Abstract
The electron deficiency and strong bonding capacity of boron have led to a vast variety of molecular structures in chemistry and materials science. Here we report the observation of highly symmetric cobalt-centered boron drum-like structures of CoB16−, characterized by photoelectron spectroscopy and ab initio calculations. The photoelectron spectra display a relatively simple spectral pattern, suggesting a high symmetry structure. Two nearly degenerate isomers with D8d (I) and C4v (II) symmetries are found computationally to compete for the global minimum. These drum-like structures consist of two B8 rings sandwiching a cobalt atom, which has the highest coordination number known heretofore in chemistry. We show that doping of boron clusters with a transition metal atom induces an earlier two-dimensional to three-dimensional structural transition. The CoB16− cluster is tested as a building block in a triple-decker sandwich, suggesting a promising route for its realization in the solid state.
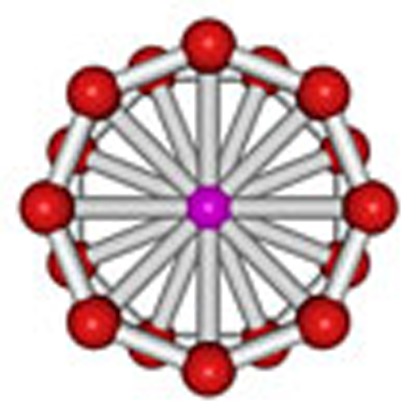 Boron is known to form a wide variety of molecular structures. Here, the authors observe the highly symmetric cobalt-centered boron drum-like structure of CoB16
−, characterized by photoelectron spectroscopy and ab initio calculations, in which the cobalt atom is sixteen-coordinate.
Boron is known to form a wide variety of molecular structures. Here, the authors observe the highly symmetric cobalt-centered boron drum-like structure of CoB16
−, characterized by photoelectron spectroscopy and ab initio calculations, in which the cobalt atom is sixteen-coordinate.
Boron, the fifth element in the periodic table, possesses such diverse chemical structures and bonding that are second only to carbon. Bulk boron consists of connected three-dimensional (3D) cages in many of its allotropes1,2 and boron-rich borides3,4. However, for isolated clusters it was computationally shown5,6 that icosahedral cage structures of B12 and B13 were unstable, even though they were initially proposed as possible candidates for these two clusters7. Over the past decade, small anionic boron clusters have been systematically characterized both experimentally and theoretically to exhibit planar or quasi-planar structures in their ground states up to B27− (refs 8, 9, 10). Recent works show that anionic boron clusters continue to be two-dimensional (2D) at B30− (ref. 11), B35− (ref. 12) and B36− (ref. 13). The 2D-to-3D transition was suggested to occur at B20 for neutral14, and at B16+ for cationic clusters15. Very recently it is shown that the transition from 2D to fullerene-like 3D structures occurs in negatively charged boron clusters at about 40 boron atoms in B39− (ref. 16) and B40− (ref. 17). Due to the nearly spherical shapes of these clusters, they have been named borospherenes. Doping boron clusters with a single metal atom opens a new avenue to create clusters with novel structures and chemical bonding. It has been experimentally observed that various transition metal atoms can be placed inside of monocyclic boron rings to form beautiful molecular wheel-type structures (M©Bn−)18, following an electronic design principle inspired by the doubly σ and π aromatic B9− cluster19. It was shown that the Nb©B10− and Ta©B10− clusters possess the record coordination number of 10 in the planar environment for the central metal atom20. These clusters have pushed the limits of structural chemistry.
Here we report the observation of a large metal-doped boron cluster of CoB16−, which is produced using a laser vaporization cluster source and characterized by photoelectron spectroscopy (PES). Extensive computational searches reveal that there are two nearly degenerate structures for CoB16−, which are indistinguishable at the highest level of theory employed. They both possess tubular double-ring framework and give similar photoelectron spectral patterns. The structures can be viewed as two B8 rings sandwiching a Co atom, reminiscent of a drum and giving rise to the highest coordination number known in chemistry thus far.
Results
Experimental results
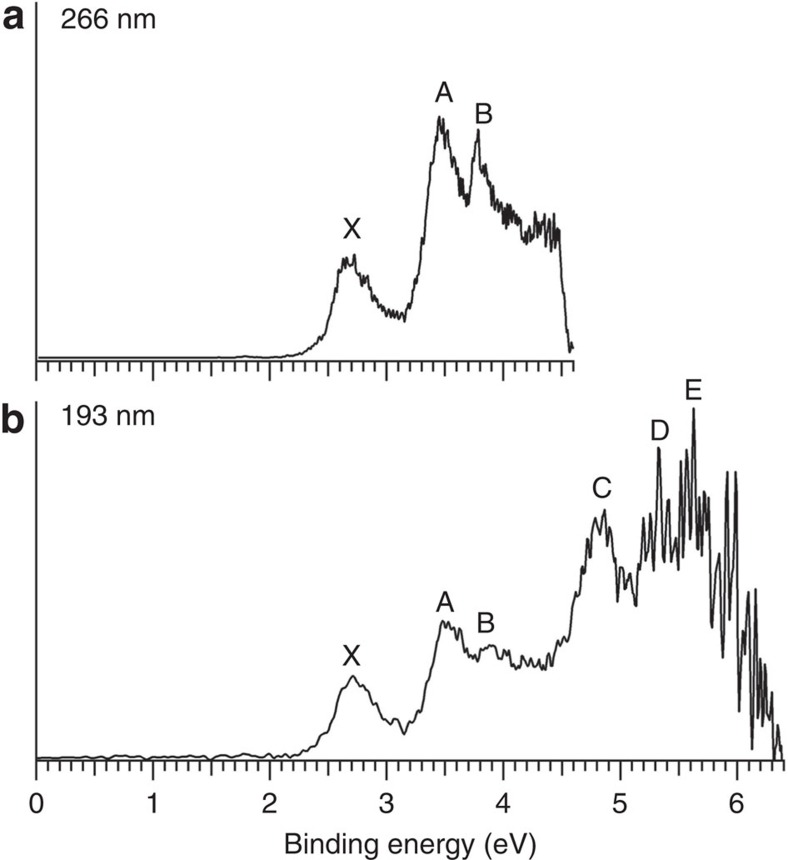
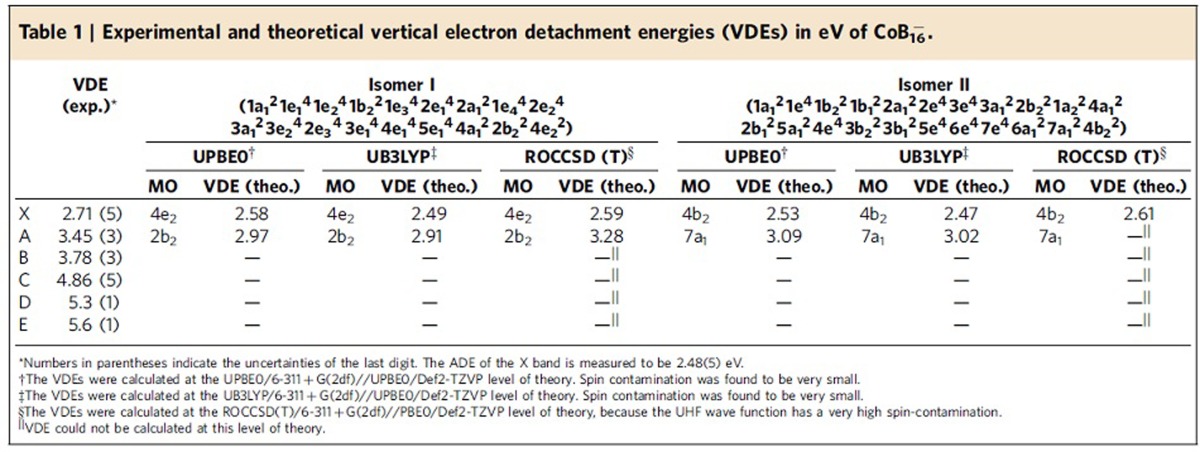
The photoelectron spectra of CoB16− at two photon energies are displayed in Fig. 1. The lowest binding energy band (X) represents the electron detachment transition from the anionic ground state to that of neutral CoB16. The higher binding energy bands, A, B, …, denote detachment transitions to the excited states of neutral CoB16. The vertical detachment energies (VDEs) for all observed bands are given in Table 1, where they are compared with the calculated VDEs.
Figure 1. Photoelectron spectra.
Photoelectron spectra (a) at 266 nm (4.661 eV) and (b) at 193 nm (6.424 eV) of CoB16−.
Table 1. Experimental and theoretical vertical electron detachment energies (VDEs) in eV of CoB16 −.
The 266 nm spectrum (Fig. 1a) reveals three well-resolved PES bands for CoB16−. The band X gives rise to a VDE of 2.71 eV. The adiabatic detachment energy (ADE) for band X was evaluated from its onset to be 2.48 eV, which also represents the electron affinity of neutral CoB16. The width of band X suggests an appreciable geometry change between the ground electronic state of CoB16− and the ground electronic state of CoB16. Following a relatively large energy gap, an intense and broad band A is observed at a VDE of 3.45 eV and a close-lying band B at a VDE of 3.78 eV. The 193 nm spectrum (Fig. 1b) shows nearly continuous signals beyond 4 eV. The sharp spikes above 5 eV in the high binding energy side of the 193 nm spectrum are due to statistical noises because of low electron counts. An intense and broad band C is clearly observed at a VDE of 4.86 eV. Two more bands can be tentatively identified at higher binding energies, D (VDE: ∼5.3 eV) and E (VDE: ∼5.6 eV). Overall, the PES spectral pattern is relatively simple, suggesting that the framework of the CoB16− cluster is likely to have high symmetry.
Theoretical results and comparison with experiment
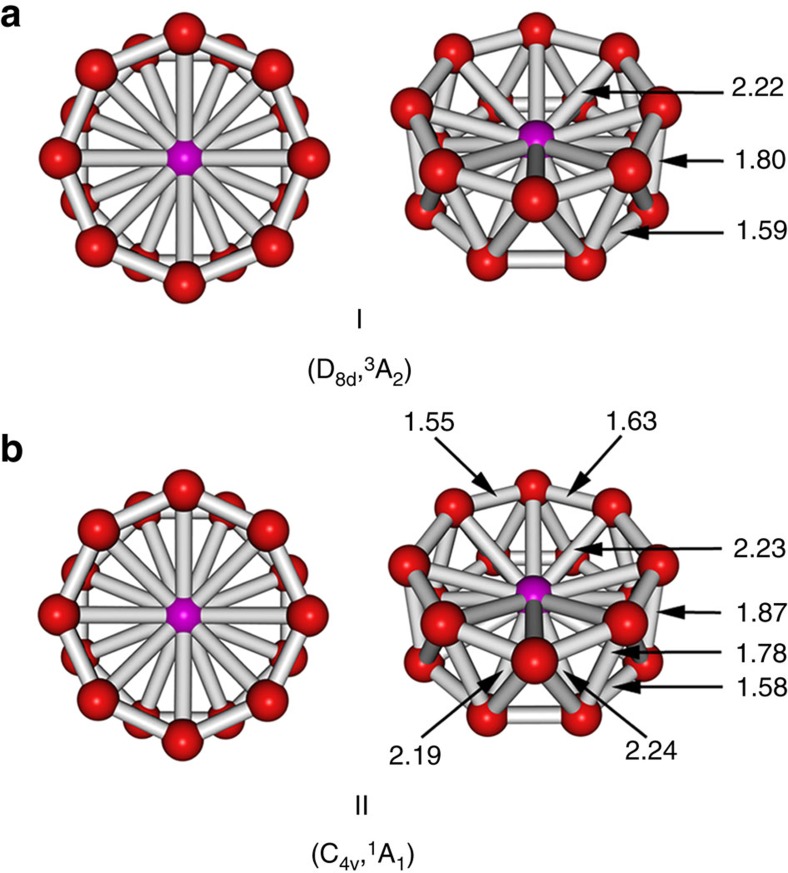
Extensive structural searches were initially done at the PBE0/3-21G level of theory with the follow-up calculations (Δ=25 kcal mol−1) at the PBE0/Def2-TZVP level of theory, which led to two similar drum-like structures: isomer I (D8d, 3A2) and isomer II (C4v, 1A1) identified as the global minima for CoB16− (Fig. 2). These two highly symmetric structures, consisting of a central Co atom sandwiched by two B8 monocyclic rings, are found to be almost degenerate at various levels of theory (Supplementary Fig. 1 and Supplementary Table 1). Clearly, the method dependency of predicting relative energies of the low-lying structures for CoB16− suggests the importance of comparison with experiment in determining the global minimum. We previously studied how optimized geometries of small boron clusters differed at density functional theory (DFT) and CCSD(T) levels of theory21,22. We found that B3LYP/6-311+G* geometries are quite close (within 0.03 Å between nearest boron atoms) to those at the CCSD(T)/6-311+G* level of theory. We also compared the geometries of boron clusters at PBE0/6-311+G* and B3LYP/6-311+G*, and found that they are also very close23. Therefore, PBE0/3-21G level of theory was used for the preliminary search and PBE0/Def2-TZVP for the final optimized geometries of CoB16−. The highest level of theory employed (ROCCSD(T)/6-311+G(2df)//PBE0/Def2-TZVP (this abbreviation means that single-point energy calculations were performed at ROCCSD(T)/6-311+G(2df) using optimized UPBE0/Def2-TZVP geometries here and elsewhere) indicates 1.4 kcal mol−1 energy difference including zero-point energy corrections (Supplementary Fig. 1). This small value is in the range of the theoretical errors for such a complex transition-metal-doped boron cluster. Therefore, isomers I and II should be considered to be degenerate based on our calculations. Figure 2 shows the small differences in bond distances between isomers I and II; the latter is not significantly distorted from the D8d symmetry. The B–B bond lengths of the B8 rings for both isomers are in the range of 1.55–1.63 Å, similar to the corresponding values (1.56 Å) in the Co©B8− molecular wheel18. The nearest isomer III (C2, 1A) is 8.7 kcal mol−1 higher in energy at the ROCCSD(T) method and represents a distorted drum-like structure composed of two B7 rings with two B atoms outside the drum (Supplementary Fig. 1). In fact, the majority of the low-lying isomers within 20 kcal mol−1 (Supplementary Fig. 1) represent various derivatives (drum-like or possessing principal geometrical features of the drum-like structure) of isomers I and II, showing the stability of the drum-like structures. It should be noted that there are significant bonding interactions between the two B8 rings and between the Co atom and all 16 B atoms in both isomers I and II (vide infra). Interestingly, the drum structure in a quintet state (isomer XIV in Supplementary Fig. 1) appears to be the most stable one out of all other quintet isomers. It should be mentioned that there were two previous DFT calculations on similar drum-like structures of neutral boron clusters doped with transition metal atoms24,25.
Figure 2. Two views of isomer I and isomer II of the CoB16− cluster.
The point group symmetries and spectroscopic states of isomer I (a) and isomer II (b) are shown in parentheses. Sticks drawn between atoms help visualization and do not necessarily represent classical 2c–2e B–B or Co–B bonds here and elsewhere. All distances are in Å.
To facilitate comparisons between the experimental and theoretical results, we calculated low-lying VDEs of isomers I and II of CoB16− using three methods (Table 1). We found that the VDEs computed using the two DFT methods are not very impressive; but we observed good agreement between the theoretical VDEs at ROCCSD(T)/6-311+G(2df) and the experimental data for the first two detachment channels (Table 1). Since isomer I is open shell, the electron detachment energy from the doubly degenerate 4e2-HOMO should lead to a doublet final state for the neutral. The computed VDE at ROCCSD(T) is 2.59 eV, compared with the experimental VDE of 2.71 eV. The next electron detachment from the non-degenerate 2b2-HOMO-1 should lead to both a quartet and a doublet final state, with the quartet being lower in energy. The calculated VDE for the quartet final state at ROCCSD(T) is 3.28 eV, compared with the VDE of the A band at 3.45 eV. Unfortunately, we were not able to calculate any higher VDEs because of the limitation of the ROCCSD(T) method. However, we believe that the good agreement between experiment and theory for the first two VDEs provides sufficient credence for the identified drum-like isomer I for the CoB16− cluster.
Isomer II gives very similar theoretical VDEs as isomer I at all three levels of theory, consistent with the similarities in their geometries. Since isomer II is a closed shell species, we were able to calculate only the first VDE value at the ROCCSD(T) method as 2.61 eV, also in good agreement with the experimental data. Furthermore, the calculated ADEs of isomer I (2.45 eV) and isomer II (2.43 eV) (PBE0/Def2-TZVP) are in excellent agreement with the experimentally measured ADE value of 2.48 eV. We should point out that there is a Jahn–Teller distortion for the neutral CoB16 drum-like structure of isomer I, consistent with the broad X band observed in the PES spectra (Fig. 1). Indeed, the calculated relaxed neutral CoB16 structure I0 (Supplementary Fig. 2 and Supplementary Table 1) has lower symmetry (C2v), as one would expect for the Jahn–Teller distorted structure due to the occupation of the doubly degenerate HOMO (4e2) of isomer I by a single electron. In fact, the HOMO (4b2) of isomer II originates from the HOMO (4e2) of isomer I when one of the doubly degenerate orbitals is doubly occupied. Therefore, the detachment of one electron from the doubly occupied HOMO (4b2) of isomer II leads to the same neutral structure I0. The high relative energy of isomer III, as well as its appreciably higher theoretical first VDE of 3.65 eV (Supplementary Table 2), makes this cluster unlikely to be populated in the molecular beam in any appreciable amount.
Discussion
Tubular (or drum-like) boron clusters have been of interest for many years, because they can be considered as the embryos for boron nanotubes14. However, such drum-like structures have never been observed experimentally for bare boron clusters, even though they have been shown to be stable computationally14,26,27,28,29. For instance, the B20 cluster was first suggested as the global minimum on the basis of theoretical calculations14, but it has not been observed or confirmed experimentally29. Tubular structures were also studied for the bare B16+, B16, B16− and B162− species15,30. For the B16+ cationic cluster, the tubular structure was suggested to be the global minimum15, whereas the tubular structures of both B16 and B16− were found to be high-energy isomers30. Clearly, the strong coordination interactions with the Co atom significantly stabilize the tubular B16 to give the drum-like global minima (structures I and II) for CoB16−. Bare anionic boron clusters are found to be 2D up to B36− (ref. 13), while some transition-metal-doped anionic boron clusters are found to preserve the planar boron framework on metal doping31,32. The largest experimentally observed metal-doped boron cluster (CoB12−) maintains a similar planar geometry for the B12 moiety32. Hence, the doping of the Co atom induces an earlier 2D-to-3D transition for boron clusters, as shown by the 3D isomers I and II of CoB16−. In fact, the CoB16− drum structure represents the highest coordination number known in chemistry today. The previous highest coordination number known experimentally was 15 for [Th(H3BNMe2BH3)4] (ref. 33), though theoretical studies have suggested the highest coordination numbers of 15 in PbHe152+ (ref. 34) and 16 in the Friauf–Laves phases in MgZn2 or MgNi2 (ref. 35). Endohedral fullerenes (M@C60) have been observed36,37, but the metal atom in those cases interacts with the C60 shell primarily ionically and it does not stay in the centre of C60.
It is interesting to point out that the B–B distances in the B8 rings of both isomers I and II of CoB16− and the bare tubular B16 are very similar (Supplementary Table 3). To gain insight into the chemical bonding of the CoB16− drums, we performed chemical bonding analyses for isomers I and II using the Adaptive Natural Density Partitioning (AdNDP) method38, which is an extension of the popular Natural Bond Orbital method39. It should be noted that the bonding in some double-ring tubular boron clusters has been discussed previously9,40,41.
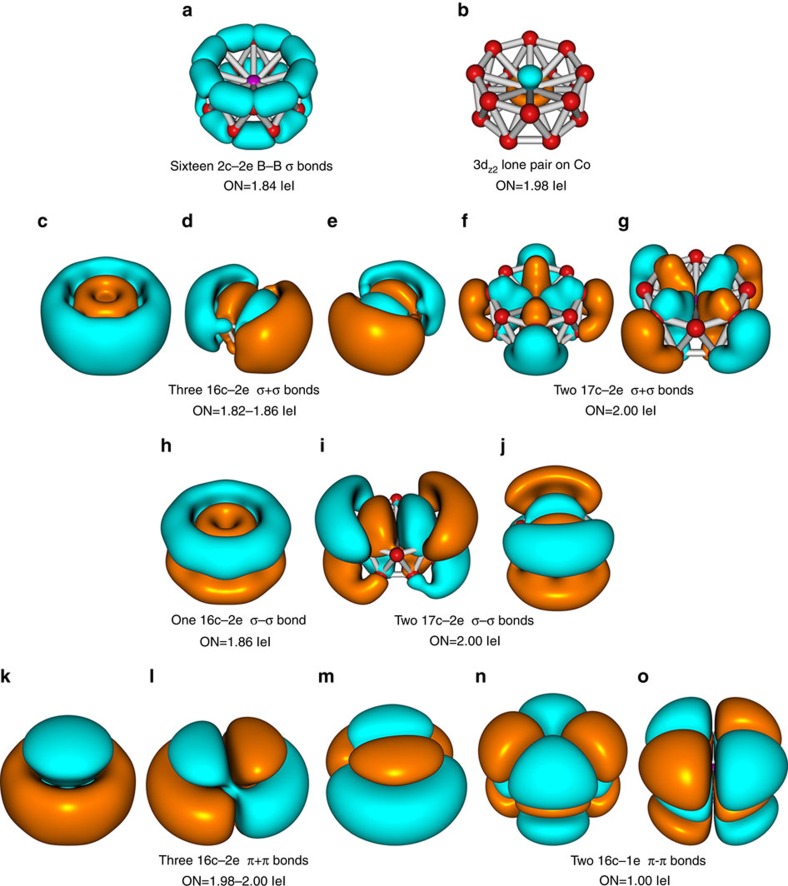
Since isomer I has two unpaired electrons, we used the unrestricted AdNDP (UAdNDP) analysis, which enables treatments of the α and β electrons separately. To obtain an averaged result for a bond (Fig. 3), we added the UAdNDP results for the α and β electrons of the same type of bonds. According to the UAdNDP analysis results, the 58 valence electrons in CoB16− can be divided into four sets. The first set (Fig. 3a,b) consists of localized bonding elements, while the other three sets (Fig. 3c–g, h–j, k–o) are composed of delocalized bonding elements. In the first set, the UAdNDP analysis for isomer I revealed the following localized bonding elements: one lone pair (1c–2e bond) (Fig. 3b) of 3dz2-type on Co with an occupation number (ON) of 1.98 |e| and sixteen 2c–2e B–B σ-bonds (Fig. 3a) with ON=1.84 |e| within each B8 ring (all superimposed onto the B16 fragment in Fig. 3), which can also be viewed as 3c–2e bonds with the ON=1.96 |e| responsible for the bonding between the boron rings. In the last case, a boron atom from the neighbouring ring contributes somewhat (0.12 |e|) to the formation of the 3c–2e σ-bond. The 2c–2e B–B σ-bonds are very similar to the peripheral B–B bonds found in all 2D boron clusters8,9,10. The second set includes five delocalized σ bonds (denoted as σ+σ), which are formed from delocalized σ bonds between the two B8 rings. Since the σ orbitals between the two boron rings overlap positively, we designate them as σ+σ in the second set, which constitutes σ-aromaticity according to the 4n+2 (n=2) Hückel rule. The three delocalized 16c–2e σ+σ bonds (Fig. 3c–e) with ON=1.82–1.86 |e| involve only σ-bonding within the boron rings, whereas the two delocalized 17c–2e σ+σ bonds (Fig. 3f,g) come primarily from the 3dxy and 3dx2–y2 AOs of Co interacting with the boron rings. It should be noted that the direct covalent interactions between Co and the B16 unit via the 3dxy and 3dx2-y2 AOs of Co are found to be around 0.6 |e| according to the AdNDP analysis. The third set (Fig. 3h–j) shows three delocalized σ–σ bonds, which represent bonding interactions within each ring, but anti-bonding interactions between the two boron rings. This set of delocalized bonds also constitutes σ-aromaticity according to the 4n+2 (n=1) Hückel rule. In the third set, the 16c–2e σ–σ bond (Fig. 3h) involves mainly the two boron rings, whereas the two 17c–2e σ–σ bonds (Fig. 3i,j) involve interactions between the 3dxz and 3dyz AOs of Co with the boron rings. The direct covalent interaction of the 3dxz and 3dyz AOs of Co with the boron kernel is assessed to be around 0.5 |e|. The last set includes five delocalized bonds, which represent π–π interactions between the boron rings: three 16c–2e π–π bonds (Fig. 3k–m) with ON=1.98–2.00 |e| and two 16c–1e π–π bonds (Fig. 3n,o) with ON=1.00 |e| (one unpaired electron on each bond). The eight π electrons in the last set suggest π-aromaticity according to the 4n rule (n=2) for triplet states. Therefore, the stability of isomer I of CoB16− can be considered to be due to the double σ- and π-aromaticity and bonding interactions of the 3d AOs of Co with the B8 rings.
Figure 3. Chemical bonding picture.
(a–o) The overall chemical bonding picture (a–o) obtained for the isomer I of the CoB16− molecular drum via the UAdNDP analysis. ON denotes occupation number here and elsewhere.
As expected, isomer II of CoB16−, which is close in energy and geometry to isomer I, has almost the same bonding pattern as that of isomer I (Supplementary Fig. 3). All the bonding elements found in isomer I are also found in isomer II except for the last set (Supplementary Fig. 3k–n). Since isomer II is closed shell, eight electrons in the last set are observed to form four 16c–e π–π bonds with ON=1.98–2.00 |e|, rendering this isomer π-antiaromatic. Hence, isomer II exhibits conflicting aromaticity (σ-aromatic and π-antiaromatic), which leads to some distortion to C4v symmetry compared to the D8d symmetry of the doubly aromatic isomer I. As was mentioned earlier, the HOMO (4b2) of isomer II originates from the HOMO (4e2) of isomer I when one of the doubly degenerate orbitals is doubly occupied. Indeed, occupation of only one degenerate MO by two electrons causes the electronic instability, which causes the geometric rearrangement of isomer II lowering the D8d symmetry to C4v.
To understand the interactions between Co and the tubular B16 host, we have performed AdNDP analyses for the neutral B16 tubular isomer (Supplementary Fig. 4). Similar to isomers I and II of CoB16−, the AdNDP analyses give 16 2c–2e B–B σ-bonds with ON values of 1.70 |e| within the two B8 rings. The encapsulation of Co strengthens the B–B σ-bonds within each B8 ring in CoB16−, but weakens the inter-ring interactions, compared with the bare B16, as reflected by their ON values (Fig. 3 and Supplementary Fig. 4) and the B–B bond lengths (Supplementary Table 3). The remaining 16 electrons in B16 participate in delocalized bonding: five 16c–2e σ+σ bonds and three 16c–2e π–π bonds, rendering the tubular B16 doubly σ- and π-aromatic. The major difference in chemical bonding between the drum-like B16 and CoB16− comes from two factors: (1) the formation of an additional set (Fig. 3h–j) of the delocalized σ–σ bonds in CoB16−; and (2) participation of Co 3d AOs in the two 17c–2e σ+σ bonds (Fig. 3f,g). Both factors are consistent with structural changes between CoB16− and B16. There are strong bonding interactions between Co and the B16 host in CoB16− to stabilize the tubular B16 structure, because the global minimum of B16 is planar30.
Isomer I of CoB16− is open shell with two unpaired electrons, whereas isomer II can be viewed as a result of Jahn–Teller distortion from isomer I. Addition of two electrons to isomers I or II would create a closed shell and doubly aromatic CoB163− species with D8d symmetry. Our calculations indeed confirmed this hypothesis: CoB163− was found to be a minimum on the potential energy surface with very similar bond distances as in isomer I (Supplementary Table 3). The triply charged CoB163− species can be electronically stabilized by external akali metal cations, such as in Na2CoB16−. Since ligation would be needed to ultimately synthesize CoB16−, we considered a triple-decked [CoB16(CaCp)2]− sandwich complex (Supplementary Fig. 5), using the divalent Ca atoms and the aromatic C5H5− (Cp−) ligands. It should be mentioned that similar [CpLiB6LiCp]2− triple-decked complex42 with the double antiaromatic B62− unit was previously suggested to be stable and viable experimentally. We found that the [CoB16(CaCp)2]− triple-decked complex was a minimum on the potential energy surface with high electronic stability. All the B–B and Co–B bond lengths were found to be almost the same as in isomers I and II of CoB16− (Supplementary Table 3). We have further performed AdNDP analyses and found that the triple-decked sandwich complex exhibits exactly the same chemical bonding pattern as the parent CoB16− (Supplementary Figs 6–8). The Natural Population Analysis (NPA) charge on Ca was found to be +1.54, consistent with the initial hypothesis and the charge-transfer nature of the triple-decked [CoB16(CaCp)2]− sandwich complex. Thus, the CoB16− molecular drum can serve as a building block for the design of novel cluster-assembled nanomaterials. The high stability of the CoB16− drum structures may also help the search for new metal-boride phases containing various boron ring units43.
We have produced and characterized a large Co-doped boron cluster, CoB16−, using photoelectron spectroscopy and quantum-chemical calculations. Extensive computational searches established two high symmetry (D8d and C4v) drum-like structures with Co sandwiched by two B8 rings as nearly degenerate global minima. The CoB16− molecular drums represent the highest coordination for a metal atom known in chemistry and opens new possibilities for designing novel boron-based nanomaterials. First, the CoB16− drums may be considered as the embryo to make filled boron nanotubes due to the significant B-B bonding between the two B8 rings. Second, there are possibilities to observe larger doped-boron clusters with even higher coordination number to further push the limit of coordination number in chemistry. Third, we have demonstrated one possibility to use CoB16− as a building block of new cluster-assembled nanomaterials in a triple-decked complex.
Methods
Experimental methods
The experiment was carried out using a magnetic-bottle PES apparatus equipped with a laser vaporization cluster source44. Briefly, the CoB16− anion clusters were produced by laser vaporization of a cold-pressed target composed of Co and isotopically enriched 11B. Bismuth was added as a binder and it also provided a convenient calibrant (Bi−) for the PES experiment. Clusters formed in the nozzle were entrained in a He carrier gas and underwent a supersonic expansion to form a collimated cluster beam. The He carrier gas was seeded with 5% Ar for better cooling of the entrained clusters22. The anionic clusters were extracted from the collimated cluster beam and analysed by a time-of-flight mass spectrometer. The CoB16− anion clusters were mass selected and decelerated before being photodetached by a laser beam at 193 nm (6.424 eV) from an ArF excimer laser or 266 nm (4.661 eV) from a Nd:YAG laser. Photoelectrons were collected at nearly 100% efficiency by a magnetic bottle and analysed in a 3.5 m long flight tube. The resolution of the apparatus, ΔEk/Ek, was about than 2.5%, that is, ∼25 meV for 1 eV electrons.
Theoretical methods
Search for the global minimum of CoB16− was performed using the Coalescence Kick program45 at the PBE0/3-21G level of theory46,47. The Coalescence Kick algorithm generated ∼10,000 trial structures for each spin multiplicity (singlet, triplet and quintet), followed by geometry optimization. Low-lying isomers within 25 kcal mol−1 were further refined at a more expansive basis set, Def2-TZVP48. For each structure, vibrational frequencies were calculated and imaginary frequencies were followed to ensure that the isomer corresponded to a true minimum on the potential energy surface. Spin contamination was found to be <10% in all DFT calculations. For selected isomers, we performed additional geometry optimization at various DFT levels, as well as more accurate single-point coupled-cluster calculations [ROCCSD(T)/6-311+G(2df)], to reliably establish the relative energy ordering. Vertical detachment energies of the three lowest energy structures were calculated at three different methods (UPBE0, UB3LYP and ROCCSD(T)) to compare with the experimental data. The VDEs were obtained as the difference in energy between the ground state of the anion and selected low-lying electronic states of the neutral molecule at the geometry of the anion. All calculations were done using GAUSSIAN-09 (ref. 49).
To understand the chemical bonding, we carried out electron localization analyses using the AdNDP method38 at the PBE0/6-31G(d) level of theory. Previously, AdNDP results have been shown to be insensitive to the level of theory or basis set used50. The AdNDP analysis is based on the concept of electron pairs as the main elements of chemical bonds. It represents the molecular electronic structure in terms of n-centre two-electron (nc–2e) bonds, recovering the familiar lone pairs (1c–2e) and localized 2c–2e bonds or delocalized nc–2e bonds (3≤n≤total number of atoms in the system). The MOLEKEL 5.4.0.8 program51 is used for molecular structure and AdNDP bond visualizations.
Additional information
How to cite this article: Popov, I. A. et al. Cobalt-centred boron molecular drums with the highest coordination number in the CoB16− cluster. Nat. Commun. 6:8654 doi: 10.1038/ncomms9654 (2015).
Supplementary Material
Supplementary Figures 1-8 and Supplementary Tables 1-3
Acknowledgments
This work was supported by the National Science Foundation (CHE-1263745 to L.S.W. and CHE-1361413 to A.I.B.). Computer, storage and other resources from the Division of Research Computing in the Office of Research and Graduate Studies at Utah State University are gratefully acknowledged.
Footnotes
Author contributions L.S.W. and A.I.B. designed the research. I.A.P. and A.I.B performed and analyzed the calculations. L.S.W., T.J. and G.V.L. designed experiments and analysed the experimental data. All authors contributed to the interpretation and discussion of the data. I.A.P. and T.J. wrote the manuscript.
References
- Albert B. & Hillebrecht H. Boron: elementary challenge for experimenters and theoreticians. Angew. Chem. Int. Ed. Engl. 48, 8640–8668 (2009). [DOI] [PubMed] [Google Scholar]
- Oganov A. R. et al. Ionic high-pressure form of elemental boron. Nature 457, 863–867 (2009). [DOI] [PubMed] [Google Scholar]
- Kuhlmann U. & Werheit H. Improved Raman effect studies on boron carbide (B4.3C). Phys. Status Solidi (b) 175, 85–92 (1993). [Google Scholar]
- Nelmes R. J. et al. Observation of inverted-molecular compression in boron carbide. Phys. Rev. Lett. 74, 2268–2271 (1995). [DOI] [PubMed] [Google Scholar]
- Kawai R. & Weare J. H. Instability of the B12 icosahedral cluster: rearrangement to a lower energy structure. J. Chem. Phys. 95, 1151–1159 (1991). [Google Scholar]
- Boustani I. A comparative study of ab initio SCF-CI and DFT. Example of small boron clusters. Chem. Phys. Lett. 233, 273 (1995). [Google Scholar]
- Hanley L., Whitten J. L. & Anderson S. L. Collision-induced dissociation and ab initio studies of boron cluster ions: determination of structures and stabilities. J. Phys. Chem. 92, 5803–5812 (1988). [Google Scholar]
- Alexandrova A. N., Boldyrev A. I., Zhai H. J. & Wang L. S. All-boron aromatic clusters as potential new inorganic ligands and building blocks in chemistry. Coord. Chem. Rev. 250, 2811–2866 (2006). [Google Scholar]
- Sergeeva A. P. et al. Understanding boron through size-selected clusters: structure, chemical bonding, and fluxionality. Acc. Chem. Res. 47, 1349–1358 (2014). [DOI] [PubMed] [Google Scholar]
- Li W. L., Pal R., Piazza Z. A., Zeng X. C. & Wang L. S. B27−: appearance of the smallest boron cluster containing a hexagonal vacancy. J. Chem. Phys. 142, 204305 (2015). [DOI] [PubMed] [Google Scholar]
- Li W. L., Zhao Y. F., Hu H. S., Li J. & Wang L. S. B30−: A quasiplanar chiral boron cluster. Angew. Chem. Int. Ed. Engl. 53, 5540–5545 (2014). [DOI] [PubMed] [Google Scholar]
- Li W. L. et al. The B35 cluster with a double-hexagonal vacancy: a new and more flexible structural motif for borophene. J. Am. Chem. Soc. 136, 12257–12260 (2014). [DOI] [PubMed] [Google Scholar]
- Piazza Z. A. et al. Planar hexagonal B36 as a potential basis for extended single-atom layer boron sheets. Nat. Commun. 5, 3113 (2014). [DOI] [PubMed] [Google Scholar]
- Kiran B. et al. Planar-to-tubular structural transition in boron clusters: B20 as the embryo of single-walled boron nanotubes. Proc. Natl Acad. Sci. USA 102, 961–964 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oger E. et al. Boron cluster cations: transition from planar to cylindrical structures. Angew. Chem. Int. Ed. Engl. 46, 8503–8506 (2007). [DOI] [PubMed] [Google Scholar]
- Chen Q. et al. Experimental and theoretical evidence of an axially chiral borospherene. ACS Nano. 9, 754–760 (2015). [DOI] [PubMed] [Google Scholar]
- Zhai H. J. et al. Observation of an all-boron fullerene. Nat. Chem 6, 727–731 (2014). [DOI] [PubMed] [Google Scholar]
- Romanescu C., Galeev T. R., Li W. L., Boldyrev A. I. & Wang L. S. Transition-metal-centered monocyclic boron wheel clusters (M©Bn): a new class of aromatic borometallic compounds. Acc. Chem. Res. 46, 350–358 (2013). [DOI] [PubMed] [Google Scholar]
- Zhai H. J., Alexandrova A. N., Birch K. A., Boldyrev A. I. & Wang L. S. Hepta- and octa-coordinated boron in molecular wheels of 8- and 9-atom boron clusters: observation and confirmation. Angew. Chem. Int. Ed. Engl. 42, 6004–6008 (2003). [DOI] [PubMed] [Google Scholar]
- Galeev T. R., Romanescu C., Li W. L., Wang L. S. & Boldyrev A. I. Observation of the highest coordination number in planar species: decacoordinated Ta©B10− and Nb©B10− anions. Angew. Chem. Int. Ed. Engl. 51, 2101–2105 (2012). [DOI] [PubMed] [Google Scholar]
- Zhai H. J., Wang L. S., Alexandrova A. N. & Boldyrev A. I. Electronic structure and chemical bonding of B5− and B5 by photoelectron spectroscopy and ab initio calculations. J. Chem. Phys. 117, 7917–7924 (2002). [Google Scholar]
- Alexandrova A. N., Boldyrev A. I., Zhai H. J. & Wang L. S. Electronic structure, isomerism, and chemical bonding in B7− and B7. J. Phys. Chem. A 108, 3509–3517 (2004). [Google Scholar]
- Piazza Z. A. et al. A photoelectron spectroscopy and ab initio study of B21−: negatively charged boron clusters continue to be planar at 21. J. Chem. Phys. 136, 104310 (2012). [DOI] [PubMed] [Google Scholar]
- Xu C., Cheng L. J. & Yang J. L. Double aromaticity in transition metal centered double-ring boron clusters M@B2n (M=Ti, Cr, Fe, Ni, Zn; n=6, 7, 8). J. Chem. Phys. 141, 124301 (2014). [DOI] [PubMed] [Google Scholar]
- Tam N. M., Pham H. T., Duong L. V., Pham-Ho M. P. & Nguyen M. T. Fullerene-like boron clusters stabilized by an endohedrally doped iron atom: B(n)Fe with n=14, 16, 18 and 20. Phys. Chem. Chem. Phys. 17, 3000–3003 (2015). [DOI] [PubMed] [Google Scholar]
- An W., Bulusu S., Gao Y. & Zeng X. C. Relative stability of planar versus double-ring tubular isomers of neutral and anionic boron cluster B20 and B20−. J. Chem. Phys. 124, 154310 (2006). [DOI] [PubMed] [Google Scholar]
- Sergeeva A. P. et al. B22− and B23−: all-boron analogues of anthracene and phenanthrene. J. Am. Chem. Soc. 134, 18065–18073 (2012). [DOI] [PubMed] [Google Scholar]
- Popov I. A., Piazza Z. A., Li W. L., Wang L. S. & Boldyrev A. I. A combined photoelectron spectroscopy and ab initio study of the quasi-planar B24− cluster. J. Chem. Phys. 139, 144307 (2013). [DOI] [PubMed] [Google Scholar]
- Romanescu C., Harding D. J., Fielicke A. & Wang L. S. Probing the structures of neutral boron clusters using IR/VUV two color ionization: B11, B16, and B17. J. Chem. Phys. 137, 014317 (2012). [DOI] [PubMed] [Google Scholar]
- Sergeeva A. P., Zubarev D. Y., Zhai H.-J., Boldyrev A. I. & Wang L. S. A photoelectron spectroscopic and theoretical study of B16− and B162−: an all-boron naphthalene. J. Am. Chem. Soc. 130, 7244–7246 (2008). [DOI] [PubMed] [Google Scholar]
- Li W. L., Romanescu C., Piazza Z. A. & Wang L. S. Geometrical requirements for transition-metal-centered aromatic boron wheels: the case of VB10−. Phys. Chem. Chem. Phys. 14, 13663–13669 (2012). [DOI] [PubMed] [Google Scholar]
- Popov I. A., Li W. L., Piazza Z. A., Boldyrev A. I. & Wang L. S. Complexes between planar boron clusters and transition metals: a photoelectron spectroscopy and ab initio study of CoB12− and RhB12−. J. Phys. Chem. A 118, 8098–8105 (2014). [DOI] [PubMed] [Google Scholar]
- Daly S. R. et al. Synthesis and properties of a fifteen-coordinate complex: the thorium aminodiboranate [Th(H3BNMe2BH3)4]. Angew. Chem. Int. Ed. Engl 49, 3379–3381 (2010). [DOI] [PubMed] [Google Scholar]
- Hermann A., Lein M. & Schwerdtfeger P. The Gregory-Newton problem of kissing sphere applied to chemistry: the search for the species with the highest coordination number. Angew. Chem. Int. Ed. Engl. 46, 2444–2447 (2007). [DOI] [PubMed] [Google Scholar]
- Komura Y. & Tokunaga K. Structural studies of stacking variants in Mg-base Friauf-Laves phases. Acta Crystallogr. Sect. B 36, 1548–1554 (1980). [Google Scholar]
- Wang L. S. et al. The electronic structure of Ca@C60. Chem. Phys. Lett. 207, 354–359 (1993). [Google Scholar]
- Popov A. A., Yang S. & Dunsch L. Endohedral fullerenes. Chem. Rev. 113, 5989–6113 (2013). [DOI] [PubMed] [Google Scholar]
- Zubarev D. Y. & Boldyrev A. I. Developing paradigms of chemical bonding: adaptive natural density partitioning. Phys. Chem. Chem. Phys. 10, 5207–5217 (2008). [DOI] [PubMed] [Google Scholar]
- Foster J. P. & Weinhold F. Natural bond orbitals. J. Am. Chem. Soc. 102, 7211–7218 (1980). [Google Scholar]
- Yuan Y. & Cheng L. J. B142+: a magic number double-ring cluster. J. Chem. Phys. 137, 044308 (2012). [DOI] [PubMed] [Google Scholar]
- Johansson M. P. On the strong ring currents in B20 and neighboring boron toroids. J. Phys. Chem. C. 113, 524–530 (2009). [Google Scholar]
- Yang L.-M., Wang J., Ding Y.-H. & Sun C.-C. Sandwich-like compounds based on bare all-boron cluster B62−. Phys. Chem. Chem. Phys. 10, 2316–2320 (2008). [DOI] [PubMed] [Google Scholar]
- Fokwa B. P. T. & Hermus M. All-boron planar B6 ring in the solid-state phase Ti7Rh4Ir2B8. Angew. Chem. Int. Ed. Engl. 51, 1702–1705 (2012). [DOI] [PubMed] [Google Scholar]
- Wang L. S., Cheng H. S. & Fan J. W. Photoelectron spectroscopy of size-selected transition metal clusters: Fen−, n=3−24. J. Chem. Phys 102, 9480–9493 (1995). [Google Scholar]
- Sergeeva A. P., Averkiev B. B., Zhai H. J., Boldyrev A. I. & Wang L. S. All-boron analogues of aromatic hydrocarbons: B17− and B18−. J. Chem. Phys. 134, 224304 (2011). [DOI] [PubMed] [Google Scholar]
- Adamo C. & Barone V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999). [Google Scholar]
- Binkley J. S., Pople J. A. & Hehre W. J. Self-consistent molecular orbital methods. 21. Small split-valence basis sets for first-row elements. J. Am. Chem. Soc. 102, 939–947 (1980). [Google Scholar]
- Weigend F. & Ahlrichs R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005). [DOI] [PubMed] [Google Scholar]
- Frisch M. J. et al. GAUSSIAN09, Revision B.01 (Gaussian, Inc., 2009).
- Sergeeva A. P. & Boldyrev A. I. The chemical bonding of Re3Cl9 and Re3Cl92− revealed by the adaptive natural density partitioning analyses. Comm. Inorg. Chem. 31, 2–12 (2010). [Google Scholar]
- Varetto U. MOLEKEL 5.4.0.8 (Swiss National Supercomputing Centre, 2009).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-8 and Supplementary Tables 1-3