Abstract
Water splitting catalysed by earth-abundant materials is pivotal for global-scale production of non-fossil fuels, yet our understanding of the active catalyst structure and reactivity is still insufficient. Here we report on the structurally reversible evolution of crystalline Co3O4 electrocatalysts during oxygen evolution reaction identified using advanced in situ X-ray techniques. At electrode potentials facilitating oxygen evolution, a sub-nanometre shell of the Co3O4 is transformed into an X-ray amorphous CoOx(OH)y which comprises di-μ-oxo-bridged Co3+/4+ ions. Unlike irreversible amorphizations, here, the formation of the catalytically-active layer is reversed by re-crystallization upon return to non-catalytic electrode conditions. The Co3O4 material thus combines the stability advantages of a controlled, stable crystalline material with high catalytic activity, thanks to the structural flexibility of its active amorphous oxides. We propose that crystalline oxides may be tailored for generating reactive amorphous surface layers at catalytic potentials, just to return to their stable crystalline state under rest conditions.
 Understanding of catalyst structure and reactivity is important for the development of water splitting catalysts. Here, the authors report reversible structural transformation of the near-surface of crystalline Co3O4 electrocatalysts to an amorphous CoOx(OH)y during oxygen evolution.
Understanding of catalyst structure and reactivity is important for the development of water splitting catalysts. Here, the authors report reversible structural transformation of the near-surface of crystalline Co3O4 electrocatalysts to an amorphous CoOx(OH)y during oxygen evolution.
Tailoring active, stable and inexpensive electrocatalysts for water splitting and oxygen evolution reaction (OER) is a major issue for the utilization of hydrogen as a chemical storage of electrical energy from intermittent renewable power sources1,2,3,4. Optimal design of these electrocatalysts requires extended knowledge about the catalytically-active site and activity-determining structural properties. Structural analysis of electrocatalysts under electrochemical reaction conditions thus is of special importance and is approached in the present investigation.
There is an ongoing controversy on the importance of structural disorder to achieve high catalytic activity (for example, special coordination or oxidation state of metal ions, defects and vacancies in the bulk of an amorphous material or at its surface, sub-nanometre-sized catalyst domains, presence of super-active sites)5,6,7,8,9,10. High water splitting activities have been reported for single crystals, (poly)crystalline and X-ray amorphous electrocatalysts based on Ru and Ir oxides working in acidic electrolytes11,12,13,14,15,16,17 as well as perovskites and NiFe layered double hydroxides under alkaline reaction conditions18,19,20,21,22.
A well-known X-ray amorphous OER electrocatalyst is the so-called cobalt-phosphate catalyst, herein denoted as CoCat2,23,24,25,26,27,28,29,30, which is catalytically active in neutral phosphate-containing electrolyte and excels by self-repair properties29. The CoCat films consist of layer fragments of ∼12–14 octahedrally coordinated (mainly) Co3+ ions connected via di-μ2/3-oxo bridges23,24,25. The CoOx fragment size depends on the deposition conditions where a lower domain size is beneficial for catalytic activity26, in line with findings for OER catalysis by iridium oxides13,17. The μ2-O(H) bridges at the edges of the catalyst domains are thought to be involved under OER catalysis26,31.
Various crystalline (double) perovskites containing Co ions exhibit water splitting activity in alkaline electrolyte20. Depending on the initial composition of lanthanides, an irreversible loss of crystallinity was detected after potential cycling covering water oxidation potentials18,32,33. Irreversible surface amorphization was shown also for other Co-containing and initially crystalline catalysts6,7,34. A popular example of a crystalline Co oxide is the Co3O4 spinel. It shares structural motifs with the CoCat because it consists of Co-deficient CoO6 Kagomé layers, which are connected via Co3+ Oh ions (Oh, octahedral coordination by six oxygen atoms) and Co2+ Td ions (Td, tetrahedral coordination by four oxygen atoms). The O2− lattice is a so-called pseudo-cubic close-packed (ccp) arrangement35,36,37,38,39,40.
Although the existence of irreversible surface amorphization during OER has been well established, reversible structural changes of electrocatalysts during gas evolution have remained completely unaddressed to date. Detection of reversible structural changes requires in situ analysis methods as approached herein. Past investigations of the active state of Co oxide-based electrocatalysts have been largely limited to electrodeposited, thus mostly X-ray amorphous films27,41,42,43,44. These studies showed that Co oxide redox features lead to oxidation state changes27,42, whereas oxygen evolution in alkaline electrolyte is accompanied by changes in Raman features indicating changes of the local atomic structure43.
In contrast to alkaline conditions, the electrocatalysis of water splitting of noble-metal-free catalysts for OER under benign reaction conditions has been receiving growing attention2,10,23,24,25,26,28,31,45,46,47. However, the structural evolution of crystalline electrocatalysts in neutral electrolyte has remained poorly understood. This is unfortunate considering that crystalline transition metal oxides (as films or supported nanoparticles) are commonly prepared using chemical precipitation methods closely related to established large-scale industrial syntheses. This study aims to change that.
Using an array of in situ bulk-sensitive X-ray scattering and spectroscopic techniques, this work unravels the local and long-range structure of the active state of crystalline Co3O4 film catalysts in neutral phosphate-containing electrolyte, with special emphasis on structural similarities and differences between the active state of amorphous and crystalline electrocatalysts. We report on a previously overlooked reversible transition between a crystalline rest state and an active amorphous state of the shell of the crystallites, which could potentially aid in the future design of more active and stable water splitting catalysts. Based on our insights, we consider it feasible that crystalline oxides form structurally flexible and hence catalytically active amorphous shells, which, upon restoring rest conditions, reversibly transform back in their thermodynamically more stable crystalline state.
Results
Morphology and local structure
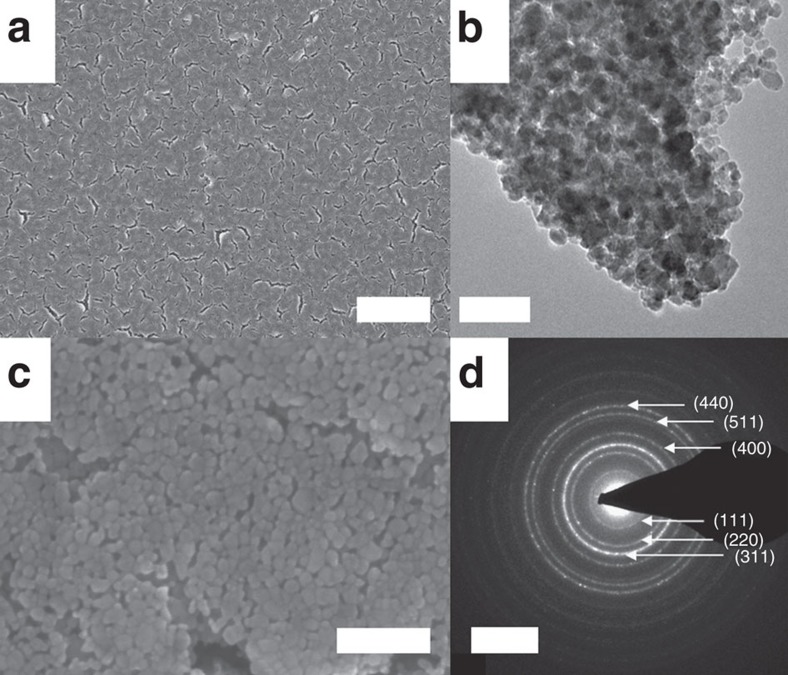
Figure 1 shows scanning and transmission electron micrographs of CoOx films as well as selected area electron diffraction (SAED). The as-prepared films exhibited a homogeneous morphology with a certain porosity leading to increased electrolyte accessibility. The porosity of Co3O4 films was ∼0.46. (cf. Supplementary Fig. 1 for further details) Thus, OER was not limited to the outer surface of the CoOx films. The metal loading was ∼960 nmolCo cm−2 on glassy carbon (GC) substrate as determined by inductively coupled plasma optical emission spectroscopy (ICP-OES). The microstructure consisted of agglomerated crystallites with size of 5–10 nm. The SAED pattern showed a typical ring structure characteristic for polycrystalline materials and agrees well with the normal spinel structure of Co3O4.
Figure 1. Structural and morphological characterization of Co3O4 films.
Scanning electron micrographs in low (a) and high magnification (c), transmission electron micrograph (b) and selected area electron diffraction (SAED) pattern (d) of as-prepared Co3O4 films. Diffraction rings of Co3O4 are indexed in the SAED pattern. The scale bars represent 2 μm, 50 nm, 100 nm and 5 nm−1 in panel a,b,c and d respectively.
Electrochemical characterization in neutral electrolyte
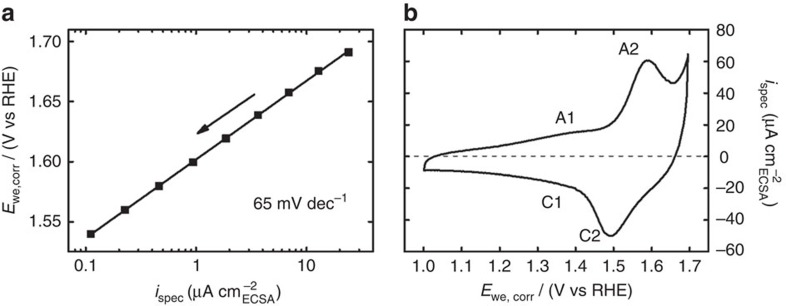
The electrocatalytic properties and Co oxide redox electrochemistry were investigated in N2-saturated 0.1 M KPi at pH 7. Tafel plot and cyclic voltammogram are shown in Fig. 2. The electrochemical surface area was determined using potentiostatic impedance spectroscopy following ref. 14. (cf. Supplementary Fig. 2 for further information). Co3O4 films exhibited a pronounced redox feature at ∼1.54 V, which was preceded by a minor feature at a potential of ∼1.4 V. According to the Pourbaix diagram48 and a previous CoOx study27, the redox feature at ∼1.54 V can be attributed to the Co3+/Co4+ redox transition, whereas the feature at about ∼1.4 V is consistent with a Co2+/Co3+ transition. Evaluation of the capacitance-corrected reductive charge of the two redox features showed that only ∼1.8% of the Co ions change their oxidation state by one equivalent during this cyclic voltammogram. Thus, we conclude that only Co ions from the surface participate in the Co oxide redox chemistry under this dynamic condition. The Co3O4 films exhibited a linear E—log(i) behaviour between 1.54 and 1.7 V with a Tafel slope of 65 mV dec−1 in N2-saturated electrolyte. The Tafel slope as determined from anodic polarization was slightly higher than that determined from cathodic quasi-stationary potential step experiment (cf. Supplementary Figs 3, 4 and 8). The Tafel slope of crystalline Co3O4 was found to be very similar compared with the one of electrodeposited Co oxide films, which exhibit ≥60 mV dec−1 in 0.1 M KPi.(refs 26, 30, 45). We note that catalytic activity and Co oxide redox behaviour showed very good stability (Supplementary Figs 3–9). Furthermore, OER did not influence morphology and Co loading (Supplementary Figs 10 and 11).
Figure 2. Electrochemical characterization of Co3O4 films.
Tafel plot (a) and cyclic voltammogram (b) of Co3O4 films deposited on glassy carbon recorded in 0.1 M KPi at pH 7. The Tafel plot was extracted from cathodic quasi-stationary potential-step rotating disc electrode experiments after equilibration for 4 min at each potential; the line corresponds to a Tafel slope and an exchange current density of 65 mV dec−1 and 2.26 × 10−9 mA cm−2ECSA, respectively. The cyclic voltammogram was recorded with a scan rate of 100 mV s−1 and shows a minor and major Co redox feature at ∼1.4 V (A1/C1) and ∼1.54 V (A2/C2), respectively. The capacitance-corrected reductive charge of the two redox features showed that only ∼1.8% of the Co ions change their oxidation state by one equivalent. Electrode potentials were corrected for Ohmic losses and are referred to reversible hydrogen electrode (RHE). The current was normalized using electrochemical surface area (ECSA) as determined by potentiostatic electrochemical impedance spectroscopy14. The electrochemical surface roughness was 26.4.
To verify electrocatalytical oxygen evolution of Co3O4 in neutral phosphate-containing electrolyte, we performed differential electrochemical mass-spectrometry (DEMS) experiments. Supplementary Fig. 12 shows a cyclic voltammogram of Co3O4 in the OER range and the corresponding ion current of m/z=32 and 44 as a measure of the evolution of oxygen and carbon dioxide, respectively. The onset potential of O2 evolution agreed well with the Co3+/Co4+ redox potential and O2 evolution followed the rise of the current density with electrode potential. Thus, we conclude that the Co3O4 films showed significant oxygen evolution activity above the Co3+/Co4+ redox couple at ∼1.54 V in neutral phosphate-containing electrolyte and exhibit typical Co oxide redox behaviour.
Long-range order under electrochemical reaction conditions
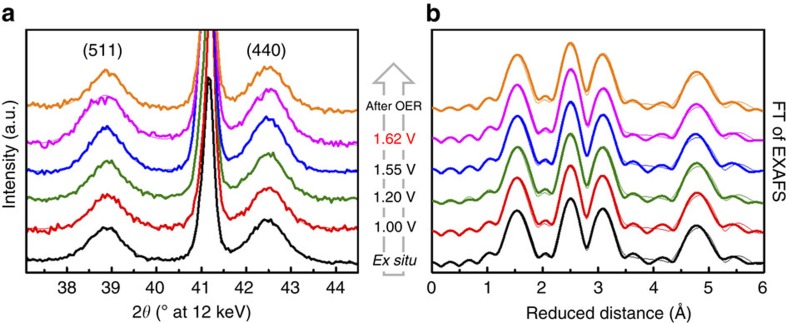
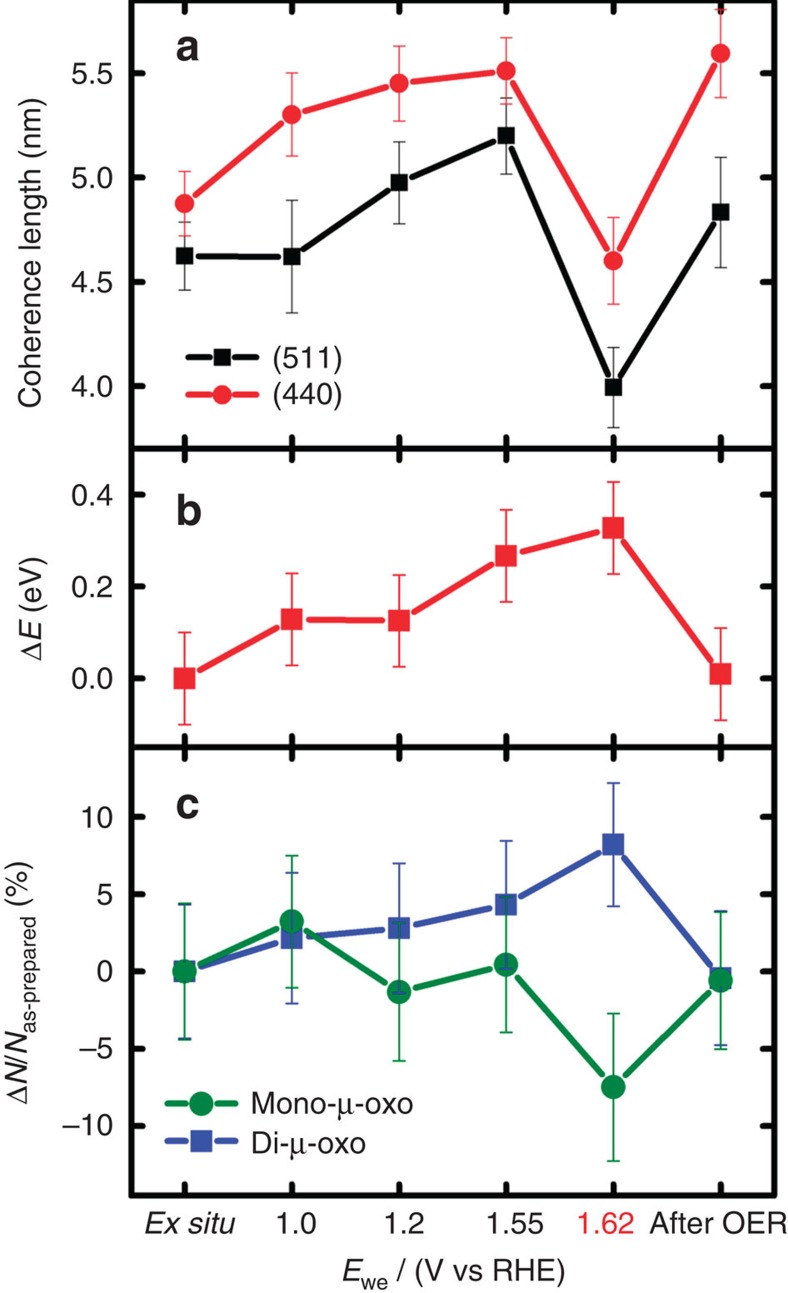
To examine atomic structure and crystallinity of Co3O4 films at different electrochemical potentials including the OER, we conducted in situ grazing-incident X-ray diffraction (GIXRD) experiments. Diffraction patterns of Co3O4 films deposited on Ti in the as-prepared state, at selected electrode potentials and after in situ characterization are shown in Supplementary Fig. 13 and Fig. 3a. Besides the reflections from the Ti substrate, Co3O4 (Space group: Fd-3m, PDF#00-042-1467) was the only detectable crystalline phase before, during, and after electrochemical reaction conditions. Thus, the catalyst film essentially retained its Co3O4 structure under the entire electrochemical potential range considered. However, a thorough analysis of the broadening of selected Co3O4 reflections (Fig. 4a and Supplementary Table 1) showed that the mean structural coherence length of the crystalline domains had changed. The structural coherence length increased after immersion into the electrolyte at +1.0 V and then increased further with more positive electrode potential. The largest structural coherence length was found at the onset of OER at +1.55 V, just anodic of the Co redox features (Fig. 2). As the electrode potential was increased further to +1.62 V, the structural coherence length of the Co3O4 domains decreased by almost 10 Å. In the dry state after OER, the structural coherence length recovered to the identical value as before OER at +1.2 V. Our observations can be plausibly explained by an initial irreversible growth of Co3O4 crystallites up to an electrode potential of +1.2 V, likely due to Ostwald ripening and/or coalescence49,50, combined with a reversible structural transformation of Co3O4 detectable at an electrode potential of +1.62 V, where catalytic oxygen evolution proceeded at elevated rates (cf. Supplementary Fig. 12). At the onset of OER (+1.55 V), the Co3O4 exhibited its largest structural coherence length. Thereafter, further increasing the oxygen evolution rate at more anodic electrode potentials resulted in a reversible structural transformation leading to a lower degree of crystallinity. This structural transformation can be explained with the formation of a CoOx shell on the crystalline Co3O4 domains, which are in contact with the electrolyte. This CoOx did not give rise to additional Bragg reflections because of its sub-nanometre size and is thus X-ray amorphous. On the reverse cathodic scan, upon leaving the potential range of sustained catalytic oxygen evolution associated with electrochemical reduction, the amorphous shell recrystallized to Co3O4. To our knowledge, this is the first time that such a reversible structural transformation of a shell on Co3O4 crystallites from crystalline to amorphous and back has been reported. We further emphasize that this unusual reversible amorphization of Co oxide appears to be linked to an elevated catalytic oxygen evolution rate.
Figure 3. In situ structural characterization of Co3O4 films.
In situ X-ray diffraction patterns (a) and Fourier-transforms (FT) of quasi-in situ EXAFS spectra collected at the Co K-edge (b) of Co3O4 catalyst films. Experimental data and fitted profiles are shown in bold and thin lines, respectively. The diffraction patterns were recorded using grazing-incident excitation at α=0.3° and 12 keV. The electrode potential was increased stepwise from +1.0 to +1.62 V versus reversible hydrogen electrode, the latter representing the catalytically active catalyst state, in 0.1 M KPi at pH 7 (cf. Fig. 2a and Supplementary Fig. 12). The state after OER is a dry state for which the electrode was removed from the electrolyte at +1.0 V rinsed with de-ionized water and dried in N2 flow. The Miller indices of selected Co3O4 reflections are indicated; the diffraction patterns were background corrected for better visualization. Fitting of the diffraction pattern was performed using pseudo-Voigt profiles. Samples for XAS were freeze-quenched under potential control using liquid N2 after 15 min at 1.62 V in 0.1 M KPi. Further details on data analysis are given in the caption of Supplementary Tables 1 and 2. See also Supplementary Figs 15 and 16.
Figure 4. Quantitative analysis of in situ structural characterization of Co3O4 films.
Structural coherence length of Co3O4 (a), shift of the Co X-ray absorption K-edge (b), change in EXAFS coordination number of di- and mono-μ-oxo bridges between Co ions (c) with respect to the as-prepared state. The state at +1.62 V corresponds to the catalytically active oxygen evolution state (cf. Fig. 2a and Supplementary Fig. 12). Electrode potentials in this particular experiment are not corrected for Ohmic losses but we note that Ohmic correction decreased the electrode potential at +1.62 V by less than ∼1 mV in the quasi-stationary experiment. The structural coherence length was calculated using the Scherrer equation from the integral breadth of the Co3O4(511) and (440) reflections65. A shape factor k of 0.89 was used. The error of the structural coherence. length was calculated from the estimated standard deviation of the fitted integral breadth. The likely error of the shift of the Co K edge was estimated to be ±0.1 eV. The coordination numbers were determined by simulations of k3-weighted EXAFS spectra. The error ranges of the EXAFS fit parameters were estimated from the covariance matrix of the fit and represent the 68% confidence intervals. Further details on data analysis are given in the caption of Supplementary Tables 1 and 2.
Local atomic structure under electrochemical reaction conditions
To determine the local atomic structure and oxidation state of the Co ions in Co3O4 under electrochemical reaction conditions and, especially, the Co coordination in the X-ray amorphous CoOx present during OER, we performed X-ray absorption spectroscopy at the Co K-edge for Co3O4 films, which were deposited on GC and freeze-quenched at selected electrode potentials. In a previous work27, we have verified that the protocol of this experiment facilitates the collection of high-quality, extended-range EXAFS data that remain unaffected by radiation-induced modifications of the solvent-exposed catalyst material. Figure 5 shows the X-ray absorption near-edge structure (XANES) spectra recorded at the Co K-edge of Co3O4 films at selected catalyst states. The energy of the Co K-edge of the as-prepared catalyst agreed well with a mean Co oxidation state between +2 and +3, as expected for Co3O4. With increasing electrode potential, the Co absorption edge shifted slightly to higher energies, which is indicative for a higher mean oxidation state of the Co ions51.
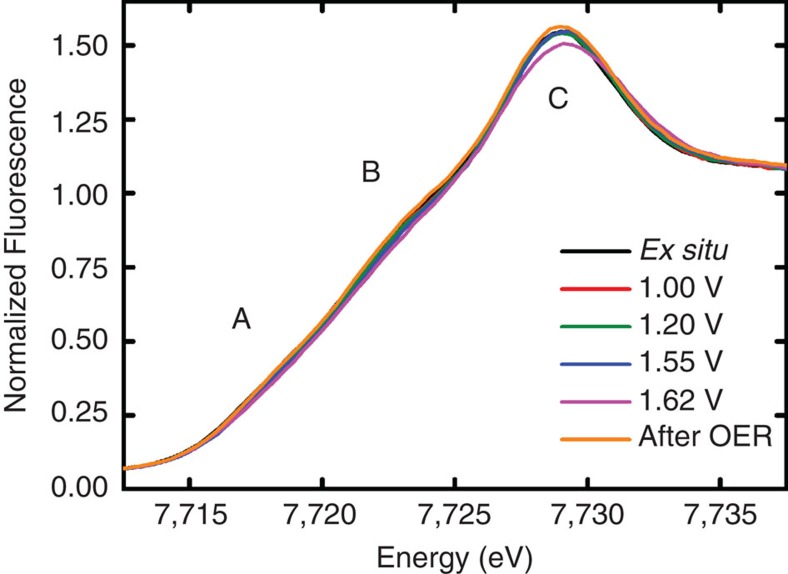
Figure 5. XANES profiles of Co3O4 under electrochemical conditions.
Fluorescence-detected quasi-in situ XANES spectra of Co3O4 films in various catalyst states. For increasingly positive potentials, the Co K-edge absorption edge shifted reversibly to higher energies suggesting oxidation of 10–15% of the cobalt ions under stationary oxygen evolution conditions. Under OER conditions, the XANES profile exhibited an almost linear increase because of significantly weaker shoulders marked with A and B. The intensity at the principal edge-maximum at (C) was clearly reduced only in the oxygen evolving active state (at +1.62 V). Samples were freeze-quenched under potential control using liquid N2 after 15 min at the given potential in 0.1 M KPi and stored in liquid N2 until XAS measurements were conducted.
During OER, the XANES profile exhibited an almost linear increase due to significantly weaker shoulders at about 7,718 (A) and 7,723 eV (B); moreover, a clearly reduced intensity at the principal edge-maximum at about 7,729 eV (C) was observed only in the OER-active state (at +1.62 V). These spectroscopic differences indicate significant structural differences in the local atomic and electronic structure of the amorphous CoOx shell formed during catalysis (at +1.62 V) as compared with the crystalline Co3O4 structure prevalent before and after catalysis. However, as expected from the in situ GIXRD results, the majority of Co ions remained in their initial state within the crystalline Co3O4.
The local atomic structure of the absorbing Co ions could be assessed qualitatively from the Fourier-transformed EXAFS and quantitatively by EXAFS simulations (Supplementary Table 2). In Fig. 3b, three major peaks representing absorber-backscatterer distances are visible for the Co3O4 films in all catalyst states. The first peak corresponds to the convolution of two Co–O distances stemming from tetrahedral and octahedral Co coordination (mean distance of ∼1.91 Å). The second and third peak is consistent with the Co–Co distances for pairs of Co ions connected via di-μ-oxo (∼2.85 Å) and mono-μ-oxo (∼3.36 Å) bridges, respectively. Di-μ-oxo bridges represent edge-sharing Co octahedra, whereas the mono-μ-oxo bridges reflect corner-sharing Co octa- and tetrahedra (cf. Fig. 6a). Comparing the magnitude of the second and third FT-peak, significant changes in the Co coordination during oxygen evolution could be identified, in particular the shift towards more di-μ-oxo-bridged, octahedrally coordinated Co ions in the OER-active state (at +1.62 V).
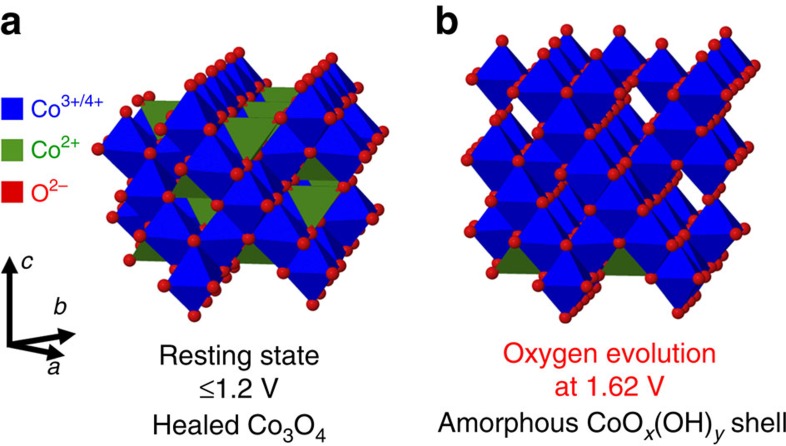
Figure 6. Possible near-surface structures on crystalline Co3O4 core under electrochemical conditions.
At potentials below Co redox features (a), Co3O4 is in a healed state at which defects in the near-surface are oxidized. At elevated O2 evolution (b), the CoOx(OH)y grows into the crystalline Co3O4 core leading to a reversible amorphization of a sub-nanometre shell. This amorphous CoOx(OH)y shell consists of di-μ-oxo-bridged Co3+/4+ ions with arbitrary site occupancy in the ideal cubic close-packed O2− lattice. Hydrogen atoms and phosphates are not shown.
Figure 4b shows the energy shift of the Co K-edge compared with the as-prepared state for various catalyst states. The edge position was identical at +1.0 and +1.2 V but was shifted by +0.1 eV compared with the as-prepared state. At +1.55 V, the edge position was +0.3 eV higher and the determined shift was maximal at high OER activity. After OER, the edge position of the Co3O4 agreed well with the as-prepared state. Thus, the evolution of the Co oxidation state is a reversible process and the mean oxidation state for electrode potentials at +1.0 V and above is higher than under dry conditions.
Simulations of k3-weighted EXAFS spectra were conducted to determine the Co coordination in Co3O4 under electrochemical reaction conditions and especially of the X-ray amorphous CoOx present during OER (Supplementary Fig. 14 and Supplementary Table 2). Figure 4c shows the relative changes in the coordination numbers assignable either to di- or mono-μ-oxo bridges at selected catalyst states. In agreement with the evolution of the structural coherence length, the number of di- and mono-μ-oxo bridges remained constant within the experimental error between the as-prepared state and +1.55 V. The reversible amorphization at elevated oxygen evolution rate, however, was accompanied by a change from mono- towards di-μ-oxo-bridged Co ions; the ex situ state after OER resembled the as-prepared state in terms of μ-oxo Co linking.
In summary, the X-ray absorption data suggest that in the OER-active state with elevated levels of oxygen evolution (at +1.62 V), tetrahedrally coordinated, mono-μ-oxo-bridged Co2+ ions are reversibly converted into octahedrally coordinated, di-μ-O(H)-bridged Co3+/4+ ions. Analysis of the X-ray diffraction data reveals a simultaneous coherence-length reduction by 5–10 Å, suggesting a smaller crystalline Co3O4 core in the OER active state. Both phenomena, the increased amount of di-μ-O(H)-bridged Co3+/4+ octahedra and the reduced size of the crystalline Co3O4 core, are reversed upon returning to the resting state of the catalyst. These observations are consistently explainable by a reversible transformation of part of the Co3O4 core into a CoOx(OH)y surface shell, as discussed in more detail further below.
Surface chemistry and electronic structure
For further insights in the elemental composition, atomic and electronic structure of the near-surface of the Co3O4 crystallites at the electrode/electrolyte interface before and after OER, we conducted synchrotron-based X-ray photoemission (XPS) and XANES spectroscopy at Co L- and O K-edge on the Co3O4 films. Supplementary Fig. 17a,b shows Co 2p XPS and the Co L3 XANES spectra of Co3O4 films, respectively. Both spectra support the notion of a Co oxide containing mainly Co3O4 in the near-surface of the crystallites52. We note that because of the more pronounced fine structure at the low-energy side of the Co L3 absorption peak, the presence of a minor fraction of Co2+ Oh in the near-surface is possible (cf. Supplementary Fig. 19). The O 1 s XPS showed that the near-surface of the as-prepared state incorporated protonated oxygen atoms in addition to lattice oxygen (cf. Supplementary Figs 17c and 20). The strong pre-edge feature of the O K-edge XANES spectrum revealed significant hybridization of Co 3d and O 2p orbitals (cf. Supplementary Fig. 17d)53.
After OER, the near-surface of the Co3O4 crystallites was largely unchanged; XPS and XANES spectra showed almost identical profiles when compared with the as-prepared catalyst material. Minor differences can be accounted for by adsorption of phosphate ions during operation of the catalyst film in the phosphate-containing electrolyte (cf. Supplementary Fig. 18). We conclude that the identified reversibility of the structural transformation observed by X-ray diffraction and X-ray absorption spectroscopy at the Co K-edge is fully supported by our surface-sensitive spectroscopic data (see Supplementary Note 1 for details).
Discussion
We have investigated the structural evolution of crystalline Co3O4 films under electrochemical potential control and during OER in neutral, phosphate-containing electrolyte in detail using in situ GIXRD and quasi-in situ XAS. For the first time, we uncover a reversible decrease in structural coherence length at electrochemical potentials facilitating elevated oxygen evolution, which is coupled to Co oxidation and a change in Co coordination from tetrahedral towards octahedral symmetry. In line with the reversibility, composition and electronic structure of the Co3O4 in the bulk volume and in the crystallites near-surface zone remained nearly identical when comparing the catalyst material before and after OER.
To explain this reversible process, we propose that the changes in Co coordination at elevated oxygen evolution rate are caused by formation of a three-dimensional (3D) cross-linked CoOx(OH)y structure. Figure 6 sketches the structural transformation of the near-surface structure of the crystallites between the resting state (below the Co redox features) and the catalytically active state (at +1.62 V). At potentials below the Co redox features (at +1.2 V), the Co3O4 is in a healed state with slightly higher mean oxidation state than in the dry state. Reduced Co sites caused by defects in the near-surface of the as-prepared state are oxidized to Co3O4. At potentials above the Co redox features at the onset of OER, Co3O4 is partially oxidized to a CoOx(OH)y without changing crystallinity and local atomic coordination. Thus, the oxidation is presumably limited to the outermost surface of the crystallites. The formation of a layered CoOOH in the near-surface can be excluded because it would diminish the structural isotropy to the Co3O4 core and thus, lead to a lower structural coherence length already at +1.55 V. Bulk oxidation of Co3O4 appears to be kinetically hindered and oxidation is thus limited to the surface of the crystallites at +1.55 V. Therefore, we conclude that the onset of the electrocatalytic oxygen evolution occurs on crystalline Co3O4, which is oxidized to CoOx(OH)y at the outermost surface (initial active surface). We propose this CoOx(OH)y represents the reaction zone of the OER. At elevated oxygen evolution rate, the crystallinity decreases and the local atomic structure of Co ions in the near-surface changes. We explain this amorphization with the growth of the CoOx(OH)y reaction zone from the surface into the crystalline Co3O4 core.
With increasing electrode potential and oxygen evolution rate, the Co2+ Td ions of the crystalline Co3O4 in the near-surface get oxidized to +3 and change their coordination from tetrahedral to octahedral. Co4+ ions can be isostructurally incorporated in the CoOx(OH)y via deprotonation of di-μ-OH bridges27. To undergo this change in coordination, Co ions have to move inside the ccp O2− lattice to vacant Oh sites of the reaction zone of the CoOx(OH)y. Site occupancy of the Co ions can be arbitrary and dynamic under the constraints of an appropriate O/Co ratio and sustained prevalence of the di-μ-oxo Co-bridging motif. As a consequence of the unified Co coordination and thus, unified Co–O and Co–Co distances in the CoOx(OH)y, a slight rearrangement of the O2− lattice towards an ideal ccp arrangement occurs. These structural changes diminish the structural isotropy to the crystalline Co3O4 core and lead to the identified amorphization. After oxygen evolution, the reaction zone transforms back to the thermodynamically favoured spinel structure.
The origin of the structural transformation of the near-surface described above can furthermore be described by participation of lattice oxygen in the OER mechanism, in which the temporary presence of O vacancies in the CoOx(OH)y also induce structural disorder in the reaction volume45,54,55. In this scheme, before OER the CoOx(OH)y surface gets oxidized but is thermodynamically frustrated when oxidation equivalents are accumulated at higher electrode potentials. Above a critical concentration of oxidation equivalents OER starts possibly under participation of lattice oxygen. The formation of oxygen vacancies then initiates structural relaxation, which leads to the described change in Co coordination and amorphization in the reaction zone. The short life-time of the O vacancies precludes the reduced Co sites from identification using quasi-in situ XANES (cf. Figs 4 and 5).
3D cross-linked Co oxides form also from Co3O4 during Li insertion, which underlines the flexibility of Co ions within the ccp O2− lattice under electrochemical conditions56,57. Under oxygen evolving conditions, octahedral Co coordination and a mean oxidation state of higher than +3 appears to be a general structural motif as it has been also found for the CoCat27,28 and layered CoOx(OH)y (refs 43, 58). The 3D cross-linked CoOx(OH)y appears also structurally similar to the rutile-type oxides such as RuO2, IrO2 and β-MnO2 (refs 11, 12, 13, 17, 31). Both structures exhibit octahedral chains of di-μ-oxo-bridged metal ions and tunnels in the oxide lattice.
We note that the uncovered amorphization of the near-surface is likely to have significant, beneficial influence on the electrocatalysis. An amorphous metal oxide provides an enhanced degree of structural flexibility, a higher number of special coordination sites possibly under participation of several metal centres and enables elevated accumulation of oxidation equivalents in the reaction zone. Vice versa, perfect crystalline order seems to prevent efficient oxygen evolution. The reported OER activity of several crystalline oxide materials may relate directly to reversible surface amorphization ‘templated' by the crystalline core of the material13,59,60. Thus, a reversible formation of an amorphous termination layer on a stable crystalline core points towards a unified common oxygen-evolving state of Co oxide (but also other) electrocatalysts—independent of its initial degree of order. This may provide a new approach towards a unified understanding of heterogeneous water oxidation catalysis. Furthermore, we propose that the design of more active and stable electrocatalysts is possible by the preparation of core-shell nanostructures in which near-surface amorphization (under electrochemical conditions) is enhanced and simultaneously the crystalline metal oxide core stabilizes the electrocatalyst from elevated metal ion dissolution.
In conclusion, using in situ X-ray diffraction and quasi-in situ X-ray absorption spectroscopy at the Co K-edge as well as XPS and XANES at Co L- and O K-edge before and after OER in neutral electrolyte, we have identified the existence of a reversible structural transformation of Co3O4, which accompanies elevated catalytic oxygen evolution. Below the Co redox features, the bulk structure of the Co3O4 is in a stable rest state with larger size of the crystalline core compared with the dry state. At the onset of the OER catalysis, the Co oxidation state increases within the pseudo-ccp O2− lattice of the reaction zone under retention of the Co coordination. As a result of this, the CoOx(OH)y structure remains limited to the surface of the crystallites. However, this catalytic reaction zone grows into the crystalline Co3O4 core at more anodic electrode potentials and enhanced oxygen evolution. This is linked to the (reversible) amorphization of a sub-nanometre shell. The amorphization is accompanied by a higher Co oxidation state and a partial change in Co coordination from tetrahedral to octahedral and can be explained by arbitrary site occupancy of Co3+/4+ ions within a slightly rearranged ccp O2− lattice. On the reverse cathodic electrode potential scan, more negative of the active catalytic regime, the amorphous shell crystallizes back to its initial state.
Structurally, we propose that the formation of an CoOx(OH)y with di-μ-oxo-bridged Co3+/4+ Oh ions at the surface is sine qua non for oxygen evolution activity on Co3O4. At elevated oxygen evolution, the amorphous 3D cross-linked CoOx(OH)y offers the structural flexibility that enables and enhances catalytic activity. The crystalline Co3O4 combines the advantages of a controlled and stable crystalline material in resting state with the required structural flexibility facilitated by a non-crystalline oxide under OER conditions. The notion of and first evidence for a reversible amorphization mechanism, in which electrocatalysts move reversibly between crystalline and amorphous regimes as a response to externally applied electrode potentials, is a new and potentially very important design concept for catalysts.
Methods
Synthesis and bulk characterization
Co oxide films were deposited on GC (HTW) plates using spin coating. Before the deposition, the substrates were polished until a mirror-like finish was achieved and cleaned stepwise in an ultrasonic bath using de-ionized water and acetone. The Ti cylinders were treated in HNO3/H2O solution for 2 h at 150 °C after polishing.
The spin coating solution consisted of 0.25 M Cobalt(II) 2,4-pentanedionate (AlfaAesar) in 15 vol.% acetic acid (Sigma-Aldrich, 99.7%), 18 vol.% de-ionized water (≥18 MΩ) and 67 vol.% ethanol (abs., AnalaR Normapur). The precursor was dissolved using sonification for 10 min. The spin coating was conducted at 2,000 r.p.m. for the GC plates and 5,000 r.p.m. for Ti cylinders. In total three and five layers were deposited on the GC and Ti substrates, respectively. After each layer deposition, the samples were calcined in air for 10 min at 300 °C to decompose the Co precursor. Finally, the samples were calcined for 15 min at 400 °C in air to achieve a crystalline Co oxide.
The Cobalt loading has been determined for a one layer deposition on a GC substrate by ICP-OES (Varian 715-ES) to be 320 nmolCo cm−2. Therefore, the samples were immersed in a HCl/HNO3 mixture overnight and the solution diluted using de-ionized water. Scanning electron microscopy images were acquired in secondary electron mode with a JEOL 7401F field emission scanning electron microscopy operated at 10 kV. Transmission electron micrographs were recorded using a FEI Tecnai G2 20S-TWIN instrument operated at 200 kV. Metal oxide films were scratched off the substrate using scalpel and transferred onto a lacy carbon-coated copper grid.
Electrochemical measurements
Electrocatalysts were tested using a three electrode rotating disk electrode setup in a custom-made glass cell using a PINE rotator with a custom-made sample holder and a Biologic SP-200 potentiostat. Platinum gauze acted as a counter electrode and a mercury/mercury sulphate electrode connected via a Haber–Luggin capillary was used as a reference electrode. The reference electrode was freshly calibrated versus a Pt/H2 electrode. Electrochemical experiments were conducted in 0.1 M phosphate buffer (KPi) at pH 7 prepared by mixing 0.1 M K2HPO4 (99.99%, Merck Suprapur) and 0.1 M KH2PO4 (99.995%, Merck Suprapur) aqueous solutions until the desired pH was obtained. Before electrochemical measurements, N2 was bubbled through the electrolyte for at least 15 min and was continuously bubbled during experiments. The working electrode was rotated at 400 r.p.m. Unless differently stated, all electrode potentials had been corrected for Ohmic losses using electrochemical impedance spectroscopy and are referred to the reversible hydrogen electrode (RHE). Impedance spectra were fitted using an equivalent electrical circuit consisting of a serial connection of an Ohmic resistance, resistor–capacitor circuit (RC) circuit and a constant phase element. A specific capacitance of 35 μF cm−2 was used to calculate electrochemical surface area from the determined capacitance14. The electrochemical protocol consisted of a dynamic OER activity test, a series of cyclic voltammograms with elevated sweep rates, an additional dynamic OER activity test, a quasi-stationary potential step OER activity test and again a dynamic OER activity test followed by a series of cyclic voltammograms with elevated sweep rates. The dynamic OER activity test consisted of a potentiostatic impedance spectroscopy recorded at 1.0 V in the frequency range between 50 kHz and 1 Hz using a modulation amplitude of 20 mV and one CV between 1.0 V and E(i=5 mA cm−2) with a sweep rate of 6 mV s−1. The first dynamic activity test consisted of two CVs at 6 mV s−1. The series of cyclic voltammograms consisted of 20 cycles with sweep rate of 500 mV s−1 followed by each three cycles of 200, 100, 50 and 20 mV s−1. For the quasi-stationary OER activity test, the electrode potential was increased from +1.50 to +1.72 V using steps of 20 mV, or vice versa. Each potential step was hold for ∼4 min and an impedance spectrum was recorded.
DEMS measurements
To investigate the reaction products, DEMS was performed. The DEMS apparatus consisted of a home-made dual thin-layer electrochemical flow cell based on a design reported elsewhere61. The flow cell was connected via separation polytetrafluoroethylene (PTFE) membrane to a Prisma quadrupole mass spectrometer (QMS 200, Pfeiffer Vacuum) equipped with two turbomolecular pumps HiPace 80 operating the MS chamber at 10−6 mbar. N2-saturated 0.1 M KPi at pH 7 acted as electrolyte and the electrolyte flow was adjusted to 5 μl s−1. The reference electrode was a reversible hydrogen electrode and a Pt wire was the counter electrode. Cyclic voltammetry measurements were conducted in the OER regime between 1.1 and 1.675 V versus reversible hydrogen electrode using a sweep rate of 6 mV s−1. Simultaneously, the ion current for O2 (m/z=32) and CO2 (m/z=44) was recorded. All electrode potentials were corrected for Ohmic losses.
In situ GIXRD measurements
Investigation of crystal structure under electrochemical reaction conditions have been conducted at the beamline of the Max-Planck-Institute for Solid State Research at the Angströmquelle Karlsruhe (ANKA)62. An home-made in situ electrochemical cell for X-ray studies based on the thin-layer concept was used63. The electrolyte was constantly circulated using a peristaltic pump and degassed with N2 during in situ GIXRD studies. A Ag/AgCl (3 M KCl, World Precision Instruments, freshly calibrated versus a Pt/H2 electrode) and a Pt wire acted as reference electrode and counter electrode, respectively. The working electrode was immersed at +1.0 V and then the electrode potential increased to the desired potential with 6 mV s−1. Before X-ray characterization, the electrode potential was hold for at least 10 min to ensure stationary conditions. The OER potential was +1.62 V, which was determined from current density of 0.5 mA cm−2 during the anodic potential scan. After OER, the electrode potential was decreased to +1.0 V and the electrode removed under potential control. Peak broadening was determined by fitting the reflections of Co3O4(511), Ti(110) and Co3O4(440) using pseudo-Voigt profiles. The structural coherence length was calculated using the Scherrer equation from the integral breadth of the fitted Co3O4 profiles using a shape factor of 0.89. Errors of the structural coherence length were calculated from the estimated s.d. of the integral breadths.
X-ray absorption measurements
Co K-edge experiments were carried out at the BESSY synchrotron radiation source operated by the Helmholtz–Zentrum Berlin. The measurements were conducted at the KMC-1 bending-magnet beamline at 20 K in a cryostat (Oxford-Danfysik) with a liquid-helium flow system. Further details are given in ref. 10. Electrochemical conditioning was conducted in analogy to the in situ GIXRD experiment. After 15 min at the desired electrode potential, the samples were freeze-quenched using liquid N2 under potential control and stored in liquid N2 until XAS measurements were conducted. Further information regarding data analysis is given in the caption of Supplementary Table 2.
XPS and XANES at Co L- and O K-edge measurements
Co, O and P core electron emission as well as Co L- and O K-edge absorption spectra were collected at the ISISS beamline64 of the synchrotron radiation facility BESSY of the Helmholtz–Zentrum Berlin. The kinetic energy of the photoelectrons recorded during XPS was 550 eV. The XANES spectra were recorded in the total electron yield mode. Electrochemical conditioning was conducted at +1.62 V for 15 min in 0.1 M KPi at pH 7. Samples were extensively washed using de-ionized water directly after removal from the electrolyte and dried in N2 flow.
Additional information
How to cite this article: Bergmann, A. et al. Reversible amorphization and the catalytically active state of crystalline Co3O4 during oxygen evolution. Nat. Commun. 6:8625 doi: 10.1038/ncomms9625 (2015).
Supplementary Material
Supplementary Figures 1-20, Supplementary Table 1-2, Supplementary Note 1 and Supplementary References
Acknowledgments
We thank S. Rudi, C. Spöri, H.N. Nong (Technische Universität Berlin) as well as Dr I. Zaharieva, J. Heitkamp and D. González-Flores (Freie Universität Berlin) for contributing to data collection at the synchrotron radiation sources. We thank Dr P. Wochner (Max-Planck-Institut für Festkörperforschung) as well as M. Mertin and Dr F. Schäfers (Helmholtz–Zentrum Berlin, HZB) for their excellent technical support at the MPI-MF beamline of ANKA, Karlsruhe as well as at the beamline KMC-1 of the BESSY II, Berlin, respectively. We thank HZB and ANKA for allocation of synchrotron radiation beamtime. We thank Dr R. Loukrakpam for recording TEM micrographs and SAED pattern at the Zelmi of Technische Universität Berlin. Financial support from the German Federal Ministry of Education and Research (BMBF) through the projects ‘MEOKATS' and ‘CO2EKAT' is gratefully acknowledged. A.B. acknowledges financial support by the Berlin Graduate school of Natural Sciences and Engineering (BIG-NSE). T.R. and D.T. acknowledge financial support by DFG through priority program SPP1613. P.C. and H.D. received support by UniCat.
Footnotes
Author contributions A.B., E.M.M., P.C. and D.T. performed the experiments and analysed the data. J.F.A. performed DEMS experiments, M.G. performed transmission electron microscopy and T.R. performed scanning electron microscopy and assisted in the synthesis. A.B., H.D. and P.S. designed the experiments and wrote the manuscript.
References
- Gray H. B. Powering the planet with solar fuel. Nat Chem 1, 7–7 (2009). [DOI] [PubMed] [Google Scholar]
- Kanan M. W. & Nocera D. G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 321, 1072–1075 (2008). [DOI] [PubMed] [Google Scholar]
- Lewis N. S. & Nocera D. G. Powering the planet: chemical challenges in solar energy utilization. Proc. Natl Acad. Sci. USA 103, 15729–15735 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsounaros I., Cherevko S., Zeradjanin A. R. & Mayrhofer K. J. J. Oxygen electrochemistry as a cornerstone for sustainable energy conversion. Angew. Chem. Int. Ed. 53, 102–121 (2013). [DOI] [PubMed] [Google Scholar]
- Over H. et al. Atomic-scale structure and catalytic reactivity of the RuO2(110). Surf. Sci. 287, 1474–1476 (2000). [DOI] [PubMed] [Google Scholar]
- Indra A. et al. Unification of catalytic water oxidation and oxygen reduction reactions: amorphous beat crystalline cobalt iron oxides. J. Am. Chem. Soc. 136, 17530–17536 (2014). [DOI] [PubMed] [Google Scholar]
- González-Flores D. et al. Heterogeneous water oxidation: surface activity versus amorphization activation in cobalt phosphate catalysts. Angew. Chem. 127, 2502–2506 (2015). [DOI] [PubMed] [Google Scholar]
- Behrens M. et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 336, 893–897 (2012). [DOI] [PubMed] [Google Scholar]
- Halck N. B., Petrykin V., Krtil P. & Rossmeisl J. Beyond the volcano limitations in electrocatalysis - oxygen evolution reaction. Phys. Chem. Chem. Phys. 16, 13682–13688 (2014). [DOI] [PubMed] [Google Scholar]
- Zaharieva I. et al. Electrosynthesis, functional, and structural characterization of a water-oxidizing manganese oxide. Energy Environ. Sci. 5, 7081–7089 (2012). [Google Scholar]
- Trasatti S. Electrocatalysis in the anodic evolution of oxygen and chlorine. Electrochim. Acta. 29, 1503–1512 (1984). [Google Scholar]
- Reier T., Oezaslan M. & Strasser P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: a comparative study of nanoparticles and bulk materials. ACS Catal. 2, 1765–1772 (2012). [Google Scholar]
- Reier T. et al. Electrocatalytic oxygen evolution on iridium oxide: uncovering catalyst-substrate interactions and active iridium oxide species. J. Electrochem. Soc. 161, F876–F882 (2014). [Google Scholar]
- McCrory C. C. L., Jung S., Peters J. C. & Jaramillo T. F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 135, 16977–16987 (2013). [DOI] [PubMed] [Google Scholar]
- Forgie R., Bugosh G., Neyerlin K. C., Liu Z. & Strasser P. Bimetallic Ru electrocatalysts for the OER and electrolytic water splitting in acidic media. Electrochem. Solid State Lett. 13, B36–B39 (2010). [Google Scholar]
- Stoerzinger K. A., Qiao L., Biegalski M. D. & Shao-Horn Y. Orientation-dependent oxygen evolution activities of rutile IrO2 and RuO2. J. Phys. Chem. Lett. 5, 1636–1641 (2014). [DOI] [PubMed] [Google Scholar]
- Bernicke M. et al. Iridium oxide coatings with templated porosity as highly active oxygen evolution catalysts: structure-activity relationships. ChemSusChem 8, 1908–1915 (2015). [DOI] [PubMed] [Google Scholar]
- Grimaud A. et al. Oxygen evolution activity and stability of Ba6Mn5O16, Sr4Mn2CoO9, and Sr6Co5O15: the influence of transition metal coordination. J. Phys. Chem. C 117, 25926–25932 (2013). [Google Scholar]
- Grimaud A. et al. Double perovskites as a family of highly active catalysts for oxygen evolution in alkaline solution. Nat. Commun 4, 2439 (2013). [DOI] [PubMed] [Google Scholar]
- Suntivich J., May K. J., Gasteiger H. A., Goodenough J. B. & Shao-Horn Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 334, 1383–1385 (2011). [DOI] [PubMed] [Google Scholar]
- Gong M. et al. An advanced Ni-Fe layered double hydroxide electrocatalyst for water oxidation. J. Am. Chem. Soc. 135, 8452–8455 (2013). [DOI] [PubMed] [Google Scholar]
- Trotochaud L., Ranney J. K., Williams K. N. & Boettcher S. W. Solution-cast metal oxide thin film electrocatalysts for oxygen evolution. J. Am. Chem. Soc. 134, 17253–17261 (2012). [DOI] [PubMed] [Google Scholar]
- Risch M. et al. Cobalt-Oxo core of a water-oxidizing catalyst film. J. Am. Chem. Soc. 131, 6936–6937 (2009). [DOI] [PubMed] [Google Scholar]
- Du P., Kokhan O., Chapman K. W., Chupas P. J. & Tiede D. M. Elucidating the domain structure of the cobalt oxide water splitting catalyst by X-ray pair distribution function analysis. J. Am. Chem. Soc. 134, 11096–11099 (2012). [DOI] [PubMed] [Google Scholar]
- Kanan M. W. et al. Structure and valency of a cobalt-phosphate water oxidation catalyst determined by in situ X-ray spectroscopy. J. Am. Chem. Soc. 132, 13692–13701 (2010). [DOI] [PubMed] [Google Scholar]
- Risch M. et al. Water oxidation by electrodeposited cobalt oxides - role of anions and redox-inert cations in structure and function of the amorphous catalyst. ChemSusChem 5, 542–549 (2012). [DOI] [PubMed] [Google Scholar]
- Risch M. et al. Water oxidation by amorphous cobalt-based oxides: in situ tracking of redox transitions and mode of catalysis. Energy Environ. Sci. 8, 661–674 (2015). [Google Scholar]
- McAlpin J. G. et al. EPR evidence for Co(IV) species produced during water oxidation at neutral pH. J. Am. Chem. Soc. 132, 6882–6883 (2010). [DOI] [PubMed] [Google Scholar]
- Lutterman D. A., Surendranath Y. & Nocera D. G. A self-healing oxygen-evolving catalyst. J. Am. Chem. Soc. 131, 3838–3839 (2009). [DOI] [PubMed] [Google Scholar]
- Risch M., Klingan K., Zaharieva I. & Dau H. in Molecular Water Oxidation Catalysis ed Llobet A. 163–185John Wiley & Sons, Ltd (2014). [Google Scholar]
- Bergmann A., Zaharieva I., Dau H. & Strasser P. Electrochemical water splitting by layered and 3D cross-linked manganese oxides: correlating structural motifs and catalytic activity. Energy Environ. Sci. 6, 2745–2755 (2013). [Google Scholar]
- May K. J. et al. Influence of oxygen evolution during water oxidation on the surface of perovskite oxide catalysts. J. Phys. Chem. Lett. 3, 3264–3270 (2012). [Google Scholar]
- Risch M. et al. Structural changes of cobalt-based perovskites upon water oxidation investigated by EXAFS. J. Phys. Chem. C 117, 8628–8635 (2013). [Google Scholar]
- Indra A. et al. Active mixed-valent mnox water oxidation catalysts through partial oxidation (Corrosion) of nanostructured MnO particles. Angew. Chem. Int. Ed. 52, 13206–13210 (2013). [DOI] [PubMed] [Google Scholar]
- Miles M. H., Huang Y. H. & Srinivasan S. The oxygen electrode reaction in alkaline solutions on oxide electrodes prepared by the thermal decomposition method. J. Electrochem. Soc. 125, 1931–1934 (1978). [Google Scholar]
- Boggio R., Carugati A. & Trasatti S. Electrochemical surface properties of Co3O4 electrodes. J. Appl. Electrochem. 17, 828–840 (1987). [Google Scholar]
- Grzelczak M. et al. Electro- and photochemical water oxidation on ligand-free Co3O4 nanoparticles with tunable sizes. ACS Catal. 3, 383–388 (2013). [Google Scholar]
- Raveau B. & Seikh M. Cobalt Oxides: From Crystal Chemistry to Physics Wiley-VCH (2012). [Google Scholar]
- Smith W. L. & Hobson A. D. The structure of cobalt oxide, Co3O4. Acta Crystallogr. B Struct. Cryst. Cryst. Chem. 29, 362–363 (1973). [Google Scholar]
- Sickafus K. E., Wills J. M. & Grimes N. W. Structure of spinel. J. Am. Chem. Soc. 82, 3279–3292 (1999). [Google Scholar]
- Subbaraman R. et al. Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nat. Mater. 11, 550–557 (2012). [DOI] [PubMed] [Google Scholar]
- Friebel D. et al. On the chemical state of Co oxide electrocatalysts during alkaline water splitting. Phys. Chem. Chem. Phys. 15, 17460–17467 (2013). [DOI] [PubMed] [Google Scholar]
- Yeo B. S. & Bell A. T. Enhanced activity of gold-supported cobalt oxide for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 133, 5587–5593 (2011). [DOI] [PubMed] [Google Scholar]
- Totir D., Mo Y., Kim S., Antonio M. R. & Scherson D. A. In situ Co K-edge X-ray absorption fine structure of cobalt hydroxide film electrodes in alkaline solutions. J. Electrochem. Soc. 147, 4594–4594 (2000). [Google Scholar]
- Surendranath Y., Kanan M. W. & Nocera D. G. Mechanistic studies of the oxygen evolution reaction by a cobalt-phosphate catalyst at neutral pH. J. Am. Chem. Soc. 132, 16501–16509 (2010). [DOI] [PubMed] [Google Scholar]
- Klingan K. et al. Water oxidation by amorphous cobalt-based oxides: volume activity and proton transfer to electrolyte bases. ChemSusChem 7, 1301–1310 (2014). [DOI] [PubMed] [Google Scholar]
- Risch M. et al. Nickel-oxido structure of a water-oxidizing catalyst film. Chem. Commun. 47, 11912–11914 (2011). [DOI] [PubMed] [Google Scholar]
- Chivot J., Mendoza L., Mansour C., Pauporté T. & Cassir M. New insight in the behaviour of Co/H2O system at 25-150 °C, based on revised Pourbaix diagrams. Corros. Sci. 50, 62–69 (2008). [Google Scholar]
- Ostwald W. Studien über die Bildung und Umwandlung fester Körper. Z. Phys. Chem. 22, 289–330 (1897). [Google Scholar]
- Borup R. et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 107, 3904–3951 (2007). [DOI] [PubMed] [Google Scholar]
- Penner-Hahn J. E. X-ray absorption spectroscopy in coordination chemistry. Coord. Chem. Rev. 190-192, 1101–1123 (1999). [Google Scholar]
- NIST. X-ray Photoelectron Spectroscopy Database, Version 4.1 National Institute of Standards and Technology (2012) http://srdata.nist.gov/xps/. [Google Scholar]
- Suntivich J. et al. Estimating hybridization of transition metal and oxygen states in perovskites from O K-edge X-ray absorption spectroscopy. J. Phys. Chem. C 118, 1856–1863 (2014). [Google Scholar]
- Willsau J., Wolter O. & Heitbaum J. Does the oxide layer take part in the oxygen evolution reaction on platinum? A DEMS study. J. Electroanal. Chem. 195, 299–306 (1985). [Google Scholar]
- Wohlfahrt-Mehrens M. & Heitbaum J. Oxygen evolution on Ru and RuO2 electrodes studied using isotope labelling and on-line mass spectrometry. J. Electroanal. Chem. 237, 251–260 (1987). [Google Scholar]
- Pralong V. Lithium intercalation into transition metal oxides: A route to generate new ordered rock salt type structure. Prog. Solid State Chem. 37, 262–277 (2009). [Google Scholar]
- Thackeray M. M., Baker S. D., Adendorff K. T. & Goodenough J. B. Lithium insertion into Co3O4: a preliminary investigation. Solid State Ionics 17, 175–181 (1985). [Google Scholar]
- Hey M. H. Cobaltic hydroxide in nature. Mineral. Mag. 32, 253–259 (1962). [Google Scholar]
- Nong H. N. et al. Oxide-supported IrNiOx core–shell particles as efficient, cost-effective, and stable catalysts for electrochemical water splitting. Angew. Chem. 127, 3018–3022 (2015). [DOI] [PubMed] [Google Scholar]
- Nong H. N., Gan L., Willinger E., Teschner D. & Strasser P. IrOx core-shell nanocatalysts for cost- and energy-efficient electrochemical water splitting. Chem. Sci. 5, 2955–2963 (2014). [Google Scholar]
- Jusys Z., Kaiser J. & Behm R. J. A novel dual thin-layer flow cell double-disk electrode design for kinetic studies on supported catalysts under controlled mass-transport conditions. Electrochim. Acta. 49, 1297–1305 (2004). [Google Scholar]
- Stierle A. et al. Dedicated Max-Planck beamline for the in situ investigation of interfaces and thin films. Rev. Sci. Instrum. 75, 5302–5307 (2004). [Google Scholar]
- Renner F. U. In-situ X-ray Study of the Initial Electrochemical Corrosion of CuAu(111), PhD thesis, Univ. Stuttgart (2004).
- Knop-Gericke A. et al. in Advances in Catalysis Vol. 52, (eds. Gates B. C., Knözinger H. 213–272Academic Press (2009). [Google Scholar]
- Zhang Z., Zhou F. & Lavernia E. J. On the analysis of grain size in bulk nanocrystalline materials via X-ray diffraction. Metall. Mater. Trans. A 34, 1349–1355 (2003). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-20, Supplementary Table 1-2, Supplementary Note 1 and Supplementary References