Abstract
Background
Mitochondrial diseases belong to the most severe inherited metabolic disorders affecting pediatric population. Despite detailed knowledge of mtDNA mutations and progress in identification of affected nuclear genes, diagnostics of a substantial part of mitochondrial diseases relies on clinical symptoms and biochemical data from muscle biopsies and cultured fibroblasts.
Methods
To investigate manifestation of oxidative phosphorylation defects in isolated lymphocytes, digitonin-permeabilized cells from 48 children were analyzed by high resolution respirometry, cytofluorometric detection of mitochondrial membrane potential and immunodetection of respiratory chain proteins with SDS and Blue Native electrophoreses.
Results
Evaluation of individual respiratory complex activities, ATP synthesis, kinetic parameters of mitochondrial respiratory chain and the content and subunit composition of respiratory chain complexes enabled detection of inborn defects of respiratory complexes I, IV and V within 2 days. Low respiration with NADH-dependent substrates and increased respiration with glycerol-3-phosphate revealed complex I defects; changes in p50 for oxygen and elevated uncoupling control ratio pointed to complex IV deficiency due to SURF1 or SCO2 mutation; high oligomycin sensitivity of state 3-ADP respiration, upregulated mitochondrial membrane potential and low content of complex V were found in lymphocytes with ATP synthase deficiency due to TMEM70 mutations.
Conclusion
Based on our results, we propose the best biochemical parameters predictive for defects of respiratory complexes I, IV and V manifesting in peripheral blood lymphocytes.
General significance
The noninvasiveness, reliability and speed of an approach utilizing novel biochemical criteria demonstrate the high potential of isolated lymphocytes for diagnostics of oxidative phosphorylation disorders in pediatric patients.
Abbreviations: ΔΨm, mitochondrial membrane potential; AA, antimycin A; BNE, Blue Native PAGE; cI–cV, respiratory chain complexes I–V; COX, cytochrome c oxidase; FCCP, carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone; GP, glycerol-3-phosphate; GPDH, mitochondrial FAD-dependent glycerophosphate dehydrogenase; OXPHOS, oxidative phosphorylation; PAGE, polyacrylamide gel electrophoresis; s3, state 3-ADP; s3u, state 3-uncoupled; s4o, state 4-oligomycin; TMPD, tetramethylphenylenediamine; TMRM, tetramethylrhodamine methyl ester
Keywords: Lymphocytes, Respirometry, Oxidative phosphorylation, Mitochondrial diseases, Diagnostics
Highlights
-
•
Analysis of inborn mitochondrial disorders in peripheral blood lymphocytes
-
•
Detection of specific defects of respiratory chain complexes I, IV and V
-
•
Manifestation of cytochrome c oxidase deficiency due to SCO2 mutations
-
•
Rapid and noninvasive diagnostics/screening appropriate for pediatric patients
1. Introduction
Mitochondrial diseases belong to most severe inherited metabolic diseases affecting pediatric population. They are caused by disorders of mitochondrial biogenesis or by mutations in the structural subunits of oxidative phosphorylation (OXPHOS) apparatus [1]. Genetics of mitochondrial OXPHOS disorders is quite unique: they can be caused either by mutations in nuclear genes or mutations in mitochondrial DNA (mtDNA), which encodes 13 of ~ 90 OXPHOS structural subunits. Mutations in the maternally-transmitted mtDNA are already well characterized. Several hundreds of them have been described to date (www.mitomap.com) and their detection and screening have become rather routine task [2]. However, according to current estimates, mtDNA mutations are responsible for only 25% of mitochondrial diseases. The remainder originates from mutations in nuclear genes, and their diagnostics still poses a significant challenge. Within the last couple of years pathogenic mutations resulting in a mitochondrial disease have been uncovered in ~ 110 nuclear genes [3] including numerous novel factors of mitochondrial biogenesis (e.g. TMEM70 [4], [5], C12orf65 [6], [7] C20orf7 [8], TACO1 [9], RMND1 [10], SDHAF1 [11], SDHAF2 [12]). Nevertheless, most of the disease-causing genes from the broad repertoire of more than thousand nuclear genes contributing to the mitochondrial proteome (www.mitocarta.com [13]) still remain to be identified. For example, among the very frequent defects of cytochrome c oxidase — complex IV (cIV), the terminal enzyme complex of the respiratory chain, the genetic basis underlying the disorder was found in less than 50% of the cases [14].
Diagnostics of a substantial part of mitochondrial diseases thus relies on clinical symptoms and biochemical analyses. Assessment of energetic function and content of individual mitochondrial proteins in patient tissues is mainly performed in bioptic samples of skeletal muscle and/or cell cultures of skin fibroblasts. Both approaches have significant drawbacks: muscle biopsy allows for an instant, single biochemical analysis of the mitochondrial quantity and function, but it is often complicated by muscle atrophy caused by mitochondrial disorders. Due to its invasive nature, it is also often refused by the parents of the patient, and the same holds true for the skin biopsy, necessary for establishing of fibroblast cell culture. While the cell culture allows for more systematic and repeated analysis of mitochondria, the diagnostic results are only obtained after a prolonged period, ranging from several weeks to months.
The analysis of mitochondrial energetic apparatus in peripheral blood lymphocytes, with the help of sensitive functional methods and detection of quantitative and qualitative changes in OXPHOS proteins, could become an attainable alternative method. Although it is practically noninvasive and easily repeatable, functional and protein analyses of mitochondrial OXPHOS system in lymphocytes or lymphoblasts have only been used sporadically for the study of mitochondrial diseases [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]. Even in this limited number of studies, lymphocytes were more often used in monitoring of tissue specific presentation of respective mutations than as a primary diagnostic tool.
To establish this approach as an alternative diagnostic tool, we adapted and optimized highly sensitive oxygraphy of digitonin-permeabilized cells for analysis of the function of mitochondrial respiratory chain in lymphocytes. Wherever feasible, we combined it with the analysis of mitochondrial membrane potential (ΔΨm) by cytofluorometry and with protein analysis of OXPHOS complexes by SDS-PAGE and BNE followed by specific immunodetection of structural subunits of mitochondrial respiratory complexes. Subsequent statistical analysis uncovered reliable and selective markers that can distinguish samples harboring deficiencies of complex I, complex IV caused by SURF1 and SCO2 mutations, and complex V on the basis of TMEM70 mutations.
2. Materials and methods
2.1. Patients
Peripheral blood samples (3–7 ml depending on patients' age) were obtained from 48 children aged 1 month to 18 years. Out of them, 35 children with neurological disturbances and/or developmental delay were referred by their local pediatricians for metabolic screening and their parents agreed to take part in our study; seven children (P1, P4–P5 and P7–P9) had already known mitochondrial disorder confirmed at biochemical and/or molecular level and six disease free children served as the age-matched controls. Altogether, 37 children were analyzed once and eleven children were analyzed 2–3 times.
Mitochondrial patient P1 at the age of 11 years had severe encephalopathy and arrest of any mental and motor development due to deficiency of respiratory chain complex I of unknown nuclear origin without any mutation in the mtDNA. Patient P2 is a 13 years old girl with intrauterine growth retardation and repeated attacks of cephalea since the age of 6 years, dissociative psychiatric disease, progressive bilateral deafness and progressive visual impairment with bilateral concentric visual field loss. The parents did not approve of skin or muscle biopsy. Molecular analyses from blood and buccal smear did not detect any mtDNA mutation responsible for any of LHON, MELAS or MERRF syndromes. Patient P3 was born at the 32nd week of gestation with a birth weight of 1990 g and a length of 42 cm. Early postnatal adaptation was uneventful, but he developed pharmaco-resistant seizures during the third week of life. MRI of the brain revealed pachygyria and cortical dysplasia. The lactic acidosis was not present. The boy died at the age of 4 months and autopsy revealed polymicrogyria focalis gyri temporalis superioris l.sin. Patients P4 and P5 had progressive encephalopathy and Leigh disease since the age of 10 and 12 months respectively due to deficiency of respiratory chain complex IV and homozygous mutations in SURF1 gene (c.845_846delCT and c.312_321del10insAT, respectively). Patient P6 presented since the age of 7 months with failure to thrive, progressive encephalomyopathy and hypertrophic cardiomyopathy. The activity of complex IV was low in lymphocytes and the consequent DNA analysis identified that the boy is compound heterozygote for mutations c.1A > G and c.418G > A in SCO2 gene. Patients P7–P9 at the age of 17, 11 and 2.5 years have encephalocardiomyopathy with deficiency of respiratory chain complex V due to homozygous c.317-2A > G mutation in TMEM70 gene (first two of them were already described as P9 and P11 in [4]).
All work involving human samples was carried out in accordance with the Declaration of Helsinki of the World Medical Association and was approved by the Ethical Committees of the participating institutions. The written informed consent was obtained from patients or patients' parents.
2.2. Lymphocytes
Within 1–2 h after collection, whole fraction of intact lymphocytes was isolated from EDTA-blood by density medium centrifugation at 4 °C, using Ficoll-Paque PLUS (GE Healthcare Bio-Sciences) following a standard protocol. Briefly, blood sample was layered on top of an equal volume of Ficoll and centrifuged at 800 g for 20 min. Separated lymphocytes were carefully collected (~ 1 ml) and resuspended in 15 ml of erythrocyte lysing buffer (30 mM NH4Cl, 2 mM NH4HCO3, 20 μM Na2EDTA, pH 8) and incubated for 20 min on ice. Lymphocytes were pelleted by 800 g centrifugation for 20 min, weighed and resuspended in PBS supplemented with protease inhibitors (1:500 protease inhibitor cocktail, Sigma) for respiration measurements and SDS-PAGE analysis. For BNE dry pellet of lymphocytes was used (see Section 2.4). Protein concentration was determined by Bradford method [26] using BSA as standard.
2.3. High resolution respirometry
Oxygen consumption was measured at 30 °C as described before [27] using Oxygraph-2k (Oroboros). Freshly isolated lymphocytes (0.6 mg protein) were suspended in 2 ml of KCl medium (80 mM KCl, 3 mM MgCl2, 1 mM EDTA, 5 mM K-Pi, 10 mM Tris–HCl pH 7.4) and digitonin 0.05 g/g protein was used to permeabilize the plasma membrane. For measurements the following concentrations of substrates and inhibitors were used: 3 mM malate, 10 mM pyruvate, 10 mM glutamate, 10 mM succinate, 10 mM glycerol 3-phosphate (GP), 1 mM ADP, 5–200 nM oligomycin, 150–200 nM FCCP, 0.25 μM antimycin A, 2 mM ascorbate, 0.6 mM TMPD and 0.5 mM KCN. The oxygen consumption was expressed in pmol oxygen/s/mg protein.
p50 value – the partial oxygen pressure (pO2), at which the cellular respiratory rate is half-maximal – was determined as before [28]. Briefly, the volume-specific rate of oxygen consumption (oxygen flux) was calculated as the negative slope of oxygen concentration recorded at 1 s time intervals. The signal was deconvoluted with the exponential time constant of the oxygen sensor (3 to 5 s) and corrected for instrumental background, which is a linear function of experimental pO2 and results from oxygen consumption by the sensor and oxygen back-diffusion from low capacity oxygen reservoirs. The p50 parameter was obtained from a hyperbolic function J02 = (Jmax · pO2) / (p50 + pO2) fitted over the low oxygen range of 0 to 1.1 kPa. All calculations were performed using routine functions of the DatLab software [29].
2.4. Electrophoresis, western blot analysis, in-gel activity assays
Tricine SDS-PAGE [30] was performed on 10% polyacrylamide minigels (MiniProtean III system, BioRad). Pelleted aliquots of isolated lymphocytes (0.1 mg protein) were resuspended in sample buffer (2% (v/v) mercaptoethanol, 4% (w/v) SDS, 50 mM Tris–HCl, pH 7.0, 10% (v/v) glycerol) using 10 × 0.5 s sonication pulses and immediately denatured at 100 °C for 10 min.
Blue Native PAGE (BNE) [31] was performed on 5–13% gradient polyacrylamide slab minigels. Pelleted lymphocytes (0.25 mg protein) were homogenized in 0.5 ml of KCl medium supplemented with protease inhibitors using a teflon-glass homogenizer (30 × 500 rpm) and centrifuged 20,000 g for 10 min at 4 °C. The sediment was resuspended in solubilization buffer (50 mM NaCl, 50 mM imidazole, 2 mM 6-aminohexanoic acid and 1 mM EDTA, pH 7), incubated with n-dodecyl β-d-maltoside (DDM) for 15 min on ice (2.5 g/g protein) and centrifuged at 30,000 g for 20 min. The protein concentration in the supernatant was determined and samples were prepared by adding 5% (v/v) glycerol and Serva Blue G 250 (0.6 g/g of DDM).
For western blot analysis, gels were blotted onto a PVDF membrane (Immobilon P, Millipore) by semidry electrotransfer for 1 h at 0.8 mA/cm2. The membranes were blocked in TBS (0.15 M NaCl, 10 mM Tris–HCl, pH 7.5) with 0.1% (v/v) Tween-20 and 5% (w/v) non-fat dry milk. Membranes were incubated overnight with antibody cocktail (MS603, Abcam, 1:250), which included antibodies against the following OXPHOS subunits: Ndufa9 (complex I), Sdha (complex II), Uqcrc2 (complex III), Cox4 (complex IV), Atp5a1 (complex V) or with antibodies against individual OXPHOS subunits, when indicated. The membranes were then incubated for 1 h with appropriate secondary antibody labeled with Alexa Fluor 680 (Life Technologies, 1:3,000). The fluorescence was detected using Odyssey Imager (Li-Cor) and the signal was quantified using Aida 3.21 Image Analyzer software (Raytest).
2.5. Cytofluorometric analysis of mitochondrial membrane potential
Measurements of ΔΨm according to Ref. [32] were adapted to lymphocytes and performed on a PAS III cytofluorometer (Partec). Cells were suspended to 1 mg protein/ml in a KCl based medium (120 mM KCl, 3 mM HEPES, 5 mM KH2PO4, 3 mM MgSO4, 1 mM EGTA, pH 7.2), permeabilized by 0.1 mg/ml digitonin (0.1 g/g protein), diluted to 20 μg/ml and incubated with 10 mM succinate and 20 nM tetramethylrhodamine methyl ester (TMRM, Life Technologies) for 10 min. ADP (1 mM) or FCCP (1 μM) was added 1 min before cytofluorometric analysis. 10 000 cells were used for each measurement. Data were acquired on a log scale and subsequently analyzed using FloMax software (Partec). TMRM fluorescence changes were calculated as percent differences between log(10) values of mean fluorescence signals. Difference between s4 and s3u was considered to be 100%.
3. Results
3.1. General outline of lymphocyte mitodiagnostics
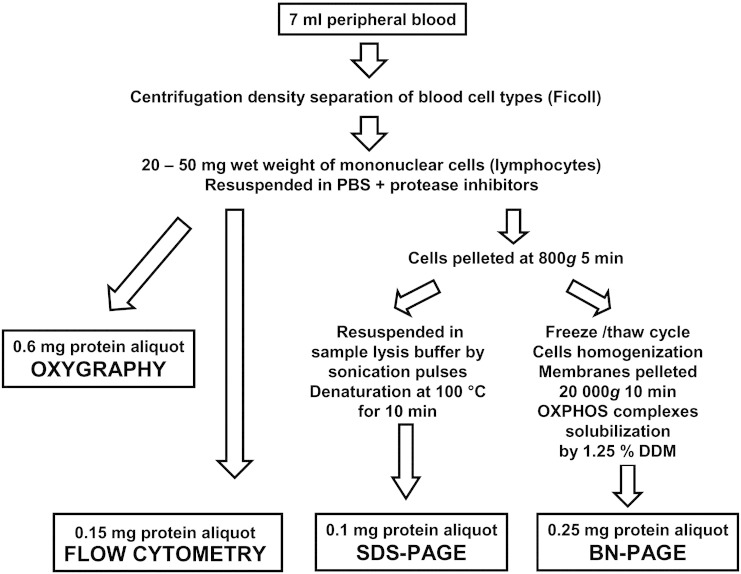
Fig. 1 summarizes the workflow of our diagnostic approach using isolated lymphocytes. Based on the detailed analysis of 60 lymphocyte preparations, obtained from 48 pediatric patients aged ~ 1m–18y (< 6m 18x, 6m–1y 9x, 1y–5y 19x, 5y–10y 5x, 10y–18y 9x), a 7 ml blood aliquot was sufficient to yield 20-50 mg (wet weight) of mononuclear cells. Cells were subsequently resuspended in PBS (~ 100 μl/50 mg wet weight) with protease inhibitors to yield a lymphocyte suspension with protein concentration ~ 10 mg/ml. The average yield of isolations was 1126 μg protein of isolated lymphocytes, corresponding to 158 μg protein of lymphocytes obtained from 1 ml of blood. The amounts of protein required for individual analytical approaches were ~ 600 μg protein for oxygraphy, ~ 150 μg protein for cytofluorometric analysis, ~ 100 μg protein for SDS-PAGE/WB, and ~ 250 μg protein for native PAGE/WB performed as described in the Materials and methods section. Therefore, in most cases a complete mitodiagnostics can be performed with lymphocytes obtained from one aliquot of peripheral blood.
Fig. 1.
Flow chart of mitochondrial oxidative phosphorylation system (OXPHOS) analysis in isolated lymphocytes.
3.2. Respiration measurements
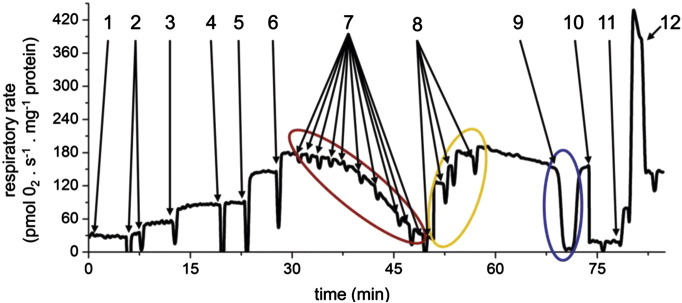
We set to design experimental workflow for respiration measurement, which will allow analysis of activities of intact respiratory chain and its individual complexes at coupled and uncoupled states, sensitivity to key inhibitors as well as affinity to oxygen. Overview of such assay is given in Fig. 2. Upon permeabilization of cells with digitonin, the addition of pyruvate and malate followed by ADP provides the measure of pyruvate dehydrogenase (PDH) and cI-dependent respiration at state 3-ADP (s3); the subsequent addition of alternative NADH-generating substrate glutamate would reveal a PDH defect, as it is capable of fully saturating complex I. Subsequent separate additions of succinate and glycerol-3-phosphate (GP) are used to assay the contributions of succinate dehydrogenase (SDH, cII) and GPDH to the maximum respiratory chain electron flux coupled to ATP synthesis achieved only after combination of these substrates. Titration with the ATP synthase inhibitor oligomycin provides the I50 value indicative of the amount of functional ATP synthase (complex V, cV) present in lymphocyte mitochondria and proton leak-driven state 4 respiration (s4o) is achieved after a complete inhibition of cV. State 3-uncoupled respiration (s3u) – a measure of electron transport chain capacity – is obtained by FCCP titration, the maximal rate being achieved in the 150–200 nM concentration range of the uncoupler. To assess the p50 value of oxygen consumption indicative of the affinity of cytochrome c oxidase (cIV) to oxygen, respiration is allowed to proceed until all oxygen is consumed. After reoxygenation the respiratory chain is blocked at the level of bc1 complex (cIII) by addition of antimycin A, and activity of cIV is assessed by ascorbate and TMPD, followed by addition of KCN that specifically inhibits cIV and provides the rate of cIV independent autooxidation of the artificial cIV substrates.
Fig. 2.
Substrate-inhibitor respiratory measurements using isolated lymphocytes. Complex analysis of respiratory chain functions includes the following steps: 1 — Cells at 0.3 mg protein.ml− 1 + permeabilizing agent digitonin at 0.05 mg per mg of protein; 2 — Malate 3 mM + pyruvate 10 mM, substrates generating NADH oxidized by complex I; 3 — ADP 1 mM, phosphorylation substrate for ATP synthase, activates respiration by utilizing ΔΨm; 4 — Glutamate 10 mM, additional substrate generating NADH; 5 — Succinate 10 mM, oxidized by complex II, additional electron input into respiratory chain; 6 — Glycerol 3-phosphate 10 mM, oxidized by GPDH, additional electron input into respiratory chain; Maximal rate at respiratory state 3 (s3);; 7 — Oligomycin, ATP synthase inhibitor, sensitive assessment of enzyme capacity by step titration in 5, 10, 20, 40, 60, 80, 120, 160 and 200 nM final concentration (red ellipse), respiratory state 4 oligomycin (s4o); 8 — FCCP, uncoupler, achievement of maximal respiratory rate at state 3 uncoupled (s3u) by step titration, usually at 150–200 nM (yellow ellipse); 9 — Full depletion of oxygen — calculation of oxygen kinetics (p50), followed by reoxygenation of medium (blue ellipse); 10 — Antimycin A 0.25 μM, complex III inhibition; 11 — Ascorbate 2 mM + TMPD 0.6 mM, artificial substrates feeding electrons directly to cytochrome c/complex IV; 12 — KCN 0.5 mM — inhibition of complex IV, KCN insensitive oxygen consumption due to ascorbate + TMPD autooxidation.
3.3. Reference charts of respiratory activities in isolated lymphocytes
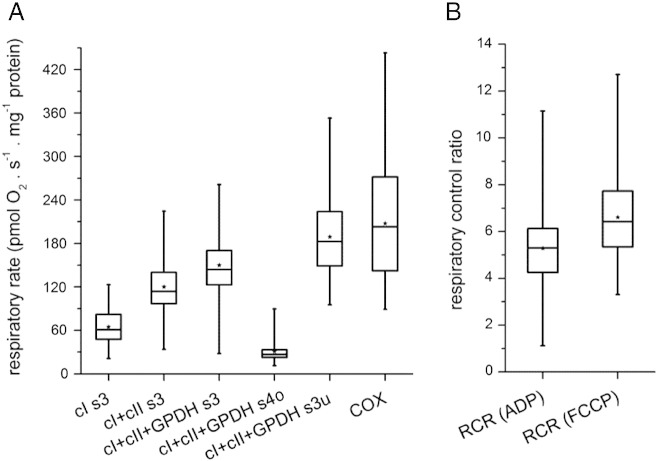
Fig. 3A summarizes the statistical analysis of respiration rates observed in isolated lymphocytes according to protocol in Fig. 2, namely state 3-ADP (s3) respiration with different substrates (cI s3, cI + cII s3, cI + cII + GPDH s3), oligomycin-induced state 4 (s4o) respiration with all substrates (cI + cII + GPDH s4o), FCCP-induced uncoupled (s3u) respiration with all substrates (cI + cII + GPDH s3u) and uncoupled respiration of cIV only (COX). The box plots show the minimum, 1st quartile median, 3rd quartile, and maximum values of specific rates of respiration calculated in pmol oxygen consumed/s/mg protein. The overall statistical analysis reveals that only the combination of electron input from complexes I, II, and GPDH is able to fully saturate the downstream components of the respiratory chain. Similar rates of s3 and s3u respiration indicate that the capacities of electron transport chain and the enzymes involved in ADP phosphorylation seem to be well matched. Also, the activity of cIV does not seem to be in huge excess over the upstream components of respiratory chain as is frequently observed in other cell types or mitochondria isolated from tissue samples.
Fig. 3.
Respiratory rates and respiratory control indexes in isolated lymphocytes. Statistical analysis of data from lymphocytes of 48 children includes controls as well as patients with mitochondrial disorders. (A) Absolute respiratory rates. The following abbreviations are used — cI, cII, cIV, respiratory complexes I, II, IV; GPDH, mitochondrial glycerophosphate dehydrogenase; s3 respiration at state 3-ADP; s3u, respiration at state 3-uncoupled; s4o, respiration at state 4 oligomycin. (B) Respiratory control ratios (RCR). Box plots represent Q1, median, Q3 (box), and minimum and maximum (whiskers), asterisks denote means.
The box plots further indicate a pronounced variation of the absolute respiratory rates, typically 3 to 4-fold differences between the minimal and maximal values in each state. Using these primary data, the following functional parameters of respiratory chain essential for evaluation of suspected OXPHOS disorders were calculated: respiratory control ratio calculated from ADP-stimulated or uncoupler-stimulated respiration (RCRADP, RCRFCCP) indicating how tightly coupled is mitochondrial OXPHOS, relative activities of state 3 ADP-coupled respiration via individual dehydrogenases indicating the capacities of these dehydrogenases (cI, cII and GPDH), sensitivity to oligomycin indicating capacity of ATP synthase to phosphorylate ADP, state 4 after oligomycin inhibition indicating the proton leak of the inner mitochondrial membrane, state 3 uncoupled respiration indicating maximum capacity of electron flow via respiratory chain, p50 oxygen value indicating cIV affinity to substrate — oxygen, and finally the activity of cIV. To evaluate how the electron transport chain activity exceeds the ADP phosphorylating activity the uncoupling control ratio (UCR) was calculated as the ratio between s3u and s3 oxidation of all substrates (cI + cII + GPDH s3u/cI + cII + GPDH s3).
Based on these data it should be possible to identify several types of mitochondrial disorders resulting from insufficient function of the respiratory chain at the level of individual respiratory chain complexes and ATP synthase as well as to indicate corresponding dysfunction in PDH and other components of ATP synthasome phosphorylating assembly, adenine nucleotide translocator and phosphate translocator.
3.4. Complex I deficiency
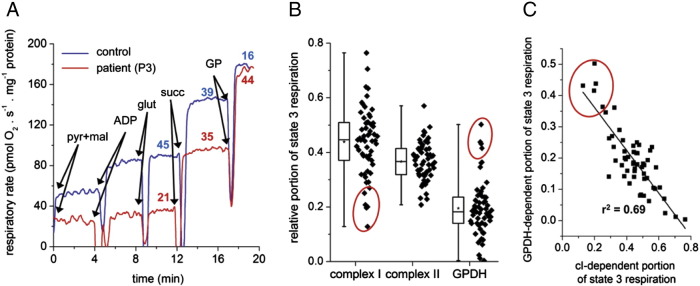
The oxygen consumption trace of lymphocytes from patient P3 represents a typical case where cI deficiency has to be considered (Fig. 4A). Low respiration with cI substrates (malate + pyruvate + glutamate) is clearly apparent, while respiration with flavoprotein-dependent substrates succinate and GP is normal or even increased. Based on the statistical analysis of all measurements performed, we identified three patients (P1–P3) with suspected mitochondrial disorder that showed low values of relative contribution of cI-dependent respiration to the s3 rate with all substrates (Fig. 4B). The mean values of the relative respiration rates for patients and controls are shown in Table 1.
Fig. 4.
Complex I deficient lymphocytes. (A) Decreased function of complex I in patient P3 lymphocytes detected as low respiration with NADH-dependent substrates. The figures above the experimental traces represent the relative portion (%) of maximal state 3 respiration contributed by individual dehydrogenases. (B) Statistical presentation of decreased relative contribution of complex I to the maximum rate of state 3-ADP respiration. Box plots represent Q1, median, Q3 (box), and minimum and maximum (whiskers), asterisks denote means, n = 60. (C) Scatter plot showing negative correlation between cI-specific and GPDH-specific respiration (n = 60), highest ratio of these parameters indicates to the suspected cI disorders. Values of patients P1–P3 are marked by red circle.
Table 1.
Diagnostic parameters for cI, cIV and cV disorders in isolated lymphocytes.
| Parameter | Patients | Controls | p |
|---|---|---|---|
| Complex I deficiency (n = 5) | |||
| cI-dependent respiration | 0.21 ± 0.070 | 0.43 ± 0.067 | < 0.01 |
| cII-dependent respiration | 0.35 ± 0.055 | 0.39 ± 0.063 | n.s. |
| GPDH-dependent respiration | 0.43 ± 0.050 | 0.17 ± 0.014 | < 0.001 |
| cI/GPDH | 0.51 ± 0.231 | 2.61 ± 0.485 | < 0.001 |
| Complex IV deficiency (n = 3) | |||
| UCR | 1.57 ± 0.113 | 1.13 ± 0.091 | < 0.01 |
| p50 (kPa) | 0.083 ± 0.0119 | 0.025 ± 0.0031 | < 0.001 |
| cIV cox4/cII SDHA content (AU/AU) | 0.31 ± 0.094 | 1.24 ± 0.288 | < 0.05 |
| Complex V deficiency (n = 6)e | |||
| Inhibition of state 3 respiration by 20 nM oligomycin (in %) | 36.5 ± 29.17 | 7.2 ± 1.36 | < 0.05 |
| UCR | 1.57 ± 0.192 | 1.13 ± 0.091 | < 0.01 |
| ADP-induced decrease of ΔΨm (in %) | 21.52 ± 0.85 | 53.73 ± 2.99 | < 0.001 |
| cVα/cII SDHA content (AU/AU) | 0.32 ± 0.053 | 0.78 ± 0.025 | < 0.01 |
Analysis of respiratory parameters and western blot detection of indicated respiratory complexes was performed as described in the Materials and methods section. cI-, cII- and GPDH-dependent respirations are expressed relative to overall cI + cII + GPDH state 3 respiration. Inhibition by oligomycin is expressed in % of the overall oligomycin-sensitive cI + cII + GPDH state 3 respiration. ADP-induced decrease of mitochondrial membrane potential ΔΨm at state 4-succinate (in %). Values represent mean ± SD of five independent controls. P1, P4, P5, P6, and P7 lymphocytes were isolated and analyzed once; P2, P3, and P8 twice; and P9 thrice, to give the total number of measurements as indicated for each patient group in the table. p represents the probability of t-test analysis used to assess the statistical significance.
Moreover, a substantially elevated GPDH-dependent respiration accompanied the decreased oxidation of substrates for cI in these samples. As depicted in Fig. 4C, analysis of all measurements performed in 60 lymphocyte preparations revealed that there is indeed a negative correlation between these parameters, i.e. between the cI-portion and GPDH-portion of the maximum state 3-ADP respiration observed with cI + cII + GPDH substrates. Interestingly, patients with low cI activity also showed the highest ratio between GPDH-dependent and cI-dependent respiration activities. Therefore, the simultaneous evaluation of cI- and GPDH-dependent portions of s3 respiration provides a sensitive measure to uncover cI defects.
3.5. Complex IV deficiency
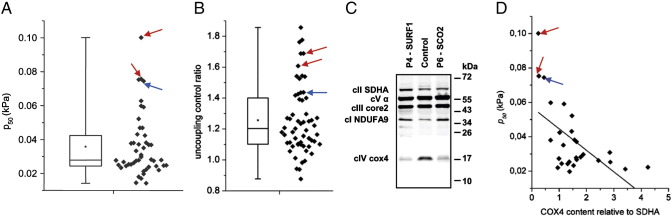
The nuclear genetic defects are rather frequent cause of cytochrome c oxidase disorders [14]. Probably the most common among them are mutations in SURF1 gene encoding cIV-specific assembly factor [33], [34]. The lack of SURF1 protein affects biogenesis of cIV and results in a decreased content of COX which is structurally and functionally altered [27], [28], [35]. Patients typically present with fatal Leigh syndrome and cIV in fibroblasts reveals a decreased proton pumping activity and decreased affinity to oxygen while the electron transport activity of cIV is generally unaffected. Fig. 5 summarizes the analysis of lymphocytes from two 2 years old patients (P4, P5), harboring SURF1 mutations and from another 7 months old patient (P6) with encephalomyopathy and hypertrophic cardiomyopathy. Subsequent DNA analysis of this patient identified mutation in SCO2, another cIV assembly factor, as a genetic cause of observed cIV deficiency.
Fig. 5.
Detection of complex IV defects in lymphocytes. (A) Oxygen p50 value (n = 47) and (B) the uncoupling control ratio (n = 60) are increased in lymphocytes with (C) isolated decrease of cIV (subunit cox4) in comparison to normal content of cI (NDUFA9 subunit), cII (SDHA subunit), cIII (core2 subunit) and cV (α subunit) detected by SDS-PAGE/WB from patients P4 and P5 harboring SURF1 mutations (red arrows) and P6 patient harboring SCO2 mutation (blue arrows), respectively; in (D) the elevated ratio of oxygen p50 relative to the content of cIV (n = 28) is shown. Box plots represent Q1, median, Q3 (box), and minimum and maximum (whiskers), asterisks denote means.
On an absolute scale, these lymphocytes displayed normal values of cI + cII + GPDH s3, cI + cII + GPDH s3u as well as COX activity. Only when related to the rate of s3u respiration, COX-dependent oxygen consumption was mildly decreased in one of the patients (not shown). However, in all three patients the p50 values for oxygen were increased to more than 3-times of the median value, and actually represented the highest values among all lymphocyte measurements performed (Fig. 5A). Besides reflecting COX Km to oxygen, the p50 value also negatively correlates with the cIV relative content to upstream respiratory chain components [36]. Western blot analysis revealed pronounced decrease in the content of subunit 4 (cox4) of cIV in P4–P6 (Fig. 5C) and when the p50 was related to the cIV content, most extreme values of both parameters (Fig. 5D) found in lymphocytes harboring SURF1 and SCO2 mutations were indicative for confident diagnosis of cIV defect. Moreover, the ratio between cI + cII + GPDH s3u and cI + cII + GPDH s3 respiration — the uncoupling control ratio (UCR) was elevated (Fig. 5B) in P4–P6 lymphocytes, indicating that electron transport activity exceeds the ADP phosphorylation capacity of OXPHOS in these cells. The mean values of parameters shown in Fig. 5 for patients and controls are shown in Table 1.
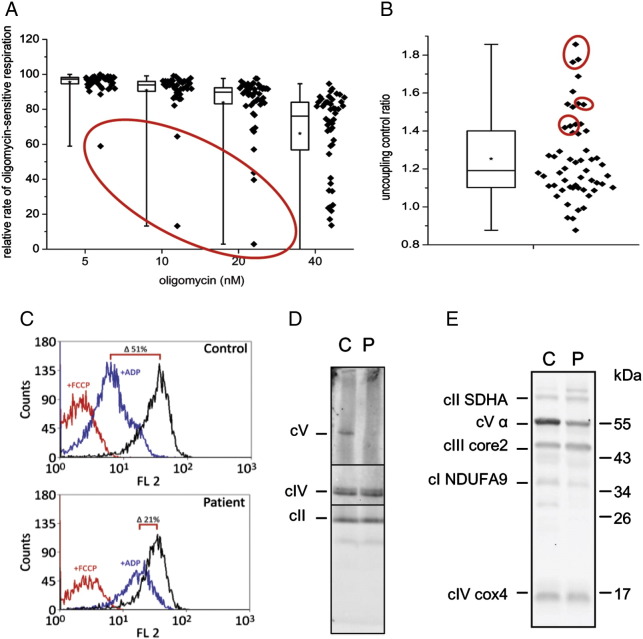
3.6. Complex V deficiency
The third type of mitochondrial disorder that we have analyzed in isolated lymphocytes was ATP synthase deficiency due to mutations in TMEM70 gene, manifesting as neonatal encephalocardiomyopathy [4], [37]. Fig. 6 summarizes the analysis of lymphocytes from 3 patients with TMEM70 mutations (P7–P9). The low content of ATP synthase in these cases limits the phosphorylating capacity of mitochondrial OXPHOS system. This was primarily observed as increased sensitivity to oligomycin, specific inhibitor of ATP synthase — a significant decrease of coupled respiration was observed at a much lower titer of the inhibitor (Fig. 6A). Statistically, this parameter appears to be particularly valuable for diagnostic purposes, as lymphocytes harboring TMEM70 mutations represent the most outlying data points in the box plots displaying the residual oligomycin-sensitive portion of s3 respiration, most apparently at 10 and 20 nM concentrations of the inhibitor. Moreover, the low content of ATP synthase limits the cI + cII + GPDH s3 respiratory rate relative to state s3u, as evidenced by increased values of UCR (Fig. 6B). In permeabilized lymphocytes with TMEM70 mutation we have also performed measurements of mitochondrial membrane potential (ΔΨm) by cytofluorometric analysis following changes in the fluorescence of cationic probe TMRM (Fig. 6C). In patient lymphocytes supplemented with succinate we have observed a much smaller decrease of ΔΨm upon addition of ADP, compared to a pronounced decrease of ΔΨm in control lymphocytes (21% versus 51% decrease of the state 4 value), indicating that the low content of ATP synthase limits the discharge of respiration-generated proton gradient by phosphorylation of added ADP. In addition, the change in the content of ATP synthase in lymphocytes was detected by native electrophoresis in combination with immunodetection. As seen in Fig. 6D, ATP synthase complex of 600 kDa was almost undetectable in patient lymphocytes with TMEM70 mutation. Corresponding specific decrease of α subunit of cV was found by SDS electrophoresis and WB, while the levels of cI, cII, cIII and cIV were unaffected (Fig. 6E). The mean values of respiration inhibition at 20 nM oligomycin, UCR, ADP-induced decrease of ΔΨm and cV α subunit content for patients and controls are shown in Table 1.
Fig. 6.
Detection of ATP synthase deficiency in lymphocytes. (A) Sensitivity of state 3-ADP respiration (with glutamate + malate + succinate + glycerol-3-phosphate) to oligomycin is increased (n = 50, box plots represent Q1, median, Q3 (box), minimum and maximum (whiskers), asterisks denote means) and (B) uncoupling control ratio (n = 60) is elevated in lymphocytes from patients (P7–P9) with ATP synthase deficiency due to TMEM70 mutation (data points of TMEM70 patients are marked by red circles). (C) High levels of mitochondrial membrane potential ΔΨm at state 3-ADP detected by TMRM (20 nM) cytofluorometry (analysis was performed using 10 mM succinate (state 4o), after addition of 1 mM ADP (state 3) or 1 μM FCCP (state 3u), decrease in ΔΨm is expressed as % change from state 4 value). (D) diminished content of ATP synthase complex (cV) in comparison to the content of other respiratory chain complexes detected by BNE/WB and by (E) SDS-PAGE/WB using subunit specific antibodies to cV (α subunit), cI (NDUFA9 subunit), cII (SDHA subunit), cIII (core2 subunit) and cIV (cox4 subunit), P — P7, C — control.
4. Discussion
Mitochondrial diseases are responsible for a significant portion of mortality and morbidity in the youngest groups of children. Due to the frequent impairment of brain function, the surviving patients represent a serious socioeconomic problem. As there is no causal therapy available for most mitochondrial diseases, the early diagnosis of biochemical and genetic defects is of utmost importance for prevention and genetic counseling in affected families.
In the present study we focused on establishing of noninvasive diagnostic methods for fast and precise identification of mitochondrial dysfunction and elucidation of molecular basis of their phenotypic manifestation. We demonstrate that the important improvement in timely diagnostics of mitochondrial disorders of OXPHOS system can be achieved by the use of lymphocytes isolated from peripheral blood. The analysis of mitochondrial functions was based on adaptation of protocols developed for mitochondrial diagnostics in cultured skin fibroblasts [25], [27], [28], [32]. The key method is the oxygraphic analysis of mitochondrial energetics in digitonin-permeabilized lymphocytes by a complex substrate-inhibitor approach which provides detailed information about electron transport activities of respiratory chain complexes, ATP synthesis as well as respiratory control, ATP synthase function or complex IV affinity to oxygen. Where feasible, the measurements can be complemented by the cytofluorometric detection of ΔΨm with TMRM. The functional analysis is further accompanied by analysis of respiratory chain protein complexes and their subunits by electrophoretic methods utilizing native and SDS electrophoreses in combination with western blot immunodetection.
Altogether, we have analyzed 60 samples of lymphocytes from children with suspected mitochondrial disorders, children with previously diagnosed OXPHOS disorders as well as children with normal function of mitochondrial OXPHOS system. Statistical analysis of all measurements established the range of values for individual respiratory parameters, covering the normal control values and their fluctuation as well as the outlying pathological values indicative of possible OXPHOS dysfunction. Rather than comparing a given case with selected control samples, we focused on the analysis of statistically extreme values in one or several parameters that are indicative for a certain OXPHOS disorder. This approach enabled us to select the best diagnostic markers for individual OXPHOS defects based on the whole dataset, without the requirement for their a priori definition. The significance of these parameters with respect to controls is summarized in Table 1. Our results indicate that this type of analysis is sensitive enough to recognize several types of OXPHOS deficiencies, namely isolated defects of complex I, complex IV and ATP synthase when they are manifested in lymphocytes.
The analysis of lymphocytes revealed a low activity of cI in three patients, P1–P3. In the first patient with severe encephalopathy, the low activity of cI was confirmed in fibroblasts but the genetic defect remains unknown — no mutation in mtDNA was found and the search for nuclear genes responsible for complex I deficiency by exome sequencing has not yet been completed. A marked decrease of cI activity was also found in patient P2, where the mitochondrial impairment was indicated by progressive sensorial defects and cephalea attacks. In the third case, P3 who died at 4 months with a pronounced brain impairment, the respirometry revealed cI deficiency in lymphocytes, while spectrophotometric analyses in cultivated fibroblasts showed a normal activity and amount of respiratory chain complexes. The muscle biopsy was not available. The mitoanalysis of lymphocytes could thus indicate a tissue specific type of disorder of respiratory chain complex I.
Importantly, in all three patients with low electron transport activity of cI we have found that besides decreased values of state 3-ADP respiration with cI-dependent substrates, they have consistently upregulated GP-supported respiration. We therefore propose that the increased GPDH-sensitive portion of the maximum respiration of cI + cII + GPDH substrates, as well as high ratio of GPDH-dependent/cI dependent respiration may serve as sensitive diagnostic criteria for cI dysfunction. Such an increase of GP respiration likely results from increased relative contribution to overall flux capacity of the respiratory chain [38], [39] when electron transport from cI is decreased. Alternatively it may reflect an adaptive increase of mitochondrial content of FAD-dependent GPDH. Together with cytosolic NAD-dependent GPDH, they constitute glycerophosphate shuttle which facilitates oxidation of cytosolic NADH and thus bypasses respiratory complex I [40].
The activity of cIV has to be significantly affected to influence respiration with different substrates due to excess capacity of COX in most mammalian cells and tissues [39]. Thus direct measurement of cIV activity, as respiration with artificial substrates ascorbate + TMPD in the presence of antimycin A, may detect the change in cIV electron transport activity more sensitively than when it is only slightly decreased. Such a situation is expected in patients with cIV dysfunction due to SURF1 mutations and our measurements showed that this also holds true in lymphocytes from these patients. However, we showed that the increase of p50 value is much more sensitive parameter for diagnostics of this type of cIV disorder. P50 serves as the measure of the affinity of respiration towards oxygen, and the observed changes in lymphocytes are in accordance with data previously observed in fibroblasts with SURF1 mutations [28]. In addition, SURF1 protein-deficient lymphocytes also displayed a high value of UCR (> 90th percentile) which is in agreement with our previous observation that in patient fibroblasts s3 respiration is decreased more significantly than s3u respiration [27].
Another important finding of our study is that all these changes, which are indicative for COX deficiency, could also be found in lymphocytes of the patient with SCO2 mutation. COX deficiency due to SCO2 dysfunction does not manifest in fibroblasts [41], [42] and thus analysis of lymphocytes may serve as an preferred diagnostic approach.
The third type of mitochondrial disorder that we have detected in isolated lymphocytes was ATP synthase deficiency, due to TMEM70 mutations, a very frequent type of autosomal recessive deficiency of ATP synthase [43]. In this case, several parameters can be used for identification of ATP synthase dysfunction in lymphocytes, namely increased sensitivity of state 3-ADP respiration to oligomycin and decreased rate of state 3-ADP respiration relative to state 3-uncoupled respiration. The low content of cV is further detected by electrophoretic analysis and the third independent approach can be cytofluorometric analysis of mitochondrial membrane potential, which shows elevated values of ΔΨm at state 3-ADP. Importantly all these changes have previously been found in patient fibroblasts with TMEM70 mutations [4], [5].
More than two decades passed since the first attempts to use blood cells, in particular lymphocytes for studying of inherited mitochondrial disorders [15], [16], [17], [18]. In subsequent years oxygraphy in combination with spectrophotometric measurements proved useful to identify different types of OXPHOS defects, mainly those caused by mtDNA mutations [19], [20], [21], [22], [23], [24], [25]. However, these approaches never became prominent tool for primary diagnostics. Today, isolated lymphocytes or lymphoblasts are only rarely utilized for routine biochemical analysis of mitochondrial respiratory chain function in patients with suspected inborn defects in oxidative phosphorylation. In part, this may reflect potential issues regarding tissue specificity of disease presentation as well as varying heteroplasmy load between tissues in case of mtDNA mutations [44], [45], [46], [47], [48], [49]. Methodical limitations in early studies, which usually focused only on a defect in the particular OXPHOS complex, as well as limited yield and viability period of intact isolated lymphocyte cells were possibly other confounding factors leading to the limited use of lymphocytes.
It should be stressed out however, that in the last couple of years, peripheral blood cells came into focus of researchers looking for disease biomarkers, primarily due to their easy accessibility. Peripheral blood cells in general or their respective subpopulations were used for evaluation of mitochondrial functions in a wide range of human pathologies [50]. While some of the pathologies studied are systemic, such as diabetes [51] and septic shock [52], others e.g. neurodegenerative diseases [53], [54], glaucoma [55] or fibromyalgia [56] display tissue specificity in their symptoms and yet manifest as mitochondrial dysfunction in peripheral blood cells. In many respects this represents analogous situation to inherited mitochondrial diseases.
Our studies clearly demonstrated that by combined approach involving sensitive functional methods (oxygraphy and cytofluorometry) and protein analysis by electrophoretic methods and western blotting it is possible to detect several types of isolated OXPHOS disorders of nuclear genetic origin. These are: (i) low respiration with cI substrates and increased respiration with GP revealed decreased activity of respiratory chain complex I, (ii) changes in p50 for oxygen and high value of UCR pointed to COX deficiency due to SURF1 mutations and (iii) high sensitivity to oligomycin, upregulated mitochondrial membrane potential and low content of ATP synthase complex were found in lymphocytes with ATP synthase deficiency due to TMEM70 mutations. Based on our current experience further systematic investigations of lymphocytes will be performed to evaluate the potential of this type of mitoanalysis for diagnostics of a broader range of mitochondrial disorders. Detection of other defects (e.g. PDH deficiency by low cI dependent respiration with pyruvate compared to glutamate plus malate) using the same experimental protocol can be envisaged but remains to be validated on respective patients.
Mitodiagnostics in isolated lymphocytes described herein represent fast and technically affordable approach. The oxygraphic analysis and mitochondrial membrane potential analysis are performed on freshly isolated lymphocytes and are completed within a day. Electrophoretic analysis in combination with immunodetection does not require fresh material and can be completed in 2 days. The key approach is the high resolution respiration measurements using Oxygraph 2k, which is sufficiently sensitive and well suited for a complex, substrate-inhibitor measurements in permeabilized lymphocytes. One such measurement usually lasts for ~ 2 h. The detailed evaluation of these measurements is subsequently performed by the data analysis using DatLab software. Cytofluorometry of mitochondrial membrane potential with TMRM is a well-established method that provides relative values of ΔΨm, rather than exact values in mV. Nevertheless, it represents a sensitive approach to detect differences between patient and control in the key parameters of ΔΨm. By detecting energization of the inner mitochondrial membrane at state 4 and state 3 we can get insight into the function of ATP synthase. The two electrophoretic methods used (SDS-PAGE and native-PAGE) represent routine tools for determination of the amount, subunit composition as well as native forms of all mitochondrial OXPHOS complexes visualized by immunodetection with commercially available, subunit specific antibodies. For quantitative analysis the detection using fluorescent secondary antibodies and laser scanners is a method of choice.
In conclusion, our study demonstrates that the use of isolated lymphocytes obtained from peripheral blood in combination with analysis of mitochondrial functions and quantitative/qualitative analysis of mitochondrial OXPHOS complexes represents a sensitive, fast and noninvasive approach for rapid screening and diagnostics of different types of mitochondrial disorders, especially of nuclear genetic origin manifesting in pediatric patients. In addition, repeated analyses of lymphocytes offer a possibility to follow the changes in mitochondrial energetic system in the course of the disease progression.
Acknowledgments
This work was supported by grants from the Grant Agency of the Ministry of Health of the Czech Republic (NT12370-5), the Grant Agency of the Czech Republic (P303/11/0970, 14-368046) and institutional support provided by the Ministry of Education, Youth and Sports of the Czech Republic (RVO:67985823).
Contributor Information
Petr Pecina, Email: petr.pecina@biomed.cas.cz.
Hana Houšťková, Email: hana.houstkova@ftn.cz.
Tomáš Mráček, Email: mracek@biomed.cas.cz.
Alena Pecinová, Email: alena.pecinova@biomed.cas.cz.
Hana Nůsková, Email: hana.nuskova@gmail.com.
Markéta Tesařová, Email: Marketa.Tesarova@lf1.cuni.cz.
Hana Hansíková, Email: HHansikova@seznam.cz.
Jan Janota, Email: jan.janota@ftn.cz.
Jiří Zeman, Email: jzem@lf1.cuni.cz.
Josef Houštěk, Email: houstek@biomed.cas.cz.
References
- 1.DiMauro S. Mitochondrial medicine. Biochim. Biophys. Acta. 2004;1659:107–114. doi: 10.1016/j.bbabio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 2.DiMauro S. Mitochondrial DNA medicine. Biosci. Rep. 2007;27:5–9. doi: 10.1007/s10540-007-9032-5. [DOI] [PubMed] [Google Scholar]
- 3.Vafai S.B., Mootha V.K. Mitochondrial disorders as windows into an ancient organelle. Nature. 2012;491:374–383. doi: 10.1038/nature11707. [DOI] [PubMed] [Google Scholar]
- 4.Cizkova A., Stranecky V., Mayr J.A., Tesarova M., Havlickova V., Paul J., Ivanek R., Kuss A.W., Hansikova H., Kaplanova V., Vrbacky M., Hartmannova H., Noskova L., Honzik T., Drahota Z., Magner M., Hejzlarova K., Sperl W., Zeman J., Houstek J., Kmoch S. TMEM70 mutations cause isolated ATP synthase deficiency and neonatal mitochondrial encephalocardiomyopathy. Nat. Genet. 2008;40:1288–1290. doi: 10.1038/ng.246. [DOI] [PubMed] [Google Scholar]
- 5.Houstek J., Kmoch S., Zeman J. TMEM70 protein — a novel ancillary factor of mammalian ATP synthase. Biochim. Biophys. Acta. 2009;1787:529–532. doi: 10.1016/j.bbabio.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Antonicka H., Ostergaard E., Sasarman F., Weraarpachai W., Wibrand F., Pedersen A.M., Rodenburg R.J., van der Knaap M.S., Smeitink J.A., Chrzanowska-Lightowlers Z.M., Shoubridge E.A. Mutations in C12orf65 in patients with encephalomyopathy and a mitochondrial translation defect. Am. J. Hum. Genet. 2010;87:115–122. doi: 10.1016/j.ajhg.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weraarpachai W., Sasarman F., Nishimura T., Antonicka H., Aure K., Rotig A., Lombes A., Shoubridge E.A. Mutations in C12orf62, a factor that couples COX I synthesis with cytochrome c oxidase assembly, cause fatal neonatal lactic acidosis. Am. J. Hum. Genet. 2012;90:142–151. doi: 10.1016/j.ajhg.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugiana C., Pagliarini D.J., McKenzie M., Kirby D.M., Salemi R., Abu-Amero K.K., Dahl H.H., Hutchison W.M., Vascotto K.A., Smith S.M., Newbold R.F., Christodoulou J., Calvo S., Mootha V.K., Ryan M.T., Thorburn D.R. Mutation of C20orf7 disrupts complex I assembly and causes lethal neonatal mitochondrial disease. Am. J. Hum. Genet. 2008;83:468–478. doi: 10.1016/j.ajhg.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weraarpachai W., Antonicka H., Sasarman F., Seeger J., Schrank B., Kolesar J.E., Lochmuller H., Chevrette M., Kaufman B.A., Horvath R., Shoubridge E.A. Mutation in TACO1, encoding a translational activator of COX I, results in cytochrome c oxidase deficiency and late-onset Leigh syndrome. Nat. Genet. 2009;41:833–837. doi: 10.1038/ng.390. [DOI] [PubMed] [Google Scholar]
- 10.Janer A., Antonicka H., Lalonde E., Nishimura T., Sasarman F., Brown G.K., Brown R.M., Majewski J., Shoubridge E.A. An RMND1 mutation causes encephalopathy associated with multiple oxidative phosphorylation complex deficiencies and a mitochondrial translation defect. Am. J. Hum. Genet. 2012;91:737–743. doi: 10.1016/j.ajhg.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghezzi D., Goffrini P., Uziel G., Horvath R., Klopstock T., Lochmuller H., D'Adamo P., Gasparini P., Strom T.M., Prokisch H., Invernizzi F., Ferrero I., Zeviani M. SDHAF1, encoding a LYR complex-II specific assembly factor, is mutated in SDH-defective infantile leukoencephalopathy. Nat. Genet. 2009;41:654–656. doi: 10.1038/ng.378. [DOI] [PubMed] [Google Scholar]
- 12.Hao H.X., Khalimonchuk O., Schraders M., Dephoure N., Bayley J.P., Kunst H., Devilee P., Cremers C.W., Schiffman J.D., Bentz B.G., Gygi S.P., Winge D.R., Kremer H., Rutter J. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325:1139–1142. doi: 10.1126/science.1175689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pagliarini D.J., Calvo S.E., Chang B., Sheth S.A., Vafai S.B., Ong S.E., Walford G.A., Sugiana C., Boneh A., Chen W.K., Hill D.E., Vidal M., Evans J.G., Thorburn D.R., Carr S.A., Mootha V.K. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bohm M., Pronicka E., Karczmarewicz E., Pronicki M., Piekutowska-Abramczuk D., Sykut-Cegielska J., Mierzewska H., Hansikova H., Vesela K., Tesarova M., Houstkova H., Houstek J., Zeman J. Retrospective, multicentric study of 180 children with cytochrome C oxidase deficiency. Pediatr. Res. 2006;59:21–26. doi: 10.1203/01.pdr.0000190572.68191.13. [DOI] [PubMed] [Google Scholar]
- 15.Rotig A., Cormier V., Blanche S., Bonnefont J.P., Ledeist F., Romero N., Schmitz J., Rustin P., Fischer A., Saudubray J.M. Pearson's marrow-pancreas syndrome. A multisystem mitochondrial disorder in infancy. J. Clin. Invest. 1990;86:1601–1608. doi: 10.1172/JCI114881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cormier V., Rotig A., Tardieu M., Colonna M., Saudubray J.M., Munnich A. Autosomal dominant deletions of the mitochondrial genome in a case of progressive encephalomyopathy. Am. J. Hum. Genet. 1991;48:643–648. [PMC free article] [PubMed] [Google Scholar]
- 17.Burgeois M., Goutieres F., Chretien D., Rustin P., Munnich A., Aicardi J. Deficiency in complex II of the respiratory chain, presenting as a leukodystrophy in two sisters with Leigh syndrome. Brain Dev. 1992;14:404–408. doi: 10.1016/s0387-7604(12)80349-4. [DOI] [PubMed] [Google Scholar]
- 18.Tatuch Y., Robinson B.H. The mitochondrial DNA mutation at 8993 associated with NARP slows the rate of ATP synthesis in isolated lymphoblast mitochondria. Biochem. Biophys. Res. Commun. 1993;192:124–128. doi: 10.1006/bbrc.1993.1390. [DOI] [PubMed] [Google Scholar]
- 19.Rustin P., Chretien D., Bourgeron T., Gerard B., Rotig A., Saudubray J.M., Munnich A. Biochemical and molecular investigations in respiratory chain deficiencies. Clin. Chim. Acta. 1994;228:35–51. doi: 10.1016/0009-8981(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 20.Chretien D., Rustin P., Bourgeron T., Rotig A., Saudubray J.M., Munnich A. Reference charts for respiratory chain activities in human tissues. Clin. Chim. Acta. 1994;228:53–70. doi: 10.1016/0009-8981(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 21.Uziel G., Moroni I., Lamantea E., Fratta G.M., Ciceri E., Carrara F., Zeviani M. Mitochondrial disease associated with the T8993G mutation of the mitochondrial ATPase 6 gene: a clinical, biochemical, and molecular study in six families. J. Neurol. Neurosurg. Psychiatry. 1997;63:16–22. doi: 10.1136/jnnp.63.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Artuch R., Colome C., Playan A., Alcaine M.J., Briones P., Montoya J., Vilaseca M.A., Pineda M. Oxygen consumption measurement in lymphocytes for the diagnosis of pediatric patients with oxidative phosphorylation diseases. Clin. Biochem. 2000;33:481–485. doi: 10.1016/s0009-9120(00)00137-5. [DOI] [PubMed] [Google Scholar]
- 23.Brown M.D., Trounce I.A., Jun A.S., Allen J.C., Wallace D.C. Functional analysis of lymphoblast and cybrid mitochondria containing the 3460, 11778, or 14484 Leber's hereditary optic neuropathy mitochondrial DNA mutation. J. Biol. Chem. 2000;275:39831–39836. doi: 10.1074/jbc.M006476200. [DOI] [PubMed] [Google Scholar]
- 24.Capkova M., Hansikova H., Godinot C., Houst'kova H., Houstek J., Zeman J. A new missense mutation of 574C>T in the SURF1 gene—biochemical and molecular genetic study in seven children with Leigh syndrome. Cas. Lek. Cesk. 2002;141:636–641. [PubMed] [Google Scholar]
- 25.Chretien D., Benit P., Chol M., Lebon S., Rotig A., Munnich A., Rustin P. Assay of mitochondrial respiratory chain complex I in human lymphocytes and cultured skin fibroblasts. Biochem. Biophys. Res. Commun. 2003;301:222–224. doi: 10.1016/s0006-291x(02)03016-4. [DOI] [PubMed] [Google Scholar]
- 26.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Pecina P., Capkova M., Chowdhury S.K., Drahota Z., Dubot A., Vojtiskova A., Hansikova H., Houst'kova H., Zeman J., Godinot C., Houstek J. Functional alteration of cytochrome c oxidase by SURF1 mutations in Leigh syndrome. Biochim. Biophys. Acta. 2003;1639:53–63. doi: 10.1016/s0925-4439(03)00127-3. [DOI] [PubMed] [Google Scholar]
- 28.Pecina P., Gnaiger E., Zeman J., Pronicka E., Houstek J. Decreased affinity for oxygen of cytochrome-c oxidase in Leigh syndrome caused by SURF1 mutations. Am. J. Physiol. Cell Physiol. 2004;287:C1384–C1388. doi: 10.1152/ajpcell.00286.2004. [DOI] [PubMed] [Google Scholar]
- 29.Gnaiger E., Steinlechner-Maran R., Mendez G., Eberl T., Margreiter R. Control of mitochondrial and cellular respiration by oxygen. J. Bioenerg. Biomembr. 1995;27:583–596. doi: 10.1007/BF02111656. [DOI] [PubMed] [Google Scholar]
- 30.Schagger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 31.Wittig I., Braun H.P., Schagger H. Blue native PAGE. Nat. Protoc. 2006;1:418–428. doi: 10.1038/nprot.2006.62. [DOI] [PubMed] [Google Scholar]
- 32.Floryk D., Houstek J. Tetramethyl rhodamine methyl ester (TMRM) is suitable for cytofluorometric measurements of mitochondrial membrane potential in cells treated with digitonin. Biosci. Rep. 1999;19:27–34. doi: 10.1023/a:1020193906974. [DOI] [PubMed] [Google Scholar]
- 33.Tiranti V., Hoertnagel K., Carrozzo R., Galimberti C., Munaro M., Granatiero M., Zelante L., Gasparini P., Marzella R., Rocchi M., Bayona-Bafaluy M.P., Enriquez J.A., Uziel G., Bertini E., Dionisi-Vici C., Franco B., Meitinger T., Zeviani M. Mutations of SURF-1 in Leigh disease associated with cytochrome c oxidase deficiency. Am. J. Hum. Genet. 1998;63:1609–1621. doi: 10.1086/302150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu Z., Yao J., Johns T., Fu K., De Bie I., Macmillan C., Cuthbert A.P., Newbold R.F., Wang J., Chevrette M., Brown G.K., Brown R.M., Shoubridge E.A. SURF1, encoding a factor involved in the biogenesis of cytochrome c oxidase, is mutated in Leigh syndrome. Nat. Genet. 1998;20:337–343. doi: 10.1038/3804. [DOI] [PubMed] [Google Scholar]
- 35.Kovarova N., Cizkova Vrbacka A., Pecina P., Stranecky V., Pronicka E., Kmoch S., Houstek J. Adaptation of respiratory chain biogenesis to cytochrome c oxidase deficiency caused by SURF1 gene mutations. Biochim. Biophys. Acta. 2012;1822:1114–1124. doi: 10.1016/j.bbadis.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Gnaiger E., Lassnig B., Kuznetsov A., Rieger G., Margreiter R. Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. J. Exp. Biol. 1998;201:1129–1139. doi: 10.1242/jeb.201.8.1129. [DOI] [PubMed] [Google Scholar]
- 37.Honzik T., Tesarova M., Mayr J.A., Hansikova H., Jesina P., Bodamer O., Koch J., Magner M., Freisinger P., Huemer M., Kostkova O., van Coster R., Kmoch S., Houstek J., Sperl W., Zeman J. Mitochondrial encephalocardio-myopathy with early neonatal onset due to TMEM70 mutation. Arch. Dis. Child. 2010;95:296–301. doi: 10.1136/adc.2009.168096. [DOI] [PubMed] [Google Scholar]
- 38.Mazat J.P., Rossignol R., Malgat M., Rocher C., Faustin B., Letellier T. What do mitochondrial diseases teach us about normal mitochondrial functions…that we already knew: threshold expression of mitochondrial defects. Biochim. Biophys. Acta. 2001;1504:20–30. doi: 10.1016/s0005-2728(00)00236-x. [DOI] [PubMed] [Google Scholar]
- 39.Rossignol R., Faustin B., Rocher C., Malgat M., Mazat J.P., Letellier T. Mitochondrial threshold effects. Biochem. J. 2003;370:751–762. doi: 10.1042/BJ20021594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mracek T., Drahota Z., Houstek J. The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues. Biochim. Biophys. Acta. 2013;1827:401–410. doi: 10.1016/j.bbabio.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Jaksch M., Ogilvie I., Yao J., Kortenhaus G., Bresser H.G., Gerbitz K.D., Shoubridge E.A. Mutations in SCO2 are associated with a distinct form of hypertrophic cardiomyopathy and cytochrome c oxidase deficiency. Hum. Mol. Genet. 2000;9:795–801. doi: 10.1093/hmg/9.5.795. [DOI] [PubMed] [Google Scholar]
- 42.Vesela K., Hansikova H., Tesarova M., Martasek P., Elleder M., Houstek J., Zeman J. Clinical, biochemical and molecular analyses of six patients with isolated cytochrome c oxidase deficiency due to mutations in the SCO2 gene. Acta Paediatr. 2004;93:1312–1317. doi: 10.1080/08035250410008761. [DOI] [PubMed] [Google Scholar]
- 43.Hejzlarova K., Mracek T., Vrbacky M., Kaplanova V., Karbanova V., Nuskova H., Pecina P., Houstek J. Nuclear genetic defects of mitochondrial ATP synthase. Physiol. Res. 2014;63(Suppl. 1):S57–S71. doi: 10.33549/physiolres.932643. [DOI] [PubMed] [Google Scholar]
- 44.Larsson N.G., Tulinius M.H., Holme E., Oldfors A., Andersen O., Wahlstrom J., Aasly J. Segregation and manifestations of the mtDNA tRNA(Lys) A→G(8344) mutation of myoclonus epilepsy and ragged-red fibers (MERRF) syndrome. Am. J. Hum. Genet. 1992;51:1201–1212. [PMC free article] [PubMed] [Google Scholar]
- 45.Holme E., Larsson N.G., Oldfors A., Tulinius M., Sahlin P., Stenman G. Multiple symmetric lipomas with high levels of mtDNA with the tRNA(Lys) A→G(8344) mutation as the only manifestation of disease in a carrier of myoclonus epilepsy and ragged-red fibers (MERRF) syndrome. Am. J. Hum. Genet. 1993;52:551–556. [PMC free article] [PubMed] [Google Scholar]
- 46.Tulinius M.H., Houshmand M., Larsson N.G., Holme E., Oldfors A., Holmberg E., Wahlstrom J. De novo mutation in the mitochondrial ATP synthase subunit 6 gene (T8993G) with rapid segregation resulting in Leigh syndrome in the offspring. Hum. Genet. 1995;96:290–294. doi: 10.1007/BF00210409. [DOI] [PubMed] [Google Scholar]
- 47.Houshmand M., Lindberg C., Moslemi A.R., Oldfors A., Holme E. A novel heteroplasmic point mutation in the mitochondrial tRNA(Lys) gene in a sporadic case of mitochondrial encephalomyopathy: de novo mutation and no transmission to the offspring. Hum. Mutat. 1999;13:203–209. doi: 10.1002/(SICI)1098-1004(1999)13:3<203::AID-HUMU4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 48.Lamantea E., Carrara F., Mariotti C., Morandi L., Tiranti V., Zeviani M. A novel nonsense mutation (Q352X) in the mitochondrial cytochrome b gene associated with a combined deficiency of complexes I and III. Neuromuscul. Disord. 2002;12:49–52. doi: 10.1016/s0960-8966(01)00244-9. [DOI] [PubMed] [Google Scholar]
- 49.Leo-Kottler B., Luberichs J., Besch D., Christ-Adler M., Fauser S. Leber's hereditary optic neuropathy: clinical and molecular genetic results in a patient with a point mutation at np T11253C (isoleucine to threonine) in the ND4 gene and spontaneous recovery. Graefes Arch. Clin. Exp. Ophthalmol. 2002;240:758–764. doi: 10.1007/s00417-002-0494-7. [DOI] [PubMed] [Google Scholar]
- 50.Chacko B.K., Kramer P.A., Ravi S., Johnson M.S., Hardy R.W., Ballinger S.W., Darley-Usmar V.M. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab. Invest. 2013;93:690–700. doi: 10.1038/labinvest.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Widlansky M.E., Wang J., Shenouda S.M., Hagen T.M., Smith A.R., Kizhakekuttu T.J., Kluge M.A., Weihrauch D., Gutterman D.D., Vita J.A. Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Transl. Res. 2010;156:15–25. doi: 10.1016/j.trsl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Japiassu A.M., Santiago A.P., d'Avila J.C., Garcia-Souza L.F., Galina A., Castro Faria-Neto H.C., Bozza F.A., Oliveira M.F. Bioenergetic failure of human peripheral blood monocytes in patients with septic shock is mediated by reduced F1Fo adenosine-5′-triphosphate synthase activity. Crit. Care Med. 2011;39:1056–1063. doi: 10.1097/CCM.0b013e31820eda5c. [DOI] [PubMed] [Google Scholar]
- 53.Leuner K., Schulz K., Schutt T., Pantel J., Prvulovic D., Rhein V., Savaskan E., Czech C., Eckert A., Muller W.E. Peripheral mitochondrial dysfunction in Alzheimer's disease: focus on lymphocytes. Mol. Neurobiol. 2012;46:194–204. doi: 10.1007/s12035-012-8300-y. [DOI] [PubMed] [Google Scholar]
- 54.Tyurina Y.Y., Winnica D.E., Kapralova V.I., Kapralov A.A., Tyurin V.A., Kagan V.E. LC/MS characterization of rotenone induced cardiolipin oxidation in human lymphocytes: implications for mitochondrial dysfunction associated with Parkinson's disease. Mol. Nutr. Food Res. 2013;57:1410–1422. doi: 10.1002/mnfr.201200801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee S., Sheck L., Crowston J.G., Van Bergen N.J., O'Neill E.C., O'Hare F., Kong Y.X., Chrysostomou V., Vincent A.L., Trounce I.A. Impaired complex-I-linked respiration and ATP synthesis in primary open-angle glaucoma patient lymphoblasts. Invest. Ophthalmol. Vis. Sci. 2012;53:2431–2437. doi: 10.1167/iovs.12-9596. [DOI] [PubMed] [Google Scholar]
- 56.Cordero M.D., De Miguel M., Moreno Fernandez A.M., Carmona Lopez I.M., Garrido Maraver J., Cotan D., Gomez Izquierdo L., Bonal P., Campa F., Bullon P., Navas P., Sanchez Alcazar J.A. Mitochondrial dysfunction and mitophagy activation in blood mononuclear cells of fibromyalgia patients: implications in the pathogenesis of the disease. Arthritis Res. Ther. 2010;12:R17. doi: 10.1186/ar2918. [DOI] [PMC free article] [PubMed] [Google Scholar]