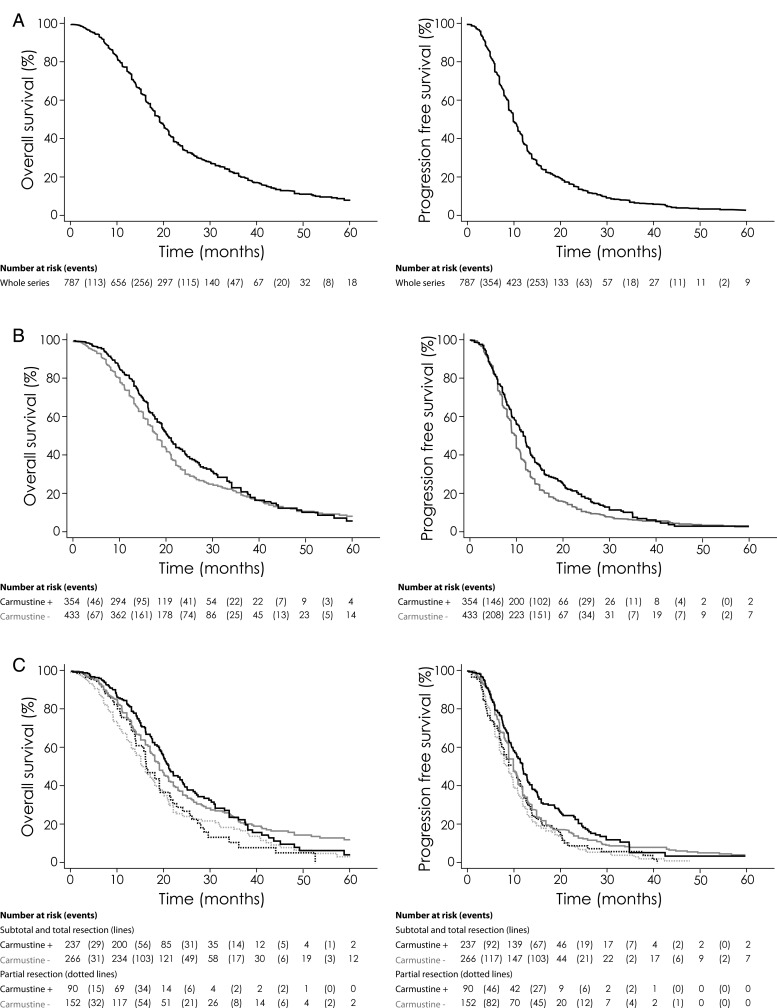
Fig. 1.
Kaplan–Meier estimates of OS and PFS according to carmustine wafer implantation and to extent of surgical resection. (A) Overall survival and PFS in the whole series (n = 787) of supratentorial newly diagnosed glioblastomas treated with surgical resection and standard chemoradiation protocol as first-line treatment. (B) Overall survival and PFS according to carmustine wafer implantation at first-line treatment. The unadjusted HR for PFS in the implantation group compared with the standard group was 0.81 (95% CI: 0.69 to 0.94; P = .005). The unadjusted HR for OS in the implantation group compared with the standard group was 0.88 (95% CI: 0.74–1.04; P = .129). (C) Overall survival and PFS according to carmustine wafer implantation and to extent of surgical resection at first-line treatment. The unadjusted HR for PFS in the subgroup with subtotal or total resection together with carmustine wafer implantation compared with the subgroup with partial resection and without carmustine wafer implantation was 0.63 (95% CI: 0.51–0.78; P < .001). The unadjusted HR for PFS in the subgroup with subtotal and total resection and without carmustine wafer implantation compared with the subgroup with partial resection and without carmustine wafer implantation was 0.77 (95% CI: 0.63–0.94; P = .011). The unadjusted HR for PFS in the subgroup with partial resection together with carmustine wafer implantation compared with the subgroup with partial resection and without carmustine wafer implantation was 0.92 (95% CI: 0.70–1.19; P = .521). The unadjusted HR for OS in the subgroup with subtotal and total resection together with carmustine wafer implantation compared with the subgroup with partial resection and without carmustine wafer implantation was 0.65 (95% CI: 0.52–0.83; P < .001). The unadjusted HR for OS in the subgroup with subtotal and total resection and without carmustine wafer implantation compared with the subgroup with partial resection and without carmustine wafer implantation was 0.71 (95% CI: 0.57–0.87; P = .001). The unadjusted HR for OS in the subgroup with partial resection together with carmustine wafer implantation compared with the subgroup with partial resection and without carmustine wafer implantation was 0.98 (95% CI: 0.73–1.31; P = .897).
After multiple adjustments using Cox models (Table 2), carmustine wafer implantation was independently associated with longer PFS (adjusted HR, 0.82 [95% CI: 0.70–0.95], P = .010). A tendency for statistical significance (.05 < P < .1) was found when testing for an interaction between carmustine wafer implantation and extent of resection in their effect on PFS (global test for interaction, P = .097): stratifying by the extent of resection, carmustine wafer implantation was still associated with a better outcome in the subgroup of patients who had a subtotal or total surgical resection (adjusted HR, 0.76 [95% CI: 0.63–0.92], P = .005), but not in the subgroup of patients who had only a partial surgical resection (adjusted HR, 1.05 [95% CI: 0.80–1.39], P = .713).
Table 2.
Multivariate predictors of progression-free survival. Adjusted HRs by Cox proportional hazards model
| Multivariate Cox Analysis of Factors Associated With PFS | Adjusted HR | 95% CI | P |
|---|---|---|---|
| Whole series (n = 787) | |||
| Age, per 10 y | 1.04 | 0.97–1.11 | .236 |
| Gender | |||
| Female | 1 (ref) | ||
| Male | 1.23 | 1.05–1.44 | .008 |
| KPS | |||
| 70 and more | 1 (ref) | ||
| Less than 70 | 1.00 | 0.78–1.28 | .915 |
| Extent of surgical resection at first-line surgery | |||
| Partial | 1 (ref) | ||
| Subtotal and total | 0.75 | 0.64–0.88 | <.0001 |
| Missing | 0.63 | 0.45–0.90 | <.0001 |
| Carmustine wafer implantation | |||
| No | 1 (ref) | ||
| Yes | 0.82 | 0.70–0.95 | .010 |
| Subgroup with partial resection (n = 242) | |||
| Age, per 10 y | 1.02 | 0.90–1.14 | .788 |
| Gender | |||
| Female | 1 (ref) | ||
| Male | 1.02 | 0.77–1.34 | .898 |
| KPS | |||
| 70 and more | 1 (ref) | ||
| Less than 70 | 1.30 | 0.85–1.99 | .226 |
| Carmustine wafer implantation | |||
| No | 1 (ref) | ||
| Yes | 1.05 | 0.80–1.39 | .713 |
| Subgroup with subtotal and total resection (n = 503) | |||
| Age, per 10 y | 1.04 | 0.96–1.13 | .348 |
| Gender | |||
| Female | 1 (ref) | ||
| Male | 1.32 | 1.09–1.61 | .005 |
| KPS | |||
| 70 and more | 1 (ref) | ||
| Less than 70 | 0.96 | 0.68–1.33 | .788 |
| Carmustine wafer implantation | |||
| No | 1 (ref) | ||
| Yes | 0.76 | 0.63–0.92 | .005 |
Unadjusted HR for OS in the implantation group compared with the standard group was 0.88 (95% CI: 0.74–1.04; P = .129) (Supplementary Table 2). One-year and 2-year OS rates were 80.8% (95% CI: 76.3–84.6) and 41.0% (95% CI: 35.2–47.2) in the implantation group compared with 71.3% (95% CI: 67.0–75.2) and 30.4% (95% CI: 26.2–34.9) in the standard group, respectively (Supplementary Table 3). Kaplan–Meier curves for OS are shown in Fig. 1, stratified by the extent of surgical resection and whether carmustine wafers were implanted. The median OS was 20.4 months (95% CI: 19.0–22.7) in the implantation group and 18.0 months (95% CI: 17.0–19.0) in the standard group. After multiple adjustments using Cox models (Table 3), carmustine wafer implantation at first surgery was not an independent predictor of OS in the whole-series analysis (adjusted HR, 0.95 [0.80–1.13], P = .574). In the subgroup of patients with treated progressions (n = 684), surgical resection, whether alone at progression or combined with carmustine wafer implantation, was independently associated with longer OS compared with no surgical resection at progression (adjusted HR, 0.58 [95% CI: 0.44–0.76], P < .0001; adjusted HR, 0.54 [95% CI: 0.41–0.70], P < .0001, respectively). Carmustine implantation at progression was not significantly associated with longer OS compared with surgical resection alone at progression (adjusted HR, 0.93 [0.66–1.32], P = .683). No statistical interaction was found between extent of resection and carmustine wafer implantation in their effect on OS, whether considering the whole series or the subgroup with treated progression (P > .5 for both).
Table 3.
Multivariate predictors of overall survival. Adjusted HRs by Cox proportional hazards model
| Multivariate Cox Analysis of Factors Associated With OS | Adjusted HR | 95% CI | P |
|---|---|---|---|
| Whole series (n = 787) | |||
| Age, per 10 y | 1.10 | 1.01–1.20 | .034 |
| Gender | |||
| Female | 1 (ref) | ||
| Male | 1.38 | 1.16–1.65 | <.001 |
| KPS | |||
| 70 and more | 1 (ref) | ||
| Less than 70 | 1.26 | 0.96–1.64 | .092 |
| RTOG-RPA classes | |||
| III + IV | 1 (ref) | ||
| V + VI | 1.21 | 1.0–1.46 | .053 |
| Extent of surgical resection at first-line surgery | |||
| Partial | 1 (ref) | ||
| Subtotal and total | 0.71 | 0.59–0.85 | <.0001 |
| Missing | 0.59 | 0.40–0.86 | .006 |
| Carmustine wafer implantation at first-line surgery | |||
| No | 1 (ref) | ||
| Yes | 0.95 | 0.80–1.13 | .574 |
| Subgroup with treated progression (n = 684) | |||
| Age, per 10 y | 1.06 | 0.97–1.17 | .208 |
| Gender | |||
| Female | 1 (ref) | ||
| Male | 1.39 | 1.16–1.67 | <.0001 |
| KPS | |||
| 70 and more | 1 (ref) | ||
| Less than 70 | 1.28 | 0.97–1.70 | .080 |
| RTOG-RPA classes | |||
| III + IV | 1 (ref) | ||
| V + VI | 1.26 | 1.03–1.54 | .025 |
| Extent of surgical resection at first-line surgery | |||
| Partial | 1 (ref) | ||
| Subtotal and total | 0.84 | 0.70–1.02 | .074 |
| Missing | 0.71 | 0.48–1.04 | .081 |
| Carmustine wafer implantation at first-line surgery | |||
| No | 1 (ref) | ||
| Yes | 0.85 | 0.70–1.02 | .085 |
| Surgical resection and carmustine wafer implantation at progression | |||
| No | 1 (ref) | ||
| Surgical resection alone | 0.58 | 0.44–0.76 | <.0001 |
| Surgical resection and carmustine wafer implantation | 0.54 | 0.41–0.70 | <.0001 |
| Radiotherapy at progression | |||
| No | 1 (ref) | ||
| Yes | 0.59 | 0.39–0.91 | .017 |
| TMZ at progression | |||
| No | 1 (ref) | ||
| Yes | 0.53 | 0.43–0.66 | <.0001 |
| Bevacizumab at progression | |||
| No | 1 (ref) | ||
| Yes | 0.57 | 0.48–0.68 | <.0001 |
Propensity-matched Analysis
After propensity score matching (n = 524), a significantly longer PFS was found for carmustine wafer implantation (HR, 0.83 [0.70–0.99], P = .048) (Table 4). Consistent with results from the whole-series analysis, a trend for statistical significance was found between carmustine wafer implantation and extent of resection (P = .091): stratifying by the extent of resection, carmustine wafer implantation was associated with longer PFS in the subgroup of patients who had a subtotal or total surgical resection (HR, 0.74 [0.60–0.92], P = .008), but not in the subgroup of patients who had only a partial surgical resection (HR, 1.09 [0.80–1.48], P = .601). The median PFS benefit was 2.0 months, with a median of 12.0 months (95% CI: 10.7–13.0) in the implantation group and 10.0 months (95% CI: 8.0–11.5) in the standard group.
Table 4.
Propensity-matched analysis
| Progression-free survival | n | Median (95% CI) | HR | 95% CI | P |
|---|---|---|---|---|---|
| Whole propensity-matched series (n = 524) | |||||
| Carmustine wafer implantation at first-line surgery | |||||
| No | 262 | 10.0 (9.1–11.0) | 1 (ref) | ||
| Yes | 262 | 11.4 (10.0–12.1) | 0.83 | 0.70–0.99 | .048 |
| Subgroup with partial surgical resection (n = 170) | |||||
| Carmustine wafer implantation at first-line surgery | |||||
| No | 85 | 10.0 (8.0–11.5) | 1 (ref) | ||
| Yes | 85 | 9.0 (7.3–11.5) | 1.09 | 0.80–1.48 | .601 |
| Subgroup with subtotal and total surgical resection (n = 354) | |||||
| Carmustine wafer implantation at first-line surgery | |||||
| No | 177 | 10.0 (8.0–11.5) | 1 (ref) | ||
| Yes | 177 | 12.0 (10.7–13.0) | 0.74 | 0.60–0.92 | .008 |
| Overall survival | |||||
| Whole propensity-matched series (n = 524) | |||||
| Carmustine wafer implantation at first-line surgery | |||||
| No | 262 | 19.0 (18.0–20.9) | 1 (ref) | ||
| Yes | 262 | 19.0 (17.1–21.1) | 1.06 | 0.87–1.29 | .561 |
| Subgroup with treated progression (n = 457) | |||||
| Carmustine wafer implantation at first-line surgery | |||||
| No | 241 | 1 (ref) | |||
| Yes | 208 | 0.91 | 0.73–1.14 | .407 | |
| Surgical resection and carmustine wafer implantation at progression | |||||
| No | 336 | 1 (ref) | |||
| Surgical resection alone | 55 | 0.56 | 0.40–0.78 | <.0001 | |
| Surgical resection and carmustine wafer implantation | 58 | 0.46 | 0.33–0.64 | <.0001 | |
| Radiotherapy at progression | |||||
| No | 430 | 1 (ref) | |||
| Yes | 19 | 0.87 | 0.51–1.47 | .591 | |
| TMZ at progression | |||||
| No | 358 | 1 (ref) | |||
| Yes | 91 | 0.50 | 0.36–0.69 | <.0001 | |
| Bevacizumab at progression | |||||
| No | 230 | 1 (ref) | |||
| Yes | 219 | 0.54 | 0.44–0.68 | <.0001 | |
The HR for OS in the implantation group compared with the standard group was 1.06 (95% CI: 0.87–1.29; P = .561) (Table 4). In the subgroup with treated progression (n = 457), surgical resection whether alone at progression or combined with carmustine wafer implantation was independently associated with longer OS compared with no surgical resection at progression (HR, 0.56 [0.40–0.78], P = .0006; HR, 0.46 [0.33–0.64], P < .0001, respectively). Carmustine wafer implantation at progression was not significantly associated with longer OS compared with surgical resection alone (HR, 0.83 [0.53–1.30], P = .415). No statistical interaction was found between extent of resection and carmustine wafer implantation in their effect on OS, whether considering the whole series or the subgroup with treated progression (P > .5 for both). Examining the potential influence of the institutions on the estimates for carmustine wafer implantation, we found neither statistical interaction nor modification of the estimates after accounting for the cluster effect in Cox models for PFS and OS. An exception was noticed for the propensity-match PFS analysis without stratification by extent of resection (P-values .036 [no cluster] vs .079 [cluster]; interaction test P < .05), whereas significant estimate for carmustine wafer implantation in the subgroup with a subtotal or total resection remained constant (P = .009 vs .002) and the interaction test strictly not significant (P = .40).
Postoperative Morbidity
Adverse postoperative event data, available for 567 patients, are detailed in Supplementary Table 4. The rate of postoperative noninfectious adverse events did not differ significantly between the implantation group and the standard group, except for raised intracranial pressure (P = .004). The rate of postoperative overall infections was significantly higher in the implantation group (7.1%) than in the standard group (1.5%) (P < .001). The occurrence of postoperative adverse events was not associated with an increased delay between surgical resection and start of radiotherapy (P = .855), with a difference in the number of administered cycles of adjuvant TMZ therapy (P = .439), with a postoperative KPS <70 (P = .419), with a KPS fall below 70 after surgery (P = .314), or with worsened outcomes (P = .285): the median OS was 17.0 months (95% CI: 14.0–20.0) with postoperative adverse events and 19.9 months (95% CI: 19.0–21.0) without.
Discussion
Only a few previous retrospective and prospective studies have analyzed the combination of carmustine wafer implantation with the combined standard chemoradiation protocol for the treatment of newly diagnosed glioblastoma.9,14–19,28–30 Unlike most previous studies,9,14,16,17,29,30 which included both glioblastoma and WHO grade III gliomas, the present study included only newly diagnosed supratentorial glioblastoma in adults. We report here the largest series assessing the impact of carmustine wafer implantation together with the combined standard chemoradiation protocol in newly diagnosed supratentorial glioblastoma in adults. To overcome the limitations inherent to retrospective observational studies and test the robustness of our findings, we performed a standard unmatched multivariate analysis (n = 787) and a confirmatory case-matched analysis using propensity score matching (n = 524), both yielding roughly similar results.
First, we demonstrated significantly increased PFS with addition of carmustine wafers at first-line resection in patients with subtotal or total surgical resection followed by combined standard chemoradiation protocol. In detail, we showed an increase in median PFS of 2 months in the whole series, yielding a relative reduction in the risk of disease progression of 18% in multivariate analysis, while a relative reduction of 24% was found in multivariate analysis in the subgroup with subtotal and total resection. Interestingly, we found no significant alteration in KPS associated with carmustine wafer implantation after completion of first-line treatment. Those results are consistent with previous studies.14 In addition, we confirm that there is no benefit for carmustine wafer implantation unless maximal resection has been achieved, carmustine wafer implantation being an independent predictor for PFSwhen associated with subtotal or total resection but not with partial resection. However, the observed 25% rate of partial removal in the implantation group underlines the difficulty of assessing intraoperatively the extent of resection.19
Second, and despite this apparent benefit on PFS, it is noticeable that we found no improved OS associated with carmustine wafer implantation at first surgery, whether it be in univariate/multivariate analysis, considering the whole series or a subgroup with treated progression. Among the elements that could explain such a discrepancy with PFS—including the absence of a true beneficial effect of carmustine wafer implantation—it should also be noted that the implantation rate of carmustine wafers at disease progression was 20.1% in the standard group against only 4.3% in the implantation group, and subsequent effective salvage therapies may have diluted the benefit of the earlier therapy.
The observed adverse postoperative event rates were similar to those previously reported, including the largest series with 250 patients13 and a large French retrospective multicenter study of 163 patients.16 We observed a significant increase in postoperative infections with carmustine wafer implantation. In addition, we observed a significant increase in postoperative edema-related changes with carmustine wafers that accounted for higher rates of raised intracranial pressure and delay of radiotherapy onset. However, this increased morbidity did not negatively impact the postoperative oncological treatment. Indeed, number of adjuvant chemotherapy cycles or worsened outcomes were not associated with carmustine wafer implantation.
The interpretation of the present results should be considered under some limitations. First, even though multiple adjustment strategies were applied to deal with confounding, our cohort study design was observational; hence, no causal conclusion can be directly made on the effects of carmustine wafer implantation in the absence of a proper randomized experimental design. Second, several variables of potential interest were not collected in our dataset, including biomarkers with known prognostic value or health-related quality of life outcomes. Finally, we lacked the ability to analyze separately the components of PFS, so as to assess whether and to what extent the benefit we observed with carmustine on PFS originated from delay in radiological progression and/or clinical symptomatic deterioration.
Overall, our findings suggest that carmustine wafer implantation in combination with maximal safe resection, followed by combined standard chemoradiation protocols, may represent a promising first-line treatment option in newly diagnosed supratentorial glioblastoma in adults. The present results warrant a multicenter randomized controlled trial to clearly assess the actual impact in terms of OS in this patient population. Such a clinical trial should ideally include: (i) molecular analyses of relevant biomarkers to identify subgroups of patients who would benefit from such combination treatment strategies and (ii) surgical techniques such as fluorescence guided resection with 5-aminolevulinic acid and intraoperative functional mapping allowing maximal resection and an intraoperative assessment of its extent.19,31,32
Supplementary Material
Funding
This work was supported by the Comité pour la Recherche Hospitalière Médicale (CRHM).
Supplementary Material
Acknowledgments
Participating centers (in alphabetical order): Amiens University Hospital – University of Amiens, Angers University Hospital – Angers University, Jean-Minjoz Hospital – University of Besançon, Pellegrin Hospital – University Victor Segalen Bordeaux 2, Morvan Hospital – University of Brest, Caen University Hospital – University Caen Lower-Normandy, Pasteur Hospital in Colmar, Limoges Hospital – University of Limoges, Pierre Wertheimer Hospital – University of Lyon, La Timone Hospital – University Aix-Marseille, Clairval Clinic in Marseille, Sainte-Anne Hospital Center - University Paris Descartes, Beaujon Hospital – University Paris Diderot, Maison Blanche Hospital – University of Reims, Pontchaillou Hospital – University of Rennes, Rouen University Hospital – Rouen University, Paul Strauss Cancer Center – University of Strasbourg, Sainte-Anne Military Teaching Hospital in Toulon.
These physicians are greatly acknowledged (in alphabetical order): Georges Abi Lahoud, Felipe Andreiuolo, Alin Borha, André Busson, Laurent Capelle, Françoise Chapon, Françine Chassoux, Isabelle Catry-Thomas, Fabrice Chrétien, Philippe Colin, Alain Czorny, Jean-Michel Derlon, Marie-Danièle Diebold, Hugues Duffau, Myriam Edjlali-Goujon, Jan Eskandari, Anne Fustier, Clément Gantois, Roberto Gadan, Julien Geffrelot, Edouard Gimbert, Joël Godard, Sylvie Godon-Hardy, Marcel Gueye, Jean-Sébastien Guillamo, N Heil, Dominique Hoffmann, Nicolas Jovenin, Michel Kalamarides, Hassan Katranji, Samih Khouri, Maria Koziak, Elisabeth Landré, V Leon, Dominique Liguoro, Emmanuel Mandonnet, Michael Mann, Eric Méary, Jean-François Meder, Charles Mellerio, Sophie Michalak, Catherine Miquel, Karima Mokhtari, Philippe Monteil, Olivier Naggara, François Nataf, Catherine Oppenheim, Isabelle Quintin-Roue, Philippe Page, Philippe Paquis, Delphine Pedenon, Philippe Peruzzi, Tanguy Riem, Valérie Rigau, Odile Rigaux-Viodé, Alain Rougier, François-Xavier Roux, Céline Salon, Etienne Théret, Baris Turak, Denis Trystram, Fanny Vandenbos, Pascale Varlet, Gabriel Viennet, Anne Vital. We would like to thank the Association des Neuro-Oncologues d′Expression Française (ANOCEF). We would like to thank Dominique Figarella-Branger, Assistance Publique-Hopitaux de Marseille (APHM biobank Authorization Number 2008/70, AC-2013–1786 and SIRIC Grant INCa-DGOS-Inserm 6038 for APHM). We would like to thank Cécile-Marie Le Reste (Department of English, University of Science of Nantes) for her help in the preparation of the manuscript.
Conflict of interest statement. Johan Pallud, Philippe Menei, Julien Duntze, Antoine Petit, and Philippe Metellus have received honoraria for consultancy from Archimedes Pharma. Johan Pallud, Johann Peltier, Thierry Faillot, Nicolas Desse, Evelyne Emery, Antoine Petit, Philippe Metellus, Vladislav Pavlov, and Olivier Langlois have done speaking engagements (including travel and accommodation) from Archimedes Pharma.
References
- 1.Louis DN, Carvenee WK. WHO Classification of Tumours of the Central Nervous System. Lyon: IARC press, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricard D, Idbaih A, Ducray F, et al. Primary brain tumours in adults. Lancet. 2012;379(9830):1984–1996. [DOI] [PubMed] [Google Scholar]
- 3.Marko NF, Weil RJ, Schroeder JL, et al. Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol. 2014;32(8):774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 6.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noël G, Huchet A, Feuvret L, et al. Waiting times before initiation of radiotherapy might not affect outcomes for patients with glioblastoma: a French retrospective analysis of patients treated in the era of concomitant temozolomide and radiotherapy. J Neurooncol. 2012;109(1):167–175. [DOI] [PubMed] [Google Scholar]
- 9.Mcgirt MJ, Than KD, Weingart JD, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110(3):583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5(2):79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westphal M, Ram Z, Riddle V, et al. Gliadel® wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien). 2006;148(3):269–275. [DOI] [PubMed] [Google Scholar]
- 12.Valtonen S, Timonen U, Toivanen P, et al. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery. 1997;41(1):44–49. [DOI] [PubMed] [Google Scholar]
- 13.Attenello FJ, Mukherjee D, Datoo G, et al. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol. 2008;15(10):2887–2893. [DOI] [PubMed] [Google Scholar]
- 14.Duntze J, Litré C-F, Eap C, et al. Implanted carmustine wafers followed by concomitant radiochemotherapy to treat newly diagnosed malignant gliomas: prospective, observational, multicenter study on 92 cases. Ann Surg Oncol. 2012;20(6):2065–2072. [DOI] [PubMed] [Google Scholar]
- 15.Affronti ML, Heery CR, Herndon JE, et al. Overall survival of newly diagnosed glioblastoma patients receiving carmustine wafers followed by radiation and concurrent temozolomide plus rotational multiagent chemotherapy. Cancer. 2009;115(15):3501–3511. [DOI] [PubMed] [Google Scholar]
- 16.Menei P, Metellus P, Parot-Schinkel E, et al. Biodegradable carmustine wafers (Gliadel) alone or in combination with chemoradiotherapy: the French experience. Ann Surg Oncol. 2010;17(7):1740–1746. [DOI] [PubMed] [Google Scholar]
- 17.La Rocca RV, Mehdorn HM. Localized BCNU chemotherapy and the multimodal management of malignant glioma. Curr Med Res Opin. 2009;25(1):149–160. [DOI] [PubMed] [Google Scholar]
- 18.Pan E, Mitchell SB, Tsai JS. A retrospective study of the safety of BCNU wafers with concurrent temozolomide and radiotherapy and adjuvant temozolomide for newly diagnosed glioblastoma patients. J Neurooncol. 2008;88(3):353–357. [DOI] [PubMed] [Google Scholar]
- 19.Pavlov V, Page P, Abi-Lahoud G, et al. Combining intraoperative carmustine wafers and Stupp regimen in multimodal first-line treatment of primary glioblastomas. Br J Neurosurgery. 2015; doi:10.3109/02688697.2015.1012051. [DOI] [PubMed] [Google Scholar]
- 20.Mirimanoff R-O, Gorlia T, Mason W, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol. 2006;24(16):2563–2569. [DOI] [PubMed] [Google Scholar]
- 21.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. [DOI] [PubMed] [Google Scholar]
- 22.Vogelbaum MA, Jost S, Aghi MK, et al. Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) working group. Neurosurgery. 2012;70(1):234–244. [DOI] [PubMed] [Google Scholar]
- 23.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 24.Macdonald DR, Cascino TL, Schold SC, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 25.Austin PC. An Introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387–398. [DOI] [PubMed] [Google Scholar]
- 28.Bock HC, Puchner MJA, Lohmann F, et al. First-line treatment of malignant glioma with carmustine implants followed by concomitant radiochemotherapy: a multicenter experience. Neurosurg Rev. 2010;33(4):441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noël G, Schott R, Froelich S, et al. Retrospective comparison of chemoradiotherapy followed by adjuvant chemotherapy, with or without prior gliadel implantation (carmustine) after initial surgery in patients with newly diagnosed high-grade gliomas. Int J Radiat Oncol Biol Phys. 2012;82(2):749–755. [DOI] [PubMed] [Google Scholar]
- 30.Miglierini P, Bouchekoua M, Rousseau B, et al. Impact of the per-operatory application of GLIADEL wafers (BCNU, carmustine) in combination with temozolomide and radiotherapy in patients with glioblastoma multiforme: efficacy and toxicity. Clin Neurol Neurosurg. 2012;114(9):1222–1225. [DOI] [PubMed] [Google Scholar]
- 31.Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. [DOI] [PubMed] [Google Scholar]
- 32.De Witt Hamer PC, Robles SG, Zwinderman AH, et al. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012;30(20):2559–2565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.