The transcription factor MYB112 in Arabidopsis promotes anthocyanin formation under conditions of salinity and high light stress.
Abstract
MYB transcription factors (TFs) are important regulators of flavonoid biosynthesis in plants. Here, we report MYB112 as a formerly unknown regulator of anthocyanin accumulation in Arabidopsis (Arabidopsis thaliana). Expression profiling after chemically induced overexpression of MYB112 identified 28 up- and 28 down-regulated genes 5 h after inducer treatment, including MYB7 and MYB32, which are both induced. In addition, upon extended induction, MYB112 also positively affects the expression of PRODUCTION OF ANTHOCYANIN PIGMENT1, a key TF of anthocyanin biosynthesis, but acts negatively toward MYB12 and MYB111, which both control flavonol biosynthesis. MYB112 binds to an 8-bp DNA fragment containing the core sequence (A/T/G)(A/C)CC(A/T)(A/G/T)(A/C)(T/C). By electrophoretic mobility shift assay and chromatin immunoprecipitation coupled to quantitative polymerase chain reaction, we show that MYB112 binds in vitro and in vivo to MYB7 and MYB32 promoters, revealing them as direct downstream target genes. We further show that MYB112 expression is up-regulated by salinity and high light stress, environmental parameters that both require the MYB112 TF for anthocyanin accumulation under these stresses. In contrast to several other MYB TFs affecting anthocyanin biosynthesis, MYB112 expression is not controlled by nitrogen limitation or an excess of carbon. Thus, MYB112 constitutes a regulator that promotes anthocyanin accumulation under abiotic stress conditions.
Anthocyanins, a subgroup of plant flavonoids, play multiple roles in higher plants: they function as photoprotective pigments in vegetative tissues to absorb UV and high light irradiation, antioxidants that scavenge reactive oxygen species (ROS), visual signals for the attraction of pollinators or seed dispersers, and agents acting against microbes in defense responses (Winkel-Shirley, 2001; Koes et al., 2005; Lepiniec et al., 2006; Pourcel et al., 2007; Mouradov and Spangenberg, 2014). Anthocyanins accumulate in plant tissues in response to different types of abiotic stresses, including osmotic stress, extreme temperature, nitrogen and phosphorous deficiency, excessive light, and salinity (Christie et al., 1994; Dixon and Paiva, 1995; Winkel-Shirley, 2002; Castellarin et al., 2007; Cominelli et al., 2008; Lillo et al., 2008; Feyissa et al., 2009).
The molecular basis of plant anthocyanin biosynthesis is well known (Koes et al., 2005; Falcone Ferreyra et al., 2012). In addition, transcription factors (TFs) regulating the expression of flavonoid biosynthesis genes have been characterized extensively in Arabidopsis (Arabidopsis thaliana) and other plants (Broun, 2005; Ramsay and Glover, 2005; Allan et al., 2008; Hichri et al., 2011; Petroni and Tonelli, 2011; Schwinn et al., 2014). Genes encoding the early flavonoid biosynthetic steps, including CHALCONE SYNTHASE (CHS), CHALCONE ISOMERASE (CHI), FLAVANONE 3-HYDROXYLASE (F3H), and FLAVONOID 3′-HYDROXYLASE (F3′H), as well as the more anthocyanin-specific genes, such as DIHYDROFLAVONOL 4-REDUCTASE (DFR), ANTHOCYANIDIN SYNTHASE (ANS), and FLAVONOID 3-O-GLYCOSYL-TRANSFERASE, are regulated by TFs of the MYB and basic helix-loop-helix (bHLH) families. TFs of the R2R3-MYB subfamily play a central role in regulating both types of genes. In particular, in Arabidopsis, the MYB TFs PRODUCTION OF ANTHOCYANIN PIGMENT1 (PAP1; also known as MYB75), PAP2 (MYB90), MYB113, and MYB114 have been shown to play important roles in activating genes of the anthocyanin biosynthesis pathway (Borevitz et al., 2000; Gonzalez et al., 2008). These TFs interact with bHLH factors, including GLABRA3 (GL3; bHLH001), ENCHANCER OF GLABRA3 (bHLH002), and TRANSPARENT TESTA8 (TT8; bHLH042; Zhang et al., 2003; Baudry et al., 2006; Gonzalez et al., 2008). The regulatory transcription complex also includes the WD40 protein TRANSPARENT TESTA GLABRA1 (TTG1), a homolog of ANTHOCYANIN11 from Petunia hybrida, which is also important for anthocyanin production (de Vetten et al., 1997; Walker et al., 1999).
The resulting MYB/bHLH/WD40 TF complex, consisting of at least these three protein members, activates anthocyanin biosynthetic genes, including ANS, DFR, F3′H, LEUCOANTHOCYANIN DIOXYGENASE, ANTHOCYANIN-3-O-GLUCOSYL TRANSFERASE (UDP-DEPENDENT GLYCOSYL TRANSFERASE78D2 [UGT78D2]), and ANTHOCYANIN-5-O-GLUCOSYL TRANSFERASE (UGT75C1; Tohge et al., 2005; Gonzalez et al., 2008). In addition, Arabidopsis PRODUCTION OF FLAVONOL GLYCOSIDE (PFG) factors from subgroup 7 of the R2R3-MYB family, namely MYB11/PFG2, MYB12/PFG1, and MYB111/PFG3, have been reported to control the flavonol branch of the flavonoid biosynthetic pathway by activating CHS, CHI, F3H and FLAVONOL SYNTHASE (FLS; Mehrtens et al., 2005; Stracke et al., 2007).
In contrast to the positive regulators of anthocyanin biosynthesis, the R3-type single MYB protein MYB-Like2 (MYBL2) functions as a negative regulator (transcriptional repressor) of this biochemical pathway. MYBL2 seems to form a protein complex with TT8 and TTG1 that functions as a transcriptional repressor of anthocyanin biosynthesis (Dubos et al., 2008; Matsui et al., 2008). Another transcriptional repressor of anthocyanin biosynthesis in Arabidopsis is the single-repeat R3 MYB protein CAPRICE (CPC; Zhu et al., 2009).
The accumulation of anthocyanins in response to environmental inputs is, to a large extent, regulated by TFs that affect the expression of genes encoding both early and late biosynthetic reactions in their production. Particularly well studied has been the effect of nutrient (mostly nitrogen) limitation on anthocyanin accumulation. Several early (CHS, F3H, and F3′H) and late (DFR and ANS) pathway flavonoid/anthocyanin biosynthesis genes are induced upon depletion of nitrogen or phosphorous (Scheible et al., 2004; Misson et al., 2005; Morcuende et al., 2007; Lillo et al., 2008). Furthermore, many genes encoding enzymes known or predicted to be involved in biosynthesis or modification of flavonoids, including various UGTs, were found to be (highly) induced by mineral nutrient depletion (Lillo et al., 2008). Light quantity and quality also strongly affect anthocyanin biosynthesis (Wade et al., 2001; Takos et al., 2006; Cominelli et al., 2008; Albert et al., 2009; Stracke et al., 2010; Azuma et al., 2012). In Arabidopsis, the bHLH TF PHYTOCHROME-INTERACTING TRANSCRIPTION FACTOR3 acts as a positive regulator of anthocyanin biosynthesis (Shin et al., 2007), and the basic Leu zipper TF ELONGATED HYPOCOTYL5, which plays a key role for light-dependent processes, positively regulates the expression of several genes of flavonoid biosynthesis, including CHS and F3H, as well as the MYB12/PFG1 TF (Stracke et al., 2010). High light enhances the expression of PAP1 and PAP2 and inhibits the expression of MYBL2 (Dubos et al., 2008). The regulatory genes PAP1 and PAP2 are induced by nitrogen and phosphorous depletion (Lillo et al., 2008). PAP1 has been shown to up-regulate the expression of 20 flavonoid biosynthesis genes (Tohge et al., 2005), and expression of almost all of these genes was also elevated by nitrogen and/or phosphorous limitation (for meta-analysis, see Lillo et al., 2008), indicating that PAP TFs play an important role in the mineral nutrition response of flavonoid/anthocyanin pathway genes. Of note, PAP1 and PAP2 are also up-regulated by exposure of plants to light (Cominelli et al., 2008). High light induces the expression of MYB12/PFG1 and MYB111/PFG3, key regulators of the flavonol pathway; this induction correlates with the strong influence that high light has on flavonol biosynthesis (Mehrtens et al., 2005). The GL3/bHLH001 TF interacts with PAP1 and PAP2 to activate the expression of the anthocyanin biosynthetic gene DFR (Zimmermann et al., 2004). Notably, expression of GL3 increases upon nitrogen limitation. Given that the nitrogen depletion-triggered accumulation of anthocyanins that is normally seen in wild-type plants was absent in the gl3 mutant, it was concluded that GL3 is important for the nutrient deprivation response (Feyissa et al., 2009). In addition, the negative regulator CPC was reported to repress anthocyanin accumulation under nitrogen limitation condition. Similarly, CPC represses anthocyanin accumulation in 35S overexpressors grown under osmotic, salinity, or cold stress; however, no difference was observed between wild-type and cpc-1 mutant plants (fig. 6 in Zhu et al., 2009). Furthermore, LATERAL ORGAN BOUNDARY DOMAIN37 (LBD37), LBD38, and LBD39, members of the LBD TF family, have been shown to negatively regulate nitrogen depletion-induced anthocyanin formation in Arabidopsis, possibly through repression of PAP1 and PAP2 (Rubin et al., 2009).
Salinity stress is yet another environmental factor known to trigger anthocyanin accumulation in a number of plant species, including crops and vegetables, such as tomato (Solanum lycopersicum), maize (Zea mays), sugarcane (Saccharum officinarum), and many others (Kaliamoorthy and Rao, 1994; Piao et al., 2001; Wahid and Ghazanfar, 2006; Keutgen and Pawelzik, 2007; Roychoudhury et al., 2008; Matus et al., 2010). Salinity leads to water deficit and ionic stress, resulting in the destabilization of membranes, inhibition of enzymes, and overproduction of ROS (Zhu, 2001; Munns, 2002; Miller et al., 2010), and the accumulation of anthocyanins has been proposed to contribute to plant tolerance against abiotic stresses (Gould et al., 2002; Nagata et al., 2003; Gould, 2004; Zeng et al., 2010). In contrast to the regulation of anthocyanin biosynthesis under nutrient deprivation, the signaling cascade and involvement of TFs in salt-triggered accumulation of flavonoids/anthocyanins have rarely been investigated so far. For example, constitutive overexpression of the Arabidopsis glycogen synthase kinase3 (GSK3)/shaggy-like protein kinase1 (AtGSK1) in transgenic plants was shown to induce salt stress responses, including the accumulation of anthocyanins, concomitant with a strong increase of CHS expression in the absence of stress, indicating that AtGSK1 is involved in a signaling cascade that controls activation of anthocyanin pathway genes upon exposure of plants to salt stress (Piao et al., 2001). Recently, MYB44, a member of subgroup 22 of the R2R3-MYB TF family, was shown to be activated by different abiotic stresses, including dehydration, low temperature, and salinity (Jung et al., 2008). After 24 h of salinity stress but not under control condition, the expression of CHS, F3H, DFR, PAP1, and PAP2 was decreased in transgenic plants overexpressing MYB44 (Jung et al., 2008). In addition, anthocyanin accumulation was less prominent in 35S:MYB44 seedlings than in wild-type plants, particularly after jasmonate treatment (Jung et al., 2010). These data indicate a negative role of MYB44 in stress-induced anthocyanin accumulation. However, a direct regulation of flavonoid/anthocyanin pathway genes by MYB44 has not, as yet, been reported.
Here, we report on MYB112, showing its role in anthocyanin biosynthesis in Arabidopsis. In contrast to several other MYB TFs regulating genes associated with anthocyanin biosynthesis, MYB112 expression is controlled by neither nitrogen limitation nor Suc excess, but rather, it is stimulated by salinity and high light stress. We show enhanced and impaired anthocyanin accumulation in MYB112 overexpressors and myb112 mutants, respectively. We further determined the binding site of the MYB112 TF by in vitro binding site selection and discovered two other MYB genes (i.e. MYB7 and MYB32) as direct downstream targets. Thus, MYB112 does not directly regulate expression of flavonoid biosynthetic genes but seems to function as a formerly unknown modulator of anthocyanin biosynthesis.
RESULTS
Tissue-Specific Expression of MYB112
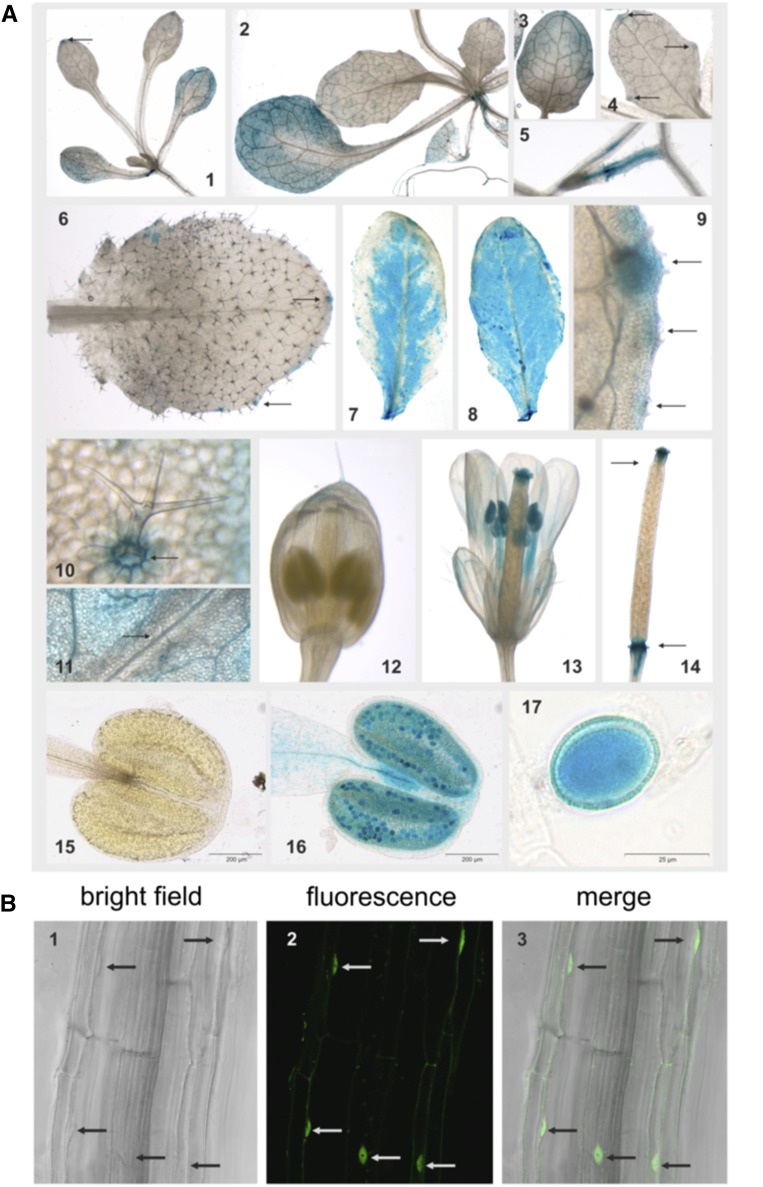
To investigate MYB112 (At1g48000) expression at the tissue level, an approximately 1.3-kb-long 5′ regulatory region upstream of the ATG was fused to the GUS reporter gene, and the resulting construct, ProMYB112:GUS, was transformed into Arabidopsis. MYB112 promoter-driven GUS activity was tested in five independent transgenic lines of T2 and T3 generations. Representative expression patterns are shown in Figure 1A. MYB112 expression was observed in most tissues, both in young seedlings and throughout plant development. At approximately 10 d after sowing, GUS activity was detected in cotyledons and the margins of the first true leaves, where hydathodes are localized (Fig. 1A1). Hydathodes enable water conduction (Candela et al., 1999). After 2 weeks, the following rosette leaves were fully expanded and displayed relatively strong GUS activity, whereas GUS staining was restricted to hydathodes in recently emerged leaves (Fig. 1, A2–A4). GUS expression was also detected in lateral roots of young seedlings (Fig. 1A5). In older plants, GUS activity was observed in both leaves and flowers. Young rosette leaves, like young seedling leaves, exhibited gene expression exclusively in hydathodes (Fig. 1, A6 and A9). In mature rosette leaves, GUS staining was also detected at the base of trichomes and around the midvein (Fig. 1, A7 and A10); however, no GUS activity was observed in the midvein itself (Fig. 1, A7 and A11). The strongest GUS signal was observed in old rosette leaves, showing that MYB112 is a senescence-associated gene (SAG; Fig. 1A8); this finding is in accordance with previous reports that found elevated MYB112 transcript abundance in senescing leaves compared with nonsenescent leaves as determined by microarray hybridizations and quantitative real-time reverse transcription (qRT)-PCR (Balazadeh et al., 2008; Breeze et al., 2011). In flowers, GUS staining was mainly localized to anthers and pollen (Fig. 1, A13, A16, and A17). Immature floral tissue showed no GUS activity (Fig. 1, A12 and A15), consistent with virtually undetectable MYB112 expression on microarrays (eFP browser; http://bar.utoronto.ca/efp). GUS expression was also noted at the stigma and bottom end (later fruit abscission zone) of the gynoecium (Fig. 1A14).
Figure 1.
Localization of MYB112 expression. A, GUS activity in Arabidopsis ProMYB112:GUS seedlings. A1, Ten-day-old seedling. GUS staining is mainly localized to cotyledons. Staining is also visible in the tip and margin regions of leaves numbers 1 and 2 (arrow). A2 to A5, Fourteen-day-old seedlings. A2 and A3, GUS activity in leaves 1 and 2. A4, GUS staining in hydathodes of leaf 6 (arrows). A5, Lateral root. A6 to A14, Three- to six-week-old plants. A6, GUS activity in a young rosette leaf is restricted to hydathodes (arrows). A7, GUS activity in mature rosette leaves is often absent from the leaf edges, with the exception of hydathodes (zoom in A9) and the trichome base (zoom in A10); also, the midvein remains unstained (zoom in A11). A8, Strong GUS staining in a senescent leaf. A12, No detectable GUS expression in a flower bud. A13, GUS activity in a mature flower. A14, GUS staining in stigma and bottom end of gynoecium (arrows). A15, No staining in young anther. A16, Intense GUS staining in mature anther and pollen grains (zoom in A17). Staining was performed for approximately 1 to 2 h. B, Subcellular localization of MYB112-GFP fusion protein in roots of transgenic Arabidopsis. A1, Bright field. A2, GFP fluorescence. A3, Merged. Arrows indicate the presence of MYB112-GFP protein in the nuclei.
MYB112 Is a Nuclear Protein
To test the subcellular localization of MYB112 protein, we expressed it as a fusion to the N terminus of GFP using the Cauliflower Mosaic Virus 35S (CaMV) promoter. The 35S:MYB112-GFP construct was transformed into Arabidopsis plants, and roots of transgenic seedlings were analyzed. GFP fluorescence was exclusively present in nuclei (Fig. 1B), consistent with the role of MYB112 as a TF.
Identification of myb112 Mutants
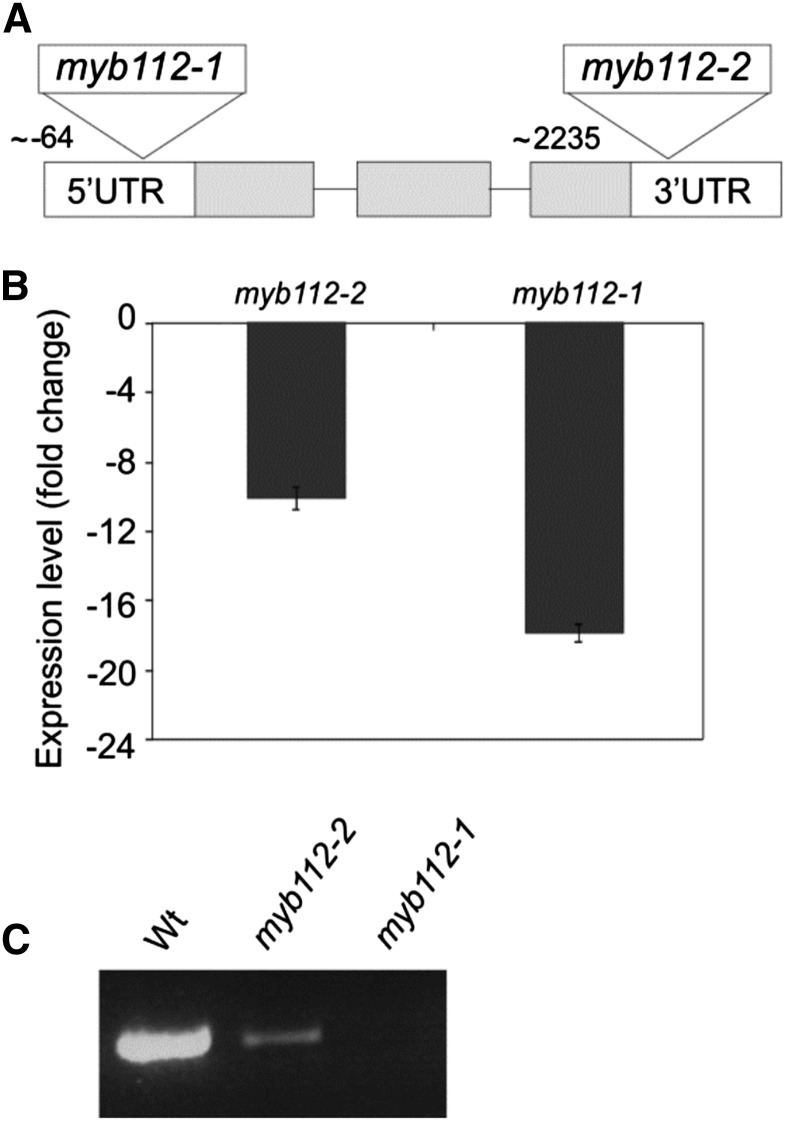
We obtained two myb112 mutants with transfer DNA (T-DNA) insertions located in the 5′ untranslated region (myb112-1; GK093E05) or the 3′ untranslated region (myb112-2; Salk098029; Fig. 2A). Expression analysis by qRT-PCR in seedlings of the two mutants revealed decreased MYB112 transcript abundance in both homozygous mutants, with a stronger reduction of expression in myb112-1 than in myb112-2 (Fig. 2B). This result was confirmed by semi-qRT-PCR, where we tested for the presence of full-length MYB112 transcript (Fig. 2C). The myb112 mutants germinated uniformly, and early vegetative growth was indistinguishable between wild-type and mutant plants. However, we observed a slightly later bolting of soil-grown myb112 mutants compared to wild-type plants under a 12-h/12-h day-night regime, whereas bolting was not seemingly affected in 35S:MYB112 overexpressors (not shown).
Figure 2.
Myb112 T-DNA insertion mutants. A, Location of T-DNA insertions in the two myb112 T-DNA insertion mutants. UTR, Untranslated region. B, Fold change decrease in MYB112 expression in myb112-1 and myb112-2 mutant seedlings compared with expression in wild-type (Wt) plants as measured by qRT-PCR. Means ± sd are shown for three biological replicates. C, Decreased level of MYB112 transcript in myb112-1 and myb112-2 mutant seedlings shown by semi-qRT-PCR (26 cycles) with primers annealing to the start and stop regions of the coding segment.
Estradiol-Inducible Overexpression of MYB112 Induces Anthocyanin Formation
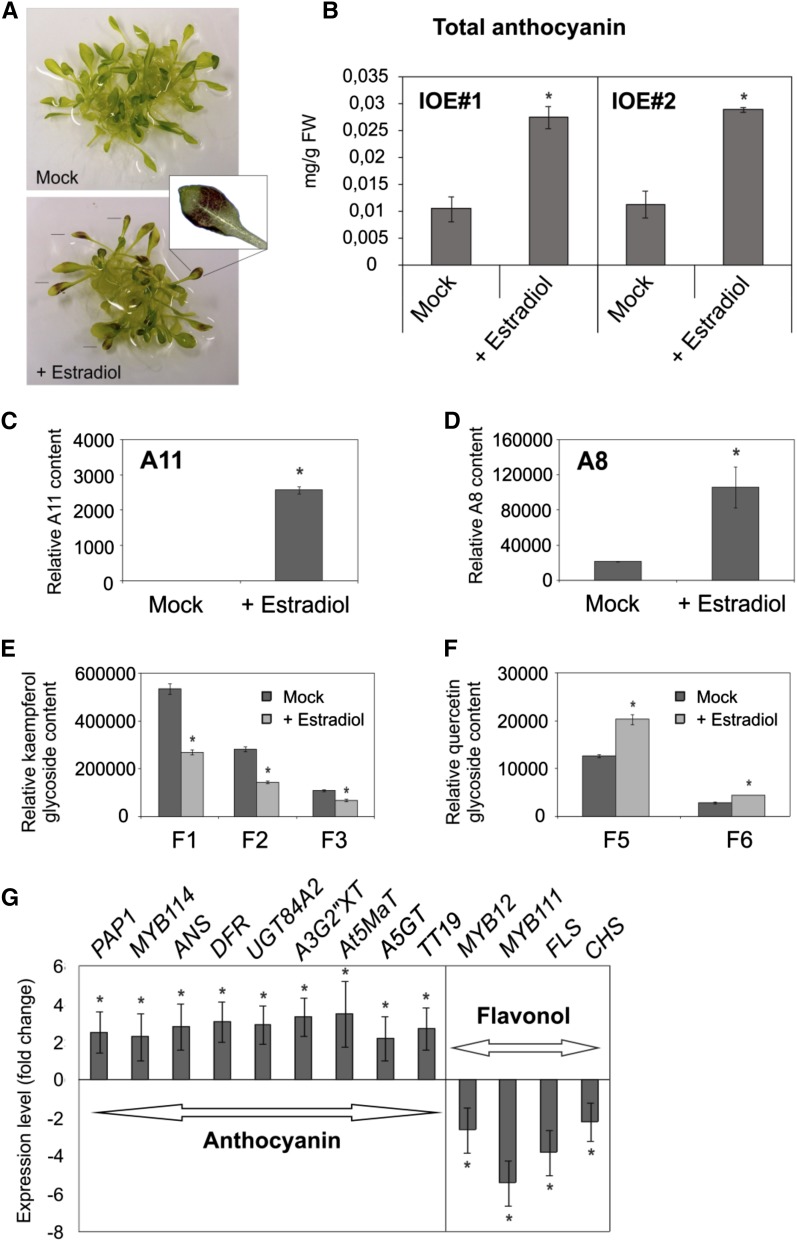
To analyze the biological role of MYB112, we generated estradiol (ESTR)-inducible overexpression (IOE; MYB112-IOE) lines using the pER8 vector developed by Zuo et al. (2000) and induced its expression with 10 μm ESTR in seedlings grown in liquid Murashige and Skoog medium (MS) supplemented with 1% (w/v) Suc. As controls, we used dimethyl sulfoxide (DMSO)-treated MYB112-IOE lines or ESTR-treated empty vector plants. After 5 d of ESTR treatment, leaves of MYB112-IOE seedlings accumulated anthocyanins, whereas mock-treated plants (DMSO; Fig. 3A) as well as ESTR-treated empty vector plants (not shown) did not. At shorter induction times (i.e. 5 h), no anthocyanin accumulation was observed in MYB112-IOE lines (not shown), perhaps suggesting that MYB112 is not immediately upstream of anthocyanin pathway genes. We extracted and measured the anthocyanin level in MYB112-IOE seedlings of two lines (IOE1 and IOE2). Interestingly, the two MYB112-IOE lines accumulated around 3 times more anthocyanins than the respective mock-treated plants (Fig. 3B). We next analyzed the flavonoid metabolite profiles of MYB112-IOE seedlings using liquid chromatography coupled to mass spectrometry (LC-MS). Compared with DMSO-treated plants, ESTR-induced seedlings showed a significant increase in the content of two cyanidin derivatives, namely cyanidin-3-O-[2-O-(2-O-(sinapoyl)-xylosyl)-6-O-(p-O-coumaroyl)-glucoside]-5-O-[6-O-(malonyl)-glucoside] (A11; Fig. 3C) and cyanidin-3-O-[2′′-O-(xylosyl)-6′′-O-(p-O-(glucosyl)-p-coumaroyl)glucoside]-5-O-[6′′′-O-(malonyl)-glucoside] (A8; Tohge et al., 2005; Fig. 3D). Other cyanidin derivatives were not reliably detected in the samples. Flavonol glycosides derived from kaempferol (named F1–F3 according to Tohge et al., 2005) were reduced (Fig. 3E), whereas the levels of flavonol glycosides derived from quercetin (named F5 and F6) accumulated in ESTR-treated plants (Fig. 3F) compared with control plants.
Figure 3.
Overexpression of MYB112 causes changes in flavonoid content. A, MYB112-IOE seedlings treated with 10 µm ESTR for 5 d accumulate red pigment in the middle of the leaves spreading to the edges (lines and magnified image). B, Total anthocyanin content was measured spectrophotometrically after extraction with HCl solution. Mean values ± sd of five biological replicates are shown. FW, Frozen weight. LC-MS analysis of A11 (C) and A8 (D) content (Tohge et al., 2005) in mock- and ESTR-treated MYB112-IOE. LC-MS analysis of flavonol content F1 (kaempferol 3-O-rhamnoside 7-O-rhamnoside), F2 (kaempferol 3-O-glucoside 7-O-rhamnoside), and F3 (kaempferol 3-O-[2′′-O-(rhamnosyl) glucoside] 7-O-rhamnoside; E) and F5 (quercetin 3-O-glucoside 7-O-rhamnoside) and F6 (quercetin 3-O-[2′′-O-(rhamnosyl) glucoside] 7-O-rhamnoside; F). For C to F, mean values ± sd are shown for three biological replicates. *, Statistically significant differences compared with DMSO-treated plants determined by Student’s t test (P < 0.05). G, Transcript levels of selected enzymatic (ANS, DFR, TT19, A3G2”XT, UGT84A2, At5MaT, A5GT, FLS, and CHS) and regulatory (PAP1, MYB114, MYB111, and MYB12) genes involved in anthocyanin and flavonol biosynthesis in MYB112-IOE seedlings treated with ESTR for 5 d. Data are represented as fold changes compared with the expression in DMSO-treated plants. Only genes showing a change in expression compared with control are shown. Mean values ± sd are shown for three biological replicates. *, Statistically significant differences compared with DMSO-treated plants determined by Student’s t test (P < 0.05).
We measured the expression of genes encoding enzymes involved in flavonoid biosynthesis as well as regulatory genes in MYB112-IOE seedlings treated with ESTR for 5 d (Fig. 3G; Supplemental Data Set S1). Transcript levels of PHE AMMONIA LYASE and CINNAMATE-4-HYDROXYLASE, genes associated with the pathway of phenylpropanoid biosynthesis, remained unchanged in the ESTR-treated seedlings. CHS and CHI were slightly down-regulated (approximately 2-fold). Transcript levels of the genes DFR and ANS (LEUCOANTHOCYANIDIN DIOXYGENASE) were significantly increased in seedlings overexpressing MYB112. DFR catalyzes the conversion of dihydroquercetin to leucoanthocyanidin, and ANS encodes a dioxygenase that operates downstream of DFR and catalyzes the conversion of leucoanthocyanidins to anthocyanidins. In addition to the anthocyanin biosynthetic genes indicated above, several genes proposed to be involved in the production of specific anthocyanin derivatives were up-regulated. These include three glycosyltransferase family genes, namely UGT79B1 (anthocyanin-3-O-glusocide-2″-O-xylosyltransferase [A3G2″XT]; Yonekura-Sakakibara et al., 2012), UGT84A2 (sinapic acid 1-O-glucosyltransferase [SGT]), and UGT75C1 (anthocyanin-5-O-glucosyltransferase [A5GT]; Tohge et al., 2005), the acyltransferase family gene anthocyanin-5-O-glucoside-6″-O-malonyltransferase (At5MaT; At3g29590; Luo et al., 2007), and the glutathione S-transferase family gene TT19. The genes encoding glycosyltransferases and acyltransferases catalyze modification reactions for the formation of the most extensively modified A11 anthocyanin (Tohge et al., 2005). TT19 is required for vacuolar sequestration of anthocyanins (Kitamura et al., 2004). The transcript level of FLS, which catalyzes the synthesis of the flavonols quercetin and kaempferol from dihydroquercetin and dihydrokaempferol, respectively, was down-regulated in the ESTR-treated MYB112-IOE seedlings. Apart from biosynthetic genes, a set of regulatory genes also displayed altered expression in MYB112-overexpressing plants. Expression of PAP1 as well as MYB114 was increased in ESTR-treated MYB112-IOE plants. It should be mentioned, however, that the MYB114 gene from the Columbia-0 (Col-0) accession encodes a protein that lacks a transcriptional activation domain because of a stop codon just after the Myb domain (at amino acid 140). The Landsberg erecta MYB114 allele encodes a full-length gene, and its function in anthocyanin accumulation was confirmed (Gonzalez et al., 2008). Interestingly, although such a truncated MYB may act as a dominant negative regulator (such as MYBL2 and CPC; Dubos et al., 2008; Matsui et al., 2008; Zhu et al., 2009) and counteract the activity of PAP1 in Col-0, up-regulation of both PAP1 and MYB114 by MYB112 overexpression triggered an increased expression of anthocyanin biosynthetic genes and an increased level of anthocyanins. MYB12 and MYB111, previously shown to control the expression of genes involved in flavonol biosynthesis, were down-regulated (Fig. 3G; Supplemental Data Set S1). This suggests that MYB112 acts through the expression of these regulatory genes.
Silencing of MYB112 Results in the Down-Regulation of Anthocyanin Pathway Genes
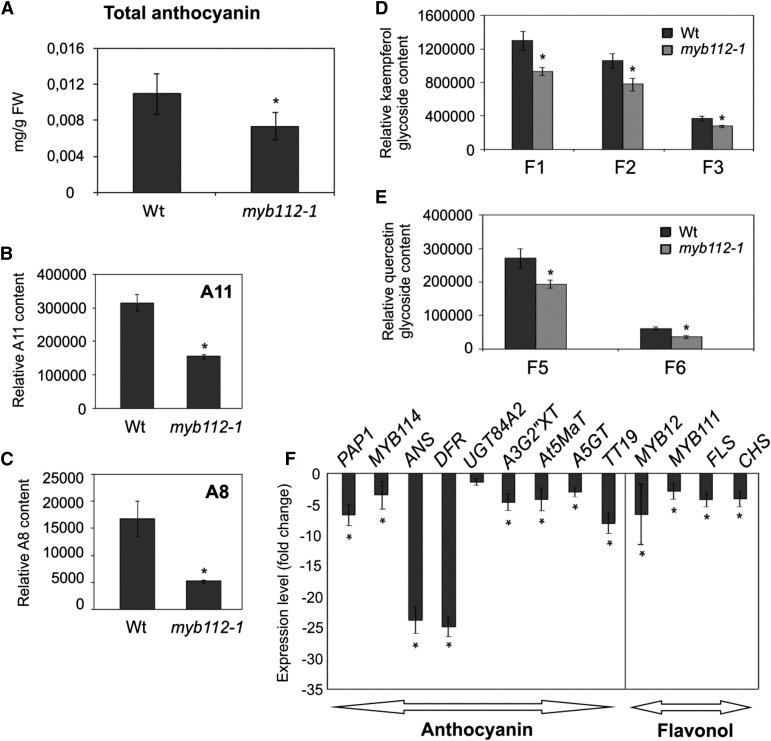
We next analyzed the relative anthocyanin content in the two myb112 T-DNA insertion mutants using spectrophotometric methods (Fig. 4A). Compared with wild-type seedlings, the total anthocyanin content was reduced by approximately 30% in the myb112-1 mutant grown for 2 weeks on MS containing 4% (w/v) Suc. No difference in total anthocyanin content was observed between wild-type and myb112-2 plants, indicating that the level of MYB112 transcript present in this knockdown mutant (Fig. 2B) is sufficient to sustain normal anthocyanin levels. LC-MS analysis revealed that the changes in the total anthocyanin content in the myb112-1 mutant are caused by decreased accumulation of A11 (Fig. 4B) and A8 (Fig. 4C) derivatives in these plants. We also detected a slight decrease of flavonol glycoside accumulation in myb112-1 seedlings compared with the wild type (Fig. 4, D and E). Expression profiling revealed decreased transcript levels of genes coding for key enzymes of flavonoid biosynthesis (ANS, DFR, A3G2″XT, At5MaT, A5GT, TT19, and FLS) as well as regulatory genes (PAP1, MYB114, and MYB12) in the myb112-1 mutant (Fig. 4F; Supplemental Data Set S1). An integrated schematic view of the transcriptomic and metabolomic shifts occurring in MYB112-modified plants compared with the wild type or DMSO-treated MYB112-IOE plants is given in Supplemental Figure S1.
Figure 4.
Reduced flavonoid content in myb112 mutants. Total anthocyanin content measured spectrophotometrically after extraction with HCl solution (A) and LC-MS analysis of A11 (B) and A8 (C) content (Tohge et al., 2005) in myb112 mutants compared with wild-type (Wt) seedlings grown on 4% (w/v) Suc. FW, Frozen weight. LC-MS analysis of flavonols content F1 (kaempferol 3-O-rhamnoside 7-O-rhamnoside), F2 (kaempferol 3-O-glucoside 7-O-rhamnoside), and F3 (kaempferol 3-O-[2′′-O-(rhamnosyl) glucoside] 7-O-rhamnoside; D) and F5 (quercetin 3-O-glucoside 7-O-rhamnoside) and F6 (quercetin 3-O-[2′′-O-(rhamnosyl) glucoside] 7-O-rhamnoside; E) in myb112 mutants compared with wild-type plants. Mean values ± sds are shown for five (A) and three (B–E) biological replicates. *, Statistically significant differences compared with wild-type plants determined by Student’s t test (P < 0.05). F, Transcript levels of selected biosynthetic (ANS, DFR, A3G2”XT, At5MaT, A5GT, TT19, FLS, and CHS) and regulatory (PAP1, MYB114, MYB111, and MYB12) genes involved in anthocyanin and flavonol biosynthesis in 2-week-old myb112-1 seedlings grown on MS and 1% (w/v) Suc. Data are represented as fold change compared with the expression in wild-type plants. Only genes showing a change in expression compared with control are shown. Mean values ± sd are shown for three biological replicates. *, Statistically significant differences compared with the wild type determined by Student’s t test (P < 0.05).
Salt-Induced Expression of MYB112
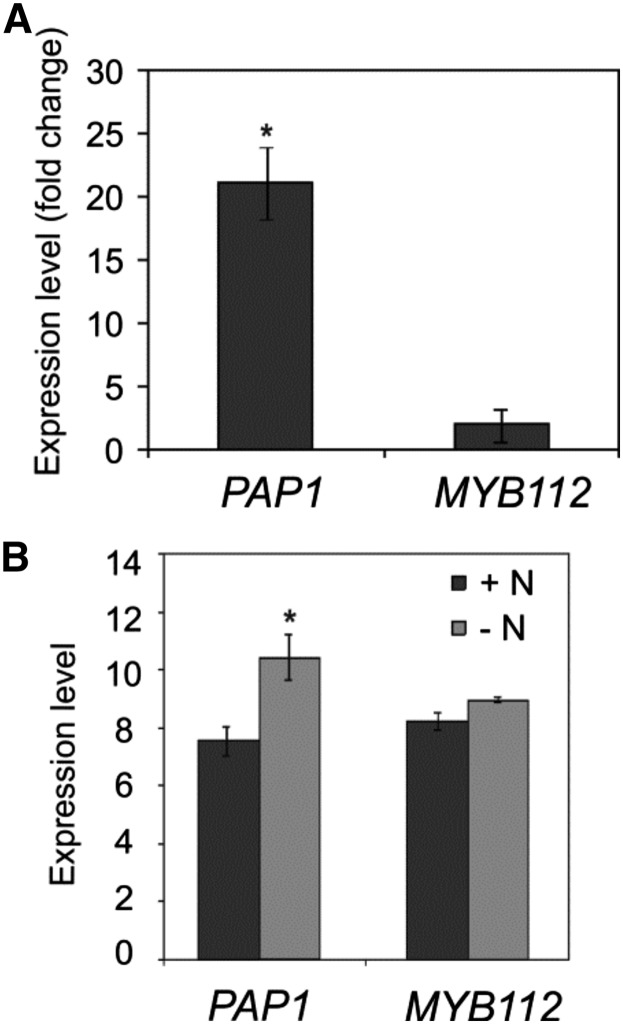
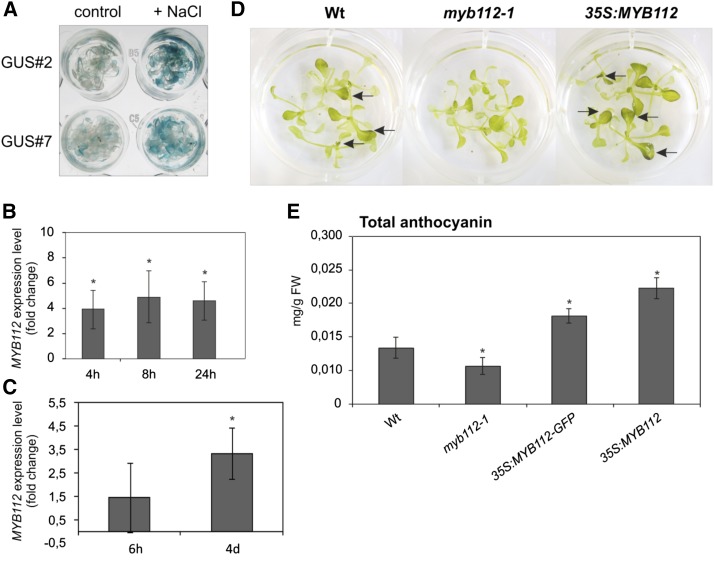
It has been previously shown that Suc is an effective inducer of anthocyanin biosynthesis in Arabidopsis seedlings and that the expression of PAP1 strongly increases upon Suc treatment (Teng et al., 2005; Solfanelli et al., 2006). To investigate the effect of Suc concentration on MYB112 expression, we measured its transcript levels in 2-week-old seedlings grown on MS supplemented with either 1% or 4% (w/v) Suc. As shown in Figure 5A, no substantial change in MYB112 expression was detected, whereas the transcript level of PAP1 increased significantly in plants grown on high-Suc medium. Our result is in agreement with previously published global transcriptome data on Suc-responsive genes (Solfanelli et al., 2006; Osuna et al., 2007). Nitrogen deficiency also enhances expression of specific MYB and bHLH TFs and accumulation of end products of the flavonoid pathway (Lea et al., 2007; Rubin et al., 2009). However, MYB112 transcript abundance in plants grown for 6 d on medium with no nitrogen source was comparable with the transcript level in control plants grown on full (nitrogen-sufficient) medium, whereas in the same experiment, as anticipated, PAP1 expression increased approximately 8-fold (Fig. 5B). According to microarray data obtained from the GENEVESTIGATOR and eFP databases, expression of MYB112 increases upon abiotic stress, namely salinity. To verify salt-induced expression of MYB112, we used the MYB112 promoter-reporter (GUS) gene fusion lines; we transferred 2-week-old ProMYB112:GUS seedlings to MS containing NaCl (150 mm for 24 h). Histochemical staining revealed enhanced GUS activity in salt-treated seedlings compared with untreated controls (Fig. 6A), showing that the MYB112 promoter faithfully recapitulated the salt-dependent expression changes observed at the transcript level. Salt-responsive MYB112 expression was further confirmed by qRT-PCR in seedlings treated with salt (150 mm NaCl) for 4, 8, and 24 h in liquid medium (Fig. 6B). In addition, we grew Arabidopsis Col-0 plants in a hydroponic culture system and subjected them to short- and long-term salt stress (150 mm NaCl) as described previously (Balazadeh et al., 2010a, 2010b). In short-term experiments, leaves of 28-d-old plants were sampled 6 h after stress treatment. In long-term stress experiments, leaves were sampled after 4 d of salt treatment at approximately 20% chlorophyll loss, indicating senescence. Expression of the senescence-specific marker gene SAG12 (Noh and Amasino, 1999) was not detected in control plants and plants subjected to short-term salt stress, whereas it was induced under long-term salt stress, which induces senescence (for data, see Balazadeh et al., 2010a). Microarray gene expression profiling revealed no change in MYB112 transcript level after short-term stress; however, after 4 d of salt treatment, the transcript level increased by over 3-fold (Fig. 6C). Similar salinity stress-triggered expression changes have previously been observed for many SAGs (Balazadeh et al., 2010a, 2010b).
Figure 5.
MYB112 expression is affected by neither Suc nor nitrogen. A, Transcript levels of PAP1 and MYB112 determined by qRT-PCR in 2-week-old seedlings grown on MS with 1% (control) or 4% (w/v) Suc. Note the absence of an effect of elevated Suc concentration on MYB112 expression. The data are represented as fold change compared with the expression in control seedlings. Means of three biological replicates ± sd are shown. *, Statistically significant difference compared with control plants as determined by Student’s t test (P < 0.01). B, Expression level of PAP1 and MYB112 in plants grown on medium with or without nitrogen. Plants were grown in a hydroponic system and after 19 d transferred to medium without a nitrogen source. Gene expression was measured 7 d after the transfer. MYB112 expression is not affected by nitrogen availability, whereas PAP1 expression increases by approximately 8-fold. Data were extracted from Affymetrix ATH1 hybridizations (Balazadeh et al., 2014) and represent the means ± sds of three biological replicates. Numbers indicate log2 values. *, Statistically significant difference compared with control plants as determined by Student’s t test (P < 0.05).
Figure 6.
MYB112 regulates anthocyanin accumulation during salt stress. A, Ten-day-old Arabidopsis ProMYB112:GUS seedlings (lines 2 and 7) were treated for 72 h with 0 mm NaCl (control) or 150 mm NaCl (+NaCl) in liquid MS. Enhanced GUS staining is visible in salt-treated seedlings (right). B, Transcript level of MYB112 in wild-type seedlings treated with 150 mm NaCl in liquid culture. Incubation times were 4, 8, and 24 h. The data are represented as fold change comparison with untreated control seedlings. Mean values of three biological replicates ± sd are shown. C, Expression profiling using Affymetrix ATH1 arrays shows elevated MYB112 transcript abundance in plants grown hydroponically for 4 d with 150 mm NaCl compared with control plants. Plants were harvested after 6 h and 4 d of treatment. The experiment was performed in three biological replications. Numbers on the y axis indicate fold change values ± sd. D, Appearance of seedlings treated with salt for 3 d. Plants were grown on MS supplemented with 1% (w/v) Suc and after 2 weeks, transferred to liquid medium containing 150 mm NaCl. Note the lack of red pigmentation in myb112-1 seedlings. E, Total anthocyanin content was measured spectrophotometrically after extraction with HCl solution. Means ± sd are shown for three biological replicates. In B, C, and E, asterisks indicate statistically significant differences compared with wild-type (Wt) plants as determined by Student’s t test (P < 0.05). FW, Frozen weight.
MYB112 Regulates Anthocyanin Accumulation under Salt Stress
To test whether MYB112 may be involved in controlling anthocyanin accumulation during salt stress, we transferred 10-d-old seedlings to liquid MS containing 150 mm NaCl. After 3 d, wild-type plants and MYB112 overexpression plants (i.e. 35S:MYB112 and 35S:MYB112-GFP lines) turned red, whereas myb112-1 mutant plants accumulated visibly less anthocyanin (Fig. 6D). Spectrophotometric analysis of anthocyanin content in these plants revealed a decrease in total anthocyanin content by 20% in the myb112-1 mutant compared with the wild type. 35S:MYB112 and 35S:MYB112-GFP plants accumulated 68% and approximately 35% more anthocyanins, respectively, than the wild-type plants (Fig. 6E). Thus, reciprocal effects on anthocyanin accumulation were observed in mutants versus overexpressors of MYB112. We therefore concluded that MYB112 contributes to regulating anthocyanin production in response to salinity stress.
Identification of MYB112 Early Target Genes
To further characterize the MYB112 regulatory network, we used 2-week-old MYB112-IOE plants and tested ESTR-dependent MYB112 expression 1, 3, and 5 h after ESTR treatment. As controls, we used either DMSO-treated MYB112-IOE lines or ESTR-treated wild-type control plants. MYB112 expression increased strongly already within 1 h of ESTR treatment, further increased after 3 h, and reached its maximum 5 h after ESTR application (Supplemental Fig. S2). To find genes responsive to MYB112, 2-week-old MYB112-IOE seedlings were transferred to liquid MS containing either 10 µm ESTR or DMSO as control. Seedlings were harvested 3 and 5 h after ESTR induction and after removal of the roots subjected to expression profiling using Affymetrix ATH1 arrays. By including the wild-type control line in our analyses, we could distinguish between genes responding solely to the ESTR treatment and those responding to elevated MYB112 expression. Two independent experiments were performed and analyzed from the 5-h time point (MYB112-IOE-5 h), and one experiment was performed and analyzed from the 3-h time point (MYB112-IOE-3 h). Statistical tests using the Limma Bioconductor package (Gentleman et al., 2004) allowed us to identify 56 genes that were significantly differentially expressed (>2-fold) upon 5 h of induction of MYB112 (Supplemental Table S1), of which 28 were up- and 28 were down-regulated (excluding the ESTR-responsive genes in wild-type plants; Supplemental Data Set S2; Gene Expression Omnibus accession no. GSE36721). Among the up-regulated genes, three encoded TFs, namely MYB32, MYB7, and MYB6. Interestingly, these three R2R3-MYB TFs are closely related and contain an ethylene-responsive factor-associated amphiphilic repression (EAR) motif within their regulatory domains (Matus et al., 2008). Moreover, MYB32 and MYB7 were classified to the same subgroup 4 of the R2R3-MYB gene family in Arabidopsis (Stracke et al., 2001). MYB factors of this subgroup were previously shown to be involved in the regulation of pollen development and second cell wall biosynthesis in Arabidopsis (Preston et al., 2004; Zhong and Ye, 2012) and the negative regulation of genes involved in lignin and flavonoid biosynthesis in Arabidopsis and maize (Jin et al., 2000; Fornalé et al., 2014). UGT84A2 is another gene with a function in phenylpropanoid biosynthesis and modification and was up-regulated after 5 h of MYB112 induction. UGT84A2 is a sinapic acid-O-glucosyltransferase (Sinlapadech et al., 2007) that plays a major role in providing sinapoyl-Glc for anthocyanin sinapoylation (therefore, being highly important in the production of the anthocyanin derivative A11; Yonekura-Sakakibara et al., 2012). Moreover, MYB32, MYB7, and UGT84A2 were previously shown to be up-regulated during age-dependent senescence in wild-type Arabidopsis (Buchanan-Wollaston et al., 2005; Balazadeh et al., 2008; GENEVESTIGATOR). Other than MYB32, MYB7, and UGT84A2, 11 other up-regulated transcripts (representing one half of the MYB112 up-regulated genes; Supplemental Table S1) were previously reported to be senescence associated (eFP browser; Balazadeh et al., 2008; Breeze et al., 2011; http://www2.warwick.ac.uk/fac/sci/lifesci/research/presta/data/senescence). This result supports the model that MYB112 is a senescence-regulatory TF and that the expression of several known SAGs (including TFs) is regulated by this MYB TF. In the single 3-h Affymetrix experiment, we identified (>4-fold) 15 differentially expressed genes (1 up- and 14 down-regulated genes; Supplemental Data Set S2; Gene Expression Omnibus accession no. GSE36721). We next tested expression of the genes up-regulated after 5 h of induction and the 10 most strongly down-regulated genes after 3 h (which also robustly responded after 5 h) by qRT-PCR; 22 of the 28 up-regulated genes and 9 of the 10 down-regulated genes could be confirmed by qRT-PCR (Supplemental Tables S1 and S2).
MYB112 Triggers the Expression of R2R3-MYB TFs of Subgroup 4
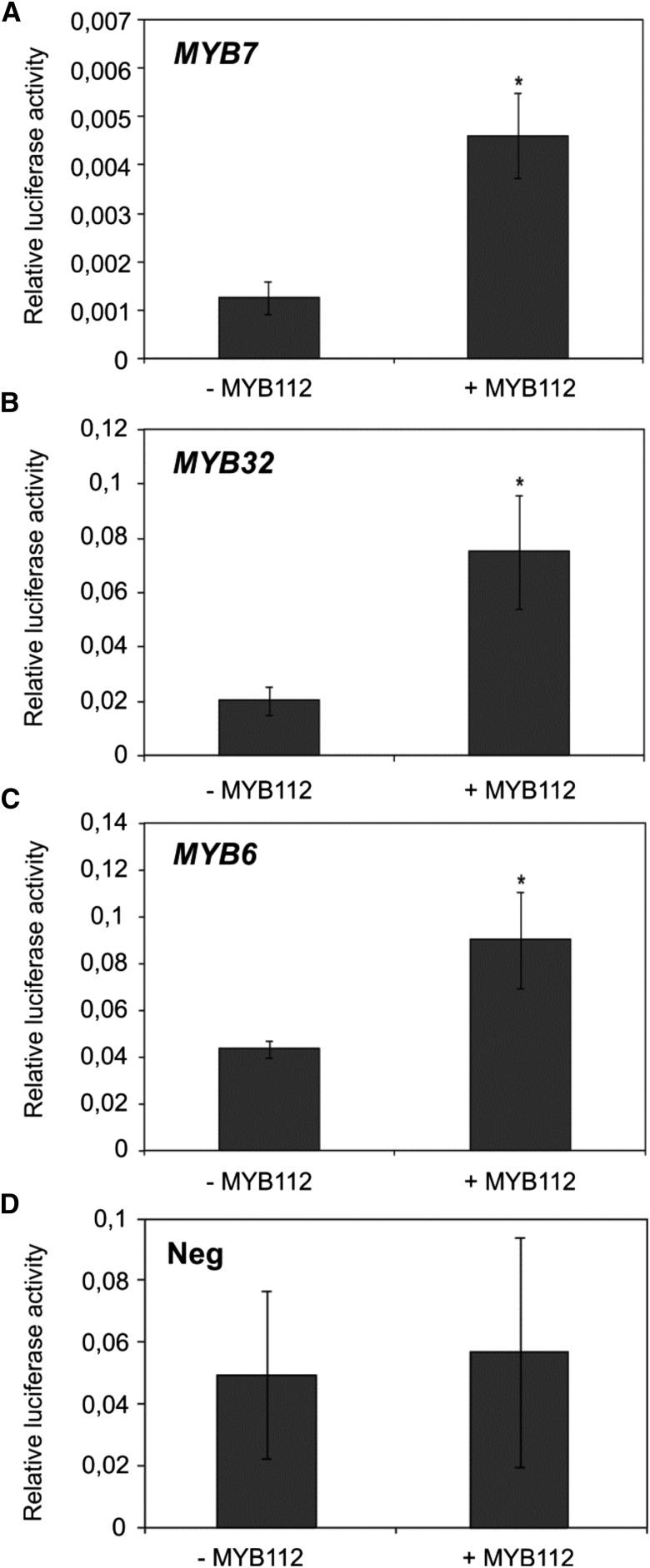
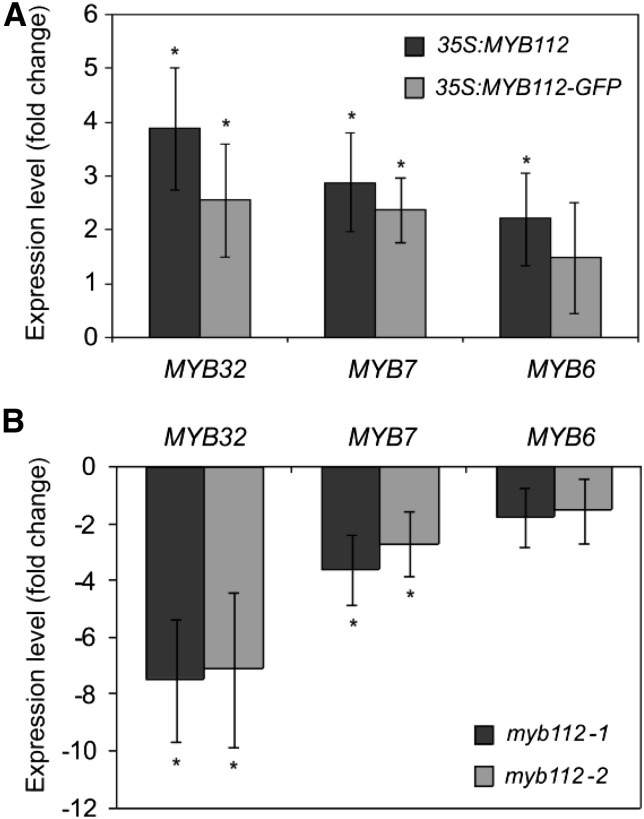
To confirm that MYB112 regulates the expression of genes identified by expression profiling, we assayed the transactivation capacity of MYB112 on the promoters of MYB32, MYB7, and MYB6. These putative downstream target genes were selected from the data set considering their predicted regulatory function in flavonoid biosynthesis. Mesophyll cell protoplasts were prepared from wild-type (Col-0) Arabidopsis leaves and transiently transformed with constructs expressing MYB112 under the control of the CaMV 35S promoter. Simultaneously, protoplasts were transfected with vectors carrying the firefly (Photinus pyralis) luciferase (fLUC) open reading frame fused to the upstream sequences of the selected MYB112 target genes. For normalization, protoplasts were additionally transformed with a third construct that expresses Renilla reniformis luciferase from the 35S promoter. Protoplasts lacking the 35S:MYB112 effector construct served as controls. MYB7 and MYB32 promoters displayed over 3-fold and MYB6 promoter displayed approximately 2-fold induction of the reporter gene when MYB112 was overexpressed in the protoplasts (Fig. 7, A–C). No transactivation was observed for the CIPK18 promoter included as negative control (Neg in Fig. 7D). In addition, we measured expression of MYB32, MYB7, and MYB6 in 35S:MYB112 and 35S:MYB112-GFP seedlings and myb112 mutants grown for 2 weeks on MS supplemented with 1% (w/v) Suc. Transcript level of MYB32 and MYB7 was elevated by approximately 4- and approximately 3-fold in the 35S:MYB112 and 35S:MYB112-GFP seedlings, respectively, compared with the wild type. Expression of MYB6 was increased by approximately 2-fold in the 35S:MYB112 seedlings, but no significant difference was observed in 35S:MYB112-GFP plants compared with the wild type (Fig. 8A). Notably, expression of MYB32 and MYB7 was reduced by approximately 9- and approximately 3-fold in the myb112-1 and myb112-2 mutants, respectively, whereas MYB6 expression was only slightly lowered (Fig. 8B). Taken together, these data confirm a regulatory function of MYB112 toward MYB32, MYB7, and MYB6, which all encode R2R3-MYB TFs of subgroup 4.
Figure 7.
MYB112 transactivates MYB7, MYB32, and MYB6 promoters. Transactivation of MYB7 (A), MYB32 (B), and MYB6 (C) expression by MYB112 in Arabidopsis mesophyll cell protoplasts. D, Absence of transactivation of the CBL-INTERACTING SERINE/THREONINE-PROTEIN KINASE18 (CIPK18) promoter (negative control [Neg]). The ProMYB7:fLUC, ProMYB32:fLUC, ProMYB6:fLUC, or ProCIPK18:fLUC constructs harboring the respective promoters (approximately 1.7 kb) upstream of the fLUC open reading frame were cotransformed with the 35S:MYB112 plasmid (omitted in control experiments). The 35S:rLUC vector was used for transformation efficiency normalization. Given are means ± sd of four biological replicates. *, Significant differences to control as determined by Student’s t test (P < 0.05).
Figure 8.
Expression of MYB32, MYB7, and MYB6 in MYB112 overexpression plants and mutants. Transcript levels of MYB32, MYB7, and MYB6 in 2-week-old 35S:MYB112 and 35S:MYB112-GFP plants (A) and myb112-1 and myb112-2 mutant seedlings (B) as measured by qRT-PCR. Data are represented as fold change compared with the expression in empty vector control plants or the wild type, respectively. Mean values ± sd are shown for three biological replicates. *, Statistically significant differences compared with controls determined by Student’s t test (P < 0.05).
Identification of the MYB112 DNA Binding Site
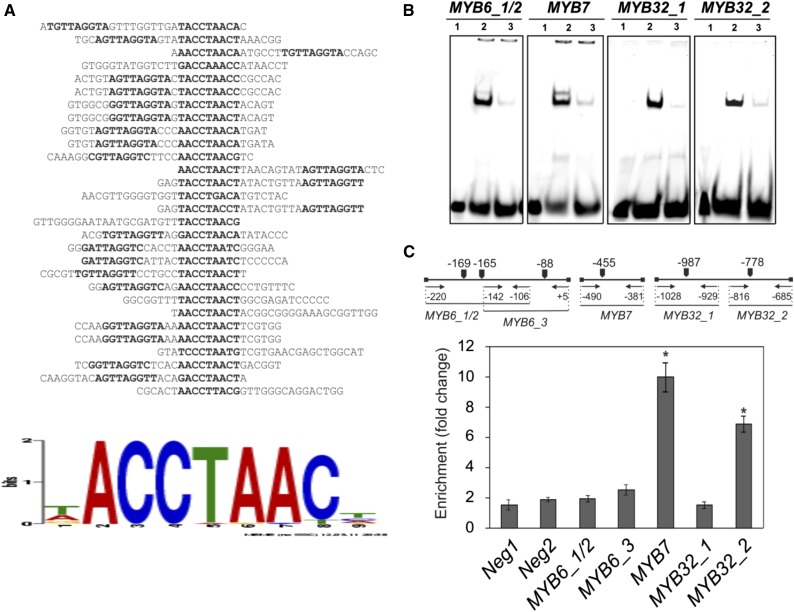
We performed an in vitro binding site selection experiment to identify sequence motifs recognized by MYB112 using the cellulase D (CELD)-TF fusion method (Xue, 2002, 2005). MYB112 was translationally fused to the catalytic domain of a 6× His-tagged CELD from Neocallimastix patriciarum and incubated with biotin-labeled random sequence oligonucleotide probes. Oligonucleotides bound by the MYB112-CELD fusion protein were recovered by means of affinity purification of the DNA-MYB112-CELD complex and amplified using PCR (Xue, 2005). Twenty-nine sequences were obtained and analyzed for binding activity. An alignment of the target sequences is shown in Figure 9A. MYB112 binds to an 8-bp DNA fragment containing the core sequence (A/T/G)(A/C)CC(A/T)(A/G/T)(A/C)(T/C). The identified recognition site is present in promoters of a number of genes controlled by MYB112 in ESTR-IOE lines (Supplemental Table S1), including the three MYB genes (within the 1-kb upstream regions): MYB32 (two binding sites), MYB7 (one binding site), and MYB6 (three binding sites).
Figure 9.
Identification of MYB112 direct target genes. A, MYB112 in vitro binding site selection. A motif common to 29 positive clones was identified using MEME. The motif is present in the promoters of putative direct target genes. B, EMSA. Purified MYB112-CELD-His protein binds specifically to the MYB112 binding sites present in the MYB6, MYB7, and MYB32 promoters. In vitro DNA binding reactions were performed with 40-bp double-stranded oligonucleotides, including the MYB112 binding sites of the respective target gene promoters. The DNA fragments were labeled with IR dye. The fragments contained two (MYB6_1/2) or one (MYB7, MYB32_1, and MYB32_2) MYB112 binding motif. 1, IR-labeled DNA fragment.; 2, MYB112 protein with labeled DNA fragment (note distinct shift indicating binding); 3, MYB112 protein plus labeled DNA fragment and 200× excess competitor (note the shift disappearance). C, ChIP-qPCR. Whole shoots of 2-week-old Arabidopsis seedlings expressing GFP-tagged MYB112 under the control of 35S CaMV promoter (35S:MYB112-GFP) and wild-type plants were harvested for the ChIP experiment. qPCR primer locations are indicated. Primers annealing to promoter regions of two Arabidopsis genes lacking MYB112 binding sites (i.e. At2g22180 [Neg1] and At3g1840 [Neg2]) were used as negative controls. Enrichment of the respective promoter fragments was quantified by qPCR. Data represent means ± sd of three experiments. Enrichment of MYB7 and MYB32 promoter fragments is detected. *, Statistically significant differences to controls as determined by Student’s t test (P < 0.05).
MYB112 Binds to the MYB7 and MYB32 Promoters
We next used an electrophoretic mobility shift assay (EMSA) to test in vitro the physical interaction between MYB112 protein and promoter fragments of MYB6, MYB7, and MYB32. Expressed and purified MYB112-CELD protein was incubated with IR dye-labeled 40-bp-long promoter fragments of the selected putative target genes containing the identified MYB112 binding site (Supplemental Material S1). The probes included the single MYB112 binding site of the MYB7 promoter (fragment MYB7_1), either of the two binding sites of the MYB32 promoter (MYB32_1 or MYB32_2), or two neighboring sites of the three present in the MYB6 promoter (both included in the MYB6_1/2 fragment). In all cases, a clear band shift was observed in the presence of MYB112-CELD fusion protein, which disappeared in the presence of unlabeled competitor DNA (Fig. 9B). These results indicate a physical interaction of MYB112 with the MYB6, MYB7, and MYB32 promoters. Finally, to verify MYB112 binding to the respective promoters in vivo, we performed chromatin immunoprecipitation (ChIP) coupled to quantitative PCR (qPCR) using plants bearing a 35S:MYB112-GFP construct (accumulating MYB112-GFP fusion protein in the nucleus; Fig. 1B). We designed primers flanking the MYB112 binding sites of the different promoters (Supplemental Material S1) and measured their abundance using qPCR. As shown in Figure 9C, the MYB32 (binding site 2) and MYB7 promoter fragments containing the selected cis-element were enriched relative to control, proving direct binding of MYB112. In contrast, we did not detect significant binding of MYB112 to the MYB6 and binding site 1 of the MYB32 promoters. It may, however, be that these two genes are targets of MYB112 in other physiological conditions that we did not test in our experiments. Taken together, our data firmly prove that MYB112 acts as a direct transcriptional regulator of MYB32 and MYB7.
MYB112 Affects Pollen Viability
MYB32, identified here as a direct MYB112 target, as well as the closely related MYB4 gene are both required for normal pollen development (Preston et al., 2004). The expression of several phenylpropanoid biosynthesis genes is altered in plants with modified expression of the MYB TFs, which likely affects the metabolite flux through the phenylpropanoid pathways. This then affects the formation of the major pollen wall component sporopollenin, which consists of polymerized phenols and fatty acid derivatives, leading to pollen distortion (Preston et al., 2004).
By analyzing MYB112 promoter activity in ProMYB112:GUS lines, we show that MYB112 is also expressed in pollen (Fig. 1, A16 and A17). We therefore tested pollen viability in MYB112 transgenic plants after staining of flowers with Alexander dye (Alexander, 1969). We found that overexpression of MYB112 leads to defective pollen; 35% and 70% of pollen was aborted in 35S:MYB112-GFP and 35S:MYB112 overexpression plants, respectively (Supplemental Fig. S3). The observed decrease in pollen viability in these lines corresponds to the level of MYB112 overexpression (approximately 5-fold increase in 35S:MYB112-GFP and approximately 15-fold in 35S:MYB112; Supplemental Fig. S4).
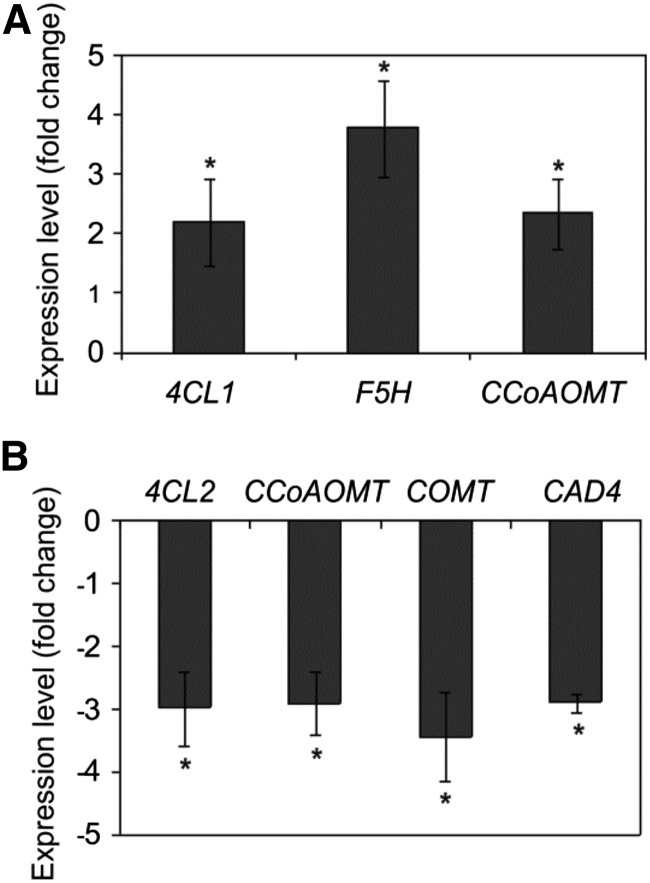
We then analyzed transcript levels of genes encoding key phenylpropanoid biosynthesis enzymes in the myb112-1 mutant and the MYB112-IOE plants treated with ESTR for 5 d (Supplemental Table S2). Figure 10 shows significant changes in the expression of a number of phenylpropanoid biosynthetic genes in MYB112 transgenic plants. For example, expression of CAFFEOYL/COENZYME A 3-O-METHYLTRANSFERASE (CCoAOMT) is increased in plants overexpressing MYB112 (Fig. 10A), similar to MYB4 overexpression plants (Jin et al., 2000). CCoAOMT is down-regulated in the myb4 knockout line (Jin et al., 2000) and the myb112-1 mutant (Fig. 10B). Thus, like in MYB32- and MYB4-modified plants, aberrant pollen observed in MYB112 transgenics is likely caused by altered phenylpropanoid biosynthesis.
Figure 10.
Changes in expression of phenylpropanoid biosynthesis genes in MYB112 transgenic plants. Expression of phenylpropanoid biosynthetic genes in MYB112-IOE seedlings treated with ESTR in liquid MS (1% [w/v] Suc) for 5 d compared with mock (DMSO)-treated plants (A) and myb112-1 seedlings compared with the wild type (B). Data are represented as fold change compared with the respective control. Only genes showing a change in expression compared with control are shown. Data are means ± sd of three biological replicates. *, Statistically significant differences to control determined by Student’s t test (P < 0.05). 4CL1, 4-COUMARATE COA LIGASE1; F5H, FERULATE 5-HYDROXYLASE; COMT, CAFFEATE O-METHYLTRANSFERASE; CAD, CINNAMOYL-ALCOHOL DEHYDROGENASE.
MYB112 Is a High Light-Induced Gene
Thylakoid-soluble Phosphoprotein of 9 kD (TSP9) is a mobile thylakoid protein that interacts with light-harvesting complex II and the peripheries of both photosystems. It was shown to regulate light harvesting by facilitating the dissociation of light-harvesting proteins from PSII. Upon phosphorylation, TSP9 is partially released from the membrane and therefore, was proposed to play a role in chloroplast signaling (Hansson et al., 2007). Global expression profiling identified 23 genes, including MYB112, that were high light dependent in wild-type plants but not in a tsp9 T-DNA insertion mutant (GK_377A12; Fristedt et al., 2009).
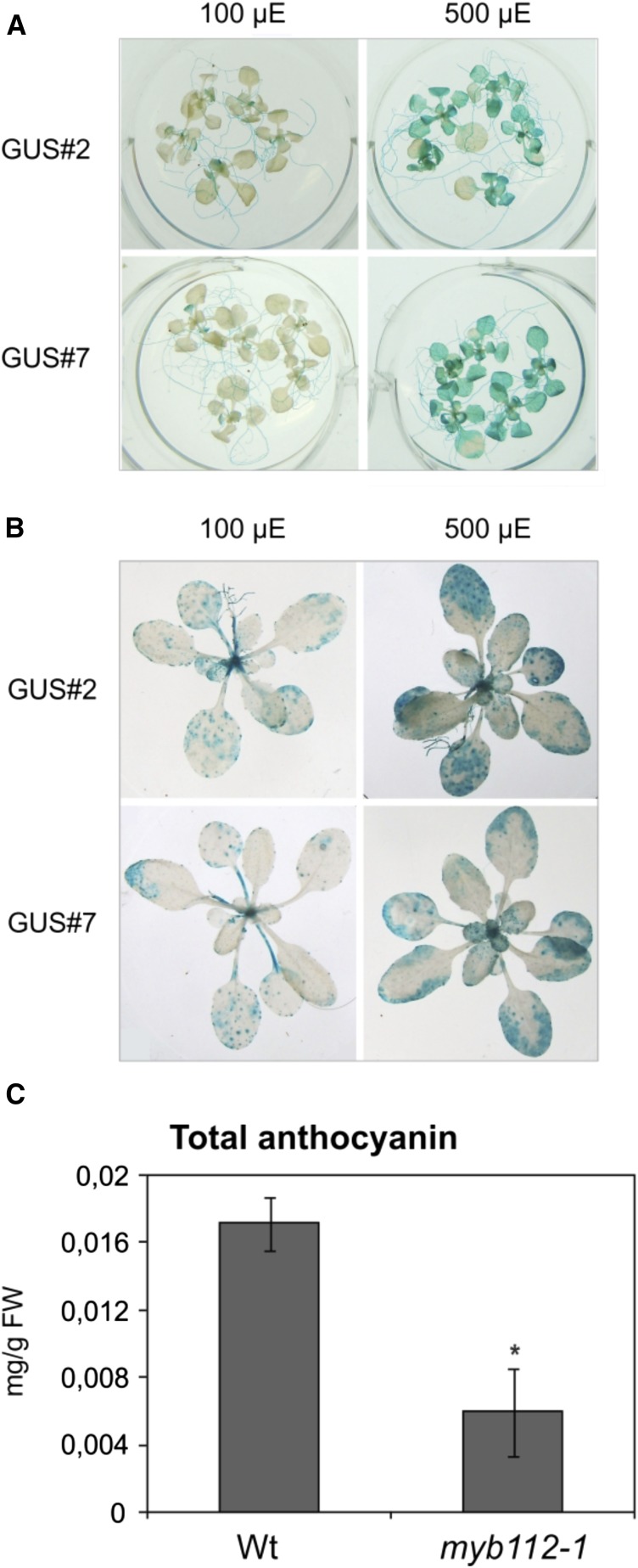
This finding suggests a function of MYB112 in TSP9-dependent high light signaling. To investigate further the relation between the two genes, we measured total anthocyanin content in two tsp9 T-DNA insertion lines under high light stress. However, anthocyanin accumulation was not affected in either mutant, suggesting that the level of MYB112 in these plants was still sufficient to sustain a wild-type phenotype. To verify high light-induced expression of MYB112, we used the ProMYB112:GUS reporter lines. Ten-day-old Arabidopsis ProMYB112:GUS seedlings as well as 3-week-old plants were treated for 6 and 20 h, respectively, with high light (500 µmol m−2 s−1). In both cases, histochemical staining revealed enhanced GUS activity in high light-treated plants compared with controls kept in normal light condition (100 µmol m−2 s−1; Fig. 11, A and B). Spectrophotometric analysis of anthocyanin content in 3-week-old myb112 mutants grown under high light condition (500 µmol m−2 s−1) for 3 d showed decreased pigment content in myb112-1 plants (Fig. 11C), suggesting that MYB112 is involved in modulating high light-induced anthocyanin accumulation.
Figure 11.
MYB112 regulates anthocyanin accumulation under high light. A, MYB112 promoter activity in 10-d-old Arabidopsis ProMYB112:GUS seedlings (lines 2 and 7) treated for 6 h with high light (500 µE; right) compared with control seedlings kept at 100 µE (left). B, GUS activity in 3-week-old ProMYB112:GUS plants treated with high light for 20 h (right) compared with control plants kept at 100 µE (left). Staining in A and B is shown for two lines (i.e. GUS#2 and GUS#7). Note the enhanced GUS staining of plants treated with high light. C, Anthocyanin content in myb112 mutants grown under high light. Plants were grown in long-day condition and after 3 weeks transferred to high light (500 µE) for 3 d. Total anthocyanin content was measured spectrophotometrically after extraction with HCl solution. Means ± sd are shown for five biological replicates. FW, Frozen weight. *, Statistically significant difference to wild-type (Wt) plants as determined by Student’s t test (P < 0.05).
DISCUSSION
We discovered MYB112 as a previously uncharacterized transcriptional regulator affecting flavonoid biosynthesis in Arabidopsis. Based on our experimental data, we suggest that MYB112 acts as a positive regulator of anthocyanin biosynthesis and a negative regulator of flavonol biosynthesis. As we show, MYB112 activates PAP1 and MYB114 but inhibits MYB12 and MYB111. PAP1 and MYB114 regulate the expression of genes encoding enzymes involved in anthocyanin formation (for example, ANS and DFR), and expression of these genes is increased upon MYB112 induction. However, note that only the Landsberg erecta MYB114 but not the Col-0 allele encodes a full-length TF (Gonzalez et al., 2008). MYB111 and MYB12 control expression of biosynthetic genes involved in flavonol biosynthesis (for example, CHS and FLS), and their transcript levels are decreased in MYB112-IOE plants after ESTR treatment. We did not obtain evidence for a direct regulation of either the known anthocyanin or flavonol biosynthetic genes or their regulators. Because the expression of PAP1 and PAP2 was not affected after short-term induction of MYB112, these transcription regulatory genes are unlikely to be direct or early targets of MYB112 and were, therefore, not included in our ChIP or transactivation assays. At this stage of analysis, it thus remains open how the regulation of the flavonoid pathway by MYB112 is achieved. However, one possible scenario is that this regulation by MYB112 occurs through the direct activation of MYB32 and MYB7, which have an EAR repressor motif and a likely role in transcriptional repression of lignin and flavonoid biosynthetic genes. Furthermore, MYB32 was previously shown to affect expression of flavonoid biosynthesis pathway genes and pollen development. Disruption of MYB32 by T-DNA insertion leads to the formation of aberrant pollen. A similar phenotype is observed when the closely related MYB4 gene is mutated or overexpressed. However, in Arabidopsis, flavonoids are not essential for pollen formation or fertility (Burbulis et al.., 1996). It was speculated that changes in the expression of flavonoid and phenylpropanoid biosynthetic genes influence the flux along these pathways, interfering with pollen development by altering structural components of the pollen wall, such as sporopollenin (Preston et al., 2004). Our results reported here reveal that overexpression of MYB112 also leads to partial pollen abortion. Expression profiling of genes encoding key phenylpropanoid biosynthesis enzymes in the myb112-1 mutant and the MYB112-IOE plants treated with ESTR for 5 d revealed significant changes in the expression of a number of phenylpropanoid biosynthetic genes in MYB112 transgenic plants. MYB32, MYB7, and MYB4 were recently reported to be direct downstream target genes of MYB46 and MYB83. These two TFs recognize the 7-bp consensus sequence ACC(A/T)A(A/C)(T/C) (Kim et al., 2012; Zhong and Ye, 2012), which is highly similar to the MYB112 binding site reported here: (A/T/G)ACC(A/T)(A/G)(A/C)(T/C). MYB46 and MYB83 function redundantly to control the production of all major secondary cell wall components in Arabidopsis fibers and vessels, such as lignin, cellulose, and xylan (Ko et al., 2009; Zhong and Ye, 2012). MYB58 and MYB63 are yet other MYB factors involved in controlling the formation of the secondary cell wall. Contrary to MYB46 and MYB83, they are specifically involved in the regulation of lignin biosynthesis (Zhou et al., 2009). Their overexpression was found to induce ectopic deposition of lignin but not cellulose and xylan, whereas their dominant repression resulted in a reduction of secondary wall thickening. In this network, expression of MYB46/83 and MYB58/63 is under the control of the NAC SECONDARY WALL-ASSOCIATED DOMAIN PROTEIN1 (NST1) and its close homologs NST2, VASCULAR-RELATED NAC-DOMAIN6 (VND6), and VND7 (Zhong and Ye, 2009; Zhou et al., 2009; Zhong et al., 2010). Zhong and Ye (2012) suggested that MYB32, MYB7, and MYB4 may be involved in fine tuning the regulation of lignin biosynthesis during secondary wall deposition. Based on microarray experiments, expression of MYB112 is neither affected by overexpression of the secondary wall NACs (SWNs; Zhong et al., 2010) nor by MYB46/83 (Ko et al., 2009), and GUS promoter activity assays revealed that MYB112 is mainly expressed in leaves and pollen, whereas SWNs and MYB46/83 factors are expressed in vascular tissue. It may, however, be that MYB112 acts as an SWN-independent regulator of lignin biosynthesis or the biosynthesis of other phenylpropanoids, leading to the observed pollen phenotype (Supplemental Fig. S3).
Further functional characterization of MYB7 and MYB32 as well as the identification of direct target genes of both TFs will be necessary to refine the MYB112 regulatory network.
Expression of MYB112 increases during senescence as well as upon salt and high light stress, conditions in which plants often receive more light energy than they can use for photochemical reactions. Excess light energy leads to photoinhibition and the formation of ROS. Anthocyanins absorb excess quanta, thereby protecting plant tissue from damaging light levels. High light activation of MYB112 expression may be regulated through TSP9 signaling. TSP9 is a thylakoid-anchored protein, which under high light, is phosphorylated and partially released from the membrane. In the absence of functional TSP9, high light-induced MYB112 expression is abolished (Fristedt et al., 2009).
In addition, transcriptome analysis revealed that the salinity stress-triggered up-regulation of MYB112 expression is strongly reduced (approximately 4- and approximately 20-fold in two independent experiments compared with the wild type) in transgenic plants overexpressing MYB44 (Jung et al., 2008). Notably, the expression of MYB32 and MYB7, the two direct MYB112 target genes identified here, was also lower in 35S:MYB44 than in wild-type plants during salt stress (Jung et al., 2008; E-ATMX-30), indicating that MYB44 negatively modulates MYB112 expression. In addition, expression of genes associated with anthocyanin biosynthesis, such as CHS, F3H, DFR, PAP1, and PAP2, is decreased in 35S:MYB44 plants upon salt stress (Jung et al., 2008; E-ATMX-30). Accordingly, 35S:MYB44 seedlings accumulate less anthocyanin than wild-type plants (Jung et al., 2010). Thus, with respect to anthocyanin accumulation, the phenotype of 35S:MYB112 seedlings (elevated anthocyanin) is opposite to that of 35S:MYB44 seedlings (low anthocyanin), whereas the myb112-1 mutant accumulates reduced levels of anthocyanin, like 35S:MYB44 (Figs. 3, 4, and 6). This finding provides further support of the negative regulation of MYB112 by MYB44. To verify the model, we created ESTR-inducible MYB44 overexpression (MYB44-IOE) plants, treated transgenic seedlings for 5 h with ESTR in the absence of salt stress, and then induced salt stress (150 mm NaCl) for 2 h (in the presence of ESTR). Using qRT-PCR, we detected a decrease of MYB112 expression in two of four tested lines (Supplemental Fig. S5), indicating that regulation of MYB112 expression by MYB44 may be indirect and may require additional factors, modulating this regulation.
Accumulation of anthocyanins has been reported to occur under a number of stresses, and the effects of nutrient (nitrogen or phosphorous) deprivation and light quality and quantity have been subject to the most attention in the past; several TFs have been shown to be involved in this response, including members of the MYB and bHLH families (Lea et al., 2007; Lillo et al., 2008; Stracke et al., 2010). However, although salinity stress is well known to lead to an accumulation of anthocyanin in a number of species, including crops and vegetables, such as tomato, maize, sugarcane, and many others (Kaliamoorthy and Rao, 1994; Piao et al., 2001; Wahid and Ghazanfar, 2006; Keutgen and Pawelzik, 2007; Roychoudhury et al., 2008; Matus et al., 2010), the regulatory mechanisms underlying this phenomena have not been studied intensively to date. Herein, we show that MYB112 expression is induced by salinity stress, whereas its expression is largely unaffected by nitrogen limitation, and sugar levels in contrast to many of the known transcriptional regulators of flavonoid/anthocyanin biosynthesis (Scheible et al., 2004; Misson et al., 2005; Lea et al., 2007; Morcuende et al., 2007; Lillo et al., 2008). We further illustrate that salinity-induced accumulation of anthocyanin is impaired in myb112 mutants but stimulated in 35S:MYB112 overexpressors, indicating that MYB112 plays a critical role during salt-induced anthocyanin accumulation. Further studies will, however, be required to reveal the details about the signaling pathways that control MYB112 expression during salinity and high light stress.
MATERIALS AND METHODS
General
Standard molecular techniques were performed as described (Sambrook and Russell, 2001; Skirycz et al., 2006). Primer sequences are given in Supplemental Table S3. For digital gene expression analyses, the online tools of GENEVESTIGATOR (https://www.genevestigator.com) and eFP browser (http://www.bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi) were used.
Plants
Arabidopsis (Arabidopsis thaliana) seedlings were grown on plates in a climate chamber with a 16-h day length provided by fluorescent light at 30 μmol m−2 s−1 and a temperature of 22°C. After 2 weeks, plants were transferred to soil and grown under controlled conditions in a greenhouse with a 16-h day length (120 μmol m−2 s−1), a 21°C /18°C day-night temperature cycle, and a 60%/75% day-night relative humidity cycle. The salt treatment experiments were performed as reported (Balazadeh et al., 2010a; Omidbakhshfard et al., 2012; Allu et al., 2014). For nitrogen depletion experiments, Arabidopsis (Col-0) seeds were surface sterilized. Plants were hydroponically grown on complete Hoagland medium containing 7 mm nitrate and 0.3 mm ammonium (+N medium). A set of plants was transferred to nitrogen-free medium (−N medium) at 19 d after sowing. After 7 d, the first two leaves that emerged after the cotyledons of 26-d-old plants (grown on +N and −N conditions) were harvested and frozen in liquid nitrogen for expression profiling (Balazadeh et al., 2014). The experiment was performed in three biological replicates, each replicate representing a pool of 24 plants.
Constructs
For ProMYB112:GUS, an approximately 1.3-kb 5′ MYB112 fragment was amplified by PCR from genomic Arabidopsis Col-0 DNA using primers MYB112:GUS-fwd. and MYB112:nested-rev., inserted into plasmid pCR2.1 (Invitrogen), reamplified by PCR using primers MYB112:GUS-fwd. and MYB112:GUS-rev., and inserted by EcoRI and NcoI sites into pCAMBIA1305.1-Hygromycin (CAMBIA). For 35S:MYB112, MYB112 open reading frame, amplified by PCR from Arabidopsis complementary DNA (cDNA) using primers MYB112-OE-fwd. and MYB112-OE-rev., was inserted into pCR2.1 and then cloned by added HindIII sites into pGreen0229-35S (Skirycz et al., 2006). For 35S:MYB112-GFP, MYB112 coding sequence was amplified by PCR from Arabidopsis cDNA using primers MYB112-fwd. and MYB112-rev. and inserted into pDONR201 (Invitrogen). Subsequently, the fragment was cloned into the GATEWAY-compatible vector pK7FWG2.0 (Plant Systems Biology, VIB, University of Ghent) by LR reaction. For MYB112-IOE, MYB112 coding region was amplified by PCR from Arabidopsis leaf cDNA using primers MYB112-IOE-fwd. and MYB112-IOE-rev., inserted into pCR2.1, and then cloned by XhoI and SpeI sites into pER8 vector (Zuo et al., 2000). Agrobacterium tumefaciens strain GV3101 (pMP90) was used for Arabidopsis (Col-0) transformations. For Pro:fLUC fusions, approximately 1.7-kb fragments upstream of the respective coding sequences of the genes MYB6, MYB7, MYB32, and CIPK18 (negative control) were amplified using PCR from Arabidopsis genomic DNA, ligated into the pENTR/D-TOPO vector (Invitrogen), and subsequently recombined into the GATEWAY-compatible vector pGWL7 (Licausi et al., 2011). For pTac:MYB112-LCELD6xHis, MYB112 sequence was amplified by PCR using primers MYB112-CELD-fwd. and MYB112-CELD-rev. and cloned into the pTacLCELD6His vector (Xue, 2002, 2005) by NheI and BamHI restriction sites.
myb112 T-DNA Insertion Lines
T-DNA insertion lines were obtained from the SALK (SALK_098029, myb112-2) or GABI-Kat (GK_093E05, myb112-1) collections. Homozygous plants were identified by PCR using the following primers: for SALK_098029, T-DNA left border primer LB, gene-specific primers SALK_LP and SALK_RP; and for GK_093E05, T-DNA left border primer LB, gene-specific primers GK_LP and GK_RP. MYB112 expression in the T-DNA insertion plants was examined by semi-qRT-PCR using primers annealing to the 5′ and 3′ ends of the MYB112 coding region.
ESTR Induction Experiments
Arabidopsis seedlings transformed with the MYB112-IOE construct were grown on solid MS and after 2 weeks, transferred to liquid MS supplemented with 10 μm ESTR for the indicated times. As controls, 0.1% (v/v) DMSO-treated MYB112-IOE lines or ESTR-treated empty vector plants were used.
Expression Profiling by qRT-PCR
Total RNA extraction, cDNA synthesis, and qRT-PCR were done as described (Caldana et al., 2007; Balazadeh et al., 2008; Wu et al., 2012). Gene expression was analyzed using the comparative Ct method. Experiments were performed using three biological replicates.
Microarray Experiments
Affymetrix ATH1 hybridizations were performed by ATLAS Biolabs. Expression of MYB112 was induced by 10 µm ESTR in 2-week-old seedlings grown in liquid MS at continuous light. Identically treated empty vector (pER8)-transformed seedlings and MYB112-IOE seedlings treated with DMSO served as controls. Seedlings were harvested after 1, 3, and 5 h, and RNA extracted from shoots was used for expression profiling. Expression data were submitted to the National Center for Biotechnology Information Gene Expression Omnibus repository (www.ncbi.nlm.nih.gov/geo/) under accession number GSE36721.
Transactivation Assays
Protoplasts were isolated from Arabidopsis Col-0 plants as described by Yoo et al. (2007) and transformed with 35S:MYB112 effector construct together with ProMYB32:fLUC, ProMYB7:fLUC, or ProMYB6:fLUC reporter constructs. For signal normalization, protoplasts were simultaneously transfected with 35S:rLUC plasmid. Luciferase activity was determined using the Dual-Luciferase Reporter Assay System (Promega) and a GloMax 20⁄20 Luminometer (Promega). Experiments were performed using four biological replicates.
DNA Binding Site Selection
Binding site selection was performed using the CELD system (Xue, 2005; Balazadeh et al., 2011) with a pTac:MYB112-LCELD6xHis construct using biotin-labeled double-stranded oligonucleotides containing a central 30-nucleotide random sequence. MYB112-selected oligonucleotides were cloned. The cloned oligonucleotides were verified for the presence of MYB112 binding motif by DNA binding activity assays and sequenced.
EMSA
MYB112-LCELD6xHis fusion protein was expressed in Escherichia coli strain XL1 Blue and purified using 1-mL nickel columns (GE Healthcare) coupled to an Äkta-Purifier FPLC System (GE Healthcare). EMSAs using 5′-DY682-labeled DNA fragments were performed as reported (Wu et al., 2012).
ChIP-qPCR
To investigate in vivo binding of MYB112 to its DNA binding site in the promoters of MYB32, MYB7, MYB6, and UGT84A2, we used ChIP-qPCR using whole shoots from long day-grown, 2-week-old Arabidopsis plants expressing GFP-tagged MYB112 protein from the CaMV 35S promoter (35S:MYB112-GFP). Wild-type plants were used as negative control. For ChIP, we followed a protocol previously described by Kaufmann et al. (2010) using anti-GFP antibody (µbeads; Miltenyi Biotec) to immunoprecipitate protein-DNA complexes. qPCR was used to test binding of MYB112 to its binding site within the selected promoters; the primers flanked the MYB112 binding site. As a negative control, we used primers annealing to promoter regions of two other Arabidopsis genes (At3g18040 and At2g22180) lacking an MYB112 binding site. Primer sequences are given in Supplemental Table S3. We analyzed ChIP-qPCR data relative to input, because these include normalization for both background levels and input chromatin going into the ChIP. The amount of genomic DNA coprecipitated by GFP antibody (ChIP signal) was calculated compared with the total input DNA used for each immunoprecipitation in the following way: cycle threshold (CT) = CT(ChIP) − CT(input). To calculate fold enrichment, normalized ChIP signals were compared between 35S:MYB112-GFP and wild-type plants, where the ChIP signal is given as the fold increase in signal relative to the background signal. Experiments were performed in three biological replicates.
Spectrophotometric Detection of Anthocyanins
To determinate the content of total anthocyanin, 30 mg of frozen plant tissue was ground in liquid nitrogen, mixed vigorously with 1 mL of extraction buffer (1% [v/v] HCl, 18% [v/v] 1-propanol, and 81% [v/v] water), and incubated at 98°C for 3 min. Samples were then incubated at room temperature in darkness for 10 to 12 h. Afterward, samples were centrifuged at 13,200g at room temperature for 20 min. For calculation, the following formula was used: ([optical density535 − 0.25 optical density650] × total volume of the extract (mL)/[ weight of the dry leaf tissue (g) × 1,000]). As a blank, extraction buffer was used.
LC-MS Analysis of Anthocyanins
Anthocyanins and flavanols were extracted and analyzed exactly as described previously (Tohge and Fernie, 2010). Metabolites were evaluated by peak area of parental ion peaks processed using Xcalibur 2.1 software (Thermo Fisher Scientific). Obtained relative peak area was normalized by internal standard (isovitexin; CAS29702-25-8) and fresh weight.
Other Methods
Histochemical GUS assays were performed as described by Plesch et al. (2001). Pollen viability was analyzed using Stereomicroscope MZ 12.5 (Leica) after Alexander staining (Alexander, 1969). Cellular location of MYB112-GFP fusion protein was analyzed by confocal laser fluorescence microscopy (Eclipse E600 Microscope; Nikon).
Statistical Analyses
Unless otherwise specified, statistical analyses were performed using Student’s t test embedded in the Microsoft Excel software. Only the return of P < 0.05 was designated as statistically significant.
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: ACTIN2 (At3g18780), MYB6 (At4g09460), MYB7 (At2g16720), MYB32 (At4g34990), MYB112 (At1g48000), At5MaT (At3g29590), UGT79B1 (A3G2″XT; At5g54060), UGT84A2 (SGT; At3g21560), UGT75C1 (A5GT; At4g14090), and TT19 (GLUTATHIONE S-TRANSFERASE26 and GLUTATHIONE S-TRANSFERASE PHI12; At5g17220). Additional accession numbers are given in Supplemental Table S3 and Supplemental Data Sets S1 and S2.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Integrated transcriptomics and metabolomics data.
Supplemental Figure S2. MYB112 expression in MYB112-IOE plants after 1, 3, and 5 h of induction with ESTR.
Supplemental Figure S3. MYB112 affects pollen viability.
Supplemental Figure S4. MYB112 expression level in MYB112 overexpression lines.
Supplemental Figure S5. Negative regulation of MYB112 expression by MYB44.
Supplemental Table S1. MYB112-dependent up-regulated genes.
Supplemental Table S2. MYB112-dependent down-regulated genes.
Supplemental Table S3. Oligonucleotide sequences.
Supplemental Data Set S1. Expression of 94 secondary metabolite-associated genes in MYB112-IOE plants induced with ESTR for 5 d compared with mock-treated seedlings and in myb112-1 seedlings compared with the wild type.
Supplemental Data Set S2. ATH1-based gene expression profiling upon short-term MYB112 induction compared with mock-treated MYB112-IOE seedlings.
Supplemental Material S1. MYB112 binding sites in target promoters.
Supplementary Material
Acknowledgments
We thank Amin Omidbakhshfard (University of Potsdam, Germany) and Annapurna Devi Allu (Max-Planck Institute of Molecular Plant Physiology, Potsdam-Golm, Germany) for providing cDNAs from salt-treated Arabidopsis plants; Karin Koehl and team (Max-Planck Institute of Molecular Plant Physiology) for plant care; Eugenia Maximova (Max-Planck Institute of Molecular Plant Physiology) for help with microscopy; Josef Bergstein (Max Planck Institute of Molecular Plant Physiology) for photographic work; Alexander V. Vener (Linköping University, Sweden) for providing tsp9 mutant seeds; Klaus Humbeck (University of Halle, Germany) for providing samples from nitrogen deprivation-stressed plants; and the European Arabidopsis Stock Centre for providing seeds of T-DNA insertion lines.
Glossary
- cDNA
complementary DNA
- ChIP
chromatin immunoprecipitation
- Col-0
Columbia-0
- CT
cycle threshold
- DMSO
dimethyl sulfoxide
- ESTR
estradiol
- LC-MS
liquid chromatography coupled to mass spectrometry
- MS
Murashige and Skoog medium
- qPCR
quantitative PCR
- qRT
quantitative real-time reverse transcription
- ROS
reactive oxygen species
- T-DNA
transfer DNA
- TF
transcription factor
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. FOR 948; MU 1199/14–1), the Bundesministerium für Bildung und Forschung (GoFORSYS Research Unit for Systems Biology Grant no. FKZ 0313924), and the European Union (Research Training Network Vacuolar Transport Equipment for Growth Regulation in Plants Grant no. MRTN–CT–2006–035833).
Articles can be viewed without a subscription.
References
- Albert NW, Lewis DH, Zhang H, Irving LJ, Jameson PE, Davies KM (2009) Light-induced vegetative anthocyanin pigmentation in Petunia. J Exp Bot 60: 2191–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander MP. (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44: 117–122 [DOI] [PubMed] [Google Scholar]
- Allan AC, Hellens RP, Laing WA (2008) MYB transcription factors that colour our fruit. Trends Plant Sci 13: 99–102 [DOI] [PubMed] [Google Scholar]
- Allu AD, Soja AM, Wu A, Szymanski J, Balazadeh S (2014) Salt stress and senescence: identification of cross-talk regulatory components. J Exp Bot 65: 3993–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma A, Yakushiji H, Koshita Y, Kobayashi S (2012) Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta 236: 1067–1080 [DOI] [PubMed] [Google Scholar]
- Balazadeh S, Kwasniewski M, Caldana C, Mehrnia M, Zanor MI, Xue GP, Mueller-Roeber B (2011) ORS1, an H₂O₂-responsive NAC transcription factor, controls senescence in Arabidopsis thaliana. Mol Plant 4: 346–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazadeh S, Riaño-Pachón DM, Mueller-Roeber B (2008) Transcription factors regulating leaf senescence in Arabidopsis thaliana. Plant Biol (Stuttg) (Suppl 1) 10: 63–75 [DOI] [PubMed] [Google Scholar]
- Balazadeh S, Schildhauer J, Araújo WL, Munné-Bosch S, Fernie AR, Proost S, Humbeck K, Mueller-Roeber B (2014) Reversal of senescence by N resupply to N-starved Arabidopsis thaliana: transcriptomic and metabolomic consequences. J Exp Bot 65: 3975–3992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazadeh S, Siddiqui H, Allu AD, Matallana-Ramirez LP, Caldana C, Mehrnia M, Zanor MI, Köhler B, Mueller-Roeber B (2010a) A gene regulatory network controlled by the NAC transcription factor ANAC092/AtNAC2/ORE1 during salt-promoted senescence. Plant J 62: 250–264 [DOI] [PubMed] [Google Scholar]
- Balazadeh S, Wu A, Mueller-Roeber B (2010b) Salt-triggered expression of the ANAC092-dependent senescence regulon in Arabidopsis thaliana. Plant Signal Behav 5: 733–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry A, Caboche M, Lepiniec L (2006) TT8 controls its own expression in a feedback regulation involving TTG1 and homologous MYB and bHLH factors, allowing a strong and cell-specific accumulation of flavonoids in Arabidopsis thaliana. Plant J 46: 768–779 [DOI] [PubMed] [Google Scholar]
- Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C (2000) Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 12: 2383–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeze E, Harrison E, McHattie S, Hughes L, Hickman R, Hill C, Kiddle S, Kim YS, Penfold CA, Jenkins D, et al. (2011) High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 23: 873–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broun P. (2005) Transcriptional control of flavonoid biosynthesis: a complex network of conserved regulators involved in multiple aspects of differentiation in Arabidopsis. Curr Opin Plant Biol 8: 272–279 [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V, Page T, Harrison E, Breeze E, Lim PO, Nam HG, Lin JF, Wu SH, Swidzinski J, Ishizaki K, et al. (2005) Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J 42: 567–585 [DOI] [PubMed] [Google Scholar]
- Burbulis IE, Iacobucci M, Shirley BW (1996) A null mutation in the first enzyme of flavonoid biosynthesis does not affect male fertility in Arabidopsis. Plant Cell 8: 1013–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldana C, Scheible WR, Mueller-Roeber B, Ruzicic S (2007) A quantitative RT-PCR platform for high-throughput expression profiling of 2500 rice transcription factors. Plant Methods 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candela H, Martínez-Laborda A, Micol JL (1999) Venation pattern formation in Arabidopsis thaliana vegetative leaves. Dev Biol 205: 205–216 [DOI] [PubMed] [Google Scholar]
- Castellarin SD, Pfeiffer A, Sivilotti P, Degan M, Peterlunger E, DI Gaspero G (2007) Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ 30: 1381–1399 [DOI] [PubMed] [Google Scholar]
- Christie PJ, Alfenito MR, Walbot V (1994) Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta 194: 541–549 [Google Scholar]
- Cominelli E, Gusmaroli G, Allegra D, Galbiati M, Wade HK, Jenkins GI, Tonelli C (2008) Expression analysis of anthocyanin regulatory genes in response to different light qualities in Arabidopsis thaliana. J Plant Physiol 165: 886–894 [DOI] [PubMed] [Google Scholar]
- de Vetten N, Quattrocchio F, Mol J, Koes R (1997) The an11 locus controlling flower pigmentation in petunia encodes a novel WD-repeat protein conserved in yeast, plants, and animals. Genes Dev 11: 1422–1434 [DOI] [PubMed] [Google Scholar]
- Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7: 1085–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos C, Le Gourrierec J, Baudry A, Huep G, Lanet E, Debeaujon I, Routaboul JM, Alboresi A, Weisshaar B, Lepiniec L (2008) MYBL2 is a new regulator of flavonoid biosynthesis in Arabidopsis thaliana. Plant J 55: 940–953 [DOI] [PubMed] [Google Scholar]
- Falcone Ferreyra ML, Rius SP, Casati P (2012) Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci 3: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyissa DN, Løvdal T, Olsen KM, Slimestad R, Lillo C (2009) The endogenous GL3, but not EGL3, gene is necessary for anthocyanin accumulation as induced by nitrogen depletion in Arabidopsis rosette stage leaves. Planta 230: 747–754 [DOI] [PubMed] [Google Scholar]
- Fornalé S, Lopez E, Salazar-Henao JE, Fernández-Nohales P, Rigau J, Caparros-Ruiz D (2014) AtMYB7, a new player in the regulation of UV-sunscreens in Arabidopsis thaliana. Plant Cell Physiol 55: 507–516 [DOI] [PubMed] [Google Scholar]
- Fristedt R, Carlberg I, Zygadlo A, Piippo M, Nurmi M, Aro EM, Scheller HV, Vener AV (2009) Intrinsically unstructured phosphoprotein TSP9 regulates light harvesting in Arabidopsis thaliana. Biochemistry 48: 499–509 [DOI] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Zhao M, Leavitt JM, Lloyd AM (2008) Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J 53: 814–827 [DOI] [PubMed] [Google Scholar]
- Gould KS. (2004) Nature’s Swiss Army Knife: the diverse protective roles of anthocyanins in leaves. J Biomed Biotechnol 2004: 314–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould KS, Neill SO, Vogelmann TC (2002) A unified explanation for anthocyanins in leaves? Adv Bot Res 37: 167–192 [Google Scholar]
- Hansson M, Dupuis T, Strömquist R, Andersson B, Vener AV, Carlberg I (2007) The mobile thylakoid phosphoprotein TSP9 interacts with the light-harvesting complex II and the peripheries of both photosystems. J Biol Chem 282: 16214–16222 [DOI] [PubMed] [Google Scholar]
- Hichri I, Barrieu F, Bogs J, Kappel C, Delrot S, Lauvergeat V (2011) Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J Exp Bot 62: 2465–2483 [DOI] [PubMed] [Google Scholar]
- Jin H, Cominelli E, Bailey P, Parr A, Mehrtens F, Jones J, Tonelli C, Weisshaar B, Martin C (2000) Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis. EMBO J 19: 6150–6161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C, Seo JS, Han SW, Koo YJ, Kim CH, Song SI, Nahm BH, Choi YD, Cheong JJ (2008) Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol 146: 623–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C, Shim JS, Seo JS, Lee HY, Kim CH, Choi YD, Cheong JJ (2010) Non-specific phytohormonal induction of AtMYB44 and suppression of jasmonate-responsive gene activation in Arabidopsis thaliana. Mol Cells 29: 71–76 [DOI] [PubMed] [Google Scholar]
- Kaliamoorthy S, Rao AS (1994) Effect of salinity on anthocyanin accumulation in the root of maize. Indian J Plant Physiol 37: 169–170 [Google Scholar]
- Kaufmann K, Muiño JM, Østerås M, Farinelli L, Krajewski P, Angenent GC (2010) Chromatin immunoprecipitation (ChIP) of plant transcription factors followed by sequencing (ChIP-SEQ) or hybridization to whole genome arrays (ChIP-CHIP). Nat Protoc 5: 457–472 [DOI] [PubMed] [Google Scholar]
- Keutgen AJ, Pawelzik E (2007) Modifications of strawberry fruit antioxidant pools and fruit quality under NaCl stress. J Agric Food Chem 55: 4066–4072 [DOI] [PubMed] [Google Scholar]
- Kim WC, Ko JH, Han KH (2012) Identification of a cis-acting regulatory motif recognized by MYB46, a master transcriptional regulator of secondary wall biosynthesis. Plant Mol Biol 78: 489–501 [DOI] [PubMed] [Google Scholar]
- Kitamura S, Shikazono N, Tanaka A (2004) TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J 37: 104–114 [DOI] [PubMed] [Google Scholar]
- Ko JH, Kim WC, Han KH (2009) Ectopic expression of MYB46 identifies transcriptional regulatory genes involved in secondary wall biosynthesis in Arabidopsis. Plant J 60: 649–665 [DOI] [PubMed] [Google Scholar]
- Koes R, Verweij W, Quattrocchio F (2005) Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci 10: 236–242 [DOI] [PubMed] [Google Scholar]
- Lea US, Slimestad R, Smedvig P, Lillo C (2007) Nitrogen deficiency enhances expression of specific MYB and bHLH transcription factors and accumulation of end products in the flavonoid pathway. Planta 225: 1245–1253 [DOI] [PubMed] [Google Scholar]
- Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, Caboche M (2006) Genetics and biochemistry of seed flavonoids. Annu Rev Plant Biol 57: 405–430 [DOI] [PubMed] [Google Scholar]
- Licausi F, Weits DA, Pant BD, Scheible WR, Geigenberger P, van Dongen JT (2011) Hypoxia responsive gene expression is mediated by various subsets of transcription factors and miRNAs that are determined by the actual oxygen availability. New Phytol 190: 442–456 [DOI] [PubMed] [Google Scholar]
- Lillo C, Lea US, Ruoff P (2008) Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ 31: 587–601 [DOI] [PubMed] [Google Scholar]
- Luo J, Nishiyama Y, Fuell C, Taguchi G, Elliott K, Hill L, Tanaka Y, Kitayama M, Yamazaki M, Bailey P, et al. (2007) Convergent evolution in the BAHD family of acyl transferases: identification and characterization of anthocyanin acyl transferases from Arabidopsis thaliana. Plant J 50: 678–695 [DOI] [PubMed] [Google Scholar]
- Matsui K, Umemura Y, Ohme-Takagi M (2008) AtMYBL2, a protein with a single MYB domain, acts as a negative regulator of anthocyanin biosynthesis in Arabidopsis. Plant J 55: 954–967 [DOI] [PubMed] [Google Scholar]
- Matus JT, Aquea F, Arce-Johnson P (2008) Analysis of the grape MYB R2R3 subfamily reveals expanded wine quality-related clades and conserved gene structure organization across Vitis and Arabidopsis genomes. BMC Plant Biol 8: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus JT, Poupin MJ, Cañón P, Bordeu E, Alcalde JA, Arce-Johnson P (2010) Isolation of WDR and bHLH genes related to flavonoid synthesis in grapevine (Vitis vinifera L.). Plant Mol Biol 72: 607–620 [DOI] [PubMed] [Google Scholar]
- Mehrtens F, Kranz H, Bednarek P, Weisshaar B (2005) The Arabidopsis transcription factor MYB12 is a flavonol-specific regulator of phenylpropanoid biosynthesis. Plant Physiol 138: 1083–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33: 453–467 [DOI] [PubMed] [Google Scholar]
- Misson J, Raghothama KG, Jain A, Jouhet J, Block MA, Bligny R, Ortet P, Creff A, Somerville S, Rolland N, et al. (2005) A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc Natl Acad Sci USA 102: 11934–11939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcuende R, Bari R, Gibon Y, Zheng W, Pant BD, Bläsing O, Usadel B, Czechowski T, Udvardi MK, Stitt M, et al. (2007) Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant Cell Environ 30: 85–112 [DOI] [PubMed] [Google Scholar]
- Mouradov A, Spangenberg G (2014) Flavonoids: a metabolic network mediating plants adaptation to their real estate. Front Plant Sci 5: 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R. (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25: 239–250 [DOI] [PubMed] [Google Scholar]
- Nagata T, Todoriki S, Masumizu T, Suda I, Furuta S, Du Z, Kikuchi S (2003) Levels of active oxygen species are controlled by ascorbic acid and anthocyanin in Arabidopsis. J Agric Food Chem 51: 2992–2999 [DOI] [PubMed] [Google Scholar]
- Noh YS, Amasino RM (1999) Identification of a promoter region responsible for the senescence-specific expression of SAG12. Plant Mol Biol 41: 181–194 [DOI] [PubMed] [Google Scholar]
- Omidbakhshfard MA, Omranian N, Ahmadi FS, Nikoloski Z, Mueller-Roeber B (2012) Effect of salt stress on genes encoding translation-associated proteins in Arabidopsis thaliana. Plant Signal Behav 7: 1095–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuna D, Usadel B, Morcuende R, Gibon Y, Bläsing OE, Höhne M, Günter M, Kamlage B, Trethewey R, Scheible WR, et al. (2007) Temporal responses of transcripts, enzyme activities and metabolites after adding sucrose to carbon-deprived Arabidopsis seedlings. Plant J 49: 463–491 [DOI] [PubMed] [Google Scholar]
- Petroni K, Tonelli C (2011) Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci 181: 219–229 [DOI] [PubMed] [Google Scholar]
- Piao HL, Lim JH, Kim SJ, Cheong GW, Hwang I (2001) Constitutive over-expression of AtGSK1 induces NaCl stress responses in the absence of NaCl stress and results in enhanced NaCl tolerance in Arabidopsis. Plant J 27: 305–314 [DOI] [PubMed] [Google Scholar]
- Plesch G, Ehrhardt T, Mueller-Roeber B (2001) Involvement of TAAAG elements suggests a role for Dof transcription factors in guard cell-specific gene expression. Plant J 28: 455–464 [DOI] [PubMed] [Google Scholar]
- Pourcel L, Routaboul JM, Cheynier V, Lepiniec L, Debeaujon I (2007) Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends Plant Sci 12: 29–36 [DOI] [PubMed] [Google Scholar]
- Preston J, Wheeler J, Heazlewood J, Li SF, Parish RW (2004) AtMYB32 is required for normal pollen development in Arabidopsis thaliana. Plant J 40: 979–995 [DOI] [PubMed] [Google Scholar]
- Ramsay NA, Glover BJ (2005) MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci 10: 63–70 [DOI] [PubMed] [Google Scholar]
- Roychoudhury A, Basu S, Sarkar SN, Sengupta DN (2008) Comparative physiological and molecular responses of a common aromatic indica rice cultivar to high salinity with non-aromatic indica rice cultivars. Plant Cell Rep 27: 1395–1410 [DOI] [PubMed] [Google Scholar]
- Rubin G, Tohge T, Matsuda F, Saito K, Scheible WR (2009) Members of the LBD family of transcription factors repress anthocyanin synthesis and affect additional nitrogen responses in Arabidopsis. Plant Cell 21: 3567–3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell DW (2001) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, New York [Google Scholar]
- Scheible WR, Morcuende R, Czechowski T, Fritz C, Osuna D, Palacios-Rojas N, Schindelasch D, Thimm O, Udvardi MK, Stitt M (2004) Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol 136: 2483–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwinn KE, Boase MR, Bradley JM, Lewis DH, Deroles SC, Martin CR, Davies KM (2014) MYB and bHLH transcription factor transgenes increase anthocyanin pigmentation in petunia and lisianthus plants, and the petunia phenotypes are strongly enhanced under field conditions. Front Plant Sci 5: 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Park E, Choi G (2007) PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J 49: 981–994 [DOI] [PubMed] [Google Scholar]
- Sinlapadech T, Stout J, Ruegger MO, Deak M, Chapple C (2007) The hyper-fluorescent trichome phenotype of the brt1 mutant of Arabidopsis is the result of a defect in a sinapic acid: UDPG glucosyltransferase. Plant J 49: 655–668 [DOI] [PubMed] [Google Scholar]
- Skirycz A, Reichelt M, Burow M, Birkemeyer C, Rolcik J, Kopka J, Zanor MI, Gershenzon J, Strnad M, Szopa J, et al. (2006) DOF transcription factor AtDof1.1 (OBP2) is part of a regulatory network controlling glucosinolate biosynthesis in Arabidopsis. Plant J 47: 10–24 [DOI] [PubMed] [Google Scholar]
- Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P (2006) Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140: 637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R, Favory JJ, Gruber H, Bartelniewoehner L, Bartels S, Binkert M, Funk M, Weisshaar B, Ulm R (2010) The Arabidopsis bZIP transcription factor HY5 regulates expression of the PFG1/MYB12 gene in response to light and ultraviolet-B radiation. Plant Cell Environ 33: 88–103 [DOI] [PubMed] [Google Scholar]
- Stracke R, Ishihara H, Huep G, Barsch A, Mehrtens F, Niehaus K, Weisshaar B (2007) Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J 50: 660–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R, Werber M, Weisshaar B (2001) The R2R3-MYB gene family in Arabidopsis thaliana. Curr Opin Plant Biol 4: 447–456 [DOI] [PubMed] [Google Scholar]
- Takos AM, Jaffé FW, Jacob SR, Bogs J, Robinson SP, Walker AR (2006) Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol 142: 1216–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng S, Keurentjes J, Bentsink L, Koornneef M, Smeekens S (2005) Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol 139: 1840–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohge T, Fernie AR (2010) Combining genetic diversity, informatics and metabolomics to facilitate annotation of plant gene function. Nat Protoc 5: 1210–1227 [DOI] [PubMed] [Google Scholar]
- Tohge T, Nishiyama Y, Hirai MY, Yano M, Nakajima J, Awazuhara M, Inoue E, Takahashi H, Goodenowe DB, Kitayama M, et al. (2005) Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over-expressing an MYB transcription factor. Plant J 42: 218–235 [DOI] [PubMed] [Google Scholar]
- Wade HK, Bibikova TN, Valentine WJ, Jenkins GI (2001) Interactions within a network of phytochrome, cryptochrome and UV-B phototransduction pathways regulate chalcone synthase gene expression in Arabidopsis leaf tissue. Plant J 25: 675–685 [DOI] [PubMed] [Google Scholar]
- Wahid A, Ghazanfar A (2006) Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J Plant Physiol 163: 723–730 [DOI] [PubMed] [Google Scholar]
- Walker AR, Davison PA, Bolognesi-Winfield AC, James CM, Srinivasan N, Blundell TL, Esch JJ, Marks MD, Gray JC (1999) The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell 11: 1337–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel-Shirley B. (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126: 485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel-Shirley B. (2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5: 218–223 [DOI] [PubMed] [Google Scholar]
- Wu A, Allu AD, Garapati P, Siddiqui H, Dortay H, Zanor MI, Asensi-Fabado MA, Munné-Bosch S, Antonio C, Tohge T, et al. (2012) JUNGBRUNNEN1, a reactive oxygen species-responsive NAC transcription factor, regulates longevity in Arabidopsis. Plant Cell 24: 482–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue GP. (2002) Characterisation of the DNA-binding profile of barley HvCBF1 using an enzymatic method for rapid, quantitative and high-throughput analysis of the DNA-binding activity. Nucleic Acids Res 30: e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue GP. (2005) A CELD-fusion method for rapid determination of the DNA-binding sequence specificity of novel plant DNA-binding proteins. Plant J 41: 638–649 [DOI] [PubMed] [Google Scholar]
- Yonekura-Sakakibara K, Fukushima A, Nakabayashi R, Hanada K, Matsuda F, Sugawara S, Inoue E, Kuromori T, Ito T, Shinozaki K, et al. (2012) Two glycosyltransferases involved in anthocyanin modification delineated by transcriptome independent component analysis in Arabidopsis thaliana. Plant J 69: 154–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zeng XQ, Chow WS, Su LJ, Peng XX, Peng CL (2010) Protective effect of supplemental anthocyanins on Arabidopsis leaves under high light. Physiol Plant 138: 215–225 [DOI] [PubMed] [Google Scholar]
- Zhang F, Gonzalez A, Zhao M, Payne CT, Lloyd A (2003) A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development 130: 4859–4869 [DOI] [PubMed] [Google Scholar]
- Zhong R, Lee C, Ye ZH (2010) Global analysis of direct targets of secondary wall NAC master switches in Arabidopsis. Mol Plant 3: 1087–1103 [DOI] [PubMed] [Google Scholar]
- Zhong R, Ye ZH (2009) Transcriptional regulation of lignin biosynthesis. Plant Signal Behav 4: 1028–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Ye ZH (2012) MYB46 and MYB83 bind to the SMRE sites and directly activate a suite of transcription factors and secondary wall biosynthetic genes. Plant Cell Physiol 53: 368–380 [DOI] [PubMed] [Google Scholar]
- Zhou J, Lee C, Zhong R, Ye ZH (2009) MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabidopsis. Plant Cell 21: 248–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu HF, Fitzsimmons K, Khandelwal A, Kranz RG (2009) CPC, a single-repeat R3 MYB, is a negative regulator of anthocyanin biosynthesis in Arabidopsis. Mol Plant 2: 790–802 [DOI] [PubMed] [Google Scholar]
- Zhu JK. (2001) Plant salt tolerance. Trends Plant Sci 6: 66–71 [DOI] [PubMed] [Google Scholar]
- Zimmermann IM, Heim MA, Weisshaar B, Uhrig JF (2004) Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. Plant J 40: 22–34 [DOI] [PubMed] [Google Scholar]
- Zuo J, Niu QW, Chua NH (2000) Technical advance: an estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J 24: 265–273 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.