Abstract
Background
We report a phase I study to examine the pharmacokinetics, safety, and recommended dosage of weekly intravenous bolus 5-fluorouracil (5-FU) in children and young adults with recurrent ependymoma.
Methods
Patients 22 years of age or less with recurrent ependymoma were treated with bolus dosage 5-FU weekly for 4 weeks followed by a 2-week rest period, defining one cycle. Patients could continue on therapy for 16 cycles. The starting 5-FU dosage was 500 mg/m2. Dose-limiting toxicity was determined after one cycle. Patients were initially enrolled according to a rolling-6 design; subsequent dose re-escalation phase was based on a 3 + 3 design.
Results
We treated patients at 400 (n = 6), 500 (n = 15), and 650 (n = 5) mg/m2, with de-escalation due to toxicity. Twenty-three of twenty-six patients enrolled were evaluable. Five patients experienced grade 4 neutropenia (n = 2: 650 mg/m2; n = 3: 500 mg/m2). One patient experienced grade 3 diarrhea. At 500 mg/m2, the median 5-FU maximal concentration, AUC0–∞, and alpha half-life were 825 µM, 205 µM × h, and 9.9 min, respectively. Interim analysis revealed an association between hematologic toxicity and prior number of chemotherapeutic regimens (P = .03). The study was amended to re-escalate the dosage in a less heavily pretreated cohort of patients.
Conclusions
These phase I clinical data provide initial pharmacokinetic parameters to describe i.v. bolus 5-FU disposition in children with recurrent ependymoma. Tumor exposures effective in preclinical testing can be achieved with tolerable bolus dosages in patients. Bolus 5-FU is well tolerated and possesses antitumor activity.
Keywords: 5-fluorouracil, pharmacokinetics, phase I, toxicity
Ependymoma accounts for ∼9% of newly diagnosed brain tumors in children <18 years of age. Standard care for ependymoma remains surgical resection followed by postoperative radiation therapy (RT). Three-year progression-free survival following irradiation has been reported as high as 75% in certain series; however, for very young children (<3 y), immediate postoperative RT is not widely accepted due to the associated neurocognitive and endocrinologic sequelae.1 Multi-agent chemotherapy has been administered in an effort to delay or avoid RT in very young patients and is currently part of ongoing trials to treat patients with newly diagnosed ependymoma. For patients with metastatic disease at diagnosis, there is currently no consensus on how to best treat their disease. Prognosis of patients with recurrent ependymoma remains poor despite advances in our understanding of tumor biology, and no convincing role for chemotherapy has been established for patients with recurrent disease. Results of studies of conventional or molecular targeted chemotherapies have proved negative.2 Thus, there is a great need for effective new chemotherapies that improve survival while preserving neurocognitive function.
Using next-generation sequencing approaches and cross-species genomics, 2 distinct subtypes of supratentorial ependymomas characterized by the presence or absence of the C11orf95-RELA translocation have been identified (hereafter termed “fusion positive” and “fusion negative” disease).3 Application of cross-species genomics also identified cerebral neural stem cells as a potential origin of supratentorial ependymoma.4 Targeting these cells in mice led to the development of the first mouse models of fusion-positive and fusion-negative disease.3,5 We are now employing these models in a series of high-throughput drug screening campaigns to select potential new treatments of ependymoma for clinical trials.6 The first of these studies identified bolus 5-fluorouracil (5-FU) as a potential treatment of supratentorial fusion-negative ependymoma.6 Specifically, high-throughput drug screening and subsequent preclinical in vivo pharmacokinetic and efficacy studies demonstrated that bolus 5-FU halved the rate of tumor growth and significantly prolonged survival of mice harboring a form of fusion-negative mouse ependymoma driven by the Ephb2 oncogene (mEPEphb2).6,7 Notably, this activity of 5-FU was supported by the relatively low expression of thymidylate synthase in mouse and human ependymomas.6 It has long been recognized that low expression of thymidylate synthase—the main target of 5-FU—sensitizes cells to the drug.8–11 Additional studies of xenografts of a human posterior fossa ependymoma suggested that 5-FU might also have activity against this form of the disease.6
Although 5-FU is a well-established chemotherapeutic for colorectal and other cancers and has activity against glioblastoma, bolus 5-FU has not been tested formally in patients with ependymoma.12–18 The primary objectives of the present study were to investigate the pharmacokinetics and safety of weekly bolus dose 5-FU in children and young adults with recurrent ependymoma. Secondary objectives included description of preliminary antitumor activity in the context of disease subtype.
Materials and Methods
Eligibility
Eligible patients were age ≤22 years with a diagnosis of recurrent intracranial or spinal ependymoma confirmed by central pathology review. Patients who received prior radiation had to be ≥6 months from craniospinal irradiation, ≥4 weeks from focal irradiation to the primary tumor, or ≥2 weeks from focal irradiation to symptomatic metastatic sites. Initially there was no restriction on the number of prior chemotherapy regimens; however, during the early phase of the study, an association was noted between toxicity and prior number of chemotherapies, prompting an amendment to the study restricting patients to a minimum of 2 prior chemotherapy regimens and 2 courses of irradiation (focal or cranial spinal).
All participants were required to undergo testing for a mutation in the dihydropyrimidine dehydrogenase (DPD) enzyme encoded by the DPYD gene, which is responsible for the degradation and inactivation of >80% of 5-FU. DPYD mutations are associated with decreased DPD activity, leading to increased risk of dose-related 5-FU sensitivity.19,20 In patients with a particular mutation termed DPYD*2, there is no activity of the enzyme and therefore patients are at risk for toxicity-related death.21 Thus patients with this mutation were not eligible for the study. No patients tested positive for this polymorphism.
Patients were required to have adequate bone marrow, renal, hepatic, and cardiac function. Those participants taking corticosteroids had to be receiving a stable or decreasing dose for at least 1 week prior to registration. Patients with seizure disorder were required to be well controlled on their current anti-epileptic regimen.
Exclusion criteria included patients having been treated with 5-FU previously, patients receiving any other anticancer or experimental therapy, patients with unstable systemic diseases, pregnant or lactating women, or any condition that potentially hindered compliance with study protocol and the follow-up schedule. The study was approved by the institutional review board according to local and national guidelines. All patients gave written informed consent to screening and study entry.
Assessment
Toxicities were graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. In regard to hematologic toxicity, the dose limiting toxicities (DLTs) for this study were defined as any grades 3 and 4 thrombocytopenia or grade 4 neutropenia of any duration during cycle 1. Additionally, any grade 3 or 4 nonhematologic toxicities were considered DLTs during the first cycle with the exception of the following: grade 3 weight gain or loss; grade 3 diarrhea lasting <3 days with optimal antidiarrheal treatment; elevations of grade 3 transaminase (aspartate aminotransferase or alanine aminotransferase) that returned to grade ≤1 (if normal at study entry) or less than or equal to baseline values within 7 days of interrupting study drug; grade 3 or 4 electrolyte abnormalities that resolved to grade ≤2 within 7 days with or without clinical intervention; and grade 3 or 4 nausea or vomiting that was controlled with anti-emetic therapy. Lastly, any grade 2 nonhematologic toxicity was considered a DLT if persisting more than 7 days and felt to cause significant symptoms or dangerous medical repercussions to warrant treatment interruption or dose reduction.
Study Design and Dosing
A “rolling-6” phase I design as introduced by Skolnik et al was used to estimate the maximum tolerated dosage (MTD), with dosage escalations planned in cohorts of 2 to 6 patients.22–24 This design allows for accrual of 2–6 patients concurrently onto a dosage level which results in reducing the overall trial duration compared with the traditional 3 plus 3 designs.24 Dosage level 1, which corresponded to 500 mg/m2, was the starting dosage for the trial. De-escalation to dosage level 0, 400 mg/m2, occurred in the event that dosage level 1 produced 2 or more DLTs. Escalation to dosage level 2, which corresponded to 650 mg/m2, occurred if 0 of 3–6 or at most 1 of 6 evaluable patients experienced a DLT while being treated at this dosage level. On the other hand, if 2 of 2–6 patients experienced DLTs, then the dosage was declared too toxic and thus above the MTD. Once a dosage was determined to be too toxic, no escalation to a higher dosage level was allowed. Upon establishment of the MTD, 6 additional patients were treated at that dosage level to better describe the toxicity profile of bolus 5-FU.
An interim analysis was conducted due to unexpected toxicity encountered early in the trial. That analysis suggested that more heavily pretreated patients were prone to DLT. Thus, the study was amended as follows: (i) exclusion of more heavily pretreated patients, defined as those with >2 prior chemotherapy regimens or 2 prior irradiation courses and (ii) reversion to a traditional 3 plus 3 design.
Dosing
Initially, 3 dosage levels of 5-FU were planned. The drug was administered intravenously on a weekly basis for 4 weeks followed by a 2-week break period, defining one cycle. The weekly infusions were given every 7 days (±1 d). A participant's 5-FU dosage was not changed during the DLT evaluation period unless a DLT occurred. For research participants who experienced DLT(s) during therapy, the 5-FU dosage was resumed at the next lower dosage level if the toxicity resolved to CTCAE v4.0 grade ≤2 within 21 days or resolution to grade ≤1 toxicity for participants who experienced grade 2 intolerable toxicity. Participants could have a maximum of 1 dosage reduction. If the drug was not tolerated after 1 dosage reduction, the participant was removed from treatment. Treatment was discontinued for participants who were already receiving 5-FU at the lowest dosage level and experienced DLT at that level. Doses held due to toxicity were not considered missed doses but rather delays in the administration schedule.
Treatment and Toxicity Monitoring
Full clinical and laboratory examinations along with MRI of the brain and spine were performed within the 2 weeks prior to starting treatment. Lumbar puncture was done prior to study entry if not contraindicated by imaging, and thereafter only upon evidence of tumor cells in the cerebrospinal fluid on initial sampling. Physical exams were required weekly during the first 4 weeks of the DLT period and then only prior to each cycle thereafter. A complete laboratory panel was conducted weekly during the first 6 weeks of therapy and then only prior to each infusion after the DLT period ended. Brain MRI was assessed at the end of the DLT evaluation period and then following every 2 cycles. Follow-up spine imaging was conducted on the same schedule only if evidence of spinal disease was present at study start or suspicion of new disease. Disease assessment by MRI was based on the modified Response Assessment in Neuro-Oncology criteria.25–27
Pharmacokinetics
Pharmacokinetic studies were performed in all patients with the first, second, and fourth 5-FU infusion during course 1 and with the first 5-FU infusion during course 2. Serial plasma pharmacokinetic samples were collected before 5-FU administration; at the end of a 5-min bolus dose; and then 15, 30, 60, 90, 120 (±10) and 180 (±10) minutes after the end of the 5-FU infusion. All blood samples were collected in heparinized tubes, centrifuged to plasma with minimal exposure to light, and stored at −80°C. Plasma concentrations of 5-FU were analyzed using a previously published high-performance liquid chromatography/ultraviolet detection method that was validated in our laboratory with slight modifications.28
The concentration–time data for 5-FU were analyzed using noncompartmental techniques. For each set of serial pharmacokinetic samples, the peak plasma concentration of drug (Cmax) and time to Cmax (Tmax) were determined from the plasma concentration–time profile. The log-linear terminal slope (β) was defined by the last 2 measurable concentration–time data points in the serial sampling window. Terminal half-life (t1/2) was calculated as t1/2 = ln(2)/β. The area under the plasma concentration versus time curve from time zero to the last measurable sampling time point (AUC0–Tlast) was calculated using the log trapezoidal rule, and the area under the curve from time zero to time infinity (AUC0–∞) was calculated by extrapolating AUC0–Tlast from the last measurable time point (Clast) using the log-linear slope: (AUC0–Tlast + Clast/β). Body surface area (BSA)–normalized apparent oral clearance (CL/F/m2) was calculated as the BSA-normalized dose divided by AUC0–∞. Dose proportionality of 5-FU was evaluated using an ANOVA on CL stratified by dosage.
Disease Subtyping
Fluorescence in situ hybridization was performed as previously described.3 Briefly, dual-color fluorescence in situ hybridization was performed on 4 µm paraffin embedded tissue sections. Probes were derived from bacterial artificial chromosome (BAC) clones (BACPAC Resources) and labeled with either AlexaFluor 488 or AlexaFluor 555 fluorochromes (Invitrogen). For the C11orf95 locus (11q13.1), BACs CH17-215P06, CH17-67K13, and CH17-388O01 were used to assess rearrangement. For RELA (11q13.1), BACs RP11-642F7 and CH17-211O12 were used to assess rearrangement. Rearrangement of both C11orf95 and RELA was interpreted as supportive of a C11orf95-RELA fusion.
Results
From January 2012 to April 2014, twenty-six patients with radiographic evidence of ependymoma progression were enrolled into the study at St Jude Children's Research Hospital. About one-quarter of patients (n = 6/23) had supratentorial ependymomas, of which 3 possessed the rearrangement for C11orf95-RELA, 2 were negative for that specific fusion, and 1 case from a referring institution was not tested. The remaining patients relapsed from primary posterior fossa disease. The dosage level distributions, toxicities, and patient characteristics are summarized in Tables 1 and 2. In particular, Table 1 is ordered by enrollment of patients according to the rolling-6 design, with the exception of the final expansion cohort listed in the last row. Three of 26 patients enrolled were deemed inevaluable. One patient on dosage level 1 completed therapy, but upon progression underwent resection of the recurrent lesion in question, which was then deemed a glioblastoma upon central review, likely secondary to re-irradiation. Another patient, on dosage level 2, clinically progressed within 2 weeks of study start, which was further confirmed on MRI. The last patient also clinically progressed within 3 weeks of starting therapy. The remaining 23 patients completed the dose finding period and were evaluable for toxicity assessment. None were lost to follow-up. One patient discontinued therapy during the seventh cycle due to compliance issues. No patients discontinued therapy due to toxicity. Discontinuation of treatment in the remaining patients was attributable to progressive disease.
Table 1.
Dosage levels, evaluable patients, and toxicities ordered by phase of enrollment
| Dosage Level | Dosage of Bolus 5-FU (mg/m2) Once Weekly | Number of Evaluable Patients Enrolled | Number of Evaluable Patients with DLTs | Number of DLTs | Description of DLTs |
|---|---|---|---|---|---|
| 1a | 500 | 6 | 1 | 1 | Grade 4 neutropenia |
| 2 | 650 | 4 | 2 | 2 | Grade 4 neutropenia |
| 1b | 500 | 2 | 2 | 3 | Grade 4 neutropenia (n = 2) |
| Grade 3 diarrhea (n = 1) | |||||
| 0 | 400 | 5 | 0 | 0 | Not applicable |
| 1c | 500 | 6 | 0 | 0 | Not applicable |
Starting dosage.
Dose de-escalation phase due to toxicity at 650 mg/m2.
Dose escalation phase in less heavily pretreated patients.
Table 2.
Evaluable patient characteristics and responses
| Patient # | Age, y, Last RT | Tumor Location | Dose Level | Prior Regimens | Months Since | No. Courses | DLT | Reason Off Study | Best Responsea |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 21 | PF, STM, SPM | 1 | 3C, 2F, 1CSI, 5S | 121 | C9W4 | No | PD | PR |
| 2 | 6.98 | PF, SPM | 1 | 3C, 2F, 3S | 24 | C2W4 | No | PD | SD |
| 3 | 6.81 | PF | 1 | 3C, 3F, 4S | 44 | C2W6 | G4 neutropenia | PD | SD |
| 4 | 14.4 | PF, STM, SPM | 1 | 2C, 3F, 2S | 2GK | C1W6 | No | PD | PD |
| 5 | 6.17 | PF, STM, SPM, L | 1 | 1C, 1CSI, 3S | 6 | C1W6 | No | PD | PD |
| 6 | 7.1 | PF, STM, SPM | 1 | 2C, 3F, 2S | 4GK | C1W6 | No | PD | PD |
| 7 | 6.25 | PF, STM, SPM | 2 | 3C, 1F, 2S | 18 | C2W4 | G4 neutropenia | Other Tx | PR |
| 8 | 17.03 | ST | 2 | 2F, 3S | 52 | C7W1 | No | Compliance | SD |
| 9 | 5.98 | PF, STM, SPM | 2 | 7C, 2F, 2CSI, 5S | 37 | C1W6 | G4 neutropenia | PD | PR |
| 10 | 8.82 | PF, STM, SPM | 2 | 4C, 4F, 3S | 1 (brain) 11 (spine) |
C5W6 | No | PD | PR |
| 11 | 7.42 | PF, STM | 1 | 4C, 7F, 1CSI, 3S | 14 | C1W6 | G3 diarrhea G4 neutropenia |
PD | PD |
| 12 | 9.98 | PF, STM, SPM | 1 | 5C, 7F, 1CSI, 8S | 4GK | C1W6 | G4 neutropenia | PD | PD |
| 13 | 8.66 | PF, L | 0 | 3C, 1F, 3S | 39 | C3W6 | No | PD | SD/PP (necrosis) |
| 14 | 17.16 | SP | 0 | 1C, 1CSI, 1S | 17 | C1W6 | No | Clinical PD | SD |
| 15 | 6.34 | PF, STM | 0 | 2F, 3S | 23 | C1W6 | No | PD | PD |
| 16 | 13.1 | ST | 0 | 1C, 2F, 3S | 6 | C3W6 | No | PD | SD/PP (necrosis) |
| 17 | 6.52 | PF | 0 | 1C, 1F, 3S | 48 | C1W6 | No | PD | PD |
| 18 | 12.84 | ST, SPM, L | 1 | 1F, 1CSI, 2S | 9 | C1W6 | No | PD | PD |
| 19 | 4.84 | PF | 1 | 1C, 1F, 1S | 22 | C1W6 | No | PD | PD |
| 20 | 13.83 | PF, SPM | 1 | 1F, 2S | 10 | C2W1 | No | Compliance | PR |
| 21 | 6.4 | ST, PFM | 1 | 2C, 1F, 1S | 23 | C1W6 | No | PD | PD |
| 22 | 12.59 | ST | 1 | 2C, 1F, 1S | 19 | C1W6 | No | PD | PD |
| 23 | 3.16 | ST, SPM | 1 | 1F, 2S | 6 | C1W6 | No | PD | PD |
Abbreviations: PF, posterior fossa; ST, supratentorial; SP, spinal; L, leptomeningeal; superscript M denotes sites of metastasis at time of enrollment; superscript GK denotes radiation in the form of gamma knife to metastatic sites; C, chemotherapy regimen; F, focal irradiation; CSI, cranial spinal irradiation; S, surgery; C#W#, course# week#; G, grade; PD, progressive disease; PR, partial response; Tx, treatment; SD, stable disease; PP, pseudoprogression.
All partial responses in metastatic sites with exception of patient 1. Bold denotes site of original primary lesion.
Toxicity in Patients
Toxicities at particular dosage levels are outlined in Table 1. For the initial phase of the study, we used a starting dosage of 500 mg/m2. One DLT was encountered in these 6 patients, and the dosage for the next cohort of patients was escalated to 650 mg/m2. At this dosage level, 2 of 4 enrolled patients experienced DLTs, prompting dosage de-escalation to 500 mg/m2. Unexpectedly, both patients who enrolled following de-escalation from dosage level 2 developed toxicity at this previously tolerated dosage, which prompted another dose de-escalation to 400 mg/m2. The grades 3 and 4 adverse events recorded were diarrhea (n = 1) and neutropenia (n = 2 at 650 mg/m2, n = 3 at 500 mg/m2), respectively. Of the 5 patients enrolled at the dosage level of 400 mg/m2, none experienced DLTs.
Expansion Cohort
Based on the preexisting 5-FU pharmacokinetic-pharmacodynamic data from murine models, we were concerned that 400 mg/m2, though tolerated, would be less likely to achieve the required CNS exposure associated with antitumor effect. Moreover, to further examine the observed toxicity rate at the 500 mg/m2 dosage level clinically, we first evaluated whether the pharmacokinetics could explain the observed toxicity. Since it was not found to be the cause or a contributing factor, we then analyzed a number of other potential contributing factors for the observed toxicity, including number of prior chemotherapies, surgeries, RT, and a combination of all 3. Specifically, we detected an association between DLTs and the number of prior chemotherapy regimens (Kruskal–Wallis test, P = .03), but not between DLTs and number of prior surgeries or various RT-related variables, which may have been due to the small number of patients and DLTs. More specifically, patients with DLTs (n = 5) had a median of 4 prior chemotherapies, whereas patients without DLTs (n = 7) had a median of 2 prior chemotherapies. We subsequently added an amendment allowing recruitment of 6 less heavily pretreated patients, restricted to 2 or fewer chemotherapy regimens and no more than 2 irradiation regimens, to determine if the 500 mg/m2 dosage level would be tolerated in the less heavily pretreated cohort. None of the 6 patients enrolled for the less heavily pretreated cohort experienced a DLT at the dosage level of 500 mg/m2.
Pharmacokinetic Objectives
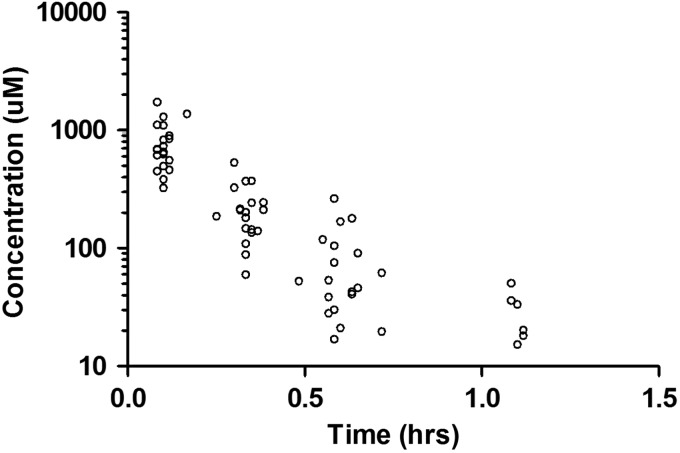
Serial pharmacokinetic studies were performed in all 26 patients. As stated earlier, 3 patients were deemed inevaluable and, for the purpose of this report, the 23 evaluable patients were included in the pharmacokinetic analysis. The course 1 day 1 concentration–time data for patients receiving 5-FU at 500 mg/m2 are presented in Fig. 1. The pharmacokinetic parameters for 5-FU in relation to dosage are presented in Table 3. The 5-FU median AUCs at 500 mg/m2 and 650 mg/m2 are greater than at 400 mg/m2, but the 650 mg/m2 group did not show a proportional increase in AUC compared with the 500 mg/m2 cohort. At the 500 mg/m2 dosage, the median (range) values of 5-FU Cmax and Tmax over the 4 pharmacokinetic studies were 825 µM (108–2076) and 6 min (4–10), respectively. Clearance of 5-FU was linear among the three 5-FU dosages across all courses of pharmacokinetic study (ANOVA P > .05; Supplementary Fig. S1). At the 500 mg/m2 dosage, no inter- or intra-occasion variability in 5-FU CL or AUC0–∞ was observed when the estimates were evaluated across all patients or within each patient (Supplementary Table S1).
Fig. 1.
5-FU concentration–time data for all patients receiving 500 mg/m2 on course 1 day 1.
Table 3.
Summary of 5-FU pharmacokinetics for 400, 500, and 650 mg/m2
| 5-FU Dosage, mg/m2, median (range) |
|||
|---|---|---|---|
| 400 | 500 | 650 | |
| N | 17 | 42 | 13 |
| Cmax, µM | 532 (324–940) | 825 (108–2076) | 711 (260–1734) |
| Tmax, min | 6 (5–7) | 6 (4–10) | 6 (5–14) |
| t1/2, min | 6.9 (5.6–7.8) | 9.9 (4.7–19.8) | 11.2 (7.7–20.7) |
| AUC0–∞, µM × h | 115 (88.9–179) | 205 (58.6–468) | 190 (157–468) |
| CL, L/h/m2 | 26.8 (17.2–34.6) | 18.8 (8.2–65.6) | 26.2 (10.7–31.9) |
Abbreviations: N, number of pharmacokinetic studies per dose group; Cmax, observed maximum plasma concentration; Tmax, time to maximum concentration; t1/2, terminal α half-life; AUC0–∞, area under the plasma concentration–time curve from time zero to infinity; CL, clearance.
Preliminary Data on Antitumor Activity in Patients
Although the study was not designed specifically to look at response rates, these data were collected (Table 2). There were no complete responses. Five patients had a partial response (duration 6–54 wk, median 12 wk). All partial responses were in metastatic sites except for that of patient 1. Four patients had stable disease (duration 6–67 wk, median 11 wk), one of whom was removed from study for clinically progressive symptoms. Two other patients had stable disease based on radiographic measurements, but unlike the other patients with stable disease, their tumors showed marked necrosis centrally. The remaining 12 patients progressed after the first course of therapy. All responders were those with posterior fossa primary disease. Of the patients with radiographically stable disease, only 1 had supratentorial fusion-positive disease. All other patients with a supratentorial primary tumor progressed.
Discussion
The present study was designed to estimate the pharmacokinetics of 5-FU given as a 5-min bolus infusion to children with recurrent ependymoma and to establish the safety profile of weekly bolus 5-FU in this cohort of patients. For the initial phase of the study, the starting 5-FU dosage was 500 mg/m2. We treated 3 cohorts of research participants (dosage level 0: 400 mg/m2; level 1: 500 mg/m2; level 2: 650 mg/m2), escalating first to 650 mg/m2 and then de-escalating the dosage of 5-FU due to toxicity, first back to the starting dosage and then to dosage level 0. During the initial portion of the study, there appeared to be an association between toxicity and prior number of therapies, as fewer heavily pretreated patients tolerated the 500 mg/m2 starting dose of 5-FU and ultimately required dosage decreases to 400 mg/m2. Further analysis revealed a significant relationship (Kruskal–Wallis test, P = .03) between prior number of chemotherapeutic regimens and toxicity. The study was subsequently amended to re-escalate the dosage to 500 mg/m2 in a less heavily pretreated cohort (n = 6) of patients. Treatment at dosage level 0 was well tolerated in all patients; dosage level 1 was well tolerated in less heavily pretreated patients. Although only the first 6 weeks of treatment were used to define the MTD in our study, treatment and evaluation continued thereafter.
At 400 mg/m2 and 500 mg/m2—the latter dosage restricted to less heavily pretreated patients—there did not seem to be a greater level of toxicity; however, concern remained that the lower dosage would be less likely to achieve the required CNS exposure associated with antitumor effect extrapolated from murine models. Specifically, our preclinical data suggested that the 5-FU systemic exposure (AUC) associated with the 400 mg/m2 dosage would be inadequate in all patients to achieve the brain extracellular fluid concentrations associated with antitumor effects. However, since we did not measure the brain extracellular fluid concentrations in our patients due to either lack of accessibility (ie, ommaya reservoir) or parental refusal, we are unable to definitively draw this conclusion. Thus, the MTD for heavily pretreated patients and, therefore, all patients, regardless of prior therapy regimens, is 400 mg/m2. However, 500 mg/m2 is the preferred recommended dosage for less heavily pretreated patients in order to potentially provide more adequate drug concentrations in the tumor. Our decision to not escalate the 5-FU dose to 650 mg/m2 in less heavily pretreated patients was twofold. Firstly, the occurrence of a DLT in 2 patients enrolled at this level who happened to be less heavily pretreated raised concern that this dosage would not be tolerated. Secondly, our goal to expediently establish a recommended phase II dose with the appropriate pharmacokinetic parameters was met with the 500 mg/m2 dosage.
We were unable to establish an association between the number of DLTs encountered as a function of radiation therapy due to the varying modalities of radiation delivery. Some patients were treated with photons, while others received proton beam therapy. There were also patients who received gamma knife radiosurgery for focal recurrences. Additionally, the targeting of involved fields varied across patients, with some opting for cranial spinal irradiation upon recurrence, others for complete spinal radiation excluding the brain, and still others for focal irradiation to new sites of metastasis.
We also analyzed the possible association between DLTs and prior number of recurrences, since this variable contributes to the degree of pretreatment. The association between DLTs and prior number of recurrences (range, 1–11; median, 2) was significant whether we analyzed the group prior to or after the study amendment. Limiting the analysis to the cohort prior to the amendment included 19 cases. Based on a Kruskal–Wallis test, we found the number of DLTs as a function of the number of recurrences to be significant, with P = .027 (5 patients with DLTs, 14 patients without DLTs). Moreover, for the entire cohort of 23 cases, the Kruskal–Wallis test showed that the number of DLTs as a function of the number of recurrences remained significant, with P = .013 (5 patients with DLTs, 18 patients without DLTs).
This study represents the first report of 5-FU pharmacokinetics after bolus administration in pediatric patients with ependymoma. Clearance estimates of 5-FU across the 3 dosages evaluated were similar to 5-FU bolus clearance values previously published in adult colorectal patients (BSA normalized; 1.73 m2).26–30 Dosage proportionality was evaluated by examining the difference in clearance across dosage by ANOVA, and no difference was found, suggesting that 5-FU exhibits linear pharmacokinetics in pediatrics over the dosage range of 400–650 mg/m2. However, our limited sample size (23 patients) and dosages evaluated (3) prevent a full evaluation of the possibility of nonlinear 5-FU elimination after bolus administration. The 5-FU starting dosage in this study of 500 mg/m2 was determined based on preclinical studies in murine models of ependymoma, which showed that 5-FU given by i.v. bolus possesses antitumor activity.6 Typical plasma AUC values in our population at the 500 mg/m2 dosage exceeded those observed in the preclinical ependymoma mouse model (112 µM × h), suggesting that efficacious exposures were likely achieved in the tumors of children receiving bolus 5-FU.
In addition to identifying a potential phase II dose and defining the pharmacokinetic properties of bolus 5-FU in children with recurrent ependymoma, this study provides at least 2 other important lessons for the development of new treatments of ependymoma. First, the close relationship between DLT and prior treatments observed in this population of patients with relapsed ependymoma may present a significant hurdle as we seek to translate potential new treatments of ependymoma from the laboratory to the clinic. Our careful preclinical pharmacokinetics and efficacy trials were conducted in mice harboring previously untreated supratentorial tumors. Better translation may require the development of relapsed disease models (something we are currently developing) of phase I populations in the clinic or the conduct of clinical trials in patients who are less heavily pretreated. Second, our preclinical development of bolus 5-FU was conducted using the mEPEphb2 model, which recapitulates fusion-negative but not fusion-positive supratentorial ependymoma. Only 2 patients in the current study presented with fusion-negative disease. Thus, the limited response in our study might also reflect a poor representation of the principal targeted subtype. In addition, 5-FU has since been tested in a patient-derived xenograft harboring the C11orf95-RELA fusion but was found to be inactive, as it had been in the patient from whom the sample was obtained for establishment of the xenograft (R. J. Gilbertson, unpublished data), further supporting the lack of responses in a small subcohort of supratentorial cases with primarily fusion-positive disease. Thus, trials that incorporate bolus 5-FU for patients with newly diagnosed fusion-negative ependymoma may identify a greater degree of drug activity. Therefore, as we move forward with the development of targeted therapies for subtype-stratified clinical trials according to identified molecular markers for an already rare disease, international collaboration will be key to not only meeting patient accrual but gaining meaningful results to better inform our treatment of patients.
Conclusion
These phase I clinical data provide initial pharmacokinetic parameters to describe the disposition of 5-FU given as an intravenous bolus to children with recurrent ependymoma. This study also provides toxicity and tolerability data to be utilized in future studies. Moreover, bolus 5-FU appears to possess antitumor activity in human ependymoma. This study has allowed for repurposing of a well-known drug by administering it in a novel manner to patients with ependymoma. Lastly and most importantly, we highlight the translational approach of applying preclinical testing in accurate mouse models of ependymoma to the human disease while gaining insight into the best way to move forward in designing future human clinical trials.
Supplementary Material
Funding
This work was supported by the Collaborative Ependymoma Research Network (CERN) US National Institutes of Health Cancer Center Support (CORE) grant P30 CA21765 and by the American Lebanese Syrian Associated Charities (ALSAC).
Acknowledgments
We thank the staff of the pharmacokinetics lab, particularly Thandranese Owens and Kristen Haddock, for their support in analyzing samples for the study. We would also like to thank the clinical staff of the neuro-oncology, neurosurgery, pathology, and neuroradiology divisions.
Conflict of interest statement. None declared.
References
- 1.Merchant TE, Mulhern RK, Krasin MJ, et al. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol. 2004;22(15):3156–3162. [DOI] [PubMed] [Google Scholar]
- 2.Bouffet E, Capra M, Bartels U. Salvage chemotherapy for metastatic and recurrent ependymoma of childhood. Childs Nerv Syst. 2009;25(10):1293–1301. [DOI] [PubMed] [Google Scholar]
- 3.Parker M, Mohankumar KM, Punchihewa C, et al. C11orf95-RELA fusions drive oncogenic NF-kB signaling in ependymoma. Nature. 2014;506(7489):451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor MD, Poppleton H, Fuller C, et al. Radial glia are candidate stem cells of ependymoma. Cancer Cell. 2005;8(4):323–335. [DOI] [PubMed] [Google Scholar]
- 5.Johnson RA, Wright KD, Poppleton H, et al. Cross-species genomics matches driver mutations and cell compartments to model ependymoma. Nature. 2010;466(7306):632–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atkinson JM, Shelat AA, Carcaboso AM, et al. An integrated in vitro and in vivo high-throughput screen identifies treatment leads for ependymoma. Cancer Cell. 2011;20(3):384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houghton JA, Cheshire PJ, Hallman JD, 2nd, et al. Evaluation of irinotecan in combination with 5-fluorouracil or etoposide in xenograft models of colon adenocarcinoma and rhabdomyosarcoma. Clin Cancer Res. 1996;2(1):107–118. [PubMed] [Google Scholar]
- 8.Santi DV, McHenry CS, Sommer H. Mechanism of interaction of thymidylate synthetase with 5-fluorodeoxyuridylate. Biochemistry. 1974;13(3):471–481. [DOI] [PubMed] [Google Scholar]
- 9.Danenberg PV. Thymidylate synthetase—a target enzyme in cancer chemotherapy. Biochem Biophys Acta. 1977;473(2):73–92. [DOI] [PubMed] [Google Scholar]
- 10.Danenberg PV, Lockshin A. Thymidylate synthetase—substrate complex formation. Mol Cell Biochem. 1982;43(1):49–57. [DOI] [PubMed] [Google Scholar]
- 11.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–338. [DOI] [PubMed] [Google Scholar]
- 12.Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):041–1047. [DOI] [PubMed] [Google Scholar]
- 13.Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet. 1995;345(8955):939–944. [PubMed] [Google Scholar]
- 14.Grunda JM, Fiveash J, Palmer CA, et al. Rationally designed pharmacogenomic treatment using concurrent capecitabine and radiotherapy for glioblastoma; gene expression profiles associated with outcome. Clin Cancer Res. 2010;16(10):2890–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto K, Tabuchi K, Furuta T, et al. Combination chemotherapy of brain tumors with ACNU and 5-FU. Neurol Med Chir. 1983;23(8):625–632. [DOI] [PubMed] [Google Scholar]
- 16.Kubo O, Himuro H, Inoue N, et al. Treatment of malignant brain tumors with slowly releasing anticancer drug-polymer composites. No Shinkei Geka. 1986;14(10):1189–1195. [PubMed] [Google Scholar]
- 17.Rodriguez LA, Prados M, Fulton D, et al. Treatment of recurrent brain stem gliomas and other central nervous system tumors with 5-fluorouracil, CCNU, hydroxyurea, and 6-mercaptopurine. Neurosurgery. 1988;22(4):691–693. [DOI] [PubMed] [Google Scholar]
- 18.Levin VA, Prados MD. Treatment of recurrent gliomas and metastatic brain tumors with a polydrug protocol designed to combat nitrosourea resistance. J Clin Oncol. 1992;10(5):766–771. [DOI] [PubMed] [Google Scholar]
- 19.Terrazzino S, Cargnin S, Del Re M, et al. DPYD IVS14+1G>A and 2846A>T genotyping for the prediction of severe fluoropyrimidine-related toxicity: a meta-analysis. Pharmacogenomics. 2013;14(11):1255–1272. [DOI] [PubMed] [Google Scholar]
- 20.Amstutz U, Froehlich TK, Largiader CR. Dihydropyrimidine dehydrogenase gene as a major predictor of severe 5-fluorouracil toxicity. Pharmacogenomics. 2011;12(9):1321–1336. [DOI] [PubMed] [Google Scholar]
- 21.Saif MW, Ezzeldin H, Vance K, et al. DPYD*2A mutation: the most common mutation associated with DPD deficiency. Cancer Chemother Pharmacol. 2007;60(4):503–507. [DOI] [PubMed] [Google Scholar]
- 22.Skolnik JM, Barrett JS, Jayaraman B, et al. Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol. 2008;26(2):190–195. [DOI] [PubMed] [Google Scholar]
- 23.Lee DP, Skolnik JM, Adamson PC. Pediatric phase I trials in oncology: an analysis of study conduct efficiency. J Clin Oncol. 2005;23(33):8431–8441. [DOI] [PubMed] [Google Scholar]
- 24.Onar-Thomas A, Xiong Z. A simulation-based comparison of the traditional method, Rolling-6 design and a frequentist version of the continual reassessment method with special attention to trial duration in pediatric Phase I oncology trials. Contemp Clin Trials. 2010;31(3):259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 26.Chinot OL, Macdonald DR, Abrey LE, et al. Response assessment criteria for glioblastoma: practical adaptation and implementation in clinical trials of antiangiogenic therapy. Curr Neurol Neurosci Rep. 2013;13(5):347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. [DOI] [PubMed] [Google Scholar]
- 28.Alsarra IA, Alarifi MN. Validated liquid chromatographic determination of 5-fluorouracil in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;804(2):435–439. [DOI] [PubMed] [Google Scholar]
- 29.Di Paolo A, Danesi R, Falcone A, et al. Relationship between 5-fluorouracil disposition, toxicity and dihydropyrimidine dehydrogenase activity in cancer patients. Ann Oncol. 2001;12(9):1301–1306. [DOI] [PubMed] [Google Scholar]
- 30.Larsson PA, Carlsson G, Gustavsson B, et al. Different intravenous administration techniques for 5-fluorouracil. Pharmacokinetics and pharmacodynamic effects. Acta Oncol. 1996;35(2):207–212. [DOI] [PubMed] [Google Scholar]