Abstract
Background
Left atrial appendage (LAA) dysfunction predisposes patients with atrial fibrillation (AF) to cardioembolic stroke. Two-dimensional (2D) speckle tracking was reported to be useful for evaluating left atrial (LA) regional function, as well as left ventricular function. However, it remains unclear whether 2D speckle tracking is useful for evaluating LAA dysfunction. Therefore, we investigated whether decreased LA strain may predict LAA dysfunction and thrombus formation in patients with acute ischemic stroke.
Methods
We performed transthoracic and transesophageal echocardiography in 120 patients (83 males, mean age 72 ± 11 years) within 7 days of onset of an acute ischemic stroke. Longitudinal LA strain was evaluated using 2D speckle tracking imaging at each LA segment, and peak systolic strain was calculated by averaging the results for each segment.
Results
Forty-eight patients had LAA dysfunction as defined by the presence of LAA thrombus and/or severe spontaneous echo contrast. LA peak systolic strain was significantly decreased in patients with LAA dysfunction compared to those without (32.3 ± 13.7% vs. 12.1 ± 7.2%, p < 0.0001). LA peak systolic strain was significantly correlated with LAA emptying flow velocity (r = 0.693, p < 0.0001). The optimum LA peak systolic strain cut-off value for predicting LAA dysfunction was 19%. Multivariate logistic regression analysis showed that LA peak systolic strain was an independent predictor of LAA dysfunction (odds ratio 0.059, 95% confidence interval 0.018–0.146; p < 0.0001).
Conclusion
Decreased LA peak systolic strain was independently associated with LAA dysfunction in patients with acute ischemic stroke.
Keywords: Left atrial appendage function, Left atrial dysfunction, 2D speckle tracking, Ischemic stroke, Non-invasive
Highlights
-
•
LA peak systolic strain can be measured easily and reproducibly.
-
•
LA peak systolic strain was independently associated with LAA dysfunction.
-
•
LA peak systolic strain is a reliable marker for LAA dysfunction.
-
•
LA peak systolic strain may be useful for stratifying the risk of stroke.
1. Introduction
Cardioembolic stroke is an important clinical issue, because it is the most common cause of death in patients with acute ischemic stroke [1], [2]. The left atrial appendage (LAA) was reported to be a major source of thromboembolism in stroke patients with atrial fibrillation (AF) [3], [4], [5]. Many clinical reports have indicated that left atrial (LA) mechanical remodeling is associated with thrombus formation in the LAA [6], [7], [8]. The presence of spontaneous echocardiographic contrast or reduced LAA peak flow velocity, as measured by transesophageal echocardiography (TEE), was reported to be useful for detecting LAA dysfunction, which causes thrombus formation in the LAA [9], [10]. While transthoracic echocardiography (TTE) is widely used as a screening tool because it is a non-invasive procedure, it is thought to be difficult to detect LAA thrombus and evaluate LAA dysfunction by TTE. Recently, we reported that LAA wall velocity (LAWV) as measured by TTE was a useful parameter for measuring LAA function in patients with AF [11], [12].
Two-dimensional (2D) speckle-tracking strain imaging is a novel method for quantitative real-time assessment of regional myocardial deformation that uses tracking of acoustic speckles or kernels rather than Doppler myocardial velocities [13]. It has been suggested that LA strain, as measured by 2D speckle tracking, can be used to evaluate dynamic LA function [14]. However, the association between LA strain and LAA function remains unclear. The aim of this study was to elucidate whether LA peak systolic strain is a novel non-invasive parameter for detecting LAA dysfunction in patients with acute ischemic stroke.
2. Methods
2.1. Patients
We performed TTE and TEE in 127 patients referred to our hospital between April 2010 and November 2012 for treatment of acute cerebral infarction within seven days of onset. The median duration from stroke onset to time of TEE was 7 days (range 1–19). Patients with advanced malignant tumors, and/or infectious disease (n = 5), and those in whom TEE had failed (n = 2), were excluded. We enrolled 120 patients in the present study. We assessed the prevalence of risk factors for cerebral infarction at admission [15], [16]. All patients underwent cerebral computed tomography and/or magnetic resonance imaging, and then neurosurgeons diagnosed clinical ischemic categories. Clinical ischemic stroke category was defined by the National Institute of Neurological Disorders and Stroke (NINDS) [17], and disease severity was assessed using the US National Institute of Health Stroke Scale (NIHSS) [18]. Patients with a history of AF prior to admission and/or documented AF on continuous electrocardiographic (ECG) monitoring during hospitalization were defined as patients with AF.
According to the NINDS clinical categorization, computed tomography findings, magnetic resonance imaging and TEE results, patients were categorized into two groups based on the incidence of cardioembolic stroke (n = 53, mean age 76 ± 9 years) or non-cardioembolic stroke (n = 67, mean age 69 ± 11 years).
2.2. Echocardiography
TTE was performed on a Vivid E9 ultrasound instrument (GE healthcare, Wauwatosa, WI, USA), equipped with a sector transducer (carrier frequency 2.5 or 3.75 MHz). A 5 MHz phased array multiplane probe was used for TEE. The following parameters were assessed using standard views and techniques [19]: left atrial dimension (LAD), left ventricular end-diastolic dimension (LVDd), left ventricular ejection fraction (LVEF), and the ratio of peak early mitral annular velocity to Ewave (E/E′), as measured by TTE. LA volume was assessed at LV end-systole by using the biplanar area-length method from 4- and 2-chamber views. Measurements of LA volume were indexed by body surface area (LA volume index; LAVI) [19]. Tissue Doppler velocities were measured at the septal and lateral annuli using spectral Doppler tissue imaging. LAWV, defined as LAA peak wall velocity, was measured using tissue Doppler imaging with the sample volume of pulsed-wave Doppler placed on the LAA tip [11], [12].
LAA thrombus was diagnosed when a fixed or mobile echogenic mass could be clearly differentiated from the wall of the LA or LAA. Spontaneous echo contrast was considered to be present when dynamic, “smoke-like” echoes were seen within the atria and could not be eliminated by changes in the gain settings [20], [21]. As previously reported by Fatkin D et al., spontaneous echo contrast was graded from 0 to 4 + by two independent echocardiologists and severe spontaneous echo contrast was judged to be 4 + [10]. LAA emptying flow velocity (eV) was measured using pulsed wave Doppler, with the sample volume placed 1 cm distal from the mouth of the appendage by TEE. LAA eV within each RR interval was determined by scanning the appendage at angles between 0° and 90° [22]. In patients with AF, echocardiographic parameters such as LAA eV and LA strain were calculated as the mean values from five cardiac cycles. We carefully measured parameters only in those cycles in which the preceding and measured cardiac cycles were nearly equivalent. All findings were evaluated by two independent experienced echocardiologists, who were blinded to the clinical and other details of the patients. LAA dysfunction was defined as the presence of LAA thrombus and/or severe spontaneous echo contrast [9], [10]. Patients were categorized into two groups based on the presence of LAA dysfunction.
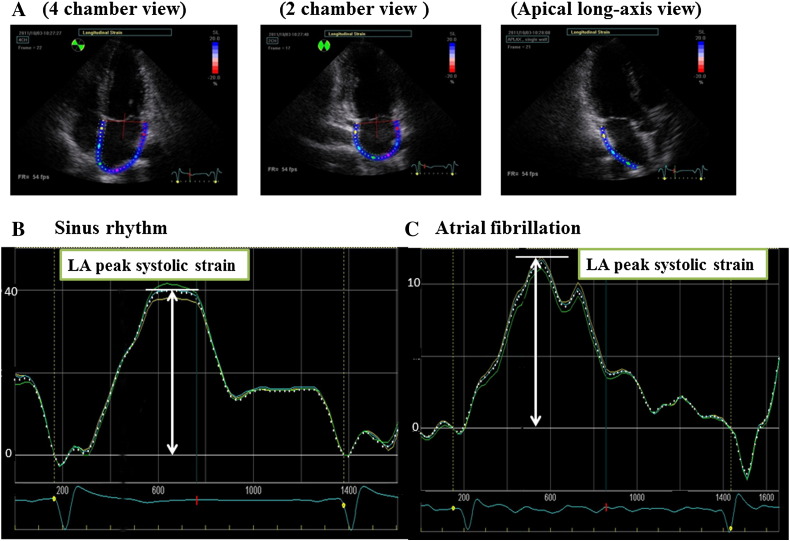
LA strain was estimated as the average of longitudinal strain data from the apical four-chamber, two-chamber and apical long axis views. The LA myocardium was divided into five regions of equal area. Five segments from the apical four- and two-chamber views were analyzed, whereas only three segments in the apical long axis view were analyzed because the remaining two segments in this view are parts of the aortic valve and ascending aorta (Fig. 1). A total of 13 LA segments were analyzed. Each regional LA strain curve was divided into component parts representing a different phase of the LA cardiac cycle. LA peak systolic strain was measured during LA relaxation and calculated in early LV diastole near the time of the Doppler E-wave [14].
Fig. 1.
Measurement of LA peak systolic strain. LA strain was estimated by averaging the longitudinal strain data obtained from the apical four-chamber, two-chamber and apical long-axis views. The LA myocardium was divided into five regions of equal area. Five segments were analyzed from the apical four- and two-chamber views, whereas only three segments were analyzed in the apical long axis view. LA, left atrium. We show good LA strain with sinus rhythm in Fig. 1B and bad LA strain with atrial fibrillation (Fig. 1C).
Reproducibility of LA peak systolic strain measurement was assessed in 20 randomly selected subjects. Intra- and inter-observer variability presented by intra-class correlation (ICC) coefficient were calculated using images independently recorded in two different occasions by the same investigator or by two different observers. Intra- and inter-observer ICCs of LA peak systolic strain were 0.99 and 0.97. Reproducibility was good.
2.3. Hemostatic markers
Blood samples were collected to determine the serum hemostatic marker levels at the time of the echocardiographic studies. General biochemical parameters were measured using routine laboratory methods.
2.4. Statistical analysis
Results are expressed as means ± standard deviation (SD) for continuous variables and as percentages of the total number of patients for categorical variables. Data for skewed variables is presented as medians with interquartile range. Statistical analyses were performed using a standard statistical program (JMP software, version 10.0, SAS Institute Inc. Cary, NC, USA). t-tests and chi-square tests were used for comparison of continuous and categorical variables, respectively. If the data was not normally distributed, the Mann–Whitney U test was used. Comparisons among three or more groups were performed by analysis of variance (ANOVA) with the Bonferroni post hoc test. P values < 0.05 were considered significant. A receiver operating characteristic (ROC) curve was constructed to determine each echocardiography parameter cut-off value giving optimum sensitivity and specificity for predicting LAA dysfunction. The area under the curve was calculated by the trapezoidal rule. Logistic regression analysis was performed to identify independent predictors for LAA dysfunction. Variables that were significant by univariate logistic regression analysis (P < 0.05) were entered into the multivariate analysis.
3. Results
Table 1 shows the clinical characteristics of the 120 patients with acute ischemic stroke. The mean age was 72 ± 11 years, 69% of the patients were men, and 74 patients had AF (36 paroxysmal AF, 38 chronic AF). The etiology was cardioembolic stroke in 44%, atherothrombotic stroke in 17%, and lacunar stroke in 12% of patients.
Table 1.
Clinical characteristics of all patients.
| Number of patients | 120 |
|---|---|
| Age, years | 72 ± 11 |
| Gender (M/F) | 83/37 |
| Atrial fibrillation, n (%) | 74 (62) |
| Paroxysmal AF, n | 36 |
| Chronic AF, n | 38 |
| Hypertension, n (%) | 88 (73) |
| Diabetes mellitus, n (%) | 35 (29) |
| Dyslipidemia, n (%) | 58 (48) |
| Smokers, n (%) | 64 (53) |
| Chronic heart failure, n (%) | 35 (29) |
| Previous stroke, n (%) | 28 (23) |
| CHA2DS2VASc scorea | 3.7 ± 1.8 |
| NIHSS | 1.0 (0.5–3.0) |
| Medications | |
| Antiplatelet drugs, n (%) | 42 (35) |
| Anticoagulants, n (%) | 54 (45) |
| Warfarin, n | 46 |
| Dabigatran, n | 8 |
| NINDS clinical categories | |
| Cardioembolic stroke, n (%) | 53 (44) |
| Atherothrombotic stroke, n (%) | 20 (17) |
| Lacunar stroke, n (%) | 14 (12) |
| Other or undetermined, n (%) | 33 (28) |
AF, atrial fibrillation; NIHSS, National Institute of Health Stroke Scale.
Data are expressed as mean ± SD, number (percentage) of subjects or median (interquartile range).
CHA2DS2VASc score before onset of acute ischemic stroke.
The clinical characteristics of the patients with or without LAA dysfunction are shown in Table 2. There were 48 patients with LAA dysfunction, including 36 LAA thrombus and 41 severe spontaneous echo contrast. Patients with LAA dysfunction were significantly older and had higher CHA2DS2VASc scores before the onset of stroke compared to those without LAA dysfunction. The prevalence of AF, hypertension, chronic heart failure or previous stroke was higher in patients with LAA dysfunction than in those without. Patients with LAA dysfunction had significantly greater LAD and LAVI values, greater E/E′ ratios, and smaller LAA eV values compared to those without LAA dysfunction. LA peak systolic strain was significantly lower in patients with than in those without LAA dysfunction (12.1 ± 7.2 vs. 32.3 ± 13.7, P < 0.0001). There were no significant differences in Simpson LVEF between patients with or without LAA dysfunction. The levels of plasma brain natriuretic peptide (BNP) and high sensitivity C-reactive protein (hs-CRP) were higher in patients with LAA dysfunction than in those without. The levels of plasma fibrinogen degradation products (FDP) and D-dimer did not differ significantly between patients with or without LAA dysfunction.
Table 2.
Comparison of the characteristics of stroke patients with or without LAA dysfunction.
| Normal LAA function |
LAA dysfunction |
P value |
|
|---|---|---|---|
| (n = 72) | (n = 48) | ||
| Age (years) | 70 ± 11 | 77 ± 9 | 0.0005 |
| Gender (M/F) | 51/21 | 32/16 | 0.6282 |
| Atrial fibrillation, n (%) | 27 (38) | 47 (98) | < 0.0001 |
| Paroxysmal AF, n | 23 | 13 | |
| Chronic AF, n | 4 | 34 | |
| Heart rate (bpm) | 69 ± 17 | 71 ± 14 | 0.4098 |
| Hypertension, n (%) | 48 (67) | 40 (83) | 0.0431 |
| Diabetes mellitus, n (%) | 23 (32) | 12 (25) | 0.4123 |
| Hyperlipidemia, n (%) | 38 (53) | 20 (42) | 0.2328 |
| Smoking, n (%) | 38 (53) | 26 (54) | 0.7128 |
| Chronic heart failure, n (%) | 12 (17) | 23 (48) | 0.0002 |
| Previous stroke, n (%) | 8 (11) | 20 (48) | 0.0001 |
| CHA2DS2VASc scorea | 3.1 ± 1.9 | 4.6 ± 1.4 | < 0.0001 |
| Medications | |||
| Antiplatelet drugs, n (%) | 22 (31) | 20 (42) | 0.2127 |
| Anticoagulants, n (%) | 25 (35) | 29 (60) | 0.0036 |
| Warfarin, n | 20 | 26 | |
| Dabigatran, n | 5 | 3 | |
| Echocardiography | |||
| LAD (mm) | 40 ± 6 | 49 ± 7 | < 0.0001 |
| LVDd (mm) | 49 ± 5 | 49 ± 7 | 0.8570 |
| Simpson LVEF (%) | 62 ± 11 | 60 ± 13 | 0.9258 |
| E/E′ | 11.0 ± 4.4 | 14.0 ± 6.3 | 0.0028 |
| LAVI (mL/m2) | 41 ± 20 | 72 ± 24 | < 0.0001 |
| LAEF (%) | 42.7 ± 15.6 | 19.5 ± 9.4 | < 0.0001 |
| LAWV (cm/s) | 15.6 ± 4.5 | 9.5 ± 3.5 | < 0.0001 |
| LAA eV (cm/s) | 56.4 ± 16.4 | 22.8 ± 15.7 | < 0.0001 |
| LA peak systolic strain | 32.3 ± 13.7 | 12.1 ± 7.2 | < 0.0001 |
| Blood markers | |||
| BNP (pg/mL) | 47.0 (14.1–128) | 211.3 (93.2–414.5) | 0.0274 |
| hs-CRP | 0.090 (0.034–0.285) | 0.190 (0.100–1.670) | 0.0312 |
| D-dimer | 0.87 (0.50–2.16) | 1.28 (0.50–3.61) | 0.4979 |
| FDP | 3.9 (2.6–6.4) | 4.3 (3.0–7.0) | 0.8865 |
| Fibrinogen | 420 ± 95 | 452 ± 115 | 0.1143 |
Abbreviations as in Table 1.
LAD, left atrial dimension; LVDd, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; E/E′, the ratio of the early transmitral flow velocity and the early mitral annular velocity; LAVI, left atrial volume index; LAEF, left atrial emptying fraction, LAWV, left atrial appendage wall velocity, LAA eV, left atrial appendage emptying flow velocity; BNP, brain natriuretic peptide; hs-CRP, high sensitivity C-reactive protein; FDP, fibrinogen degradation products.
Data are expressed as mean ± SD, number (percentage) of subjects or median (interquartile range).
CHA2DS2VASc score before onset of acute ischemic stroke.
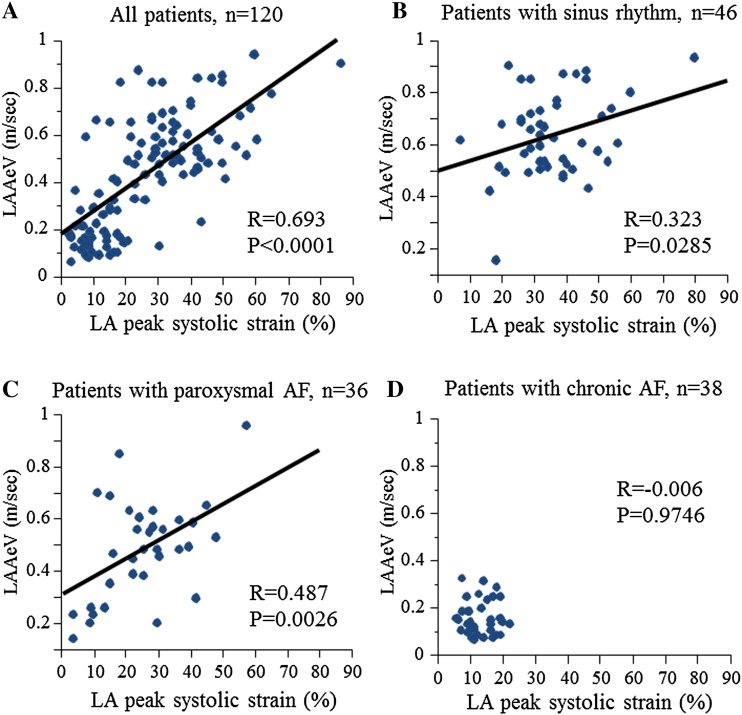
Representative figures of both a patient with preserved LA strain without LAA dysfunction and a patient with decreased LA strain and LAA dysfunction are shown in Fig. 1B and C, respectively. There was fair correlation between LA peak systolic strain and LAA eV in all patients (R = 0.693, P < 0.001, Fig. 2A). Although LA peak systolic strain correlated with LAA eV in patients with sinus rhythm and paroxysmal AF (R = 0.323, P = 0.0285, R = 0.487, P = 0.0026, respectively, Fig. 2B, C), there was no significant association between LA peak systolic strain and LAA eV in patients with chronic AF (Fig. 2D).
Fig. 2.
Relationship between LA peak systolic strain and LAA eV in all patients. LA peak systolic strain was significantly correlated with LAA eV (R = 0.693, P < 0.0001) (A). Relationship between LA peak systolic strain and LAA eV in patients with sinus rhythm (B), paroxysmal AF (C), or chronic AF (D). LAA, left atrial appendage; eV, LAA emptying flow velocity; AF, atrial fibrillation.
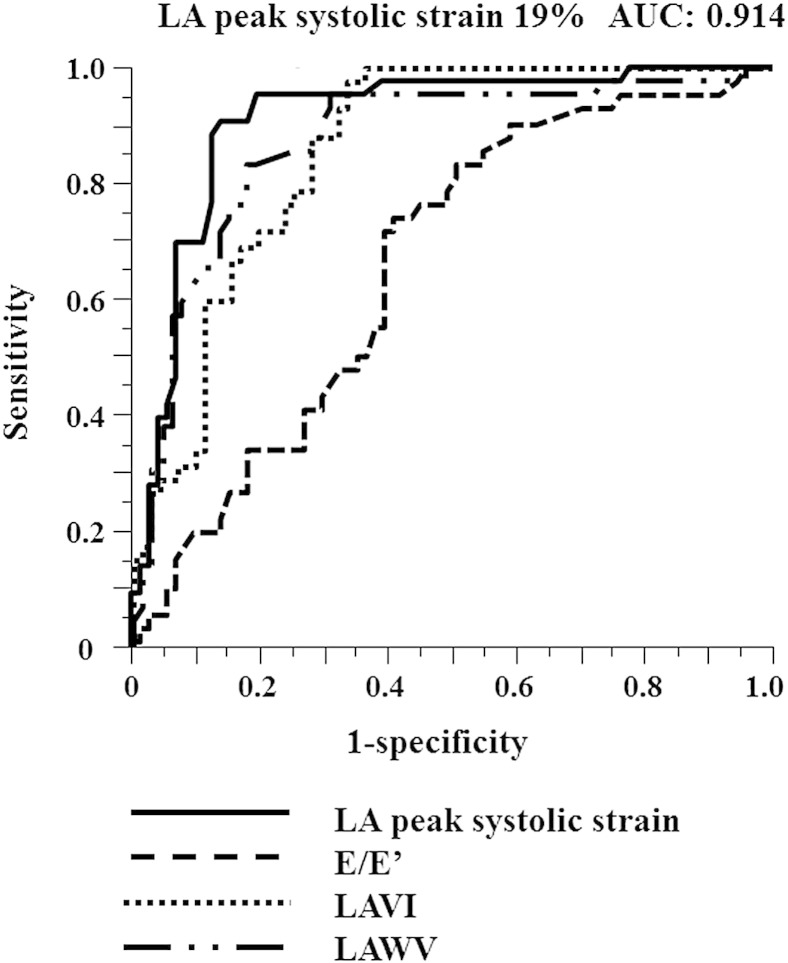
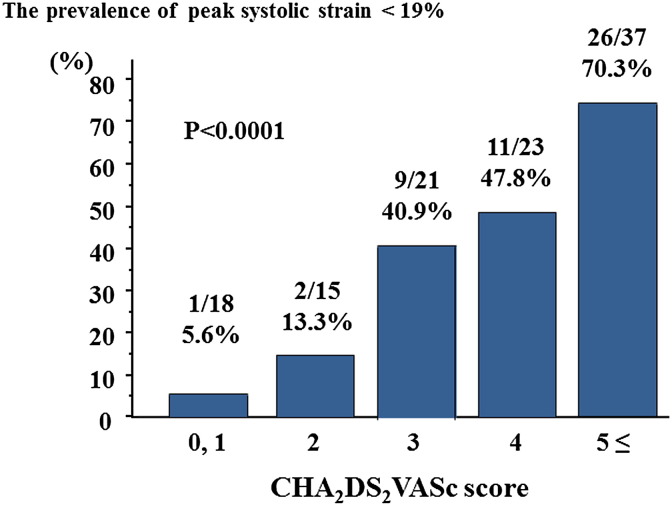
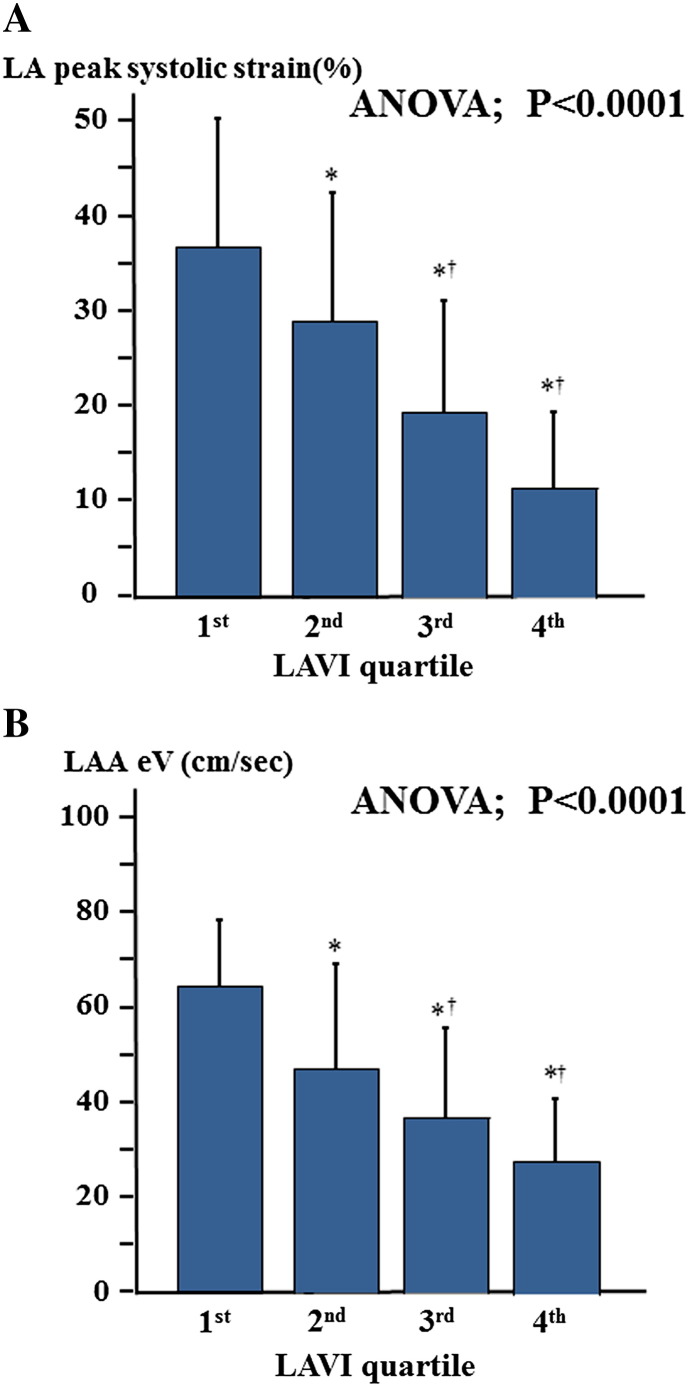
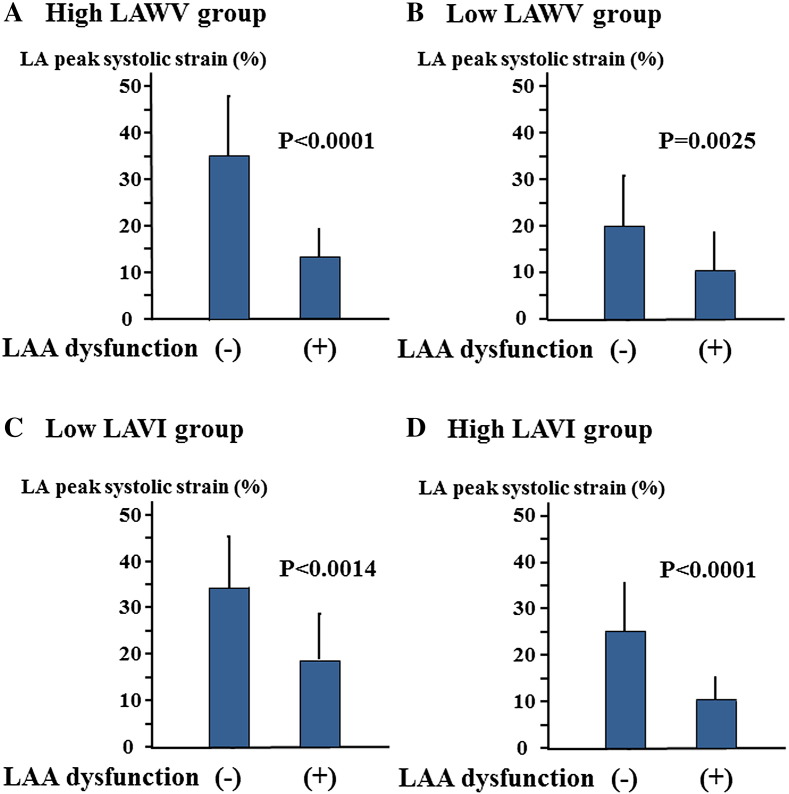
The optimum cut-off value of LA peak systolic strain for predicting LAA dysfunction was determined by ROC curve analysis; LA peak systolic strain of < 19% had a sensitivity of 92% and a specificity of 86% (Fig. 3). The area under the ROC curve (AUC) of LA peak systolic strain was 0.914. AUC of LA peak systolic strain was larger than those of LAWV, LAVI and E/E′. As shown in Fig. 4, the incidence of low LA peak systolic strain increased with advancing CHA2DS2VASc score. The patients were categorized into quartiles according to LAVI values. Both LA peak systolic strain and LAA eV decreased with increasing LAVI (Fig. 5). In addition, the patients were categorized into two groups based on LAWV values (median 11.5 cm/s) to assess the sensitivity of LA peak systolic strain. As shown in Fig. 6, LA peak systolic strain was significantly decreased in patients with LAA dysfunction, even in the high LAWV group (B). We divided the study patients into two groups based on LAVI value (median 46.9 mL/m2). In both low and high LAVI groups, LA peak systolic strain was significantly lower in patients with than in those without LAA dysfunction (low LAVI group: 18.5 ± 9.5 vs. 34.1 ± 12.6, P = 0.0014, high LAVI group: 10.1 ± 5.1 vs. 25.2 ± 9.9, P < 0.0001, respectively) (Fig. 6C, D).
Fig. 3.
Receiver operating characteristic (ROC) curve analysis of LA peak systolic strain, LAWV, LAVI and septal E/E′ as predictors for LAA dysfunction. The area under the ROC curve for LA peak systolic strain was 0.914. LA peak systolic strain > 19% had a sensitivity of 92% and a specificity of 86%.
Fig. 4.
The association between CHA2DS2VASc score and the percentages of patients with low peak systolic strain (< 19%).
Fig. 5.
The associations between LAVI and LA peak systolic strain (A), and LAA eV (B). LAVI, left atrial volume index.*p < 0.05 vs. 1st tertile, †p < 0.05 vs. 2nd tertile.
Fig. 6.
The association between LA peak systolic strain and LAA dysfunction in patients with (A) high LAWV (≥ 11.5 cm/s) or (B) low LAWV (< 11.5 cm/s). The association between LA peak systolic strain and LAA dysfunction in patients with (C) low LAVI (< 46.9 mL/m2) or (D) high LAVI (≥ 46.9 mL/m2).
Logistic regression analysis was performed to identify independent non-invasive predictors of LAA dysfunction (Table 3). In the univariate logistic regression analysis, age, E/E′, LAVI, LAEF, LAWV, and LA peak systolic strain were significantly associated with LAA dysfunction. In the multivariate logistic regression analysis, LA peak systolic strain was an independent predictor for LAA dysfunction (Risk ratio 0.061, 95% confidence interval 0.089–0.217, P < 0.0001) after adjustment for age, E/E′, LAVI and LAWV. We evaluated left ventricular longitudinal strain in the limited patients (98 patients among 120 patients) to investigate association between LA peak systolic strain and LV systolic function. Consistent with the previous reports [23], there was a significant linear relationship between LA peak systolic strain and LV global longitudinal strain (r = − 0.483, P < 0.001). However, LA peak systolic strain was still an independent predictor for LAA dysfunction in the multivariate logistic regression analysis after adjustment for age, E/E′, LAVI, LAWV and LV global longitudinal strain (risk ratio 0.184, 95% confidence interval 0.010–0.096, P < 0.0001, Supplemental Table).
Table 3.
Univariate and multivariate logistic regression analyses for LAA dysfunction.
| Variables | Risk ratio | 95% CI | P value |
|---|---|---|---|
| Univariate analysis | |||
| Age (per 1 year increase) | 1.071 | 1.028–1.121 | 0.0007 |
| Female | 1.063 | 0.464–2.508 | 0.8850 |
| Simpson LVEF (per 1 SD increase) | 0.800 | 0.545–1.166 | 0.2454 |
| E/E′ (per 1 SD increase) | 1.605 | 1.084–2.453 | 0.0219 |
| LAVI (per 1 SD increase) | 4.808 | 2.706–9.231 | < 0.0001 |
| LAEF (per 1 SD increase) | 0.115 | 0.047–0.235 | < 0.0001 |
| LAWV (per 1 SD increase) | 0.123 | 0.051–0.254 | < 0.0001 |
| LA peak systolic strain (per 1 SD increase) | 0.059 | 0.018–0.146 | < 0.0001 |
| Multivariate analysis | |||
| Age (per 1 year increase) | 1.002 | 0.924–1.085 | 0.9528 |
| E/E′ (per 1 SD increase) | 1.465 | 0.616–3.010 | 0.4511 |
| LAVI (per 1 SD increase) | 1.279 | 0.636–3.890 | 0.3338 |
| LAWV (per 1 SD increase) | 0.573 | 0.157–1.161 | 0.1007 |
| LA peak systolic strain (per 1 SD increase) | 0.061 | 0.089–0.217 | < 0.0001 |
Abbreviations as in Table 1. CI, confidence interval.
4. Discussion
The results from the current study demonstrated that there is a strict relationship between LA peak systolic strain and LAA function. LA peak systolic strain was significantly correlated with LAA eV in patients with sinus rhythm and paroxysmal AF, but not in those with chronic AF. LA peak systolic strain decreased with increasing LA enlargement. Multivariate logistic regression analysis showed that a decrease in LA peak systolic strain was an independent predictor of LAA dysfunction in patients with acute ischemic stroke.
It is well known that TEE parameters for evaluating LAA dysfunction can predict LAA thrombus formation [6], [7], [8]. The presence of spontaneous echo contrast and low LAA eV was reported to be reliable markers for stratifying patients with AF for the risk of thromboembolism [7]. A large variation of LAA eV was observed in paroxysmal AF patients with similar level of LA systolic strain, because 22 of 36 patients with paroxysmal AF were in sinus rhythm at the time of TEE. In addition, TTE parameters such as increased LAD, decreased LA fractional shortening, and increased trans-mitral inflow velocities (E/A) were useful predictors for LAA thrombus. However their predictive values for detecting LAA thrombus were much lower than those of TEE parameters [24], [25].
4.1. Assessment of regional LA function by 2D speckle-tracking echocardiography
Although tissue Doppler imaging is the conventional method of assessing regional myocardial contractility, the calculated parameters and observable regions are limited by the angle dependence inherent in all Doppler techniques [26], [27]. Myocardial strain analysis by 2-dimensional speckle tracking echocardiography is less angle-dependent than tissue Doppler echocardiography [28], [29]. Although it was initially applied to the quantification of LV function, 2D speckle-tracking imaging has recently been used to quantify LA function [14]. Several reports indicated that the normal range of LA peak systolic strain is 42.2 ± 6.1% [30], and that LA peak systolic strain decreases with age [29]. It was also reported that LA peak systolic strain is an independent predictor of paroxysmal AF [31], [32]. The burden of LA fibrosis, as analyzed by magnetic resonance imaging (MRI), was shown to correlate inversely with LA strain [33].
We recently reported that LAWV as measured by TTE can be used to stratify patients with AF for the risk of cardiac stroke [11], [12]. Since tissue Doppler imaging is resistant to noise, LAWV can be measured in most patients, no matter how low the image quality. However, LAWV cannot necessarily be accurately measured because of the inherent angle dependence. On the other hand, a relatively good image is required for 2D speckle tracking. These two parameters can be used appropriately depending on the quality of the image obtained. Fortunately, we were able to measure LA peak systolic strain and LAWV in all patients in the present study. Since a decreased LA peak systolic strain can be used to detect the presence of LAA dysfunction even in patients with normal LAWV (Fig. 6), LA peak systolic strain may be a more sensitive parameter than LAWV. It is known that there are three types of LA mechanical function: reservoir, conduit and booster pump function [34]. LA peak systolic strain reflects LA reservoir function. Since LAA clamping during cardiac surgery markedly increases LA pressure and volume, it has been suggested that the LAA may contribute to LA reservoir function, especially in the presence of LA pressure and/or volume overload [35].
It was reported that LA strain is useful for evaluating the therapeutic benefit on LA reservoir function in hypertensive patients [36]. In the present study, LA peak systolic strain decreased with LA enlargement, which is associated with LA dysfunction and is a predictor for poor cardiac outcomes [37]. These results suggested that LA peak systolic strain is associated with mechanical and structural remodeling of the LA. Saha et al. reported that LA strain is an integrated marker of dynamic LA function that shows high reproducibility and identifies patients who are at high clinical risk of thromboembolism as defined by CHADS2 score [38]. Shin et al. also reported that LA strain and strain rate are independently associated with stroke in patients with permanent AF [39]. However, both studies revealed a relationship between LA strain and CHADS2 score but not thromboembolic events. For the first time, we have shown a relationship between LA strain and LAA dysfunction such as thrombus formation, in patients with acute ischemic stroke. LA functional parameter such as LA peak systolic strain may be more accurate for detecting LAA dysfunction compared to LV morphologic parameter such as LAVI.
Ersbøll et al. reported that LA peak systolic strain was associated with LV global longitudinal strain and was not an independent prognostic parameter in patients with acute myocardial infarction [23]. However, in the present study LA peak systolic strain is an independent predictor for LAA dysfunction after adjustment of LV global longitudinal strain. This discrepancy may result from different study populations. Since the present study subjects were the patients with acute cerebral infarction who had normal LV systolic function, LA peak systolic strain may be mostly affected by LA dysfunction rather than LV systolic and diastolic dysfunction.
4.2. LA strain and CHA2DS2VASc score
The CHA2DS2VASc score is a modification of the CHADS2 score that aims to improve the prediction of stroke risk in patients with atrial fibrillation. Patients with a high CHA2DS2VASc score have a high incidence of ischemic stroke despite receiving anticoagulation therapy [20], [40]. We demonstrated that LAA dysfunction was associated with increasing CHA2DS2VASc score. There was only one patient with low LA peak systolic strain (< 19%) among the low risk patients (CHA2DS2VASc score 0–1). The finding that there is a relationship between CHA2DS2VASc score and LA strain is consistent with previous reports [38], [39], which suggested that LA strain is correlated with the risk of thromboembolism as defined by CHADS2 score [38], [39], [40]. TEE was performed in all patients and could provide accurate information about vascular diseases, which are assigned one point in the CHA2DS2VASc score. Decreased LA strain due to LA overload may effectively detect LAA dysfunction rather than decreased LAA wall velocity. Decreased LA peak systolic strain may be a promising marker for detecting patients at high risk of acute ischemic stroke. Further prospective study with large study population is required to elucidate that LA peak systolic strain can risk-stratify the patients with AF at high risk of cardioembolic stroke.
4.3. Limitations
This study had several limitations. First, some patients in this study were receiving low-dose anticoagulation therapy. However, despite this anticoagulation therapy, LAA eV and spontaneous echo contrast are well established parameters indicating LAA dysfunction. Second, the observed differences in LA strain values nevertheless underscore the robustness of LA strain for five beat measurements of strain values in patients with AF. This variability is reduced if the cycle length in the preceding beat and the measured cycle lengths are similar. We were careful to measure strain values only in those cycles in which the preceding and measured cardiac cycles were nearly equivalent. Third, since these study participants were consecutive patients with acute cerebral infarction who underwent TEE, the proportion of cardioembolic stroke was relatively high. Therefore, study sample size was adequate to analyze independent predictors for LAA dysfunction in the multivariate analysis. Fourth, we did not measure LA fractional shortening in the present study. We could not determine the superiority of LA peak systolic strain over LA fractional shortening.
5. Conclusion
LA peak systolic strain may be a reliable marker for LAA dysfunction and thrombus formation in patients with acute ischemic stroke. LA peak systolic strain may be a useful non-invasive parameter for stratifying the risk of cardioembolic stroke. Further prospective study is needed to elucidate whether LA peak systolic strain can predict cardioembolic stroke in patients with AF.
The following are the supplementary data related to this article.
Multivariate logistic regression analyses for LAA dysfunction.
Acknowledgments
This work was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan (No. 22790683 and No. 25461040).
References
- 1.National Institute of Neurological Disorders and Stroke Special report: classification of cerebrovascular diseases III. Stroke. 1990;21:637–676. doi: 10.1161/01.str.21.4.637. [DOI] [PubMed] [Google Scholar]
- 2.Cooper R., Culter J., Desivigne-Nickens P., Fortmann S.P., Friedman L., Havlik R. Trend and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: findings of the national conference on cardiovascular disease prevention. Circulation. 2000;102:3137–3147. doi: 10.1161/01.cir.102.25.3137. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin E.J., Wolf P.A., D'Agostino R.B., Silbershatz H., Kannel W.B., Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 4.Takada T., Yasaka M., Nagatsuka K., Minematsu K., Yamaguchi T. Blood flow in the left atrial appendage and embolic stroke in non-valvular atrial fibrillation. Eur. Neurol. 2001;46:148–152. doi: 10.1159/000050788. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Fernandez M.A., Torrecilla E.G., San Roman D., Azevedo J., Bueno H., Moreno M.N. Left atrial appendage Doppler flow patterns: implications on thrombus formation. Am. Heart J. 1992;124:955–961. doi: 10.1016/0002-8703(92)90978-5. [DOI] [PubMed] [Google Scholar]
- 6.Verhorst P.M., Kamp O., Visser C.A., Verheugt F.W. Left atrial appendage flow velocity assessment using transesophageal echocardiography in nonrheumatic atrial fibrillation and systemic embolism. Am. J. Cardiol. 1993;71:192–196. doi: 10.1016/0002-9149(93)90737-w. [DOI] [PubMed] [Google Scholar]
- 7.Kamp O., Verhorst P.M., Welling R.C., Visser C.A. Importance of left atrial appendage flow as a predictor of thromboembolic events in patients with atrial fibrillation. Eur. Heart J. 1999;20:979–985. doi: 10.1053/euhj.1998.1453. [DOI] [PubMed] [Google Scholar]
- 8.Sparks P.B., Mond H.G., Vohra J.K., Yapanis A.G., Grigg L.E., Kalman J.M. Mechanical remodeling of the left atrium after loss of atrioventricular synchrony: a long-term study in humans. Circulation. 1999;100:1714–1721. doi: 10.1161/01.cir.100.16.1714. [DOI] [PubMed] [Google Scholar]
- 9.Zabalgoitia M., Halperin J.L., Pearce L.A., Blackshear J.L., Asinger P.W., Hart R.G. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. J. Am. Coll. Cardiol. 1998;31:1622–1626. doi: 10.1016/s0735-1097(98)00146-6. [DOI] [PubMed] [Google Scholar]
- 10.Fatkin D., Kelly R.P., Feneley M.P. Relationship between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J. Am. Coll. Cardiol. 1994;23:961–969. doi: 10.1016/0735-1097(94)90644-0. [DOI] [PubMed] [Google Scholar]
- 11.Tamura H., Watanabe T., Hirono O., Nishiyama S., Sasaki S., Shishido T. Low wall velocity of left atrial appendage measured by trans-thoracic echocardiography predicts thrombus formation caused by atrial appendage dysfunction. J. Am. Soc. Echocardiogr. 2010;23:545–552. doi: 10.1016/j.echo.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Tamura H., Watanabe T., Nishiyama S., Sasaki S., Wanezaki M., Arimoto T. Prognostic value of low left atrial appendage wall velocity in patients with ischemic stroke and atrial fibrillation. J. Am. Soc. Echocardiogr. 2012;25:576–583. doi: 10.1016/j.echo.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Leitman M., Lysyansky P., Sidenko S., Shir V., Peleg E., Binenbaum M. Two-dimensional strain — a novel software for real-time quantitative echocardiographic assessment of myocardial function. J. Am. Soc. Echocardiogr. 2004;17:1021–1029. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Vianna-Pinton R., Moreno C.A., Baxter C.M., Lee K.S., Tsang T.S., Appleton C.P. Two-dimensional speckle-tracking echocardiography of the left atrium. Feasibility and regional contraction and relaxation differences in normal subjects. J. Am. Soc. Echocardiogr. 2009;22:299–305. doi: 10.1016/j.echo.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Gage B.F., Waterman A.D., Shannon W., Boechler M., Rich M.W., Radford M.J. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 16.Masaki N., Suzuki M., Iwatsuka R., Mizukami A., Kumasaka L., Nagahori W. Effectiveness of risk stratification according to CHADS2 score in Japanese patients with nonvalvular atrial fibrillation. Int. Heart J. 2009;50:323–329. doi: 10.1536/ihj.50.323. [DOI] [PubMed] [Google Scholar]
- 17.Special report from the National Institute of Neurological Disorders and Stroke. Classification of cerebrovascular disease III. Stroke. 1990;21:637–676. doi: 10.1161/01.str.21.4.637. [DOI] [PubMed] [Google Scholar]
- 18.Brott T., Adams H.P., Jr., Olinger C.P., Marler J.R., Barsan W.G., Biller J. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 19.Lang R.M., Bierig M., Devereux R.B., Flachskampf F.A., Foster E., Pellikka P.A., Chamber Quantification Writing Group, American Society of Echocardiography's Guidelines and Standards Committee, European Association of Echocardiography Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Beppu S., Nimura Y., Sakakibara H., Nagata S., Park Y.D., Izumi S. Smoke-like echo in the left atrial cavity in mitral valve disease: its features and significance. J. Am. Coll. Cardiol. 1985;6:744–749. doi: 10.1016/s0735-1097(85)80476-9. [DOI] [PubMed] [Google Scholar]
- 21.Siostrzonek P., Koppensteiner R., Gössinger H., Zangeneh M., Heinz G., Kreiner G. Hemodynamic and hemorheologic determinants of left atrial spontaneous echo contrast and thrombus formation in patients with idiopathic dilated cardiomyopathy. Am. Heart J. 1993;125:430–434. doi: 10.1016/0002-8703(93)90022-2. [DOI] [PubMed] [Google Scholar]
- 22.Shinokawa N., Hirai T., Takashima S., Kameyama T., Nakagawa K., Asanoi H. A transesophageal echocardiographic study on risk factors for stroke in elderly patients with atrial fibrillation: a comparison with younger patients. Chest. 2001;120:840–846. doi: 10.1378/chest.120.3.840. [DOI] [PubMed] [Google Scholar]
- 23.Ersbøll Mads, Andersen Mads J., Valeur Nana, Mogensen Ulrik Madvig. The prognostic value of left atrial peak reservoir strain in acute myocardial infarction is dependent on left ventricular longitudinal function and left atrial size. Circ. Cardiovasc. Imaging. 2013;6:26–33. doi: 10.1161/CIRCIMAGING.112.978296. [DOI] [PubMed] [Google Scholar]
- 24.Ling L., Hirono O., Okuyama H., Takeishi Y., Kayama T., Kubota I. Ratio of peak early to late diastolic filling velocity of the left ventricular inflow is associated with left atrial appendage thrombus formation in elderly patients with acute ischemic stroke and sinus rhythm. J. Cardiol. 2006;48:75–84. [PubMed] [Google Scholar]
- 25.Korinek J., Wang J., Sengupta P.P., Miyazaki C., Kjaergaard J., McMahon E. Two-dimensional strain. A Doppler-independent ultrasound method for quantitation of regional deformation. Validation in vitro and in vivo. J. Am. Soc. Echocardiogr. 2005;18:1247–1253. doi: 10.1016/j.echo.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 26.Yamada H., Oki T., Tabata T., Iuchi A., Ito S. Assessment of left ventricular systolic wall motion velocity with pulsed tissue Doppler imaging: comparison with peak dP/dt of the left ventricular pressure curve. J. Am. Soc. Echocardiogr. 1998;11:442–449. doi: 10.1016/s0894-7317(98)70024-0. [DOI] [PubMed] [Google Scholar]
- 27.Miyatake K., Yamagishi M., Tanaka N., Uematsu M., Yamazaki N., Mine Y. New method for evaluating left ventricular wall motion by color-coded tissue Doppler imaging: in vitro and in vivo studies. J. Am. Coll. Cardiol. 1995;25:717–724. doi: 10.1016/0735-1097(94)00421-L. [DOI] [PubMed] [Google Scholar]
- 28.Amundsen B.H., Helle-Valle T., Edvardsen T., Torp H., Crosby J., Lyseggen E. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J. Am. Coll. Cardiol. 2006;47:789–793. doi: 10.1016/j.jacc.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 29.Saraiva R.M., Demirkol S., Buakhamsri A., Greenberg N., Popovic Z.B., Thomas J.D. Left atrial strain measured by two-dimensional speckle tracking represents a new tool to evaluate left atrial function. J. Am. Soc. Echocardiogr. 2010;23:172–180. doi: 10.1016/j.echo.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Cameli M., Caputo M., Mondillo S., Ballo P., Palmerini E., Lisi M. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc. Ultrasound. 2009;7:6. doi: 10.1186/1476-7120-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai W.C., Lee C.H., Lin C.C., Liu Y.W., Huang Y.Y., Li W.T. Association of left atrial strain and strain rate assessed by speckle tracking echocardiography with paroxysmal atrial fibrillation. Echocardiography. 2009;26:1188–1194. doi: 10.1111/j.1540-8175.2009.00954.x. [DOI] [PubMed] [Google Scholar]
- 32.Schneider C., Malisius R., Krause K., Lampe F., Bahlmann E., Boczor S. Strain rate imaging for functional quantification of the left atrium: atrial deformation predicts the maintenance of sinus rhythm after catheter ablation of atrial fibrillation. Eur. Heart J. 2008;29:1397–1409. doi: 10.1093/eurheartj/ehn168. [DOI] [PubMed] [Google Scholar]
- 33.Kuppahally S.S., Akoum N., Burgon N.S., Badger T.J., Kholmovski E.G., Vijayakumar S. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation. Relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ. Cardiovasc. Imaging. 2010;3:231–239. doi: 10.1161/CIRCIMAGING.109.865683. [DOI] [PubMed] [Google Scholar]
- 34.To A.C., Flamm S.D., Marwick T.H., Klein A.L. Clinical utility of multimodality LA imaging: assessment of size, function, and structure. JACC Cardiovasc. Imaging. 2011;4:788–798. doi: 10.1016/j.jcmg.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 35.Tabata T., Oki T., Yamada H., Iuchi A., Ito S., Hori T. Role of left atrial appendage in left atrial reservoir function as evaluated by left atrial appendage clamping during cardiac surgery. Am. J. Cardiol. 1998;81:327–332. doi: 10.1016/s0002-9149(97)00903-x. [DOI] [PubMed] [Google Scholar]
- 36.Kokubu N., Yuda S., Tsuchihashi K., Hashimoto A., Nakata T., Miura T. Noninvasive assessment of left atrial function by strain rate imaging in patients with hypertension: a possible beneficial effect of renin–angiotensin system inhibition on left atrial function. Hypertens. Res. 2007;30:13–21. doi: 10.1291/hypres.30.13. [DOI] [PubMed] [Google Scholar]
- 37.Tamura H., Watanabe T., Nishiyama S., Sasaki S., Arimoto T., Takahashi H. Increased left atrial volume index predicts a poor prognosis in patients with heart failure. J. Card. Fail. 2011;17:210–216. doi: 10.1016/j.cardfail.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Saha S.K., Anderson P.L., Caracciolo G., Kiotsekoglou A., Wilansky S., Govind S. Global left atrial strain correlates with CHADS2 risk score in patients with atrial fibrillation. J. Am. Soc. Echocardiogr. 2011;24:506–512. doi: 10.1016/j.echo.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Shih J.Y., Tsai W.C., Huang Y.Y., Liu Y.W., Lin C.C., Huang Y.S. Association of decreased left atrial strain and strain rate with stroke in chronic atrial fibrillation. J. Am. Soc. Echocardiogr. 2011;24:513–519. doi: 10.1016/j.echo.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 40.Naccarelli G.V., Panaccio M.P., Cummins G., Tu N. CHADS2 and CHA2DS2–VASc risk factors to predict first cardiovascular hospitalization among atrial fibrillation/atrial flutter patients. Am. J. Cardiol. 2012;109:1526–1533. doi: 10.1016/j.amjcard.2012.01.371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multivariate logistic regression analyses for LAA dysfunction.