Abstract
Background
Technological advances including high-throughput sequencing have identified numerous tumor-specific genetic changes in pediatric and adolescent cancers that can be exploited as targets for novel therapies.
Scope of review
This review provides a detailed overview of recent advances in the application of target-specific therapies for childhood cancers, either as single agents or in combination with other therapies. The review summarizes preclinical evidence on which clinical trials are based, early phase clinical trial results, and the incorporation of predictive biomarkers into clinical practice, according to cancer type.
Major conclusions
There is growing evidence that molecularly targeted therapies can valuably add to the arsenal available for treating childhood cancers, particularly when used in combination with other therapies. Nonetheless the introduction of molecularly targeted agents into practice remains challenging, due to the use of unselected populations in some clinical trials, inadequate methods to evaluate efficacy, and the need for improved preclinical models to both evaluate dosing and safety of combination therapies.
General significance
The increasing recognition of the heterogeneity of molecular causes of cancer favors the continued development of molecularly targeted agents, and their transfer to pediatric and adolescent populations.
Abbreviations: ALK, anaplastic lymphoma kinase; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ARMS, alveolar rhabdomyosarcoma; AT/RT, atypical teratoid/rhabdoid tumor; AURKA, aurora kinase A; AURKB, aurora kinase B; BET, bromodomain and extra terminal; CAR, chimeric antigen receptor; CML, chronic myeloid leukemia; DFMO, difluoromethylornithine; DIPG, diffuse intrinsic pontine glioma; EGFR, epidermal growth factor receptor; ERMS, embryonal rhabdomyosarcoma; HDAC, histone deacetylases; Hsp90, heat shock protein 90; IGF/IGFR, insulin-like growth factor/receptor; IGF-1R, insulin-like growth factor type 1 receptor; mAb, monoclonal antibody; mAbs, monoclonal antibodies; mTOR, mammalian target of rapamycin; NSCLC, non-small cell lung cancer; ODC1, ornithine decarboxylase 1; PARP, poly(ADP-ribose) polymerase; PDGFRA/B, platelet derived growth factor alpha/beta; Ph +, Philadelphia chromosome-positive; PI3K, phosphatidylinositol 3′-kinase; PLK1, polo-like kinase 1; RMS, rhabdomyosarcoma; SHH, sonic hedgehog; SMO, smoothened; SYK, spleen tyrosine kinase; TOP1/TOP2, DNA topoisomerase 1/2; TRAIL, TNF-related apoptosis-inducing ligand; VEGF/VEGFR, vascular endothelial growth factor/receptor
Keywords: Childhood cancer, Molecular diagnostics, Targeted therapy, Biomarkers
Highlights
-
•
Increasing numbers of targeted therapies are being tested for pediatric cancers.
-
•
Molecularly targeted therapies are proving most effective in combination regimes.
-
•
More rigorous preclinical testing should further improve clinical trial results.
1. Introduction
Childhood cancers are rare, representing less than 1% of new cancer diagnoses, however, they are still the leading cause of disease-related death in children in industrialized countries such as the US [1]. Children's malignancies are generally treated with a combination of cytotoxic chemotherapy, radiation, surgery, and occasionally bone marrow transplant, which have markedly improved the overall 5-year survival rate over the past decades from 50 to 60% for cases diagnosed during the 1970s, to over 80% today [2].
The greatest contribution to better outcomes comes from advances in our understanding of the genetics of cancer [3], [4], [5], and the discovery of molecular biomarkers and incorporation of novel targeted agents has led to improved outcomes for cancer patients, and decreases in both short- and long-term toxicities [6]. Some biomarkers are routinely used for diagnostics (such as Ki-67 staining as proliferation index) [7], risk-stratification (MYCN amplification in neuroblastoma) [8] and monitoring (S100-beta in melanoma) [9]. Others are used to direct the use of targeted therapy, such as the fusion tyrosine–kinase protein BCR–ABL for the use of imatinib in chronic myeloid leukemia (CML) and Philadelphia chromosome positive (Ph +) acute lymphoblastic leukemia (ALL) [10], [11] or anaplastic lymphoma kinase (ALK) mutations (especially EML4–ALK rearranged cancers) for the use of crizotinib, the ALK inhibitor approved for treatment of non-small cell lung cancer (NSCLC) [12], [13]. In summary, the genetic heterogeneity now recognized to underpin pediatric and adolescent cancers may be exploited by agents targeting these specific molecular/genetic lesions. A principal challenge is to now further expand the number of molecular targets investigated, and integrate conventional practices with the best target-matched therapies. The aim of this review is to give an overview of recent advances in the application of “druggable” biomarkers, and the use of target-specific therapies for childhood cancers.
2. Relevant biomarkers in childhood malignancies and novel therapies
The role of germ-line mutations in explaining susceptibility to childhood cancer and genetic predisposition to familial malignancies is well established [14], whereas the roles of somatic, acquired mutations have become a central subject of study in more recent years. Cancer biomarkers are now increasingly used to characterize human tumors and to explain the heterogeneity that exists between different tumors. Such heterogeneity is reflected by the wide range of sub-classifications (diagnostic markers) and risk-stratifications (prognostic markers) existing for many cancer types, as well as by the increasing number of molecules able to forecast the response of patients to personalized therapies (predictive markers) [15].
The following sections represent an overview of relevant markers and matched therapies in pediatric tumors. A full list of the molecular inhibitors reviewed is available in Table 1, while a schematic summary of cancer pathway inhibition is shown in Fig. 1. Table 2 reports the frequency of mutation/overexpression of some biomarkers relevant in childhood cancer.
Table 1.
List of targets in childhood cancer discussed in this review with matching relevant targeted therapies.
| Activity | Target | Agent | Activity | Target | Agent |
|---|---|---|---|---|---|
| Monoclonal antibodies | ERBB2 | Pertuzumab | Tyrosine kinase inhibitors | ALK | Crizotinib |
| Trastuzumab | NVP-TAE684 | ||||
| EGFR | Cetuximab | BCR–ABL | Imatinib | ||
| Nimotuzumab | Dasatinib | ||||
| VEGFR | Bevacizumab | c-KIT | Dasatinib | ||
| IGF-1R | Cixutumumab | Sorafenib | |||
| Figitumumab | Pazopanib | ||||
| Teprotumumab | MET | Foretinib | |||
| Robatumumab | Cabozantinib | ||||
| Alpha IR-3 | SU11274 | ||||
| AMG 479 | K252a | ||||
| R1507 | EGFR/ERBB2 | Erlotinib | |||
| EM164 | Gefitinib | ||||
| IMC-A12 | Lapatinib | ||||
| SCH 717454 | Afatinib | ||||
| Ser/Thr kinase inhibitors | PI3K | XL147 | Canertinib | ||
| NVP-BEZ235 | AEE788 | ||||
| NVP-BKM120 | JAK/STAT3 | AZD1480 | |||
| GDC-0941 | Ruxolitinib | ||||
| IC87114 | AG-490 | ||||
| PI103 | PDGFRA | Imatinib | |||
| AKT | Perifosine | Dasatinib | |||
| SF1126 | Sorafenib | ||||
| GSK690693 | Sunitinib | ||||
| MK-2206 | Pazopanib | ||||
| Aurora kinase | Alisertib | Foretinib | |||
| Tozasertib | PDGFRB | Dasatinib | |||
| AT9283 | Sorafenib | ||||
| BRAF | Sorafenib | Sunitinib | |||
| Vemurafenib | Pazopanib | ||||
| Selumetinib | CP-673,451 | ||||
| mTOR | Rapamycin | VEGFR | Sunitinib | ||
| Temsirolimus | Dasatinib | ||||
| PI103 | Sorafenib | ||||
| PP242 | Vandetanib | ||||
| Hedgehog pathway inhibitors | SHH | Vismodegib | Cediranib | ||
| SEN450 | Pazopanib | ||||
| PF-5274857 | PLK1 | BI2536 | |||
| SMO | LDE225 | BI6727 | |||
| γ-Secretase inhibitors | Notch | RO4929097 | LFM-A13 | ||
| Histone deacetylase inhibitors | HDAC | Vorinostat | GW843682X | ||
| Panobinostat | SYK | Fostamatinib | |||
| Entinostat | C-61 | ||||
| Valproic acid | TrkB | Lestaurtinib | |||
| Romidepsin | AZ64 | ||||
| Trichostatin A | AZ623 | ||||
| Topoisomerase inhibitors | TOP1 | Topotecan | FLT3 | Lestaurtinib | |
| Irinotecan | Linifanib | ||||
| Namitecan | Sorafenib | ||||
| Gimatecan | Midostaurin | ||||
| EZN-2208 | AC220 | ||||
| Genz-644282 | SU11657 | ||||
| TOP2 | Etoposide | IGF-1R | BMS-754807 | ||
| (R +)XK469 | NVP-AEW541 | ||||
| MAP kinase pathway inhibitors | MAP2K1–2 | AZD6244 | Chaperone inhibitors | Hsp90 | Geldanamycin |
| Sorafenib | Tanespimycin | ||||
| KRAS | Tipifarnib | Ganetespib | |||
| MEK | Selumetinib | AT13387 | |||
| U0126 | NVP-AUY922 | ||||
| ERK | U0126 | Alvespimycin | |||
| PARP inhibitors | PARP | Olaparib | SNX-2112 | ||
| Veliparib | 17-AAG | ||||
| ABT-888 | 17DMAP-GA | ||||
| AG-014700 | 17AEP-GA |
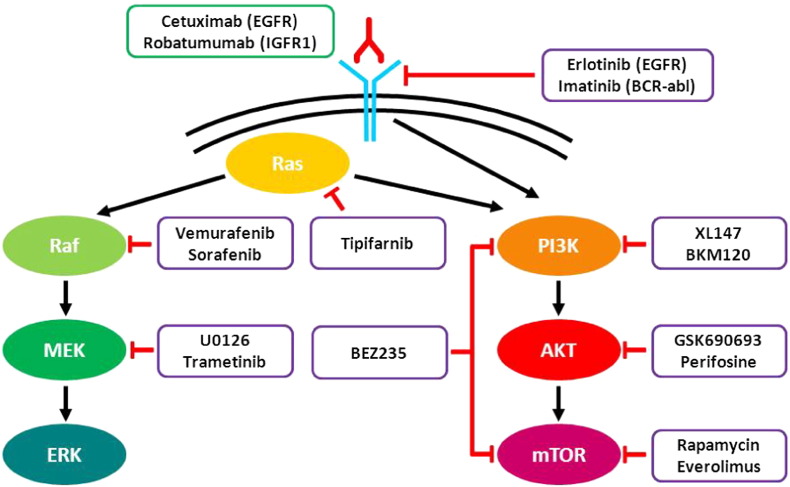
Fig. 1.
Schematic representation of two main pathways aberrantly activated in pediatric tumors and corresponding targeted therapies. Molecular inhibitors and monoclonal antibodies are circled in purple and green, respectively.
Table 2.
Frequency (%) of positivity by immunohistochemistry or mutation of relevant biomarkers in childhood cancers. References are listed in Supplemental document (S1).
| Target | Childhood cancer | IHC (%) | Reference | Mutation (%) | Reference |
|---|---|---|---|---|---|
| ALK | Neuroblastoma | 23.5% (stage 1–2) 77% (stage 3–4) | [I] | 10.4% | [II] |
| Rhabdomyosarcoma | 53% (ARMS) 23% (ERMS) | [III] | 16% (deletion) | [IV] | |
| Ewing sarcoma | 69% | [V] | 16% | [V] | |
| Glioblastoma | 82% (in cell lines) | [VI] | – | ||
| BRAF | Low-grade glioma | – | 15% (V600E) | [VII] | |
| cMET | Glioblastoma | 29% (pediatric and adult cohort) | [VIII] | – | |
| Rhabdomyosarcoma | 35% | [IX] | – | ||
| Ewing sarcoma | 86% | [V] | 9% | [V] | |
| EGFR | Glioma | 80% | [X] | 17% (deletion) | [XI] |
| Neuroblastoma | 95% | [XII] | infrequent | [XII] | |
| Osteosarcoma | 59% | [XIII] | – | ||
| Wilms tumor | 38% | [XIV] | – | ||
| Rhabdomyosarcoma | 47% | [XV] | 13% | [XVI] | |
| ERBB2 | Medulloblastoma/ATRT | 57.5% | [XVII] | – | |
| Rhabdomyosarcoma | 33% | [XV] | – | ||
| Wilms tumor | 39.1% | [XVIII] | – | ||
| Osteosarcoma | 18.8% | [XIX] | – | ||
| MGMT | Glioblastoma | 11% | [XX] | – | |
| PDGFRA | DIPG | 36% | [XXI] | 5% | [XXII] |
| Glioblastoma | 18% | [XXIII] | 14% | [XXII] | |
| Neuroblastoma | 100% | [XXIV] | – | ||
| Ependymoma | 29.2% | [XXV] | – | ||
| Rhabdomyosarcoma | > 40% | [XXVI] | – | ||
| PDGFRB | Rhabdomyosarcoma | > 40% | [XXVI] | – | |
| Glioma | 78.9% | [X] | 0% | [XI] | |
| Ewing sarcoma | 79% | [XXVII] | 0% | [XXVIII] | |
| Astrocytoma | 31% | [XXIX] | – | ||
| Neuroblastoma | 72.5% | [XXX] | – | ||
| PIK3CA | Neuroblastoma | 92% | [XII] | Infrequent | [XII] |
| PTEN | Osteosarcoma | – | 20% (deletion) | [XIII] | |
| Neuroblastoma | 100% | [XII] | 39% (deletion) | [XII] | |
| SPARC | Osteosarcoma | 96.3% | [XXXI] | – | |
| VEGF | ALL | 21% | [XXXII] | – | |
| AML | 38% | [XXIV] | – |
– indicates not studied.
2.1. Leukemias
Leukemias are the most common type of childhood cancers, accounting for one-third of all cancers diagnosed in children. Among them, ALL accounts for approximately three-quarters of all childhood leukemia diagnoses, while acute myelogenous leukemia (AML) is 5 times less frequent. Chronic myeloid leukemia (CML) and chronic lymphocytic leukemia are very rare in the pediatric population [16]. Several chromosomal abnormalities have been identified in pediatric leukemias, including translocations such as t(9;22) BCR–ABL, t(12;21) TEL–AML1, t(8;21) AML1–ETO and t(15;17) PML–RARA [17].
2.1.1. Tyrosine kinase inhibitors
The tyrosine kinase inhibitor imatinib (Gleevec) is a rare example of an approved first-line targeted treatment in adults and children, which is indicated for the treatment of Philadelphia chromosome-positive CML. Imatinib used in combination with cytotoxic chemotherapy was also demonstrated to dramatically improve the 3-year event-free survival of children and adolescents with Ph + ALL [11]. Although BCR–ABL kinase domain mutations have been reported in Ph + ALL patients relapsing after imatinib, this may occur less frequently than in adults treated with imatinib [18].
Following the success of imatinib, a number of other tyrosine kinase inhibitors have emerged as potential therapies in pediatric leukemias. Dasatinib is an oral multi-BCR–ABL and Src family inhibitor (also active against c-KIT, platelet derived growth factor alpha/beta (PDGFRA/B) and vascular endothelial growth factor (VEGF)/VEGFR, but not epidermal growth factor receptor (EGFR)/ERBB2) that was recently granted approval for adult Ph-CML [19]. Dasatinib showed encouraging results in a phase I trial in pediatric CML patients, with 6/8 evaluable patients achieving partial or complete cytogenetic responses [20], and is currently in phase II study (NIH trial NCT01460160). Sorafenib is a small molecule that inhibits several tyrosine (VEGFR and PDGFR) and serine/threonine kinases (MAP kinases), and has been approved for the treatment of renal cell and hepatocellular carcinoma [21]. In a phase 1 study of single-agent sorafenib, two acute myeloid leukemia (AML) patients with FLT3 internal tandem duplication achieved dramatic reductions in bone marrow blasts, and proceeded to bone marrow transplantation [22]. Sorafenib is currently being evaluated for incorporation into standard chemotherapy regimens in a Children's Oncology Group multi-center study [22]. Other tyrosine kinase inhibitors directed against FLT3 such as AC220 and midostaurin (PKC412) are in phase I or I/II trials for relapsed or refractory pediatric leukemia (NCT01411267 and NCT00866281NCT01411267NCT00866281, respectively), while SU11657 is in preclinical development [23]. Overall, primary pediatric AML samples with FLT3 or KIT mutations were significantly more sensitive to SU11657 than wild-type AML samples [23].
In 2011, the JAK/STAT inhibitor ruxolitinib was approved for the treatment of intermediate or high-risk myelofibrosis [24]. However, recent results demonstrated its in vivo activity in Ph-ALL xenograft models, when administered in combination with the mammalian target of rapamycin (mTOR) inhibitor rapamycin [25]. Fostamatinib is an experimental drug targeting spleen tyrosine kinase (SYK), and is in clinical trial for rheumatoid arthritis (NCT01242514), autoimmune thrombocytopenia (NCT00706342) and lymphoma (NCT00798096). Dietary fostamatinib was reported to reduce the burden of leukemic blasts in mice injected intrafemorally with primary B-ALL samples [26]. Recently, a nanoscale liposomal formulation of another selective SYK inhibitor C61 exhibited potent anti-leukemic activity against patient-derived ALL xenografts in vivo [27]. The same authors described the in vivo chemosensitizing and apoptosis-promoting activity of LFM-A13, a dual-function inhibitor of Bruton's tyrosine kinase and polo-like kinase 1 (PLK1), against pediatric ALL [28].
2.1.2. Serine/threonine kinase inhibitors
A second class of molecular inhibitors that has been employed in the treatment of pediatric leukemias is one directed against serine/threonine kinases such as MAP kinase, phosphatidylinositol 3′-kinase (PI3K) and Aurora kinase. The MAP kinase pathway is often activated in pediatric malignancies [29] and other inhibitors have been developed to target this specifically. Among them, the farnesyl transferase inhibitor tipifarnib was tested in a phase I clinical trial of pediatric patients with advanced, heavily pretreated leukemia, where no objective responses were observed in 23 evaluable patients [30]. GDC-0941 and IC87114, two inhibitors of PI3K directed against the alpha and delta subunits, respectively, reduced granulocyte macrophage-colony stimulating factor (GM-CSF) hypersensitivity in a mouse model of juvenile myelomonocytic leukemia [31]. However the pan-PI3K inhibitor XL147 (SAR245408) significantly improved event-free survival in only 2/7 pediatric leukemia xenografts, and produced no objective responses [32].
Aurora kinase inhibitors have produced more encouraging results in childhood leukemia in the preclinical setting. The pan-Aurora kinase inhibitor AT9283 produced target kinase inhibition [33] and inhibited the growth and survival of pediatric leukemia cell lines [34], although a significant correlation between AT9283 and target protein expression could not be established [34]. Similarly, the Aurora kinase A (AURKA) inhibitor alisertib (MLN8237) produced complete responses in 6/6 ALL xenograft models, although there was again no correlation between AURKA copy number or expression [35]. Objective responses were subsequently demonstrated in 3 additional ALL xenografts with low AURKA expression [36]. AT9283 reached phase I/II clinical trial for pediatric relapsed or refractory acute leukemia (NCT01431664), which is still in progress.
2.1.3. Histone deacetylase (HDAC) and heat shock protein 90 (Hsp90) inhibitors
Small molecules directed against other targets, including histone deacetylases (HDAC) and heat shock protein 90 (Hsp90), have been evaluated in the preclinical setting. Vorinostat (SAHA), an HDAC inhibitor approved for the treatment of refractory cutaneous T-cell lymphoma, produced no objective responses in 8 ALL xenografts [37]. No objective responses were similarly obtained in 6 pediatric leukemia patients in a phase I clinical trial [38]. Vorinostat is now being evaluated in combination with the demethylating agent decitabine and chemotherapy in relapsed ALL patients (NCT01483690). Hsp90 inhibitors reduce the stability of associated client proteins, and may thereby exert anti-oncogenic effects. Tanespimycin (17-AAG), a member of the geldanamycin family of antibiotics, rendered a pre-B ALL patient-derived cell line significantly more sensitive to imatinib, and reduced the IC50 values for a number of other chemotherapeutic agents [39]. However ganetespib, another Hsp90 inhibitor, showed no significant activity as a single agent towards 4 JAK-mutated ALL xenografts [40].
2.1.4. DNA topoisomerase inhibitors
Among the DNA topoisomerase 1 (TOP1) inhibitors, topotecan is one of the best characterized and one of the first to enter clinical trials. Topotecan is a derivative of the natural alkaloid camptothecin, and is currently approved for the treatment of adult ovarian and cervical cancer, as well as small cell lung cancer [41]. Topotecan has also been successful in clinical trials against relapsed pediatric ALL [42] and AML [43] in combination with a variety of chemotherapy agents. Irinotecan is another TOP1 inhibitor that has been approved for the treatment of colorectal cancer [44]. A panel of 6 patient-derived pediatric ALL cell lines was shown to be more sensitive to irinotecan than topotecan, based on measured IC50 values [45]. Recently, a combination of irinotecan, the TOP2 inhibitor etoposide and cytarabine entered a phase I clinical trial for pediatric refractory or relapsed ALL and AML (NCT01239485). Etoposide in combination with clofarabine and cyclophosphamide has previously been tested in phase I and II studies of refractory or relapsed ALL or AML, resulting in complete remissions (with or without platelet count recovery) in 16/25 (64%) patients in the phase I trial [46], and in 11/25 (44%) patients in the phase II trial [47]. This treatment regimen therefore produced encouraging response rates, and sustained remission in refractory or relapsed patients [47].
2.1.5. Proteasome inhibitors
The proteosome inhibitor bortezomib exerts anti-tumor activity by affecting a number of physiological processes, including preventing degradation of pro-apoptotic factors. Bortezomib treatment as a single agent showed encouraging preclinical results, significantly increasing event-free survival in 4/7 ALL xenograft models [48], but produced no objective clinical responses in a phase I clinical trial of 12 refractory or relapsed ALL or AML patients [49]. Because of bortezomib's distinctive mechanism of action, there was interest in combining this with standard anti-leukemia agents, and synergistic or additive interactions were demonstrated with range of chemotherapeutic agents in T-ALL and pre-B ALL cell lines in vitro [50]. In a phase I trial, bortezomib added to standard therapy for relapsed pediatric ALL induced complete responses in 6/9 patients [51], with 16/22 (73%) relapsed ALL patients achieving complete remission in the subsequent phase II trial, which met the pre-defined criteria for early trial closure [52]. The study authors recommended evaluating the addition of bortezomib in randomized studies on childhood pre-B ALL protocols [52].
2.1.6. Immunotherapy
Understanding the role of the immune system in tumor surveillance has led to novel approaches for cancer treatment, such as unconjugated and conjugated monoclonal antibodies (mAbs), and chimeric antigen receptors (CARs) [53]. Hematopoietic differentiation antigens represent a major tumor antigen class for the treatment of leukemia and lymphoma, which may be relevant to both adult and pediatric diseases. In a phase II window trial, 36/87 (41.4%) pediatric B-cell non-Hodgkin's lymphoma patients responded to the anti-CD20 mAb rituximab as a single agent [54], producing at least objective responses at an index site, and no other signs of progression. Combining rituximab with chemotherapy produced a 60% response rate in 12/20 patients with relapsed and refractory pediatric B-cell non-Hodgkin's lymphoma or mature B-cell leukemia [55]. In contrast, the anti-CD52 mAb alemtuzumab induced complete remission in only 1/13 (7.6%) relapsed ALL patients, and was not recommended for further use as a single agent [56]. However, in combination with busulfan and fludarabine, alemtuzumab improved the engraftment rate in pediatric patients undergoing hematopoietic stem cell transplantation [57]. As unconjugated antibodies have been approved to treat a number of adult malignancies [58], there may be scope for further translation of these reagents to pediatric oncology.
Payload-delivery mAbs conjugated with cytotoxic agents, including antibody–immunotoxin and antibody–drug conjugates, and radio-immunoconjugates, could all improve the potency of unconjugated mAbs, particularly in immunocompromised patients [59], [60]. Gemtuzumab ozogamicin is an anti-CD33 mAb conjugated to a derivative of the cytotoxic antibiotic calicheamicin, and has been comprehensively studied in adults. In a phase I study as a single agent, 8/29 (28%) pediatric AML patients with relapsed or refractory disease achieved complete remission, with or without platelet count recovery [61]. Higher proportions of AML patients (40% and 55%) achieved complete remissions in a larger subsequent dose de-escalation study where gemtuzumab ozogamicin was combined with standard chemotherapy [62]. In relapsed and refractory pediatric ALL patients, the anti-CD22 immunotoxin moxetumomab pasudotox produced complete responses in 4/17 (24%) patients [63], and the anti-CD22 calicheamicin conjugate inotuzumab ozogamicin produced complete responses in 3/5 relapsed pre-B ALL patients, all of which expressed CD22 [64]. There is further scope for testing other conjugated mAbs in pediatric leukemia, as the anti-CD19 mAb–drug conjugate SAR3419 delayed progression in 4/4 pre B-ALL and 3/3 infant mixed lineage leukemia xenograft models as a single agent [65].
T-cell engaging antibodies represent new therapeutic approaches for treating leukemia and lymphoma, with the bi-specific T-cell engaging (or BiTE®) antibody blinatumomab now being tested in pediatric leukemia patients, following encouraging results in adults [66]. Blinatumomab is a fusion of two single chain antibodies to CD19 and CD3, and thereby brings malignant B cells into close proximity with CD3-positive T cells [66]. Blinatumomab treatment of 3 pediatric patients with refractory pre-B ALL rapidly induced complete remissions, which was maintained in one case for over 23 months [67]. Preliminary data from the first phase I clinical trial of blinatumomab indicate complete remissions in 11/34 (32%) relapsed/refractory pre-B ALL patients [68], although the associated side effect profile, which can include cytokine release syndrome, requires careful management [66]. The phase II NCT01471782 clinical trial to investigate the pharmacokinetics, pharmacodynamics and safety of escalating doses of blinatumomab in pediatric and adolescent patients with relapsed/refractory pre-B ALL is ongoing, and estimated to complete in 2016.
Chimeric antigen receptors (CARs) represent an exciting new form of targeted therapy that confers the targeting specificity of mAbs to cytotoxic T cells, the first of which were reported some 25 years ago [69]. Second and third generations of CARs have been developed by the addition of one or two co-stimulatory molecules [70], with the goal of improving the in vivo persistence of the transferred T cells [71]. B-cell malignancies are particularly amenable to CAR therapy, as mAbs are available to target B-cell surface antigens, and temporary loss of mature B-cells is an acceptable on-target, off-tumor effect [70]. A recently reported trial of CD19-targeting CAR T-cells showed the feasibility and safety of this approach [72], while two pediatric patients with relapsed B-ALL treated with CD19-specific CAR T-cells were reported to achieve remission [73]. Numerous trials of T cells engineered with CD19-directed CARs are currently undergoing in ALL patients (NCT00608270, NCT01593696, see also [70] for other examples).
2.1.7. Leukemia summary
Over the past 50 years, outcomes for pediatric leukemia patients have dramatically improved by varying the schedules and doses of established cytotoxic drugs, albeit at the cost of severe toxicity [53], [74]. However, there are encouraging signs that this situation is changing, largely through the exploitation of targets such as protein kinases known from adult oncology [74]. The presence of tissue restricted antigens on hematopoietic cells also provides numerous options for targeting B-cell malignancies in children, with acceptable on-target side effects, and the accessibility of leukemic cells within the circulation makes leukemias particularly suited to antibody-based treatment strategies [53], [70]. Evolution of resistant disease may be avoided by the use of less selective kinase inhibitors, and of targeted agents that can access sanctuary sites such as the CNS [18].
2.2. Neuroblastoma
Neuroblastoma originates from primordial neural crest cells and is the most common extra-cranial malignant childhood tumor. It is a heterogeneous malignancy classified into low-, intermediate-, or high-risk categories based upon clinical and biological features correlating with prognosis. Low- and intermediate-risk patients have an overall survival exceeding 90%, while newly diagnosed high-risk neuroblastoma and patients whose disease recurs show survival rates of 50% and less than 10%, respectively [75].
2.2.1. MYCN
MYCN is a member of the MYC transcription factor family, with MYCN gene amplification being strongly associated with poor outcome in neuroblastoma. MYCN amplification is present in approximately 20% of neuroblastomas and nearly half of high-risk cases [8]. As such, MYCN represents a prime therapeutic target in neuroblastoma, and small molecules have been developed to target MYC family members as well as MYC DNA binding functions, transcription and protein–protein interactions. 10058-F4, a novel drug that inhibits MYC–MAX interactions, produced modest survival benefit in a MYCN-dependent neuroblastoma mouse model (TH-MYCN) [76]. Recently, the bromodomain and extra terminal (BET) family adaptor proteins were shown to localize to MYC promoters [77], with BET proteins binding acetylated histones associated with open chromatin and transcriptional activation [78]. Use of the small molecule BET inhibitor JQ1 induced MYC down-regulation [77], [79] and improved survival of mice bearing MYCN-amplified BE(2)-C neuroblastoma or primary human neuroblastoma xenografts, and of TH-MYCN transgenic mice [80]. A JQ1 derivative has now entered phase I/II clinical trial for the treatment of MYCN-amplified cancers, including neuroblastoma (NCT01587703). Myc was also shown to cause DNA damage through induction of replication stress, with the resulting replication arrest being partially overcome by Chk1 inhibition [81]. CHK1 transcript levels were subsequently found to be significantly higher in MYCN-amplified versus non-amplified neuroblastomas, and neuroblastoma cell lines were more sensitive to CHK1 inhibition than controls [82]. CCT244747, a highly selective, orally active CHK1 inhibitor, showed single-agent activity in the TH-MYCN transgenic mouse neuroblastoma model, as evidenced by significantly reduced tumor weights after 7 days of treatment [83]. Future clinical studies will now determine whether agents targeting MYCN activity or downstream cellular processes could improve outcomes in MYCN-amplified neuroblastoma patients.
2.2.2. Differentiating agents and immunotherapy
In children with high-risk neuroblastoma, treatment with the differentiating agent 13-cis-retinoic acid reduces the risk of recurrence after high-dose chemotherapy and stem cell transplant [84]. Fenretinide, a synthetic retinoid, was shown to induce apoptosis in neuroblastoma cells in vitro [85] and, as a consequence, entered phase I and II clinical trials for the treatment of refractory or recurrent neuroblastoma. However, the best reported response was stable disease, potentially due to the scarce bioavailability of this molecule [86], [87]. Novel fenretinide formulations with improved bioavailability have been evaluated in preclinical studies [88] and are currently in phase I clinical trials in recurrent or refractory neuroblastoma patients (NCT00295919).
In the last decade, the addition of disialoganglioside (GD2) immunotherapy to retinoid maintenance therapy for high-risk neuroblastoma has constituted a major advance, where typically 50% of this group of patients die from their disease [89]. Disialoganglioside GD2 is a surface glycolipid antigen normally found on neurons, peripheral pain fibers and skin melanocytes that is also abundantly expressed on neuroblastoma cells. Clinical use of the murine anti-GD2 mAb 3F8 was frustrated by severe side effects, and the development of human anti-mouse antibodies [89]. A subsequent phase III trial of a chimeric human–murine anti-GD2 mAb ch14.18 in high-risk neuroblastoma patients who had previously received intensive multi-model therapy, demonstrated significantly improved event-free and overall survival in the immunotherapy group, and met the criteria for early stopping [90]. To improve the side-effect profile associated with ch14.18, a humanized anti-GD2 mAb was developed with a single point mutation (K322A) to reduce complement-dependent lysis [91]. In summary, anti-GD2 antibodies are likely to provide a major shift in the treatment of minimal residual disease in high-risk neuroblastoma patients, once these are commercially available [90].
2.2.3. PI3K/AKT/mTOR inhibitors
The PI3K/AKT/mTOR pathway is one of the most potent pro-survival signaling cascades [92] and its aberrant activation is a common event in high-risk neuroblastoma patients [93]. Perifosine is an alkylphospholipid inhibitor of the PI3K/AKT pathway, which when used as a single agent, significantly improved the survival of mice bearing human neuroblastoma xenografts (AS, NGP, BE2, and KCNR) [94], and showed synergistic effects in combination with etoposide [95]. Results from these studies paved the way for a phase I clinical trial of perifosine in refractory or recurrent pediatric solid tumors (NCT00776867). Preliminary results indicate encouraging perifosine activity in neuroblastoma patients, in the absence of significant toxicity [96].
Rapamycin (sirolimus) is a natural macrolide antibiotic with immunosuppressive and antiproliferative properties. Its anti-cancer activity derives from its ability to bind the cytosolic protein FKBP12, inhibit the mTOR complex 1, and as a consequence, inhibit VEGF and angiogenesis [97]. Temsirolimus, a derivate of rapamycin, is an approved drug for the treatment of renal cell carcinoma, and has been tested as a single agent in phase I and II trials for pediatric solid tumors [98], [99]. After 12 weeks of temsirolimus treatment, 6/19 (32%) neuroblastoma patients showed stable disease, supporting further exploration of temsirolimus in combination with other therapies [99].
Recent in vivo studies reported the anti-proliferative ability of four novel drugs active against the PI3K/AKT/mTOR pathway, namely SF1126, XL147, PI103 and NVP-BEZ235 [32], [100], [101], [102], [103], [104]. The peptidic pro-drug inhibitor SF1126 was demonstrated to interfere with the pAKT–MDM2 axis, and to markedly inhibit the growth of neuroblastoma xenografts [100]. SF1126 is also in phase II trial for adult solid tumors (NCT00907205). XL147, which is in phase I and II trials for adult cancers (NCT01587040), significantly improved event-free survival in 6/6 neuroblastoma xenografts, but produced no objective responses [32]. PI103 is a dual PI3K/mTOR inhibitor that was demonstrated to cooperate with TNF-related apoptosis-inducing ligand (TRAIL) to synergistically induce apoptosis in neuroblastoma cells (SH-EP, LAN-5 and CHP-212) [101] and to delay growth of MYCN-amplified SK-N-BE(2) neuroblastoma xenografts [102]. Another dual PI3K/mTOR inhibitor NVP-BEZ235 showed increased sensitivity towards MYCN-amplified neuroblastoma cells in vitro, and reduced tumor angiogenesis through destabilization of MYCN protein levels in vivo, thereby significantly improving the survival of tumor-bearing TH-MYCN mice [103]. Synergistic interactions were subsequently demonstrated between NVP-BEZ235 and the lysosomotropic agent chloroquine in vitro, triggering lysosome-mediated apoptosis through lysosome enlargement and the generation of reactive oxygen species (NVP-BEZ235) and lysosomal membrane permeabilization (chloroquine) [104]. NVP-BEZ235 is yet to enter clinical trial for neuroblastoma, with phase I trials involving adult patients currently ongoing. As chloroquine is in clinical use, this could be feasibly be combined with NVP-BEZ235 for neuroblastoma therapy [104], following preclinical testing in vivo.
2.2.4. Tyrosine kinase and HDAC inhibitors
Some 8% of all neuroblastoma cases and over 12% of high-risk patients harbor alterations in the tyrosine kinase ALK sequence [105], [106], and 23.5% of stage 1–2 and 77% of stage 3–4 patients show high-level ALK immunoreactivity [107]. Crizotinib, an ALK and MET inhibitor, entered a phase I/II clinical trial as single agent for pediatric refractory solid tumors, producing several complete responses and prolonged stable disease in both ALK-positive and unknown ALK status neuroblastoma patients [108]. A combination phase I trial of crizotinib and conventional chemotherapy is currently ongoing (NCT01606878). Among other ALK inhibitors, LDK378 demonstrated greater potency over crizotinib, and anti-tumor activity in crizotinib-resistant adult tumors [109]. A pediatric phase I clinical trial with LDK378 as a single agent is also underway (NCT01742286).
Several drugs synthesized to specifically inhibit other tyrosine kinases have also been tested in clinical trials for neuroblastoma. The ERBB2/EGFR inhibitors erlotinib and gefitinib have completed phase I clinical trials in combination with temozolomide [110] or topotecan and cyclophosphamide [111], respectively, with both trials reporting prolonged stable disease in some neuroblastoma patients. A phase II trial of erlotinib in combination with pertuzumab (a mAb against ERBB2) was terminated due to high toxicities (NCT00947167), while a phase II trial of gefitinib in combination with irinotecan reported partial responses in only 5/19 (26%) high-risk neuroblastoma patients [112]. Canertinib (CI-1033), a pan-ERBB inhibitor, reduced the growth and size of SK-N-SH neuroblastoma xenografts to a greater extent than erlotinib [113].
Neurotrophin receptors of the tyrosine kinase (Trk) family play important roles in neuroblastoma etiology, with TrkB expression being associated with unfavorable outcomes and MYCN amplification. A phase I trial of lestaurtinib (a FLT3 and TrkB inhibitor) supported its safe use as a single agent in children with refractory neuroblastoma, warranting further evaluation [114]. Similarly, Iyer and colleagues reported significant improvements in the survival of mice bearing SH-SY5Y xenografts when lestaurtinib was combined with topotecan plus cyclophosphamide [115]. Two other TrkB inhibitors AZ64 and AZ623 have also been tested in neuroblastoma xenografts. AZ64 enhanced the response to irinotecan [116], while AZ623 inhibited tumor growth, and prolonged the inhibition of tumor regrowth when combined with topotecan [117]. The same authors found that the VEGFR inhibitor, vandetanib (ZD6474), in combination with 13-cis-retinoic acid, reduced tumor vascularity and induced apoptosis in neuroblastoma xenografts [118]. Vandetanib also reduced the growth of SK-N-SH neuroblastoma xenografts by 85% as a single agent [119]. VEGFR is also the target of cediranib (AZD2171), an investigational drug in phase III clinical trial for a number of adult malignancies including glioblastoma (NCT00777153), NSCLC (NCT00245154) and colorectal cancer (NCT00384176). Two separate in vivo studies demonstrated that cediranib delayed the growth of neuroblastoma xenografts as a single agent [120], but reduced the event-free survival of NB-EBc1 neuroblastoma xenografts associated with cyclophosphamide treatment alone when used in combination [121].
Polo-like 1 (PLK1) is another kinase recently identified as a potential therapeutic target in neuroblastoma [122]. BI2536 and BI6727, two PLK1 inhibitors in clinical trial for adult malignancies (NCT00526149 and NCT01022853NCT00526149NCT01022853, respectively), were shown to be cytotoxic towards neuroblastoma cells (NB12, NB88R2, and NB122R), and BI2536 significantly inhibited the growth of neuroblastoma xenografts as a single agent, and in combination with irinotecan [122]. The PLK1 inhibitor GW843682X also blocked the proliferation of a panel of pediatric cancer cell lines in vitro, including IMR-5 and SMS-KCN MYCN-amplified neuroblastoma cells [123].
PDGFR inhibitors are a family of novel drugs that have been widely tested in neuroblastoma cell lines and mouse models. For example, dasatinib showed cytostatic and anti-migratory activity in vitro [124] but only partial tumor growth inhibition in vivo [125]. More encouraging results have come from in vivo testing of pazopanib, sorafenib and sunitinib. Pazopanib, approved for renal cell carcinoma and soft tissue sarcomas in adults [126], showed high anti-tumor and anti-angiogenic activity when administered as metronomic chemotherapy in combination with topotecan in SK-N-BE and SH-SY5Y neuroblastoma xenografts [127]. Sorafenib inhibited the growth of neuroblastoma xenografts by targeting both cell proliferation and angiogenesis [128]. Sunitinib, a PDGFR (and VEGFR) inhibitor approved for renal cell carcinoma and imatinib-resistant gastrointestinal stromal tumor [129], showed anti-tumor activity both in early stage tumor formation and in progressive metastatic neuroblastoma in SK-N-BE and SH-SY5Y xenografts [130].
Other tyrosine kinase inhibitors with activity against neuroblastoma are those directed against the JAK/STAT axis and Aurora kinase. AZD1480, a JAK/STAT3 inhibitor [131], was recently demonstrated to show anti-tumor activity, and to decrease expression of STAT3 and downstream targets in KCNR and SH-SY5Y neuroblastoma xenografts [132]. Alisertib, an AURKA inhibitor, disrupted mitotic spindle assembly and the mitotic checkpoint in neuroblastoma cell lines (NB-1643, NB-EBC1, CHLA-90 and CHLA-136), and induced maintained complete responses in a panel of 7 neuroblastoma xenograft models [35]. These results provided the preclinical rationale for alisertib to enter phase II clinical trials, either as a single agent (NCT01154816, for neuroblastoma and sarcomas), or in combination with temozolomide and irinotecan (NCT01601535, for neuroblastoma). Thus far, the only reported result for neuroblastoma patients indicated stable disease as the best response to alisertib single-agent therapy [133]. In IMR-32 neuroblastoma cells, alisertib showed additive cytotoxic effects in combination with the HDAC inhibitor vorinostat [134]. This supported the inclusion of vorinostat in clinical trials for refractory or recurrent pediatric solid tumors including neuroblastoma. Either as a single agent [38] or combined with the proteasome inhibitor bortezomib [135], vorinostat was demonstrated to be safe, and produced stable disease or complete response in some neuroblastoma patients. Other HDAC inhibitors including romidepsin and entinostat have been tested in KCNR neuroblastoma xenografts, resulting in tumor growth inhibition and apoptosis [136], [137].
2.2.5. DNA topoisomerase inhibitors
Topoisomerase poisons such as irinotecan have been successfully used to treat neuroblastoma patients [112] and proved active in phase I and II trials in combination with temozolomide [138], [139]. The combination of irinotecan with temozolomide is considered standard therapy in relapsed high-risk neuroblastoma patients [140]. Recent studies have reported the NF-κB pathway and the B-MYB/MYCN axis as targets of topotecan in neuroblastoma cells [141], [142] and clinical trials have shown encouraging responses to topotecan in neuroblastoma patients. For example, topotecan and etoposide administered together induced partial or complete remissions in 17/36 (47%) patients [143], with the addition of cyclophosphamide inducing partial or complete responses in 19/31 (61%) relapsed and 8/11 (72%) newly diagnosed neuroblastoma patients [144]. In a more recent trial, topotecan in combination with cyclophosphamide produced significantly better progression-free survival than topotecan as a single regimen [145]. Etoposide has also been employed in numerous trials for refractory and relapsed neuroblastoma, with encouraging results [146], while preclinical studies have examined other TOP1 and TOP2 inhibitors including namitecan, gimatecan and the investigational drugs EZN-2208, and (R +)XK469 [147], [148], [149], [150], [151]. Namitecan demonstrated antitumor activity in SK-N-AS neuroblastoma xenografts, inducing apoptosis and tumor shrinkage, and a significant enhancement of platinum agent activity [147], whereas gimatecan produced significant regression of SK-N-DZ neuroblastoma xenografts in vivo [148]. This latter result supported the superior activity of gimatecan versus irinotecan and topotecan, in terms of producing growth arrest in 5 human neuroblastoma cell lines (SK-NDZ, BE(2)M17, LAN-1, RNGA, SK-N-BE(2)c) [149]. Similarly, EZN-2208, a PEGylated version of irinotecan, was superior to standard irinotecan in inducing tumor regression and preventing tumor relapse in a panel of neuroblastoma mouse xenografts [150], while (R +)XK469 induced growth inhibition of neuroblastoma xenografts and produced disease stabilization in a case-report of a neuroblastoma patient [151].
2.2.6. Polyamine inhibitors
Polyamines are multifunctional polycations that are indispensible for cancer cell survival, influencing a range of biological processes including DNA synthesis and stability, replication, transcription and translation, ribosome biogenesis, and protein phosphorylation [152]. Ornithine decarboxylase 1 (ODC1), the first and rate limiting enzyme in polyamine biosynthesis is a well characterized Myc and MYCN target gene, and high ODC1 levels were significantly associated with reduced event-free survival in neuroblastoma patients [153]. Difluoromethylornithine (DFMO), a suicide inhibitor of ODC1, demonstrated inhibition of tumor growth in TH-MYCN mice and synergized with chemotherapy in treating established tumors [154]. Clinical trials employing DFMO as a single agent or as part of combination treatment for neuroblastoma are ongoing (NCT01059071; NANT 2012-01). In addition to DFMO, a number of other polyamine depletion agents have been identified [152], although their clinical potential remains to be determined.
2.2.7. Hsp90 inhibitors
A final category of targeted therapies tested on neuroblastoma models are those directed against Hsp90, including geldanamycin, SNX-2112 and ganetespib. Geldanamycin treatment depleted key anti-apoptotic proteins in SK-N-SH neuroblastoma cells [154], and both geldanamycin and SNX-2112 demonstrated marked single-agent activity, and synergistic [154] or greater than additive activity with cisplatin [155] in BE(2)-C [154] or SK-N-SH and IMR-32 cells, respectively [155]. However, ganetespib demonstrated limited activity as a single agent against NB-1643 neuroblastoma xenografts in vivo [40].
2.2.8. Neuroblastoma summary
Neuroblastoma accounts for 8–10% of all childhood cancers, but is responsible for more than 15% of childhood cancer deaths [156]. In order to improve this latter statistic, many targeted agents are being tested in neuroblastoma patients, and their clinical trial outcomes are eagerly awaited. To date, ALK inhibitors show promise in the patient subset whose tumors bear ALK alterations, and substantial efforts are being made to target MYCN and its downstream targets. The use of anti-GD2 antibodies in combination with immunomodulators represents a major advance in the treatment of high-risk neuroblastoma, and the control of minimal residual disease [89], [90].
2.3. CNS tumors
Pediatric brain tumors represent the second most common neoplastic disease of childhood. The main categories of childhood CNS tumors are gliomas, including low- and high-grade astrocytomas and ependymomas, and embryonal tumors, such as medulloblastoma, CNS primitive neuro-ectodermal tumors and atypical teratoid/rhabdoid tumors (AT/RTs) [157]. Of all childhood cancers, malignant brain tumors are the most significant in terms of mortality and morbidity, and would greatly benefit from more targeted therapeutic approaches with less long-term toxicities [158].
2.3.1. Temozolomide
Temozolomide is an oral DNA damaging (alkylating) agent approved for the treatment of astrocytoma and melanoma in adult patients [159]. Testing for epigenetic silencing of O-6-methylguanine-DNA methyltransferase (MGMT, encoding a DNA repair protein) predicts which adult patients will receive the greatest benefit from temozolomide treatment [160]. Randomized trials in adult glioblastoma multiforme patients have shown a small but significant survival advantage when temozolomide was added to radiation therapy [161]. Similar studies have not been performed in the pediatric setting, but given the poor prognosis of high grade gliomas, and the results demonstrated in adult patients, the use of temozolomide has become standard of care.
Temozolomide has shown anti-tumor activity in pediatric low grade gliomas, where single-agent temozolomide induced disease stabilization in children with progressive low-grade glioma [162]. However, no significant activity was demonstrated for temozolomide in the most aggressive glioma of childhood, diffuse intrinsic pontine glioma (DIPG) [163]. Moreover, temozolomide in combination with the MGMT inhibitor O-6-benzylguanine (O6-BG), although safe [164], did not achieve the target response rate for activity in pediatric patients with recurrent or progressive gliomas [165]. To allow dose escalation of temozolomide while minimizing bone marrow toxicity, a phase I trial (NCT01269424) has been initiated for adult glioblastoma multiforme patients. Here, O6-BG will be combined with infusion of autologous hematopoietic stem cells transduced with a lentiviral vector encoding a mutant form of MGMT P140K resistant to O6-BG, which may reduce the bone marrow side-effects of temozolomide. Favorable results using a similar approach have already been reported for 3 glioblastoma multiforme patients, who all exceeded the median expected survival [166]. A similar phase I trial enrolling patients aged 1–21 years with recurrent or progressive brain tumors, or DIPG, is now underway in Australia (ACTRN12612000535875). These trials will provide valuable indications as to whether gene therapy approaches can improve outcomes in CNS tumor patients treated with temozolomide.
2.3.2. Poly(ADP-ribose) polymerase (PARP) inhibitors
Inhibitors of poly(ADP-ribose) polymerase (PARP) prevent the repair of single-stranded DNA breaks, and may produce synthetic lethality in cells with DNA repair defects, or other forms of DNA damage. Temozolomide in combination with the PARP inhibitor AG-014700 proved active in medulloblastoma xenografts, with sustained tumor regression being achieved in the D425Med MGMT-negative model [167]. The PARP inhibitor olaparib also sensitized pediatric high-grade glioma, medulloblastoma and ependymoma cell lines to radiotherapy in vitro, albeit at micromolar concentrations [168]. Clinical trials involving pediatric brain tumor patients are now combining radiotherapy and/or temozolomide with the PARP inhibitors veliparib (NCT01514201) or ABT-888 (NCT00946335).
2.3.3. Sonic hedgehog (SHH) inhibitors
The secreted SHH ligand is a morphogenic protein that signals through the smoothened (SMO) receptor, inducing proliferation of neuronal precursor cells. As medulloblastoma has been classified into Wnt, sonic hedgehog (SHH), Group 3 and Group 4 subgroups, according to molecular features [169], SHH antagonists represent exciting novel prospects for treating a sub-set of medulloblastoma patients. LDE-225 (erismodegib) is a potent and selective SMO antagonist that also led to acquired resistance during treatment of medulloblastoma xenografts [170]. However, LDE-225 used in combination with the PI3K inhibitor NVP-BKM120, or the dual PI3K–mTOR inhibitor NVP-BEZ235, markedly delayed the development of resistant disease, and may provide an efficient way to treat refractory and relapsed medulloblastoma patients [170]. Vismodegib (GDC-0449) is another SHH inhibitor that has induced tumor regression in mouse medulloblastomas and xenografts [171], [172], [173], [174], and dramatic anti-tumor effects in a relapsed medulloblastoma adult patient [175]. More recent preclinical in vivo studies highlighted potential roles for novel SHH inhibitors such as SEN450 and PF-5274857 in treating gliomas and medulloblastoma [176], [177]. SEN450 showed anti-proliferative activity as a single agent, and further reduced tumor size after temozolomide pretreatment in an adult glioblastoma xenograft model [176]. PF-5274857 effectively penetrated the blood–brain barrier in mice bearing medulloblastoma xenografts, and produced better survival rates than vehicle-treated mice [177]. Vismodegib is now in phase I (NCT00822458) and phase II (NCT00939484, NCT01239316) clinical trials for medulloblastoma treatment, and in phase II trial for DIPG (NCT01774253). Temozolomide and LDE-225 are currently in phase III trial for the treatment of relapsed medulloblastoma (NCT01708174).
2.3.4. PI3K/mTOR inhibitors
Several trials have been reported or are ongoing for the use of rapamycin and other derivatives for childhood brain tumors. Rapamycin in combination with erlotinib induced prolonged disease stabilization in 2/19 (10.5%) low-grade glioma patients [178], while everolimus as a single agent produced tumor volume reductions in 65–79% tuberose sclerosis patients with subependymal giant cell astrocytoma [179]. After obtaining encouraging results in a pre-clinical medulloblastoma mouse model, temsirolimus entered phase I and II trials for pediatric solid tumors [98], [99], where it was well tolerated as a monotherapy, and induced disease stabilization in 7/17 (41%) high-grade glioma patients [99]. Other molecules evaluated for the treatment of pediatric CNS tumors and active against the PI3K/mTOR pathway include perifosine, XL147 (SAR245408) and torkinib (PP242). Perifosine was tested in a PDGFRA-overexpressing mouse model of high-grade brainstem glioma as either a monotherapy, or in combination with radiation, but did not significantly improve survival relative to either no treatment, or radiation alone [180]. Similarly, XL147 was tested against 8 CNS tumor xenografts but demonstrated at best modest activity [32].
2.3.5. MAP kinase pathway inhibitors
The Ras/Raf/MEK/ERK axis is abnormally activated in pediatric cancers by mutations in BRAF and KRAS genes. Multiple inhibitors of this pathway, including selumetinib and tipifarnib, have been tested in the context of pediatric CNS tumors since the BRAF V600E mutation was recently identified as a driving factor in subsets of pediatric low grade gliomas [181]. Although selumetinib showed limited in vitro and in vivo activity as a single agent in a panel of 44 pediatric xenograft models [182], it was highly active against a juvenile pilocytic astrocytoma xenograft (BT-40) harboring the BRAF V600E mutation, causing complete regressions [182]. Selumetinib is now in phase I clinical trial for recurrent or refractory low-grade gliomas (NCT01089101). Tipifarnib, a Ras farnesyltransferase inhibitor, was evaluated in a phase I trial for refractory sarcomas and brain tumors or neurofibromatosis, where it was well tolerated in children but showed limited activity [183]. In a more recent trial of newly diagnosed DIPG patients, tipifarnib administered with irradiation offered no clinical advantage above irradiation alone [184], although Ras status (presence of mutations, or aberrant activity in tumors) was not reported.
2.3.6. Monoclonal antibody therapy
Cetuximab is a mAb directed against EGFR, and approved for treatment of KRAS wild-type metastatic colorectal cancer, and squamous cell carcinoma of the head and neck. The combination of cetuximab and irinotecan proved safe in children and active against CNS tumors [185], leading to ongoing phase II evaluation in high-grade astrocytomas and DIPG, in combination with radiotherapy (NCT01012609). Recently, a case report described intra-arterial cerebral infusion of cetuximab and bevacizumab (a monoclonal VEGF antibody) for multiply recurrent pediatric ependymoma, producing stable residual disease [186]. Another EGFR mAb used to treat pediatric brain tumors is nimotuzumab, which could be safely administered over long periods, and was associated with improved survival in 23 high-grade glioma patients [187]. The potential use of nimotuzumab in radioimmunotherapy (when conjugated to (177)Lu) was also recently tested in A431 epithelial carcinoma xenografts, where locoregional application demonstrated antitumor activity [188]. Less encouragingly, discordant responses in adult versus pediatric high-grade glioma patients have been reported for bevacizumab. Adults with recurrent high-grade glioma treated with bevacizumab and irinotecan achieved radiographic responses in 20/32 (63%) cases [189], whereas only 2/12 (16.7%) pediatric patients achieved a partial response, and 5/12 (45.5%) experienced diffuse invasive recurrence [190]. Similarly, a phase II study of the combination of bevacizumab and irinotecan in pediatric cases of recurrent ependymoma showed disease stabilization in 2/13 (15.4%) patients [191]. On the other hand, the same combined therapy produced objective responses with clinical improvements in 7/9 pediatric low-grade gliomas [192].
2.3.7. Tyrosine kinase inhibitors
A number of small molecule EGFR inhibitors have been tested in children with CNS tumors. Erlotinib in combination with rapamycin produced prolonged disease stabilization in 2/19 (10.5%) low-grade glioma patients [178]. Erlotinib was also tested in combination with radiotherapy in two independent phase I trials for high-grade gliomas and astrocytomas. The first study reported no objective radiologic responses to therapy, but prolonged disease stabilization in 16/23 (70%) patients [193], while the second reported that 4/18 (22%) patients achieved partial responses [194]. A phase I trial of erlotinib in combination with temozolomide produced stable disease or partial response in 2/13 (15%) patients with CNS tumors [110]. Gefitinib is an EGFR/ERBB2 inhibitor that has been proposed for the treatment of malignant gliomas, brain stem tumors and medulloblastoma following preclinical results using mouse xenografts [195]. Phase I trials have indicated that gefitinib is well tolerated in children as a single agent, and in combination with irinotecan or radiotherapy [196]. A more recent phase II clinical trial of gefitinib and radiotherapy in brainstem gliomas indicated partial responses in 6/43 (14%) patients, and long-term progression-free survival in this usually fatal disease [197]. Another dual EGFR/ERBB2 inhibitor lapatinib proved safe and induced prolonged stable disease in 13/59 (22%) CNS tumor patients [198]. Through a cytotoxicity screening approach, lapatinib also demonstrated antitumor activity against AT/RT cell lines in vitro and xenografts in vivo [199]. Another EGFR/ERBB2 inhibitor, AEE788, produced prolonged survival and tumor growth suppression in xenograft models of ependymoma [200] and medulloblastoma [201], respectively.
Other tyrosine kinases such as VEGFR, PDGFR and MET are potential therapeutic targets for pediatric CNS malignancies, due to their aberrant activation in pediatric brain tumors [202], [203]. However, the VEGFR inhibitors sunitinib and vandetanib gave disappointing results in childhood brain cancer clinical trials. In particular, sunitinib showed cardiotoxicity in children with previous exposure to anthracyclines or cardiac radiation [204], whereas vandetanib in combination with radiotherapy induced longer progression-free survival in only 3/35 (8.5%) DIPG patients [205]. Recently, a combination of VEGFR and PDGFR inhibitors (vandetanib and dasatinib) did not improve the poor prognosis of newly diagnosed DIPG patients, who showed 2-year survival rates of less than 10% [206]. The VEGFR inhibitor cediranib (AZD2171) produced tumor growth delay in 1/3 medulloblastoma xenografts when administered as a monotherapy [120], but showed additive effects with rapamycin and vincristine towards D645 xenografts [121]. A phase I clinical trial in children with recurrent or progressive CNS tumors is nonetheless underway (NCT00326664). Foretinib, a multi-kinase inhibitor of MET and PDGFRA, reduced tumor growth and metastasis in xenograft models of disseminated medulloblastoma [207], whereas the polo-like kinase (PLK1) inhibitor BI2536 suppressed cell growth and enhanced radiation sensitivity in 5 medulloblastoma cell lines (ONS-76, Daoy, D283, D425 and D458) [208].
2.3.8. DNA topoisomerase inhibitors
A number of DNA topoisomerase inhibitors have shown activity in the treatment of childhood CNS malignancies. Topotecan in particular produced complete or partial responses in 10/36 (28%) newly diagnosed high-risk medulloblastoma patients [209], and consolidated remission of poor prognosis pediatric CNS tumors when combined with carboplatin [210]. Two phase II trials of topotecan as part of combination therapy in high-risk pediatric brain tumors are currently underway (NCT01342237, NCT01756989). Similarly, irinotecan as a monotherapy proved active in children with relapsed CNS tumors, producing partial or complete responses in subsets of glioma [211] and medulloblastoma patients [212], and objective responses in children with recurrent medulloblastoma when combined with bevacizumab [213]. Etoposide in combination therapy has also successfully completed phase I [214] and II trials [215] for pediatric CNS tumors, with 22/28 (79%) patients showing complete or partial responses to carboplatin, etoposide, and high-dose methotrexate [215]. As described for neuroblastoma, investigational topoisomerase poisons such as namitecan and Genz-644282 have produced some encouraging results in the preclinical setting. In the PFSK primitive neuroectodermal tumor xenograft model, namitecan treatment induced complete responses and prevented tumor relapse, but only slowed tumor growth in medulloblastoma Daoy xenografts [147], whereas Genz-644282 produced partial regression of D645 glioblastoma xenografts, but progressive disease in mice bearing BT-45 medulloblastoma xenografts [216].
2.3.9. HDAC and Aurora kinase inhibitors
As in leukemia and neuroblastoma, HDAC inhibitors have also demonstrated activity against pediatric CNS tumors. The anticonvulsant valproic acid was tested in a phase I study in patients with refractory solid or CNS tumors, and gave partial or minor responses in single patients with glioblastoma multiforme and brainstem glioma, respectively [217]. This led to further phase II studies in combination with radiotherapy and bevacizumab (NCT00879437). Entinostat decreased proliferation and potentiated the effects of ionizing radiation in AT/RT tumor cell lines [218], while both panobinostat and vorinostat showed tumor growth inhibition and radio-sensitization in the HD-MB03 medulloblastoma mouse model [219]. Phase I trials of vorinostat as either a single agent [38], or in combination with temozolomide [220] or bortezomib [135] produced cases of disease stabilization, but no objective responses. Vorinostat was also tested in combination with the AURKA inhibitor alisertib, showing additive cytotoxicity in Daoy medulloblastoma cells [134]. The importance of Aurora kinases as potential therapeutic targets for childhood brain malignancies is highlighted by AURKB being highly and consistently overexpressed in the majority of high-grade glioma (8/11) and DIPG (6/9) patients [221]. Alisertib alone also produced objective responses in medulloblastoma and glioblastoma xenografts, including a maintained complete response (BT-46 cells) and a partial response (D456 cells) [35].
2.3.10. Hsp90 inhibitors
Hsp90 inhibitors represent candidate agents for the treatment of pediatric brain tumors. In the preclinical setting, geldanamycin and its analog 17AEP-GA showed potent inhibition of adult LN18, LN229 and T98G glioblastoma cell proliferation, survival, migration and invasion in vitro [222]. NVP-AUY922, a synthetic Hsp90 inhibitor, also strongly inhibited the proliferation of pediatric SF188 and KNS42 glioblastoma cell lines [223]. Recently, another Hsp90 inhibitor ganetespib was tested on the BRAF-mutant BD-40 astrocytoma xenograft model, but showed no antitumor activity [40]. In a phase I trial for 17AAG in patients with relapsed/refractory solid tumors, the best response was stable disease in 5/17 (29%) patients, including 1/4 ependymoma patients [224].
2.3.11. CNS tumor summary
In addition to poor survival rates, CNS tumors are associated with significant long-term neurologic effects in survivors that result from radio- and chemotherapy. Targeted agents with an improved therapeutic window could spare normal cells in the developing brain, and even agents delaying radiotherapy treatment could significantly reduce adverse long-term effects [225]. Although anti-tumor activity in pre-clinical models has not always been confirmed in early phase clinical trials, these results may not reflect those that could be obtained at initial diagnosis. In summary, the significant recent advances in our understanding of the molecular changes driving pediatric CNS tumors require more time to fulfill their promise in the clinic.
2.4. Sarcomas
Pediatric sarcomas are a heterogeneous group of neoplasms characterized by specific chromosomal translocations (Ewing sarcoma, synovial sarcoma and ARMS) [226], or complex karyotypes and gains of whole chromosomes (osteosarcoma and ERMS) [227], [228].
2.4.1. Ewing sarcoma
Ewing sarcoma is a rare tumor of bone or soft tissues, and is characterized by the fusion of the EWS gene to those encoding the transcription factors FLI1 or ERG in 85% and 10% of cases, respectively [229]. Multiple targets have been demonstrated for EWS/FLI1, including IGF binding protein 3 (IGFBP-3) [230], which increases IGF-1 bioavailability, and the Aurora kinases [231].
2.4.1.1. Insulin-like growth factor type 1 receptor (IGF-1R) inhibitors
As EWS translocations activate the IGF pathway, inhibition of insulin-like growth factor type 1 receptor (IGF-1R) function may target Ewing sarcoma [232]. However, despite encouraging pre-clinical data [233], [234], clinical trial results for IGF-1R targeted agents have been disappointing [232]. A phase I trial of AMG 479, a mAb targeting IGF-1R induced partial or complete responses in Ewing sarcoma patients [235], but more recent phase II trials using similar agents such as R1507 [236], figitumumab [237] and cixutumumab [238] reported only partial responses in patients with recurrent or refractory disease. A limitation of these trials however was that patients were not selected on the basis of IGF-1R expression. Several trials are currently evaluating the activity of novel IGF-1R antibodies such as teprotumumab as a monotherapy (NCT00642941), or in combination with mTOR inhibitors (NCT00880282, NCT00927966).
2.4.1.2. Aurora kinase inhibitors
Although the AURKA inhibitor alisertib induced complete responses in 1/5 Ewing sarcoma xenograft models (SK-NEP-1) [35], single-agent alisertib only stabilized disease in a phase I trial that included Ewing sarcoma patients [133]. A phase II trial has recently been completed, although results are not yet available (NCT01154816). The pan-Aurora kinase inhibitor tozasertib was shown to selectively and potently reduce the viability of Ewing sarcoma cell lines, and demonstrated synergies with etoposide and doxorubicin [239]. Dual inhibition of AURKA and AURKB in Ewing sarcoma may therefore be more potent than AURKA inhibition alone [239].
2.4.1.3. Other tyrosine kinase inhibitors
Surface expression of c-KIT and PDGFRΒ in Ewing sarcoma cell lines predicted that Ewing sarcoma may respond to imatinib, which produced concentration-dependent inhibition of cell growth in vitro in multiple Ewing sarcoma cell lines, and of tumor growth in vivo [240]. However, single-agent imatinib produced disappointing results in phase II clinical trials, with only 1/24 (4%) [241] or 1/5 [242] Ewing sarcoma/PNET patients achieving partial responses. This occurred despite the immunohistochemical detection of KIT or PDGFRΑ being a requirement for patient selection in one trial [242]. The multi-targeted kinase inhibitor pazopanib induced tumor growth delay as the best response in 5 Ewing sarcoma xenografts [243], while cediranib showed modest anti-tumor activity as a single agent, but additive effects against EW5 cells in combination with rapamycin [121]. Dasatinib inhibited proliferation without inducing apoptosis in 3 Ewing sarcoma cell lines [124], whereas gefitinib and vandetanib inhibited cell line proliferation at only micromolar concentrations [244], indicating that Ewing sarcomas may not depend on EGFR and VEGFR pathways for survival [244]. More recently, MET and ALK mutations were demonstrated in Ewing sarcomas, and in vitro treatment of five patient-derived cell lines with MET and/or ALK inhibitors produced IC50 values within the low micromolar range [245]. Further research will be required to determine whether MET/ALK inhibitors could be used to treat a subset of Ewing sarcoma patients [245].
2.4.1.4. HDAC and Hsp90 inhibitors
The HDAC inhibitor entinostat (MS-27-275) showed in vitro cytotoxicity in Ewing sarcoma cell lines, and marked regression of established TC71 Ewing sarcoma xenografts [137]. More recently, vorinostat induced significant tumor growth delay in 3/5 Ewing xenograft models, but no objective responses [246], and no objective responses in combination with bortezomib in a subsequent phase I clinical trial [135]. Similarly, both the Hsp90 inhibitors ganetespib [40] and AT13387 [247] produced little anti-tumor activity and no objective responses in 2 and 5 Ewing sarcoma xenograft models, respectively [40], [247].
2.4.1.5. DNA topoisomerase inhibitors
Encouraging results have come from the testing of TOP1 and TOP2 inhibitors, where irinotecan and etoposide were both well-tolerated and active in high-risk or refractory/relapsed Ewing sarcoma patients in combination regimes [248], [249]. Topotecan in combination with cyclophosphamide induced stable disease or partial responses in 4/13 (31%) and 3/13 (23%) relapsed Ewing sarcoma patients, respectively [250], while objective responses were attained in 7/14 (50%) patients with recurrent or progressive disease when vincristine was added to the protocol [251]. In vivo studies also revealed that Genz-644283 [216] and namitecan [252] produced maintained complete responses in SK-NEP-1 [216] and TC-71 [252] Ewing sarcoma xenografts at well-tolerated doses.
2.4.1.6. Ewing sarcoma summary
A number of challenges emerge from a consideration of targeted therapy use in Ewing sarcoma. While targets and pathways downstream of oncogenic fusion proteins provide a wealth of therapeutic targets in Ewing sarcoma [226], the clinical benefits from targeted therapy have been limited, and not always been explained by target expression [242], [253]. Limitations to drug diffusion and penetration in bone may reduce the activity of high molecular weight agents such as mAbs [253], and the relevance of preclinical results obtained in subcutaneous xenograft models. Intra-tumor heterogeneity also makes a strong case for the use of multiple targeted therapies in combination regimes [253].
2.4.2. Rhabdomyosarcoma
Rhabdomyosarcoma (RMS) is a group of malignancies that resemble developing skeletal muscle, and is the most common soft-tissue sarcoma in children and adolescents [228]. RMS is divided into two major histologic subgroups, ERMS and ARMS, which account for 60% and 30% of cases, respectively [228]. ERMS is characterized by chromosome gains (chromosomes 2, 8, 12, 13) and loss of heterozygosity for chromosome 11p15.5 [228]. In contrast, ARMS is characterized by fusions of the transcription factors PAX3 and PAX7 with FOXO1 in 70% and 10% ARMS patients, respectively [254]. Great efforts have been made to directly alter the expression or function of these fusion proteins, through the use of antisense oligonucleotides [255], transcriptional repressors [256] and vaccines against the fusion region [257]. As for neuroblastoma (Section 2.2.2), differentiation therapy has been attempted in RMS cell lines in vitro. Treatment of RD and Rh30 cells with retinoic acid induced stereo-specific growth inhibition, but not differentiation [258], whereas 12-O-tetradecanoylphorbol-13-acetate treatment of RD cells induced myogenic differentiation [259]. More recently, fenretinide was shown to reduce PAX3–FOXO1 fusion protein levels in ARMS cell lines, and to significantly delay in vivo tumor growth in ARMS Rh4 xenografts, compared to untreated controls [260].
2.4.2.1. Tyrosine kinase and PI3K/mTOR inhibitors
PAX3/7-FOXO1 downstream targets include MET, PDGFR and IGF/IGFR, so therapies targeting these molecules or pathways may be useful for RMS treatment. Transgenic expression of the MET ligand hepatocyte growth factor in Ink4a/Arf−/−mice induced RMS with extremely high penetrance and short latency [261], while shRNA silencing of MET inhibited RD18 proliferation and invasiveness in vitro, and induced regression of RD18 xenografts [262]. High levels of phosphorylated MET were detected in 14/24 (58%) RMS patient samples and CW9019 and Rh30 ARMS cell lines, where treatment with the MET inhibitor SU11274 significantly inhibited cell proliferation and migration in vitro [263].
Following the demonstration that PDGFRA is a transcriptional target of PAX3–FOXO1 [264], PDGFRΑ inhibition using RNA interference or imatinib reduced mouse ARMS cell line proliferation in vitro, and imatinib or PDGFRA neutralizing antibody treatment led to disease regression or stabilization in vivo [265]. The selective PDGFR inhibitor CP-673,451 also reduced tumor volume, Ki67 staining and PDGFR phosphorylation of RUCH2 xenografts in vivo [266]. The multi-target tyrosine kinase inhibitor pazopanib delayed growth of Rh30 xenografts and enhanced survival, both as a single agent and in combination with topotecan [127]. However when used at a lower dose, single-agent pazopanib significantly improved event-free survival in only 2/5 ARMS/ERMS xenograft models, and produced no objective responses [243].
Targeting IGF/IGFR as downstream effectors of PAX3/7–FOXO1 has been tested using neutralizing antibodies and small molecule inhibitors. IGF-1R antibodies such as alpha IR-3 [267], IMC-A12 [268], and the IGF-1R inhibitor BMS-754807 in combination with cetuximab [233] commonly inhibited growth of RMS xenografts. In RMS, IGFR survival signaling is primarily maintained through the AKT pathway, and activation of PI3K/mTOR signaling [269]. Concomitant use of IGFR antibodies and rapamycin resulted in additive inhibition of Rh30 and RD cell growth and survival [270], and the PI3K inhibitor XL147 induced tumor growth delay as a single agent, and significantly improved event-free survival in 6/6 RMS xenografts [32]. However a phase II trial of single-agent temsirolimus induced prolonged stable disease in 1/16 (6%) RMS patients [99]. IGF-1R can also signal through the MEK/ERK cascade, and the specific MEK inhibitor U0126 reduced anchorage-dependent and -independent RD cell proliferation [271]. U0126 also showed synergistic interactions with radiotherapy in 3 RMS cell lines in vitro, and significantly improved progression free survival of mice bearing TE671 RMS xenografts [272].
Over 80% of ARMS patients carry altered ALK gene copy number [273], identifying ALK as a potential therapeutic target in ARMS patients. ALK and IGF-1R were subsequently shown to be co-expressed in the majority of ARMS patient samples examined, and simultaneous targeting of ALK and IGF-1R (using NVP-TAE684 and the R1507 antibody, respectively) produced synergistic effects in the ARMS cell line Rh41 and Rh30 [274]. A phase I trial (NCT01742286) is now open to evaluate the safety profile of the ALK inhibitor LDK378 in pediatric cancer patients with known ALK alterations. Similarly, a phase II trial (NCT01524926) is underway to investigate the efficacy of crizotinib in locally advanced or metastatic tumors, including ARMS patients harboring specific alterations leading to ALK and/or MET activation.
Finally, a number of other kinase inhibitors have been tested in RMS cell lines and xenografts, such as erlotinib, alisertib and AZD1480, which are EGFR, Aurora kinase and JAK/STAT inhibitors, respectively. While primary tumors from a transgenic ARMS model expressed EGFR, these were unaffected by erlotinib treatment [275]. In contrast, alisertib induced maintained complete responses in Rh65 ARMS xenografts [35], [36], and significantly improved event-free survival in 4/5 other RMS xenografts, including Rh18 [35]. Similarly, AZD1480 reduced Rh18 ERMS cell viability in vitro, significantly inhibited tumor growth in vivo, and significantly improved the survival of mice bearing Rh18 xenografts [132].
2.4.2.2. Hsp90 inhibitors
It is worth discussing the role of Hsp90 inhibitors as potential therapies for RMS, as despite demonstrating little in vivo antitumor activity against other pediatric tumor types, alvespimycin induced objective responses in Rh30 ARMS xenografts [276]. Hsp90 was highly expressed in RMS (SMS-CTR and Rh30) but not in control cell lines, and geldanamycin and its analogs tanespimycin, 17AEP-GA and 17DMAP-GA reduced RMS cell proliferation, migration and invasion, and induced apoptosis [277]. In contrast, ganetespib and AT13387 demonstrated only low activity against a number of RMS xenografts, and produced no objective responses [40], [247].
2.4.2.3. DNA topoisomerase inhibitors
As previously described (2.1.4, 2.2.5), topoisomerase inhibitors such as etoposide have been studied in clinical trials as reinduction or maintenance therapy for pediatric malignancies [278], [279]. In relapsed or refractory RMS patients, irinotecan showed anti-tumor activity and a good tolerance profile as a single agent [280], as well as additive effects when administered with vincristine [281]. Similarly, the combination of topotecan with vincristine and doxorubicin induced objective responses in a small cohort of 6 patients with refractory or recurrent RMS, and was well tolerated [282]. More recent in vivo studies also indicated that namitecan, Genz-644282 and gimatecan were highly active in a number of RMS xenograft models [148], [216], [252]. As described for Ewing sarcoma (Section 2.4.1.5), namitecan produced RD/TE-671 RMS xenograft regression at low doses and additive effects with the antiangiogenic agents bevacizumab and sunitinib [252], while Genz-644282 induced complete responses in Rh18 and Rh28 ARMS and Rh30 ERMS xenografts [216]. Prolonged daily treatment with low doses of gimatecan also produced significant tumor regression in RD/TE-671 RMS tumor xenografts [148].
2.4.2.4. RMS summary
Outcomes in children with RMS vary greatly, from 95% to 15% 5 year event-free survival for patients with low- or high-risk disease, respectively [283]. Future clinical trials using targeted therapy must therefore focus upon improving outcomes for intermediate- and high-risk patients [283]. While many agents have been tested in the preclinical RMS setting, patient numbers limit those that can progress to clinical trial, particularly in the case of low frequency targets. However there is optimism that agents such as IGF-1R mAbs and ALK inhibitors may shortly deliver long-awaited improvements in outcomes for RMS patients.
2.4.3. Synovial sarcoma
Synovial sarcoma is the most common non-RMS soft tissue sarcoma in adolescents, and is characterized by translocations that fuse SS18 (SYT) to SSX1, SSX2, and, more rarely, to SSX4 [226]. As such, SS18 fusion proteins represent a major therapeutic target in synovial sarcoma, and siRNA down-regulation of SS18–SSX transcripts reduced anchorage-independent growth of synovial sarcoma cell lines in vitro, and significantly delayed tumor formation in vivo [284]. Expression microarray profiling of synovial sarcomas bearing SS18–SSX translocations identified IGFBP2, ERBB2 and BCL-2 up-regulation [285], with overexpression of these and other proteins such as EGFR being confirmed by immunohistochemistry [285], [286], [287]. Whereas treatment of synovial sarcoma cell lines with the IGF-1R inhibitor NVP-AEW541 impaired cell growth and significantly increased apoptosis [288], a phase II trial of gefitinib in patients with advanced EGFR-positive synovial sarcoma produced only stable disease as the best response [289]. Following frequent BCL-2 detection in synovial sarcoma [285], [286], [287], the BCL-2 antisense oligonucleotide (G3139) was tested against FU-SY-1 synovial sarcoma cells in vitro, resulting in significantly increased sensitivity to doxorubicin treatment [290]. In a phase I trial, G3139 induced prolonged stable disease in 2/5 synovial sarcoma patients in combination with doxorubicin and cyclophosphamide [291]. In summary, these results suggest that continued attempts to target either SS18–SSX fusion proteins and/or their downstream targets are likely to improve outcomes in patients with this rare tumor type [226].
2.4.4. Osteosarcoma
Osteosarcoma is a primary bone malignancy characterized by heterogeneous genetic complexity [227], but as with other childhood sarcomas, may also be driven by altered IGFR signaling, as well as EGFR/ERBB2 and Wnt pathways [292].
2.4.4.1. Tyrosine kinase inhibitors
Several studies have evaluated the effects of IGF-1R inhibition on childhood osteosarcoma cell lines and xenografts. The IGF-1R small molecule inhibitor picropodophyllin dramatically reduced in vitro colony formation by osteosarcoma cell lines [293], while NVP-AEW541 [234] and the mAb SCH-717454 (Robatumumab) delayed osteosarcoma xenograft growth either as monotherapy [294], [295], or in combination with cyclophosphamide [295]. The IGF-1 receptor antagonist RG1507 subsequently produced stable disease in 2/3 osteosarcoma patients during a larger phase I trial in children with refractory or relapsed solid tumors [296].
EGFR inhibitors have also been tested in osteosarcoma cell lines, where the EGFR inhibitor 324674 (a 4,6-disubstituted pyrimidine) reduced migration and colony formation of 5/5 and 3/3 osteosarcoma cell lines, respectively, and the invasiveness of 143B cells [293]. However, the EGFR inhibitors gefitinib and afatinib did not reduce the viability of 4 osteosarcoma cell lines in a dose-responsive manner [297]. Although ERBB2 expression has been variably reported in pediatric osteosarcoma [298], ERBB2 positivity has been associated with reduced event-free and overall survival in osteosarcoma patients [299]. However, trastuzumab in combination with cytotoxic chemotherapy did not improve outcomes in metastatic osteosarcoma patients with ERBB2-positive disease, relative to those of ERBB2-negative patients not receiving trastuzumab [300].
Osteosarcoma primary cultures [301] and cell lines [302] have been reported to express PDGFRA and/or PDGFRB and to respond to PDGF stimulation in vitro [301,301]. Nonetheless, imatinib inhibited cell proliferation in vitro at only micromolar concentrations in PDGFR-expressing patient-derived osteosarcoma cells [301] and MG-63 cells [302], and a phase I trial of single-agent imatinib produced no responses in 10 patients with refractory/relapsed osteosarcoma [241]. Other PDGFR inhibitors have been tested in osteosarcoma, with pazopanib in combination with topotecan inhibiting the growth of KHOS xenografts [127], whereas in a phase I study, sunitinib induced stable disease in one of 2 osteosarcoma patients, but no objective responses [204]. Cediranib (a VEGFR inhibitor) produced complete responses in 1/5 osteosarcoma xenografts [120] and showed additive effects with cisplatin and rapamycin [121].
Other kinases may have particular significance in osteosarcoma, and further therapeutic application. For example, MET overexpression converted primary human osteoblasts into osteosarcoma cells that displayed the transformed phenotype in vitro, and distinguishing features of human osteosarcomas in vivo [303]. The MET inhibitors K252a [304] and SU11274 [293] reduced HGF-induced cell proliferation and migration of Saos-2 and U2OS osteosarcoma cell lines [304], and reduced MNNG cell migration, and colony formation by TE85, 143B and MNNG cells [293], respectively. siRNA screening demonstrated the sensitivity of osteosarcoma cell lines towards PLK1 depletion [305], and the PLK1 inhibitor GW843682X inhibited proliferation of MNNG–HOS and OST osteosarcoma cell lines that were described as highly resistant to standard anticancer drugs such as doxorubicin and cisplatin [123].
2.4.4.2. Notch and Wnt pathway inhibitors
Notch signaling is upregulated in primary osteosarcoma samples and cell lines, and genetic or chemical down-regulation of Notch signaling both significantly reduced the growth of SJSA-1 xenografts in vivo [306]. The γ-secretase and Notch pathway inhibitors RO4929097 and PF-03084014 delayed in vivo tumor growth in 4/6 [307] and 5/5 [308] osteosarcoma xenografts, but produced no objective responses. Wnt signaling controls bone development and regeneration, and overexpression of numerous Wnt pathway components have been reported in osteosarcoma [309]. For example, Wnt10b ligand was detected in 33/44 (75%) osteosarcoma cases and showed a trend towards association with reduced overall survival [310]. Inhibition of Wnt/β-catenin signaling with CCT036477 enhanced Saos2 cell line sensitivity to methotrexate, and showed synergistic effects with Notch inhibition [311]. Targeting the Wnt pathway using recombinant proteins, small molecules, antibodies, or via downstream transcriptional targets are all in preclinical development [309], although no agents are yet in clinical trial for osteosarcoma.
2.4.4.3. PI3K/mTOR inhibitors
Inhibition of the PI3K/AKT/mTOR axis has also been proposed for osteosarcoma patients, and PI3K/AKT inhibitors such as GSK690693 and MK-2206 have been tested against osteosarcoma xenograft panels. Both agents significantly improved event-free survival in 6/6 osteosarcoma xenografts in vivo as single agent therapy, but produced no objective responses [312], [313]. More encouragingly, rapamycin induced maintained complete responses in OS-33 osteosarcoma xenografts in vivo [314]. Its derivative everolimus, in combination with sorafenib, also showed antiproliferative and proapoptotic effects, producing tumor growth inhibition, antiangiogenic and antimetastatic effects in the MNNG–HOS xenograft model [315]. Phase II studies of everolimus in children and adolescents with refractory or relapsed osteosarcoma (NCT01216826) are ongoing, and of sorafenib and everolimus in relapsed and non-resectable osteosarcoma (NCT01804374).
2.4.4.4. Hsp90, HDAC and DNA topoisomerase inhibitors
As for neuroblastoma [154], Hsp90 inhibitors have shown interesting results towards osteosarcoma cells in vitro. Geldanamycin inhibited osteosarcoma U-2OS and Saos-2 cell proliferation at micromolar concentrations [154], although U-2OS cells were markedly more sensitive to the analog SNX-2112 [155]. Both agents demonstrated synergism with cisplatin [154], [155]. HDAC inhibitors such as FR901228 and trichostatin A demonstrated activity towards osteosarcoma cells in vitro and in vivo, particularly when used in combination therapy [316], [317]. The HDAC inhibitor MS-27-275 also increased apoptosis and promoted regression of lung metastases in osteosarcoma xenografted mice, with no evidence of significant toxicity [318]. Vorinostat produced prolonged stable disease in one of 2 osteosarcoma patients in a phase I trial, without major toxicities [38], and is under further evaluation in pediatric oncology patients (NCT01422499). Investigational TOP1 and TOP2 inhibitors recently demonstrated high activity in osteosarcoma xenografts, with objective responses to Genz-644284 in 4/5 osteosarcoma xenografts that included 2 maintained complete responses [216], and complete responses to namitecan in U2-OS xenografts [252].
2.4.4.5. Osteosarcoma summary
Osteosarcoma patient outcomes have failed to improve substantially over the past decades, due to low disease prevalence combined with high degrees of tumor heterogeneity [214], [319]. Most targets will therefore be present at low frequency, and primary and secondary resistance to targeted agents will be common. Disease heterogeneity also presents difficulties in extrapolating preclinical results obtained in small numbers of cell lines or patient xenografts, although limited drug penetration may also explain why encouraging preclinical results from subcutaneous osteosarcoma xenografts have not been recapitulated in subsequent phase I trials. More rigorous pre-clinical testing prior to phase I trial initiation is therefore required [319]. Targeting metastatic progression as opposed to existing lesions may also improve outcomes in osteosarcoma patients [320]. Such targeted agents might show no activity against established lesions, and would require the use of different experimental and clinical trial end-points, but could potentially be applied to a number of solid tumors [320].
3. Conclusions
The use of tailored treatments has already proven successful in the management of adult cancer patients. Von Hoff and colleagues demonstrated the achievement of longer progression free survival in 27% of patients with refractory solid tumors treated with drugs suggested by molecular profiling [321]. More recently, Kim et al. described the importance of tumor profiling in “real-time” biopsies that predicted tumor responsiveness in heavily pre-treated advanced NSCLC patients [322].
Results from early clinical trials demonstrate that the use of molecularly targeted therapies is generally safe in the pediatric population [30], [38], [72], [114], [135], [164], [185], [187], [198], and that particular drugs have shown promising results. Among them, imatinib, dasatinib and sorafenib were found active in Ph + ALL, CML and ALL pediatric patients, respectively [11], [20], [22]. A large number of tyrosine kinase inhibitors including crizotinib, erlotinib, gefitinib and lestaurtinib have been used to treat children with refractory/relapsed neuroblastoma [108], [110], [111], [114]. Erlotinib and gefitinib have been successfully employed to treat high-grade gliomas [193], [194], [197], while IGFR mAbs show promising results in pediatric sarcomas [235]. These encouraging results indicate that molecularly targeted therapies have great further potential for the treatment of childhood cancers.
Despite these promising results, the literature published to date also highlights problems that need to be overcome, to fulfill the promise of molecularly targeted therapies for childhood cancer. A large number of biomarker-matched drugs are tested in clinical trials, and then dismissed. In general, 90% of novel targeted therapies do not transition to routine use, despite good results in the preclinical setting [323]. This can be due to unexpectedly high toxicities, lack of objective efficacy, and/or because agents present no measurable advantages over those already in use [323]. In terms of preclinical studies, the assumption that childhood cancer in vivo models recapitulate the actual disease has not always proved correct [225], [240], [241], [242]. Efficacy of targeted therapies will be greatly influenced by intra-tumoral heterogeneity, meaning that more homogenous models such as cell line xenografts may overestimate the efficacy subsequently demonstrated in patients [319]. Other findings may reflect the fact that the efficacy of targeted therapies cannot always be evaluated using the standard methods used for conventional chemotherapy, due to off-target effects, requirements for prolonged dosing at lower levels, and general cytostatic rather than cytotoxic effects [324]. In other words, tumor growth inhibition could represent a more adequate measurement of activity rather than tumor regression in the assessment of single agent efficacy, highlighting the importance of combination trials. This is particularly important in the pediatric population where the goal must be to eradicate disease in the long term.
Another challenge is the low frequency at which predictive markers are often detected in tumors (Table 2), meaning that only a minority of patients may benefit from a specific targeted therapy [325], [326]. This problem can become particularly acute in rare diseases such as childhood cancers. Efficacy of targeted therapies may be underestimated if preclinical models do not represent the full range of clinical disease, or if patients are not appropriately selected in clinical trials [323]. Another consideration is the presence or development of drug resistance, due to either activation of alternative pathways, or the presence or acquisition of further mutations that confer a selection advantage to a subgroup of cancer cells [327]. In this context, it is important to consider the combination of multiple agents and/or the use of less selective drugs, in order to inhibit multiple pathways at the same time, or the same pathways at multiple levels [328].
In summary, our understanding of altered signaling pathways responsible for childhood malignancies is changing treatment approaches and improving the outcomes of pediatric patients. A huge effort has been made to prove the safety of molecular targeted therapies in the pediatric population, but patient results have sometimes been considered disappointing. It is clear that conventional methods to evaluate clinical efficacy of novel therapies do not always apply to targeted agents, due to the genetic complexity and heterogeneity of tumors, and the modes of action of these novel drugs. Therefore, the testing of targeted agents in combination with traditional chemotherapeutics needs further investment, in order to produce long-lasting clinical benefits. At the same time, preclinical models need to be improved for the assessment of optimal dosing to obtain additive effects in combination therapy, while clinical trials with patient selection based on molecular profiling will avoid administering target-specific agents to patients who are unlikely to benefit. Finally, of the 12 known pathways that are aberrantly activated in cancer, at least 140 different genes have been described to carry driver mutations [329], but only a small percentage of these are currently “actionable” in the context of targeted therapy. Identifying new actionable targets will be essential to expand the benefits of targeted therapies to a broader range of patients.
Funding
This publication was supported by funding to the Kids Cancer Alliance from the Cancer Institute NSW (Grant ID: 11/TRC/1-03) and by grants from Cancer Council New South Wales (Grant ID: PG 11-06), Cancer Institute New South Wales (Grant ID: 10/TPG/1-03) and the National Health and Medical Research Council (NHMRC) of Australia (Grant ID: 1016699).
The following are the supplementary data related to this article.
Supplemental references for Table 2.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bbacli.2014.06.003.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
The authors thank past and present group members and external collaborators for their support.
References
- 1.American Cancer Society . American Cancer Society; Atlanta, Ga: 2014. Cancer Facts & Figs. 2014. [Google Scholar]
- 2.Siegel R. Cancer treatment and survivorship statistics, 2012. CA Cancer J. Clin. 2012;62(4):220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 3.Sardi I., Cavalieri D., Massimino M. Emerging treatments and gene expression profiling in high-risk medulloblastoma. Paediatr. Drugs. 2007;9(2):81–96. doi: 10.2165/00148581-200709020-00002. [DOI] [PubMed] [Google Scholar]
- 4.Owens C., Irwin M. Neuroblastoma: the impact of biology and cooperation leading to personalized treatments. Crit. Rev. Clin. Lab. Sci. 2012;49(3):85–115. doi: 10.3109/10408363.2012.683483. [DOI] [PubMed] [Google Scholar]
- 5.Loh M.L., Mullighan C.G. Advances in the genetics of high-risk childhood B-progenitor acute lymphoblastic leukemia and juvenile myelomonocytic leukemia: implications for therapy. Clin. Cancer Res. 2012;18(10):2754–2767. doi: 10.1158/1078-0432.CCR-11-1936. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein M.L. Targeted therapy in pediatric and adolescent oncology. Cancer. 2011;117(10 Suppl.):2268–2274. doi: 10.1002/cncr.26050. [DOI] [PubMed] [Google Scholar]
- 7.Scholzen T., Gerdes J. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 2000;182(3):311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Westermark U.K. The MYCN oncogene and differentiation in neuroblastoma. Semin. Cancer Biol. 2011;21(4):256–266. doi: 10.1016/j.semcancer.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Henze G. Serum S100—a marker for disease monitoring in metastatic melanoma. Dermatology. 1997;194(3):208–212. doi: 10.1159/000246103. [DOI] [PubMed] [Google Scholar]
- 10.Stegmeier F. Targeted cancer therapies in the twenty-first century: lessons from imatinib. Clin. Pharmacol. Ther. 2010;87(5):543–552. doi: 10.1038/clpt.2009.297. [DOI] [PubMed] [Google Scholar]
- 11.Schultz K.R. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children's oncology group study. J. Clin. Oncol. 2009;27(31):5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.La Madrid A.M. Targeting ALK: a promising strategy for the treatment of non-small cell lung cancer, non-Hodgkin's lymphoma, and neuroblastoma. Target. Oncol. 2012;7(3):199–210. doi: 10.1007/s11523-012-0227-8. [DOI] [PubMed] [Google Scholar]
- 13.Ou S.H. Crizotinib: a novel and first-in-class multitargeted tyrosine kinase inhibitor for the treatment of anaplastic lymphoma kinase rearranged non-small cell lung cancer and beyond. Drug Des. Dev. Ther. 2011;5:471–485. doi: 10.2147/DDDT.S19045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shannon K. Genetic predispositions and childhood cancer. Environ. Health Perspect. 1998;106(Suppl. 3):801–806. doi: 10.1289/ehp.98106801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel J.N., Mandock K., McLeod H.L. Clinically relevant cancer biomarkers and pharmacogenetic assays. J. Oncol. Pharm. Pract. 2014;20:65–72. doi: 10.1177/1078155212473862. [DOI] [PubMed] [Google Scholar]
- 16.Belson M., Kingsley B., Holmes A. Risk factors for acute leukemia in children: a review. Environ. Health Perspect. 2007;115(1):138–145. doi: 10.1289/ehp.9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith M.T. Molecular biomarkers for the study of childhood leukemia. Toxicol. Appl. Pharmacol. 2005;206(2):237–245. doi: 10.1016/j.taap.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 18.Chang B.H. Imatinib resistant BCR–ABL1 mutations at relapse in children with Ph + ALL: a Children's Oncology Group (COG) study. Br. J. Haematol. 2012;157(4):507–510. doi: 10.1111/j.1365-2141.2012.09039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gnoni A. Dasatinib: an anti-tumour agent via Src inhibition. Curr. Drug Targets. 2011;12(4):563–578. doi: 10.2174/138945011794751591. [DOI] [PubMed] [Google Scholar]
- 20.Aplenc R. Pediatric phase I trial and pharmacokinetic study of dasatinib: a report from the children's oncology group phase I consortium. J. Clin. Oncol. 2011;29(7):839–844. doi: 10.1200/JCO.2010.30.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keating G.M., Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69(2):223–240. doi: 10.2165/00003495-200969020-00006. [DOI] [PubMed] [Google Scholar]
- 22.Widemann B.C. A phase I trial and pharmacokinetic study of sorafenib in children with refractory solid tumors or leukemias: a Children's Oncology Group Phase I Consortium report. Clin. Cancer Res. 2012;18(21):6011–6022. doi: 10.1158/1078-0432.CCR-11-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goemans B.F. FLT3 and KIT mutated pediatric acute myeloid leukemia (AML) samples are sensitive in vitro to the tyrosine kinase inhibitor SU11657. Leuk. Res. 2010;34(10):1302–1307. doi: 10.1016/j.leukres.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Deisseroth A. U.S. Food and Drug Administration approval: ruxolitinib for the treatment of patients with intermediate and high-risk myelofibrosis. Clin. Cancer Res. 2012;18(12):3212–3217. doi: 10.1158/1078-0432.CCR-12-0653. [DOI] [PubMed] [Google Scholar]
- 25.Maude S.L. Targeting JAK1/2 and mTOR in murine xenograft models of Ph-like acute lymphoblastic leukemia. Blood. 2012;120(17):3510–3518. doi: 10.1182/blood-2012-03-415448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perova T. AACR 103rd Annual Meeting, Abstract 867: Therapeutic potential of small molecule SYK inhibitors for treatment of primary B cell acute lymphoblastic leukemia. Cancer Res. 2012;72(8, Supp 1) [Google Scholar]
- 27.Uckun F.M. Nanoscale liposomal formulation of a SYK P-site inhibitor against B-precursor leukemia. Blood. 2013;121(21):4348–4354. doi: 10.1182/blood-2012-11-470633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uckun F. In vitro and in vivo chemosensitizing activity of LFM-A13, a dual-function inhibitor of Bruton's tyrosine kinase and polo-like kinases, against human leukemic B-cell precursors. Arzneimittelforschung. 2011;61(4):252–259. doi: 10.1055/s-0031-1296196. [DOI] [PubMed] [Google Scholar]
- 29.Macy M.E. Pediatric developmental therapies: interesting new drugs now in early-stage clinical trials. Curr. Oncol. Rep. 2008;10(6):477–490. doi: 10.1007/s11912-008-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Widemann B.C. Phase 1 trial and pharmacokinetic study of the farnesyl transferase inhibitor tipifarnib in children and adolescents with refractory leukemias: a report from the Children's Oncology Group. Pediatr. Blood Cancer. 2011;56(2):226–233. doi: 10.1002/pbc.22775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodwin C.B. Genetic disruption of the PI3K regulatory subunits, p85 alpha, p55 alpha, and p50 alpha, normalizes mutant PTPN11-induced hypersensitivity to GM-CSF. Haematologica. 2012;97(7):1042–1047. doi: 10.3324/haematol.2011.046896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds C.P. Initial testing (stage 1) of the phosphatidylinositol 3′ kinase inhibitor, SAR245408 (XL147) by the pediatric preclinical testing program. Pediatr. Blood Cancer. 2013;60(5):791–798. doi: 10.1002/pbc.24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Podesta J.E. Adaptation of the plasma inhibitory activity assay to detect Aurora, ABL and FLT3 kinase inhibition by AT9283 in pediatric leukemia. Leuk. Res. 2011;35(9):1273–1275. doi: 10.1016/j.leukres.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Jayanthan A. Occurrence and modulation of therapeutic targets of Aurora kinase inhibition in pediatric acute leukemia cells. Leuk. Lymphoma. 2013;54(7):1505–1516. doi: 10.3109/10428194.2012.752079. [DOI] [PubMed] [Google Scholar]
- 35.Maris J.M. Initial testing of the aurora Kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP) Pediatr. Blood Cancer. 2010;55(1):26–34. doi: 10.1002/pbc.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carol H. Efficacy and pharmacokinetic/pharmacodynamic evaluation of the Aurora kinase A inhibitor MLN8237 against preclinical models of pediatric cancer. Cancer Chemother. Pharmacol. 2011;68(5):1291–1304. doi: 10.1007/s00280-011-1618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keshelava N. Initial testing (stage 1) of vorinostat (SAHA) by the pediatric preclinical testing program. Pediatr. Blood Cancer. 2009;53(3):505–508. doi: 10.1002/pbc.21988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fouladi M. Pediatric phase I trial and pharmacokinetic study of vorinostat: a Children's Oncology Group phase I consortium report. J. Clin. Oncol. 2010;28(22):3623–3629. doi: 10.1200/JCO.2009.25.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hawkins L.M., Jayanthan A.A., Narendran A. Effects of 17-allylamino-17-demethoxygeldanamycin (17-AAG) on pediatric acute lymphoblastic leukemia (ALL) with respect to Bcr–Abl status and imatinib mesylate sensitivity. Pediatr. Res. 2005;57(3):430–437. doi: 10.1203/01.PDR.0000153871.45184.19. [DOI] [PubMed] [Google Scholar]
- 40.Lock R.B. Initial testing (stage 1) of ganetespib, an Hsp90 inhibitor, by the pediatric preclinical testing program. Pediatr. Blood Cancer. 2013;60(7):E42–E45. doi: 10.1002/pbc.24451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garst J. Safety of topotecan in the treatment of recurrent small-cell lung cancer and ovarian cancer. Expert Opin. Drug Saf. 2007;6(1):53–62. doi: 10.1517/14740338.6.1.53. [DOI] [PubMed] [Google Scholar]
- 42.Hijiya N. Phase II study of topotecan in combination with dexamethasone, asparaginase, and vincristine in pediatric patients with acute lymphoblastic leukemia in first relapse. Cancer. 2008;112(9):1983–1991. doi: 10.1002/cncr.23395. [DOI] [PubMed] [Google Scholar]
- 43.Inaba H. Combination of cladribine plus topotecan for recurrent or refractory pediatric acute myeloid leukemia. Cancer. 2010;116(1):98–105. doi: 10.1002/cncr.24712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varadhachary G.R., Hoff P.M. Front-line therapy for advanced colorectal cancer: emphasis on chemotherapy. Semin. Oncol. 2005;32(6 Suppl. 9):S40–S42. doi: 10.1053/j.seminoncol.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 45.Goto H. Chemo-sensitivity in a panel of B-cell precursor acute lymphoblastic leukemia cell lines, YCUB series, derived from children. Leuk. Res. 2009;33(10):1386–1391. doi: 10.1016/j.leukres.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Hijiya N. A multi-center phase I study of clofarabine, etoposide and cyclophosphamide in combination in pediatric patients with refractory or relapsed acute leukemia. Leukemia. 2009;23(12):2259–2264. doi: 10.1038/leu.2009.185. [DOI] [PubMed] [Google Scholar]
- 47.Hijiya N. Phase 2 trial of clofarabine in combination with etoposide and cyclophosphamide in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. Blood. 2011;118(23):6043–6049. doi: 10.1182/blood-2011-08-374710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Houghton P.J. Initial testing (stage 1) of the proteasome inhibitor bortezomib by the pediatric preclinical testing program. Pediatr. Blood Cancer. 2008;50(1):37–45. doi: 10.1002/pbc.21214. [DOI] [PubMed] [Google Scholar]
- 49.Horton T.M. A phase 1 study of the proteasome inhibitor bortezomib in pediatric patients with refractory leukemia: a Children's Oncology Group study. Clin. Cancer Res. 2007;13(5):1516–1522. doi: 10.1158/1078-0432.CCR-06-2173. [DOI] [PubMed] [Google Scholar]
- 50.Horton T.M. Bortezomib interactions with chemotherapy agents in acute leukemia in vitro. Cancer Chemother. Pharmacol. 2006;58(1):13–23. doi: 10.1007/s00280-005-0135-z. [DOI] [PubMed] [Google Scholar]
- 51.Messinger Y. Phase I study of bortezomib combined with chemotherapy in children with relapsed childhood acute lymphoblastic leukemia (ALL): a report from the therapeutic advances in childhood leukemia (TACL) consortium. Pediatr. Blood Cancer. 2010;55(2):254–259. doi: 10.1002/pbc.22456. [DOI] [PubMed] [Google Scholar]
- 52.Messinger Y.H. Bortezomib with chemotherapy is highly active in advanced B-precursor acute lymphoblastic leukemia: Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Study. Blood. 2012;120(2):285–290. doi: 10.1182/blood-2012-04-418640. [DOI] [PubMed] [Google Scholar]
- 53.Vedi, A., and D.S. Ziegler, Antibody therapy for pediatric leukemia. Front Oncol, 4: 82. [DOI] [PMC free article] [PubMed]
- 54.Meinhardt A. Phase II window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin's lymphoma and Burkitt leukemia. J. Clin. Oncol. 2010;28(19):3115–3121. doi: 10.1200/JCO.2009.26.6791. [DOI] [PubMed] [Google Scholar]
- 55.Griffin T.C. A study of rituximab and ifosfamide, carboplatin, and etoposide chemotherapy in children with recurrent/refractory B-cell (CD20 +) non-Hodgkin lymphoma and mature B-cell acute lymphoblastic leukemia: a report from the Children's Oncology Group. Pediatr. Blood Cancer. 2009;52(2):177–181. doi: 10.1002/pbc.21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Angiolillo A.L. A phase II study of Campath-1H in children with relapsed or refractory acute lymphoblastic leukemia: a Children's Oncology Group report. Pediatr. Blood Cancer. 2009;53(6):978–983. doi: 10.1002/pbc.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Law J. Busulfan, fludarabine, and alemtuzumab as a reduced toxicity regimen for children with malignant and nonmalignant diseases improves engraftment and graft-versus-host disease without delaying immune reconstitution. Biol. Blood. Marrow. Transplant. 2012;18(11):1656–1663. doi: 10.1016/j.bbmt.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Scott A.M., Wolchok J.D., Old L.J. Antibody therapy of cancer. Nat. Rev. Cancer. 2012;12(4):278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 59.FitzGerald D.J. Treatment of hematologic malignancies with immunotoxins and antibody–drug conjugates. Cancer Res. 2011;71(20):6300–6309. doi: 10.1158/0008-5472.CAN-11-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharkey R.M., Goldenberg D.M. Cancer radioimmunotherapy. Immunotherapy. 2011;3(3):349–370. doi: 10.2217/imt.10.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arceci R.J. Safety and efficacy of gemtuzumab ozogamicin in pediatric patients with advanced CD33 + acute myeloid leukemia. Blood. 2005;106(4):1183–1188. doi: 10.1182/blood-2004-10-3821. [DOI] [PubMed] [Google Scholar]
- 62.Aplenc R. Safety and efficacy of gemtuzumab ozogamicin in combination with chemotherapy for pediatric acute myeloid leukemia: a report from the Children's Oncology Group. J. Clin. Oncol. 2008;26(14):2390–3295. doi: 10.1200/JCO.2007.13.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wayne A.S. A novel anti-CD22 immunotoxin, moxetumomab pasudotox: phase I study in pediatric acute lymphoblastic leukemia (ALL) Blood. 2011:118. doi: 10.1182/blood-2017-02-749101. Abstract 248, (ASH Annual Meeting Abstracts) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rytting M. Initial experience with CMC-544 (inotuzumab ozogamicin) in pediatric patients with relapsed B-cell acute lymphocytic leukaemia. Pediatr. Blood Cancer. 2014;61(2):369–372. doi: 10.1002/pbc.24721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carol H. The anti-CD19 antibody–drug conjugate SAR3419 prevents hematolymphoid relapse postinduction therapy in preclinical models of pediatric acute lymphoblastic leukemia. Clin. Cancer Res. 2013;19(7):1795–1805. doi: 10.1158/1078-0432.CCR-12-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoffman L.M., Gore L. Blinatumomab, a bi-specific anti-CD19/CD3 BiTE® antibody for the treatment of acute lymphoblastic leukemia: perspectives and current pediatric applications. Front. Oncol. 2014;4:63. doi: 10.3389/fonc.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Handgretinger R. Complete remission after blinatumomab-induced donor T-cell activation in three pediatric patients with post-transplant relapsed acute lymphoblastic leukemia. Leukemia. 2011;25(1):181–184. doi: 10.1038/leu.2010.239. [DOI] [PubMed] [Google Scholar]
- 68.von Stackelberg A. A phase 1/2 study of blinatumomab in pediatric patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Blood. 2013;122(21):70. [Google Scholar]
- 69.Gross G., Waks T., Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. U. S. A. 1989;86(24):10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee D.W. The future is now: chimeric antigen receptors as new targeted therapies for childhood cancer. Clin. Cancer Res. 2012;18(10):2780–2790. doi: 10.1158/1078-0432.CCR-11-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Till B.G. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112(6):2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Curran K.J. ASH Annual Meeting, Abstracts 120: CD19 targeted allogeneic EBV-specific T cells for the treatment of relapsed ALL in pediatric patients post HSCT. Blood. 2012:120. Abstract 353, (ASH Annual Meeting Abstracts) [Google Scholar]
- 73.Grupp S.A. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Napper, A.D. and Watson V.G., Targeted drug discovery for pediatric leukemia. Front Oncol, 3: 170 [DOI] [PMC free article] [PubMed]
- 75.Park J.R. Children's Oncology Group's 2013 blueprint for research: neuroblastoma. Pediatr. Blood Cancer. 2013;60(6):985–993. doi: 10.1002/pbc.24433. [DOI] [PubMed] [Google Scholar]
- 76.Zirath H. MYC inhibition induces metabolic changes leading to accumulation of lipid droplets in tumor cells. Proc. Natl. Acad. Sci. U. S. A. 2013;110(25):10258–10263. doi: 10.1073/pnas.1222404110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mertz J.A. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc. Natl. Acad. Sci. U. S. A. 2011;108(40):16669–16674. doi: 10.1073/pnas.1108190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mujtaba S., Zeng L., Zhou M.M. Structure and acetyl-lysine recognition of the bromodomain. Oncogene. 2007;26(37):5521–5527. doi: 10.1038/sj.onc.1210618. [DOI] [PubMed] [Google Scholar]
- 79.Zuber J. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478(7370):524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Puissant A. Targeting MYCN in neuroblastoma by BET bromodomain inhibition. Cancer Discov. 2013;3(3):308–323. doi: 10.1158/2159-8290.CD-12-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dominguez-Sola D. Non-transcriptional control of DNA replication by c-Myc. Nature. 2007;448(7152):445–451. doi: 10.1038/nature05953. [DOI] [PubMed] [Google Scholar]
- 82.Cole K.A. RNAi screen of the protein kinome identifies checkpoint kinase 1 (CHK1) as a therapeutic target in neuroblastoma. Proc. Natl. Acad. Sci. U. S. A. 2011;108(8):3336–3341. doi: 10.1073/pnas.1012351108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Walton M.I. CCT244747 is a novel potent and selective CHK1 inhibitor with oral efficacy alone and in combination with genotoxic anticancer drugs. Clin. Cancer Res. 2012;18(20):5650–5661. doi: 10.1158/1078-0432.CCR-12-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reynolds C.P. Retinoid therapy of high-risk neuroblastoma. Cancer Lett. 2003;197(1–2):185–192. doi: 10.1016/s0304-3835(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 85.Maurer B.J. Increase of ceramide and induction of mixed apoptosis/necrosis by N-(4-hydroxyphenyl)- retinamide in neuroblastoma cell lines. J. Natl. Cancer Inst. 1999;91(13):1138–1146. doi: 10.1093/jnci/91.13.1138. [DOI] [PubMed] [Google Scholar]
- 86.Garaventa A. Phase I trial and pharmacokinetics of fenretinide in children with neuroblastoma. Clin. Cancer Res. 2003;9(6):2032–2039. [PubMed] [Google Scholar]
- 87.Villablanca J.G. Phase II study of oral capsular 4-hydroxyphenylretinamide (4-HPR/fenretinide) in pediatric patients with refractory or recurrent neuroblastoma: a report from the Children's Oncology Group. Clin. Cancer Res. 2011;17(21):6858–6866. doi: 10.1158/1078-0432.CCR-11-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Di Paolo D. Enhanced anti-tumor and anti-angiogenic efficacy of a novel liposomal fenretinide on human neuroblastoma. J. Control. Release. 2013;170(3):445–451. doi: 10.1016/j.jconrel.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 89.Parsons K. Targeted immunotherapy for high-risk neuroblastoma—the role of monoclonal antibodies. Ann. Pharmacother. 2013;47(2):210–218. doi: 10.1345/aph.1R353. [DOI] [PubMed] [Google Scholar]
- 90.Yu A.L. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010;363(14):1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Navid F. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J. Clin. Oncol. 2014;32(14):1445–1452. doi: 10.1200/JCO.2013.50.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fulda S. The PI3K/Akt/mTOR pathway as therapeutic target in neuroblastoma. Curr. Cancer Drug Targets. 2009;9(6):729–737. doi: 10.2174/156800909789271521. [DOI] [PubMed] [Google Scholar]
- 93.Izycka-Swieszewska E. Analysis of PI3K/AKT/mTOR signalling pathway in high risk neuroblastic tumours. Pol. J. Pathol. 2010;61(4):192–198. [PubMed] [Google Scholar]
- 94.Li Z. In vitro and in vivo inhibition of neuroblastoma tumor cell growth by AKT inhibitor perifosine. J. Natl. Cancer Inst. 2010;102(11):758–770. doi: 10.1093/jnci/djq125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Z. Perifosine-induced inhibition of Akt attenuates brain-derived neurotrophic factor/TrkB-induced chemoresistance in neuroblastoma in vivo. Cancer. 2011;117(23):5412–5422. doi: 10.1002/cncr.26133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kushner B.H. 2012. ANR 2012, Abstract POC27: Targeting the PI3K/Akt Pathway: Perifosine Monotherapy for Resistant Neuroblastoma (NB) in a Phase I/Ib Study. [Google Scholar]
- 97.Karar J., Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front. Mol. Neurosci. 2011;4:51. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Spunt S.L. Phase I study of temsirolimus in pediatric patients with recurrent/refractory solid tumors. J. Clin. Oncol. 2011;29(21):2933–2940. doi: 10.1200/JCO.2010.33.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Geoerger B. Phase II trial of temsirolimus in children with high-grade glioma, neuroblastoma and rhabdomyosarcoma. Eur. J. Cancer. 2012;48(2):253–262. doi: 10.1016/j.ejca.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peirce S.K. The PI-3 kinase–Akt–MDM2–survivin signaling axis in high-risk neuroblastoma: a target for PI-3 kinase inhibitor intervention. Cancer Chemother. Pharmacol. 2011;68(2):325–335. doi: 10.1007/s00280-010-1486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Opel D. Targeting aberrant PI3K/Akt activation by PI103 restores sensitivity to TRAIL-induced apoptosis in neuroblastoma. Clin. Cancer Res. 2011;17(10):3233–3247. doi: 10.1158/1078-0432.CCR-10-2530. [DOI] [PubMed] [Google Scholar]
- 102.Segerström L. Effects of small molecule inhibitors of PI3K/Akt/mTOR signaling on neuroblastoma growth in vitro and in vivo. Int. J. Cancer. 2011;129(12):2958–2965. doi: 10.1002/ijc.26268. [DOI] [PubMed] [Google Scholar]
- 103.Chanthery Y.H. Paracrine signaling through MYCN enhances tumor–vascular interactions in neuroblastoma. Sci. Transl. Med. 2012;4(115) doi: 10.1126/scitranslmed.3002977. (115ra3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Seitz C. The dual PI3K/mTOR inhibitor NVP-BEZ235 and chloroquine synergize to trigger apoptosis via mitochondrial-lysosomal cross-talk. Int. J. Cancer. 2013;132(11):2682–2693. doi: 10.1002/ijc.27935. [DOI] [PubMed] [Google Scholar]
- 105.Mosse Y.P. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455(7215):930–935. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Azarova A.M., Gautam G., George R.E. Emerging importance of ALK in neuroblastoma. Semin. Cancer Biol. 2011;21(4):267–275. doi: 10.1016/j.semcancer.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Passoni L. Mutation-independent anaplastic lymphoma kinase overexpression in poor prognosis neuroblastoma patients. Cancer Res. 2009;69(18):7338–7346. doi: 10.1158/0008-5472.CAN-08-4419. [DOI] [PubMed] [Google Scholar]
- 108.Mosse Y.P. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children's Oncology Group phase 1 consortium study. Lancet Oncol. 2013;14(6):472–480. doi: 10.1016/S1470-2045(13)70095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mehra R. ASCO Annual Meeting, Abstract 3007: First-in-human phase I study of the ALK inhibitor LDK378 in advanced solid tumours. J. Clin. Oncol. 2012;30(15 Suppl.) [Google Scholar]
- 110.Jakacki R.I. Pediatric phase I and pharmacokinetic study of erlotinib followed by the combination of erlotinib and temozolomide: a Children's Oncology Group Phase I Consortium Study. J. Clin. Oncol. 2008;26(30):4921–4927. doi: 10.1200/JCO.2007.15.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Donfrancesco A. Gefitinib in combination with oral topotecan and cyclophosphamide in relapsed neuroblastoma: pharmacological rationale and clinical response. Pediatr. Blood Cancer. 2010;54(1):55–61. doi: 10.1002/pbc.22219. [DOI] [PubMed] [Google Scholar]
- 112.Furman W.L. A single-arm pilot phase II study of gefitinib and irinotecan in children with newly diagnosed high-risk neuroblastoma. Invest. New Drugs. 2012;30(4):1660–1670. doi: 10.1007/s10637-011-9724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Richards K.N. Signaling of ERBB receptor tyrosine kinases promotes neuroblastoma growth in vitro and in vivo. Cancer. 2010;116(13):3233–3243. doi: 10.1002/cncr.25073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Minturn J.E. Phase I trial of lestaurtinib for children with refractory neuroblastoma: a new approaches to neuroblastoma therapy consortium study. Cancer Chemother. Pharmacol. 2011;68(4):1057–1065. doi: 10.1007/s00280-011-1581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Iyer R. Lestaurtinib enhances the antitumor efficacy of chemotherapy in murine xenograft models of neuroblastoma. Clin. Cancer Res. 2010;16(5):1478–1485. doi: 10.1158/1078-0432.CCR-09-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Iyer R. AZ64 inhibits TrkB and enhances the efficacy of chemotherapy and local radiation in neuroblastoma xenografts. Cancer Chemother. Pharmacol. 2012;70(3):477–486. doi: 10.1007/s00280-012-1879-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zage P.E. The selective Trk inhibitor AZ623 inhibits brain-derived neurotrophic factor-mediated neuroblastoma cell proliferation and signaling and is synergistic with topotecan. Cancer. 2011;117(6):1321–1391. doi: 10.1002/cncr.25674. [DOI] [PubMed] [Google Scholar]
- 118.Zage P.E. A novel therapeutic combination for neuroblastoma: the vascular endothelial growth factor receptor/epidermal growth factor receptor/rearranged during transfection inhibitor vandetanib with 13-cis-retinoic acid. Cancer. 2010;116(10):2465–2475. doi: 10.1002/cncr.25017. [DOI] [PubMed] [Google Scholar]
- 119.Beaudry P. Potent antitumor effects of ZD6474 on neuroblastoma via dual targeting of tumor cells and tumor endothelium. Mol. Cancer Ther. 2008;7(2):418–424. doi: 10.1158/1535-7163.MCT-07-0568. [DOI] [PubMed] [Google Scholar]
- 120.Maris J.M. Initial testing of the VEGFR inhibitor AZD2171 by the pediatric preclinical testing program. Pediatr. Blood Cancer. 2008;50(3):581–587. doi: 10.1002/pbc.21232. [DOI] [PubMed] [Google Scholar]
- 121.Morton C.L. Combination testing of cediranib (AZD2171) against childhood cancer models by the pediatric preclinical testing program. Pediatr. Blood Cancer. 2012;58(4):566–571. doi: 10.1002/pbc.23159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Grinshtein N. Small molecule kinase inhibitor screen identifies polo-like kinase 1 as a target for neuroblastoma tumor-initiating cells. Cancer Res. 2011;71(4):1385–1395. doi: 10.1158/0008-5472.CAN-10-2484. [DOI] [PubMed] [Google Scholar]
- 123.Spaniol K., Boos J., Lanvers-Kaminsky C. An in-vitro evaluation of the polo-like kinase inhibitor GW843682X against paediatric malignancies. Anticancer Drugs. 2011;22(6):531–542. doi: 10.1097/CAD.0b013e3283454526. [DOI] [PubMed] [Google Scholar]
- 124.Timeus F. In vitro antiproliferative and antimigratory activity of dasatinib in neuroblastoma and Ewing sarcoma cell lines. Oncol. Rep. 2008;19(2):353–359. [PubMed] [Google Scholar]
- 125.Vitali R. Activity of tyrosine kinase inhibitor dasatinib in neuroblastoma cells in vitro and in orthotopic mouse model. Int. J. Cancer. 2009;125(11):2547–2555. doi: 10.1002/ijc.24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Keisner S.V., Shah S.R. Pazopanib: the newest tyrosine kinase inhibitor for the treatment of advanced or metastatic renal cell carcinoma. Drugs. 2011;71(4):443–454. doi: 10.2165/11588960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 127.Kumar S. Metronomic oral topotecan with pazopanib is an active antiangiogenic regimen in mouse models of aggressive pediatric solid tumor. Clin. Cancer Res. 2011;17(17):5656–5667. doi: 10.1158/1078-0432.CCR-11-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kakodkar N.C. Sorafenib inhibits neuroblastoma cell proliferation and signaling, blocks angiogenesis, and impairs tumor growth. Pediatr. Blood Cancer. 2012;59(4):642–647. doi: 10.1002/pbc.24004. [DOI] [PubMed] [Google Scholar]
- 129.Rock E.P. Food and Drug Administration drug approval summary: sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist. 2007;12(1):107–113. doi: 10.1634/theoncologist.12-1-107. [DOI] [PubMed] [Google Scholar]
- 130.Zhang L. In vivo antitumor and antimetastatic activity of sunitinib in preclinical neuroblastoma mouse model. Neoplasia. 2009;11(5):426–435. doi: 10.1593/neo.09166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ioannidis S. Discovery of 5-chloro-N2-[(1S)-1-(5-fluoropyrimidin-2-yl)ethyl]-N4-(5-methyl-1H-pyrazol-3-yl)p yrimidine-2,4-diamine (AZD1480) as a novel inhibitor of the Jak/Stat pathway. J. Med. Chem. 2011;54(1):262–276. doi: 10.1021/jm1011319. [DOI] [PubMed] [Google Scholar]
- 132.Yan S., Li Z., Thiele C.J. Inhibition of STAT3 with orally active JAK inhibitor, AZD1480, decreases tumor growth in neuroblastoma and pediatric sarcomas in vitro and in vivo. Oncotarget. 2013;4(3):433–445. doi: 10.18632/oncotarget.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mosse Y.P. Pediatric phase I trial and pharmacokinetic study of MLN8237, an investigational oral selective small-molecule inhibitor of Aurora kinase A: a Children's Oncology Group Phase I Consortium study. Clin. Cancer Res. 2012;18(21):6058–6064. doi: 10.1158/1078-0432.CCR-11-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Muscal J.A. Additive effects of vorinostat and MLN8237 in pediatric leukemia, medulloblastoma, and neuroblastoma cell lines. Invest. New Drugs. 2013;31(1):39–45. doi: 10.1007/s10637-012-9831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Muscal J.A. A phase I trial of vorinostat and bortezomib in children with refractory or recurrent solid tumors: a Children's Oncology Group phase I consortium study (ADVL0916) Pediatr. Blood Cancer. 2013;60(3):390–395. doi: 10.1002/pbc.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Panicker J. Romidepsin (FK228/depsipeptide) controls growth and induces apoptosis in neuroblastoma tumor cells. Cell Cycle. 2010;9(9):1830–1838. doi: 10.4161/cc.9.9.11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jaboin J. MS-27-275, an inhibitor of histone deacetylase, has marked in vitro and in vivo antitumor activity against pediatric solid tumors. Cancer Res. 2002;62(21):6108–6115. [PubMed] [Google Scholar]
- 138.Wagner L.M. Phase I trial of oral irinotecan and temozolomide for children with relapsed high-risk neuroblastoma: a new approach to neuroblastoma therapy consortium study. J. Clin. Oncol. 2009;27(8):1290–1296. doi: 10.1200/JCO.2008.18.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bagatell R. Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: a Children's Oncology Group study. J. Clin. Oncol. 2011;29(2):208–213. doi: 10.1200/JCO.2010.31.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kushner B.H. Irinotecan plus temozolomide for relapsed or refractory neuroblastoma. J. Clin. Oncol. 2006;24(33):5271–5276. doi: 10.1200/JCO.2006.06.7272. [DOI] [PubMed] [Google Scholar]
- 141.Tsang P.S. Synthetic lethal screen identifies NF-kappaB as a target for combination therapy with topotecan for patients with neuroblastoma. BMC Cancer. 2012;12:101. doi: 10.1186/1471-2407-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sottile F. A chemical screen identifies the chemotherapeutic drug topotecan as a specific inhibitor of the B-MYB/MYCN axis in neuroblastoma. Oncotarget. 2012;3(5):535–545. doi: 10.18632/oncotarget.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Simon T. Topotecan and etoposide in the treatment of relapsed high-risk neuroblastoma: results of a phase 2 trial. J. Pediatr. Hematol. Oncol. 2007;29(2):101–106. doi: 10.1097/MPH.0b013e3180320b48. [DOI] [PubMed] [Google Scholar]
- 144.Simon T. Topotecan, cyclophosphamide, and etoposide (TCE) in the treatment of high-risk neuroblastoma. Results of a phase-II trial. J. Cancer Res. Clin. Oncol. 2007;133(9):653–661. doi: 10.1007/s00432-007-0216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.London W.B. Phase II randomized comparison of topotecan plus cyclophosphamide versus topotecan alone in children with recurrent or refractory neuroblastoma: a Children's Oncology Group study. J. Clin. Oncol. 2010;28(24):3808–3815. doi: 10.1200/JCO.2009.27.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kushner B.H. Ifosfamide, carboplatin, and etoposide for neuroblastoma: a high-dose salvage regimen and review of the literature. Cancer. 2013;119(3):665–671. doi: 10.1002/cncr.27783. [DOI] [PubMed] [Google Scholar]
- 147.Meco D. Preclinical evaluation of the novel 7-substituted camptothecin Namitecan (ST1968) in paediatric tumour models. Cancer Chemother. Pharmacol. 2012;70(6):811–822. doi: 10.1007/s00280-012-1973-0. [DOI] [PubMed] [Google Scholar]
- 148.Zucchetti M. Antitumor activity and pharmacokinetics of oral gimatecan on pediatric cancer xenografts. Cancer Chemother. Pharmacol. 2010;66(4):635–641. doi: 10.1007/s00280-009-1201-8. [DOI] [PubMed] [Google Scholar]
- 149.Di Francesco A.M. The novel lipophilic camptothecin analogue gimatecan is very active in vitro in human neuroblastoma: a comparative study with SN38 and topotecan. Biochem. Pharmacol. 2005;70(8):1125–1136. doi: 10.1016/j.bcp.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 150.Pastorino F. Tumor regression and curability of preclinical neuroblastoma models by PEGylated SN38 (EZN-2208), a novel topoisomerase I inhibitor. Clin. Cancer Res. 2010;16(19):4809–4821. doi: 10.1158/1078-0432.CCR-10-1354. [DOI] [PubMed] [Google Scholar]
- 151.Kakodkar N.C. The quinoxaline anti-tumor agent (R +)XK469 inhibits neuroblastoma tumor growth. Pediatr. Blood Cancer. 2011;56(1):164–167. doi: 10.1002/pbc.22639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gamble L.D. Polyamine pathway inhibition as a novel therapeutic approach to treating neuroblastoma. Front. Oncol. 2012;2:162. doi: 10.3389/fonc.2012.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hogarty M.D. ODC1 is a critical determinant of MYCN oncogenesis and a therapeutic target in neuroblastoma. Cancer Res. 2008;68(23):9735–9745. doi: 10.1158/0008-5472.CAN-07-6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bagatell R. Hsp90 inhibitors deplete key anti-apoptotic proteins in pediatric solid tumor cells and demonstrate synergistic anticancer activity with cisplatin. Int. J. Cancer. 2005;113(2):179–188. doi: 10.1002/ijc.20611. [DOI] [PubMed] [Google Scholar]
- 155.Chinn D.C. Anti-tumor activity of the HSP90 inhibitor SNX-2112 in pediatric cancer cell lines. Pediatr. Blood Cancer. 2012;58(6):885–890. doi: 10.1002/pbc.23270. [DOI] [PubMed] [Google Scholar]
- 156.Castel V., Segura V., Berlanga P. Emerging drugs for neuroblastoma. Expert Opin. Emerg. Drugs. 2013;18(2):155–171. doi: 10.1517/14728214.2013.796927. [DOI] [PubMed] [Google Scholar]
- 157.Fleming A.J., Chi S.N. Brain tumors in children. Curr. Probl. Pediatr. Adolesc. Health Care. 2012;42(4):80–103. doi: 10.1016/j.cppeds.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 158.Heath J.A., Zacharoulis S., Kieran M.W. Pediatric neuro-oncology: current status and future directions. Asia Pac. J. Clin. Oncol. 2012;8(3):223–231. doi: 10.1111/j.1743-7563.2012.01558.x. [DOI] [PubMed] [Google Scholar]
- 159.Mutter N., Stupp R. Temozolomide: a milestone in neuro-oncology and beyond? Expert. Rev. Anticancer. Ther. 2006;6(8):1187–1204. doi: 10.1586/14737140.6.8.1187. [DOI] [PubMed] [Google Scholar]
- 160.Hegi M.E. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 161.Stupp R. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J. Clin. Oncol. 2002;20(5):1375–1382. doi: 10.1200/JCO.2002.20.5.1375. [DOI] [PubMed] [Google Scholar]
- 162.Gururangan S. Temozolomide in children with progressive low-grade glioma. Neuro Oncol. 2007;9(2):161–168. doi: 10.1215/15228517-2006-030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cohen K.J. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children's Oncology Group. Neuro Oncol. 2011;13(4):410–416. doi: 10.1093/neuonc/noq205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Broniscer A. Phase I trial of single-dose temozolomide and continuous administration of o6-benzylguanine in children with brain tumors: a pediatric brain tumor consortium report. Clin. Cancer Res. 2007;13(22 Pt 1):6712–6718. doi: 10.1158/1078-0432.CCR-07-1016. [DOI] [PubMed] [Google Scholar]
- 165.Warren K.E. A phase II study of O6-benzylguanine and temozolomide in pediatric patients with recurrent or progressive high-grade gliomas and brainstem gliomas: a Pediatric Brain Tumor Consortium study. J. Neurooncol. 2012;106(3):643–649. doi: 10.1007/s11060-011-0709-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Adair J.E. Extended survival of glioblastoma patients after chemoprotective HSC gene therapy. Sci. Transl. Med. 2012;4(133):133ra57. doi: 10.1126/scitranslmed.3003425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Daniel R.A. Central nervous system penetration and enhancement of temozolomide activity in childhood medulloblastoma models by poly(ADP-ribose) polymerase inhibitor AG-014699. Br. J. Cancer. 2010;103(10):1588–1596. doi: 10.1038/sj.bjc.6605946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.van Vuurden D.G. PARP inhibition sensitizes childhood high grade glioma, medulloblastoma and ependymoma to radiation. Oncotarget. 2011;2(12):984–996. doi: 10.18632/oncotarget.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Northcott P.A. Molecular subgroups of medulloblastoma. Expert. Rev. Neurother. 2012;12(7):871–884. doi: 10.1586/ern.12.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Buonamici S. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci. Transl. Med. 2010;2(51):51ra70. doi: 10.1126/scitranslmed.3001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Romer J.T. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell. 2004;6(3):229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 172.Sasai K. Medulloblastomas derived from Cxcr6 mutant mice respond to treatment with a smoothened inhibitor. Cancer Res. 2007;67(8):3871–3877. doi: 10.1158/0008-5472.CAN-07-0493. [DOI] [PubMed] [Google Scholar]
- 173.De Smaele E., Ferretti E., Gulino A. Vismodegib, a small-molecule inhibitor of the hedgehog pathway for the treatment of advanced cancers. Curr. Opin. Investig. Drugs. 2010;11(6):707–718. [PubMed] [Google Scholar]
- 174.Wong H. Pharmacokinetic–pharmacodynamic analysis of vismodegib in preclinical models of mutational and ligand-dependent Hedgehog pathway activation. Clin. Cancer Res. 2011;17(14):4682–4692. doi: 10.1158/1078-0432.CCR-11-0975. [DOI] [PubMed] [Google Scholar]
- 175.Rudin C.M. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N. Engl. J. Med. 2009;361(12):1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ferruzzi P. In vitro and in vivo characterization of a novel Hedgehog signaling antagonist in human glioblastoma cell lines. Int. J. Cancer. 2012;131(2):E33–E44. doi: 10.1002/ijc.27349. [DOI] [PubMed] [Google Scholar]
- 177.Rohner A. Effective targeting of Hedgehog signaling in a medulloblastoma model with PF-5274857, a potent and selective Smoothened antagonist that penetrates the blood–brain barrier. Mol. Cancer Ther. 2012;11(1):57–65. doi: 10.1158/1535-7163.MCT-11-0691. [DOI] [PubMed] [Google Scholar]
- 178.Yalon M. A feasibility and efficacy study of rapamycin and erlotinib for recurrent pediatric low-grade glioma (LGG) Pediatr. Blood Cancer. 2013;60(1):71–76. doi: 10.1002/pbc.24142. [DOI] [PubMed] [Google Scholar]
- 179.Krueger D.A. Everolimus long-term safety and efficacy in subependymal giant cell astrocytoma. Neurology. 2013;80(6):574–580. doi: 10.1212/WNL.0b013e3182815428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Becher O.J. Preclinical evaluation of radiation and perifosine in a genetically and histologically accurate model of brainstem glioma. Cancer Res. 2010;70(6):2548–2557. doi: 10.1158/0008-5472.CAN-09-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dougherty M.J. Activating mutations in BRAF characterize a spectrum of pediatric low-grade gliomas. Neuro Oncol. 2010;12(7):621–630. doi: 10.1093/neuonc/noq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kolb E.A. Initial testing (stage 1) of AZD6244 (ARRY-142886) by the Pediatric Preclinical Testing Program. Pediatr. Blood Cancer. 2010;55(4):668–677. doi: 10.1002/pbc.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Widemann B.C. Phase I trial and pharmacokinetic study of the farnesyltransferase inhibitor tipifarnib in children with refractory solid tumors or neurofibromatosis type I and plexiform neurofibromas. J. Clin. Oncol. 2006;24(3):507–516. doi: 10.1200/JCO.2005.03.8638. [DOI] [PubMed] [Google Scholar]
- 184.Haas-Kogan D.A. Phase II trial of tipifarnib and radiation in children with newly diagnosed diffuse intrinsic pontine gliomas. Neuro Oncol. 2011;13(3):298–306. doi: 10.1093/neuonc/noq202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Trippett T.M. Phase I and pharmacokinetic study of cetuximab and irinotecan in children with refractory solid tumors: a study of the pediatric oncology experimental therapeutic investigators' consortium. J. Clin. Oncol. 2009;27(30):5102–5108. doi: 10.1200/JCO.2008.20.8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rajappa P. Super-selective basilar artery infusion of bevacizumab and cetuximab for multiply recurrent pediatric ependymoma. Interv. Neuroradiol. 2011;17(4):459–465. doi: 10.1177/159101991101700410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cabanas R. Treatment of children with high grade glioma with nimotuzumab: a 5-y institutional experience. MAbs. 2013;5(2):202–207. doi: 10.4161/mabs.22970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Vera D.R. Preparation and preclinical evaluation of 177Lu-nimotuzumab targeting epidermal growth factor receptor overexpressing tumors. Nucl. Med. Biol. 2012;39(1):3–13. doi: 10.1016/j.nucmedbio.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 189.Vredenburgh J.J. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin. Cancer Res. 2007;13(4):1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 190.Narayana A. Bevacizumab in recurrent high-grade pediatric gliomas. Neuro Oncol. 2010;12(9):985–990. doi: 10.1093/neuonc/noq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gururangan S. Lack of efficacy of bevacizumab + irinotecan in cases of pediatric recurrent ependymoma—a Pediatric Brain Tumor Consortium study. Neuro Oncol. 2012;14(11):1404–1412. doi: 10.1093/neuonc/nos213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Packer R.J. Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr. Blood Cancer. 2009;52(7):791–795. doi: 10.1002/pbc.21935. [DOI] [PubMed] [Google Scholar]
- 193.Broniscer A. Phase I and pharmacokinetic studies of erlotinib administered concurrently with radiotherapy for children, adolescents, and young adults with high-grade glioma. Clin. Cancer Res. 2009;15(2):701–707. doi: 10.1158/1078-0432.CCR-08-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Geoerger B. Innovative Therapies for Children with Cancer pediatric phase I study of erlotinib in brainstem glioma and relapsing/refractory brain tumors. Neuro Oncol. 2011;13(1):109–118. doi: 10.1093/neuonc/noq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Meco D. Antitumor effect in medulloblastoma cells by gefitinib: ectopic HER2 overexpression enhances gefitinib effects in vivo. Neuro Oncol. 2009;11(3):250–259. doi: 10.1215/15228517-2008-095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Freeman B.B., III Evaluation of gefitinib for treatment of refractory solid tumors and central nervous system malignancies in pediatric patients. Cancer Invest. 2006;24(3):310–317. doi: 10.1080/07357900600632058. [DOI] [PubMed] [Google Scholar]
- 197.Pollack I.F. A phase II study of gefitinib and irradiation in children with newly diagnosed brainstem gliomas: a report from the Pediatric Brain Tumor Consortium. Neuro Oncol. 2011;13(3):290–297. doi: 10.1093/neuonc/noq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fouladi M. Phase I trial of lapatinib in children with refractory CNS malignancies: a Pediatric Brain Tumor Consortium study. J. Clin. Oncol. 2010;28(27):4221–4227. doi: 10.1200/JCO.2010.28.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Singh A. Profiling pathway-specific novel therapeutics in preclinical assessment for central nervous system atypical teratoid rhabdoid tumors (CNS ATRT): favorable activity of targeting EGFR–ErbB2 signaling with lapatinib. Mol. Oncol. 2013;7(3):497–512. doi: 10.1016/j.molonc.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Servidei T. Effects of epidermal growth factor receptor blockade on ependymoma stem cells in vitro and in orthotopic mouse models. Int. J. Cancer. 2012;131(5):E791–E803. doi: 10.1002/ijc.27377. [DOI] [PubMed] [Google Scholar]
- 201.Meco D. Dual inhibitor AEE788 reduces tumor growth in preclinical models of medulloblastoma. Transl. Oncol. 2010;3(5):326–335. doi: 10.1593/tlo.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sikkema A.H. Kinome profiling in pediatric brain tumors as a new approach for target discovery. Cancer Res. 2009;69(14):5987–5995. doi: 10.1158/0008-5472.CAN-08-3660. [DOI] [PubMed] [Google Scholar]
- 203.Gilbertson R.J. Mutational analysis of PDGFR-RAS/MAPK pathway activation in childhood medulloblastoma. Eur. J. Cancer. 2006;42(5):646–649. doi: 10.1016/j.ejca.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 204.Dubois S.G. Phase I and pharmacokinetic study of sunitinib in pediatric patients with refractory solid tumors: a Children's Oncology Group study. Clin. Cancer Res. 2011;17(15):5113–5122. doi: 10.1158/1078-0432.CCR-11-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Broniscer A. Phase I study of vandetanib during and after radiotherapy in children with diffuse intrinsic pontine glioma. J. Clin. Oncol. 2010;28(31):4762–4768. doi: 10.1200/JCO.2010.30.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Broniscer A. Phase I trial, pharmacokinetics, and pharmacodynamics of vandetanib and dasatinib in children with newly diagnosed diffuse intrinsic pontine glioma. Clin. Cancer Res. 2013;19(11):3050–3058. doi: 10.1158/1078-0432.CCR-13-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Faria C.C. AACR 103rd Annual Meeting, Abstract 847: Foretinib, a multi-kinase inhibitor of cMET and PDGFRα, in the treatment of disseminated medulloblastoma. Cancer Res. 2012;72(8 Suppl. 847) [Google Scholar]
- 208.Harris P.S. Polo-like kinase 1 (PLK1) inhibition suppresses cell growth and enhances radiation sensitivity in medulloblastoma cells. BMC Cancer. 2012;12:80. doi: 10.1186/1471-2407-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Stewart C.F. Results of a phase II upfront window of pharmacokinetically guided topotecan in high-risk medulloblastoma and supratentorial primitive neuroectodermal tumor. J. Clin. Oncol. 2004;22(16):3357–3365. doi: 10.1200/JCO.2004.10.103. [DOI] [PubMed] [Google Scholar]
- 210.Gilheeney S.W. Thiotepa/topotecan/carboplatin with autologous stem cell rescue in recurrent/refractory/poor prognosis pediatric malignancies of the central nervous system. Pediatr. Blood Cancer. 2010;54(4):591–595. doi: 10.1002/pbc.22347. [DOI] [PubMed] [Google Scholar]
- 211.Turner C.D. Phase II study of irinotecan (CPT-11) in children with high-risk malignant brain tumors: the Duke experience. Neuro Oncol. 2002;4(2):102–108. doi: 10.1093/neuonc/4.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bomgaars L.R. Phase II trial of irinotecan in children with refractory solid tumors: a Children's Oncology Group study. J. Clin. Oncol. 2007;25(29):4622–4627. doi: 10.1200/JCO.2007.11.6103. [DOI] [PubMed] [Google Scholar]
- 213.Aguilera D. Response to bevacizumab, irinotecan, and temozolomide in children with relapsed medulloblastoma: a multi-institutional experience. Childs Nerv. Syst. 2013;29(4):589–596. doi: 10.1007/s00381-012-2013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ruggiero A. Phase I study of temozolomide combined with oral etoposide in children with recurrent or progressive medulloblastoma. Eur. J. Cancer. 2010;46(16):2943–2949. doi: 10.1016/j.ejca.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 215.Kellie S.J. Activity of postoperative carboplatin, etoposide, and high-dose methotrexate in pediatric CNS embryonal tumors: results of a phase II study in newly diagnosed children. Med. Pediatr. Oncol. 2002;39(3):168–174. doi: 10.1002/mpo.10137. [DOI] [PubMed] [Google Scholar]
- 216.Houghton P.J. Testing of the topoisomerase 1 inhibitor Genz-644282 by the pediatric preclinical testing program. Pediatr. Blood Cancer. 2012;58(2):200–209. doi: 10.1002/pbc.23016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Su J.M. Phase 1 study of valproic acid in pediatric patients with refractory solid or CNS tumors: a children's oncology group report. Clin. Cancer Res. 2011;17(3):589–597. doi: 10.1158/1078-0432.CCR-10-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Knipstein J.A. Histone deacetylase inhibition decreases proliferation and potentiates the effect of ionizing radiation in atypical teratoid/rhabdoid tumor cells. Neuro Oncol. 2012;14(2):175–183. doi: 10.1093/neuonc/nor208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Milde T. HD-MB03 is a novel Group 3 medulloblastoma model demonstrating sensitivity to histone deacetylase inhibitor treatment. J. Neurooncol. 2012;110(3):335–348. doi: 10.1007/s11060-012-0978-1. [DOI] [PubMed] [Google Scholar]
- 220.Hummel T.R. A pediatric phase 1 trial of vorinostat and temozolomide in relapsed or refractory primary brain or spinal cord tumors: a Children's Oncology Group phase 1 consortium study. Pediatr. Blood Cancer. 2013;60(9):1452–1457. doi: 10.1002/pbc.24541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Buczkowicz P. Aurora kinase B is a potential therapeutic target in pediatric diffuse intrinsic pontine glioma. Brain Pathol. 2013;23(3):244–253. doi: 10.1111/j.1750-3639.2012.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Miekus K. 17AEP-GA, an HSP90 antagonist, is a potent inhibitor of glioblastoma cell proliferation, survival, migration and invasion. Oncol. Rep. 2012;28(5):1903–1909. doi: 10.3892/or.2012.1996. [DOI] [PubMed] [Google Scholar]
- 223.Gaspar N. Mechanistic evaluation of the novel HSP90 inhibitor NVP-AUY922 in adult and pediatric glioblastoma. Mol. Cancer Ther. 2010;9(5):1219–1233. doi: 10.1158/1535-7163.MCT-09-0683. (210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Weigel B.J. A phase I study of 17-allylaminogeldanamycin in relapsed/refractory pediatric patients with solid tumors: a Children's Oncology Group study. Clin. Cancer Res. 2007;13(6):1789–1793. doi: 10.1158/1078-0432.CCR-06-2270. [DOI] [PubMed] [Google Scholar]
- 225.Nageswara Rao A.A. Biologically targeted therapeutics in pediatric brain tumors. Pediatr. Neurol. 2012;46(4):203–211. doi: 10.1016/j.pediatrneurol.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Anderson J.L. Pediatric sarcomas: translating molecular pathogenesis of disease to novel therapeutic possibilities. Pediatr. Res. 2012;72(2):112–121. doi: 10.1038/pr.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Martin J.W., Squire J.A., Zielenska M. The genetics of osteosarcoma. Sarcoma. 2012;2012:627254. doi: 10.1155/2012/627254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wang C. Childhood rhabdomyosarcoma: recent advances and prospective views. J. Dent. Res. 2012;91(4):341–350. doi: 10.1177/0022034511421490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ludwig J.A. Ewing sarcoma: historical perspectives, current state-of-the-art, and opportunities for targeted therapy in the future. Curr. Opin. Oncol. 2008;20(4):412–418. doi: 10.1097/CCO.0b013e328303ba1d. [DOI] [PubMed] [Google Scholar]
- 230.Prieur A. EWS/FLI-1 silencing and gene profiling of Ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3. Mol. Cell. Biol. 2004;24(16):7275–7283. doi: 10.1128/MCB.24.16.7275-7283.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wakahara K. EWS-Fli1 up-regulates expression of the Aurora A and Aurora B kinases. Mol. Cancer Res. 2008;6(12):1937–1945. doi: 10.1158/1541-7786.MCR-08-0054. [DOI] [PubMed] [Google Scholar]
- 232.Wager M.J., Maki R.G. Type 1 insulin growth factor receptor targeted therapies in pediatric cancer. Front. Oncol. 2013;3:9. doi: 10.3389/fonc.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Carboni J.M. BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol. Cancer Ther. 2009;8(12):3341–3349. doi: 10.1158/1535-7163.MCT-09-0499. [DOI] [PubMed] [Google Scholar]
- 234.Scotlandi K. Antitumor activity of the insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 in musculoskeletal tumors. Cancer Res. 2005;65(9):3868–3876. doi: 10.1158/0008-5472.CAN-04-3192. [DOI] [PubMed] [Google Scholar]
- 235.Tolcher A.W. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J. Clin. Oncol. 2009;27(34):5800–5807. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 236.Pappo A.S. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research through Collaboration study. J. Clin. Oncol. 2011;29(34):4541–4547. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Juergens H. Preliminary efficacy of the anti-insulin-like growth factor type 1 receptor antibody figitumumab in patients with refractory Ewing sarcoma. J. Clin. Oncol. 2011;29(34):4534–4540. doi: 10.1200/JCO.2010.33.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Malempati S. Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: a report from the Children's Oncology Group. J. Clin. Oncol. 2012;30(3):256–262. doi: 10.1200/JCO.2011.37.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Winter G.E. An integrated chemical biology approach identifies specific vulnerability of Ewing's sarcoma to combined inhibition of Aurora kinases A and B. Mol. Cancer Ther. 2011;10(10):1846–1856. doi: 10.1158/1535-7163.MCT-11-0100. [DOI] [PubMed] [Google Scholar]
- 240.Merchant M.S. Potential use of imatinib in Ewing's Sarcoma: evidence for in vitro and in vivo activity. J. Natl. Cancer Inst. 2002;94(22):1673–1679. doi: 10.1093/jnci/94.22.1673. [DOI] [PubMed] [Google Scholar]
- 241.Bond M. A phase II study of imatinib mesylate in children with refractory or relapsed solid tumors: a Children's Oncology Group study. Pediatr. Blood Cancer. 2008;50(2):254–258. doi: 10.1002/pbc.21132. [DOI] [PubMed] [Google Scholar]
- 242.Chao J. Phase II clinical trial of imatinib mesylate in therapy of KIT and/or PDGFRalpha-expressing Ewing sarcoma family of tumors and desmoplastic small round cell tumors. Anticancer Res. 2010;30(2):547–552. [PubMed] [Google Scholar]
- 243.Keir S.T. Initial testing of the multitargeted kinase inhibitor pazopanib by the Pediatric Preclinical Testing Program. Pediatr. Blood Cancer. 2012;59(3):586–588. doi: 10.1002/pbc.24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Andersson M.K., Aman P. Proliferation of Ewing sarcoma cell lines is suppressed by the receptor tyrosine kinase inhibitors gefitinib and vandetanib. Cancer Cell Int. 2008;8:1. doi: 10.1186/1475-2867-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Fleuren E.D. Expression and clinical relevance of MET and ALK in Ewing sarcomas. Int. J. Cancer. 2013;133(2):427–436. doi: 10.1002/ijc.28047. [DOI] [PubMed] [Google Scholar]
- 246.Keshelava N. Initial testing (stage 1) of vorinostat (SAHA) by the pediatric preclinical testing program. Pediatr. Blood Cancer. 2009;53(3):505–508. doi: 10.1002/pbc.21988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kang M.H. Initial testing (Stage 1) of AT13387, an HSP90 inhibitor, by the pediatric preclinical testing program. Pediatr. Blood Cancer. 2012;59(1):185–188. doi: 10.1002/pbc.23154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Milano G.M. High histologic and overall response to dose intensification of ifosfamide, carboplatin, and etoposide with cyclophosphamide, doxorubicin, and vincristine in patients with high-risk Ewing sarcoma family tumors: the Bambino Gesu Children's Hospital experience. Cancer. 2006;106(8):1838–1845. doi: 10.1002/cncr.21780. [DOI] [PubMed] [Google Scholar]
- 249.Casey D.A. Irinotecan and temozolomide for Ewing sarcoma: the Memorial Sloan–Kettering experience. Pediatr. Blood Cancer. 2009;53(6):1029–1034. doi: 10.1002/pbc.22206. [DOI] [PubMed] [Google Scholar]
- 250.Farhat R. Cyclophosphamide and topotecan as first-line salvage therapy in patients with relapsed Ewing sarcoma at a single institution. J. Pediatr. Hematol. Oncol. 2012;35(5):356–360. doi: 10.1097/MPH.0b013e318270a343. [DOI] [PubMed] [Google Scholar]
- 251.Kebudi R. A modified protocol with vincristine, topotecan, and cyclophosphamide for recurrent/progressive Ewing sarcoma family tumors. Pediatr. Hematol. Oncol. 2013;30(3):170–177. doi: 10.3109/08880018.2013.767868. [DOI] [PubMed] [Google Scholar]
- 252.Cassinelli G. The curative efficacy of namitecan (ST1968) in preclinical models of pediatric sarcoma is associated with antiangiogenic effects. Biochem. Pharmacol. 2012;84(2):163–171. doi: 10.1016/j.bcp.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 253.Fleuren E.D.G. Targeting receptor tyrosine kinases in osteosarcoma and Ewing sarcoma: current hurdles and future perspectives. Biochim. Biophys. Acta. 2014;1845(2):266–276. doi: 10.1016/j.bbcan.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 254.Gallego Melcon S., Sanchez de Toledo Codina J. Molecular biology of rhabdomyosarcoma. Clin. Transl. Oncol. 2007;9(7):415–419. doi: 10.1007/s12094-007-0079-3. [DOI] [PubMed] [Google Scholar]
- 255.Bernasconi M. Induction of apoptosis in rhabdomyosarcoma cells through down-regulation of PAX proteins. Proc. Natl. Acad. Sci. U. S. A. 1996;93(23):13164–13169. doi: 10.1073/pnas.93.23.13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Fredericks W.J., Ayyanathan K., Rauscher F.J., III Regulating the neoplastic phenotype using engineered transcriptional repressors. Cancer Lett. 2001;162(Suppl.):S23–S32. doi: 10.1016/s0304-3835(00)00649-2. [DOI] [PubMed] [Google Scholar]
- 257.Rodeberg D.A. Lack of effective T-lymphocyte response to the PAX3/FKHR translocation area in alveolar rhabdomyosarcoma. Cancer Immunol. Immunother. 2005;54(6):526–534. doi: 10.1007/s00262-004-0625-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Crouch G.D., Helman L.J. All-trans-retinoic acid inhibits the growth of human rhabdomyosarcoma cell lines. Cancer Res. 1991;51(18):4882–4887. [PubMed] [Google Scholar]
- 259.Bouche M. TPA-induced differentiation of human rhabdomyosarcoma cells: expression of the myogenic regulatory factors. Exp. Cell Res. 1993;208(1):209–217. doi: 10.1006/excr.1993.1239. [DOI] [PubMed] [Google Scholar]
- 260.Herrero Martin D. A. Boro, and B.W. Schafer, Cell-based small-molecule compound screen identifies fenretinide as potential therapeutic for translocation-positive rhabdomyosarcoma. PLoS One. 2013;8(1):e55072. doi: 10.1371/journal.pone.0055072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sharp R. Synergism between INK4a/ARF inactivation and aberrant HGF/SF signaling in rhabdomyosarcomagenesis. Nat. Med. 2002;8(11):1276–1280. doi: 10.1038/nm787. [DOI] [PubMed] [Google Scholar]
- 262.Taulli R. Validation of met as a therapeutic target in alveolar and embryonal rhabdomyosarcoma. Cancer Res. 2006;66(9):4742–4749. doi: 10.1158/0008-5472.CAN-05-4292. [DOI] [PubMed] [Google Scholar]
- 263.Hou J. Inhibition of phosphorylated c-Met in rhabdomyosarcoma cell lines by a small molecule inhibitor SU11274. J. Transl. Med. 2011;9:64. doi: 10.1186/1479-5876-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Epstein J.A. Tumor-specific PAX3-FKHR transcription factor, but not PAX3, activates the platelet-derived growth factor alpha receptor. Mol. Cell. Biol. 1998;18(7):4118–4130. doi: 10.1128/mcb.18.7.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Taniguchi E. PDGFR-A is a therapeutic target in alveolar rhabdomyosarcoma. Oncogene. 2008;27(51):6550–6560. doi: 10.1038/onc.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Ehnman M. Distinct effects of ligand-induced PDGFRalpha and PDGFRbeta signaling in the human rhabdomyosarcoma tumor cell and stroma cell compartments. Cancer Res. 2013;73(7):2139–2149. doi: 10.1158/0008-5472.CAN-12-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kalebic T., Tsokos M., Helman L.J. In vivo treatment with antibody against IGF-1 receptor suppresses growth of human rhabdomyosarcoma and down-regulates p34cdc2. Cancer Res. 1994;54(21):5531–5534. [PubMed] [Google Scholar]
- 268.Houghton P.J. Initial testing of a monoclonal antibody (IMC-A12) against IGF-1R by the Pediatric Preclinical Testing Program. Pediatr. Blood Cancer. 2010;54(7):921–926. doi: 10.1002/pbc.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Mayeenuddin L.H. Insulin-like growth factor 1 receptor antibody induces rhabdomyosarcoma cell death via a process involving AKT and Bcl-x(L) Oncogene. 2010;29(48):6367–6377. doi: 10.1038/onc.2010.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wan X. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26(13):1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 271.Marampon F., Ciccarelli C., Zani B.M. Down-regulation of c-Myc following MEK/ERK inhibition halts the expression of malignant phenotype in rhabdomyosarcoma and in non muscle-derived human tumors. Mol. Cancer. 2006;5:31. doi: 10.1186/1476-4598-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Marampon F. MEK/ERK inhibitor U0126 affects in vitro and in vivo growth of embryonal rhabdomyosarcoma. Mol. Cancer Ther. 2009;8(3):543–551. doi: 10.1158/1535-7163.MCT-08-0570. [DOI] [PubMed] [Google Scholar]
- 273.van Gaal J.C. Anaplastic lymphoma kinase aberrations in rhabdomyosarcoma: clinical and prognostic implications. J. Clin. Oncol. 2012;30(3):308–315. doi: 10.1200/JCO.2011.37.8588. [DOI] [PubMed] [Google Scholar]
- 274.van Gaal J.C. Simultaneous targeting of insulin-like growth factor-1 receptor and anaplastic lymphoma kinase in embryonal and alveolar rhabdomyosarcoma: a rational choice. Eur. J. Cancer. 2013;49(16):3462–3470. doi: 10.1016/j.ejca.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 275.Abraham J. Preclinical testing of erlotinib in a transgenic alveolar rhabdomyosarcoma mouse model. Sarcoma. 2011;2011:130484. doi: 10.1155/2011/130484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Smith M.A. Stage 1 testing and pharmacodynamic evaluation of the HSP90 inhibitor alvespimycin (17-DMAG, KOS-1022) by the pediatric preclinical testing program. Pediatr. Blood Cancer. 2008;51(1):34–41. doi: 10.1002/pbc.21508. [DOI] [PubMed] [Google Scholar]
- 277.Lukasiewicz E. High anti tumor activity against rhabdomyosarcoma cells and low normal cells cytotoxicity of heat shock protein 90 inhibitors, with special emphasis on 17-[2-(pyrrolidin-1-yl)ethyl]-aminno-17-demethoxygeldanamycin. J. Physiol. Pharmacol. 2009;60(3):161–166. [PubMed] [Google Scholar]
- 278.Van Winkle P. Ifosfamide, carboplatin, and etoposide (ICE) reinduction chemotherapy in a large cohort of children and adolescents with recurrent/refractory sarcoma: the Children's Cancer Group (CCG) experience. Pediatr. Blood Cancer. 2005;44(4):338–347. doi: 10.1002/pbc.20227. [DOI] [PubMed] [Google Scholar]
- 279.Klingebiel T. Treatment of children with metastatic soft tissue sarcoma with oral maintenance compared to high dose chemotherapy: report of the HD CWS-96 trial. Pediatr. Blood Cancer. 2008;50(4):739–745. doi: 10.1002/pbc.21494. [DOI] [PubMed] [Google Scholar]
- 280.Vassal G. Phase II trial of irinotecan in children with relapsed or refractory rhabdomyosarcoma: a joint study of the French Society of Pediatric Oncology and the United Kingdom Children's Cancer Study Group. J. Clin. Oncol. 2007;25(4):356–361. doi: 10.1200/JCO.2006.06.1960. [DOI] [PubMed] [Google Scholar]
- 281.Pappo A.S. Two consecutive phase II window trials of irinotecan alone or in combination with vincristine for the treatment of metastatic rhabdomyosarcoma: the Children's Oncology Group. J. Clin. Oncol. 2007;25(4):362–369. doi: 10.1200/JCO.2006.07.1720. [DOI] [PubMed] [Google Scholar]
- 282.Meazza C. Efficacy of topotecan plus vincristine and doxorubicin in children with recurrent/refractory rhabdomyosarcoma. Med. Oncol. 2009;26(1):67–72. doi: 10.1007/s12032-008-9085-8. [DOI] [PubMed] [Google Scholar]
- 283.Hawkins D.S. Children's Oncology Group's 2013 blueprint for research: soft tissue sarcomas. Pediatr. Blood Cancer. 2013;60(6):1001–1008. doi: 10.1002/pbc.24435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Takenaka S. Downregulation of SS18–SSX1 expression in synovial sarcoma by small interfering RNA enhances the focal adhesion pathway and inhibits anchorage-independent growth in vitro and tumor growth in vivo. Int. J. Oncol. 2010;36(4):823–831. doi: 10.3892/ijo_00000559. [DOI] [PubMed] [Google Scholar]
- 285.Allander S.V. Expression profiling of synovial sarcoma by cDNA microarrays: association of ERBB2, IGFBP2, and ELF3 with epithelial differentiation. Am. J. Pathol. 2002;161(5):1587–1595. doi: 10.1016/S0002-9440(10)64437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Nielsen T.O. Tissue microarray validation of epidermal growth factor receptor and SALL2 in synovial sarcoma with comparison to tumors of similar histology. Am. J. Pathol. 2003;163(4):1449–1456. doi: 10.1016/S0002-9440(10)63502-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Krskova L. Molecular and immunohistochemical analyses of BCL2, KI-67, and cyclin D1 expression in synovial sarcoma. Cancer Genet. Cytogenet. 2009;193(1):1–8. doi: 10.1016/j.cancergencyto.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 288.Friedrichs N. Insulin-like growth factor-1 receptor acts as a growth regulator in synovial sarcoma. J. Pathol. 2008;216(4):428–439. doi: 10.1002/path.2438. [DOI] [PubMed] [Google Scholar]
- 289.Ray-Coquard I. A phase II study of gefitinib for patients with advanced HER-1 expressing synovial sarcoma refractory to doxorubicin-containing regimens. Oncologist. 2008;13(4):467–473. doi: 10.1634/theoncologist.2008-0065. [DOI] [PubMed] [Google Scholar]
- 290.Joyner D.E. G3139 antisense oligonucleotide directed against antiapoptotic Bcl-2 enhances doxorubicin cytotoxicity in the FU-SY-1 synovial sarcoma cell line. J. Orthop. Res. 2006;24(3):474–480. doi: 10.1002/jor.20087. [DOI] [PubMed] [Google Scholar]
- 291.Rheingold S.R. Phase I trial of G3139, a bcl-2 antisense oligonucleotide, combined with doxorubicin and cyclophosphamide in children with relapsed solid tumors: a Children's Oncology Group Study. J. Clin. Oncol. 2007;25(12):1512–1518. doi: 10.1200/JCO.2006.09.5125. [DOI] [PubMed] [Google Scholar]
- 292.Geryk-Hall M., Hughes D.P. Critical signaling pathways in bone sarcoma: candidates for therapeutic interventions. Curr. Oncol. Rep. 2009;11(6):446–453. doi: 10.1007/s11912-009-0061-z. [DOI] [PubMed] [Google Scholar]
- 293.Messerschmitt P.J. Specific tyrosine kinase inhibitors regulate human osteosarcoma cells in vitro. Clin. Orthop. Relat. Res. 2008;466(9):2168–2175. doi: 10.1007/s11999-008-0338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kolb E.A. Initial testing (stage 1) of a monoclonal antibody (SCH 717454) against the IGF-1 receptor by the pediatric preclinical testing program. Pediatr. Blood Cancer. 2008;50(6):1190–1197. doi: 10.1002/pbc.21450. [DOI] [PubMed] [Google Scholar]
- 295.Wang Y. A fully human insulin-like growth factor-I receptor antibody SCH 717454 (robatumumab) has antitumor activity as a single agent and in combination with cytotoxics in pediatric tumor xenografts. Mol. Cancer Ther. 2010;9(2):410–418. doi: 10.1158/1535-7163.MCT-09-0555. [DOI] [PubMed] [Google Scholar]
- 296.Bagatell R. Pharmacokinetically guided phase 1 trial of the IGF-1 receptor antagonist RG1507 in children with recurrent or refractory solid tumors. Clin. Cancer Res. 2011;17(3):611–619. doi: 10.1158/1078-0432.CCR-10-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Lee J.A. Epidermal growth factor receptor: is it a feasible target for the treatment of osteosarcoma? Cancer Res. Treat. 2012;44(3):202–209. doi: 10.4143/crt.2012.44.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Somers G.R. HER2 amplification and overexpression is not present in pediatric osteosarcoma: a tissue microarray study. Pediatr. Dev. Pathol. 2005;8(5):525–532. doi: 10.1007/s10024-005-0044-5. [DOI] [PubMed] [Google Scholar]
- 299.Yalçin B. C-erbB-2 expression and prognostic significance in osteosarcoma. Pediatr. Blood Cancer. 2008;51(2):222–227. doi: 10.1002/pbc.21576. [DOI] [PubMed] [Google Scholar]
- 300.Ebb D. Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: a report from the Children's Oncology Group. J. Clin. Oncol. 2012;30(20):2545–2551. doi: 10.1200/JCO.2011.37.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kubo T. Platelet-derived growth factor receptor as a prognostic marker and a therapeutic target for imatinib mesylate therapy in osteosarcoma. Cancer. 2008;112(10):2119–2129. doi: 10.1002/cncr.23437. [DOI] [PubMed] [Google Scholar]
- 302.McGary E.C. Inhibition of platelet-derived growth factor-mediated proliferation of osteosarcoma cells by the novel tyrosine kinase inhibitor STI571. Clin. Cancer Res. 2002;8(11):3584–3591. [PubMed] [Google Scholar]
- 303.Patane S. MET overexpression turns human primary osteoblasts into osteosarcomas. Cancer Res. 2006;66(9):4750–4757. doi: 10.1158/0008-5472.CAN-05-4422. [DOI] [PubMed] [Google Scholar]
- 304.Coltella N. Role of the MET/HGF receptor in proliferation and invasive behavior of osteosarcoma. FASEB J. 2003;17(9):1162–1164. doi: 10.1096/fj.02-0576fje. [DOI] [PubMed] [Google Scholar]
- 305.Yamaguchi U. Functional genome screen for therapeutic targets of osteosarcoma. Cancer Sci. 2009;100(12):2268–2274. doi: 10.1111/j.1349-7006.2009.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Engin F. Notch signalling contributes to the pathogenesis of human osteosarcomas. Hum. Mol. Genet. 2009;18(8):1464–1470. doi: 10.1093/hmg/ddp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Kolb E.A. Initial testing (stage 1) by the pediatric preclinical testing program of RO4929097, a gamma-secretase inhibitor targeting notch signaling. Pediatr. Blood Cancer. 2012;58(5):815–818. doi: 10.1002/pbc.23290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Carol H. Initial testing (stage 1) of the notch inhibitor PF-03084014, by the pediatric preclinical testing program. Pediatr. Blood Cancer. 2014;61(8):1493–1496. doi: 10.1002/pbc.25026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Cai Y. Wnt pathway in osteosarcoma, from oncogenic to therapeutic. J. Cell. Biochem. 2014;115(4):625–631. doi: 10.1002/jcb.24708. [DOI] [PubMed] [Google Scholar]
- 310.Chen K. Wnt10b induces chemotaxis of osteosarcoma and correlates with reduced survival. Pediatr. Blood Cancer. 2008;51(3):349–355. doi: 10.1002/pbc.21595. [DOI] [PubMed] [Google Scholar]
- 311.Ma Y. Inhibition of the Wnt-beta-catenin and Notch signaling pathways sensitizes osteosarcoma cells to chemotherapy. Biochem. Biophys. Res. Commun. 2013;431(2):274–279. doi: 10.1016/j.bbrc.2012.12.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Carol H. Initial testing (stage 1) of the Akt inhibitor GSK690693 by the pediatric preclinical testing program. Pediatr. Blood Cancer. 2010;55(7):1329–1337. doi: 10.1002/pbc.22710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Gorlick R. Testing of the Akt/PKB inhibitor MK-2206 by the Pediatric Preclinical Testing Program. Pediatr. Blood Cancer. 2012;59(3):518–524. doi: 10.1002/pbc.23412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Houghton P.J. Initial testing (stage 1) of the mTOR inhibitor rapamycin by the pediatric preclinical testing program. Pediatr. Blood Cancer. 2008;50(4):799–805. doi: 10.1002/pbc.21296. [DOI] [PubMed] [Google Scholar]
- 315.Pignochino Y. The combination of sorafenib and everolimus abrogates mTORC1 and mTORC2 upregulation in osteosarcoma preclinical models. Clin. Cancer Res. 2013;19(8):2117–2131. doi: 10.1158/1078-0432.CCR-12-2293. [DOI] [PubMed] [Google Scholar]
- 316.Imai T. FR901228 induces tumor regression associated with induction of Fas ligand and activation of Fas signaling in human osteosarcoma cells. Oncogene. 2003;22(58):9231–9242. doi: 10.1038/sj.onc.1207184. [DOI] [PubMed] [Google Scholar]
- 317.Roh M.S. Mechanism of histone deacetylase inhibitor Trichostatin A induced apoptosis in human osteosarcoma cells. Apoptosis. 2004;9(5):583–589. doi: 10.1023/B:APPT.0000038037.68908.6e. [DOI] [PubMed] [Google Scholar]
- 318.Rao-Bindal K. The histone deacetylase inhibitor, MS-275 (entinostat), downregulates c-FLIP, sensitizes osteosarcoma cells to FasL, and induces the regression of osteosarcoma lung metastases. Curr. Cancer Drug Targets. 2013;13(4):411–422. doi: 10.2174/1568009611313040005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Botter S.M., Neri D., Fuchs B. Recent advances in osteosarcoma. Curr. Opin. Pharmacol. 2014;16:15–23. doi: 10.1016/j.coph.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 320.Khanna C. Towards a drug development path that targets metastatic progression in osteosarcoma. Clin. Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-13-2574. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Von Hoff D.D. Pilot study using molecular profiling of patients' tumors to find potential targets and select treatments for their refractory cancers. J. Clin. Oncol. 2010;28(33):4877–4883. doi: 10.1200/JCO.2009.26.5983. [DOI] [PubMed] [Google Scholar]
- 322.Kim E.S. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov. 2011;1(1):44–53. doi: 10.1158/2159-8274.CD-10-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Von Hoff D.D. There are no bad anticancer agents, only bad clinical trial designs—twenty-first Richard and Hinda Rosenthal Foundation Award Lecture. Clin. Cancer Res. 1998;4(5):1079–1086. [PubMed] [Google Scholar]
- 324.Gutierrez M.E., Kummar S., Giaccone G. Next generation oncology drug development: opportunities and challenges. Nat. Rev. Clin. Oncol. 2009;6(5):259–265. doi: 10.1038/nrclinonc.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Sargent D.J. Clinical trial designs for predictive marker validation in cancer treatment trials. J. Clin. Oncol. 2005;23(9):2020–2027. doi: 10.1200/JCO.2005.01.112. [DOI] [PubMed] [Google Scholar]
- 326.Savas S., Liu G. Genetic variations as cancer prognostic markers: review and update. Hum. Mutat. 2009;30(10):1369–1377. doi: 10.1002/humu.21078. [DOI] [PubMed] [Google Scholar]
- 327.Gasparini G., Longo R. The paradigm of personalized therapy in oncology. Expert Opin. Ther. Targets. 2012;16(Suppl. 1):S7–S16. doi: 10.1517/14728222.2011.637921. [DOI] [PubMed] [Google Scholar]
- 328.Al-Lazikani B., Banerji U., Workman P. Combinatorial drug therapy for cancer in the post-genomic era. Nat. Biotechnol. 2012;30(7):679–692. doi: 10.1038/nbt.2284. [DOI] [PubMed] [Google Scholar]
- 329.Vogelstein B. Cancer genome landscapes. Science. 2013;339(6127):1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental references for Table 2.