Abstract
It is now well established that the immune system can control and eliminate cancer cells. Adoptive T cell transfer has the potential to overcome the significant limitations associated with vaccine-based strategies in patients who are often immune compromised. Application of the emerging discipline of synthetic biology to cancer, which combines elements of genetic engineering and molecular biology to create new biological structures with enhanced functionalities, is the subject of this overview. Various chimeric antigen receptor designs, manufacturing processes and study populations, among other variables, have been tested and reported in recent clinical trials. Many questions remain in the field of engineered T cells, but the encouraging response rates pave a wide road for future investigation into fields as diverse as cancer and chronic infections.
Keywords: immunotherapy, synthetic biology, cancer
1. Introduction
It is widely accepted that the immune system has evolved cellular and humoral mechanisms that can evoke natural immune responses to tumours [1]. However, in most instances, vaccines fail to induce rejection of established tumours [2]. Adoptive T-cell transfer, a term coined by Billingham et al. [3], has the potential to overcome one of the significant limitations associated with vaccine-based strategies, and specifically the requirement to de novo activate and expand a tumour antigen-specific T-cell response in patients, who are often immune compromised. Mitchison [4] first reported the targeting of cancer through the adoptive transfer of lymphocytes in rodent models over 50 years ago.
Application of the emerging discipline of synthetic biology to cancer, which combines elements of genetic engineering and molecular biology to create new biological structures with enhanced functionalities [5], is the focus of this volume. In 1989, Eshhar and co-workers [6] reported the first synthetic receptor expressed in lymphocytes. Shortly thereafter, Irving & Weiss [7] reported that a chimeric antigen receptor (CAR) comprised CD8, and the CD3ζ chain was sufficient to activate T cells. A coalescence of pre-clinical and clinical data supports the premise that the principles of gene transfer combined with adoptive cellular therapy are poised to overcome the fundamental limitations associated with central and peripheral tolerance and enable the potent and efficient at-will targeting of tumours.
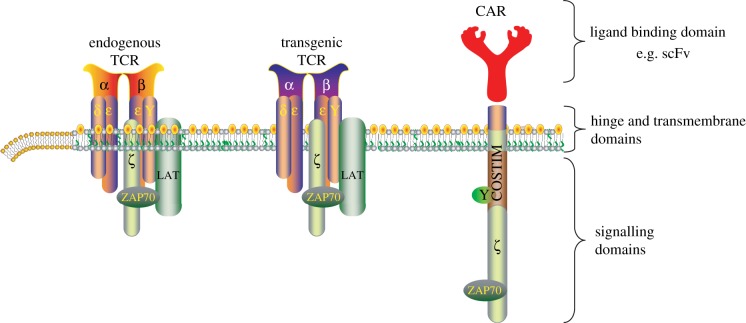
There are many mechanisms that prevent the immune system from eliminating tumours in most patients [8]. One major issue is the relatively low affinity of T-cell receptors (TCRs) for self-antigens compared with foreign antigens. In humans, comparative analyses have revealed that the TCRs from T cells that recognize self-tumour antigens have a substantially lower affinity (approx. 1.5 logs) for cognate major histocompatibility complex (MHC) : peptide complexes compared with their virus-specific TCRs [9]. Adoptive transfer using engineered TCRs and CARs is a promising approach to overcome this obstacle (figure 1). The adoptive transfer of T cells with endogenous TCRs is an effective therapy for virally induced tumours. As reviewed by Rooney and co-workers [10], the fraction of cancer known to be caused by tumour-associated viruses continues to increase. Because cytomegalovirus (CMV) appears to infect glioblastoma [11], clinical studies are using CMV-specific T cells as a potential therapy [10,12].
Figure 1.
T cells can be engineered to have retargeted specificity for tumours. Bispecific T cells are created by introduction of genes that encode T-cell receptors (TCRs) and chimeric antigen receptors (CARs) of desired specificity and affinities for tumours. CARs target surface antigens in an MHC-independent fashion. The T cells retain expression of the endogenous TCR, unless this is knocked down by various approaches. Costim, cosignalling domain such as CD28, ICOS or 4-1BB; LAT, linker for activation of T cells; scFv, single-chain variable fragment; ZAP70, ζ chain associated protein kinase 70 kDa. (Online version in colour.)
Despite anti-retroviral therapies (ARTs), HIV-1/AIDS continues to cause a considerable medical and economic burden, and there continues to be a pressing need for an HIV-1 cure. The goal of engineered T-cell therapy for HIV is to generate an immune system that can resist HIV-1 infection, control viral replication below the limit of detection and persist at high functional competency in the absence of ART. A number of recent technical, conceptual and clinical trial advances now make the goal of a HIV-1 cure tangibly within reach. Our group has recently infused CD4 T cells rendered HIV-1 resistant by deletion of CCR5 using zinc finger nucleases into HIV-1-infected individuals [13]. These modified T cells not only persisted during ART interruption, but also exerted some control of HIV-1 replication in vivo. In an unpublished follow-up study supported by Sangamo Biosciences, two subjects controlled HIV-1 replication off ART, one maintaining control for 48 weeks.
2. Tumour-infiltrating lymphocytes
Adoptive transfer of tumour-infiltrating lymphocytes (TILs) following harvest from tumour and ex vivo expansion was pioneered by a group at the National Cancer Institute, under the premise that lymphocytic infiltrates at tumours are enriched for tumour antigen-specific T cells. As reviewed by Hinrichs & Rosenberg [14], many factors influence the success of this approach, including culture technology and host conditioning with chemotherapy and ionizing radiation. TIL cultures for adoptive transfer typically are generated via short-term ex vivo expansion and screening for anti-tumour activity. TIL-based approaches have been primarily evaluated in the setting of melanoma, in part because melanoma biopsies are readily obtainable and in part because melanoma has long been considered to be an ‘immunogenic’ tumour. TIL therapy has been shown to result in durable tumour regression in a subset of patients with advanced metastatic melanoma [15]. As reviewed by Linnemann et al. [16], the mechanisms of responses of patients treated with TILs are the result of T cells reacting to shared antigens as well as neo-antigens created by tumour-specific mutations or by epitopes that are encoded by alternative open reading frames [17,18]. Preliminary data suggest that some T-cell responses against neo-antigens may be of a higher magnitude than T-cell responses against shared self-antigens [19,20]. We believe that the major issue facing the field that prevents the widespread use of TIL therapy has been the infusions of high dose IL-2 and the attendant off target toxicities. A secondary obstacle is the challenging logistics of tumour harvest and TIL culture that has prevented investigators from conducting randomized clinical trials analysed with intent to treat endpoints.
3. Chimeric antigen receptors
CARs are modular polypeptides typically consisting of three distinct modules: an extracellular target-binding module, a transmembrane module anchoring the CAR into the cell membrane and an intracellular signalling module. The extracellular target-binding module is usually derived from scFv determinants isolated from antibodies, linked in a single chain through linker polypeptide sequences. Transmembrane modules are usually derived from molecules involved in T-cell function such as the CD8 and CD4 coreceptor molecules [21]. Recent contributions by Chmielewski et al. [22], Cheadle et al. [23], Ruella & Kalos [24] and Jensen & Riddell [25] focus on the status of CARs in clinical trials. The principal advantage of CAR-based strategies is that the target-binding moiety is derived from antibodies with affinities several orders of magnitude higher than TCRs. In addition, because CARs recognize intact cell surface proteins, targeting of target cells is neither MHC restricted nor dependent on processing and effective presentation of target epitopes, and therefore, CAR-based approaches are insensitive to tumour escape mechanisms related to MHC loss variants. At this point, many groups have shown that CAR T cells have potent anti-tumour effects against a variety of advanced haematologic malignancies of the B-cell lineage. The central issue facing the field is whether the technology can be extended to non-B-cell derived malignancies, and in particular, can this strategy work for carcinomas?
There are a number of limitations and challenges, both practical and theoretical, associated with CAR-based strategies. In terms of practical limitations, CAR-based approaches are restricted to the targeting of cell surface determinants to which antibodies can be generated in heterologous species. In addition, because CARs are chimeric molecules composed of distinct combinatorial modules that include unique junctional fragments, there is reasonable potential for CAR-modified T cells to be targeted by patient humoral and cellular immune responses, which may be a clinically silent event or in rare instances can provoke anaphylaxis [26,27]. In terms of theoretical limitations, because CARs are engineered to deliver TCR and costimulation-mediated signals independently from the physiological complex through which natural signalling occurs, it is possible that the signalling cascades initiated through CAR engagement are qualitatively and/or quantitatively distinct from those evoked by native TCR signalling. This could result in adverse effects such as uncontrolled lymphoproliferation, an event which fortunately has not occurred. However, the non-physiologic signalling modules in CARs could also have beneficial effects. An example is that CAR T cells may be less susceptible to regulation, and therefore, may have improved function in the tumour microenvironment [28]. Abken and co-workers [22] describe a clever strategy of targeting the tumour stroma by recruiting innate immunity following the adoptive transfer of CAR T cells engineered to secrete transgenic cytokines such as IL-12.
A number of toxicities have emerged from the trials with CAR T cells. These include B-cell aplasia, cytokine release syndrome, macrophage activation syndrome and neurologic toxicity. The cause of the neurologic toxicity remains enigmatic but may be a class effect with drugs targeting CD19, because it has also been observed with blinatumomab [29]. The management of cytokine release syndrome has recently been reviewed [30].
4. T-cell receptor engineering
The feasibility of transferring T-cell specificity into primary T cells through transfer of TCR α and β chains was demonstrated almost 20 years ago [31,32]. Tumour-antigen-specific T cells, expanded from both cancer patients and healthy volunteers, have been a primary source for isolating tumour-specific heterodimeric TCRs, and over the years, a large variety of approaches using both peptides and whole antigen have been implemented to expand such T cells. Because of the low frequency of such T cells in peripheral blood, the lack of effective culture and expansion methodologies, and the impact of central tolerance on the repertoire, T cells have only be isolated with considerable difficulty using these approaches; furthermore, such T cells are in general of low affinity and demonstrate weak anti-tumour activity. A number of approaches to overcome these issues and generate more potent tumour antigen-specific T cells have been developed. One recent and promising approach to overcome the issue of the intrinsically low-affinity of TCR to self-antigens has been to enhance the affinity of the TCR isolated from such T cells by mutagenesis of α and β receptor chains. Recent technological advances have facilitated elegant molecular and rational high-throughput genetic approaches to affinity enhance TCRs [33–35], and such efforts have resulted in the ability to reproducibly generate TCR with substantially higher affinities for target antigens [36]. An alternative strategy to enhance TCR affinity follows from observations of enhanced functional avidity and improved recognition of tumour cells following introduction of mutations that reduced N-glycosylation on TCR chains [37].
As reviewed by Hinrichs & Rosenberg [14], and Ruella & Kalos [24], there are promising early results in a variety of tumours treated with T cells expressing TCRs engineered by various approaches. However, there have also been on-target and off-target toxicities with engineered TCRs. In one trial, T cells were engineered to express a TCR generated in HLA-A*0201 transgenic mice (i.e. not subjected to selection by the human immune system) and that recognized an epitope shared between MAGE-A3, -A9 and -A12. Of nine patients treated, five demonstrated objective clinical responses, but three patients demonstrated serious adverse events associated with neural toxicity, including two deaths. Post-mortem analysis revealed rare and previously unrecognized expression of MAGE-A12 in brain tissue [38]. Two trials that evaluated the use of affinity enhanced HLA-A*01-restricted and MAGE-A3-specific TCR to target melanoma and myeloma were reported recently. The first treated patient in each of these trials experienced severe cardiac toxicity, and each patient died within 7 days of T-cell infusion [39]. Retrospective analysis demonstrated that the affinity enhancement of the TCR resulted in the off target recognition of a related HLA-A*01-restricted epitope from the protein titin expressed in cardiac cells [40]. These results highlight the potency of adoptively transferred T cells with redirected specificity and the need to develop improved methods for pre-clinical screening of engineered TCRs.
A potential toxicity following the introduction of engineered TCRs is the production of mixed dimers comprised chains from the endogenous TCR with chains from the transgenic TCR [41]. As reviewed by Torikai et al. [42], a particularly elegant approach to prevent this complication involves TCR gene editing with zinc finger nucleases. Expression of the endogenous TCR α and β chains can be permanently abrogated using this approach, resulting in improved expression and function of the transgenic TCRs and CARs [42,43].
5. Bridging success in cancer to HIV
It is interesting to note from a historical perspective that some of the first forms of adoptive cell transfer (ACT) involving gene-modified T cells were conducted two decades previously in patients with advanced HIV-1/AIDS [44], and that many of the results from trials conducted in patients with AIDS have informed current concepts in the field of cancer [45]. The initial trials were done in order to control drug-resistant forms of HIV-1 infection. However, the current challenge in the field is to develop cellular therapies with the potential to eliminate the reservoir of HIV-1 that is resistant to current antiviral therapies [46]. The field has been energized by an extraordinary experiment conducted by Gero Hütter and co-workers in Berlin in a patient who has apparently been cured of HIV infection following an allogeneic haematopoietic stem cell (HSC) transplant and ACT from a homozygous CCR5 delta32 donor [47]. There are a number of approaches to induce a cell-intrinsic resistance to HIV-1 infection and to target the reservoir of HIV-1 by gene-modified ACT [48].
6. Cellular engineering
In addition to receptor engineering, optimizing the effector function of engineered T cells can also increase clinical efficacy. Previous disappointing results with adoptive transfer strategies were due to the use of cell culture approaches that resulted in a population of terminally differentiated effector cells. Recent results with CAR T cells indicate that proliferative capacity of the infused T cells is a predictive biomarker of clinical responses, as reviewed by Kalos and co-workers [24]. It is now well recognized that stimulation of T cells via their TCR without a second costimulatory signal induces tolerance and more recent CAR-based technologies have focused on overcoming this limitation. Thus, while first-generation CARs depended on intracellular transduction of the recognition signal via the CD3ζ chain alone, second- and third-generation CAR constructs have incorporated costimulatory signalling domains such as those derived from CD27, CD28, CD134 or CD137. In addition, culture systems that provide costimulation by immobilized ligands on beads have improved the function of adoptively transferred T cells [49]. Sophisticated artificial antigen-presenting cells that provide arrays of selected costimulatory molecules and cytokines have been developed [50,51], as reviewed by Butler & Hirano [52].
A major controversy in the field is defining the optimal cell product for infusion. At issue is whether to purify selected subsets of cells for culture and subsequent genetic engineering or, more straightforward, to use bulk cell products that contain mixtures of CD4+ helper, CD8+ cytotoxic, naive, central memory, effector memory and other subsets? For example, cell culture conditions can be optimized to promote the expansion of T-central memory cells using anti-CD3 and anti-CD28 coated beads with IL-7 and IL-15 [53]. As summarized by Fowler [54], the blockade of the mechanistic target of rapamycin (mTOR) during culture has the potential to enhance adoptive therapy approaches. Manipulation of metabolic pathways with rapamycin and other mTOR kinase inhibitors can change the fate and function of adoptively transferred T cells [55]. Furthermore, CAR T cells encoding a rapamycin-resistant mutant of mTOR have enhanced anti-tumour effects in pre-clinical models [56]. The factors related to the desired composition of the adoptively transferred cells are reviewed by Jensen & Riddell [25]. T cells with stem cell-like properties have been described [57,58]; however, it is not yet known if these cells are superior to central memory or naive T cells. Ghosh et al. [59] have focused on the development of T-cell-based immunotherapy for use in the context of allogeneic HSC transplantation. They have reviewed some recent studies on the development of ‘off the shelf’ cellular immunotherapies across MHC barriers, highlighting the key milestones in their development and use. In particular, they show that the adoptive transfer of precursor T cells enhances T-cell reconstitution after allogeneic stem cell transplantation [60].
A major issue with clinical adoptive cell transfer therapy is the avoidance of senescent and exhausted states in the infused cells. This issue was not predicted in mouse models because of substantial differences in telomere biology between the mouse and human immune systems [61]. With TIL therapy, the telomere length of the transferred lymphocytes correlates with in vivo persistence and tumour regression in melanoma patients receiving cell transfer therapy [62]. CD28 costimulation can augment telomerase activity and enhance telomere length during in vitro culture [63,64]. One approach to circumvent this issue is the use of HSCs or induced pluripotent stem cells [65,66], as reviewed by Gschweng et al. [67]. Another approach to prevent terminal differentiation during culture is to uncouple cell proliferation from effector differentiation. Crompton et al. [68] have reviewed the cellular mechanisms that lead to progressive differentiation during the physiologic immune response and they propose the use of synthetic biology to uncouple proliferation from differentiation.
A potential safety concern related to the infusion of engineered T cells is virus integration-related insertional mutagenesis and cellular transformation, which has been demonstrated with the genetic engineering of HSCs [69]. This issue may also occur with non-viral-based integration using sleeping beauty, as described by Cooper [42,43]. In patients with congenital and acquired immunodeficiency, genetically modified T cells have been shown to persist after adoptive transfer in humans for more than a decade without adverse effects [45,70], indicating that the approach to genetically modify mature human T cells is fundamentally safe, at least in part, because lentiviral integration sites are not random and do not favour proto-oncogenes [71]. Furthermore, unlike B cells, T cells are subject to clonal competition at the TCR level, which may explain the rarity of T-cell leukaemia and the relative resistance of T cells to transformation [72].
The development of mechanisms to control the lifespan of the transferred T cells is yet another challenge for the field. Initial approaches attempted to introduce ‘suicide genes’ such as the herpes simplex virus thymidine kinase (TK) gene; however, these efforts revealed the strong potential for immunologic rejection based on targeting of TK-derived sequences [73]. More recently, an elegant and potentially powerful inducible system based on the use of a modified human caspase-9 fused to a human FK506 binding protein permits conditional dimerization and delivery of apoptotic signals in response to small molecules that can permeate the T-cell plasma membrane is currently being evaluated in clinical trials [74]. Approaches to regulate the persistence of engineered T cells are discussed by Dotti and co-workers, Gottschalk, Savoldo and Brenner [75] and by Jensen & Riddell [25].
7. Conclusion
In this review, we have highlighted two basic gene-transfer approaches that are being pursued to bypass the effects of central and peripheral tolerance on the T-cell repertoire. Clinical data from the group at the University of Pennsylvania and elsewhere generated principally over the past 5 years suggest that we are at the threshold of a golden era for adoptive T-cell therapy, with a number of recent profound examples of the potency and promise of this approach to target cancer. Recent reports, using CAR T cells with CD137 and CD3ζ signalling domains, which documented long-term functional persistence of T cells engineered to target CD19, along with long-lasting clinical remissions and ongoing B-cell aplasia, have highlighted the potential for adoptive T-cell transfer to induce a profound long-term functional anti-tumour activity [76,77]. Despite these early successes, a number of fundamental and important questions still remain to be resolved for the broad, reproducible and effective implementation of this approach to treat cancer beyond B-cell malignancies.
A few common themes have emerged as the principle challenges to the field. First, identification of the optimal composition of the transferred cellular product requires clarification. Second, in ongoing clinical studies with CAR-engineered cells that target CD19, patients remain disease free with persisting engineered T cells for more than 4 years post-treatment but also with ongoing B-cell aplasia owing to targeting of normal CD19-positive B cells, highlighting the practical necessity to eventually ablate engineered cells and enable normal B-cell reconstitution. Therefore, a central issue facing the field is the design and implementation of various approaches to control the fate of adoptively transferred cells. These findings are being translated into the clinic at a rapid pace, and it is likely that engineered T-cell transfer will become established as an effective cancer therapy during the next decade. Finally, a challenge for adoptive T-cell therapy will be the necessity and rationale to combine the therapy with other anti-tumour therapies. In particular, we will require information to rationally combine with therapeutic vaccination, checkpoint inhibition, agonistic antibodies, small molecule inhibitors of tumours and the targeting of tumour stroma and neo-vasculature, as discussed by Yee [78].
Acknowledgements
Our work in this area is supported by grants from the NIH (5R01CA120409) and the Leukemia and Lymphoma Society (SCOR grant no. 7007).
Competing interests
The University of Pennsylvania has licensed technology developed in the laboratories of the authors to Novartis.
Funding
We received no funding for this study.
References
- 1.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. 2002. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3, 991–998. ( 10.1038/ni1102-991) [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Yang JC, Restifo NP. 2004. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 10, 909–915. ( 10.1038/nm1100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billingham RE, Brent L, Medawar PB. 1954. Quantitative studies on tissue transplantation immunity. II. The origin, strength and duration of actively and adoptively acquired immunity. Proc. R. Soc. Lond. B 143, 58–80. ( 10.1098/rspb.1954.0054) [DOI] [PubMed] [Google Scholar]
- 4.Mitchison NA. 1955. Studies on the immunological response to foreign tumor transplants in the mouse. I. The role of lymph node cells in conferring immunity by adoptive transfer. J. Exp. Med. 102, 157–177. ( 10.1084/jem.102.2.157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen YY, Galloway KE, Smolke CD. 2012. Synthetic biology: advancing biological frontiers by building synthetic systems. Genome Biol. 13, 240 ( 10.1186/gb-2012-13-2-240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gross G, Waks T, Eshhar Z. 1989. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl Acad. Sci. USA 86, 10 024–10 028. ( 10.1073/pnas.86.24.10024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irving BA, Weiss A. 1991. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell 64, 891–901. ( 10.1016/0092-8674(91)90314-O) [DOI] [PubMed] [Google Scholar]
- 8.Mellman I, Coukos G, Dranoff G. 2011. Cancer immunotherapy comes of age. Nature 480, 480–489. ( 10.1038/nature10673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aleksic M, Liddy N, Molloy PE, Pumphrey N, Vuidepot A, Chang K-M, Jakobsen BK. 2012. Different affinity windows for virus and cancer-specific T-cell receptors: implications for therapeutic strategies. Eur. J. Immunol. 42, 3174–3179. ( 10.1002/eji.201242606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghazi A. et al. 2012. Generation of polyclonal CMV-specific T cells for the adoptive immunotherapy of glioblastoma. J. Immunother. 35, 159–168. ( 10.1097/CJI.0b013e318247642f) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampson JH, Mitchell DA. 2011. Is cytomegalovirus a therapeutic target in glioblastoma? Clin. Cancer Res. 17, 4619–4621. ( 10.1158/1078-0432.CCR-11-0992) [DOI] [PubMed] [Google Scholar]
- 12.Crough T, Beagley L, Smith C, Jones L, Walker DG, Khanna R. 2012. Ex vivo functional analysis, expansion and adoptive transfer of cytomegalovirus-specific T-cells in patients with glioblastoma multiforme. Immunol. Cell Biol. 90, 872–880. ( 10.1038/icb.2012.19) [DOI] [PubMed] [Google Scholar]
- 13.Tebas P. et al. 2014. Gene editing of CCR5 in autologous CD4 T-cells of persons infected with HIV. N. Engl. J. Med. 370, 901–910. ( 10.1056/NEJMoa1300662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinrichs CS, Rosenberg SA. 2014. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol. Rev. 257, 56–71. ( 10.1111/imr.12132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudley ME. et al. 2008. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 26, 5233–5239. ( 10.1200/JCO.2008.16.5449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linnemann C, Mezzadra R, Schumacher TN. 2014. TCR repertoires of intratumoral T-cell subsets. Immunol. Rev. 257, 72–82. ( 10.1111/imr.12140) [DOI] [PubMed] [Google Scholar]
- 17.Andersen RS. et al. 2012. Dissection of T-cell antigen specificity in human melanoma. Cancer Res. 72, 1642–1650. ( 10.1158/0008-5472.CAN-11-2614) [DOI] [PubMed] [Google Scholar]
- 18.Robbins PF. et al. 2013. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat. Med. 19, 747–752. ( 10.1038/nm.3161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castle JC. et al. 2012. Exploiting the mutanome for tumor vaccination. Cancer Res. 72, 1081–1091. ( 10.1158/0008-5472.CAN-11-3722) [DOI] [PubMed] [Google Scholar]
- 20.Matsushita H. et al. 2012. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 482, 400–404. ( 10.1038/nature10755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadelain M, Brentjens R, Riviere I. 2013. The basic principles of chimeric antigen receptor design. Cancer Discov. 3, 388–398. ( 10.1158/2159-8290.CD-12-0548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chmielewski M, Hombach AA, Abken H. 2014. Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol. Rev. 257, 83–90. ( 10.1111/imr.12125) [DOI] [PubMed] [Google Scholar]
- 23.Cheadle EJ, Gornall H, Baldan V, Hanson V, Hawkins RE, Gilham DE. 2014. CAR T cells: driving the road from the laboratory to the clinic. Immunol. Rev. 257, 91–106. ( 10.1111/imr.12126) [DOI] [PubMed] [Google Scholar]
- 24.Ruella M, Kalos M. 2014. Adoptive immunotherapy for cancer. Immunol. Rev. 257, 14–38. ( 10.1111/imr.12136) [DOI] [PubMed] [Google Scholar]
- 25.Jensen MC, Riddell SR. 2014. Design and implementation of adoptive therapy with chimeric antigen receptor-modified T cells. Immunol. Rev. 257, 127–144. ( 10.1111/imr.12139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamers C. et al. 2011. Immune responses to transgene and retroviral vector in patients treated with ex vivo engineered T cells. Blood 117, 72–82. ( 10.1182/blood-2010-07-294520) [DOI] [PubMed] [Google Scholar]
- 27.Maus MV, Haas AR, Beatty GL, Albelda SM, Levine BL, Liu X, Zhao Y, Kalos M, June CH. 2013. T Cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol. Res. 1, 26–31. ( 10.1158/2326-6066.CIR-13-0006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, Brentjens RJ. 2012. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 119, 4133–4141. ( 10.1182/blood-2011-12-400044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teachey DT. et al. 2013. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine directed therapy. Blood 121, 5154–5157. ( 10.1182/blood-2013-02-485623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. 2014. How I treat: current concepts in the diagnosis and management of cytokine release syndrome. Blood 124, 188–195. ( 10.1182/blood-2014-05-552729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, Nishimura MI. 1999. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J. Immunol. 163, 507–513. [PubMed] [Google Scholar]
- 32.Cooper LJ, Kalos M, Lewinsohn DA, Riddell SR, Greenberg PD. 2000. Transfer of specificity for human immunodeficiency virus type 1 into primary human T lymphocytes by introduction of T-cell receptor genes. J. Virol. 74, 8207–8212. ( 10.1128/JVI.74.17.8207-8212.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y. et al. 2005. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat. Biotechnol. 23, 349–354. ( 10.1038/nbt1070) [DOI] [PubMed] [Google Scholar]
- 34.Chervin AS, Aggen DH, Raseman JM, Kranz DM. 2008. Engineering higher affinity T cell receptors using a T cell display system. J. Immunol. Methods. 339, 175–184. ( 10.1016/j.jim.2008.09.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Udyavar A, Alli R, Nguyen P, Baker L, Geiger TL. 2009. Subtle affinity-enhancing mutations in a myelin oligodendrocyte glycoprotein-specific TCR alter specificity and generate new self-reactivity. J. Immunol. 182, 4439–4447. ( 10.4049/jimmunol.0804377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louie KA, Weiner LP, Du J, Kochounian HH, Fling SP, Wei W, McMillan M. 2005. Cell-based gene therapy experiments in murine experimental autoimmune encephalomyelitis. Gene Ther. 12, 1145–1153. ( 10.1038/sj.gt.3302503) [DOI] [PubMed] [Google Scholar]
- 37.Kuball J, Hauptrock B, Malina V, Antunes E, Voss R-H, Wolfl M, Strong R, Theobald M, Greenberg PD. 2009. Increasing functional avidity of TCR-redirected T cells by removing defined N-glycosylation sites in the TCR constant domain. J. Exp. Med. 206, 463–475. ( 10.1084/jem.20082487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morgan RA. et al. 2013. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 36, 133–151. ( 10.1097/CJI.0b013e3182829903) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linette GP. et al. 2013. Cardiovascular toxicity and titin cross-reactivity of affinity enhanced T cells in myeloma and melanoma. Blood 122, 863–871. ( 10.1182/blood-2013-03-490565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cameron BJ. et al. 2013. Identification of a titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci. Transl. Med. 5, 197ra103. ( 10.1126/scitranslmed.3006034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bendle GM. et al. 2010. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat. Med. 16, 565–570. ( 10.1038/nm.2128) [DOI] [PubMed] [Google Scholar]
- 42.Torikai H. et al. 2012. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood 119, 5697–5705. ( 10.1182/blood-2012-01-405365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Provasi E. et al. 2012. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat. Med. 18, 807–815. ( 10.1038/nm.2700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riddell SR. et al. 1996. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat. Med. 2, 216–223. ( 10.1038/nm0296-216) [DOI] [PubMed] [Google Scholar]
- 45.Scholler J. et al. 2012. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med. 4, 132RA153. ( 10.1126/scitranslmed.3003761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ho YC. et al. 2013. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155, 540–551. ( 10.1016/j.cell.2013.09.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hütter G. et al. 2009. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 360, 692–696. ( 10.1056/NEJMoa0802905) [DOI] [PubMed] [Google Scholar]
- 48.Rossi JJ, June CH, Kohn DB. 2007. Genetic therapies for HIV/AIDS. Nat. Biotechnol. 25, 1444–1454. ( 10.1038/nbt1367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levine BL, Bernstein W, Craighead N, Lindsten T, Thompson CB, June CH. 1997. Effects of CD28 costimulation on long term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J. Immunol. 159, 5921–5930. [PubMed] [Google Scholar]
- 50.Hirano N. et al. 2006. Engagement of CD83 ligand induces prolonged expansion of CD8+ T cells and preferential enrichment for antigen specificity. Blood 107, 1528–1536. ( 10.1182/blood-2005-05-2073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suhoski MM, Golovina TN, Aqui NA, Tai VC, Varela-Rohena A, Milone MC, Carroll RG, Riley JL, June CH. 2007. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol. Ther. 15, 981–988. ( 10.1038/mt.sj.6300134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Butler MO, Hirano N. 2014. Human cell-based artificial antigen-presenting cells for cancer immunotherapy. Immunol. Rev. 257, 191–209. ( 10.1111/imr.12129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaneko S. et al. 2009. IL-7 and IL-15 allow the generation of suicide gene-modified alloreactive self-renewing central memory human T lymphocytes. Blood 113, 1006–1015. ( 10.1182/blood-2008-05-156059) [DOI] [PubMed] [Google Scholar]
- 54.Fowler DH. 2014. Rapamycin-resistant effector T-cell therapy. Immunol. Rev. 257, 210–225. ( 10.1111/imr.12127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pearce EL, Pearce EJ. 2013. Metabolic pathways in immune cell activation and quiescence. Immunity 38, 633–643. ( 10.1016/j.immuni.2013.04.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huye LE, Nakazawa Y, Patel MP, Yvon E, Sun J, Savoldo B, Wilson MH, Dotti G, Rooney CM. 2011. Combining mTor inhibitors with rapamycin-resistant T cells: a two-pronged approach to tumor elimination. Mol. Ther. 19, 2239–2248. ( 10.1038/mt.2011.179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lugli E. et al. 2013. Superior T memory stem cell persistence supports long-lived T cell memory. J. Clin. Invest. 123, 594–599. ( 10.1172/jci66327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gattinoni L. et al. 2011. A human memory T cell subset with stem cell-like properties. Nat. Med. 17, 1290–1297. ( 10.1038/nm.2446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghosh A, Holland AM, van den Brink MR. 2014. Genetically engineered donor T cells to optimize graft-versus-tumor effects across MHC barriers. Immunol. Rev. 257, 226–236. ( 10.1111/imr.12142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zakrzewski JL. et al. 2006. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat. Med. 12, 1039–1047. ( 10.1038/nm1463) [DOI] [PubMed] [Google Scholar]
- 61.Weng NP. 2006. Aging of the immune system: how much can the adaptive immune system adapt?. Immunity 24, 495–499. ( 10.1016/j.immuni.2006.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. 2005. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J. Immunol. 175, 7046–7052. ( 10.4049/jimmunol.175.10.7046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weng NP, Levine BL, June CH, Hodes RJ. 1997. Regulation of telomerase RNA template expression in human T lymphocyte development and activation. J. Immunol. 158, 3215–3220. [PubMed] [Google Scholar]
- 64.Weng NP, Palmer LD, Levine BL, Lane HC, June CH, Hodes RJ. 1997. Tales of tails: regulation of telomere length and telomerase activity during lymphocyte development, differentiation, activation, and aging. Immunol. Rev. 160, 43–54. ( 10.1111/j.1600-065X.1997.tb01026.x) [DOI] [PubMed] [Google Scholar]
- 65.Giannoni F. et al. 2013. Allelic exclusion and peripheral reconstitution by TCR transgenic T cells arising from transduced human hematopoietic stem/progenitor cells. Mol. Ther. 21, 1044–1054. ( 10.1038/mt.2013.8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Themeli M, Kloss CC, Ciriello G, Fedorov VD, Perna F, Gonen M, Sadelain M. 2013. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat. Biotechnol. 31, 928–933. ( 10.1038/nbt.2678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gschweng E, De Oliveira S, Kohn DB. 2014. Hematopoietic stem cells for cancer immunotherapy. Immunol. Rev. 257, 237–249. ( 10.1111/imr.12128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crompton JG, Sukumar M, Restifo NP. 2014. Uncoupling T-cell expansion from effector differentiation in cell-based immunotherapy. Immunol. Rev. 257, 264–276. ( 10.1111/imr.12135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hacein-Bey-Abina S. et al. 2008. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 118, 3132–3142. ( 10.1172/JCI35700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muul LM. et al. 2003. Persistence and expression of the adenosine deaminase gene for 12 years and immune reaction to gene transfer components: long-term results of the first clinical gene therapy trial. Blood 101, 2563–2569. ( 10.1182/blood-2002-09-2800) [DOI] [PubMed] [Google Scholar]
- 71.Bushman FD. 2007. Retroviral integration and human gene therapy. J. Clin. Invest. 117, 2083–2086. ( 10.1172/JCI32949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Newrzela S. et al. 2012. T-cell receptor diversity prevents T-cell lymphoma development. Leukemia 26, 2499–2507. ( 10.1038/leu.2012.142) [DOI] [PubMed] [Google Scholar]
- 73.Marktel S. et al. 2003. Immunologic potential of donor lymphocytes expressing a suicide gene for early immune reconstitution after hematopoietic T-cell-depleted stem cell transplantation. Blood 101, 1290–1298. ( 10.1182/blood-2002-08-2351) [DOI] [PubMed] [Google Scholar]
- 74.Di Stasi A. et al. 2011. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 365, 1673–1683. ( 10.1056/NEJMoa1106152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou X. et al. 2014. Long-term outcome and immune reconstitution after haploidentical stem cell transplant in recipients of allodepleted-T-cells expressing the inducible caspase-9 safety transgene. Blood 123, 3896–3905. ( 10.1182/blood-2014-01-551671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. 2011. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 3, 95ra73 ( 10.1126/scitranslmed.3002842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grupp SA. et al. 2013. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 368, 1509–1518. ( 10.1056/NEJMoa1215134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yee C. 2014. The use of endogenous T cells for adoptive transfer. Immunol. Rev. 257, 250–263. ( 10.1111/imr.12134) [DOI] [PubMed] [Google Scholar]