Axial shoot-to-root and radial cell-to-cell transport of jasmonates in leaves plays a major role in the Arabidopsis wound response.
Abstract
Jasmonates are oxygenated lipids (oxylipins) that control defense gene expression in response to cell damage in plants. How mobile are these potent mediators within tissues? Exploiting a series of 13-lipoxygenase (13-lox) mutants in Arabidopsis (Arabidopsis thaliana) that displays impaired jasmonic acid (JA) synthesis in specific cell types and using JA-inducible reporters, we mapped the extent of the transport of endogenous jasmonates across the plant vegetative growth phase. In seedlings, we found that jasmonate (or JA precursors) could translocate axially from wounded shoots to unwounded roots in a LOX2-dependent manner. Grafting experiments with the wild type and JA-deficient mutants confirmed shoot-to-root oxylipin transport. Next, we used rosettes to investigate radial cell-to-cell transport of jasmonates. After finding that the LOX6 protein localized to xylem contact cells was not wound inducible, we used the lox234 triple mutant to genetically isolate LOX6 as the only JA precursor-producing LOX in the plant. When a leaf of this mutant was wounded, the JA reporter gene was expressed in distal leaves. Leaf sectioning showed that JA reporter expression extended from contact cells throughout the vascular bundle and into extravascular cells, revealing a radial movement of jasmonates. Our results add a crucial element to a growing picture of how the distal wound response is regulated in rosettes, showing that both axial (shoot-to-root) and radial (cell-to-cell) transport of oxylipins plays a major role in the wound response. The strategies developed herein provide unique tools with which to identify intercellular jasmonate transport routes.
Both animals and plants produce potently active lipid-derived mediators in response to wounding. These oxylipins (oxygenated lipid derivatives) include leukotrienes and prostaglandins in animals (Funk, 2001) and jasmonates in plants (Wasternack and Hause, 2013). Although these regulators frequently show structural similarities (many are cyclopentenone and cyclopentanone lipids), they operate through different signaling pathways often involving large protein complexes. For example, prostaglandins signal in part through G protein-coupled receptor complexes (Furuyashiki and Narumiya, 2011; Kalinski, 2012), and plant jasmonate signaling operates through the Skp/Cullin/F-box CORONATINE INSENSITIVE1 complex (Browse, 2009). Many oxylipins produced in response to tissue damage in metazoans act as paracrine signals to elicit defense responses in distal undamaged cells (Funk, 2001). Similarly, it is possible that jasmonates, including the biologically active derivative jasmonoyl-Ile (JA-Ile; Fonseca et al., 2009), might be transported from cell to cell in plants. However, to date, the majority of studies on oxylipin transport in plants have used exogenous jasmonates, and it remains unclear to what extent these compounds are transported between cells and tissues when produced endogenously.
Based on the fact that jasmonic acid (JA) or methyl jasmonate treatments can affect defense gene expression at a distance to the sites of their application, JA was proposed to operate as a paracrine signal capable of being transported from cell to cell in tomato (Solanum lycopersicum) leaves (Farmer et al., 1992). Similar conclusions were drawn for JA in wild tobacco (Nicotiana sylvestris; Zhang and Baldwin, 1997). Isotope-labeling experiments using exogenous jasmonates have indicated JA/JA-Ile transport away from the site of application to distal tissues and even distal organs (Zhang and Baldwin, 1997; Thorpe et al., 2007; Sato et al., 2011). Additionally, grafting experiments in tomato were consistent with long-distance transport of JA/JA precursors (Li et al., 2002; Schilmiller and Howe, 2005), although other studies did not find evidence for JA transport from wounded leaves to distal unwounded leaves (Strassner et al., 2002). Concerning Arabidopsis (Arabidopsis thaliana), Koo et al. (2009) concluded that JA-Ile accumulation detected in leaves distal to the wound site resulted mainly from de novo synthesis in undamaged leaves rather than from the transport of JA/JA-Ile from the wound site. Recently, a transporter (GLUCOSINOLATE TRANSPORTER1) capable of importing JA-Ile (but not JA) into Xenopus laevis oocytes has been described (Saito et al., 2015), further supporting the possibility that jasmonates move between cells.
In addition to the transport of jasmonates, there is much evidence consistent with other wound signaling mechanisms that lead to JA synthesis and JA-mediated defense responses at various distances from wounds. That is, wound-activated signaling pathways can be classified as those working near the damage site (i.e. local responses) and those operating distal to it (Rhodes et al., 2006; Wu et al., 2007). Both these types of wound responses can be difficult to study, because several types of events (including the transport of jasmonates) may contribute to JA signaling. However, there has been some progress in understanding long-distance signaling leading to distal wound responses. These mechanisms include electrical and potentially, hydraulic signaling (for review, see Koo and Howe, 2009; Farmer et al., 2014). Membrane hyperpolarizations have been recorded in wounded plants (Zimmermann et al., 2009); however, their relationship with JA synthesis or JA responses has not yet been reported. In Arabidopsis, wounding of adult-phase rosettes stimulates the leaf-to-leaf propagation of signals that (1) can be detected with surface electrodes as cell membrane depolarizations; (2) are propagated from leaf to leaf in a mechanism that requires several clade 3 GLUTAMATE RECEPTOR-LIKE (GLR) genes, including GLR3.3 and GLR3.6; and (3) can induce JA and JA-Ile accumulation in distal unwounded sites (Mousavi et al., 2013). However, even when electrical signals were compromised in both the wounded and distal leaves of a glr3.3 glr3.6 double mutant, JA responses were affected only in the distal leaf; local responses in the damaged leaf itself were similar to the wild type (Mousavi et al., 2013). Therefore, certain clade 3 GLRs operate in rosette-stage plants to extend the range of the wound response, and even if these genes are mutated, wounded rosette leaves still produce jasmonates. In summary, both jasmonates made near wounds and jasmonates made far from wounds in response to distal signals might be subject to transport within the plant.
This study focused on the mobility of endogenous jasmonates produced in response to wounding. Here, we ask: how mobile are endogenous jasmonates generated in aboveground tissues in response to wounding? Our analysis was conducted throughout the vegetative phase and included different tissues that ranged from embryonic leaves (cotyledons) and roots to expanded rosette leaves. We investigated whether a glr3.3 glr3.6 double mutant that reduces leaf-to-leaf signal propagation in the adult phase (Mousavi et al., 2013) could also reduce cotyledon-to-root wound signaling in seedlings. Results from these electrophysiology experiments then led us to investigate whether JA (or JA precursors) can translocate from wounded cotyledons to roots. To do this, we used two approaches. One was based on mutants in 13-LIPOXYGENASEs (13-LOXs) that are necessary for an early step in the synthesis of the JA precursor oxophytodienoic acid. All four 13-LOXs in Arabidopsis (LOX2, LOX3, LOX4, and LOX6) are known to contribute to JA synthesis in vivo (Chauvin et al., 2013). First, LOX2 is responsible for the synthesis of a large pool of JA in wounded leaves (Bell et al., 1995), and it also produces precursors for the synthesis of arabidopside defense-related metabolites (Glauser et al., 2009). Second, LOX3 and LOX4 act together to produce the JA required for full male fertility (Caldelari et al., 2011). Third, LOX6 produces jasmonates in roots that are first separated from aerial tissues and then wounded (Grebner et al., 2013). We tested the impact of mutations in the different 13-LOXs on root JA signaling after cotyledon wounding. This was followed by grafting experiments between the wild type and the JA-deficient mutant allene oxide synthase (aos; Park et al., 2002) to test whether JA (or JA precursors) could translocate axially from wounded shoots into undamaged roots.
In addition to its role in wounded roots (Grebner et al., 2013), LOX6 has been implicated in long-distance wound signaling in the adult-phase rosette, where it is necessary for most of the rapid distal expression of the JA-responsive gene JASMONATE ZIM-DOMAIN10 (JAZ10) when another leaf is wounded (Chauvin et al., 2013). This and the fact that the LOX6 promoter is active principally in xylem contact cells (Chauvin et al., 2013) provided us with the opportunity to investigate oxylipin transport within leaves. We confirmed the cellular localization of the LOX6 polypeptide with a LOX6-GUS fusion protein. We then used a lox234 triple mutant expressing a JAZ10 reporter to test whether jasmonates could be exported from xylem contact cells. These experiments led to unique insights into the transport of jasmonates across different leaf cell layers.
RESULTS
GLR3.3/3.6 Are Not Necessary for Cotyledon-to-Root Jasmonate Signaling in Seedlings
In the adult rosette phase, functional clade 3 GLR genes are necessary for efficient leaf-to-leaf signal propagation, leading to JA accumulation in distal unwounded leaves on wounded plants (Mousavi et al., 2013). To test whether the same GLRs are also involved in cotyledon-to-root wound signaling in seedlings, we compared basal and wound-induced levels of the early wound response JA marker JAZ10 (Acosta et al., 2013) between the wild type and the glr3.3 glr3.6 double mutant. The double mutant had no significant impact on basal or wound-inducible JAZ10 levels in aerial or root tissues after cotyledon wounding (Supplemental Fig. S1). To test whether cotyledon wounding or laser stimulation was able to induce surface electrical potential changes in undamaged cotyledons and roots of stimulated 5-d-old seedlings, we developed a unique electrophysiology protocol (Supplemental Fig. S2A). In agreement with Acosta et al. (2013), puncturing one cotyledon with a needle induced a strong activity of the secretable JAZ10p:GUSPlussec (JGP) reporter in the wounded cotyledon as well as in the root compared with control unwounded plants (Supplemental Fig. S2, B and C). In contrast, the stimulation of cotyledons with a high-energy laser did not induce a visible JGP reporter expression (Supplemental Fig. S2D). Moreover, under the experimental conditions tested, we could not detect differences in wound-activated surface potential (WASP) changes between the wild type and the glr3.3 glr3.6 double mutant in either undamaged cotyledons or roots (Supplemental Fig. S2E). However, rapid laser wounding of cotyledons induced robust surface potential changes (depolarizations) in undamaged cotyledons and roots, serving as positive control for the experimental setup (Supplemental Fig. S2F). For needle wounding, the recorded WASP changes for both genotypes were very low (<5 mV) and variable, whereas for laser stimulation, the observed depolarization patterns were uniform (20–30 mV). Nevertheless, amplitudes of surface potential changes were the same between the wild type and the glr3.3 glr3.6 double mutant for both treatments (Supplemental Fig. S2, G and H). Although surface potential changes could be measured in cotyledons and roots after laser stimulation, their propagation was not dependent on GLR3.3/3.6. In addition, wound signaling from cotyledons to roots seemed to work independently of WASPs, because the effects of needle wounding were efficiently transduced to roots to activate JA responses, but they only weakly and unreliably induced WASPs. Therefore, WASP-independent mechanisms must be responsible for efficient JAZ10 induction in roots of aerially wounded plants. Alternative approaches were used to test if JA (or JA precursors) could translocate to undamaged roots.
LOX2 Is Required to Induce Full JAZ10 Expression in Roots after Cotyledon Wounding
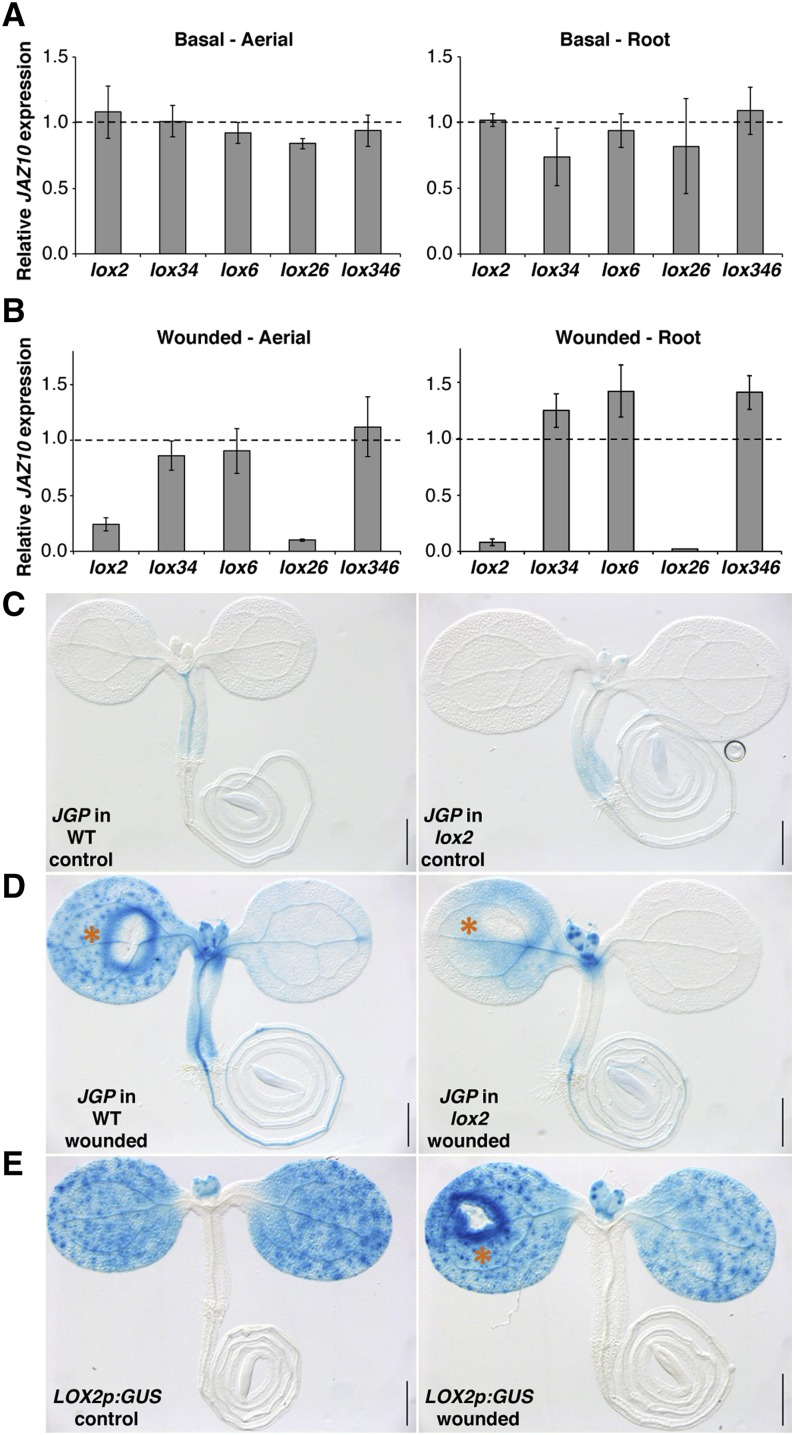
LOX6 is known to produce the bulk of JA and JA-Ile in roots when they are first separated from aerial tissues and then wounded (Grebner et al., 2013). Here, we analyzed the contribution of 13-LOXs to JAZ10 expression in roots after cotyledon wounding in intact seedlings. The wild type, lox2, lox6, lox26 double mutant, and lox346 triple mutant (retaining only LOX2 activity) were wounded with a needle on one cotyledon, and after 1 h, shoots and roots were separated and analyzed by quantitative real-time (qRT)-PCR. None of the mutants were significantly different from the wild type in basal JAZ10 levels (Fig. 1A). In contrast, wound-induced JAZ10 levels were decreased in the aerial tissue of the lox2 mutant (to approximately 25% of those seen in the wounded wild type), and they were further reduced in the lox26 double mutant (Fig. 1B). A similar but even more striking pattern was observed for root tissues where lox2 and lox26 double mutants reduced postwound JAZ10 expression to less than 10% of wild-type levels. The lox6, lox34, and lox346 seedlings did not exhibit a compromised wound response in aerial and root tissues (Fig. 1B). In accordance with the qRT-PCR results, secretable JGP reporter activity was strongly attenuated in the lox2 mutant background compared with the wild type after cotyledon wounding (Fig. 1, C and D).
Figure 1.
LOX2 is required to induce JAZ10 expression in roots after cotyledon wounding. qRT-PCR of JAZ10 expression basally (A) and 1 h after cotyledon wounding (B) in 5-d-old aerial organs and roots of lox2, lox34, lox6, lox26, and lox346 mutants. JAZ10 transcript levels were normalized to those of UBC21 and are displayed relative to the expression of wild-type controls that are set to one and indicated with dashed lines. Bars represent the means of three biological replicates (±sd), each containing a pool of organs from approximately 60 seedlings. Complete qRT-PCR data are in Supplemental Data Set S1, data set 1. JGP reporter activity in control (C) and wounded wild-type (WT) and lox2 (D) 5-d-old seedlings. E, LOX2p:GUS reporter in control and wounded wild-type seedlings. Detection of GUS activity was performed 2 h after wounding. Orange asterisks indicate cotyledon wounding sites. Bars = 0.5 mm.
The lox2 and lox346 data indicated that a functional LOX2 is necessary and sufficient to ensure near wild-type levels of wound-induced JAZ10 expression in both aerial organs and roots of young seedlings (Fig. 1B). However, LOX2 transcripts have not been detected in unwounded or wounded Arabidopsis roots (Grebner et al., 2013). Consistent with the work by Vellosillo et al. (2007), we observed LOX2p:GUS reporter activity only in cotyledons and emerging leaves and not in roots (Fig. 1E). In our experimental conditions, promoter activity for the other three 13-LOX constructs was also not detected in roots of control or cotyledon-wounded seedlings (Supplemental Fig. S3). In contrast, LOX6 transcripts were detected by qRT-PCR in adult roots under basal conditions, and LOX3 and LOX4 transcripts were induced when the roots were first separated from the shoots and then wounded (Grebner et al., 2013). The LOX2p:GUS reporter displayed induction only in the vicinity of a wound (Fig. 1E). Overall, our results suggested that JA and/or JA precursors might be transported from wounded aerial tissues to roots where they activated JAZ10 expression.
Micrografting Reveals Shoot-to-Root Oxylipin Transport
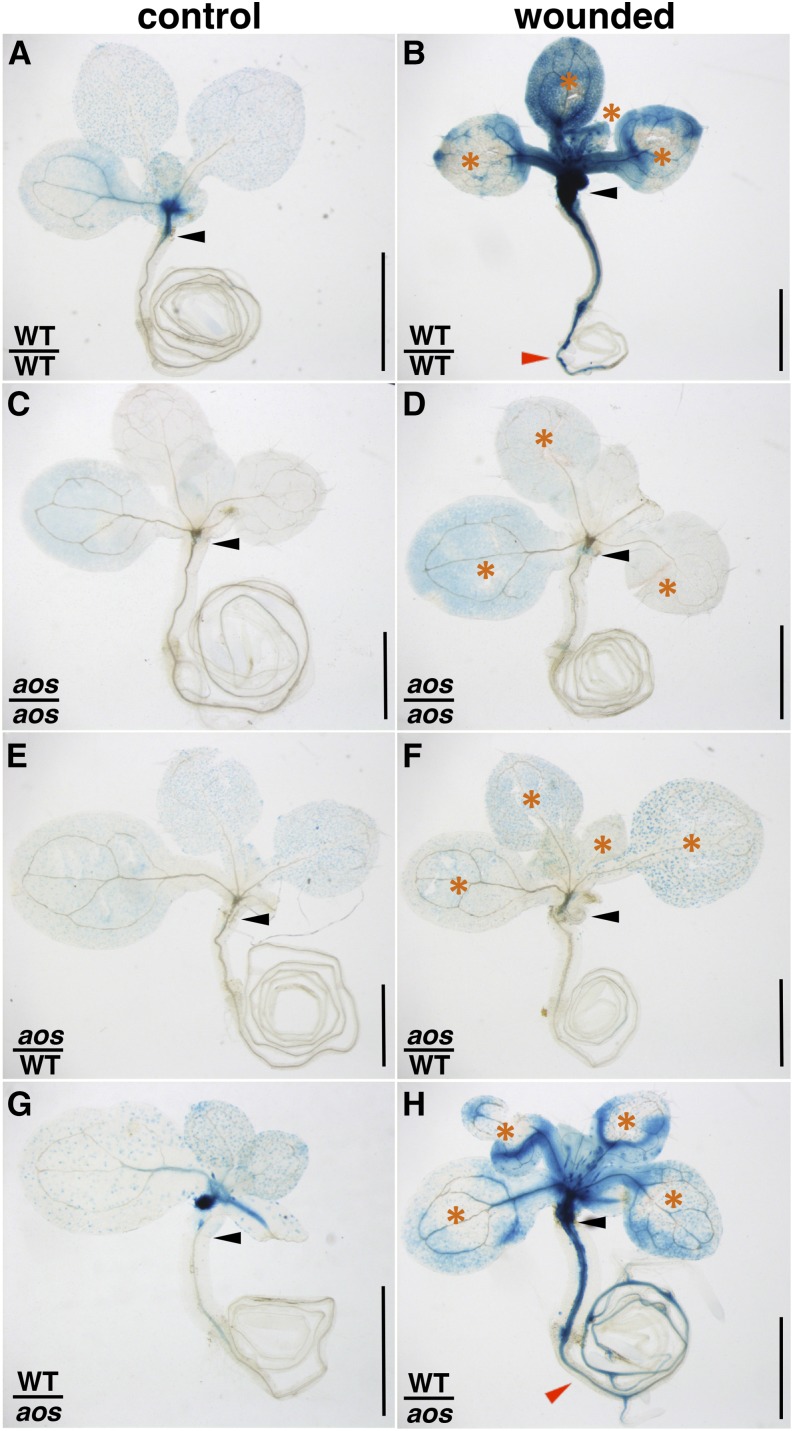
To test the hypothesis that oxylipins made in shoots could be transported to roots, reciprocal grafts were made between the wild type and the JA-deficient mutant aos. Both genetic backgrounds contained a JAZ10p:GUS reporter to visualize sites of JA signaling. A wild-type scion grafted onto a wild-type rootstock showed weak basal reporter activity and strong activation in both aerial organs and root vasculature after cotyledon and leaf wounding (Fig. 2, A and B). GUS activity encompassed approximately 59% of the wild-type rootstock length, extending for 5 ± 3 mm (n = 13) from the grafting site. In contrast, aos/aos grafted seedlings exhibited low basal JAZ10p:GUS levels, which were not further induced by wounding treatments (Fig. 2, C and D). These results validated the JA dependency of the reporter line and our grafting system. In the aos/wild-type graft combination, the JAZ10p:GUS reporter exhibited low basal activity and failed to be induced in the wild-type rootstock after aerial wounding of the aos scion (Fig. 2, E and F). In contrast, although basal reporter activity was low in wild-type/aos grafted seedlings, the reporter was strongly induced after cotyledon or leaf wounding in both wild-type scion and JA-deficient aos rootstock (Fig. 2, G and H). Similar to wild-type/wild-type wounded seedlings, reporter activity encompassed approximately 68% of the aos rootstock length, extending for 6 ± 2 mm (n = 10) from the grafting site. These data provide evidence for axial oxylipin transport from the wounded wild-type scion into the aos rootstock, where JAZ10 promoter activity is consequently activated.
Figure 2.
Evidence for axial shoot-to-root transport of jasmonate (or jasmonate precursors). JAZ10p:GUS reporter activity in grafted 13-d-old seedlings of the indicated scion/rootstock genotypes: WT/WT grafts (A and B), aos/aos grafts (C and D), aos/WT grafts (E and F), and WT/aos grafts (G and H). One of the two cotyledons was excised before grafting. GUS staining was performed 3 h after wounding aerial organs with a needle at sites indicated by orange asterisks (B, D, F, and H). Note the presence of reporter activity in the rootstock of the wild-type (WT)/aos grafted plant. Black arrowheads indicate grafting sites, and red arrowheads indicate reporter activity in the root. Bars = 1 mm.
LOX6 and LOX2 Activate JAZ10 Expression in Wounded and Distal Leaves of Adult Rosette Plants
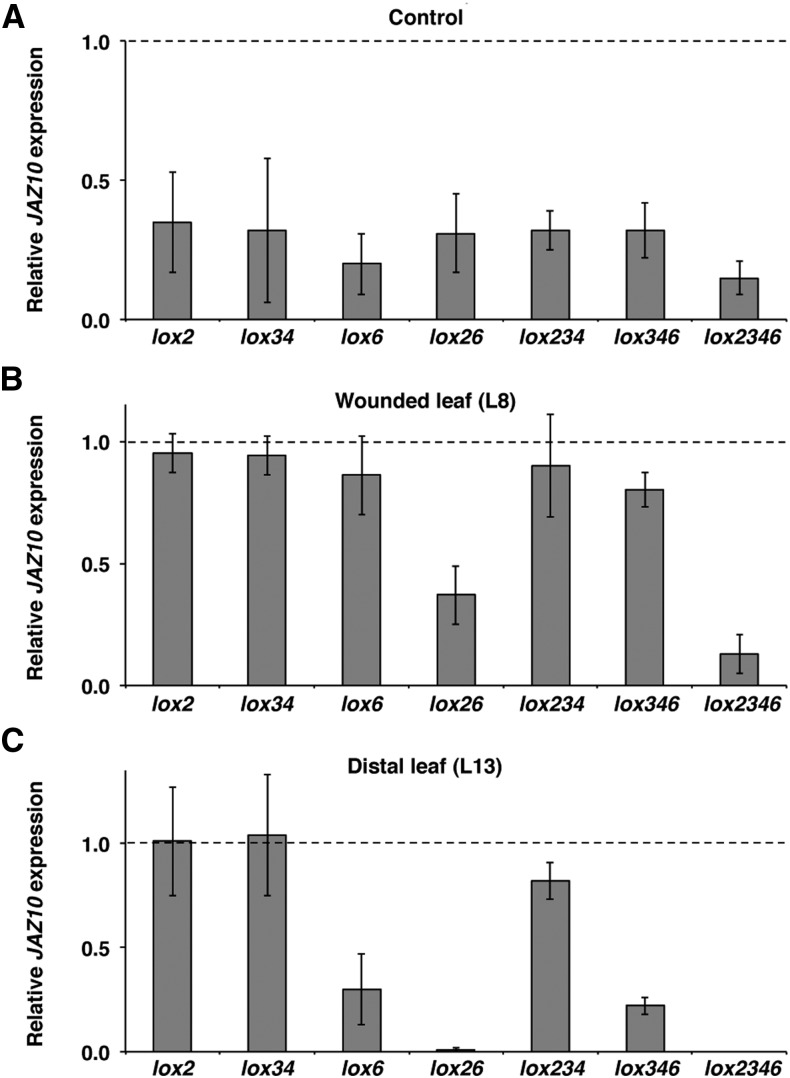
Severely wounding a leaf causes the generation of multiple signals that are transmitted axially along veins to activate JA synthesis in leaves distal to the wound (Rhodes et al., 2006; Koo and Howe, 2009; Farmer et al., 2014). However, for defenses to be activated fully, radial signals that reach the mesophyll are also likely to occur. Could the transport of jasmonates from veins be a part of these radial signaling events? To test for the export of jasmonates from veins, we conducted an extensive qRT-PCR analysis of JAZ10 expression 1 h after wounding in single, double, triple, and quadruple 13-lox mutants in adult-stage rosettes. Basal JAZ10 levels were consistently lower than the wild type in all of the mutants examined (Fig. 3A). Single 13-lox mutants and the lox34 double mutant did not significantly affect JAZ10 expression in the wounded leaf 8 (L8; Fig. 3B). However and in agreement with Chauvin et al. (2013), the lox6 mutant reduced JAZ10 expression in a distal unwounded leaf 13 (L13; Fig. 3C). Lack of LOX2 and LOX6 activity in the lox26 double mutant strongly reduced JAZ10 expression in wounded L8 and totally abolished the marker gene induction in distal L13. Analysis of lox234 (retaining only LOX6 activity) and lox346 (retaining only LOX2 activity) triple mutants showed that the individual activities of LOX6 or LOX2 could ensure most of the JAZ10 expression in wounded L8 but that LOX6 played the major role in promoting near wild-type JAZ10 expression in distal L13 of a wounded plant. However, the almost abolished JAZ10 induction in distal leaves of lox26 wounded plants and the capability of lox346 to slightly induce JAZ10 expression in distal sites (Fig. 3C) suggest that LOX2 also contributes (to a lesser extent than LOX6) to JA synthesis at a distance from wounds in adult-phase plants. As expected, the lox2346 quadruple mutant was severely compromised in JAZ10 induction in wounded L8, and JAZ10 expression was abolished in distal L13 (Fig. 3, B and C). Taken together, the data indicate that the activity of LOX6 is sufficient to induce near wild-type JAZ10 expression in wounded and distal leaves of adult rosette plants. We therefore focused on LOX6 and using a LOX6p:LOX6-GUS protein fusion reporter, began by identifying the cells in which the LOX6 protein was present.
Figure 3.
LOX6 activity is sufficient to activate near wild-type JAZ10 expression in wounded and distal leaves of adult rosette plants. qRT-PCR of JAZ10 expression in unwounded L8 (A) and 1 h after wounding L8 (B) and distal L13 (C) in indicated genotypes. Basal JAZ10 levels are similar between L8 and L13 (Mousavi et al., 2013); thus, L8 was used as the unwounded control. Note that, after wounding, the lox234 mutant harboring only LOX6 activity reaches near wild-type JAZ10 expression levels in both L8 and L13. JAZ10 transcript levels were normalized to those of UBC21 and are displayed relative to the expression of wild-type control (L8) or wounded (L8 or L13) levels that are set to one and indicated with dashed lines. Bars represent the means of three to four biological replicates (±sd) from individual 4.5-week-old plants. Complete qRT-PCR data are in Supplemental Data Set S1, data set 2.
LOX6-Derived Jasmonates Are Exported from the Vasculature into the Leaf Blade
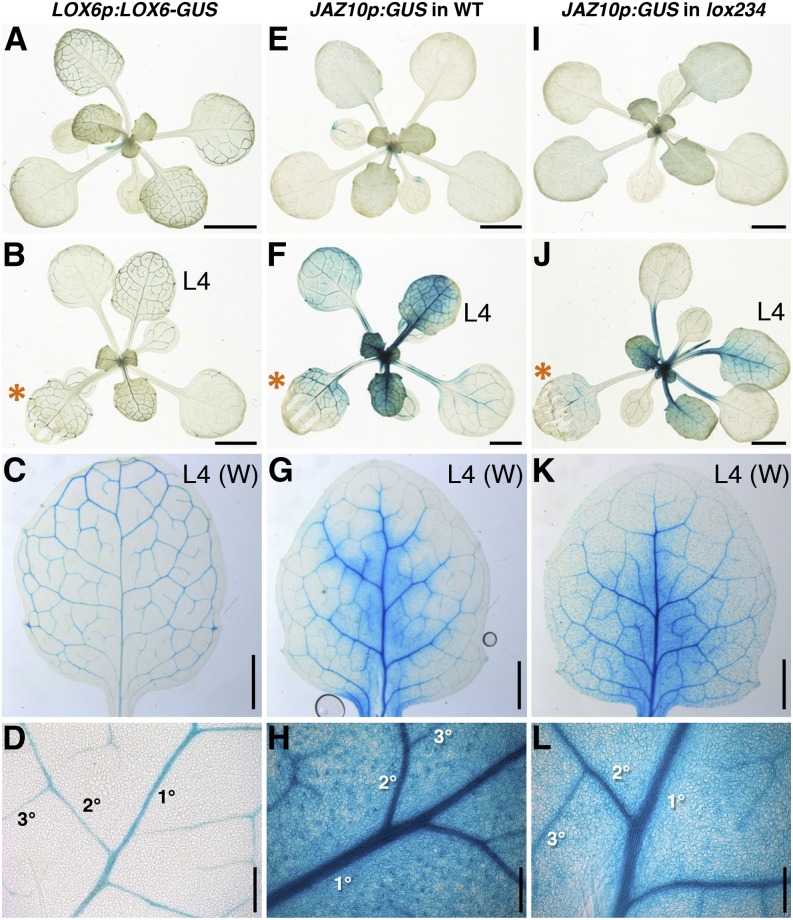
LOX6p:LOX6-GUS reporter activity was confined to the leaf vasculature of adult rosette plants (Fig. 4A), and in agreement with LOX6 transcript levels (Grebner et al., 2013; Supplemental Fig. S4), the LOX6 protein fusion reporter was also not wound inducible (Fig. 4, B–D). As in the wild type (Chauvin et al., 2013), the LOX6p:GUS expression pattern was restricted to the vasculature in the lox234 triple-mutant background (Supplemental Fig. S5). We previously noted the activity of a transcriptional LOX6p:GUS reporter in xylem contact cells and occasionally, subtrichomal mounds (Chauvin et al., 2013). However, here, we did not observe LOX6-GUS protein expression in subtrichomal mounds in any rosette leaves, including leaf 4 (L4) from control or wounded plants. These LOX6 protein localization findings suggested a strategy to investigate whether LOX6-derived jasmonates could act as part of the radial cell-to-cell wound-signaling component. Specifically, we followed the expression of the JA-responsive JAZ10p:GUS reporter in the leaf lamina of the lox234 triple mutant, in which the activity of all 13-LOXs, except LOX6, is abolished.
Figure 4.
LOX6-derived oxylipins are exported from the vasculature into the leaf blade. A to D, LOX6p:LOX6-GUS protein fusion activity in rosettes before (A) and after (B–D) wounding. E to L, JAZ10p:GUS activity in the wild type (WT) and lox234 triple mutant before (E and I) and after (F–H and J–L) wounding. In each case, L3 of 21-d-old plants was wounded (orange asterisks), and GUS staining was performed 6 h later. L4 from a wounded (W) plant is shown in C, G, and K, and details of the primary order (1°), secondary order (2°), and tertiary order (3°) veins in L4 are shown in D, H, and L. Bars = 0.2 cm (A, B, E, F, I, and J), 1 mm (C, G, and K), and 200 μm (D, H, and L).
Basal JAZ10p:GUS activity in adult wild-type rosettes was low (Fig. 4E) but readily induced in wounded leaf 3 (L3) as well as in distal L4 in both the vasculature and extraveinal regions (Fig. 4, F–H). Wounding of aos rosettes harboring the JAZ10p:GUS reporter did not increase GUS activity with respect to control plants (Supplemental Fig. S6), validating the JA dependency of the reporter. Similar to the wild type, basal JAZ10p:GUS activity was very weak in the lox234 triple-mutant background (Fig. 4I), but it was significantly induced after wounding L3 in both local and distal leaves (Fig. 4, J and K). The JA signaling reporter was activated in both veins and extraveinal cells in the lox234 mutant retaining only LOX6 activity (Fig. 4L). In addition to mechanical wounding, the JAZ10p:GUS reporter was activated in extraveinal regions in both the wild type and lox234 mutant challenged with larvae of the generalist herbivore Spodoptera littoralis (Supplemental Fig. S7).
Evidence for Cell-to-Cell Oxylipin Transport in Leaves
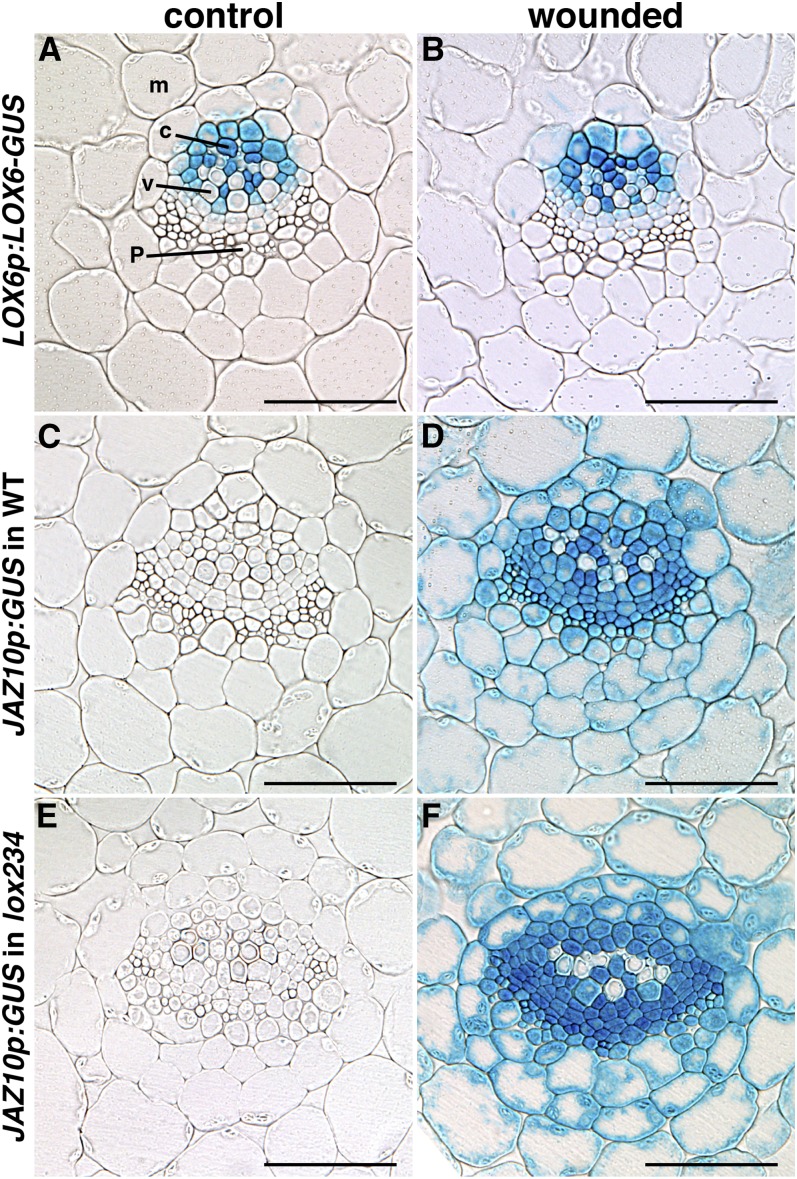
To determine which cell types responded to JA in a lox234 triple mutant retaining LOX6 activity only in the vasculature, we sectioned L4 transversally from control (unwounded) plants and plants wounded on L3. Activity of the LOX6p:LOX6-GUS fusion reporter remained confined to the xylem pole, was predominantly in contact cells, was not wound inducible, and did not translocate to other vascular cells in response to wounding (Fig. 5, A and B). Next, we examined the activity of the JAZ10p:GUS reporter. Basal JAZ10p:GUS expression was undetectable in cells of the epidermis, mesophyll, and vascular bundles in both the wild-type and lox234 backgrounds. However, sections from wounded plants showed robust reporter activation extending from xylem contact cells into cells of the whole vasculature, the mesophyll, and the epidermis in both genetic backgrounds (Fig. 5, C–F; Supplemental Fig. S8). Reporter activity extended 125 ± 15 μm (n = 10) from xylem contact cells to the abaxial leaf epidermis in the wild type and 132 ± 22 μm (n = 10) in the lox234 triple mutant.
Figure 5.
Oxylipins produced in xylem contact cells activate JAZ10 expression throughout the vascular cylinder and in extraveinal cells. A and B, Cross sections through the midvein showing the localization of LOX6-GUS fusion protein expressed under the LOX6 promoter before (A) and after (B) wounding. C and D, JAZ10p:GUS activity in the wild type (WT) before (C) and after (D) wounding. E and F, JAZ10p:GUS activity in the lox234 triple mutant before (E) and after (F) wounding. L4 of 21-d-old plants was stained for GUS activity in control samples and 6 h after wounding L3. c, Xylem contact cell; m, mesophyll; p, phloem region including phloem parenchyma; v, xylem vessel. Bar = 50 μm.
DISCUSSION
How do signals that originate in spatially restricted wounds lead to responses elsewhere in the plant body? Key to resolving this longstanding question will be to decipher both axial and radial signaling mechanisms. Here, and bearing in mind that mechanistic aspects and cell types involved in long-distance wound signaling may differ during plant development, we set the goal of characterizing the mobility of endogenous wound-induced jasmonates.
Axial Translocation of JA (or JA Precursors) from Shoots to Roots in Seedlings
Wounding of a single Arabidopsis cotyledon with a needle causes a strong jasmonate response in roots (Acosta et al., 2013; Gasperini et al., 2015; Larrieu et al., 2015). Having found that several clade 3 GLR genes (GLR3.1, GLR3.2, GLR3.3, and GLR3.6) play a role in propagating membrane depolarizations in the adult-phase rosette (Mousavi et al., 2013), we expected to find the same mechanism in wounded seedlings. However, our results suggest that two GLRs (GLR3.3 and GLR3.6) that contribute to the propagation of wound signals in adult-phase leaves are not required for cotyledon-to-root wound signaling that leads to root JA responses. Moreover, in seedlings wounded on one cotyledon, we were able to break the correlation between electrical events measured as surface potentials and JA responses in roots. These observations agree with the work by Rhodes et al. (2006), which concluded that (1) readily detectable electrical signals are not produced from small wounds and that (2) they are not necessary for defense signaling over short distances. Other mechanisms, therefore, contribute to activating JA responses in the roots of seedlings when aerial tissues are wounded.
Our studies of root JAZ10 expression from aerially wounded plants in a variety of 13-lox mutants suggested that cotyledon-expressed LOXs (chiefly LOX2) can produce JA (or JA precursors) that could be then translocated to roots. This finding is in agreement with the work by Zhang and Baldwin (1997), which found that radiolabeled JA applied to wild tobacco leaves can be transported from aerial tissues to roots. Our grafting experiments established that this was also the case for endogenous JA (or JA precursors). Grafting wild-type scions onto aos JA biosynthesis mutant rootstocks revealed that, similar to a wild-type/wild-type graft, JA and/or its precursors can be translocated at least 5 mm from the graft junction into the primary root. In doing so, these translocated oxylipins must pass axially through many cells. Phloem is known to be involved in the transport of several plant hormones (Ham and Lucas, 2014). JAZ10p:GUS expression was visible along the root vascular bundle, suggesting that shoot-to-root oxylipin transport might use the phloem route.
In wild tobacco, Zhang and Baldwin (1997) found that JA pools in roots increased 90 min after damage to aerial tissues. The kinetics of the major phase of JA accumulation signaling in Arabidopsis roots after the injury of aerial tissues are similar. In Arabidopsis, shoot-to-root wound signals generated in response to a puncture wound on one cotyledon caused an initial rapid but minor wave of root JA signaling that takes place in the first 20 min after damage (Larrieu et al., 2015). Subsequently, after an apparent delay lasting approximately 20 min, a second major wave representing approximately 85% of total jasmonate signaling output occurs in the root between 40 and 120 min after cotyledon wounding (Larrieu et al., 2015). Because grafting experiments revealed a very strong contribution of shoot-derived JA (or JA precursors) to root JA signaling, we speculate that jasmonate translocation may correlate with the second major burst of JA signaling observed by Larrieu et al. (2015). That is, the axial transport of jasmonates may be the predominant mechanism of shoot-to-root JA signaling at the seedling stage. This leaves open the possibility of an initial and more rapid shoot-to-root signaling event that is not necessarily explained by the translocation of JA (or JA precursors) that we observed. This will require further attention. As stated in Rhodes et al., 2006, there is perhaps no need for electrical signals (i.e. transmembrane ion fluxes that can be detected with surface electrodes) to activate JA responses within short distances (here defined as <1 cm) of a wound (1 cm corresponds to the length of an entire 5-d-old Arabidopsis seedling). Finding evidence of oxylipin transport in seedlings led us to test whether this also occurred in leaves.
Radial Transport of Jasmonates from Veins into Interveinal Leaf Regions
Organ-to-organ wound signaling has been studied extensively in adult-phase plants. To date, much of the literature has implicated two tissues as principal routes for long-distance wound signals: phloem and xylem (for review, see Koo and Howe, 2009; Farmer et al., 2014; van Bel et al., 2014). The phloem was long ago identified as an excitable tissue that propagates damage signals through Mimosa pudica (Bose, 1926). Recent evidence from a variety of other plants supports this (for review, see van Bel et al., 2014) along with real-time recordings of wound-induced electrical events in Arabidopsis sieve cells (Salvador-Recatalà et al., 2014). Moreover, sites of JA synthesis in tomato are associated with the phloem (Hause et al., 2003). In parallel, the xylem may also play a crucial role in axial wound signal transmission by allowing the propagation of pressure waves (Stahlberg and Cosgrove, 1997; Farmer et al., 2014), and sites of JA synthesis are present adjacent to xylem vessels in Arabidopsis (Chauvin et al., 2013). Although LOX6 contributes to JA production in the vicinity of the wound, it has a dominant impact on promoting JA/JA-Ile synthesis at a distance from wounds in distal undamaged leaves (Chauvin et al., 2013). It is possible that, during leaf-to-leaf wound signaling, some jasmonates produced by LOX6 in xylem contact cells are then transported into phloem sieve elements or xylem vessels, where they could be further translocated. Such scenarios remain to be tested.
By using an antibody-based approach, Mielke et al. (2011) found that jasmonates are distributed within all cells near the site of damage of mechanically wounded tomato leaves. Herein, we showed that wounding a leaf of the wild type or the lox234 triple mutant harboring LOX6 activity in xylem contact cells induced JAZ10 expression in cells outside the region of LOX6 protein accumulation. In fact, we were able to detect GUS staining from the wound-activated expression of JAZ10p:GUS in a region encompassing at least six cell types other than contact cells: xylem parenchyma, vascular cambium, cells in the phloem pole, bundle sheath cells, mesophyll cells, and epidermis. LOX6-derived jasmonates are, therefore, transported radially (outward) from xylem contact cells. The distance from xylem contact cells to the abaxial epidermis was about 130 µm. Under our experimental conditions, it is evident that contact cell-produced oxylipins can be transported radially over at least eight cell layers (Supplemental Fig. S8).
The lox234 mutant (retaining only LOX6 activity) also expressed near wild-type levels of the JAZ10p:GUS reporter in leaves challenged with S. littoralis. Both the wild type and the lox234 triple mutant showed strong reporter activation in the center of insect-fed rosettes (which are often the most defended tissues in insect feeding experiments), including in plants that express only LOX6 (Chauvin et al., 2013). However, the early wound response marker gene JAZ10 is not always a suitable marker for defense responses (Gasperini et al., 2015), and it is not surprising that the lox234 mutant is more susceptible than the wild type to herbivorous insects (Chauvin et al., 2013). In fact, the lox234 mutant lacks LOX2 activity, which is known to contribute to leaf defense against S. littoralis (Glauser et al., 2009). Additionally, the transport of jasmonates from LOX6-expressing cells may not be sufficient to cover areas of the leaf distant from the site of damage.
Which Oxylipins Are Mobile in Wounded Arabidopsis?
After mechanical damage, JA and JA-Ile are abundant oxylipins in many leaf cell types (Mielke et al., 2011), and both compounds are possible candidates for mobile jasmonates as suggested previously (Farmer et al., 1992, 2014; Zhang and Baldwin, 1997; Li et al., 2002; Schilmiller and Howe, 2005; Thorpe et al., 2007; Wu et al., 2007; Mielke et al., 2011; Sato et al., 2011). In Arabidopsis seedlings, the gene encoding the JASMONIC ACID RESISTANT1 (JAR1) enzyme (Staswick and Tiryaki, 2004) that converts JA to JA-Ile was found to be indispensable for the activation JA signaling in roots after wounding cotyledons, although JA signaling occurred in the wounded aerial organs of the jar1-1 mutant (Acosta et al., 2013). This evidence makes JA-Ile an unlikely candidate for a mobile jasmonate in shoot-to-root translocation under our experimental conditions. For Arabidopsis leaves, it is at present not possible to conclude which forms of jasmonate are transported, although oxophytodienoic acid was excluded from leaf-to-leaf transport in experiments by Koo et al. (2009). Interestingly, lesion-bearing leaves of the accelerated death2 mutant (Greenberg et al., 1994), in which oxylipin synthesis is hyperactivated, accumulate JA and to a lesser extent, its intermediate precursor 3-oxo-2-(2-(Z)-pentenyl)-cyclopentane-1-butanoic acid (OPC4; Mueller et al., 2006). In the same study, JA predominates over OPC intermediates in wounded wild-type Arabidopsis plants. JA and 3-oxo-2-(2-(Z)-pentenyl)-cyclopentane-1-butanoic acid are considered here as the most likely transported oxylipins; however, this hypothesis will require careful verification.
CONCLUSION
Our current understanding of oxylipin transport is still rudimentary, but herein, we show that the transport of endogenous wound-induced jasmonates can occur in two vectors relative to organ growth: axial and radial. In the timeframe tested, we present evidence that JA (or JA precursors) can travel a minimum of 0.5 cm from wounded shoots to undamaged roots. This and the finding of radial oxylipin transport in leaves help to explain why the treatment of plants with exogenous jasmonates is so effective in stimulating defense gene expression in distal untreated tissues. This work shows an interesting parallel to prostanoid transport in metazoans.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) accession Columbia-0 was the wild type and the background for previously described mutants and reporter lines used in this study: glr3.3 glr3.6 (glr3.3a glr3.6a; SALK_099757 SALK_091801; Mousavi et al., 2013), JGP reporter line (Acosta et al., 2013), aos (Park et al., 2002), lox2 (lox2-1; Glauser et al., 2009), lox3 (lox3B; SALK_147830), lox4 (lox4A; SALK_071732), lox6 (lox6A; SALK_138907), lox26, lox34, lox234, lox346, and lox2346 (Caldelari et al., 2011; Chauvin et al., 2013). After seed stratification for 2 d at 4°C, plants were grown at 21°C under 100 μE m−2 s−1 light, with a photoperiod depending on the application (seedlings, 14-h-light/10-h-dark cycle; rosette plants, 10-h-light/14-h-dark cycle). For seedling growth, seeds were surfaced sterilized and grown on one-half-strength solid Murashige and Skoog medium (MS; 2.15 g L−1, pH 5.7; Duchefa) supplemented with 0.5 g L−1 MES hydrate (Sigma) and 0.7% (w/v) agar (for horizontally grown seedlings) or 0.85% (w/v) agar (for vertically grown seedlings) as described (Acosta et al., 2013).
Plant Treatments
Wounding of 5-d-old and grafted seedlings was performed with a 25-gauge × 5/8-in needle (0.5 × 16 mm) under a stereomicroscope. For wounding of adult rosettes, leaves were numbered from oldest to youngest, and 50% of selected leaves were crushed with metal forceps in distal parts from the petiole. For insect feeding experiments, three neonate Spodoptera littoralis larvae were placed on individually grown 21-d-old plants located in Plexiglas boxes for 24 h before GUS staining.
Electrophysiology
Seedlings were grown vertically in petri dishes on sterile 11- × 11-cm, 3-mm CHR Whatman Paper (GE Healthcare) placed on solid medium (one-half-strength MS, 0.5 g L−1 MES hydrate, pH 5.7, and 0.85% [w/v] agar) for 4 d; 1 d before the experiment, short root portions were gently lifted with a sterile toothpick from the paper without disturbing the hypocotyl or root tip that remained in contact with the moist paper support. Ag/AgCl electrodes were used for surface potential measurements on 5-d-old seedlings. Electrodes were prepared as follows: 4-mm-long pieces of silver wire (0.25 mm in diameter; World Precision Instruments) serving as electrode tips were soldered to 10-mm-long spiral copper wires (0.1 mm in diameter) that were twisted into spirals to serve as movement absorbers. The copper spirals were in turn soldered onto 0.7-mm-diameter copper wires acting as holders between the electrode tip and the amplifier probes. Soldered connections between silver and copper wires were isolated with Parafilm, and the nonisolated part of the silver wire was chloridized by electrolysis in 0.1 m HCl. Contacts between plant surfaces and measurement electrodes were enhanced by the addition of approximately 1 μL of 10 mm KCl in 50% (v/v) glycerol. Ag/AgCl reference electrodes (0.5 mm in diameter) were placed in the side of the petri dish in direct contact with the growth medium. Contact between the chloridized silver wire and the growth medium was provided by an agar bridge composed of a 6-mm-wide silicon tube half filled with 1 m KCL in 1% (w/v) agar in contact with the medium. The upper part of the silicon tube was filled with aqueous 1 m KCl in which the chloridized silver wire was immersed. Electrophysiological measurements were conducted at a stable temperature of 22°C and light intensity of 55 μmol m−2 s−1 photosynthetically active radiation. Measurement electrodes were mounted on high-resistance probes on a two-channel amplifier FD223a (World Precision Instruments), which together with InstruTECH LIH 8+8 Acquisition Interface (HEKA Electronic) and Chartmaster software (Heka Electronic), was used to record surface potentials at a 100-Hz sampling frequency. Laser wounding used an IR (808-nm) light-emitting diode (LED) laser system (MDL-N-808-W LED laser head coupled with the PSU-H-LED Power Supply; Changchun New Industries Optoelectronics Technology Co.) controlled by the InstruTECH LIH 8+8 Acquisition Interface; 0.5-s impulses of light with maximum laser power (approximately 8 W) were supplied to one cotyledon using 0.4-mm-diameter fiber optics (Changchun New Industries Optoelectronics Technology Co.). This punctured a hole in the cotyledon in less than 1 s. Seedling wounding with a needle was performed as above. Data analysis used the Chartmaster software. Amplitudes of depolarizations were automatically calculated with a homemade protocol as the difference between the depolarization minimum recorded in the 200 s after wounding and the average surface potential in the 10 s before wounding.
Grafting
Grafts were performed as in Melnyk et al. (2015) with minor modifications. Vertically grown 5-d-old seedlings were grafted under sterile conditions using a dissecting microscope. Seedlings were transferred to square petri dishes containing one layer of 2.5- × 11-cm sterilized Hybond C Super Nitrocellulose Membrane (RPN.203G; Amersham) on top of sterile 11- × 11-cm, 3-mm CHR Whatman Paper (GE Healthcare) placed on solid medium (one-half-strength MS, 0.5 g L−1 MES hydrate, pH 5.7, and 1.5% agar) to guarantee adequate humidity and nourishment. One cotyledon was removed to facilitate handling, and a transverse cut was made through the hypocotyl as close as possible to the shoot using a stainless steel 23 surgical blade (Heinz Herenz Medizinalbedarf GMBH). Grafts were assembled by butt alignment of the two cut halves (Turnbull et al., 2002). After grafting, petri dishes were sealed with micropore tape and kept horizontal for 8 d. Successful graft formation was evaluated by the attachment of scion to rootstock and the resumption of root growth.
Gene Expression
Root and shoot samples of 5-d-old seedlings were collected as described (Acosta et al., 2013), and leaves from adult rosettes were sampled as in Chauvin et al. (2013). RNA and complementary DNA were prepared as in Gfeller et al. (2011). qRT-PCR was performed as described (Chauvin et al., 2013). Primers for qRT-PCR of JAZ10 (At5g13220) and UBIQUITIN-CONJUGATING ENZYME21 (UBC21; At5g25760) have been previously reported in Gfeller et al. (2011). To quantify LOX6 transcripts (At1g67560), the following primers were used: gaagaaatcatagctcgagaagttg and aatggcaagagtatgtcatggtaat.
Transgenic Lines
Promoters were amplified from wild-type genomic DNA with indicated oligonucleotides for LOX2 (cggggtacctgacaacaaaattcccgaaa and ttcccccccgggggcttacattcctctcatatcca; 2,904 bp; At3g45140), LOX3 (cggggtacccttgatacgcgataatcttacaagc and ttcccccccgggttagtgaagaagaaggagttgagga; 2,875 bp; At1g17420), and LOX4 (cggggtaccgaggccaaggacactaggaa and ttcccccccgggcaagactgagtttgagagctttctt; 2,719 bp; At1g72520) and cloned by restriction with XmaI and KpnI into a modified pUC57 (Chauvin et al., 2013) to create pEN-L4-promoter-R1 clones. Underlined sequences represent XmaI and KpnI sites. Fusions to GUS were obtained by Gateway Cloning Technology as described (Chauvin et al., 2013). Promoters for LOX6 (Chauvin et al., 2013) and JAZ10 (Acosta et al., 2013) were described previously. A unique nonsecretable version of the JAZ10p:GusPlus (JAZ10p:GUS) reporter was generated in wild-type, aos, and lox234 backgrounds and used in all experiments described in Figures 2, 4, and 5 and Supplemental Figures S1 to S8. The coding DNA sequence of LOX6 was amplified from wild-type complementary DNA with (atgttcgtagcatctccggtaa and aatggaaatgctgttgggaata) oligonucleotides containing the appropriate recombination adaptors and cloned into pDONR221 (Invitrogen). The LOX6p:LOX6-GUS protein fusion construct was generated by Multisite Gateway Technology of entry clones into pH7m34gw. All constructs were introduced into Arabidopsis backgrounds by floral dip Agrobacterium tumefaciens-mediated transformation (Clough and Bent, 1998). For promoter fusions, transformed seeds expressing red fluorescence protein in T1, T2, and T3 lines were selected by fluorescence microscopy, whereas for the protein fusion reporter, lines were selected on medium containing 40 mg L−1 hygromycin. A minimum of two independent transgenic lines was used for each construct to perform experiments and verify reproducibility.
GUS Staining, Sectioning, and Light Microscopy
For GUS staining, samples were prefixed for a minimum of 30 min in 90% acetone, washed two times with 50 mm sodium phosphate buffer (pH 7.2), vacuum infiltrated in the staining solution [10 mm Na2EDTA, 50 mm sodium phosphate buffer, pH 7.2, 3 mm K4Fe(CN)6, 3 mm K3Fe(CN)6, 0.1% Triton X-100, and 0.5 mg mL−1 5-bromo-4-chloro-3-indolyl-β-glucuronic acid], and incubated at 37°C in the dark for 2 h (5-d-old seedlings), 4 h (grafted plants), or overnight (adult rosettes). Five-day-old seedlings and grafted plants were cleared in 70% ethanol, mounted in chloral hydrate:glycerol:water solution (8:2:1), and imaged with a Leica MZ16A Stereomicroscope fitted with a DFC310FX Camera. Adult rosettes were cleared in 70% ethanol and imaged with a Keyence Digital Microscope VHX-500F. Transversal sections of leaves were performed as described (Chauvin et al., 2013), mounted in 10% glycerol, and imaged with a Leica DM5500 Microscope fitted with a DFC420 Camera.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. qRT-PCR of JAZ10 expression 1 h after cotyledon wounding in aerial organs and roots of wild-type and glr3.3 glr3.6 5-d-old seedlings.
Supplemental Figure S2. Surface potentials recorded from cotyledons and roots of stimulated seedlings.
Supplemental Figure S3. LOX3p:GUS, LOX4p:GUS, and LOX6p:GUS expression in control and 2 h after cotyledon wounding wild-type 5-d-old seedlings.
Supplemental Figure S4. LOX6 transcripts are not wound inducible.
Supplemental Figure S5. Basal (control) and 6 h after wounding LOX6p:GUS expression in 3-week-old rosettes of the lox234 triple mutant.
Supplemental Figure S6. Basal (control) and 6 h after wounding JAZ10p:GUS expression in 3-week-old rosettes of the JA-deficient mutant aos.
Supplemental Figure S7. JAZ10p:GUS expression in 21-d-old rosettes of the wild type and the lox234 triple mutant in control conditions or after 24 h of treatment with neonate S. littoralis larvae.
Supplemental Figure S8. Oxylipins produced in xylem contact cells activate JAZ10 expression throughout the vascular cylinder and into the mesophyll and epidermis.
Supplemental Data Set S1. Complete qRT-PCR data.
Supplementary Material
Acknowledgments
We thank Niko Geldner for critical reading of the article and for the plasmids; Aurore Lenglet for advice in using the laser setup; Philippe Reymond for insect eggs; and Charles W. Melnyk, Evangelia Vogiatzaki, and Variluska Fragoso for advice on grafting.
Glossary
- JA
jasmonic acid
- JA-Ile
jasmonoyl-Ile
- L3
leaf 3
- L4
leaf 4
- L8
leaf 8
- L13
leaf 13
- MS
Murashige and Skoog medium
- qRT
quantitative real-time
- WASP
wound-activated surface potential
Footnotes
This work was supported by the Swiss National Science Foundation (grant nos. 200020–146200 to J.-L.W. and E.E.F. and 31003A–138235 to E.E.F).
Articles can be viewed without a subscription.
References
- Acosta IF, Gasperini D, Chételat A, Stolz S, Santuari L, Farmer EE (2013) Role of NINJA in root jasmonate signaling. Proc Natl Acad Sci USA 110: 15473–15478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E, Creelman RA, Mullet JE (1995) A chloroplast lipoxygenase is required for wound-induced jasmonic acid accumulation in Arabidopsis. Proc Natl Acad Sci USA 92: 8675–8679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose JC. (1926) The Nervous Mechanism of Plants. Longmans, Green & Co., London [Google Scholar]
- Browse J. (2009) Jasmonate passes muster: a receptor and targets for the defense hormone. Annu Rev Plant Biol 60: 183–205 [DOI] [PubMed] [Google Scholar]
- Caldelari D, Wang G, Farmer EE, Dong X (2011) Arabidopsis lox3 lox4 double mutants are male sterile and defective in global proliferative arrest. Plant Mol Biol 75: 25–33 [DOI] [PubMed] [Google Scholar]
- Chauvin A, Caldelari D, Wolfender JL, Farmer EE (2013) Four 13-lipoxygenases contribute to rapid jasmonate synthesis in wounded Arabidopsis thaliana leaves: a role for lipoxygenase 6 in responses to long-distance wound signals. New Phytol 197: 566–575 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Farmer EE, Gasperini D, Acosta IF (2014) The squeeze cell hypothesis for the activation of jasmonate synthesis in response to wounding. New Phytol 204: 282–288 [DOI] [PubMed] [Google Scholar]
- Farmer EE, Johnson RR, Ryan CA (1992) Regulation of expression of proteinase inhibitor genes by methyl jasmonate and jasmonic acid. Plant Physiol 98: 995–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, Miersch O, Wasternack C, Solano R (2009) (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol 5: 344–350 [DOI] [PubMed] [Google Scholar]
- Funk CD. (2001) Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294: 1871–1875 [DOI] [PubMed] [Google Scholar]
- Furuyashiki T, Narumiya S (2011) Stress responses: the contribution of prostaglandin E(2) and its receptors. Nat Rev Endocrinol 7: 163–175 [DOI] [PubMed] [Google Scholar]
- Gasperini D, Chételat A, Acosta IF, Goossens J, Pauwels L, Goossens A, Dreos R, Alfonso E, Farmer EE (2015) Multilayered organization of jasmonate signalling in the regulation of root growth. PLoS Genet 11: e1005300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gfeller A, Baerenfaller K, Loscos J, Chételat A, Baginsky S, Farmer EE (2011) Jasmonate controls polypeptide patterning in undamaged tissue in wounded Arabidopsis leaves. Plant Physiol 156: 1797–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser G, Dubugnon L, Mousavi SA, Rudaz S, Wolfender JL, Farmer EE (2009) Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded Arabidopsis. J Biol Chem 284: 34506–34513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebner W, Stingl NE, Oenel A, Mueller MJ, Berger S (2013) Lipoxygenase6-dependent oxylipin synthesis in roots is required for abiotic and biotic stress resistance of Arabidopsis. Plant Physiol 161: 2159–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JT, Guo A, Klessig DF, Ausubel FM (1994) Programmed cell death in plants: a pathogen-triggered response activated coordinately with multiple defense functions. Cell 77: 551–563 [DOI] [PubMed] [Google Scholar]
- Ham BK, Lucas WJ (2014) The angiosperm phloem sieve tube system: a role in mediating traits important to modern agriculture. J Exp Bot 65: 1799–1816 [DOI] [PubMed] [Google Scholar]
- Hause B, Hause G, Kutter C, Miersch O, Wasternack C (2003) Enzymes of jasmonate biosynthesis occur in tomato sieve elements. Plant Cell Physiol 44: 643–648 [DOI] [PubMed] [Google Scholar]
- Kalinski P. (2012) Regulation of immune responses by prostaglandin E2. J Immunol 188: 21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo AJ, Gao X, Jones AD, Howe GA (2009) A rapid wound signal activates the systemic synthesis of bioactive jasmonates in Arabidopsis. Plant J 59: 974–986 [DOI] [PubMed] [Google Scholar]
- Koo AJ, Howe GA (2009) The wound hormone jasmonate. Phytochemistry 70: 1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrieu A, Champion A, Legrand J, Lavenus J, Mast D, Brunoud G, Oh J, Guyomarc’h S, Pizot M, Farmer EE, et al. (2015) A fluorescent hormone biosensor reveals the dynamics of jasmonate signalling in plants. Nat Commun 6: 6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li C, Lee GI, Howe GA (2002) Distinct roles for jasmonate synthesis and action in the systemic wound response of tomato. Proc Natl Acad Sci USA 99: 6416–6421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnyk CW, Schuster C, Leyser O, Meyerowitz EM (2015) A developmental framework for graft formation and vascular reconnection in Arabidopsis thaliana. Curr Biol 25: 1306–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke K, Forner S, Kramell R, Conrad U, Hause B (2011) Cell-specific visualization of jasmonates in wounded tomato and Arabidopsis leaves using jasmonate-specific antibodies. New Phytol 190: 1069–1080 [DOI] [PubMed] [Google Scholar]
- Mousavi SA, Chauvin A, Pascaud F, Kellenberger S, Farmer EE (2013) GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 500: 422–426 [DOI] [PubMed] [Google Scholar]
- Mueller MJ, Mène-Saffrané L, Grun C, Karg K, Farmer EE (2006) Oxylipin analysis methods. Plant J 45: 472–489 [DOI] [PubMed] [Google Scholar]
- Park JH, Halitschke R, Kim HB, Baldwin IT, Feldmann KA, Feyereisen R (2002) A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J 31: 1–12 [DOI] [PubMed] [Google Scholar]
- Rhodes JD, Thain JF, Wildon DC (2006) Signals and Signalling Pathways in Plant Wound Responses. Springer, Berlin, pp 391–401 [Google Scholar]
- Saito H, Oikawa T, Hamamoto S, Ishimaru Y, Kanamori-Sato M, Sasaki-Sekimoto Y, Utsumi T, Chen J, Kanno Y, Masuda S, et al. (2015) The jasmonate-responsive GTR1 transporter is required for gibberellin-mediated stamen development in Arabidopsis. Nat Commun 6: 6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador-Recatalà V, Tjallingii WF, Farmer EE (2014) Real-time, in vivo intracellular recordings of caterpillar-induced depolarization waves in sieve elements using aphid electrodes. New Phytol 203: 674–684 [DOI] [PubMed] [Google Scholar]
- Sato C, Aikawa K, Sugiyama S, Nabeta K, Masuta C, Matsuura H (2011) Distal transport of exogenously applied jasmonoyl-isoleucine with wounding stress. Plant Cell Physiol 52: 509–517 [DOI] [PubMed] [Google Scholar]
- Schilmiller AL, Howe GA (2005) Systemic signaling in the wound response. Curr Opin Plant Biol 8: 369–377 [DOI] [PubMed] [Google Scholar]
- Stahlberg R, Cosgrove DJ (1997) The propagation of slow wave potentials in pea epicotyls. Plant Physiol 113: 209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I (2004) The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16: 2117–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassner J, Schaller F, Frick UB, Howe GA, Weiler EW, Amrhein N, Macheroux P, Schaller A (2002) Characterization and cDNA-microarray expression analysis of 12-oxophytodienoate reductases reveals differential roles for octadecanoid biosynthesis in the local versus the systemic wound response. Plant J 32: 585–601 [DOI] [PubMed] [Google Scholar]
- Thorpe MR, Ferrieri AP, Herth MM, Ferrieri RA (2007) 11C-imaging: methyl jasmonate moves in both phloem and xylem, promotes transport of jasmonate, and of photoassimilate even after proton transport is decoupled. Planta 226: 541–551 [DOI] [PubMed] [Google Scholar]
- Turnbull CG, Booker JP, Leyser HM (2002) Micrografting techniques for testing long-distance signalling in Arabidopsis. Plant J 32: 255–262 [DOI] [PubMed] [Google Scholar]
- van Bel AJ, Furch AC, Will T, Buxa SV, Musetti R, Hafke JB (2014) Spread the news: systemic dissemination and local impact of Ca²⁺ signals along the phloem pathway. J Exp Bot 65: 1761–1787 [DOI] [PubMed] [Google Scholar]
- Vellosillo T, Martínez M, López MA, Vicente J, Cascón T, Dolan L, Hamberg M, Castresana C (2007) Oxylipins produced by the 9-lipoxygenase pathway in Arabidopsis regulate lateral root development and defense responses through a specific signaling cascade. Plant Cell 19: 831–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot (Lond) 111: 1021–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Hettenhausen C, Meldau S, Baldwin IT (2007) Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. Plant Cell 19: 1096–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann MR, Maischak H, Mithöfer A, Boland W, Felle HH (2009) System potentials, a novel electrical long-distance apoplastic signal in plants, induced by wounding. Plant Physiol 149: 1593–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZP, Baldwin IT (1997) Transport of [2-14C] jasmonic acid from leaves to roots mimics wound-induced changes in endogenous jasmonic acid pools in Nicotiana sylvestris. Planta 203: 436–441 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.