Supplemental Digital Content is available in the text.
Keywords: epidemiology, patient outcome assessment, peripheral vascular diseases, risk factors
Background—
There are few published data on the incidence and long-term outcomes of critical limb ischemia, acute limb ischemia, or acute visceral ischemia with which to inform health service planning, to monitor prevention, and to enable risk prediction.
Methods and Results—
In a prospective population-based study (Oxfordshire, UK; 2002–2012), we determined the incidence and outcome of all acute peripheral arterial events in a population of 92 728. Risk factors were assessed by comparison with the underlying population. A total of 510 acute events occurred in 386 patients requiring 803 interventions. Two hundred twenty-one patients (59.3%) were ≥75 years of age, and 98 (26.3%) were ≥85 years old. Two hundred thirty patients (62.3%) were independent before the event, but 270 (73.4%) were dead or dependent at the 6-month follow-up, and 328 (88.9%) were dead or dependent at 5 years. The 30-day survival was lowest for patients with acute visceral ischemia (28.2%) compared with acute limb ischemia (75.3%) and critical limb ischemia (92.6%; P<0.001). Risk factors (all P<0.001) were hypertension (age- and sex-adjusted risk ratio, 2.75; 95% confidence interval, 1.95–3.90), smoking (adjusted risk ratio, 2.14; 95% confidence interval, 1.37–3.34), and diabetes mellitus (adjusted risk ratio, 3.01; 95% confidence interval, 1.69–5.35), particularly for critical limb ischemia (adjusted risk ratio, 5.96; 95% confidence interval, 3.15–11.26). Two hundred eighty-eight patients (77.2%) had known previous cardiovascular disease, and 361 (96.8%) had vascular risk factors, but only 203 (54.4%) were on an antiplatelet and only 166 (44.5%) were on a statin. Although 260 patients (69.7%) were taking antihypertensives, 42.9% of all blood pressures recorded during the 5 years before the event were >140/90 mm Hg. Of 88 patients (23.6%) with incident cardioembolic events, 62 had known atrial fibrillation (diagnosed before the event), of whom only 14.5% were anticoagulated despite 82.3% having a CHA2DS2VASC score ≥2 without contraindications.
Conclusions—
The clinical burden of peripheral arterial events is substantial. Although the vast majority of patients have known vascular disease in other territories and multiple treatable risk factors, premorbid control is poor.
Vascular disease is the leading cause of death and disability worldwide.1–3 Peripheral arterial disease (PAD) has a poor prognosis4,5 but has been neglected in terms of both research and early detection and primary prevention.6,7 Previous epidemiological studies have concentrated on the prevalence of stable PAD (intermittent claudication or subclinical disease),8–19 and studies of critical limb ischemia (CLI) have been limited to selected cohorts20–22 or based on hospital coding data of interventions or amputation outcome,23–27 cross-sectional surveys,28 or randomized, interventional trials with various inclusion/exclusion criteria.29–31 Epidemiological studies of acute limb ischemia (ALI) and acute visceral ischemia (AVI) are lacking, with current estimates of incidence and outcome being based on hospital registries,31,32 interventional trials,33,34 and autopsy studies.35,36 The comparative epidemiology and relative clinical burdens of all acute PAD syndromes have not been determined in the same population.
Clinical Perspective on p 1815
PAD events also tend not to be reported as outcomes in cardiovascular disease prevention trials,37 or reporting is limited.38–40 Of the 91 largest blood pressure (BP)–lowering treatment trials, only 11 included PAD as a discrete outcome (Table I in the online-only Data Supplement). However, several strategies for primary and secondary prevention of PAD events in at-risk groups are evidence based, including antiplatelet, statin, and antihypertensive therapy; smoking cessation; and supervised exercise program.41–43 In addition, anticoagulation is highly effective in the prevention of atrial fibrillation–related peripheral embolic events.44 Population-based data on the incidence, outcome, risk factors, and current levels of preventive treatment are needed to improve the prevention of PAD events, to enable risk stratification, to inform patients about risks and prognosis, to facilitate health service planning, and to direct future research.
We prospectively ascertained all acute PAD events in patients presenting to medical attention, regardless of age, in a population of 92 728 in Oxfordshire, UK, during 2002 to 2012 (Oxford Vascular Study [OXVASC])45 and determined the incidence, outcome, risk factors, premorbid preventive treatment, and long-term prognosis.
Methods
The OXVASC study population comprises all individuals (10-year average, 92 728), regardless of age, registered with ≈100 family physicians in 9 primary care practices in Oxfordshire, UK.45,46 In the United Kingdom, the vast majority of individuals register for primary health care, which provides a lifelong record of all medical consultations and details of medications, BP measurements, and investigations. All participating practices held accurate age/sex patient registers and allowed regular searches of their computerized diagnostic coding systems. All practices refer patients to only 1 major secondary care center.
The OXVASC study population is 94% white, 3.1% Asian, 1.5% Chinese, and 1.4% Afro-Caribbean.46 On the basis of the Index of Multiple Deprivation,47 the electoral wards covering our population are less deprived than the rest of England (mean Index of Multiple Deprivation score, 8.69 versus 16.98; t test, P<0.001) but have a broad range of deprivation, with 22% of wards ranking in the lower third nationally. OXVASC was approved by our local research ethics committee.
Case ascertainment was by prospective daily searches for acute events in hospital (hot pursuit), supplemented by searches of discharge and primary care diagnostic coding data (cold pursuit). Full details are given in Methods in the online-only Data Supplement. A study clinician assessed patients acutely in hospital (or at home if ascertained late). Informed consent was sought when possible, or assent was obtained from a relative. Standardized clinical history and examination were recorded, with details of medication, history, and all investigations and interventions occurring subsequent to the event. All diagnoses were reviewed by a vascular surgeon. If a patient died before assessment or was identified only by cold pursuit, eyewitness accounts were obtained and relevant records reviewed. If death occurred outside the hospital or before investigation, autopsy results were reviewed. Clinical details were sought from primary care physicians or other clinicians on all deaths resulting from a possible vascular cause.
Cardiovascular examination included assessment of the peripheral pulses, the Buerger test, and absolute ankle pressure and ankle-brachial pressure index recordings. For patients with incompressible ankle signals, pressures were estimated by pole test. For patients in whom clinical vascular assessment was not possible by the study clinician before urgent revascularization or death, the assessments made by the admitting clinician were used.
All survivors were followed up by a research nurse at 6 months and subsequently by their family doctor, with recurrent events also identified by the ongoing study ascertainment. If a vascular event was suspected, the patient was reassessed by a study physician. Premorbid disability and disability on follow-up were assessed with the modified Rankin Scale.48
To assess key vascular risk factors and levels of premorbid control, we extracted individual data from primary healthcare records for all cases and compared these data with group average data for the underlying unaffected population. The accuracy of these data was tested in cases through direct questioning of patients and relatives and review of records. All premorbid BP measurements were extracted from primary care records of incident cases. BP was generally taken with automated sphygmomanometers by a primary care physician or practice nurse. A cutoff value of 140/90 mm Hg was used to define hypertension.
All patients with acute PAD events from April 1, 2002, to March 31, 2012, from the study registered practices were included. Events were defined as any acute arterial event that affected a limb or an organ other than the heart or the brain/eye and led to hospital assessment/admission or caused death in the community. The likely cause (atherosclerotic/in situ thrombosis, embolic, diabetic microvascular disease, iatrogenic, graft/stent thrombosis, or multifactorial) was determined by a vascular surgeon taking into account clinical findings and subsequent investigations.
ALI was defined as an arterial event of sudden onset and <2 weeks in duration resulting in symptomatic limb ischemia.5 Degree of limb ischemia was graded by the Rutherford classification of severity as viable, threatened-marginal, threatened-immediate, or irreversible.49 AVI was defined as acute arterial events of sudden onset and <2 weeks in duration resulting in symptomatic visceral ischemia (including bowel, liver, spleen, and renal end-organ compromise). Severity of ischemia was graded by the presence or absence of lactic acidosis on admission. CLI has several overlapping diagnostic classification systems.5,41,49,50 The original Fontaine and Rutherford classification systems describe symptoms persisting for >2 weeks and delineate intermittent claudication from ischemic rest pain and ulceration. More recent defining criteria from the European Union,50 Society of Vascular Surgery (United States),49 and Trans-Atlantic Inter-Society Consensus (TASC) steering committee5 require both clinical and objective assessment of absolute ankle pressure, ankle-brachial pressure index, or toe pressure (eg, plethysmographic or laser Doppler techniques). As pointed out in the latest TASC consensus statement,5 strict objective criteria exclude some patients with imminently threatened ischemic limbs in need of urgent revascularization. We therefore included all patients whose symptoms had been present for >2 weeks with ischemic rest pain or tissue loss of sufficient severity to warrant urgent hospital admission and thought to be secondary to large- or small-vessel arterial disease. Objective measurements, although performed, were not required for inclusion. Incidence was compared on the basis of all current diagnostic criteria.
Analysis
Event rates were defined as the total number of PAD events that led to separate clinical presentations during the study period. Individual patients could have multiple separate events in the same arterial territory. All events were categorized as first-ever incident or recurrent and specific to territory. An incident event implied the first-ever event of that type that a patient experienced in a given vascular territory. A patient could theoretically have 1 incident event of each type (AVI, CLI, and ALI). However, second events of the same type were always classified as recurrent, even if affecting a different limb.
Patients who had an event while temporarily away from Oxfordshire were assessed on their return and included in the analysis, but visitors to Oxfordshire who were not registered with one of the study practices were excluded. The underlying study population was based on the mean of the 10 midyear population age/sex structures. Age-, sex-, and risk factor–specific rates (per 100 000 population per year) were calculated for all incident events of each type. Analyses were done with SPSS (version 20.0; SPSS Inc, Chicago, IL). Numerical data were expressed as means (standard deviations) or proportions as appropriate. Group differences in continuous parametric variables (eg, age) were examined with the Student t test or 1-way ANOVA as appropriate. Group differences in categorical variables were examined with the Fisher exact test or χ2 test as appropriate. Risk ratios were adjusted for age and sex and corrected for overdispersion. Odds ratios were calculated by univariable and multivariable binary logistic regression analyses. In multivariable analysis, odds ratios were adjusted for all other risk predictors that were assessed. Outcome was reported in terms of functional status, amputation, amputation-free survival, and overall survival by Kaplan-Meier analysis. Values of P<0.05 were considered statistically significant.
Results
A total of 510 acute PAD events occurred in 386 patients requiring 803 interventions. Of 373 incident events, 202 were incident CLI events (22 per 100 000 per year; 95% confidence interval [CI], 19–25; median age,75.2 years; 54.0% male), 93 were incident ALI events (10 per 100 000 per year; 95% CI, 8–12; age, 76.3 years; 52.7% male), and 78 were incident AVI events (8 per 100 000 per year; 95% CI, 7–11; age, 79.8 years; 35.9% male; Tables II and III in the online-only Data Supplement). Event incidence and distribution did not vary significantly across the practices over the 12-year period
Twenty-two patients had incident events of differing types (3 had an incident CLI event during follow-up after an incident ALI event, 12 had an ALI event after a previous CLI event, and 7 had an AVI event after a CLI event.) The remaining events were episodes of the same type as the first event and thus were classified as recurrent (many were CLI events in the contralateral lower limb). Lower-limb ischemia was significantly more common than upper-limb ischemia (78:15 for ALI and 202:0 for CLI). Of 373 patients, 94 (25.2%) underwent emergency intervention within 48 hours, 14 (18.1%) of whom had multiple interventions (Table IV in the online-only Data Supplement).
Table 1 shows the demographics, risk factors, and causes of all incident events by type and sex. Mean age at event was similar for both ALI and CLI at 76.3 years (SD, 11.9 years) and 75.2 years (SD, 10.9 years), respectively, but higher for AVI events at 79.8 years (SD, 14.1 years; P=0.02). Age at event was significantly higher for women than for men for all types of event (79.3 years [SD, 10.8 years] versus 73.6 years [12.5 years]; P<0.001; Table 1). Incidence rates for all event types increased steeply with age, with 221 events (59.3%) occurring at ≥75 years of age and 98 (26.3%) at ≥85 years of age (Figure 1 and Table II in the online-only Data Supplement).
Table 1.
Demographics, Pathogenesis, and Risk Factors for Incident Events in All Territories

Figure 1.
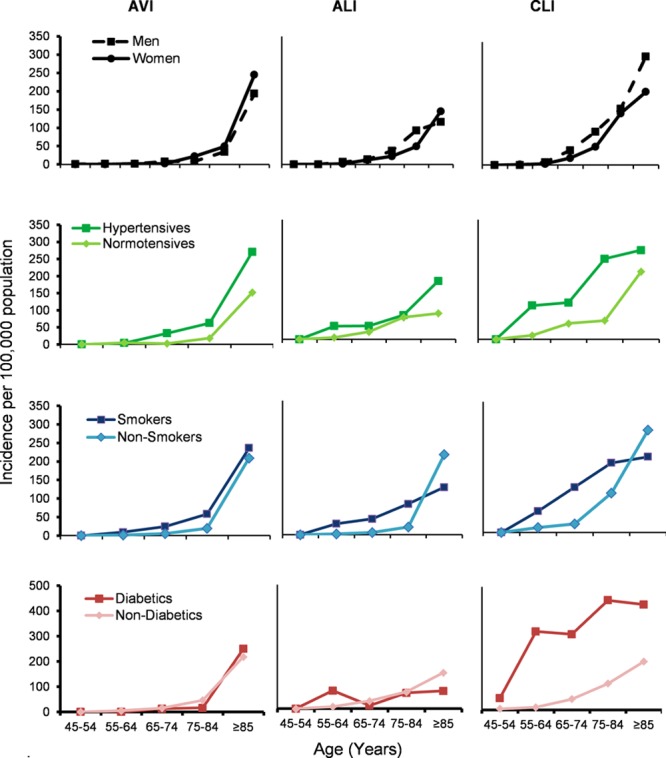
Age-, sex-, and risk factor–specific rates per 100 000 population (2002–2012) for all incident acute peripheral arterial events. ALI indicates acute limb ischemia; AVI, acute visceral ischemia; and CLI, critical limb ischemia.
For CLI, the Society of Vascular Surgery consensus criteria were the strictest and resulted in the lowest age-specific incidence (Figure 2), particularly in the older age groups, whereas the 2007 Trans-Atlantic Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) clinical criteria are similar to the OXVASC inclusion criteria and thus resulted in similar incidence rates. Routine hospital episode and death coding data missed 192 of 373 incident events (51.5%; Figure 3) and 165 of 346 events (47.7%) apparently identified by routine coding were not acute PAD events (They were either elective admissions for stable disease such as intermittent claudication or incorrectly coded admissions for sepsis or venous disease).
Figure 2.
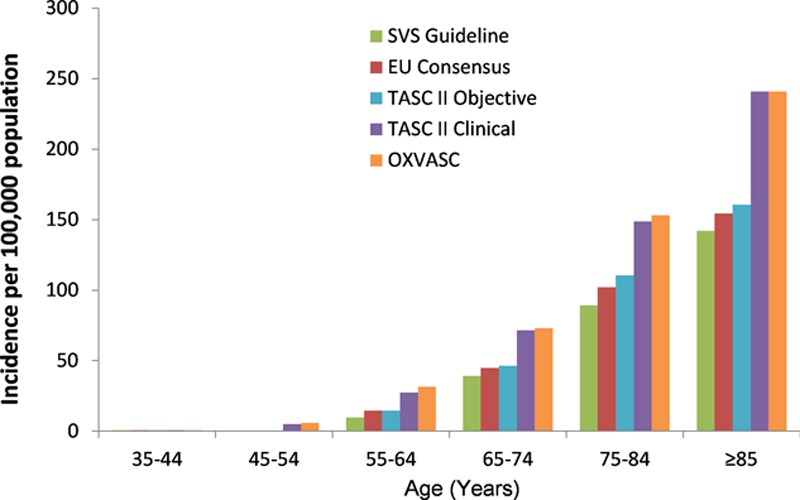
Comparative age-specific rates for incident episodes of critical limb ischemia as defined by current recognized classification systems. EU indicates European Union; OXVASC, Oxford Vascular Study; SVS, Society for Vascular Surgery; and TASC II, Trans-Atlantic Inter-Society Consensus for the Management of Peripheral Arterial Disease.
Figure 3.

The efficacy of routine coding (hospital discharge, hospital death, and primary care death coding) in identifying ischemic peripheral arterial events compared with Oxford Vascular Study (OXVASC) ascertainment.
Overall, 288 patients (77.2%) with incident PAD events had previous vascular disease in any territory, with stable PAD being most common in those with CLI events (70.8%) versus ALI (41.9%) and AVI (20.5%) events (P<0.001; Table 1). Three hundred sixty-one patients (96.8%) had known vascular risk factors. Risk factors for atherosclerosis (hypertension, smoking, diabetes mellitus, hyperlipidemia, and male sex) were most prevalent in patients with CLI events (≥3 risk factors: 61.9% for CLI, 44.9% for AVI, and 39.8% for ALI; P=0.001). In contrast, previous atrial fibrillation was most common in patients with AVI and ALI events and least prevalent in those with CLI events (37.2% for AVI, 38.7% for ALI, and 20.8% for CLI events; P=0.001; Table 1 and Table III in the online-only Data Supplement). The most prevalent underlying cause was embolism for AVI and ALI events (55.1% and 46.2%) and atherosclerosis for CLI events (70.6%; the remainder being multifactorial, most commonly [18.8%] atherosclerosis plus diabetic microvascular disease; Table 1 and Table III in the online-only Data Supplement).
Compared with the underlying study population, incident PAD events were associated with known previous hypertension (risk ratio, 2.75; 95% CI, 1.95–3.90; P<0.001), ever smoking (risk ratio, 2.14; 95% CI, 1.37–3.34; P<0.001), and diabetes mellitus (risk ratio, 3.01; 95% CI, 1.69–5.35; P<0.001; Table 2, Figure 1, and Figure I and Table V in the online-only Data Supplement). Previous diabetes mellitus was more prevalent in those with CLI events (44.1%) compared with ALI (12.9%) and AVI (11.5%) events (P<0.001). Mean age at the incident event was highly correlated with the number of underlying atherosclerotic risk factors, especially for CLI. Patients with CLI with >3 risk factors had events ≈8 years younger than those with <2 risk factors (P=0.007; Table 3).
Table 2.
Age- and Sex-Adjusted Risk Ratios for the Presence of Key Risk Factors in Those With Acute Events Compared With the Remaining Underlying Study Population (Controls) Stratified by Event Type
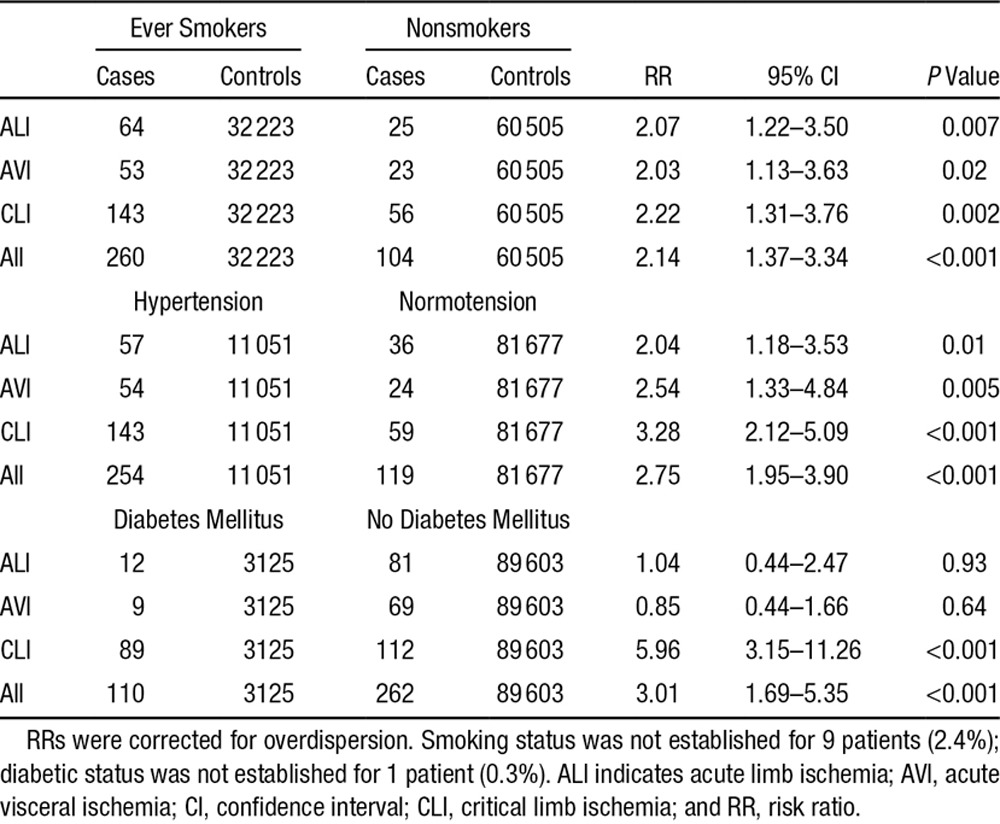
Table 3.
Mean Age at Event Stratified by the Presence of Multiple Atherosclerotic Risk Factors (Smoking History, Hypertension, Diabetes Mellitus, Hyperlipidemia, and Male Sex)

Despite the high prevalence of cardiovascular risk factors and known arterial disease, only 203 patients (54.4%) were on an antiplatelet drug, 166 (44.5%) were on a statin, and 260 (69.7%) were on antihypertensive medication. Of 373 patients (93.0%) with incident events, 347 had premorbid BP measurements taken in primary care with a mean of 22.6 (SD, 20.4) readings per patient. Of all BPs recorded in all patients during the 5 years before the incident event, 42.9% (SD, 36.1%) were >140/90 mm Hg (43.7% [SD, 32.6%] for AVI; 42.1% [SD, 38.6%] for ALI; 43.0% [SD, 36.4%] for CLI; P=0.96). During the 15 years before the event, 56.1% (30.8) of readings were >140/90 mm Hg, with a greater proportion of hypertensive readings in women (60.7% [SD, 27.9%] versus 51.3% [SD, 32.9%] in men; P=0.004). Mean systolic BP was greatest for ALI cases (151.2 mm Hg [SD,17.3 mm Hg] compared with 139.7 mm Hg [SD, 37.1 mm Hg] for AVI and 142.2 mm Hg [33.7 mm Hg] CLI) and was greater in women (148.2 mm Hg [SD, 27.5 mm Hg] versus 139.3 mm Hg [SD, 34.9]; P=0.008). Among those patients who had been started on antihypertensive treatment, 50.2% of subsequent BP readings still exceeded 140/90 mm Hg despite the majority being on combination therapy (30.0% monotherapy, 34.2% dual therapy, 35.8% triple or greater therapy).
Of 88 patients (23.6%) with incident cardioembolic events (Table VI in the online-only Data Supplement), 62 had known atrial fibrillation (diagnosed before the event), of whom only 9 (14.5%) were anticoagulated despite 51 (82.3%) having CHA2DS2VASC scores ≥2 without contraindications to anticoagulation.
Figure 4 and Table VII in the online-only Data Supplement show the premorbid and 6-month postevent disability status. Premorbid levels of disability (modified Rankin Scale score >2) was similar for AVI (43.6%), ALI (40.7%), and CLI (34.0%; P=0.24). Of 230 of 369 patients (62.3%; modified Rankin Scale score missing in 4 cases) who were independent before the event (modified Rankin Scale score ≤2), only 99 (26.6%) remained alive and independent at 6 months and only 41 (11.1%) at 5 years after the event, with outcomes being worst for AVI.
Figure 4.

Changes in disability status for patients with incident acute events stratified by event type. ALI indicates acute limb ischemia; AVI, acute visceral ischemia; CLI, critical limb ischemia; and mRS, modified Rankin Scale.
Figure 5 and Figure II in the online-only Data Supplement show amputation rates, amputation-free survival, and overall survival. In patients with CLI and ALI events, there was a significant risk of future limb loss, which was similar at 1 year (6.6% and 7.5%, respectively) but greater for CLI events at 5 years (43.4% versus 16.9%; P=0.01). Amputation-free survival was initially lower for ALI compared with CLI at 3 months (59.1% versus 75.7%; P=0.001), but by 5 years, the curves had crossed (36.7% versus 27.1% for CLI). Overall survival at 30 days and 5 years was lowest for AVI events (28.2% and 24.4%) compared with ALI (75.3% and 55.9%) and CLI (92.6% and 70.8%; P<0.001). The out-of-hospital death rates (deaths in the community before hospital admission) were low but were higher for patients with AVI events (6.4%) compared with those with ALI (5.4%) and CLI (0.0%) events (P=0.002).
Figure 5.
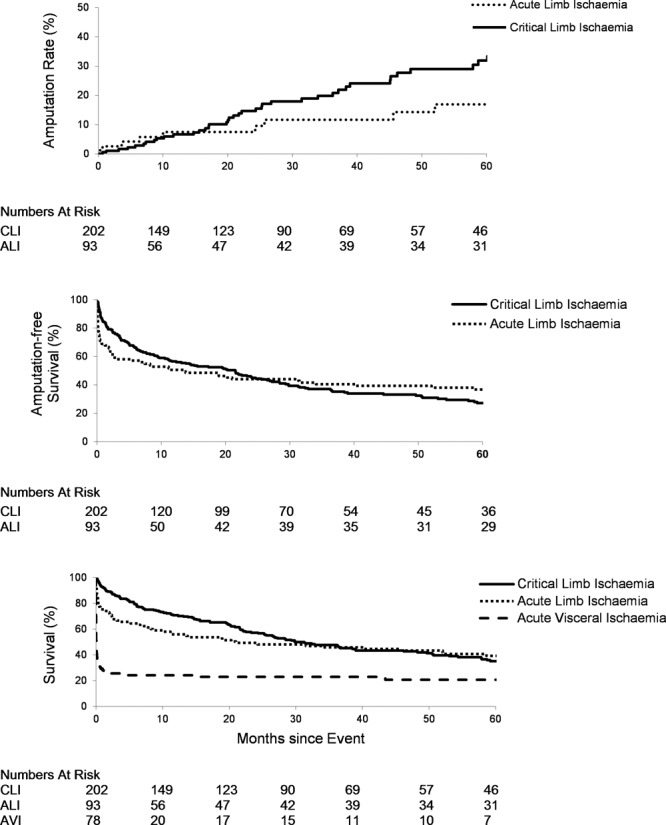
Five-year rates of major amputation, amputation-free survival, and overall survival for incident acute peripheral arterial events by subtype with numbers at risk tabled below. ALI indicates acute limb ischemia; and CLI, critical limb ischemia.
Severity of ischemia on admission was associated with 30-day amputation or death and 1-year mortality for all event types (Table 4 and Table VIII in the online-only Data Supplement). For patients with ALI, male sex, renal dysfunction, and previous coronary artery disease were independently associated with 30-day amputation or death, and age and renal dysfunction were associated with 1-year mortality. For AVI, age and hypertension were associated with 1-year mortality, whereas for CLI, previous heart failure and renal dysfunction were related. In addition, for CLI, admission ankle-brachial pressure index was associated with 1-year amputation-free survival (risk ratio, 2.4; 95% CI, 1.7–8.6; P=0.04). The 30-day amputation-free survival rate stratified by severity of ischemia was 100% for viable, 79.5% for threatened, and 4.8% for irreversible ischemia for ALI and 100% for rest pain only (Rutherford 4), 83.7% for minor tissue loss (Rutherford 5), and 41.7% for major tissue loss (Rutherford 6; Table IX in the online-only Data Supplement) for CLI. The severity of event and 1-year fatality increased significantly with age for all event types (Table X in the online-only Data Supplement). AVI has the highest 1-year fatality of all acute vascular events (Figure III in the online-only Data Supplement).
Table 4.
Potential Risk Factors for 30-Day Amputation/Organ Loss/Death and 1-Year Mortality Stratified by Event Type (Multivariable Analysis)

Discussion
OXVASC is a comprehensive, population-based study of all acute vascular events without exclusion of patients by age or sex,45 including the first-ever large-scale prospective study of all acute ischemic PAD events. We showed that prospectively collected incidence data are substantially more accurate than routine hospital episode and death coding data. Because there is no International Classification of Diseases, 10th Revision code for CLI, previous estimates of incidence have been limited to codes that include stable vascular disease (intermittent claudication) or outcome codes such as amputation and death. In addition, our access to the lifelong medical records allowed us to reliably assess premorbid risk factors and their control.
We have several main findings. First, acute PAD events are a substantial disease burden with high case fatality, poor functional outcome, and high rates of emergency and subsequent vascular intervention to prevent death or limb loss. Second, AVI, ALI, and CLI differ with respect to risk factors, functional outcomes, and survival. Third, age of onset is correlated with degree of atherosclerotic risk factor burden. Fourth, major adverse outcomes (amputation and death) are associated with severity of disease-grading criteria and comorbidities such as renal dysfunction, diabetes mellitus, and premorbid cardiac failure. Finally, the vast majority of patients presenting with acute events had preexisting vascular disease or known vascular risk factors, highlighting a great potential for early recognition of risk and prevention.
The overall incidence of CLI was approximately twice that of ALI and AVI, but age trends in rates were similar for all event types, with the majority of events occurring at ≥75 years of age. Unlike coronary artery disease and acute aortic disease, the incidence of acute peripheral arterial events was similar in men and women, although women had events at an older age. However, the majority of patients were independent before the event, but only a quarter remained so at 6 months after the event. Early mortality increased with age for all event types.
The high rates of previous vascular disease in individuals presenting with acute peripheral vascular events suggest that limb ischemia and visceral ischemia are usually late-stage manifestations of vascular disease and that individuals suffering PAD events are highly likely to have systemic atherosclerosis affecting other vascular beds. There is therefore great potential for early recognition of risk. Risk factors strongly associated with atherosclerosis and previous atherosclerotic vascular diseases (acute coronary syndrome and intermittent claudication) were most prevalent in patients with CLI events, whereas previous atrial fibrillation was prevalent in those with AVI and ALI. Degree of atherosclerotic risk factor burden was also associated with prematurity of disease. For acute events of cardioembolic origin and known atrial fibrillation, premorbid levels of anticoagulation were very low despite the vast majority of patients being at high risk for thromboembolism (CHA2DS2VASC scores ≥2) without contraindications to anticoagulation.
AVI events had a 30-day and 1-year survival of only 28.2% and 24.4%, respectively. ALI and CLI also have a significant mortality at 1 year, and in surviving patients, there is a significant risk of amputation in both forms of limb disease, being greatest for CLI cases at 5 years (43.4%). The 30-day major amputation or death rate and 1-year mortality rate were associated with severity of ischemia on admission, renal dysfunction, previous cardiac failure, and age, whereas diabetes mellitus was associated with both short- and long-term risk of limb loss. These factors could be used to inform clinical management, particularly for diabetic patients who are at high risk of future amputation, so more aggressive early intervention may be warranted.
The potential limitations of OXVASC have been previously noted.45 First, by not recording the prevalence of patients with stable intermittent claudication, we are unable to estimate the total burden of PAD, although all acute events in such patients were studied. Second, the OXVASC population is predominantly white, so it is difficult to extrapolate the results to other ethnic groups. Third, the population has relatively lower deprivation indexes than the UK average. However, the general practices within OXVASC include the full range of deprivation. Fourth, for some specific age/sex subgroups, there were a limited number of cases. This will limit the ability to precisely estimate the incidence rates in these groups. Finally, we purposely avoided the use of objective measurements to exclude patients with possible CLI from the study and instead included all patients with documented limb ischemia with rest pain or tissue loss of sufficient severity to warrant urgent hospital admission. On one hand, this may have led to an overestimation of CLI incidence compared with studies using strict consensus criteria, but on the other, this has enabled us to perform the first fully inclusive population-based study of all acute peripheral vascular events.
Conclusions
The clinical burden of peripheral arterial events is substantial, with high case fatality, poor functional outcome, and high rates of emergency and subsequent vascular intervention to prevent death or limb loss. However, although premorbid function is good in the majority of patients and although the vast majority have known vascular disease in other territories and multiple treatable risk factors, premorbid control of risk factors is poor. It is likely that much of the current clinical burden of acute PAD is preventable by more effective use of existing preventive treatments.
Acknowledgments
We are grateful to all the staff in the Oxfordshire general practices that collaborated in OXVASC.
Sources of Funding
OXVASC is funded by the Wellcome Trust, UK Medical Research Council, Dunhill Medical Trust, Stroke Association, National Institute for Health Research (NIHR), and the NIHR Biomedical Research Center, Oxford. Dr Howard is funded by the NIHR Biomedical Research Center, Oxford. Dr Rothwell has received a Wellcome Trust Senior Investigator Award and an NIHR Senior Investigator Award.
Disclosures
None.
Supplementary Material
Footnotes
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.115.016424/-/DC1.
CLINICAL PERSPECTIVE
This is the first prospective, population-based study of all acute ischemic peripheral arterial events over a 10-year period in a population of 92 728 in Oxfordshire, UK. We have shown that the clinical burden of peripheral arterial events is substantial, with high case fatality, poor functional outcome, and high rates of emergency and subsequent vascular intervention to prevent death or limb loss. However, although premorbid function is good in the majority of patients and although the vast majority have known vascular disease in other territories and multiple treatable risk factors, premorbid control of risk factors is poor. It is likely that much of the current clinical burden of acute peripheral arterial disease is preventable by more effective use of existing preventive treatments.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez-Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, AlMazroa MA, Memish ZA. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Dwyer-Lindgren L, Lofgren KT, Rajaratnam JK, Marcus JR, Levin-Rector A, Levitz CE, Lopez AD, Murray CJ. Age-specific and sex-specific mortality in 187 countries, 1970-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2071–2094. doi: 10.1016/S0140-6736(12)61719-X. doi: 10.1016/S0140-6736(12)61719-X. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 5.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 6.Tomson J, Lip GY. Peripheral arterial disease: a high risk–but neglected–disease population. BMC Cardiovasc Disord. 2005;5:15. doi: 10.1186/1471-2261-5-15. doi: 10.1186/1471-2261-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaff MR. Why patients know more about cars than peripheral artery disease. Circulation. 2014;130:1778–1779. doi: 10.1161/CIRCULATIONAHA.114.012872. doi: 10.1161/CIRCULATIONAHA.114.012872. [DOI] [PubMed] [Google Scholar]
- 8.Widmer LK, da Silva A. Historical perspectives and the Basel study. In: Fowkes FGR, editor. Epidemiology of Peripheral Vascular Disease. London, UK: Springer-Verlag; 1991. pp. 69–83. [Google Scholar]
- 9.Leng GC, Lee AJ, Fowkes FG, Whiteman M, Dunbar J, Housley E, Ruckley CV. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1996;25:1172–1181. doi: 10.1093/ije/25.6.1172. [DOI] [PubMed] [Google Scholar]
- 10.Bainton D, Sweetnam P, Baker I, Elwood P. Peripheral vascular disease: consequence for survival and association with risk factors in the Speedwell prospective heart disease study. Br Heart J. 1994;72:128–132. doi: 10.1136/hrt.72.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowlin SJ, Medalie JH, Flocke SA, Zyzanski SJ, Goldbourt U. Epidemiology of intermittent claudication in middle-aged men. Am J Epidemiol. 1994;140:418–430. doi: 10.1093/oxfordjournals.aje.a117264. [DOI] [PubMed] [Google Scholar]
- 12.Murabito JM, D’Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication: a risk profile from the Framingham Heart Study. Circulation. 1997;96:44–49. doi: 10.1161/01.cir.96.1.44. [DOI] [PubMed] [Google Scholar]
- 13.Hughson WG, Mann JI, Garrod A. Intermittent claudication: prevalence and risk factors. Br Med J. 1978;1:1379–1381. doi: 10.1136/bmj.1.6124.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Backer G, Kornitzer M, Sobolski J, Denolin H. Intermittent claudication: epidemiology and natural history. Acta Cardiol. 1979;34:115–124. [PubMed] [Google Scholar]
- 15.Reunanen A, Takkunen H, Aromaa A. Prevalence of intermittent claudication and its effect on mortality. Acta Med Scand. 1982;211:249–256. doi: 10.1111/j.0954-6820.1982.tb01939.x. [DOI] [PubMed] [Google Scholar]
- 16.Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991;20:384–392. doi: 10.1093/ije/20.2.384. [DOI] [PubMed] [Google Scholar]
- 17.Stoffers HE, Rinkens PE, Kester AD, Kaiser V, Knottnerus JA. The prevalence of asymptomatic and unrecognized peripheral arterial occlusive disease. Int J Epidemiol. 1996;25:282–290. doi: 10.1093/ije/25.2.282. [DOI] [PubMed] [Google Scholar]
- 18.Smith WCS, Woodward M, Tunstall-Pedoe H. Intermittent claudication in Scotland. In: Fowkes FGR, editor. Epidemiology of Peripheral Vascular Disease. London, UK: Springer-Verlag; 1991. pp. 117–123. [Google Scholar]
- 19.Novo S, Avellone G, Di Garbo V, Abrignani MG, Liquori M, Panno AV, Strano A. Prevalence of risk factors in patients with peripheral arterial disease: a clinical and epidemiological evaluation. Int Angiol. 1992;11:218–229. [PubMed] [Google Scholar]
- 20.Catalano M. Epidemiology of critical limb ischaemia: north Italian data. Eur J Med. 1993;2:11–14. [PubMed] [Google Scholar]
- 21.Faglia E, Clerici G, Clerissi J, Gabrielli L, Losa S, Mantero M, Caminiti M, Curci V, Lupattelli T, Morabito A. Early and five-year amputation and survival rate of diabetic patients with critical limb ischemia: data of a cohort study of 564 patients. Eur J Vasc Endovasc Surg. 2006;32:484–490. doi: 10.1016/j.ejvs.2006.03.006. doi: 10.1016/j.ejvs.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Dormandy JA, Murray GD. Reprinted article “The fate of the claudicant: a prospective study of 1969 claudicants.”. Eur J Vasc Endovasc Surg. 2011;42(suppl 1):S4–S6. doi: 10.1016/j.ejvs.2011.06.014. doi: 10.1016/j.ejvs.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Feiring AJ, Krahn M, Nelson L, Wesolowski A, Eastwood D, Szabo A. Preventing leg amputations in critical limb ischemia with below-the-knee drug-eluting stents: the PaRADISE (PReventing Amputations using Drug eluting StEnts) trial. J Am Coll Cardiol. 2010;55:1580–1589. doi: 10.1016/j.jacc.2009.11.072. doi: 10.1016/j.jacc.2009.11.072. [DOI] [PubMed] [Google Scholar]
- 24.Bosiers M, Scheinert D, Peeters P, Torsello G, Zeller T, Deloose K, Schmidt A, Tessarek J, Vinck E, Schwartz LB. Randomized comparison of everolimus-eluting versus bare-metal stents in patients with critical limb ischemia and infrapopliteal arterial occlusive disease. J Vasc Surg. 2012;55:390–398. doi: 10.1016/j.jvs.2011.07.099. doi: 10.1016/j.jvs.2011.07.099. [DOI] [PubMed] [Google Scholar]
- 25.Melillo E, Nuti M, Bongiorni L, Golgini E, Balbarini A. Major and minor amputation rates and lower critical limb ischemia: the epidemiological data of western Tuscany [in Italian]. Ital Heart J Suppl. 2004;5:794–805. [PubMed] [Google Scholar]
- 26.Eskelinen E, Lepäntalo M, Hietala EM, Sell H, Kauppila L, Mäenpää I, Pitkänen J, Salminen-Peltola P, Leutola S, Eskelinen A, Kivioja A, Tukiainen E, Lukinmaa A, Brasken P, Railo M. Lower limb amputations in Southern Finland in 2000 and trends up to 2001. Eur J Vasc Endovasc Surg. 2004;27:193–200. doi: 10.1016/j.ejvs.2003.10.011. doi: 10.1016/j.ejvs.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Luther M, Kantonen I, Lepäntalo M, Salenius J FINNVASC Study Group. Arterial intervention and reduction in amputation for chronic critical leg ischaemia. Br J Surg. 2000;87:454–458. doi: 10.1046/j.1365-2168.2000.01354.x. doi: 10.1046/j.1365-2168.2000.01354.x. [DOI] [PubMed] [Google Scholar]
- 28.Critical limb ischaemia: management and outcome. report of a national survey: the Vascular Surgical Society of Great Britain and Ireland. Eur J Vasc Endovasc Surg. 1995;10:108–113. doi: 10.1016/s1078-5884(05)80206-0. [DOI] [PubMed] [Google Scholar]
- 29.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, Raab G, Ruckley CV. Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery-first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease: the Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial. Health Technol Assess. 2010;14:1–210, iii. doi: 10.3310/hta14140. doi: 10.3310/hta14140. [DOI] [PubMed] [Google Scholar]
- 30.Cull DL, Langan EM, Gray BH, Johnson B, Taylor SM. Open versus endovascular intervention for critical limb ischemia: a population-based study. J Am Coll Surg. 2010;210:555–561. doi: 10.1016/j.jamcollsurg.2009.12.019. doi: 10.1016/j.jamcollsurg.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 31.Earnshaw JJ, Whitman B, Foy C. National Audit of Thrombolysis for Acute Leg Ischemia (NATALI): clinical factors associated with early outcome. J Vasc Surg. 2004;39:1018–1025. doi: 10.1016/j.jvs.2004.01.019. doi: 10.1016/j.jvs.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Eliason JL, Wainess RM, Proctor MC, Dimick JB, Cowan JA, Jr, Upchurch GR, Jr, Stanley JC, Henke PK. A national and single institutional experience in the contemporary treatment of acute lower extremity ischemia. Ann Surg. 2003;238:382–389. doi: 10.1097/01.sla.0000086663.49670.d1. doi: 10.1097/01.sla.0000086663.49670.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs: Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N Engl J Med. 1998;338:1105–1111. doi: 10.1056/NEJM199804163381603. doi: 10.1056/NEJM199804163381603. [DOI] [PubMed] [Google Scholar]
- 34.Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity: the STILE trial. Ann Surg. 1994;220:251–266. doi: 10.1097/00000658-199409000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acosta S, Ogren M, Sternby NH, Bergqvist D, Björck M. Incidence of acute thrombo-embolic occlusion of the superior mesenteric artery: a population-based study. Eur J Vasc Endovasc Surg. 2004;27:145–150. doi: 10.1016/j.ejvs.2003.11.003. doi: 10.1016/j.ejvs.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Acosta S, Ogren M, Sternby NH, Bergqvist D, Björck M. Fatal nonocclusive mesenteric ischaemia: population-based incidence and risk factors. J Intern Med. 2006;259:305–313. doi: 10.1111/j.1365-2796.2006.01613.x. doi: 10.1111/j.1365-2796.2006.01613.x. [DOI] [PubMed] [Google Scholar]
- 37.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 38.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 39.Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45:645–654. doi: 10.1016/j.jvs.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 40.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of the randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke among high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:1425–1443. doi: 10.1161/CIR.0b013e31828b82aa. doi: 10.1161/CIR.0b013e31828b82aa. [DOI] [PubMed] [Google Scholar]
- 42.Board JBS. Joint British Societies’ consensus recommendations for the prevention of cardiovascular disease (JBS3). Heart. 2014;100(suppl 2):ii1–ii67. doi: 10.1136/heartjnl-2014-305693. [DOI] [PubMed] [Google Scholar]
- 43.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvänne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG) European guidelines on cardiovascular disease prevention in clinical practice (version 2012): the Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 44.You JJ, Singer DE, Howard PA, Lane DA, Eckman MH, Fang MC, Hylek EM, Schulman S, Go AS, Hughes M, Spencer FA, Manning WJ, Halperin JL, Lip GY American College of Chest Physicians. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e531S–e575S. doi: 10.1378/chest.11-2304. doi: 10.1378/chest.11-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, Redgrave JN, Bull LM, Welch SJ, Cuthbertson FC, Binney LE, Gutnikov SA, Anslow P, Banning AP, Mant D, Mehta Z Oxford Vascular Study. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 46.Office for National Statistics. 2001 and 2011 Census area statistics. http://www.ons.gov.uk/ons/guide-method/census/2011/index.html. Accessed January 2, 2015. [Google Scholar]
- 47.UK Department for Communities and Local Government. English indices of deprivation 2000 and 2010. https://www.gov.uk/government/publications/english-indices-of-deprivation-2010.Accessed January 2, 2015.
- 48.Farrell B, Godwin J, Richards S, Warlow C. The United Kingdom Transient Ischaemic Attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry. 1991;54:1044–1054. doi: 10.1136/jnnp.54.12.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, Jones DN. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–538. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 50.Second European consensus document on chronic critical leg ischaemia. Eur J Vasc Surg. 1992;6:1–28. [PubMed] [Google Scholar]


