Abstract
The clinical trials with intrastriatal transplantation of human fetal mesencephalic tissue, rich in dopaminergic neurons, in Parkinson's disease (PD) patients show that cell replacement can work and in some cases induce major, long-lasting improvement. However, owing to poor tissue availability, this approach can only be applied in very few patients, and standardization is difficult, leading to wide variation in functional outcome. Stem cells and reprogrammed cells could potentially be used to produce dopaminergic neurons for transplantation. Importantly, dopaminergic neurons of the correct substantia nigra phenotype can now be generated from human embryonic stem cells in large numbers and standardized preparations, and will soon be ready for application in patients. Also, human induced pluripotent stem cell-derived dopaminergic neurons are being considered for clinical translation. Available data justify moving forward in a responsible way with these dopaminergic neurons, which should be tested, using optimal patient selection, cell preparation and transplantation procedures, in controlled clinical studies.
Keywords: Parkinson's disease, dopaminergic neurons, neural grafts, stem cells, transplantation, reprogramming
1. Introduction
Until the late 1970s, it was generally believed that repairing the central nervous system in humans, which had never been possible in the past, would never be possible in the future. However, two articles with obvious clinical implications were published in 1979 [1,2], showing that intracerebral grafts of fetal mesencephalic dopamine (DA)-rich tissue in rats could ameliorate signs of experimental Parkinson's disease (PD). In humans, this chronic neurodegenerative disorder is characterized by impairment of movement. The main pathology underlying the motor symptoms is a progressive loss of dopaminergic neurons in the substantia nigra. Motor symptoms can be successfully treated by dopaminergic drugs for several years but over time, the drugs become less effective and are associated with side effects such as involuntary movements (dyskinesias). The findings in the animal models raised the possibility of a novel therapeutic approach for PD patients based on replacing the dead dopaminergic neurons by healthy ones through transplantation.
Although there was a lot of enthusiasm about the preclinical data, the first clinical transplantations in PD were not performed with human fetal mesencephalic tissue. In pioneering work performed by Olson, Seiger and Backlund and their co-workers [3,4], autologous adrenal medulla cells were implanted into striatum of four PD patients to provide a local catecholamine source but the beneficial effects were minimal. Similarly, after an initial hype with open microsurgery and pieces of adrenal medulla tissue placed in the caudate nucleus [5], this method was abandoned because of lack of efficacy and adverse effects. In 1987, the first intrastriatal implantations of human fetal mesencephalic tissue, rich in dopaminergic neuroblasts, were performed in PD patients. Clinical studies were then ongoing until the late 1990s. Improvements were reported in open-label studies (for references see, e.g. [6]), but two double-blind trials [7,8] demonstrated no significant changes compared with sham-operated controls. Moreover, troublesome dyskinesias developed in a subgroup of patients [8]. The lack of efficacy and occurrence of dyskinesias following transplantation of human fetal mesencephalic tissue stopped clinical activities with cell therapy in PD for a decade.
Clinical cell therapy research for PD has now entered a new, exciting phase and recent developments in the field give reason for optimism. Three sources of dopaminergic neurons are being planned for clinical application, i.e. human fetal mesencephalic tissue, human embryonic stem (ES) cells and human induced pluripotent stem (iPS) cells. In this study, I will describe how far the clinical translation has reached for these different approaches and what are the major challenges. Types of stem cells which have been used for transplantation in animal models of PD but do not work by dopaminergic cell replacement, such as mesenchymal stem cells, will not be discussed here.
2. Transplantation of human fetal mesencephalic tissue
Despite three decades of experimental and clinical work, transplantation of human fetal mesencephalic tissue has not yet been developed into a clinically competitive treatment for PD patients. However, these studies have provided valuable insight into the basic principles of cell therapy in PD. Implantation of human fetal DA-rich mesencephalic tissue is, therefore, regarded as a gold standard when moving dopaminergic neurons derived from other sources towards clinical application.
It is well established, from a large number of studies, that the fetal dopaminergic neurons can survive and grow after intrastriatal transplantation into the PD patient's brain. Positron emission tomography (PET) has demonstrated increased 18F-DOPA uptake in the grafted putamen (for references see, e.g. [6]), and histopathological studies have shown survival of implanted dopaminergic neurons and reinnervation of the striatum [9–17]. Four patients with major clinical improvement, in whom 18F-DOPA uptake was normalized in the grafted putamen from 10 to 16 years post-surgery, showed normal DA release, as assessed by 11C-raclopride binding, at 7–10 years after transplantation [18,19]. Providing evidence for their functional integration into host neuronal circuitry, the fetal mesencephalic grafts reversed the deficits in movement-related cortical activation with a time course paralleling that of the clinical improvement [20].
Clinical benefits have been observed in several open-label trials (for references see, e.g. [6]), and the most successful cases have had l-DOPA treatment withdrawn, exhibiting major recovery for several years [18,19,21]. The strongest evidence that human fetal dopaminergic grafts can give rise to clinically competitive improvement comes from two patients subjected to bilateral intrastriatal transplantation of human fetal mesencephalic tissue [19,21]. The motor improvement in both patients was sustained up to 18 years after grafting, more than 10 years after withdrawal of dopaminergic medication [19,21]. Improvements in these patients are most probably because of the restoration of striatal dopaminergic function as evidenced by normalization of putaminal 18F-DOPA uptake and 11C-raclopride binding.
A major problem for the field emerged when two sham surgery-controlled clinical studies with bilateral intraputaminal grafts did not confirm the positive findings in the open-label trials. In the first trial, Freed et al. [7] demonstrated only very modest improvement of motor function at 12 months. The lack of efficacy could be explained by the low number of surviving, grafted dopaminergic neurons. In an open-label follow-up of these patients, Ma et al. [22] observed clinical benefit and surviving grafts up to 4 years after transplantation. In the second trial [8], PD symptoms did not differ between grafted and sham groups at 24 months. Improvement was observed only during the first six to nine months after transplantation. The function of the graft may have been impaired because of immune reaction, as suggested by the deterioration after withdrawal of immunosuppression at six months [8]. The lack of efficacy could also be explained by the patients in this controlled trial being more severely disabled prior to grafting as compared to those in the open-label trials [23–25]. The dopaminergic denervation in areas not reached by intraputaminal grafts, such as ventral striatum, has been shown to negatively influence the efficacy of transplantation [22,26]. Therefore, it is unlikely that advanced PD patients with widespread denervation will exhibit successful outcome following intraputaminal transplantation. In accordance, Olanow et al. [8] found improvement only in less severely disabled patients.
One concern for the field of cell therapy in PD has been whether the disease process would also destroy the transplanted dopaminergic neurons and prevent long-term graft function. Lewy bodies (LBs), which are the pathological hallmark of PD, have been detected in a fraction of the grafted dopaminergic neurons which survive for 10 years or longer in PD patients [9,10,14,15,27]. This finding may indicate spread of pathology from the host cells and has triggered a new research field hypothesizing that PD is a prion-like disorder. Long-term surviving grafts in humans have been reported to exhibit reduced expression of the DA transporter (DAT) [9,10,27], which suggests that α-synuclein pathology is associated with synaptic dysfunction. However, in one case DAT binding increased after grafting and remained unchanged at 14 years post-transplantation [28]. Moreover, Hallett et al. [29] reported robust DAT expression and normal mitochondrial localization in grafted dopaminergic neurons in five PD patients at 4–14 years after transplantation. Thus, the degree of graft pathology varies between patients. In agreement, Mendez et al. [17] did not detect LBs in the grafts of their PD patients up to 14 years. In summary, even if pathology spreads to the grafted cells, dopaminergic cell therapy is a viable therapeutic option because (i) disease propagation is slow, (ii) the majority of grafted neurons are unaffected after a decade, and (iii) patients experience long-term improvement.
The dyskinesias which have developed in a subgroup of grafted PD patients can be effectively treated with deep-brain stimulation (DBS) of the globus pallidus [30,31]. Several mechanisms which probably underlie graft-induced dyskinesias have been identified in animal models, such as post-synaptic supersensitivity, established by chronic l-DOPA treatment prior to transplantation [32], and small transplants forming ‘hot-spots’ of DA release while the surrounding striatum remains supersensitive [33,34]. Clinical observations provide strong evidence that graft-derived serotonergic hyperinnervation of the striatum causes dyskinesias after transplantation (see, however, [35]). Three patients with major clinical improvement, who developed moderately severe graft-induced dyskinesias, exhibited excessive serotonergic innervation in the grafted striatum [19,28]. The dyskinesias were abolished by administration of a 5-HT1A receptor agonist, which dampens transmitter release from serotonergic neurons.
In the ongoing EU-sponsored TRANSEURO clinical trial (www.transeuro.org.uk), human fetal mesencephalic tissue will be implanted into striatum with optimized patient selection and tissue preparation procedures. Patients will be younger and earlier in their disease, as compared to previous trials, and they have not developed significant l-DOPA-induced dyskinesias. The outcome in the grafted group (20 patients are planned) will be compared with the behaviour in a matched natural history control group. This trial could provide valuable information contributing to the further development of a clinically competitive cell therapy for PD. However, human fetal mesencephalic tissue can be obtained only in limited amounts and is difficult to standardize, which makes it unlikely that it will become useful for transplantation in large numbers of patients. Therefore, there is a need for new sources of dopaminergic neurons. The generation of dopaminergic neurons from stem/progenitor cells or by reprogramming somatic cells could be the solution.
3. Transplantation of dopaminergic neurons derived from stem cells or somatic cells
(a). General aspects
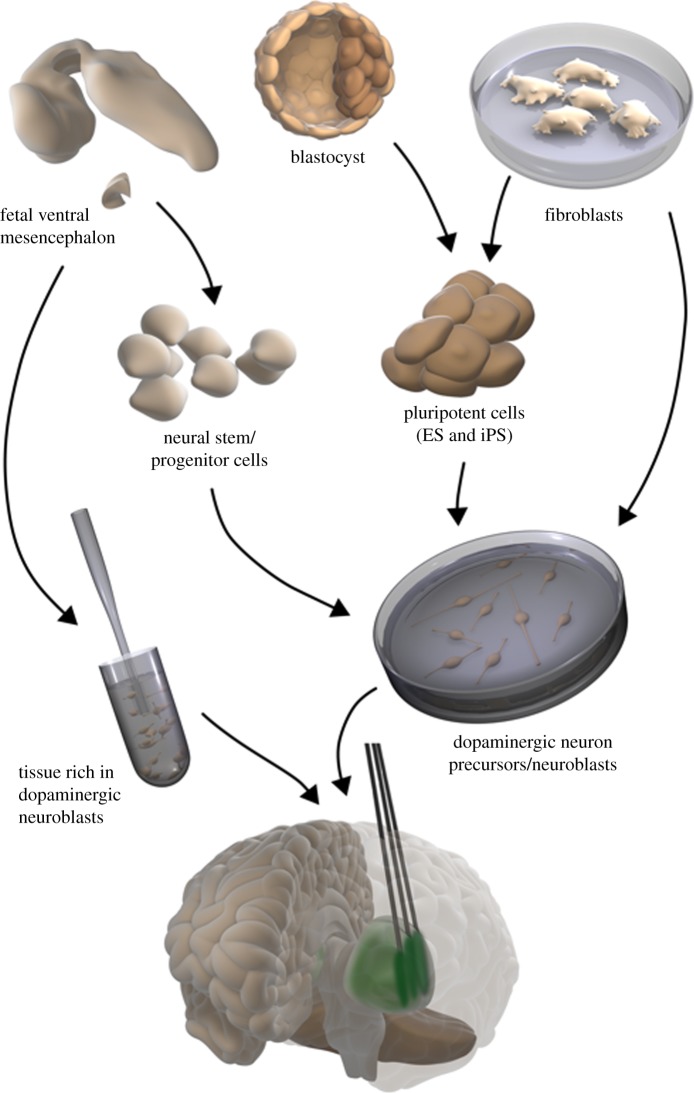
Several potential sources of cells have been proposed for the generation of transplantable dopaminergic neurons in PD, including neural stem cells, pluripotent cells such as ES cells and iPS cells, and somatic cells which are directly converted to dopaminergic neurons (figure 1). The experimental data and clinical studies clearly show that not any DA-producing cell will be useful to induce substantial symptomatic relief. A clinical candidate cell must be of human origin and have the properties of a substantia nigra neuron to be able to induce maximum functional recovery [36]. This means that the candidate cell should express genes/transcription factors and phenotypic markers typical for substantia nigra dopaminergic neurons, be electrophysiologically excitable, and capable of DA release. Not only the candidate cell but also other cell types generated from the stem cells or reprogrammed cells need to be known, i.e. the number of non-nigral dopaminergic neurons, serotonin neurons, other non-dopaminergic neurons, glial elements, undifferentiated precursor/stem cells and non-neural cells.
Figure 1.
Schematic of potential sources of dopaminergic neurons for transplantation in PD patients. (i) Human fetal mesencephalic tissue, rich in dopaminergic neuroblasts, made into a cell suspension; (ii) neural stem/progenitor cells from human fetal mesencephalon, expanded and differentiated to dopaminergic neuroblasts; (iii) pluripotent cells generated from human blastocysts (ES cells) or fibroblasts (iPS cells), expanded and differentiated to dopaminergic neuron precursors/neuroblasts; (iv) human fibroblasts directly converted to dopaminergic neuron precursors/neuroblasts. The dopaminergic cells are implanted stereotaxically into the striatum, primarily putamen.
For the further development of standardized, safe and effective stem cell-based clinical procedures for dopaminergic neuron transplantation, methods for cell sorting will most probably be of major importance. Such methods should ideally allow (i) the enrichment of dopaminergic neurons of the correct mesencephalic phenotype and at the optimum stage of differentiation and (ii) the removal of unwanted cells. Several recent studies provide evidence supporting the idea that cell sorting could become a useful strategy for the transplantation of well-characterized dopaminergic cell preparations in PD patients [37–40]. It will be important to demonstrate, prior to clinical application, that the cell sorting does not significantly compromise the survival and functional capacity of the dopaminergic neurons after transplantation.
The candidate cells must be able to survive long-term and differentiate to the appropriate neuronal phenotype, i.e. nigral dopaminergic neurons, after transplantation into an animal model of PD. Ongoing cell division must not be present beyond 1–2 months following transplantation. A major portion of the striatum should be evenly innervated by graft-derived dopaminergic fibres. The grafts should be able to induce a substantial functional recovery in behavioural tests relevant for the clinical condition.
It should be emphasized that the requirements for efficacy of a cell-based cell therapy for PD are high. Several treatment options are now available for patients with PD, even in advanced stages of the disease, e.g. l-DOPA, dopamine agonists, enzyme inhibitors, and DBS. Therefore, to be clinically competitive, a cell-based therapy should give rise to (i) long-lasting, major improvement (>60–70%) of mobility and suppression of dyskinesias and/or (ii) improvement of symptoms resistant to other treatments or modification of disease progression. So far, no scientifically based clinical studies with transplantation of dopaminergic neurons derived from stem cells or reprogrammed cells have been performed.
(b). Dopaminergic grafts derived from human embryonic stem cells
Graft survival and behavioural improvements following intrastriatal transplantation of human ES cell-derived dopaminergic neurons in a rat PD model were first reported almost a decade ago [41]. The grafts showed large numbers of dopaminergic neurons of substantia nigra phenotype but also contained potentially tumourigenic, mitotic undifferentiated neuroepithelial cells. There was a breakthrough in the field in 2011 when Kriks and co-workers [42] described a new protocol, guided by developmental principles, which efficiently converts human ES cells to dopaminergic neurons. Floor plate cells are derived in vitro using dual inhibition of SMAD signalling and high levels of Sonic Hedgehog. A midbrain floor plate identity is then induced by activation of Wnt signalling, and cells are differentiated to dopaminergic precursors. After intrastriatal transplantation of these precursors in rodents, high numbers of substantia nigra dopaminergic neurons survived long-term. No tumours were observed. The grafts could reinnervate a major portion of striatum also in a larger (non-human primate) brain, and improved functional deficits in rodents relevant for the clinical condition. In parallel, a very similar protocol was developed by Kirkeby and co-workers [43].
There are several advantages with the generation of dopaminergic neurons from human ES cells using these new protocols. High numbers of human-derived dopaminergic neurons of the correct (substantia nigra) phenotype can be produced and these cells survive transplantation, reinnervate the denervated striatum, and improve clinically relevant behavioural deficits. Using optogenetics and electrophysiological and pharmacological approaches, Steinbeck and co-workers [44] recently demonstrated that the motor recovery induced by the human ES cell-derived dopaminergic neurons, implanted into the DA-denervated mouse striatum, was dependent on neuronal activity and DA release. The grafts modulated glutamatergic transmission in the host striatum similarly to endogenous substantia nigra neurons. Importantly, Grealish and co-workers [45] have reported that grafts of human ES cell-derived dopaminergic neurons implanted in a rat model of PD have the capacity for axonal growth and long-term survival as well as functional efficacy similar to that of human fetal mesencephalic dopaminergic neurons. Thus, the potency of the human ES cell-derived dopaminergic neurons seems to be comparable with the potency of fetal dopaminergic neurons, which have induced major, long-lasting symptomatic relief in PD patients. However, even if no tumours have been detected [42,43,45], safety is still an important issue.
(c). Dopaminergic grafts derived from human somatic cells
Human dopaminergic neurons can be generated by reprogramming fibroblasts through a pluripotent stage, so-called iPS cells [38,46–50]. With this technology, patient-specific cells can be produced and used for transplantation, avoiding immune reactions and the ethical issues associated with human ES cells. Potential problems are tumourigenesis and variability of reprogramming. There is also a risk that the patient-specific cells may exhibit increased susceptibility to the pathological process in PD. This is unlikely to occur if the technology is used to create a cell bank with human leukocyte antigen haplotype-matching iPS cells to be used in allografting experiments [51].
Extensive axonal outgrowth from the human iPS cell-derived dopaminergic neurons implanted into the denervated rodent striatum has not been convincingly demonstrated [38,46–50], and their capacity to improve behavioural deficits relevant for the clinical condition is incompletely known. A rich dopaminergic terminal network extending throughout the striatum will definitely be needed for clinical efficacy. Although functional recovery after implantation of iPS cell-derived dopaminergic neurons into rodent striatum has been described, mostly in tests for drug-induced rotational asymmetry, the underlying mechanisms are unclear. Since density of fibres extending from the graft has been low, it seems possible that DA may not have acted via synaptic release but through diffuse volume transmission. This can work in a smaller rodent brain but will it work in the larger human brain? It should also be pointed out that stem/progenitor cells which are transplanted into the brain can induce functional improvement not only by cell replacement but also through other mechanisms such as trophic support, immunomodulation, and stimulation of neural plasticity [52]. Recently, Hallett et al. [53] reported that autologous iPS cell-derived dopaminergic neurons implanted into striatum of a non-human primate model of PD can survive in large numbers for 2 years, give rise to extensive reinnervation, and improve motor function. Although these findings were observed only in one out of three grafted monkeys, they provide important preclinical evidence supporting the further clinical translation of iPS cell-derived dopaminergic neurons for transplantation in PD.
Functional dopaminergic neurons can also be generated by direct conversion of human fibroblasts [54,55], which is potentially of interest in a clinical perspective. Dopaminergic neurons directly converted from mouse fibroblasts were initially shown to survive transplantation [56] but the fibre outgrowth and functional effects were modest. A recent study [57] reported more extensive axonal outgrowth from grafts of mouse fibroblast-derived dopaminergic neurons implanted into denervated rat striatum. Deficits in apomorphine-induced rotational asymmetry and stepping were improved and, interestingly, performance could be further enhanced by pharmacological activation of the dopaminergic neurons. However, much work is needed before the clinical application of dopaminergic neurons generated by direct conversion can be considered. As a first step, it has to be demonstrated that such cells of human origin can survive in large numbers, show extensive reinnervation of the denervated striatum and give rise to substantial improvement of clinically relevant behavioural deficits in animal PD models.
4. Prospects for treatment of Parkinson's disease using cell transplantation
Degeneration of the nigrostriatal dopaminergic system is the main pathology underlying motor symptoms in PD. In addition, patients can exhibit disabling non-motor symptoms because the disease process also affects other neuronal systems. Non-motor symptoms originating from degeneration outside striatum or in non-dopaminergic systems are unlikely to be alleviated by implantation of dopaminergic cells into putamen. This notion is illustrated by the work of Politis et al. [58], who studied three PD patients at 13–16 years after intrastriatal transplantation of human fetal mesencephalic tissue. Dopaminergic innervation was normalized in the basal ganglia associated with major relief of motor symptoms. However, the patients developed non-motor symptoms such as fatigue, anxiety, mood swings and sleep problems. Using PET, a serotonergic neuron marker was found to be reduced in raphe nuclei and regions receiving serotonergic projections. Most probably, this serotonergic degeneration, which occurs concomitantly with the graft-induced dopaminergic regeneration, underlies some non-motor symptoms in these patients.
These findings underscore that intrastriatal dopaminergic cell therapy will never provide a cure for PD. The three patients have declared that the advantages with the motor improvements outweigh the worsening of non-motor symptoms. It is likely that if dopaminergic grafts induce major relief of motor symptoms for more than a decade, as observed in these patients, the cell therapeutic approach will be clinically competitive despite occurrence of non-motor symptoms. Several therapeutic options exist for PD patients also in advanced stages of the disease. However, a therapy giving rise to robust, long-lasting improvement of motor function is still highly warranted and will be valuable for the treatment of PD.
There are three major challenges for the successful clinical application of stem cells and reprogrammed cells as a treatment for PD. The first challenge is to demonstrate the potency of the generated dopaminergic neurons after transplantation in an animal PD model. The new cells have to work as well as human fetal dopaminergic neurons in order to induce a clinically competitive improvement. Potency tests should include assessment of capacity for axonal growth and DA release as well as magnitude of improvement of clinically relevant behavioural deficits. The potency, including growth capacity, has to be known in order to determine the number of cells for transplantation and the number of implants in each patient. The second challenge is to demonstrate safety. The risk for tumour formation and graft-induced dyskinesias, which have been observed after implantation of fetal mesencephalic tissue, has to be minimized. Tumourigenicity should be assessed rigorously. As part of this evaluation, it will be important to determine the identity of all cell types in the implants. Use of cell sorting to eliminate tumour-forming cells may be necessary to improve safety. One strategy to avoid graft-induced dyskinesias could be to minimize the number of serotonergic neurons in the implants. The third challenge is to select the most suitable patient for the first clinical trials with dopaminergic neurons derived from stem cells or reprogrammed cells. To demonstrate safety, patients have to be in a relatively advanced stage of disease but there should also be a chance of therapeutic benefit. To demonstrate efficacy, patients must be in relatively early stages of the disease, when the dopaminergic denervation is largely restricted to caudate-putamen and not widespread in the forebrain.
To conclude, there has been a steady scientific progress in the field of cell therapy for PD since the first clinical trials were performed three decades ago. Human fetal mesencephalic tissue implantation shows proof-of-principle for the cell replacement strategy but is not a competitive treatment. Human ES and iPS cell-derived dopaminergic neurons are now being moved towards clinical application. For the further development of a useful treatment for PD, these new cells should be tested, using optimal patient selection, cell preparation and transplantation procedures, in well-controlled clinical studies.
Acknowledgement
I thank Bengt Mattsson for the illustration.
Competing interests
I have no competing interests.
Funding
This work was supported by grants from the Swedish Research Council and Swedish Government Initiative for Strategic Research Areas (StemTherapy).
References
- 1.Bjorklund A, Stenevi U. 1979. Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Res. 177, 555–560. ( 10.1016/0006-8993(79)90472-4) [DOI] [PubMed] [Google Scholar]
- 2.Perlow MJ, Freed WJ, Hoffer BJ, Seiger A, Olson L, Wyatt RJ. 1979. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science 204, 643–647. ( 10.1126/science.571147) [DOI] [PubMed] [Google Scholar]
- 3.Backlund EO, Granberg PO, Hamberger B, Knutsson E, Mårtensson A, Sedvall G, Seiger A, Olson L et al. . 1985. Transplantation of adrenal medullary tissue to striatum in parkinsonism. First clinical trials. J. Neurosurg. 62, 169–173. ( 10.3171/jns.1985.62.2.0169) [DOI] [PubMed] [Google Scholar]
- 4.Lindvall O, Backlund EO, Farde L, Sedvall G, Freedman R, Hoffer B, Nobin A, Seiger A, Olson L. 1987. Transplantation in Parkinson's disease: two cases of adrenal medullary grafts to the putamen. Ann. Neurol. 22, 457–468. ( 10.1002/ana.410220403) [DOI] [PubMed] [Google Scholar]
- 5.Madrazo I, Drucker-Colín R, Díaz V, Martínez-Mata J, Torres C, Becerril JJ. 1987. Open microsurgical autograft of adrenal medulla to the right caudate nucleus in two patients with intractable Parkinson's disease. N. Engl. J. Med. 316, 831–834. ( 10.1056/NEJM198704023161402) [DOI] [PubMed] [Google Scholar]
- 6.Lindvall O. 2013. Developing dopaminergic cell therapy for Parkinson's disease---give up or move forward? Mov. Disord. 28, 268–273. ( 10.1002/mds.25378) [DOI] [PubMed] [Google Scholar]
- 7.Freed CR. et al. 2001. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N. Engl. J. Med. 344, 710–719. ( 10.1056/NEJM200103083441002) [DOI] [PubMed] [Google Scholar]
- 8.Olanow CW. et al. 2003. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann. Neurol. 54, 403–414. ( 10.1002/ana.10720) [DOI] [PubMed] [Google Scholar]
- 9.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. 2008. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat. Med. 14, 504–506. ( 10.1038/nm1747) [DOI] [PubMed] [Google Scholar]
- 10.Kordower JH, Chu Y, Hauser RA, Olanow CW, Freeman TB. 2008. Transplanted dopaminergic neurons develop PD pathologic changes: a second case report. Mov. Disord. 23, 2303–2306. ( 10.1002/mds.22369) [DOI] [PubMed] [Google Scholar]
- 11.Kordower JH, Freeman TB, Chen EY, Mufson EJ, Sanberg PR, Hauser RA, Snow B, Olanow CW. 1998. Fetal nigral grafts survive and mediate clinical benefit in a patient with Parkinson's disease. Mov. Disord. 13, 383–393. ( 10.1002/mds.870130303) [DOI] [PubMed] [Google Scholar]
- 12.Kordower JH. et al. 1995. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson's disease. N. Engl. J. Med. 332, 1118–1124. ( 10.1056/NEJM199504273321702) [DOI] [PubMed] [Google Scholar]
- 13.Kordower JH. et al. 1996. Functional fetal nigral grafts in a patient with Parkinson's disease: chemoanatomic, ultrastructural, and metabolic studies. J. Comp. Neurol. 370, 203–230. () [DOI] [PubMed] [Google Scholar]
- 14.Li JY. et al. 2008. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat. Med. 14, 501–503. ( 10.1038/nm1746) [DOI] [PubMed] [Google Scholar]
- 15.Li JY, Englund E, Widner H, Rehncrona S, Bjorklund A, Lindvall O, Brundin P. 2010. Characterization of Lewy body pathology in 12- and 16-year-old intrastriatal mesencephalic grafts surviving in a patient with Parkinson's disease. Mov. Disord. 25, 1091–1096. ( 10.1002/mds.23012) [DOI] [PubMed] [Google Scholar]
- 16.Mendez I, Sanchez-Pernaute R, Cooper O, Vinuela A, Ferrari D, Björklund L, Dagher A, Isacson O. 2005. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson's disease. Brain 128, 1498–1510. ( 10.1093/brain/awh510) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendez I. et al. 2008. Dopamine neurons implanted into people with Parkinson's disease survive without pathology for 14 years. Nat. Med. 14, 507–509. ( 10.1038/nm1752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piccini P. et al. 1999. Dopamine release from nigral transplants visualized in vivo in a Parkinson's patient. Nat. Neurosci. 2, 1137–1140. ( 10.1038/16060) [DOI] [PubMed] [Google Scholar]
- 19.Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, Rehncrona S, Bjorklund A, Lindvall O, Piccini P. 2010. Serotonergic neurons mediate dyskinesia side effects in Parkinson's patients with neural transplants. Sci. Transl. Med. 2, 38ra46. ( 10.1126/scitranslmed.3000976) [DOI] [PubMed] [Google Scholar]
- 20.Piccini P. et al. 2000. Delayed recovery of movement-related cortical function in Parkinson's disease after striatal dopaminergic grafts. Ann. Neurol. 48, 689–695. () [DOI] [PubMed] [Google Scholar]
- 21.Kefalopoulou Z. et al. 2014. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 71, 83–87. ( 10.1001/jamaneurol.2013.4749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma Y, Tang C, Chaly T, Greene P, Breeze R, Fahn, Freed C, Dhawan V, Eidelberg D. 2010. Dopamine cell implantation in Parkinson's disease: long-term clinical and 18F-FDOPA PET outcomes. J. Nucl. Med. 51, 7–15. ( 10.2967/jnumed.109.066811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brundin P. et al. 2000. Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson's disease. Brain 123, 1380–1390. ( 10.1093/brain/123.7.1380) [DOI] [PubMed] [Google Scholar]
- 24.Hagell P. et al. 1999. Sequential bilateral transplantation in Parkinson's disease: effects of the second graft. Brain 122, 1121–1132. ( 10.1093/brain/122.6.1121) [DOI] [PubMed] [Google Scholar]
- 25.Wenning GK. et al. 1997. Short- and long-term survival and function of unilateral intrastriatal dopaminergic grafts in Parkinson's disease. Ann. Neurol. 42, 95–107. ( 10.1002/ana.410420115) [DOI] [PubMed] [Google Scholar]
- 26.Piccini P, Pavese N, Hagell P, Reimer J, Björklund A, Oertel WH, Quinn NP, Brooks DJ, Lindvall O. 2005. Factors affecting the clinical outcome after neural transplantation in Parkinson's disease. Brain 128, 2977–2986. ( 10.1093/brain/awh649) [DOI] [PubMed] [Google Scholar]
- 27.Kurowska Z, Englund E, Widner H, Lindvall O, Li J-Y, Brundin P. 2011. Signs of degeneration in 12–22-year old grafts of mesencephalic dopamine neurons in patients with Parkinson's disease. J. Parkinson's Dis. 1, 83–92. ( 10.3233/JPD-2011-11004) [DOI] [PubMed] [Google Scholar]
- 28.Politis M, Oertel WH, Wu K, Quinn NP, Pogarell O, Brooks DJ, Bjorklund A, Lindvall O, Piccini P. 2011. Graft-induced dyskinesias in Parkinson's disease: high striatal serotonin/dopamine transporter ratio. Mov. Disord. 26, 1997–2003. ( 10.1002/mds.23743) [DOI] [PubMed] [Google Scholar]
- 29.Hallett PJ, Cooper O, Sadi D, Robertson H, Mendez I, Isacson O. 2014. Long-term health of dopaminergic neuron transplants in Parkinson's disease patients. Cell Rep. 7, 1755–1761. ( 10.1016/j.celrep.2014.05.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graff-Radford J, Foote KD, Rodriguez RL, Fernandez HH, Hauser RA, Sudhyadhom A, Rosado CA, Sanchez JC, Okun MS. 2006. Deep brain stimulation of the internal segment of the globus pallidus in delayed runaway dyskinesia. Arch. Neurol. 63, 1181–1184. ( 10.1001/archneur.63.8.1181) [DOI] [PubMed] [Google Scholar]
- 31.Herzog J, Pogarell O, Pinsker MO, Kupsch A, Oertel WH, Lindvall O, Deuschl G, Volkmann J. 2008. Deep brain stimulation in Parkinson's disease following fetal nigral transplantation. Mov. Disord. 23, 1293–1296. ( 10.1002/mds.21768) [DOI] [PubMed] [Google Scholar]
- 32.Lane EL, Vercammen L, Cenci MA, Brundin P. 2009. Priming for L-DOPA-induced abnormal involuntary movements increases the severity of amphetamine-induced dyskinesia in grafted rats. Exp. Neurol. 219, 355–358. ( 10.1016/j.expneurol.2009.04.010) [DOI] [PubMed] [Google Scholar]
- 33.Carlsson T, Winkler C, Lundblad M, Cenci MA, Bjorklund A, Kirik D. 2006. Graft placement and uneven pattern of reinnervation in the striatum is important for development of graft-induced dyskinesia. Neurobiol. Dis. 21, 657–668. ( 10.1016/j.nbd.2005.09.008) [DOI] [PubMed] [Google Scholar]
- 34.Maries E, Kordower JH, Chu Y, Collier TJ, Sortwell CE, Olaru E, Shannon K, Steece-Collier K. 2006. Focal not widespread grafts induce novel dyskinetic behavior in parkinsonian rats. Neurobiol. Dis. 21, 165–180. ( 10.1016/j.nbd.2005.07.002) [DOI] [PubMed] [Google Scholar]
- 35.Shin E, Garcia J, Winkler C, Bjorklund A, Carta M. 2012. Serotonergic and dopaminergic mechanisms in graft-induced dyskinesia in a rat model of Parkinson's disease. Neurobiol. Dis. 47, 393–406. ( 10.1016/j.nbd.2012.03.038) [DOI] [PubMed] [Google Scholar]
- 36.Lindvall O, Barker RA, Brustle O, Isacson O, Svendsen CN. 2012. Clinical translation of stem cells in neurodegenerative disorders. Cell Stem Cell 10, 151–155. ( 10.1016/j.stem.2012.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bye CR, Jonsson ME, Bjorklund A, Parish CL, Thompson LH. 2015. Transcriptome analysis reveals transmembrane targets on transplantable midbrain dopamine progenitors. Proc. Natl Acad. Sci. USA 112, E1946–E1955. ( 10.1073/pnas.1501989112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doi D. et al. 2014. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Rep. 2, 337–350. ( 10.1016/j.stemcr.2014.01.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganat YM. et al. 2012. Identification of embryonic stem cell-derived midbrain dopaminergic neurons for engraftment. J. Clin. Invest. 122, 2928–2939. ( 10.1172/JCI58767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hedlund E, Pruszak J, Lardaro T, Ludwig W, Vinuela A, Kim KS, Isacson O. 2008. Embryonic stem cell-derived Pitx3-enhanced green fluorescent protein midbrain dopamine neurons survive enrichment by fluorescence-activated cell sorting and function in an animal model of Parkinson's disease. Stem Cells 26, 1526–1536. ( 10.1634/stemcells.2007-0996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. 2006. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat. Med. 12, 1259–1268. ( 10.1038/nm1495) [DOI] [PubMed] [Google Scholar]
- 42.Kriks S. et al. 2011. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature 480, 547–551. ( 10.1038/nature10648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M, Lindvall O, Parmar M. 2012. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 1, 703–714. ( 10.1016/j.celrep.2012.04.009) [DOI] [PubMed] [Google Scholar]
- 44.Steinbeck JA, Choi SJ, Mrejeru A, Ganat Y, Deisseroth K, Sulzer D, Mosharov EV, Studer L. 2015. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson's disease model. Nat. Biotechnol. 33, 204–209. ( 10.1038/nbt.3124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grealish S. et al. 2014. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson's disease. Cell Stem Cell 15, 653–665. ( 10.1016/j.stem.2014.09.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hargus G. et al. 2010. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc. Natl Acad. Sci. USA 107, 15 921–15 926. ( 10.1073/pnas.1010209107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kikuchi T, Morizane A, Doi D, Onoe H, Hayashi T, Kawasaki T, Hidemoto S, Susumu M, Jun T. 2011. Survival of human induced pluripotent stem cell-derived midbrain dopaminergic neurons in the brain of a primate model of Parkinson's disease. J. Parkinson's Dis. 1, 395–412. ( 10.3233/JPD-2011-11070) [DOI] [PubMed] [Google Scholar]
- 48.Rhee YH. et al. 2011. Protein-based human iPS cells efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease. J. Clin. Invest. 121, 2326–2335. ( 10.1172/JCI45794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sundberg M. et al. 2013. Improved cell therapy protocols for Parkinson's disease based on differentiation efficiency and safety of hESC-, hiPSC-, and non-human primate iPSC-derived dopaminergic neurons. Stem Cells 31, 1548–1562. ( 10.1002/stem.1415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swistowski A, Peng J, Liu Q, Mali P, Rao MS, Cheng L, Zeng X. 2010. Efficient generation of functional dopaminergic neurons from human induced pluripotent stem cells under defined conditions. Stem Cells 28, 1893–1904. ( 10.1002/stem.499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner M. et al. 2013. Toward the development of a global induced pluripotent stem cell library. Cell Stem Cell 13, 382–384. ( 10.1016/j.stem.2013.08.003) [DOI] [PubMed] [Google Scholar]
- 52.Kokaia Z, Martino G, Schwartz M, Lindvall O. 2012. Cross-talk between neural stem cells and immune cells: the key to better brain repair? Nat. Neurosci. 15, 1078–1087. ( 10.1038/nn.3163) [DOI] [PubMed] [Google Scholar]
- 53.Hallett PJ. et al. 2015. Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson's disease. Cell Stem Cell 16, 269–274. ( 10.1016/j.stem.2015.01.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caiazzo M. et al. 2011. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 476, 224–227. ( 10.1038/nature10284) [DOI] [PubMed] [Google Scholar]
- 55.Pfisterer U. et al. 2011. Direct conversion of human fibroblasts to dopaminergic neurons. Proc. Natl Acad. Sci. USA 108, 10 343–10 348. ( 10.1073/pnas.1105135108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim J. et al. 2011. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell Stem Cell 9, 413–419. ( 10.1016/j.stem.2011.09.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dell'Anno MT. et al. 2014. Remote control of induced dopaminergic neurons in parkinsonian rats. J. Clin. Invest. 124, 3215–3229. ( 10.1172/JCI74664) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, Oertel WH, Bjorklund A, Lindvall O, Piccini P. 2012. Serotonin neuron loss and nonmotor symptoms continue in Parkinson's patients treated with dopamine grafts. Sci. Transl. Med. 4, 128ra41 ( 10.1126/scitranslmed.3003391) [DOI] [PubMed] [Google Scholar]