Abstract
Background
Exogenous or endogenous hydrogen peroxide (H2O2) is a reactive oxygen species (ROS) that can lead to oxidation of cellular nucleophiles, particularly cysteines in proteins. Commercial mouthwashes containing H2O2 provide the opportunity to determine clinically whether changes in S-glutathionylation of susceptible proteins in buccal mucosa cells can be used as biomarkers of ROS exposure.
Methods
Using an exploratory clinical protocol, 18 disease-free volunteers rinsed with a mouthwash containing 1.5% H2O2 (442 mM) over four consecutive days. Exfoliated buccal cell samples were collected prior and post-treatment and proteomics were used to identify S-glutathionylated proteins.
Results
Four consecutive daily treatments with the H2O2-containing mouthwash induced significant dose and time-dependent increases in S-glutathionylation of buccal cell proteins, stable for at least 30 min following treatments. Elevated levels of S-glutathionylation were maintained with subsequent daily exposure. Increased S-glutathionylation preceded and correlated with transcriptional activation of ROS sensitive genes, such as ATF3, and with the presence of 8-hydroxy deoxyguanosine. Data from a human buccal cell line TR146 were consistent with the trial results. We identified twelve proteins that were S-glutathionylated following H2O2 exposure.
Conclusions
Buccal cells can predict exposure to ROS through increased levels of S-glutathionylation of proteins. These post-translationally modified proteins serve as biomarkers for the effects of H2O2 in the oral cavity and in the future, may be adaptable as extrapolated pharmacodynamic biomarkers for assessing the impact of other systemic drugs that cause ROS and/or impact redox homeostasis.
General significance
S-glutathionylation of buccal cell proteins can be used as a quantitative measure of exposure to ROS.
Keywords: Buccal cells, Cysteine, Hydrogen peroxide, Kinase signaling, Glutathione, Glutathione S-transferase, Reactive oxygen species, S-glutathionylation
Highlights
-
•
Hydrogen peroxide is both a toxin and an endogenous signaling molecule.
-
•
The use of hydrogen peroxide mouthwash causes ROS mediated damage in cheek cells.
-
•
S-glutathionylated proteins are biomarkers for this damage.
-
•
S-glutathionylated proteins may be of future value as pharmacodynamic markers.
1. Introduction
Hydrogen peroxide (H2O2) is both a chemical toxicant and at lower concentrations an endogenous physiological signaling molecule [1]. Intracellular concentrations can manifest thresholds that determine the precise nature of the signal. However, the transmission of the signaling events generally occurs through the oxidation of cellular nucleophiles, particularly susceptible cysteine residues that can be found in certain clusters of target proteins [2]. Emerging evidence confirms that cellular sensing of redox changes is mediated through post-translational modifications (PTMs) of cysteine residues. While there is debate as to what constitutes redox “sensing” versus redox “signaling” [3], cysteine residues at various oxidation states are at the center of the process. Cysteine is one of the least coded amino acids in the human genome (~ 200,000), implying restricted usage, but evolutionary importance [4]. S-glutathionylation is a PTM that occurs when a cysteine in a low pK environment forms a disulfide bond with glutathione (GSH) [5], [6]. S-glutathionylation is a dynamic and reversible cycle that can serve as a secondary level of regulation for a number of important cellular processes in protein cluster functionalities including, kinases and phosphatases; glycolytic enzymes; calcium transport proteins; cytoskeletal structural proteins; protein folding pathways; transcription factors [7]. S-glutathionylation introduces a negative charge and frequently alters tertiary and quaternary structure and a variety of protein–protein interactions [2], [6]. S-glutathionylation can serve to protect against further oxidative damage and reversal can restore the protein to its native state [5], [7], a circumstance well suited to redox-mediated regulatory control.
Direct exposure to H2O2 can occur through the use of oral hygiene and cosmetic tooth whiteners, and commercially available products can contain concentrations that range from 0.1 to 6.0%. Various reports have outlined adverse health risks associated with localized effects of increased cell exposure to reactive oxygen species (ROS; [8], [9]). For example, DNA damage in buccal mucosa cells has been associated with etiology of oral cancers and suggested as a biomarker to assess exposure to oxidative stress or environmental toxins [10]. Indirect exposure to H2O2 can occur as a consequence of metabolism of a wide range of drugs that have electrophilic centers. This is particularly relevant for a number of anticancer drugs and irradiation.
Recent trends in oncology drug development have moved towards targeted therapies that allow optimizing treatment for specific groups of cancer patients. The application of biomarkers that predict therapy efficacy and/or toxicity for individual patients and malignancies would reduce incidence of ineffective treatment protocols and unnecessary side effects, as well as optimize effective treatments. In diagnosis or prognosis of specific diseases, assessments of matrix combinations of proteins, nucleic acids or metabolites may in some cases provide the best correlations. However, in measuring drug response, a focused biomarker can lend itself to quantitative measurements through dose–response studies. Preclinical studies in mice have suggested that S-glutathionylated serine protease inhibitors (serpins A1 and A3) in blood could be used as correlates to drug exposure [11]. Extension of these data to humans suggested that the evaluation of S-glutathionylated protein profiles in plasma when animals are exposed to agents that cause ROS may provide useful biomarkers [11]. Collection and monitoring of buccal cells provides a relatively non-invasive technique to monitor biomarkers that may assess systemic impact of drug or xenobiotic exposure. It is challenging to obtain sequential biopsy samples from patients with solid tumors and as such, surrogate tissues especially blood or cheek (buccal) cells can provide practical alternatives. To this end we enacted a clinical trial to consider the predictive value of measuring S-glutathionylated proteins from buccal cell samples taken from normal volunteers exposed to H2O2 containing mouthwash. This approach may prove applicable to pharmacodynamic studies that involve other drugs or radiation.
2. Participants and methods
2.1. Participants
Eighteen healthy adult (18–75 years old), English-speaking, non-cognitively impaired volunteers, representative of all races and genders were recruited at the Medical University of South Carolina between October 2012 and June 2013. Subjects provided written informed consent and self reported data on sex, age, race, smoking, dental procedures, and alcohol consumption are summarized in Table 1.
Table 1.
Demographic characteristics of participants.
| Demographic variable | Number in each category (%) (n = 18) |
|---|---|
| Age | |
| Mean (SD) | 30.9 (13.3) |
| 18 to 30 years | 10 (56%) |
| 31 to 65 years | 8 (44%) |
| Gender | |
| Female | 14 (78%) |
| Male | 4 (22%) |
| Ethnicity | |
| White | 11 (62%) |
| Black | 1 (5%) |
| Native American | 1 (5%) |
| Hispanic | 2 (11%) |
| Asian | 3 (17%) |
| Overall health statusa | |
| High = excellent or very good | 7 (39%) |
| Medium = good | 10 (56%) |
| Low = fair or poor | 0 (0%) |
| Alcohol consumptiona | |
| No | 6 (33%) |
| Occasionally (not consistently) | 2 (11%) |
| 1 to 5 drinks/week | 6 (33%) |
| 5 to 10 drinks/week | 3 (17%) |
| More than 10 drinks/week | 0 (0%) |
| Smoking habitsa | |
| No | 17 (100%) |
| Yes | 0 (0%) |
| Mouthwash usagea | |
| No | 8 (44%) |
| 1 to 3 times/day | 8 (44%) |
| More than 4 times/day | 1 (5%) |
| Currently taking medicationsa | |
| No | 11 (61%) |
| Yes | 6 (33%) |
| Currently taking vitaminsa | |
| No | 11 (61%) |
| Yes | 6 (33%) |
| Recent dental procedures (within last month)a | |
| No | 16 (89%) |
| Yes | 1 (5%) cleaning |
One volunteer declined to answer.
2.2. Buccal cell collection
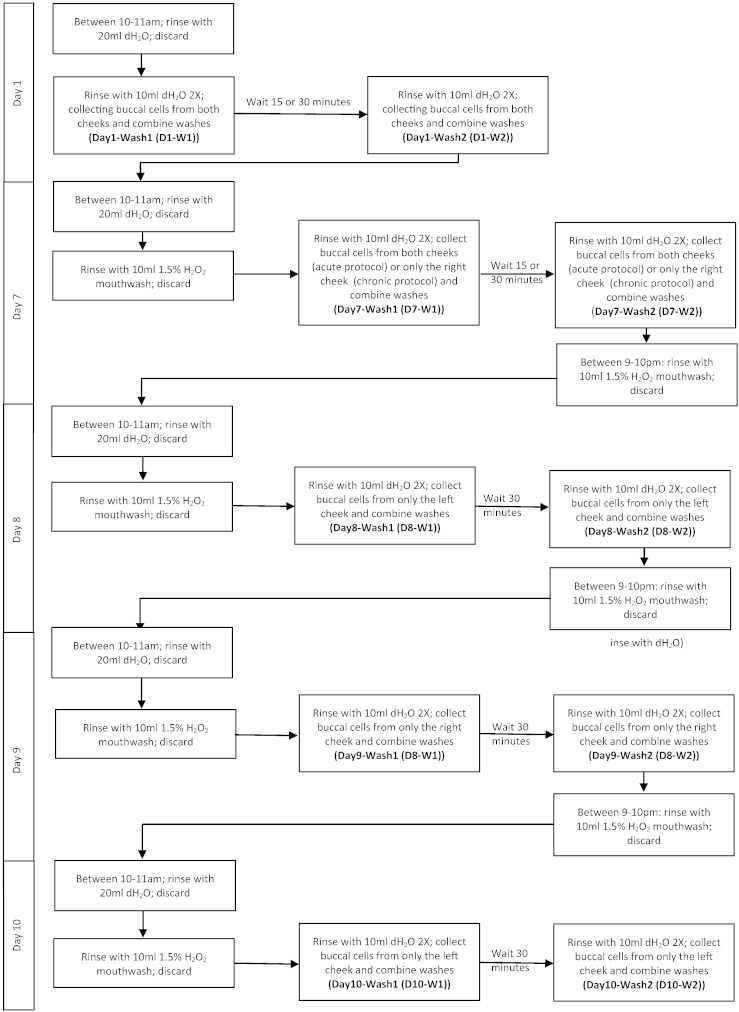
The study protocol (Fig. 1) was designed to evaluate the induction of S-glutathionylation and recovery associated with both acute and chronic ROS exposure. Since thiol homeostasis in mammals may be subject to diurnal variations [12], buccal cells were collected between 10 and 11 a.m. to limit inconsistencies that may be associated to variations in sensitivity to oxidative stress. Our estimates suggest that, contingent upon the extent of the ROS exposure (time and concentration effects), the half-life of S-glutathionylation approximates 3–6 h [13]. This formed part of the rationale for the timing of sample collection. Because the S-glutathionylation cycle has a reversible component, longer term samples to assess the degree of de-glutathionylation were incorporated [2], [14].
Fig. 1.
Protocol study design.
Prior to collection participants pre-rinsed the oral cavity with 20 mL deionized (dH2O) water for 2 min, followed by a 1–5 min exposure to (a) 10 mL dH2O or (b) 10 mL H2O2 (442 mM; 1.5% H2O2) containing commercial mouthwash (Colgate, New York, USA). Participants brushed both cheeks vigorously using 20 strokes with a sterile toothbrush followed by two-2 min rinses (10 mL dH2O). Rinses were collected in 50 mL tubes and washes were combined and labeled as Wash 1 (W1). After a ‘recovery period’ of 15–30 min 2 washes were repeated using a new sterile toothbrush, labeled as Wash 2 (W2). Toothbrushes were rinsed in their respective washes to recover any further buccal cells. Complete turnover of buccal epithelium occurs within 5–7 days [15]. Between all control (collected on Day 1 (D1)) and treatment protocols (initiating on Day 7 (D7)) a waiting period of 6 days allowed recovery and avoided bias towards collection of differentially oxidized cell populations (cell surface vs. underlying cells) [10], [15].
In extended exposure protocols, following an initial buccal cell collection (rinsing with dH2O (Day 1-Wash 1 (D1-W1) and Day 1-Wash 2 (D1-W2))), participants waited 6 days for epithelium recovery and then began an exposure protocol of two H2O2 mouthwash washes/day for 2 min. H2O2 rinses were performed between 10–11 AM and 9–10 PM. Buccal cells were collected following each H2O2 exposure, with a recovery period of 30 min between W1 and W2. Participants continued this protocol for 4 days (D7–10) with collections from alternating cheeks each day (Fig. 1).
Buccal cells were centrifuged (800 × g for 5 min at 4 °C) and washed three times (chilled 1× PBS), and the cell pellets were flash frozen and stored at − 80 °C. Unless subject to freeze thawing (not used in this protocol), our previous experiences suggest that S-glutathionylated proteins are stable at − 80 °C for > 2 years. This has been confirmed by others [16].
2.3. Cell culture
The human buccal cell line, TR146 (Sigma-Aldrich, St. Louis, USA) was maintained at 37 °C and 5% CO2/95% air in 98% humidity, in DMEM supplemented with 10% FCS, 100 IU/mL penicillin, and 100 μg/mL streptomycin. To mimic the growth of cells in the oral cavity, 24,000 cells/cm2 were cultured on Falcon® 4.2 cm2 permeable polyethylene terephthalate inserts with a pore size of 0.4 μm (Corning, MA, USA) for 7 days prior to oxidative stress studies. Cells were rinsed twice with 1 × PBS prior to H2O2 and ‘recovery periods’ were in the presence of growth medium.
2.4. Protein preparation
Cell pellets were suspended in lysis buffer (20 mM Tris–HCl, pH 7.5, 15 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, and 1 mM β-glycerophosphate with freshly added protease and phosphatase inhibitors, 5 mM NaF and 1 mM Na3VO4) and incubated for 30 min on ice. Lysates were sonicated for 10 s and centrifuged for 30 min at 10,000 g at 4 °C. Protein concentrations were assayed with Bradford reagent (Bio-Rad Laboratories, Hercules, CA) using IgG as a standard.
2.5. Immunoblot analysis
Equal amounts of total protein were electrophoretically resolved under non-reducing conditions on 10% SDS-polyacrylamide gels (SDS-PAGE); unmodified proteins were separated under reducing conditions. Proteins were transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA). Non-specific binding was reduced in blocking buffer (20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.1% Tween 20, 1 μM protease inhibitors, 5 mM NaF, and 1 mM Na3VO4) containing 10% non-fat dried milk, for 1 h. Membranes were incubated with monoclonal anti-glutathione antibodies (Virogen, Watertown, MA) to detect protein S-glutathionylation (PSSG), or polyclonal antibodies for actin or ATF3 (Abcam, Cambridge, MA) in blocking buffer containing 5% non-fat dried milk overnight at 4 °C, washed 3× with PBS for 15 min, and incubated with the appropriate secondary antibody (Amersham Biosciences, Piscataway, NJ) conjugated to horseradish peroxidase for 1 h. Membranes were washed 3 × and developed with enhanced chemiluminescence detection reagents (Bio-Rad). Blots were scanned with a BioRad ChemiDoc system and visualized with a transilluminator and evaluated using Quantity One software (version 4.5.2; Bio-Rad) and normalized to actin.
2.6. Immunoprecipitation and identification of S-glutathionylated proteins
Immunoprecipitations of S-glutathionylated proteins were performed using the anti-glutathione antibody as previously described [11]. Human buccal cell samples (2 mg) were incubated overnight at 4 °C with 5 μg of the antibody and separated by non-reducing SDS-PAGE and bands corresponding to S-glutathionylated proteins were excised, trypsin digested and analyzed by matrix-assisted laser desorption/ionization, time-of-flight (MALDI-TOF) mass spectrometry at the Proteomics Core Facility of the Medical University of South Carolina. Protein identification was performed using software from the National Center for Biotechnology Information protein database. Automated database searching was performed with BioWorks software running TurboSequest. Accuracy of the peptide assignments was assessed with Peptide prophet and Protein prophet algorithms from the Institute for Systems Biology (Seattle, WA). Automated database searching used GPS Explorer software using Mascot. Only peptides and proteins with a reported confidence > 95% were considered identified.
2.7. Cell viability and DNA damage evaluation
TR146 cell viability was evaluated using Trypan Blue solution (Sigma-Aldrich) and assessing the number of live (translucent) and dead (blue) cells using a hemocytometer under phase-contrast microscopy. DNA damage was evaluated using OxiSelect Oxidative DNA ELISA kit (Cell BioLabs, San Diego, CA) in an ELISA format based on comparison to a predetermined 8-OHdG standard curve.
2.8. RNA collection and qPCR analyses
Frozen buccal cell pellets were resuspended in 250 μL TE Buffer (10 mM Tris–HCl, pH 8.0, 10 mM EDTA) containing 200 mM NaOH and 1% SDS. Pellets were then incubated for 5 min at room temperature and then 250 μL 3 M potassium acetate, pH 5.5 was added for an additional 5 min at 4 °C. The supernatant was collected by centrifuging at 18,000 g for 10 min and the RNA processed using the Qiagen RNeasy kit (Qiagen, Valencia, CA) and stored at − 80 °C for future analyses. cDNA was generated from 2 μg total RNA and real-time PCR (qPCR) reactions and data analyses were performed using iQ SYBR Green Supermix and the MyiQ thermal cycler (Bio-Rad, Hercules, CA) (40 cycles, 58 °C annealing, 81 °C real-time data collection). Each oligonucleotide primer was synthesized by OriGene (Rockville, MD). Results of experiments were verified by repetition of RT-PCR with RNA extracted from different aliquots of cells (at least three independent reactions performed per template/primer combination). For relative quantification in qPCR, a mathematical model was used that incorporated the effects of the efficiency of amplification for each primer pair over a 104 range of template dilutions and starting template concentrations were normalized by comparing to β-actin amplification. qPCR reactions were run in triplicate for each sample, and at least three independent experiments were performed. Overall results were mean of results from eight individuals' samples.
2.9. Statistical analyses
Statistical analyses were performed in Prism version 5.0 (GraphPad Software, Inc., San Diego, CA). p values lower than 0.05 were considered significant. Differences in the induction of total S-glutathionylation and ATF3 proteins from participants and cell culture following control or H2O2 exposure were analyzed by analysis of variance (ANOVA) followed by a Bonferroni's multiple comparison post-hoc test. For the induction of S-glutathionylation over time and with increasing doses in buccal cells in vivo and in TR146 cells, a Dunnett's multiple comparison post hoc test against baseline controls was used (S1-W1 or 0 H2O2, no recovery). The induction of 8-OHdG in buccal cells following treatment with H2O2 was analyzed by ANOVA with a Dunnett's multiple comparison post-hoc test against baseline control (S1-W1). Corrections were applied based on program recommendations.
3. Results
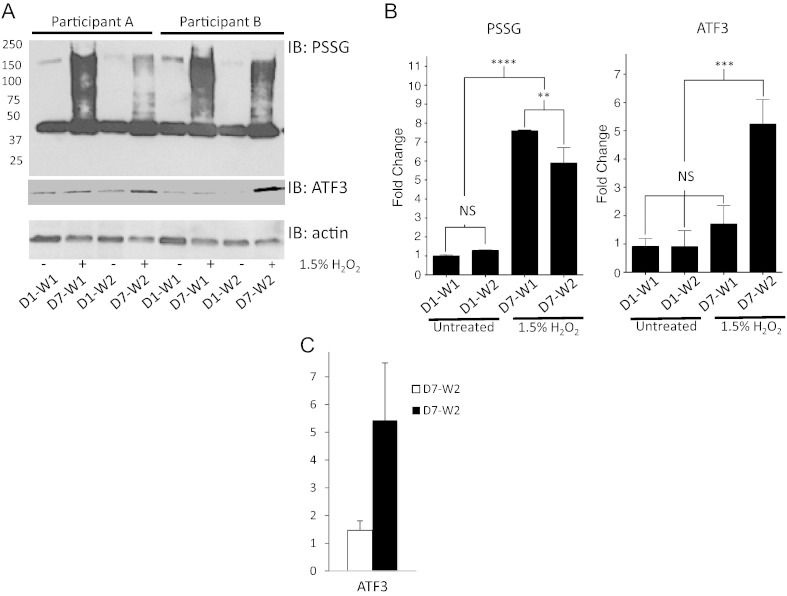
Oral exposure to 1.5% H2O2 rapidly led to a significant increase in S-glutathionylation of numerous proteins from human buccal samples relative to the individual baseline untreated control samples (p < 0.0001, Fig. 2A–B). S-glutathionylation levels decreased significantly (p < 0.01) after 15 min, but did not return to baseline (p < 0.0001). There were no significant differences in protein S-glutathionylation levels between initial and recovery samples collected in the control and baseline samples (Fig. 2B). The sensitivity of the antibodies and the conditions utilized in the development of the blots likely minimized detection of S-glutathionylated proteins that were in low abundance. This likely underestimates the basal level of post-translationally modified proteins in samples not exposed to H2O2, but these levels are usually quite low. Basal levels of S-glutathionylation are also dependent on cell and tissue type. However, a ~ 40 kDa protein identified as actin, commonly found to be S-glutathionylated in the absence of external ROS [2] was present in baseline samples. Accompanying immunoblots showed significant increases in protein levels of the oxidative stress-responsive transcription factor ATF3 in buccal cells collected 15 min after H2O2 (p < 0.001) (Fig. 2A–B), confirming that H2O2 activated a general stress associated transcription factor. Real-time qPCR (Fig. 2C) confirmed that ATF3 was also transcriptionally upregulated.
Fig. 2.
H2O2 induces protein S-glutathionylation of human buccal cells through stress response pathways.
Buccal cells were collected following 5 min oral rinses with dH20 (D1-W1). After a recovery period of 15 min, buccal cells were collected in an additional dH20 wash (D1-W2). After 6 days buccal cells were similarly collected from the same participant following 5 min oral rinses with a mouthwash containing 1.5% H2O2 (D7-W1 and D7-W2). Buccal cell pellets were lysed and 40 μg protein was loaded and evaluated by immunoblot for S-glutathionylation (PSSG) or ATF3 levels. Actin levels were used as loading controls. Representative blots from two participants are shown (panel A). Averaged values and statistical comparisons are shown in panel B, where fold change is calculated relative to D1-W1. D = day; W = wash; NS = not significant; ** = p < 0.01; *** = p < 0.001; **** = p < 0.0001; n = 3, ± SD. Panel C shows the qPCR results for ATF3 samples prepared from eight individuals.
S-glutathionylated proteins identified by MALDI-TOF mass spectrometry (Table 2) fell into three functional clusters: (a) redox regulatable enzymes. For example, activities of GSTP1 [14] and various cysteine dependent serine protease inhibitors [11] have been shown to be impacted by S-glutathionylation. Inter-α-trypsin inhibitor is one example of this family [17], but until now there has been no indication that it is subject to S-glutathionylation. (b) A group of structural proteins influencing cell shape and motility, also typically subject to redox regulation [5]. (c) Lactotransferrin, complement and albumin are cysteine rich proteins known to be subject to this PTM [11]. Given the sensitivity restrictions of the antibody pull down and detection methodologies used, it is possible that some less abundant proteins were not identified. These proteins represent those that are in S-glutathionylated at high levels and thus readily detected. Moreover, while additional peptide fragments were found, the algorithms used in the proteomic detection procedures prevent identification of false positives.
Table 2.
MALDI-TOF identification of S-glutathionylated buccal cell proteins following H2O2 exposure.
| Protein | MW (kDa) | Protein function |
|---|---|---|
| GSTP1 | 24 | Phase II metabolism; S-glutathionylation. |
| Inter-α trypsin inhibitor | 75 | Plasma protease inhibitor. |
| B4GALNT2 | 20 | β-1,4-N-acetyl-galactosaminyl transferase 2 isoform c; protein glycosylation; negative regulation of cell adhesion. |
| GRAF | 52 | rho GTPase activating protein 26; associates with focal adhesion kinase. |
| PAR6 | 65 | Partitioning defective 6 homolog γ; cell division and polarization. |
| Flaggrin | 125 | Epithelial structure; mucosal S100 fusion type protein. |
| Plakoglobin | 125 | γ-Catenin; complexes with cadherins. |
| Reticulon-2 | 65, 20 | ER protein promotes membrane curvature, nuclear pore complex and vesicle formation. |
| Actin | 42 | Microfilament formation. |
| Histone H1 | 20 | Chromatin structure. |
| Lactotransferrin | 65 | Antimicrobial activity; part of the innate defense at the mucoses. |
| Complement | 52, 42 | Innate immunity. |
| Albumin | 75, 65, 42 | Blood globular protein. |
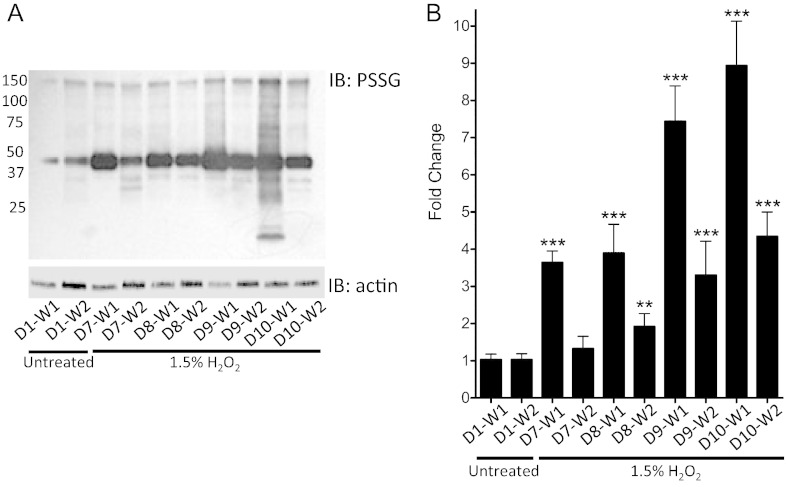
The S-glutathionylation response was evaluated following chronic exposure outlined in Fig. 1. Data show that twice daily exposure for 4 days significantly potentiated the induction of S-glutathionylation in buccal cells (Fig. 3A–B) (p < 0.001) as compared to participants' baseline control samples (D1-W1), in addition to sustaining the time periods that cells take to recover to basal levels (p < 0.01 at D8 in W2 and p < 0.001 at D9 and D10 in W2, compared to D1-W1).
Fig. 3.
H2O2 mediated protein S-glutathionylation and recovery from human buccal cells.
H2O2 induced protein S-glutathionylation and recovery in human buccal cells is dose dependent. Buccal cells were collected following oral rinses for 2 min with dH20 (D1-W1). After a recovery period of 30 min, buccal cells were collected in an additional dH20 wash (D1-W2). Repeat H2O2 exposure protocols were initiated 6 days later to allow for oral epithelium recovery. Beginning on Day 7, 2 min 1.5% H2O2 treatments were conducted twice daily (in the AM and PM) to represent habitual mouthwash usage. All samples were collected from each participant daily in the morning immediately following treatments (D7-W1; D8-W1; D9-W1; D10-W1) and then again 30 min after exposure (D7-W2; D8-W2; D9-W2; D10-W2). Buccal cell pellets were lysed, and 40 μg total protein was loaded and total protein S-glutathionylation (PSSG) levels evaluated by immunoblots (a representative immunoblot from a participant is shown in Panel A). Densitometry levels from all participants were normalized to actin and averaged values and statistical comparisons are shown in panel B, where fold change is calculated relative to D1-W1. D = day; W = wash; ** = p < 0.01; *** = p < 0.001; n = 11, ± SD.
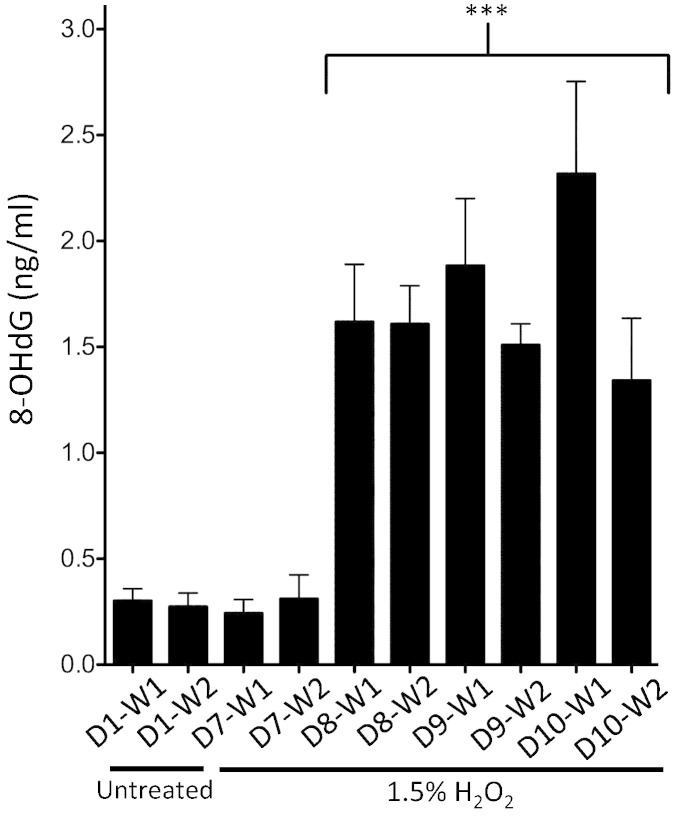
As a correlative biomarker of oxidative stress, levels of 8-OHdG were measured in the human buccal samples exposed to the chronic H2O2 time course (Fig. 4). In participants completing an extended repeat-exposure protocol, H2O2 exposure significantly increased 8-OHdG levels by the second day as compared to the participants' baseline control (D1-W1) samples (p < 0.001). Significantly elevated levels of the damaged DNA markers were maintained throughout the remainder of the study (through protocol Day 10) in both initial and recovery (after 30 min) samples compared to baseline control (D1-W1) (p < 0.001).
Fig. 4.
H2O2 induced DNA damage in human buccal cell samples estimated using 8-OHdG as a marker.
Buccal cells were collected following oral rinses for 2 min with dH20 (D1-W1). After a recovery period of 30 min, buccal cells were collected in an additional dH20 wash (D1-W2). To allow for oral epithelial recovery, repeat H2O2 exposure protocols were initiated 6 days later. Beginning on Day 7, 2 min 1.5% H2O2 treatments were conducted twice daily (in the AM and PM) to represent habitual mouthwash usage. All samples were collected from each participant daily in the morning immediately following treatment (D7-W1; D8-W1; D9-W1; D10-W1) and then again 30 min after exposure (D7-W2; D8-W2; D9-W2; D10-W2). 8-OHdG levels were calculated against a standard curve. D = day; W = wash; *** = p < 0.001; n = 3, ± SD.
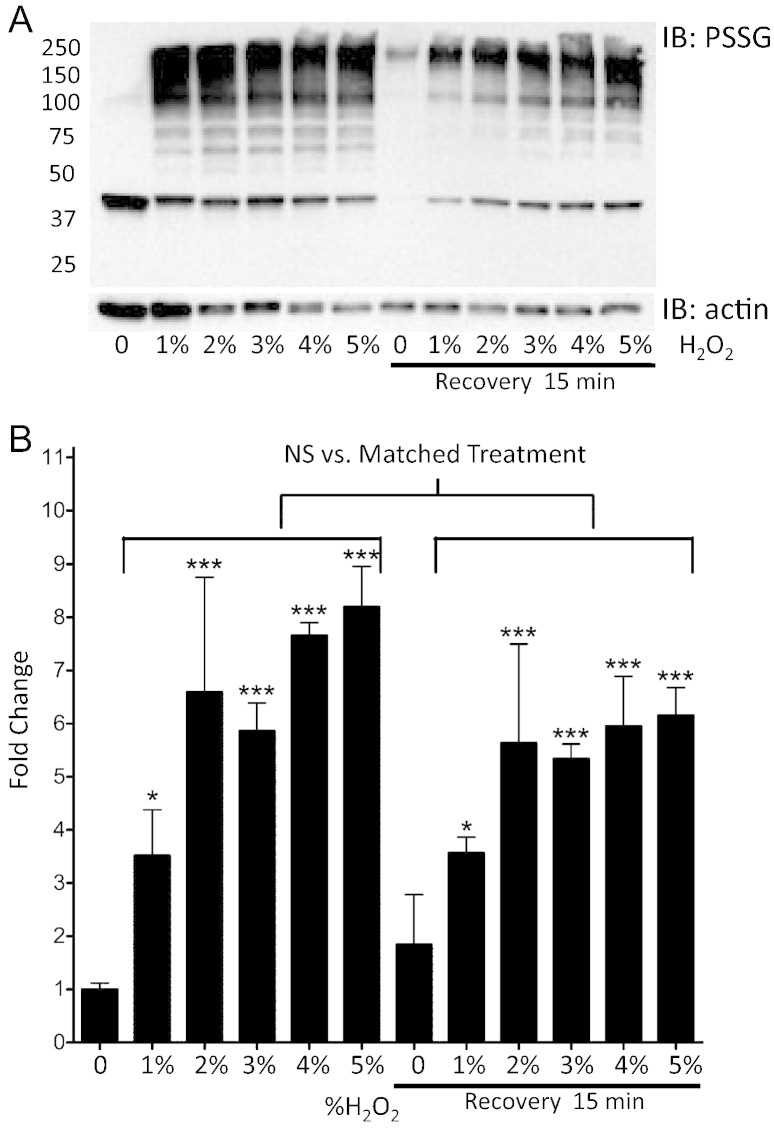
TR146 is an immortalized human buccal cell line (isolated from a neck metastasis) forming undifferentiated, non-keratinized, stratified epithelium that shares many morphological and functional characteristics of normal oral mucosa [18], [19]. TR146 cells were exposed to increasing concentrations of H2O2 for 2.5 min (concentrations of > 5% resulted in significant cell death). Immunoblot analyses showed that higher H2O2 concentrations significantly increased total protein S-glutathionylation levels compared to untreated controls (1% p < 0.5; 2%–5% p < 0.001; Fig. 5A–B). This dose–response plateaus at ~ 4% H2O2, likely due to saturation or cytotoxicity. Following a 15 min recovery, significantly elevated levels of S-glutathionylated proteins remained in all treated samples compared to untreated controls (1% p < 0.5; 2%–5% p < 0.001). Analyses of S-glutathionylation levels in matched samples (immediately following H2O2 exposure compared to after 15 min recovery) showed that recovery (as a result of deglutathionylation) had not occurred by 15 min (p > 0.05) and there was a negative correlation with H2O2 dosage (Fig. 5B). A prominent band corresponding to S-glutathionylated actin was detected in untreated samples, supportive that the cell model mimics that of the human data (Fig. 5A).
Fig. 5.
H2O2 mediated protein S-glutathionylation in TR146 cells is concentration dependent.
TR146 cells grown in transwells were treated with various concentrations of H2O2 followed by removal and a recovery period of 15 min. Panel A shows a representative S-glutathionylation (PSSG) immunoblot. Densitometry levels were normalized to actin and averaged values and statistical comparisons are shown in panel B, where fold change is calculated relative to no H2O2. Statistical comparisons of H2O2 induced S-glutathionylation levels compared to baseline untreated control samples (0% no recovery), as well as between matched treatments pre- and post-15 min recovery are shown. NS = not significant; * = p < 0.05; *** = p < 0.001; n = 3, ± SD.
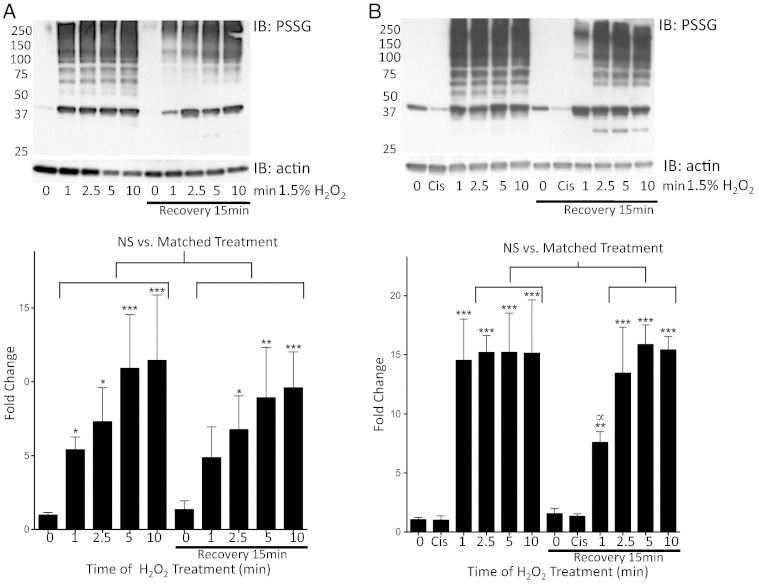
Treatment of TR146 cells with 1.5% H2O2 yielded a time-dependent increase in S-glutathionylation that became saturated by 5 min (1 and 2.5 min p < 0.05; 5 and 10 min p < 0.001) as compared to untreated controls. Following 15 min recovery S-glutathionylation levels were maintained in cells that had been exposed to 1.5% H2O2 (2.5 min p < 0.05; 5 min p < 0.01; 10 min p < 0.001). Comparison of protein S-glutathionylation levels in matched samples (immediately following H2O2 exposure compared to after 15 min recovery) showed that significant deglutathionylation had not occurred by 15 min (p > 0.05). Extended exposures to H2O2 mildly increased recovery to baseline S-glutathionylation levels (Fig. 6A). Treatment of cells with cisplatin did not induce direct S-glutathionylation (Fig. 6B). However, treatment of TR146 cells with 2.43 μM cisplatin for 48 h (IC50) potentiated total levels of (and sustained) S-glutathionylation following H2O2 (p < 0.001) compared to untreated controls. This combination also caused saturation of S-glutathionylation levels within a min, which was further sustained and less reversible (Fig. 6A–B). Analyses of matched samples (immediately following H2O2 compared to 15 min recovery) showed that only low levels of S-glutathionylation had occurred by 15 min with 1.5% H2O2 for 2.5, 5 and 10 min (p > 0.05). Cells exposed to 1.5% H2O2 for 1 min showed significant S-glutathionylation levels during the 15 min recovery period (p < 0.05) Fig. 6B.
Fig. 6.
H2O2 induced protein S-glutathionylation in TR146 cells in buccal cells is time-dependent and cisplatin pretreatment potentiates and sustains S-glutathionylation levels.
TR146 cells grown in transwells were treated with 1.5% H2O2 for various time periods followed by removal and a recovery period of 15 min without any pretreatment (panel A) or with cell pretreatment for 48 h with 2.43 μM cisplatin (panel B). Representative S-glutathionylation (PSSG) immunoblots are shown in the upper panels. Densitometry levels were normalized to actin and averaged values and statistical comparisons are shown graphically in the bottom of panels A and B, where fold change is calculated relative to untreated (0 min) cells. Statistical comparisons of H2O2 induced S-glutathionylation levels as compared to baseline untreated control samples (0 min, no recovery), as well as between matched treatments pre- and post-15 min recovery are shown. NS = not significant; * = p < 0.05; ** = p < 0.01; *** = p < 0.001; ∝ = p < 0.05 where significant deglutathionylation occurred after a 15 min recovery period in cells treated for 1 min with 1.5% H2O2; n = 4, ± SD.
4. Discussion
Aerobic metabolism is the most efficient way to create energy, however, reactive oxygen species (ROS) such as superoxide (O2•−) and hydroxyl radicals (•OH) are byproducts that are non-specifically reactive with cellular nucleophiles and can cause toxicity. Exogenous hydrogen peroxide can be a precursor of these, but it is generally stable (t1/2, months), can move freely within and between cells and has evolved as an endogenous second messenger, frequently signaling through redox regulation of reactive cysteine residues in target proteins [20]. Reversible S-glutathionylation of cysteines provides a framework for a cycle that facilitates such regulation [5]. The biological effects of H2O2 are concentration dependent with a threshold effect that influences response. The present study was designed as an exploratory clinical trial to consider whether protein S-glutathionylation might be adaptable as a quantitative/qualitative measure of H2O2 (ROS) exposure. Extrapolating these results may lead to incorporation of similar biomarkers into other protocols to monitor pharmacokinetic/dynamic measurements of other drugs that produce ROS.
The importance of salivary oxidant state has been considered in oral inflammatory diseases and cancer etiology [21]. However, there have been no studies investigating the impact of ROS on PTM of proteins from buccal cells. In the present trial, the use of the H2O2 mouthwash rapidly induced both a time- and dose-dependent increase in protein S-glutathionylation that remained for extended periods. Chronic H2O2 both potentiated induction of S-glutathionylation and extended the period of recovery (deglutathionylation). In these samples, H2O2 also enhanced expression of ATF3, a transcription factor specifically linked with ROS stress response pathways [22]. In addition, H2O2 characteristically causes nucleic acid damage [23] and our data confirm a dose/time response for the presence of 8-OHdG in buccal cell samples. Establishing whether these levels of DNA damage cause precancerous dysplasia, leukoplakia or oral cancer was beyond the scope of this study. Previous multi-center, case-controlled studies have reported a link between daily mouthwash use and head and neck or esophageal cancers; however, these reports did not consider the relevance of ROS on cell events [24]. H2O2 containing bleaching products have also been associated with genotoxic effects including micronuclei formation and DNA damage in buccal mucosa. These have been suggested as biomarkers to assess ROS exposure or genetic damage in chemoprevention trials [10], [25], [26], [27]. However, levels of DNA damage may not always be high enough to provide a reliable marker for this type of exposure. In the present trial, our data support linkage between protein S-glutathionylation and DNA damage in buccal cells.
Proteomic analysis of buccal cell protein S-glutathionylation following H2O2 revealed some previously characterized, but a number that are novel. For example, GSTP1 has been implicated in cancer etiology, drug resistance, and kinase signaling and can carry out the forward reaction in the S-glutathionylation cycle [28]. Its activity is subject to auto-regulation by S-glutathionylation at cysteines 47 and 101 [14]. Previously, altered GST expression was linked with differentiation and tumor stage in buccal mucosal cancers [29], [30], implying a plausible link with S-glutathionylation. S-glutathionylated plasma protease inhibitors (serpins A1 and A3), similar to inter-α trypsin inhibitor, not only regulate mobilization of bone marrow progenitor cells [31], [32] but also act as serum biomarkers for exposure to ROS [11]. Specific protein clusters are susceptible to S-glutathionylation [2], [5] and those identified here can be classified accordingly. For example, structural (B4GALNT2, GRAF, PAR6, Flaggrin-2, Plakoglobin, Reticulon-2, actin, histone H1), or for lactotransferrin, complement and albumin, cysteine rich blood proteins that are sensitive to redox. One (or more) of these S-glutathionylated proteins may progress as a plausible biomarker for exposure to H2O2 or other ROS. The antibody pull-down techniques used in this study have some limitations, particularly with regard to sensitivity (for example, antibody detection can vary depending on the conformation of the glutathione adduct and the environment of the thiolated cysteine) and may have limited the number of S-glutathionylated proteins identified. Nevertheless, those proteins shown in Table 2 represent primary S-glutathionylation targets.
In general terms, buccal cells provide an accessible pool of epithelial cells that can mirror systemic health status, whether influenced by exposure to ROS [33] or environmentally genotoxic agents [34], [35]. Deleterious effects of chemotherapy and radiation can directly manifest in the oral mucosa, frequently producing compromised epithelial proliferation and mucosal ulceration [36]. Because a large part of the highly vascularized mucosa is non-keratinized, exposure of the oral mucosa to redox altering agents may also leave deeper tissues at risk. Protein S-glutathionylation is induced in rats, where high levels of protein bound GSH were detected in squamous cell carcinomas of the tongue and adjacent tissues [37]. Significant levels of S-glutathionylated proteins were present in the oral cavity long before the presence of clinically observable lesions implying that they may serve in the determination of cancer susceptibility and early etiology.
In parallel with the human clinical trial, we analyzed the effects of H2O2 in a transformed human buccal cell line. TR146 cells have characteristically high levels of expression of GSTP (data not shown). Induced and sustained levels of S-glutathionylation in TR146 cells were similar to those in human buccal cell samples. We compared the results with cells exposed to cisplatin, a drug known to cause damage to both nucleic acids and proteins [38]. While cisplatin did not cause significant S-glutathionylation, in combination with H2O2, the drug combination potentiated it and delayed deglutathionylation. Since the platinum alkylating species [39] do not cause the PTM, some selective degree of electrophilic selectivity is required.
In the present study, the S-glutathionylation profile of proteins from buccal cells is predictive of exposure to H2O2. As such, buccal cell samples should be useful as a surrogate tissue source for biomarker analysis to define the effects of specific drugs that can cause ROS, enabling development of candidate biomarkers of response to redox-altering therapeutics. Furthermore, persistent levels of ROS may activate pro-inflammatory events causing toxicity. The identification of biomarkers that evaluate the effects of ROS in the oral cavity may define at-risk populations for oral cancer and be useful in clinical trials to measure the efficacy or toxicity of drugs that influence redox homeostasis. Current efforts validating the prognostic potential of protein S-glutathionylation profiles in large cohorts of patients are currently underway.
References
- 1.Rhee S.G. Redox signaling: hydrogen peroxide as intracellular messenger. Exp. Mol. Med. 1999;31:53–59. doi: 10.1038/emm.1999.9. [DOI] [PubMed] [Google Scholar]
- 2.Townsend D.M. S-glutathionylation: indicator of cell stress and regulator of the unfolded protein response. Mol. Interv. 2007;7:313–324. doi: 10.1124/mi.7.6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones D.P. Redox sensing: orthogonal control in cell cycle and apoptosis signalling. J. Intern. Med. 2010;268:432–448. doi: 10.1111/j.1365-2796.2010.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grek C.L., Zhang J., Manevich Y., Townsend D.M., Tew K.D. Causes and consequences of cysteine s-glutathionylation. J. Biol. Chem. 2013;288:26497–26504. doi: 10.1074/jbc.R113.461368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong Y., Uys J.D., Tew K.D., Townsend D.M. S-glutathionylation: from molecular mechanisms to health outcomes. Antioxid. Redox Signal. 2011;15:233–270. doi: 10.1089/ars.2010.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tew K.D., Townsend D.M. Glutathione-s-transferases as determinants of cell survival and death. Antioxid. Redox Signal. 2012;17:1728–1737. doi: 10.1089/ars.2012.4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tredwin C.J., Naik S., Lewis N.J., Scully C. Hydrogen peroxide tooth-whitening (bleaching) products: review of adverse effects and safety issues. Br. Dent. J. 2006;200:371–376. doi: 10.1038/sj.bdj.4813423. [DOI] [PubMed] [Google Scholar]
- 9.Naik S., Tredwin C.J., Scully C. Hydrogen peroxide tooth-whitening (bleaching): review of safety in relation to possible carcinogenesis. Oral Oncol. 2006;42:668–674. doi: 10.1016/j.oraloncology.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Borthakur G., Butryee C., Stacewicz-Sapuntzakis M., Bowen P.E. Exfoliated buccal mucosa cells as a source of DNA to study oxidative stress. Cancer Epidemiol. Biomarkers Prev. 2008;17:212–219. doi: 10.1158/1055-9965.EPI-07-0706. [DOI] [PubMed] [Google Scholar]
- 11.Grek C.L., Townsend D.M., Uys J.D., Manevich Y., Coker W.J., III, Pazoles C.J. S-glutathionylated serine proteinase inhibitors as plasma biomarkers in assessing response to redox-modulating drugs. Cancer Res. 2012;72:2383–2393. doi: 10.1158/0008-5472.CAN-11-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neuschwander-Tetri B.A., Rozin T. Diurnal variability of cysteine and glutathione content in the pancreas and liver of the mouse. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996;114:91–95. doi: 10.1016/0305-0491(96)83706-0. [DOI] [PubMed] [Google Scholar]
- 13.Townsend D.M., Manevich Y., He L., Xiong Y., Bowers R.R., Jr., Hutchens S. Nitrosative stress-induced s-glutathionylation of protein disulfide isomerase leads to activation of the unfolded protein response. Cancer Res. 2009;69:7626–7634. doi: 10.1158/0008-5472.CAN-09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townsend D.M., Manevich Y., He L., Hutchens S., Pazoles C.J., Tew K.D. Novel role for glutathione S-transferase pi. Regulator of protein S-glutathionylation following oxidative and nitrosative stress. J. Biol. Chem. 2009;284:436–445. doi: 10.1074/jbc.M805586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris D., Robinson J.R. Drug delivery via the mucous membranes of the oral cavity. J. Pharm. Sci. 1992;81:1–10. doi: 10.1002/jps.2600810102. [DOI] [PubMed] [Google Scholar]
- 16.Rubino F., Della Noce C., Vigna L., De Giuseppe R., Novembrino C., de Liso F., Maiavacca R., Patrini L., Riboldi L., Bamonti F. Measurement of glutathionylated haemoglobin by MALDI-ToF mass spectrometry as a biomarker of oxidative stress in heavy smokers and in occupational obese subjects. Int. J. Anal. Mass Spectrom. Chromatogr. 2013;1:22–30. [Google Scholar]
- 17.Zhuo L., Hascall V.C., Kimata K. Inter-alpha-trypsin inhibitor, a covalent protein–glycosaminoglycan–protein complex. J. Biol. Chem. 2004;279:38079–38082. doi: 10.1074/jbc.R300039200. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen H.M., Verhoef J.C., Ponec M., Rassing M.R. TR146 cells grown on filters as a model of human buccal epithelium: permeability of fluorescein isothiocyanate-labelled dextrans in the presence of sodium glycocholate. J. Control. Release. 1999;60:223–233. doi: 10.1016/s0168-3659(99)00081-4. [DOI] [PubMed] [Google Scholar]
- 19.Morck Nielsen H., Romer Rassing M. TR146 cells grown on filters as a model of human buccal epithelium: V. Enzyme activity of the TR146 cell culture model, human buccal epithelium and porcine buccal epithelium, and permeability of leu-enkephalin. Int. J. Pharm. 2000;200:261–270. doi: 10.1016/s0378-5173(00)00394-x. [DOI] [PubMed] [Google Scholar]
- 20.Rhee S.G., Kang S.W., Jeong W., Chang T.S., Yang K.S., Woo H.A. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr. Opin. Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Greabu M., Totan A., Battino M., Mohora M., Didilescu A., Totan C. Cigarette smoke effect on total salivary antioxidant capacity, salivary glutathione peroxidase and gamma-glutamyltransferase activity. BioFactors. 2008;33:129–136. doi: 10.1002/biof.5520330205. [DOI] [PubMed] [Google Scholar]
- 22.Shaulian E., Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 23.Zunino A., Degan P., Vigo T., Abbondandolo A. Hydrogen peroxide: effects on DNA, chromosomes, cell cycle and apoptosis induction in Fanconi's anemia cell lines. Mutagenesis. 2001;16:283–288. doi: 10.1093/mutage/16.3.283. [DOI] [PubMed] [Google Scholar]
- 24.Guha N., Boffetta P., Wunsch Filho V., Eluf Neto J., Shangina O., Zaridze D. Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: results of two multicentric case–control studies. Am. J. Epidemiol. 2007;166:1159–1173. doi: 10.1093/aje/kwm193. [DOI] [PubMed] [Google Scholar]
- 25.Klaric E., Par M., Profeta I., Kopjar N., Rozgaj R., Kasuba V. Genotoxic effect of two bleaching agents on oral mucosa. Cancer Genomics Proteomics. 2013;10:209–215. [PubMed] [Google Scholar]
- 26.Holland N., Bolognesi C., Kirsch-Volders M., Bonassi S., Zeiger E., Knasmueller S. The micronucleus assay in human buccal cells as a tool for biomonitoring DNA damage: the HUMN project perspective on current status and knowledge gaps. Mutat. Res. 2008;659:93–108. doi: 10.1016/j.mrrev.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Majer B.J., Laky B., Knasmuller S., Kassie F. Use of the micronucleus assay with exfoliated epithelial cells as a biomarker for monitoring individuals at elevated risk of genetic damage and in chemoprevention trials. Mutat. Res. 2001;489:147–172. doi: 10.1016/s1383-5742(01)00068-0. [DOI] [PubMed] [Google Scholar]
- 28.Tew K.D., Manevich Y., Grek C.L., Xiong Y., Uys J.D., Townsend D.M. The role of glutathione S-transferase P in signaling pathways and S-glutathionylation in cancer. Free Radic. Biol. Med. 2011;15:299–313. doi: 10.1016/j.freeradbiomed.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y.K., Lin L.M. Evaluation of glutathione S-transferase activity in human buccal epithelial dysplasias and squamous cell carcinomas. Int. J. Oral Maxillofac. Surg. 1997;26:205–209. doi: 10.1016/s0901-5027(97)80820-x. [DOI] [PubMed] [Google Scholar]
- 30.Patel B.P., Rawal U.M., Shah P.M., Prajapati J.A., Rawal R.M., Dave T.K. Study of tobacco habits and alterations in enzymatic antioxidant system in oral cancer. Oncology. 2005;68:511–519. doi: 10.1159/000086995. [DOI] [PubMed] [Google Scholar]
- 31.Grek C.L., Townsend D.M., Tew K.D. The impact of redox and thiol status on the bone marrow: pharmacological intervention strategies. Pharmacol. Ther. 2011;129:172–184. doi: 10.1016/j.pharmthera.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Pel M., van Os R., Velders G.A., Hagoort H., Heegaard P.M., Lindley I.J. Serpina1 is a potent inhibitor of IL-8-induced hematopoietic stem cell mobilization. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1469–1474. doi: 10.1073/pnas.0510192103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nair U.J., Friesen M., Richard I., MacLennan R., Thomas S., Bartsch H. Effect of lime composition on the formation of reactive oxygen species from areca nut extract in vitro. Carcinogenesis. 1990;11:2145–2148. doi: 10.1093/carcin/11.12.2145. [DOI] [PubMed] [Google Scholar]
- 34.Feng Z., Xia Y., Tian D., Wu K., Schmitt M., Kwok R.K. DNA damage in buccal epithelial cells from individuals chronically exposed to arsenic via drinking water in Inner Mongolia, China. Anticancer Res. 2001;21:51–57. [PubMed] [Google Scholar]
- 35.Pastor S., Gutierrez S., Creus A., Xamena N., Piperakis S., Marcos R. Cytogenetic analysis of Greek farmers using the micronucleus assay in peripheral lymphocytes and buccal cells. Mutagenesis. 2001;16:539–545. doi: 10.1093/mutage/16.6.539. [DOI] [PubMed] [Google Scholar]
- 36.Squier C.A., Kremer M.J. Biology of oral mucosa and esophagus. J. Natl. Cancer Inst. Monogr. 2001:7–15. doi: 10.1093/oxfordjournals.jncimonographs.a003443. [DOI] [PubMed] [Google Scholar]
- 37.Huang Z., Komninou D., Kleinman W., Pinto J.T., Gilhooly E.M., Calcagnotto A. Enhanced levels of glutathione and protein glutathiolation in rat tongue epithelium during 4-NQO-induced carcinogenesis. Int. J. Cancer J. Int. Cancer. 2007;120:1396–1401. doi: 10.1002/ijc.22525. [DOI] [PubMed] [Google Scholar]
- 38.Wozniak K., Czechowska A., Blasiak J. Cisplatin-evoked DNA fragmentation in normal and cancer cells and its modulation by free radical scavengers and the tyrosine kinase inhibitor STI571. Chem. Biol. Interact. 2004;147:309–318. doi: 10.1016/j.cbi.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Farrell N. Nonclassical platinum antitumor agents: perspectives for design and development of new drugs complementary to cisplatin. Cancer Invest. 1993;11:578–589. doi: 10.3109/07357909309011676. [DOI] [PubMed] [Google Scholar]