Abstract
Background
We aimed to identify a brief screening measure for detection of cognitive deficit in children treated for cerebellar tumors that would be useful in clinical practice.
Methods
A sample of 72 children, aged 8–14 years, and within 3 years post diagnosis for standard-risk medulloblastoma (n = 37) or low-grade cerebellar astrocytoma (n = 35) and 38 children in a nontumor group were assessed using teacher-, parent-, and child-report of the Behavior Rating Inventory of Executive Function (BRIEF), Strengths and Difficulties Questionnaire (SDQ), and Pediatric Quality of Life Inventory (PedsQL). The accuracy of these scores as a screen for a full-scale Intelligence Quotient (FSIQ) < 80 on the Wechsler Intelligence Scale for Children (WISC-IV UK) was assessed using their receiver operating characteristic (ROC) curves.
Results
The questionnaires with the highest areas under the ROC curves were the child- and parent-report PedsQL, the teacher-report BRIEF, and the SDQ. At optimal cutoff scores, their sensitivities (95% CIs) to cases of FSIQ < 80 were 84 (60–96)%, 65 (41–84)%, 79 (54–93)%, and 84 (60–96)%, and their specificities (95% CIs) were 79 (68–86)%, 87 (77–93)%, 77 (66–86)%, and 71 (64–84)% respectively. All cases of FSIQ < 80 screened positive on either teacher-report SDQ or self-report PedsQL.
Conclusions
The PedsQL child- and parent-report and the teacher-report BRIEF and SDQ have moderately good accuracy for discriminating between children with and without a FSIQ < 80. The PedsQL could be used in a clinical setting, and the BRIEF and SDQ in an educational setting, to screen for cases with FSIQ < 80 in children treated for brain tumors.
Keywords: cerebellar tumors, children, cognitive deficit, health-related quality of life, screening
Brain tumors are second in incidence to leukemia among the neoplasms of childhood and constitute 23% of all tumors that develop before the age of 15 years.1 Actuarial 5-year survival for all CNS tumors combined was 72%–75% among those diagnosed in the United Kingdom during 2001–20102 and 72% (95% CI, 71%–73%) in the SEER-18 cancer registries in the United States during 1995–2010.3
About half of long-term survivors of childhood brain tumors experience significant neurocognitive impairment4,5 attributed to the tumor itself, hydrocephalus, neurosurgery,6 adjuvant radiotherapy,7 or radiotherapy and chemotherapy in combination.8 They achieve significantly lower educational attainment than the general population9 and suffer long-term socio-economic and work place disadvantage.10,11 There is international agreement among experts that cognitive and psychosocial deficits affect health-related quality of life (HRQoL) for child and adolescent survivors of cancer, and monitoring of them should be a priority.12 To address these issues, there is a need for early and continuing systematic assessment of these children to identify the need to expedite implementation of clinical psychology and other services that would enable timely rehabilitation9,13–15 to improve their life chances.
Systematic assessment, however, is not always achieved in practice,9,13,16,17 even though guidelines advocate such access for all families,17,18 due to limited access to clinical psychology services that might provide this service.16,17 Prior attempts to screen specifically for cognitive deficits have used direct assessments requiring face-to-face administration,19–22 which is resource-intensive. These screening meaures typically need to be executed by a trained assessor19,20 or psychologist21,22 in a designated quiet room in a hospital setting, and the additional time needed may prolong the hospital visit for the patient by up to 75 minutes.21 Alternatively, screening for cognitive deficit using brief, accurate, psychometrically robust self-report measures in a clinical setting, initially without the need to engage psychological services, might suffice when limited resources preclude face-to-face psychometric assessment. Those children falling above or below specified cutoff scores, indicating clinical risk, could then undergo full rehabilitation assessment and be considered for intervention. Self-report paper-and-pencil measures have been successfully applied in clinical settings to screen for psychosocial difficulties in children treated for cancer,23–27 and a measure of parent-perceived cognitive function showed good ability to discriminate between childhood cancer survivors with and without a brain tumor.28
We found that cognition and emotion accounted for more than half of the variance in HRQoL scores in a representative sample of children treated for cerebellar tumors in the UK,15 and many other studies have found cognitive function to be associated with HRQoL.29–31 Our observations15 led us to hypothesize that poor scores on self-report measures of HRQoL, executive function, and behavioral function might be sufficiently accurate to be usable as a screen for deficits in those domains and also as screening tests for deficits in full-scale intelligence quotient (FSIQ) in these children. We therefore examine here the accuracy of 3 widely available questionnaire measures with good psychometric properties as screens for detecting children with borderline or greater cognitive deficit (defined as a FSIQ < 80).32 As far as we are aware, this is the first time that the accuracy of self-report measures have been studied as screening tests for detecting cognitive deficits, as defined by direct psychometric assessment, in children with cerebellar tumors.
Materials and Methods
Design
The present study was part of a multicenter prospective longitudinal HRQoL study that was undertaken from February 2005 to January 2010.
Patients
We have previously reported the population studied and the methods used to obtain both questionnaire responses and the FSIQ assessments from them in our report on factors predicting their HRQoL 2 years after enrollment in the study.15 Briefly, the participants were children aged 8–14 years with either “standard risk” medulloblastoma (ie, <1.5 cm3 residual tumor and no evidence of metastatic disease) or low-grade cerebellar astrocytoma diagnosed within the preceding 3 years. They were recruited from 11 of the 20 Children's Cancer and Leukaemia Group (CCLG) Children's Cancer Treatment Centres (CCTCs) in England and Wales over a period of 20 months. A nontumor comparison group was randomly selected from the same year groups of the schools attended by children in the tumor groups. The only noninclusion criteria in each group were premorbid disability or inability to communicate in the English language, but these criteria were not met in any child referred to the study. For the present study of the accuracy of questionnaires as screens for concurrent deficits in FSIQ, nonavailability of a FSIQ score at enrollment to the study was an exclusion criterion that was applied to 6 of the 110 participants in the HRQoL study.
All participating children diagnosed with cerebellar tumor had undergone neurosurgical removal of the tumor. Those with medulloblastoma also received adjuvant treatment comprising 6 weeks of daily craniospinal radiotherapy of 23.4 Gy with a boost to 55.8 Gy at the posterior fossa and Packer regimen chemotherapy (weekly vincristine for 8 weeks followed 6 weeks later by eight 6-week cycles of chemotherapy using CCNU and cisplatin plus vincristine given weekly for 3 weeks).33 There were no major deviations from this standard treatment.
Measures
We selected the following measures for their good psychometric properties, brevity, and applicability to children with brain tumors:15,22,37–39 parent- and teacher-report of the child's executive function in everyday life using the Behavior Rating Inventory of Executive Function (BRIEF);34 parent-, teacher-, and child-report of the child's behavior using the Strengths and Difficulties Questionnaire (SDQ);35 and parent- and child-report of the child's HRQoL using the Pediatric Quality of Life Inventory (PedsQL).15,22,36,37–39 These additional 7 questionnaires are of relatively low cost and are widely used in departments of clinical psychology. The Wechsler Intelligence Scale for Children-4th UK Edition (WISC-IV UK)32 was administered as a gold standard measure of cognitive function. We chose a FSIQ score < 80 as the defining threshold below which participants were classified as having borderline or greater cognitive deficit according to the WISC-IV UK manual.32 In a typically developing population, 9% of children and young people would be expected to be “cases” (ie, produce a FSIQ score <80) because this is the percentage of a normal distribution expected to fall more than 1.33 SD below the population mean. (Nine percent has been used in previous descriptions of cognitive deficits in similar populations.40,41) In our sample of children treated for cerebellar tumors, this degree of deficit was present in 19 of 66 (29%) children, and identifying them would therefore be a way to identif the proportion of the population in whom cognitive evaluation was most likely to lead to interventions to support learning.
Procedure
Children fulfilling inclusion criteria were identified from hospital discharge and clinic lists and referred to the study center by the treating clinicians. Written informed consent was obtained from all participating parents and children. Assessments were undertaken in the family home, to which questionnaires had been sent by post in advance, while the WISC was administered at the visit itself. Parents provided information on premorbid socio-economic status (SES) classified according to the UK Office for National Statistics Socio-economic Classification (ONS 2004). Teacher questionnaires were mailed to schools following the home visit. The protocol for this study was approved by the UK CCLG. Ethical approval was obtained from the Trent Multi-Centre Research Ethics Committee, UK.
Statistical Analyses
All available FSIQ scores, assessed at enrollment into the study, were included in the analyses. Screening accuracy was evaluated by plotting receiver operating characteristic (ROC) curves for each measure. The ROC curve is an X-Y graph of the accuracy of a screening test for a target condition. Sensitivities at all possible screening test threshold scores are plotted on the Y-axis against 1–specificity on the X-axis. A 45° diagonal line indicates a screening test operating at a chance level of separating true positive from true negative cases of the target condition. Youden's index, the maximum orthogonal distance between the 45° diagonal line and the ROC curve, identifies the optimal cutoff score that maximizes the extent to which the test separates true positives from true negatives.42 The area under the ROC curve (AUC) gives an overall summary of the screening test's accuracy for identifying the target condition: AUCs of >0.90, 0.70–0.90, and 0.50–0.70 are commonly taken to indicate high, moderate, and low accuracy, respectively ,while an AUC of 0.50 indicates a chance result.42 Having identified the optimal screen threshold score using Youden's index, the sensitivity (proportion of true positives that screen positive), specificity (proportion of true negatives that screen negative), likelihood ratio for a screen positive (LR+, the ratio of the probability of a true positive to the probability of a false positive), and for a screen negative (LR−, the ratio of the probability of a false negative to the probability of a true negative) for that threshold score were calculated for the total sample. A LR+ >7.00 and a LR− <0.30 indicate high screening accuracy.43 Youden's index, the AUC, sensitivity, specificity, LR+, and LR− are independent of the prevalence of a condition, whereas positive and negative predictive values are not.43 The AUCs, sensitivities, and specificities were calculated and their 95% confidence intervals used to define the precision of the estimates of accuracy of the screening tests. All analyses were conducted using IBM SPSS version 21.
Results
Sample Characteristics
Seventy-six children treated for cerebellar tumors were referred to the study center. Of these, 72 (95%) children (37 with medulloblastoma and 35 with astrocytoma) were enrolled over a 20-month period, of which FSIQ data obtained at the first assessment were available in 32 and 34 children in their respective groups. The annual rate of enrollment into the study over the 1.8-year recruitment period was 104% for medulloblastoma and 87% for astrocytoma of the expected number of diagnoses of eligible cases at the participating centers over that time, estimated from the relevant figures for disease incidence and time trends in the UK population.15 Of the 38 participants in the nontumor group, 25 were the first random choice, and 7 were the second random choice (the first family declined to participate). FSIQ data obtained at the first assessment were available for all of these children. In the present study, the 66 children treated for cerebellar tumors had a mean (range) time interval from tumor diagnosis of 16.3 (1–35) months (Table 1). Child and parent demographic characteristics were similar in the 2 tumor groups and the nontumor group at recruitment, except for an excess of lone parents, only children, lower parental educational qualifications, and occupations other than managerial or professional in families of children treated for medulloblastoma (Table 1). Mean scores for each measure showed poorer functioning in the tumor groups compared with the nontumor group (Table 2).
Table 1.
Child and parent characteristics by tumor group
| Medulloblastoma n = 32 | Astrocytoma n = 34 | Nontumor n = 38 | |
|---|---|---|---|
| Mean age in years (range) | 10.2 (8–14) | 10.4 (8–14) | 10.4 (8–14) |
| Mean age in years at diagnosis (range) | 8.8 (6–13) | 9.2 (5–14) | N/A |
| Mean months from diagnosis (range) | 17.6 (1–35) | 15.0 (1–35) | N/A |
| Parent mean age in years (SD) | 38.7 (5.1) | 40.8 (8.2) | 40.5 (5.3) |
| n (%) | n (%) | n (%) | |
| Female | 13 (41) | 23 (68) | 19 (50) |
| Mother respondent | 31 (97) | 31 (91) | 33 (87) |
| Lone-parent family | 6 (19) | 3 (9) | 5 (13) |
| Only child | 6 (19) | 3 (9) | 4 (11) |
| Parent education | |||
| None | 1 (3) | 2 (6) | 2 (5) |
| School | 12 (38) | 5 (15) | 7 (18) |
| College | 14 (44) | 18 (53) | 21 (55) |
| University | 4 (13) | 9 (27) | 8 (21) |
| Unknown | 1 (3) | 0 | 0 |
| SES prediagnosis: | |||
| Managerial/professional | 9 (28) | 21 (62) | 18 (47) |
| Intermediate | 12 (38) | 8 (24) | 7 (18) |
| Routine and manual | 7 (22) | 5 (15) | 10 (26) |
| Not working | 3 (9) | 0 | 3 (8) |
| Unknown | 1 (3) | 0 | 0 |
Abbreviations: N/A, not applicable; SD, standard deviation; SES, socio-economic status.
Table 2.
Mean (SD) scores for each screening measure by tumor group
| Measure | Medulloblastoma n = 32a | Astrocytoma n = 34a | Nontumor n = 38a |
|---|---|---|---|
| BRIEF (T score mean = 50, SD = 10)b | |||
| Parent | 55.3 (12.5) | 56.3 (11.4) | 51.2 (10.0) |
| Teacher | 60.1 (13.2) | 56.9 (14.4) | 51.0 (9.0) |
| SDQ (possible range 0–40)b | |||
| Parent | 10.7 (6.7) | 10.0 (6.0) | 8.1 (5.3) |
| Child | 9.7 (4.8) | 10.0 (5.8) | 8.8 (5.5) |
| Teacher | 9.0 (5.2) | 6.2 (5.1) | 4.7 (5.0) |
| PedsQL (possible range 0–100)c | |||
| Parent | 51.5 (20.8) | 68.2 (23.9) | 84.3 (11.0) |
| Child | 61.2 (18.2) | 71.3 (20.4) | 82.1 (12.3) |
BRIEF, Behavior Rating Inventory of Executive Functioning;34 PedsQL, Pediatric Quality of Life Inventory;36 SD, standard deviation; SDQ, Strengths and Difficulties Questionnaire.35
Numbers varied slightly for each measure and informant; higher scores.
Higher scores indicate increased dysfunction.
Higher scores indicate better quality of life.
Screening Accuracy of Each Measure
Thirteen (41%) of the children with medulloblastoma and 6 (18%) of those with cerebellar astrocytoma had a FSIQ < 80, compared with 1 (3%) in the nontumor group (Table 3 and Supplementary Table 1). Among the 18 children treated for cerebellar tumors who had FSIQ < 80 and for whom we had information about special education services, 3 (17%) were not attending school regularly, 3 (17%) were receiving no extra help, 5 (28%) were receiving help commensurate with their class mates (typically with reading and mathematics), and 7 (39%) were receiving specific individual help. Evaluation of the suitability of all 7 questionnaires as a screen for FSIQ < 80 demonstrated that they performed significantly better than chance for detecting cognitive deficit (P < .001) and with moderate accuracy indicated by AUCs that ranged between 0.73 and 0.85 (sensitivities ranging from 0.55 to 0.84, specificities ranging from 0.71 to 0.87; and LR+ values ranging from 2.74 to 4.90) (Table 4). The 95% CI of 4 of the 7 questionnaires fell entirely within the high-to-moderate accuracy range. These were the child- and parent-report PedsQL and the teacher-report BRIEF and SDQ (Table 4).
Table 3.
Screening for cognitive deficit following medulloblastoma, low-grade cerebellar astrocytoma, and nontumor comparison group: performance of 3 self- and proxy-report questionnaires
| Target Condition of WISC FSIQ < 80 Present (+) or Absent (−) |
||||||
|---|---|---|---|---|---|---|
| Medulloblastoma |
Astrocytoma |
Nontumor |
||||
| + | − | + | − | + | − | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| FSIQ < 80 n (%) | 13 (41) | 19 (59) | 6 (18) | 28 (82) | 1 (3) | 37 (97) |
| Screen positive (+) or screen negative (−) at optimal cut-off score point | ||||||
| BRIEF | ||||||
| Parent (>57) | ||||||
| + | 8 (62) | 4 (21) | 6 (100) | 10 (36) | 1 (100) | 7 (19) |
| − | 5 (38) | 15 (79) | 0 | 18 (64) | 0 | 30 (81) |
| Teacher (>59) | ||||||
| + | 11 (85) | 3a (19) | 3b (60) | 8b (30) | 1 (100) | 7b (19) |
| − | 2 (15) | 13a (81) | 2b (40) | 19b (70) | 0 | 29b (81) |
| SDQ | ||||||
| Child (>11) | ||||||
| + | 9b (75) | 4 (21) | 3 (50) | 10 (36) | 1 (100) | 7 (19) |
| − | 3b (25) | 15 (79) | 3 (50) | 18 (64) | 0 | 30 (81) |
| Parent (>14) | ||||||
| + | 6 (46) | 3 (16) | 5 (83) | 4 (14) | 1 (100) | 4 (11) |
| − | 7 (54) | 16 (84) | 1 (17) | 24 (86) | 0 | 33 (89) |
| Teacher (>7) | ||||||
| + | 12 (92) | 6c (35) | 3b (60) | 8b (30) | 1 (100) | 6b (17) |
| − | 1 (8) | 11c (65) | 2b (40) | 19b (70) | 0 | 30b (83) |
| PedsQL | ||||||
| Child (<65) | ||||||
| + | 9b (75) | 9 (47) | 6 (100) | 6 (21) | 1 (100) | 3 (8) |
| − | 3b (25) | 10 (53) | 0 | 22 (79) | 0 | 34 (92) |
| Parent (<51) | ||||||
| + | 8 (62) | 9b (50) | 5 (83) | 2 (7) | 0 | 0 |
| − | 5 (38) | 9b (50) | 1 (17) | 26 (93) | 1 (100) | 37 (100) |
Table 4.
Screening accuracy to detect a full scale IQ < 80 based on maximum Youden's Index for each measure
| Measure | N | Informant | Cutoff Score | AUC (95% CI) | Sens% (95% CI) | Spec% (95% CI) | LR+ | LR− | J |
| BRIEFa | 104 | Parent | >57 | 0.76 (0.61–0.90) | 75 (51–90) | 75 (64–84) | 3.00 | 0.33 | 0.50 |
| 98 | Teacher | >59 | 0.82 (0.70–0.94) | 79 (54–93) | 77 (66–86) | 3.46 | 0.27 | 0.56 | |
| SDQa | 103 | Child | >11 | 0.73 (0.63–0.84) | 68 (43–86) | 75 (64–84) | 2.74 | 0.42 | 0.43 |
| 104 | Parent | >14 | 0.78 (0.67–0.89) | 55 (36–80) | 87 (77–93) | 4.20 | 0.52 | 0.42 | |
| 99 | Teacher | >7 | 0.80 (0.70–0.94) | 84 (60–96) | 71 (64–84) | 2.93 | 0.22 | 0.56 | |
| PedsQLb | 103 | Child | <65 | 0.85 (0.77–0.93) | 84 (60–96) | 79 (68–86) | 3.93 | 0.20 | 0.63 |
| 103 | Parent | <51 | 0.82 (0.72–0.92) | 65 (41–84) | 87 (77–93) | 4.90 | 0.40 | 0.52 |
Abbreviations: AUC, area under the curve; BRIEF, Behavior Rating Inventory of Executive Functioning;34 J, Youden Index; LR+, likelihood ratio for a positive test; LR−, likelihood ratio for a negative test; PedsQL, Pediatric Quality of Life Inventory;36 SDQ, Strengths and Difficulties Questionnaire;35 Sens, sensitivity; Spec, specificity.
All AUCs were significant at P < 001.
Higher scores indicate increased dysfunction.
Higher scores indicate better quality of life.
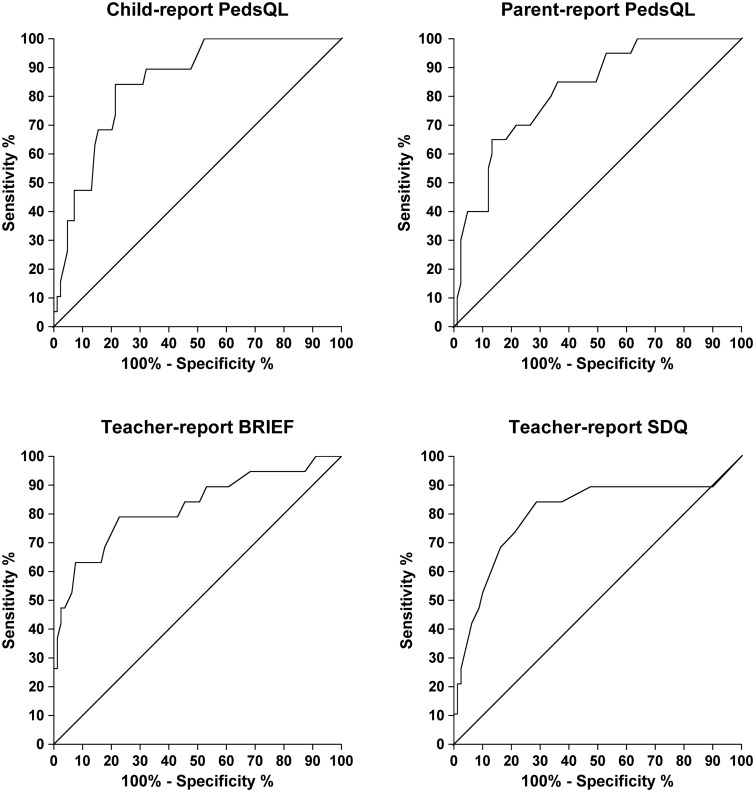
Youden's index identified the optimum screen threshold score. Child-report PedsQL had the greatest AUC (score <65 = positive screen, sensitivity 0.84, specificity 0.79, and LR+ of 3.93). Parent-report PedsQL (score <51 = positive screen, sensitivity 0.65, specificity 0.87, LR+ 4.90), teacher-report BRIEF (score >59 = positive screen, sensitivity 0.79, specificity 0.77, LR+ 3.46), and SDQ (score >7 = positive screen, sensitivity 0.84, specificity 0.71) had AUCs that were slightly lower but with 95% CIs that overlapped with those of the AUC for child-report PedsQL (Fig. 1; Table 4).
Fig. 1.
Receiver operating characteristic curves showing percentages of sensitivity and specificity for the 3 measures that show the highest accuracy in detecting a FSIQ < 80. PedsQL (n = 103), Pediatric Quality of Life Inventory;36 BRIEF (n = 98), Behavior Rating Inventory of Executive Functioning;34 SDQ (n = 99), Strengths and Difficulties Questionnaire.35
Screening by using a score beyond the threshold value on either the teacher-report SDQ (sensitivity 0.84, specificity 0.71) or the self-report PedsQL (sensitivity 0.84, specificity 0.79) increased sensitivity (95% CI) to cases of FSIQ < 80 to 1.00 (0.79–1.00) (Supplementary Table 1) but reduced specificity (95% CI) from the above figures to 0.65 (0.53–0.75) (figures not tabulated).
Discussion
All screening measures correctly identified 55%–84% of children with borderline or greater deficit in FSIQ and correctly identified 71%–87% of children without a deficit. The precision of these estimates was sufficient to indicate that the child- and parent-report PedsQL and the teacher-report BRIEF and SDQ were moderately or highly accurate screens. Screening positive on any one of the child-report PedsQL or the teacher-report SDQ correctly identified all cases with FSIQ < 80 but decreased the specificity of the screen.
Our findings are likely to be generalizable to the great majority of children with cerebellar tumors for 2 reasons. First, we included the 2 most common tumor types and the 2 most common combinations of treatment modalities, namely surgery alone and surgery combined with both craniospinal radiation therapy and chemotherapy. Second, the population base comprising the catchment area populations of half (11 of 22) of all UK CCTCs was large, and the number of children in the tumor groups who were enrolled was close to the total number of cases predicted (from UK national figures for incidence and time trends) to present over the 20-month recruitment period.44
The inclusion of an unbiased sample of children of the same age (but without tumors) in the general population enabled us to increase the sample size and therefore the precision of our estimates of accuracy as indicated by likelihood ratios and similar measures of accuracy that are independent of the population prevalence of the target condition unlike predictive values, which are prevalence dependent.43,45
The use of the WISC as a gold standard measure for cognitive function is a strength of this study, and the choice of threshold of FSIQ < 80 (below which 9% of a typically developing population and 29% of our sample of children treated for cerebellar tumors falls) is a reasonable pragmatic decision, especially when resources only allow direct assessment of a minority of cases.
The added benefit of the 3 questionnaires that we used is that they also provide a screen for problems with executive function, behavioral and emotional problems, and HRQoL, and their constituent domains of functioning, which is another strength of the approach described in the present study as it thus avoids focusing too narrowly on those who display low IQ. In fact, 2 of these 3 measures are included in the short battery of assessments devised and shown to be deliverable in the setting of a US Children's Oncology Group trial.22 The PedsQL has been shown to have an impact on clinical intervention decision-making in pediatric clinic settings for children with rheumatic, cardiac, and orthopedic problems.27 An et al (2013)29 reported strong correlations between child-report PedsQL and FSIQ in children aged 6–13 years treated for brain tumors. This is supported by the present study.
Conversely, one potential limitation of a screening approach is its reduction of cognitive ability to any single number and the accompanying narrowing of the scope of the cognitive deficits to which it is sensitive. This is to some extent unavoidable in the quest for a simple short screening test that requires definition of a unitary target condition. Many survivors of CNS tumors have problems with specific skills (eg, attention and processing speed) that will impair their academic performance significantly without leading to a decrease in their FSIQ to <80.8 If access to a clinical psychologist can be obtained routinely, a full psychological evaluation (including tests of attention, processing speed, working memory, and executive function,4,7,8,46,47) is preferable to any single screening test. When, on the other hand, it is not possible for a child to gain access to a psychometric assessment, there are some cases in which there the specific cognitive deficits in which the BRIEF score was not sufficiently abnormal to constitute a positive screen for FSIQ < 80, would nevertheless come to light by closer examination of BRIEF scores and sub-scores by a psychologist who has the knowledge and training to interpret BRIEF profiles. For these reasons, the screening approach that we propose does not only provide a simple indication of cognitive deficit but also permits consideration of the executive, emotional, physical, and psychosocial aspects that impinge on cognitive function. Examination of subscale scores might be seen as defeating the purpose of screening, which needs to divide those screened into screen-positive and screen-negative groups, but future work could examine incorporating these simple reports into the screening process, based on automated on-line scoring in which subscale scores that identify neurocognitive dysfunction evident before FSIQ scores are affected (eg, processing speed, working memory) could be categorized by scoring centile as green, amber, or red.
A second limitation of our study, which was designed primarily to assess HRQoL in children old enough to provide reliable self-report and young enough to remain within the pediatric age range after 24 months of follow-up, was the fact that the age range was restricted to 8–14 years. Our findings may not apply to younger children, although children as young as 5 years can reliably and validly self-report using the PedsQL.48 Further studies are also needed in children treated for tumors in other locations (particularly supratentorial) to examine the performance of these screening measures in those contexts.
Teacher-report of executive function and behavioral difficulties proved to be an accurate source of information about a child's cognitive and behavioral functioning in our study and was relatively strongest in those treated for medulloblastoma, in whom it correctly identified 85%–92% of true cases of FSIQ < 80 compared with only 60% of true cases in the astrocytoma group. In contrast, parent- and child-report HRQoL correctly identified 83%–100% of true cases in the astrocytoma group but only 67%–75% of cases in the medulloblastoma group. This may indicate differences in the variation in screening accuracy of the measures within a clinical context or in the sensitivity of teacher-, child-, and parent-report to cognitive deficits or both. This could be explored in future research with a larger sample of children treated for low-grade astrocytoma because this group contained few cases of FSIQ < 80 in the present study.
Our finding that accuracy of 100% was obtainable by accepting a score beyond the threshold value for either the self-report PedsQL or the teacher-report SDQ was adopted as a strategy post hoc, and this needs to be tested by an independent sample in future research. If high accuracy were confirmed, the use of teacher-reports as an approach to screening would only succeed in an educational context in which teachers are willing to provide their responses to health providers. Such success in liaising with teachers would itself constitute an important step towards aligning clinical and educational perspectives on the child's needs.
It is important to stress that we would recommend repeated annual screening through the acute phase of survival into the longer-term during the school years for the detection of cognitive deficits as problems emerge over time.29,41 Participants in the present study were all less than 3 years from diagnosis at enrollment. Both tumor groups showed an increase in their group mean FSIQ over the 24 months they remained in the study,15 but it is well established that failure to acquire new skills may lead to a fall in FSIQ over time in children treated with cranial radiotherapy8 as well as those treated with neurosurgery alone,46 with some problems not being fully manifested until more than 5 years from diagnosis.4 It is possible that the screening battery used here may be sensitive to neuropsychological difficulties related to medical factors known to affect cognitive processes more acutely in the perioperative period (eg, complications from treatment of hydrocephalus, perioperative infections, or hemorrhage) rather than those that emerge over time. Nonetheless, 85% of the sample had been diagnosed more than 6 months previously and were therefore well past the perioperative period.
In clinical settings where access to pediatric neuropsychologists is readily available, we support use of a short battery, such as those proposed by Embry et al22 in the United States or by Ottensmeier et al (2014)49 in Germany,and have made recommendations (together with colleagues from 10 other countries across Europe) on the domains and assessments of those domains that should be prioritized when assessing survivors of childhood brain tumors.39 These psychometric assessments are, however, often not available outside Europe, North America, or some countries within Europe, even in the context of treatment trials and are not undertaken in most children treated in any country outside the setting of a clinical trial (eg, many children with low grade cerebellar astrocytomas). By contrast, the use of questionnaires has been substantiated across several European countries in a treatment trial for medulloblastoma,37 having been achieved with web-based versions of the questionnaires in a current European treatment trial and potentially being applicable over a wider geographical area and clinical context. The present study suggests that, where access to psychometric assessment is limited, questionnaires may provide a pragmatic screen for prioritizing direct psychometric assessment of those most likely to have cognitive deficits in FSIQ and are therefore ery likely to need support to ameliorate these difficulties.
The fact that only 39% of the children treated for cerebellar tumors had a FSIQ < 80 and were identified as having specific educational needs at school reflects the schools' lack of experience meeting the needs of the relatively rare child with newly acquired (rather than developmental) cognitive deficits. This further suggests that screening children for low FSIQ by those delivering their health care could help alert schools to the need of assessing individual special needs in order to characterize their developing pattern of difficulties and initiate treatment or services before the problems worsen and cause greater functional impairment These survivors may actually be most in need of neuropsychological follow-up in order to characterize their developing pattern of difficulties and to initiate treatment or services before the problems worsen and cause greater functional impairment. The extent to which this applies to countries other than the UK merits further study.
In summary, the child- and parent-report PedsQL and the teacher-reports BRIEF and SDQ have demonstrated moderately good discriminative power for identifying children with and without a FSIQ < 80, including 79%–84% sensitivity to FSIQ < 80. The PedsQL could be used in a clinical setting, and the BRIEF and SDQ in an educational setting, to detect cognitive deficits as well as problems with emotional and behavioral disorders, executive dysfunction, and poor HRQoL in children treated for a brain tumor and indicate the need for referral to more comprehensive psychological evaluation at an early stage.
Supplementary Material
Funding
The Brain Tumour Charity (formerly the Samantha Dickson Brain Tumour Trust and Brain Tumour UK) funded the study, which was conducted with the support of the Children′s Cancer and Leukaemia Group (CCLG).
Supplementary Material
Acknowledgments
We are indebted to the many individuals who helped to facilitate this study, including all of the consultants, research nurses, and other hospital staff who made this study possible and, above all, to the children, their schools, and their parents. JLP is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas′ NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Conflict of interest statement. None declared.
References
- 1.Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev. 2010;36(4):277–285. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Intelligence Network. National Registry of Childhood Tumours Progress Report, 2012. http://www.ncin.org.uk/cancer_type_and_topic_specific_work/cancer_type_specific_work/cancer_in_children_teenagers_and_young_adults/. Accessed December 4, 2014.
- 3.Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15:1–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong GT, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101(13):946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roncadin C, Dennis M, Greenberg ML, et al. Adverse medical events associated with childhood cerebellar astrocytomas and medulloblastomas: natural history and relation to very long-term neurobehavioral outcome. Childs Nerv Syst. 2008;24(9):995–1002. [DOI] [PubMed] [Google Scholar]
- 6.Turner CD, Chordas CA, Liptak CC, et al. Medical, psychological, cognitive and educational late-effects in pediatric low-grade glioma survivors treated with surgery only. Pediatr Blood Cancer. 2009;53(3):417–423. [DOI] [PubMed] [Google Scholar]
- 7.Tonning Olsson I, Perrin S, Lundgren J, Hjorth L, Johanson A. Long-term cognitive sequelae after pediatric brain tumor related to medical risk factors, age, and sex. Pediatr Neurol. 2014;51(4):515–521. [DOI] [PubMed] [Google Scholar]
- 8.Duffner PK. Risk factors for cognitive decline in children treated for brain tumors. Eur J Paediatr Neurol. 2010;14(2):106–115. [DOI] [PubMed] [Google Scholar]
- 9.Lancashire ER, Frobisher C, Reulen RC, et al. Educational attainment among adult survivors of childhood cancer in Great Britain: a population-based cohort study. J Natl Cancer Inst. 2010;102(4):254–270. [DOI] [PubMed] [Google Scholar]
- 10.Boman KK, Lindblad F, Hjern A. Long-term outcomes of childhood cancer survivors in Sweden: a population-based study of education, employment, and income. Cancer. 2010;116(5):1385–1391. [DOI] [PubMed] [Google Scholar]
- 11.Olson R, Hung G, Bobinski MA, Goddard K. Prospective evaluation of legal difficulties and quality of life in adult survivors of childhood cancer. Pediatr Blood Cancer. 2011;56(3):439–443. [DOI] [PubMed] [Google Scholar]
- 12.Kremer LCM, Mulder RL, Oeffinger KC, et al. A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer. 2013;60(4):543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aukema EJ, Last BF, Schouten-van Meeteren AYN, Grootenhuis MA. Explorative study on the aftercare of pediatric brain tumor survivors: a parents' perspective. Support Care Cancer. 2011;19(10):1637–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eshelman-Kent D, Kinahan KE, Hobbie W, et al. Cancer survivorship practices, services, and delivery: a report from the Children's Oncology Group (COG) nursing discipline, adolescent/young adult, and late effects committees. J Cancer Surviv. 2011;5(4):345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bull KS, Liossi C, Culliford D, et al. Child-related characteristics predicting subsequent health-related quality of life in 8- to 14-year-old children with and without cerebellar tumors: a prospective longitudinal study. Neurooncol Pract. 2014;1(3):114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selove R, Kroll T, Coppes M, Cheng Y. Psychosocial services in the first 30 days after diagnosis: results of a web-based survey of Children's Oncology Group (COG) member institutions. Pediatr Blood Cancer. 2012;58(3):435–440. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell W, Clarke S, Sloper P. Survey of psychosocial support provided by UK paediatric oncology centres. Arch Dis Child. 2005;90(8):796–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Children's Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers, Version 4.0. 2013. http://www.survivorshipguidelines.org Accessed March 27, 2015.
- 19.Krull KR, Okcu MF, Potter B, et al. Screening for neurocognitive impairment in pediatric cancer long-term survivors. J Clin Oncol. 2008;26(25):4138–4143. [DOI] [PubMed] [Google Scholar]
- 20.Castellino SM, Tooze JA, Flowers L, Parsons SK. The peabody picture vocabulary test as a pre-screening tool for global cognitive functioning in childhood brain tumor survivors. J Neurooncol. 2011;104(2):559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pejnovic LP, De Luca CR, Gentle E, et al. Feasibility of neurobehavioral screening following diagnosis of pediatric cancer. Pediatr Blood Cancer. 2012;59(2):295–300. [DOI] [PubMed] [Google Scholar]
- 22.Embry L, Annett RD, Kunin-Batson A, et al. Implementation of multi-site neurocognitive assessments within a pediatric cooperative group: can it be done? Pediatr Blood Cancer. 2012;59(3):536–539. [DOI] [PubMed] [Google Scholar]
- 23.Liptak C, Manley P, Recklitis CJ. The feasibility of psychosocial screening for adolescent and young adult brain tumor survivors: the value of self-report. J Cancer Surviv. 2012;6(4):379–387. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg-Kushnir N, Freedman S, Eshel R, et al. Screening tool for late-effect pediatric neuro-oncological clinics: a treatment-oriented questionnaire. Pediatr Blood Cancer. 2013;60(8):1369–1374. [DOI] [PubMed] [Google Scholar]
- 25.Kazak AE, Barakat LP, Hwang WT, et al. Association of psychosocial risk screening in pediatric cancer with psychosocial services provided. Psychooncology. 2011;20(7):715–723. [DOI] [PubMed] [Google Scholar]
- 26.Pai AL, Patiño-Fernandez AM, McSherry M, et al. The Psychosocial Assessment Tool (PAT2.0): psychometric properties of a screener for psychosocial distress in families of children newly diagnosed with cancer. J Pediatr Psychol. 2008;33(1):50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varni JW, Seid M, Knight TS, et al. The PedsQL (TM) 4.0 Generic Core Scales: sensitivity, responsiveness, and impact on clinical decision-making. J Behav Med. 2002;25(2):175–193. [DOI] [PubMed] [Google Scholar]
- 28.Lai J-S, Zelko F, Krull KR, et al. Parent-reported cognition of children with cancer and its potential clinical usefulness. Qual Life Res. 2014;23(4):1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.An KJ, Joung YS, Sung KW, et al. Health-related quality of life and cognitive functioning at on- and off-treatment periods in children aged between 6-13 years old with brain tumors: a prospective longitudinal study. Yonsei Med J. 2013;54(2):306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhlthau KA, Pulsifer MB, Yeap BY, et al. Prospective study of health-related quality of life for children with brain tumors treated with proton radiotherapy. J Clin Oncol. 2012;30(17):2079–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reimers TS, Mortensen EL, Nysom K, Schmiegelow K. Health-related quality of life in long-term survivors of childhood brain tumors. Pediatr Blood Cancer. 2009;53(6):1086–1091. [DOI] [PubMed] [Google Scholar]
- 32.Wechsler D. Wechsler Intelligence Scale for Children. 4th ed London, UK: Harcourt Assessment; 2004. [Google Scholar]
- 33.Packer RJ, Goldwein J, Nicholson HS, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: a Children's Cancer Group study. J Clin Oncol. 1999;17(7):2127–2136. [DOI] [PubMed] [Google Scholar]
- 34.Gioia GA, Isquith PK, Guy SC, et al. Behavior Rating Inventory of Executive Function™ Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc, 2000. [Google Scholar]
- 35.Goodman R. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry. 1997;38(5):581–586. [DOI] [PubMed] [Google Scholar]
- 36.Varni JW, Seid M, Rode CA. The PedsQL™: measurement model for the pediatric quality of life inventory. Med Care. 1999;37(2):126–139. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy C, Bull K, Chevignard M, et al. Quality of survival and growth in children and young adults in the PNET4 European controlled trial of hyperfractionated versus conventional radiation therapy for standard-risk medulloblastoma. Int J Radiat Oncol Biol Phys. 2014;88(2):292–300. [DOI] [PubMed] [Google Scholar]
- 38.Bull KS, Spoudeas HA, Yadegarfar G, et al. Reduction of health status 7 years after addition of chemotherapy to craniospinal irradiation for medulloblastoma: a follow-up study in PNET 3 trial survivors - On behalf of the CCLG (formerly UKCCSG). J Clin Oncol. 2007;25(27):4239–4245. [DOI] [PubMed] [Google Scholar]
- 39.Limond JA, Bull KS, Calaminus G, et al. Quality of survival assessment in European childhood brain tumour trials, for children aged 5 years and over. Eur J Paediatr Neurol. 2015;19(2):202–210. [DOI] [PubMed] [Google Scholar]
- 40.Duffner PK, Cohen ME, Thomas P. Late effects of treatment on the intelligence of children with posterior-fossa tumors. Cancer. 1983;51(2):233–237. [DOI] [PubMed] [Google Scholar]
- 41.Grill J, Renaux VK, Bulteau C, et al. Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int J Radiat Oncol Biol Phys. 1999;45(1):137–145. [DOI] [PubMed] [Google Scholar]
- 42.Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr. 2007;96(5):644–647. [DOI] [PubMed] [Google Scholar]
- 43.Fischer JE, Bachmann LM, Jaeschke R. A readers' guide to the interpretation of diagnostic test properties: clinical example of sepsis. Intensive Care Med. 2003;29(7):1043–1051. [DOI] [PubMed] [Google Scholar]
- 44.Kroll ME, Carpenter LM, Murphy MF, et al. Effects of changes in diagnosis and registration on time trends in recorded childhood cancer incidence in Great Britain. Br J Cancer. 2012;107(7):1159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akobeng AK. Understanding diagnostic tests 1: sensitivity, specificity and predictive values. Acta Paediatr. 2007;96(3):338–341. [DOI] [PubMed] [Google Scholar]
- 46.Aarsen FK, Paquier PF, Arts WF, et al. Cognitive deficits and predictors 3 years after diagnosis of a pilocytic astrocytoma in childhood. J Clin Oncol. 2009;27(21):3526–3532. [DOI] [PubMed] [Google Scholar]
- 47.Palmer SL, Armstrong C, Onar-Thomas A, et al. Processing speed, attention, and working memory after treatment for medulloblastoma: an international, prospective, and longitudinal study. J Clin Oncol. 2013;31(28):3494–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varni JW, Limbers C, Burwinkle TM. Literature review: health-related quality of life measurement in pediatric oncology: hearing the voices of the children. J Pediatr Psychol. 2007;32(9):1151–1163. [DOI] [PubMed] [Google Scholar]
- 49.Ottensmeier H, Zimolong B, Wolff JE, et al. Neuropsychological short assessment of disease- and treatment-related intelligence deficits in children with brain tumours. Eur J Paediatr Neurol. 2015;19(3):298–307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.