Shade avoidance increases indeterminacy in the initiating leaf primordium, increasing leaf complexity and serration through a heteroblasty-independent mechanism.
Abstract
Plants sense the foliar shade of competitors and alter their developmental programs through the shade-avoidance response. Internode and petiole elongation, and changes in overall leaf area and leaf mass per area, are the stereotypical architectural responses to foliar shade in the shoot. However, changes in leaf shape and complexity in response to shade remain incompletely, and qualitatively, described. Using a meta-analysis of more than 18,000 previously published leaflet outlines, we demonstrate that shade avoidance alters leaf shape in domesticated tomato (Solanum lycopersicum) and wild relatives. The effects of shade avoidance on leaf shape are subtle with respect to individual traits but are combinatorially strong. We then seek to describe the developmental origins of shade-induced changes in leaf shape by swapping plants between light treatments. Leaf size is light responsive late into development, but patterning events, such as stomatal index, are irrevocably specified earlier. Observing that shade induces increases in shoot apical meristem size, we then describe gene expression changes in early leaf primordia and the meristem using laser microdissection. We find that in leaf primordia, shade avoidance is not mediated through canonical pathways described in mature organs but rather through the expression of KNOTTED1-LIKE HOMEOBOX and other indeterminacy genes, altering known developmental pathways responsible for patterning leaf shape. We also demonstrate that shade-induced changes in leaf primordium gene expression largely do not overlap with those found in successively initiated leaf primordia, providing evidence against classic hypotheses that shaded leaf morphology results from the prolonged production of juvenile leaf types.
Not only is the shape of a single leaf highly multivariate, but the shape of leaves within and between plants is influenced by evolutionary, genetic, developmental, and environmental factors (Chitwood et al., 2012a, 2012b, 2013, 2014; Chitwood and Topp, 2015). Over a lifetime, a plant will produce numerous leaf shapes, influenced by the development of individual leaves as their blades unequally expand (allometric expansion; Hales, 1727; Remmler and Rolland-Lagan, 2012; Rolland-Lagan et al., 2014) and the different types of leaf shapes a plant produces at successive nodes, a result of the temporal development of the shoot apical meristem (SAM; heteroblasty; Goebel, 1900; Ashby, 1948; Poethig, 1990, 2010; Kerstetter and Poethig, 1998). Therefore, leaf shape in a single plant cannot be reduced to a single shape, as shapes are ephemeral, changing from one moment to the next in individual leaves, and the shapes of leaves emerging from successive nodes are not necessarily constant.
When environmental conditions induce changes in leaf shape (plasticity), it is within the above-mentioned developmental context that morphology must be considered (Diggle, 2002). For example, a once prevailing hypothesis was that changes in leaf shape across successive nodes were dependent on nutrition. The rationale for this premise rested on the unique (often irregular) shapes of first emerging leaves, thought to result from abortive development because of reduced photosynthetic support from any previous leaves. Similarly, many plants produce juvenile-looking leaves when shaded, interpreted again as resulting from reduced photosynthate (Goebel, 1908; Allsopp, 1954). This hypothesis has recently been revisited, as sugar has been found to be a signal mediating vegetative phase change (Yang et al., 2013; Yu et al., 2013). Careful morphological studies of leaf development can separate the different effects of shade and heteroblasty, refuting these ideas, at least at a morphological level in some species. In Cucurbita spp., the changes in successively emerging leaves are morphologically observable in early leaf primordia. Despite light intensity-induced changes in the heteroblastic progression of mature leaf morphology, leaf primordia initiated under low light resemble those initiated in sun. This suggests that, in Cucurbita spp., light intensity-induced morphology results from plastic responses later in leaf development after initiation rather than through changes in heteroblasty and timing (Jones, 1995).
In addition to the responses to decreased light intensity discussed above, plants can also sense changes in light quality. Phytochrome proteins, which sense decreases in the ratio of red light to far-red light (R:FR), initiate the shade-avoidance response upon detecting deflected light from competitors (Smith and Whitelam, 1997). Shade-avoiding plants typically exhibit increases in internode and petiole length, reduced leaf mass per area, alterations in stomatal patterning, and shoot/root resource reallocation as an adaptive response to overgrow competitors and better intercept light (Casal, 2012). The changes in leaf shape in response to shade are more ambiguous and can be radically different based on morphological context (such as simple versus complex leaves) and species. For example, in Arabidopsis (Arabidopsis thaliana), shade avoidance is typified by greater increases in petiole length relative to the blade region and inhibited blade outgrowth (Tsukaya et al., 2002; Kim et al., 2005; Kozuka et al., 2005), but in wild relatives of domesticated tomato (Solanum lycopersicum), both the petiole and rachis region expand equally and blade outgrowth is increased. These shade-avoiding responses inversely correlate with the amount of vegetation present in the native locale of an accession, implying adaptive significance in tomato (Chitwood et al., 2012a).
Here, we begin by characterizing the effects of simulated foliar shade on leaf morphology in domesticated tomato and its wild relatives through a meta-analysis of more than 18,000 previously published leaflets (Chitwood et al., 2012a, 2012b, 2014). We find that the effects of decreased R:FR on leaf shape are strong, but only when multiple morphometric parameters are considered across leaflets, both within leaves and across the leaf series, of individual plants. Circularity (a measure of leaflet serration in tomato) and leaf complexity are the most strongly affected individual traits during the shade-avoidance response. We then seek to determine when different shade-avoiding traits manifest during leaf development by swapping plants between light treatments during early leaf development. Leaves will plastically increase their blade area late into development if moved into simulated foliar shade from an initial sunlight treatment, but other traits, such as stomatal patterning, are irrevocably specified earlier during development. Observing increases in SAM size under low R:FR conditions, we then perform laser-capture microdissection to analyze the effects of simulated foliar shade on gene expression in the first emerging leaf primordium (P1) and the meristem. The most conspicuous change in gene expression is increased expression of LeT6 (the tomato ortholog of SHOOTMERISTEMLESS) and other indeterminacy-related genes in the P1, consistent with the increases in leaflet serration and leaf complexity observed in tomato during the shade-avoidance response. Finally, to determine the developmental context of our observations, we compare gene expression changes during shade avoidance with heteroblastic gene expression (i.e. successively initiating leaves at the same developmental stage) in the SAM and young leaf primordia. Gene expression induced by decreased R:FR in light and that which changes with progression through the heteroblastic series in leaf primordia are largely distinct, suggesting not only that the shade-avoidance response is not mediated through heteroblastic changes but also that increases in leaf complexity in these two contexts employ distinct suites of genes.
RESULTS
Morphology of Shade-Avoiding Tomato Leaves
Increases in internode and petiole length induced during the shade-avoidance response are well known and occur in tomato (Fig. 1A). With regard to leaf shape, we previously described increases in blade area, and elongation throughout all parts of the leaf, petiole, and rachis alike, in shade-avoiding tomato plants (Chitwood et al., 2012b). When more comprehensive measures of leaflet contours are assessed using elliptical Fourier descriptors (EFDs; a method to globally describe closed contours as a Fourier series; Kuhl and Giardina, 1982; Iwata and Ukai, 2002) across successively emerging leaves and the leaf proximal-distal axis, shade avoidance does not induce shade morphology per se. Rather, the developmental trajectory of leaf shapes throughout the leaf series takes on qualities associated with more adult leaves with accelerated development (Chitwood et al., 2012b). These shape changes are subtle and may ultimately reflect small accelerations in the transition to reproduction. Shape changes induced by shade are so subtle that when we assessed leaf shape in a set of 76 near-isogenic introgression lines (ILs) harboring tiled genomic segments from the desert-adapted tomato relative Solanum pennellii, shade effects for univariate traits in individual leaflets were not significant and were subsequently ignored in models (Chitwood et al., 2014).
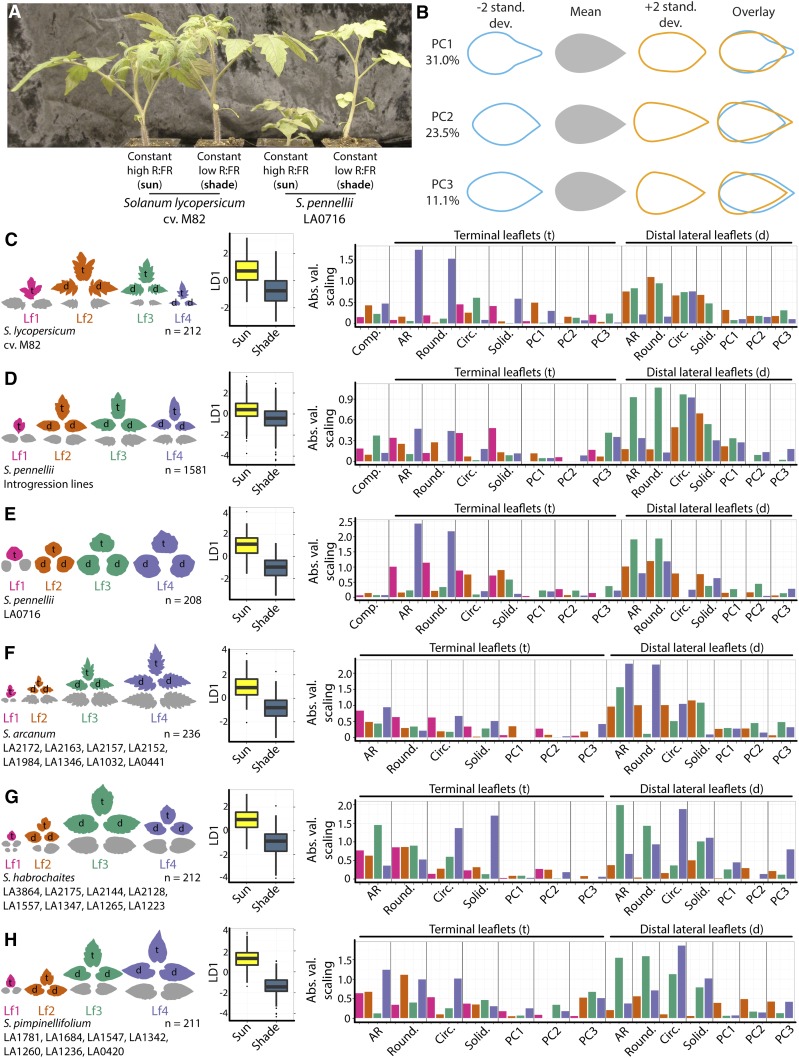
Figure 1.
The morphology of shade-avoiding leaves in domesticated tomato and wild relatives. A, S. lycopersicum ‘M82’ (domesticated tomato) and S. pennellii LA0716 grown under high (simulated sun) and low (simulated foliar shade) R:FR conditions. S. pennellii has an exaggerated shade-avoidance response compared with domesticated tomato, characterized by increased internode and petiole lengths. B, Eigenleaves representing theoretical leaf shapes found ±2 sd along each PC. The percentage variance explained by each PC is indicated. PC space and other morphometric parameters were calculated from previously published data on wild relatives of tomato (Chitwood et al., 2012a, 2012b) and field-grown (Chitwood et al., 2013) and chamber-grown (Chitwood et al., 2014) S. pennellii ILs and parents. Eigenleaves were reproduced here from Chitwood et al. (2014) for meta-analysis purposes. C to H, Separate LDAs by light treatment performed for leaflets across the leaf series for S. lycopersicum ‘M82’ (C), S. pennellii ILs (D), S. pennellii LA0716 (E), S. arcanum (F), S. habrochaites (G), and S. pimpinellifolium (H) accessions. Leaflets used in the analyses are indicated by color (magenta, leaf 1; orange, leaf 2; teal, leaf 3; and lavender, leaf 4) and letters (t, terminal; and d, distal lateral). Box plots show resulting LD1 values for each analysis by light treatment (yellow, simulated sun; and dark blue, simulated foliar shade). Bar plots show the absolute values of scalings resulting from LDAs, which indicate the relative contributions of traits to the discrimination of leaflets by light treatment. AR, Aspect ratio.
Here, before embarking upon describing the developmental gene regulatory networks in the SAM and early leaf primordia that are activated during shade avoidance in domesticated tomato, we wish to fully describe the morphological changes in tomato leaf shape in a meta-analysis. We draw upon (1) more than 11,000 sampled leaflets from up to eight accessions each of Solanum arcanum, Solanum habrochaites, and Solanum pimpinellifolium (Chitwood et al., 2012a, 2012b) and (2) more than 33,000 leaflets from the S. pennellii ILs and their two parents, S. lycopersicum ‘M82’ and S. pennellii LA0716 (Chitwood et al., 2014; Supplemental Data Set S1). Leaflets from both data sets were grown under simulated sun and foliar shade conditions with equal photosynthetically active radiation but lower R:FR in shade conditions created by alternating white light and far-red fluorescent bulbs (see “Materials and Methods”). A principal component analysis (PCA) describing EFDs of these leaflets and field-grown leaves from the ILs (Chitwood et al., 2013) was calculated previously to describe the overall tomato leaf morphospace (Chitwood et al., 2014), and we use the previously published principal components (PCs) here in our analysis (Fig. 1B). In addition to PCs, shape is described by measures influenced by length-to-width ratio, including aspect ratio ([Major axis]/[Minor axis]) of the best-fitted ellipse) and roundness (4 × [Area]/{π × [Major axis]2}), inversely related to aspect ratio), and traits that largely reflect serrations and lobing, including circularity (4π × [Area]/[Perimeter]2) and solidity ([Area]/[Convex area]).
Because shade effects on leaf morphology are so subtle for individual shape features in each measured leaflet (Chitwood et al., 2014), we have used linear discriminant analysis (LDA) to maximize the discrimination of leaf shapes from simulated sun and foliar shade conditions. We began by considering each trait alone across seven different leaflet types (the terminal leaflets of the first four leaves and averages of distal lateral leaflets from leaves 2–4; more than 18,000 leaflets). Because of extremely high replication within single genotypes or classes of genotypes, the resulting linear discriminant 1 (LD1) is significantly different between sun and shade treatments for nearly all traits in all genotype classes (Table I). However, the ability to discriminate shade and sun leaves is not nearly as powerful for single traits when all traits are considered in combination across the leaf series (Table I). Previous analyses suggested that the morphological effects of shade avoidance on tomato leaves were either subtle (Chitwood et al., 2012b) or not significant (Chitwood et al., 2014) when individual traits were considered alone for particular leaflets. However, using the resultant linear discriminant space defined for all measured traits across the leaf series in a predictive fashion, a majority of plants, across tomato and its wild relatives, can be correctly identified as growing under simulated sun or foliar shade conditions (Table II). These results suggest that, although morphological changes induced by shade for any single shape feature for a particular leaflet are weak, the signature across the leaf series and over many shape traits is combinatorially quite strong.
Table I. P values for differences in LD1 values between light treatments.
LDAs were performed for the given traits for leaflets across the leaf series for the indicated genotypes. Shown are P values for differences in LD1 values between simulated sun and foliar shade light treatments as calculated using the Mann-Whitney U test. Results from an LDA performed using all traits are also provided. NA, Not applicable.
| Trait | S. lycopersicum ‘M82’ (n = 212) | S. pennellii ILs (n = 1,581) | S. pennellii (n = 208) | S. arcanum (n = 236) | S. habrochaites (n = 212) | S. pimpinellifolium (n = 211) |
|---|---|---|---|---|---|---|
| Leaf complexity | 0.002164 | 5.19E-09 | 0.4715 | NA | NA | NA |
| Aspect ratio | 0.001312 | 2.06E-05 | 5.69E-05 | 5.40E-05 | 2.20E-08 | 0.0009972 |
| Circularity | 2.17E-08 | 2.62E-11 | 0.001226 | 0.003227 | 3.57E-06 | 1.27E-07 |
| Roundness | 0.002222 | 3.15E-05 | 8.71E-05 | 0.000302 | 4.38E-08 | 0.0009275 |
| Solidity | 3.93E-05 | 3.91E-05 | 1.26E-08 | 0.0003157 | 2.11E-06 | 1.27E-10 |
| PC1 | 0.0002014 | 2.77E-07 | 0.01267 | 2.44E-05 | 0.001133 | 5.32E-07 |
| PC2 | 0.001799 | 1.07E-06 | 6.39E-07 | 7.46E-05 | 4.04E-07 | 0.001266 |
| PC3 | 4.46E-05 | <2.2e-16 | 0.1103 | 9.62E-05 | 3.72E-11 | 4.05E-14 |
| All traits | <2.2e-16 | <2.2e-16 | <2.2e-16 | <2.2e-16 | <2.2e-16 | <2.2e-16 |
Table II. Predicted identities of leaflets.
Using calculated linear discriminants, plants were predicted as originating from simulated sun or foliar shade treatments using morphometric trait information for leaflets across the leaf series. Shown are numbers for the actual and predicted light treatments for plants and the percentage correct identification for each light treatment.
| Species | No. of Plants | Shade Predicted as Shade | Sun Predicted as Sun | Shade Predicted as Sun | Sun Predicted as Shade | Correctly Predicted Shade | Correctly Predicted Sun |
|---|---|---|---|---|---|---|---|
|
% |
|||||||
| S. lycopersicum ‘M82’ | 212 | 82 | 80 | 25 | 25 | 76.6 | 76.2 |
| S. pennellii ILs | 1,581 | 506 | 548 | 278 | 249 | 64.5 | 68.8 |
| S. pennellii | 208 | 97 | 89 | 8 | 14 | 92.4 | 86.4 |
| S. arcanum | 236 | 101 | 87 | 23 | 25 | 81.4 | 77.7 |
| S. habrochaites | 212 | 86 | 89 | 18 | 19 | 82.7 | 82.4 |
| S. pimpinellifolium | 211 | 87 | 103 | 13 | 8 | 87.0 | 92. |
What are the discriminants that separate sun and shade-avoiding leaflets? An analysis of the absolute values of the LD1 scalings (coefficients that when multiplied by trait values additively produce the resultant linear discriminant values) for each genotypic class (Fig. 1, C–H) shows that shape attributes describing length-to-width ratio (aspect ratio and roundness) and serration and lobing (circularity and solidity), and to a lesser extent PCs derived from EFDs, all contribute to the discrimination of shade and sun plants (Supplemental Data Set S2). These contributions vary across the leaf series, sometimes strongly, for different genotype classes. From these results, to say that a particular aspect of shape, or particular leaflets in the leaf series, predominantly contribute to shade-avoiding leaf morphology would be premature. Rather, shade avoidance strongly affects leaf shape in a way that touches upon many metrics commonly used to quantify morphology.
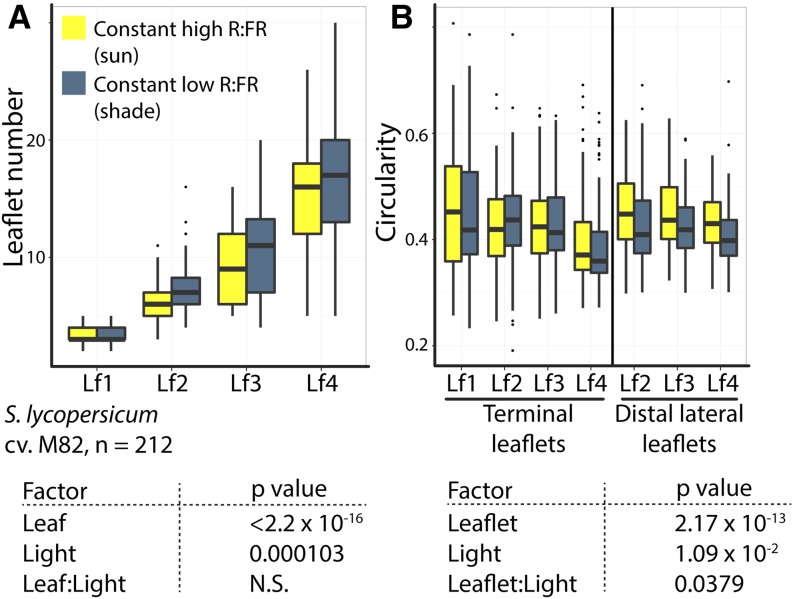
To demonstrate the subtle contributions of individual morphometric parameters to shade-avoiding leaf morphology, leaf complexity, aspect ratio, roundness, circularity, and solidity were modeled for S. lycopersicum ‘M82’ data as a function of leaflet type, light treatment, and their interaction using ANOVA. All morphometric traits strongly vary across leaflet types (or the leaf series, for leaf complexity), but only leaf complexity and circularity (a measure of serration in tomato leaflets) vary significantly by light treatment in domesticated tomato, as defined by an α level of P < 0.05 (Table III). Additionally, the interaction term between leaflet type and light treatment is significant for circularity as well. Box plots for leaf complexity and circularity demonstrate the subtlety of the effects of light treatment on domesticated leaf morphology when considered within the greater context of shape changes induced by the leaf series itself (Fig. 2). The directions of these effects are such that shade avoidance increases leaf complexity (Fig. 2A) and increases leaflet serration (lower circularity values), especially in distal lateral leaflets compared with terminal leaflets (Fig. 2B). We first describe the morphogenetic window during which the shade-avoidance response manifests developmentally in leaves and then describe gene expression changes in the SAM and early leaf primordia induced by shade consistent with these morphological effects.
Table III. Light affects domesticated tomato leaf complexity and serration.
For each of the indicated traits, an ANOVA was performed considering leaflet type (or leaf, for leaf complexity), light treatment, and a leaflet-treatment interaction effect. Only significant terms were included in the final model, for which P values are indicated. NS, Not significant at the 0.05 α level.
| Trait | Leaflet | Light Treatment | Interaction |
|---|---|---|---|
| Leaf complexity | <2.2E-16 | 0.000103 | NS |
| Aspect ratio | <2.2E-16 | NS | NS |
| Roundness | <2.2E-16 | NS | NS |
| Circularity | 2.17E-13 | 1.09E-02 | 0.0379 |
| Solidity | <2.2E-16 | NS | NS |
Figure 2.
Leaf complexity and serration are increased during the shade-avoidance response in domesticated tomato. Box plots show leaflet number, a measure of leaf complexity (A), and circularity, decreased values of which indicate increased serration in tomato leaflets (B), across leaves and leaflet types in domesticated tomato. Not only does shade avoidance increase leaf complexity and serration but also the heteroblastic series (developmental differences in successively emerging leaves). For significance values of light and developmental effects on leaf shape, see Table III. Significance values for different factors affecting leaf shape for leaf complexity and circularity are provided below the graphs for convenience. Light treatment is indicated by color (yellow, simulated sun; and dark blue, simulated foliar shade). N.S., Not significant.
Early Versus Late Developmental Responses to Shade
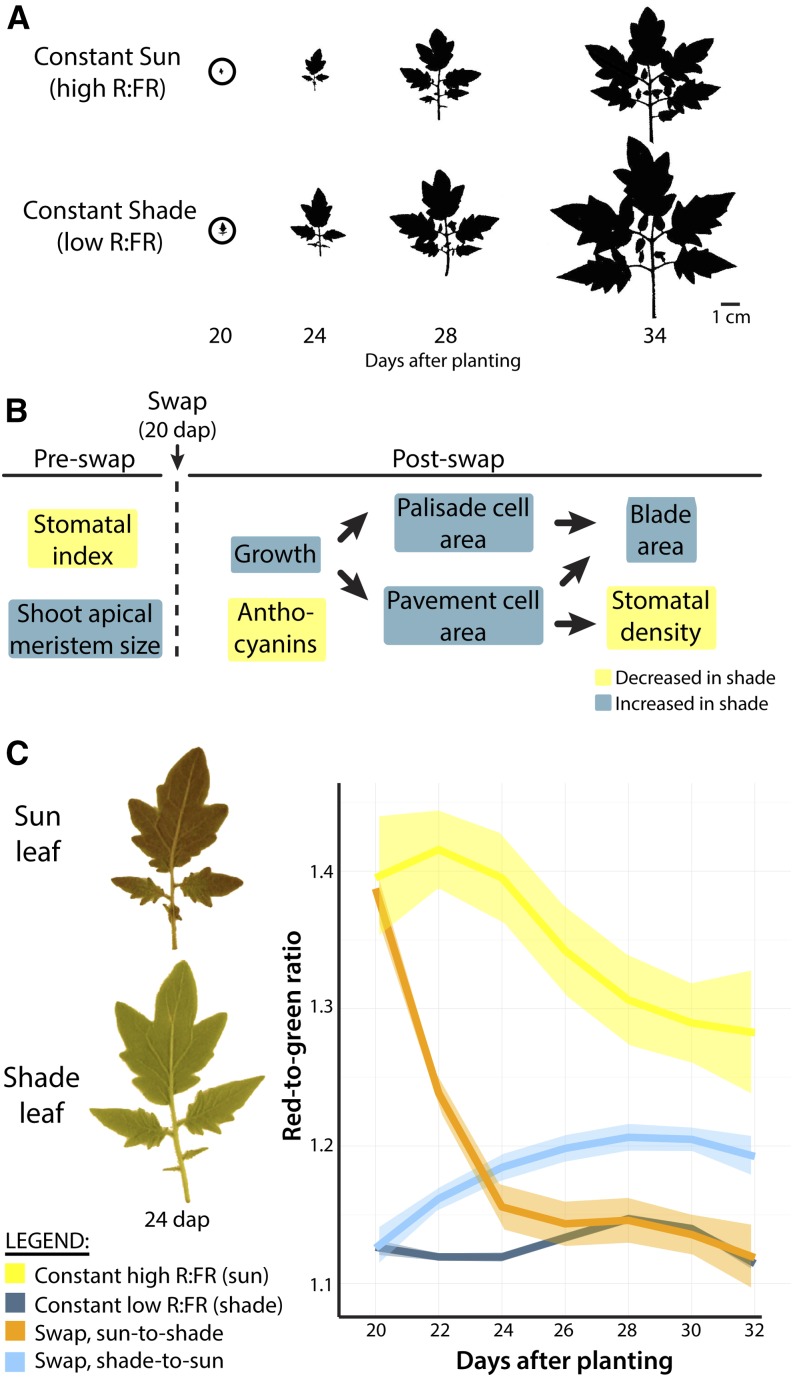
To resolve the developmental window during which shade-avoidance traits manifest in tomato leaves, plants were reciprocally swapped between simulated sun (high R:FR) and foliar shade (low R:FR) conditions at 20 d after planting (DAP). At 20 DAP, leaf 2 has initiated but has yet to fully expand in plants grown under either constant sun or shade conditions (Fig. 3A). Comparing plants grown under constant sun (yellow) and shade (dark blue) conditions reveals shade-induced phenotypes. If plants swapped from sun into shade (orange) or from shade into sun (cyan) retain the phenotype associated with their initial growth conditions, it suggests that shade alters the trait during early leaf development and that it persists regardless of subsequent changes in light quality. Traits that are altered by the new environment postswap indicate that a trait can be changed late in development (after laminar expansion). As described below, examples of shade-induced traits determined preswap include stomatal patterning (stomatal index, the ratio of stomata to other epidermal cells, distinct from stomatal density) and the size of the SAM, which are irrevocably altered by preswap light conditions (Fig. 3B). Postswap, shade-induced increases in leaf size are achieved through increased palisade and pavement cell size, which increase blade area and reduce absolute stomatal density (Fig. 3B; arrows indicate the developmental progression of postswap shade-induced phenotypic changes [e.g. cellular expansion leads to increased blade area]). The ability of a postinitiated leaf from one light condition to achieve the attributes of leaves from the other condition indicates that the trait can be influenced late in development, after blade expansion. An excellent example of this scenario is anthocyanin accumulation under simulated sun conditions, which dissipates upon shifting a mature leaf into shade (Fig. 3C).
Figure 3.
Swap experiments resolve the shade-avoidance responses before and after laminar expansion. A, Outlines of leaf 2, in constant simulated sun (high R:FR) and constant simulated shade (low R:FR) treatments, show the exponential increases in growth that occur between 20 and 34 DAP, during which traits were recorded. In swapped light conditions, plants are swapped into different light treatments at 20 DAP, at the onset of laminar expansion in leaf 2. B, Traits specified preswap and postswap. Arrows indicate the developmental progression of traits: that is, the effects of one trait (e.g. cell expansion) on subsequent traits (e.g. blade area and stomatal density). Yellow indicates decreased trait values upon swap to shade, and dark blue indicates increased values. C, Red-to-green ratio in leaves is influenced by shade 20 DAP. Sun leaves swapped into shade can dissipate anthocyanins such that levels comparable to constant shade plants are attained. Transparent bands indicate 95% confidence intervals of Loess regression. Yellow, constant sun; dark blue, constant shade; orange, sun-to-shade swap; cyan, shade-to-sun swap.
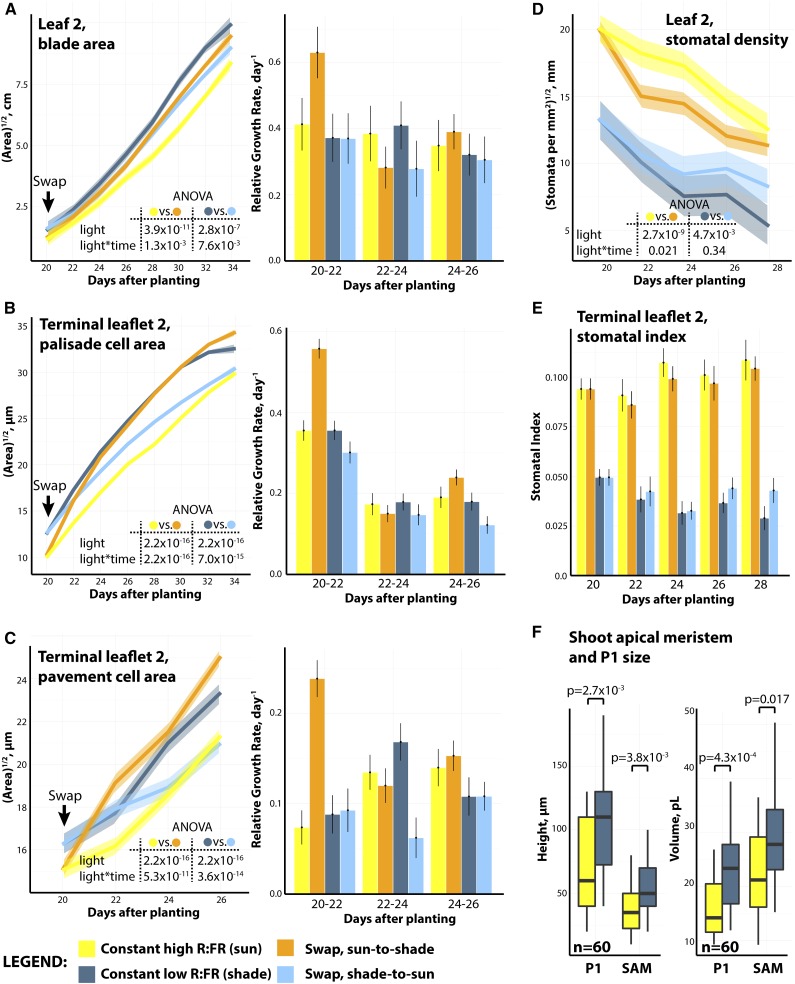
Overall blade area in leaves is remarkably labile late into development. Leaves swapped from sun into shade (orange) increase significantly in overall blade area compared with leaves that remain in sun (yellow) and vice versa (Fig. 4A). Importantly, the blade area of swapped leaves does not fully assume the area of leaves grown under constant conditions, suggesting that, although labile, a fraction of the size of leaf 2 is fixed before 20 d, when blade expansion had yet to occur. Like blade area, cell sizes (both palisade and pavement cells) in swapped leaves rapidly adjust to the postswap light condition, but unlike blade area, the cells attain the size of cells in leaves constantly grown under the postswap light condition (Fig. 4, B and C).
Figure 4.
Shade avoidance is characterized by rapid increases in relative growth rate upon acute shade exposure. A to C, Swap results for leaf 2 overall blade area (A), palisade cell area (B), and pavement cell area (C). Indicated for each trait are P values for factors from fitted ANOVA models comparing constant sun with sun-to-shade treatments and constant shade with shade-to-sun treatments. For each trait, relative growth rates for each condition over 2-d intervals are shown. D and E, Swap results for leaf 2 stomatal traits: absolute stomatal density (D) and stomatal index (patterning) from the terminal leaflet (E). F, Increases in P1 and SAM height and volume as measured from reconstructed histological sections. P values indicate significant differences between constant sun and shade treatments for each trait measured. Transparent bands indicate 95% confidence intervals of Loess regression. Yellow, constant sun; dark blue, constant shade; orange, sun-to-shade swap; cyan, shade-to-sun swap.
Calculation of relative growth rate (the change in trait value over time normalized for the magnitude of the trait) shows that growth under all conditions occurred at similar relative rates, except for a burst of growth when plants are first swapped from sun into shade (Fig. 4, A–C). That is, despite plants grown under constant shade conditions being larger, they still grow at equivalent relative growth rates to sun plants once size is taken into account. The burst in relative growth rate upon first exposure to foliar shade is rapid, occurring within the first 2 d after the swap, and transient, subsiding soon after. The rapid expansion of cells introduced to shade, in fully patterned leaves in which most cell division has subsided (Donnelly et al., 1999), contributes toward the increases in blade area observed in postswap shade-avoiding plants. Further experiments will determine for how long during development a leaf can respond to shade after laminar expansion, but the response may ultimately be associated with the initial exponential growth rate of leaves and begin to subside as leaf growth plateaus and ultimately ceases.
Some leaf traits are fixed before the 20-d swap and irrevocably change despite any postswap changes in light quality. Stomatal density (the number of stomata per given leaf surface area) is higher in sun compared with shade conditions and inversely tracks pavement cell size in swapped plants, suggesting that shade-induced increases in pavement cell area push stomata away from each other after the swap (Fig. 4D). However, stomatal index (the ratio of stomata to all epidermal cell types, a measure of stomatal patterning), which is also higher in sun compared with shade, remains constant after swapping, indicating that the ratio of stomata to total epidermal cell number had been previously, and irrevocably, specified previous to the swap during early leaf development (Fig. 4E).
Previous experiments, purposefully altering either the CO2 concentration (Lake et al., 2001) or irradiance (Thomas et al., 2004) of mature leaves, have observed non-cell-autonomous effects on stomatal patterning, epidermal cell morphology, and overall size of developing leaves. Such an observation is consistent with mature leaves sensing changes in light quality and transmitting signals (whether metabolites, hormones, transcripts, or proteins or a combination thereof) to alter development in young leaf primordia, similar in concept to the preswap changes in leaf morphology we observe (Figs. 3 and 4). Shade avoidance may require a similar mechanism: although the SAM of tomato is relatively exposed compared with other species, it is thoroughly encapsulated by leaf primordia, preventing the direct reception of light possible in mature leaves. An important first step toward understanding the connection between shade perception and developmental change is to ask whether leaf primordia in the SAM are altered during the shade-avoidance response.
Analysis of the volume and height of the P1 and SAM (in this case referring to the meristem and the incipient leaf [P0]) shows that both are significantly larger in continuous shade compared with sun (Fig. 4F). As the SAM includes the P0, this observation shows that, in addition to the shade-induced changes we observe later during development, shade likely alters morphology from the very beginnings of leaf development as well. As was shown recently by Nito et al. (2015), gene expression likely underlies such morphological changes in the SAM, and shade alters gene expression more strongly in the SAM compared with the cotyledons in Arabidopsis. In the next section, we explore how shade alters gene expression from the beginnings of tomato leaf development in both the meristem and young leaf primordia.
Shade Alters Developmental Programs in the SAM
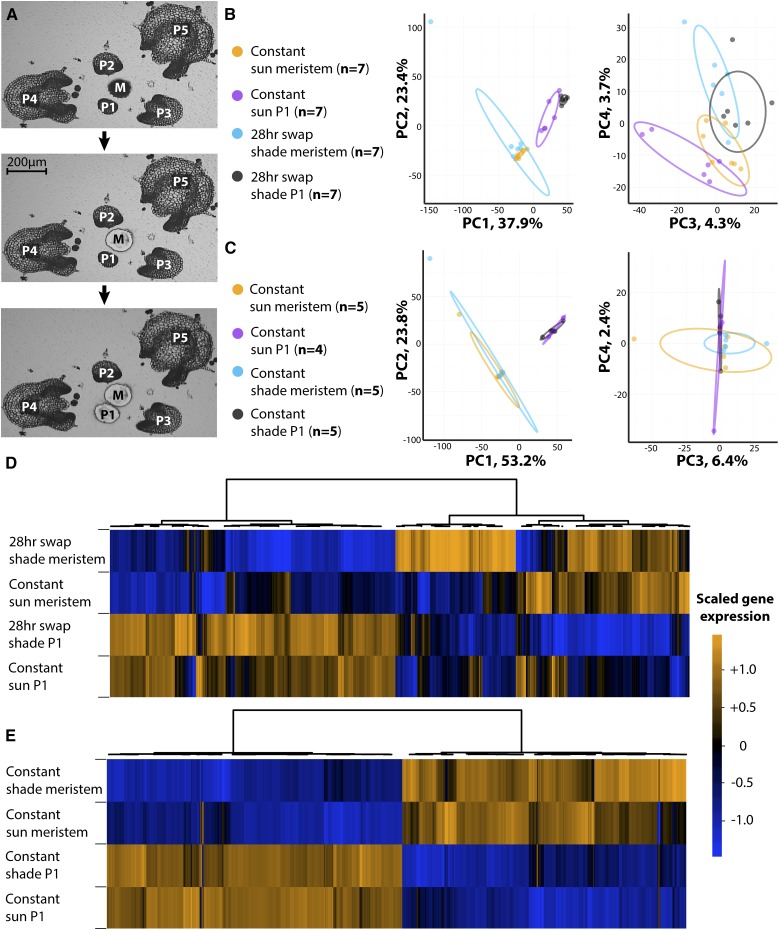
To determine the gene expression changes underlying (1) the global changes in leaf morphology we observe (Fig. 1), especially those increasing leaflet serration and leaf complexity (Fig. 2; Table III), and (2) increases in leaf primordium and meristem size induced by shade (Fig. 4F), we captured the SAM (which contains both the meristem proper and the P0) and P1 using laser microdissection (Fig. 5A). Given the rapid and temporary burst of growth observed in relative growth rate (Fig. 4, A–C), we measured gene expression in the meristem and organ primordia from plants grown in constant high R:FR (sun) and counterparts shifted from high R:FR to low R:FR light (shade) for 28 h, assuming that the largest changes in gene expression would correspond to the acute changes in relative growth rate we observe (Fig. 4). Using PCA, PCs PC1 and PC2 cluster RNA sequencing (RNA-Seq) replicates more strongly by organ type (SAM or P1) than light treatment (constant sun or 28 h of shade), indicating that the differentiation of a leaf from the meristem alters gene expression more strongly than light conditions (Fig. 5B). The effects of light treatment are still observable, however, as PC3 and PC4 cluster samples more closely by light treatment than organ type.
Figure 5.
Transcriptional responses to shade in the SAM. A, Steps of laser microdissection, capturing the SAM (denoted M for meristem) followed by P1. B and C, PCA on replicate gene expression values: constant sun and 28-h swap to shade (B) and constant sun and constant shade conditions (C). Note the separation by light conditions in PCs PC3 and PC4 under the swap experiment absent under constant conditions. Ovals indicate 95% confidence ellipses. Orange, constant sun SAM; magenta, constant sun P1; cyan, constant/28-h shade SAM; black, constant/28-h shade P1. D and E, Hierarchical clustering of those genes differentially expressed either between tissues or by light treatments: constant sun and 28-h swap to shade (D) and constant sun and constant shade conditions (E). Note that under constant light conditions, most differential expression separates between meristem and primordium, whereas in the swap experiment, light conditions contribute to gene expression differences as well. Color indicates averaged, scaled gene expression levels across tissues and treatments (orange, high expression; and blue, low expression).
Acute changes in SAM gene expression upon exposure to 28 h of shade relative to constant sun-grown controls are modest. Only 54 genes are differentially expressed in the SAM, and a majority of those (52) are down-regulated upon the shift to low R:FR (Supplemental Data Set S3). Among these genes are LIGHT-SENSITIVE HYPOCOTYL (LSH) and TORNADO (TRN) homologs (as identified through reciprocal BLAST searches with Arabidopsis genes, described in Chitwood et al., 2013). LSH genes mediate light-regulated development, meristem maintenance, and organogenesis (through boundary specification; Zhao et al., 2004; Cho and Zambryski, 2011). In particular, in tomato, the LSH6 homolog TERMINATING FLOWER leads to premature meristem growth arrest and the LSH3b homolog is implicated in modulating leaf complexity across the tomato clade (MacAlister et al., 2012; Ichihashi et al., 2014), although neither of these genes is the LSH homolog we observe differentially expressed. Both tomato LSH6 and LSH3b homologs interact with BLADE-ON-PETIOLE a (BOPa), which patterns primary leaflet specification across the tomato rachis, and LSH3b directly binds the promoter of PETROSELINUM that regulates the tomato ortholog of SHOOTMERISTEMLESS (LeT6), which modulates leaf complexity (Janssen et al., 1998; Kimura et al., 2008; Ichihashi et al., 2014). trn mutations disrupt auxin regulation and promote senescence (Cnops et al., 2006). TRN function is epistatic to PHANTASTICA/ASYMMETRIC LEAVES1 (PHAN/AS1), which down-regulates KNOTTED1-LIKE HOMEOBOX (KNOX) gene expression in the P0. Thus, meristems acutely exposed to low R:FR light conditions (shade) for 28 h exhibit changes in the expression of genes intimately associated with leaf development and complexity in tomato, which are altered by the shade-avoidance response (Figs. 1 and 2; Table III).
The P1 response to acute shade is far more robust than that of the SAM. Genes are both significantly down- and up-regulated (290 down-regulated and 355 up-regulated) in the P1 (Supplemental Data Set S4). Up-regulated genes in the P1 upon acute shade exposure are significantly enriched for an auxin Gene Ontology (GO) term and numerous transcription-related terms (Supplemental Data Set S5). Many of the genes described by transcriptional GO terms are developmental regulators associated with meristem identity and, in tomato, leaf complexity. Notably, of the 10 most differentially expressed genes in the shade P1, five are KNOX and KNOX-related, including LeT6 (Supplemental Data Set S4). Also highly expressed in the shade P1 are WUSCHEL (WUS), CLAVATA1 (CLV1), and HANABA TANARU as well as PLETHORA (PLT) and EPIDERMAL PATTERNING FACTOR-LIKE (EPFL) homologs, the former consistent with increases in leaf indeterminacy and complexity (Fig. 2; Table III) and the latter consistent with shade-induced changes in stomatal patterning (Fig. 4, D and E). Commensurately, genes promoting leaf differentiation are down-regulated, including PHAN/AS1, SAWTOOTH (SAW), JAGGED/LYRATE (JAG/LYR), and TCPs (TEOSINTE BRANCHED1, CYCLOIDEA, and PROLIFERATING CELL FACTOR1 [PCF1] and PCF2; Supplemental Data Set S4). In the P1, acute shade promotes the expression of genes associated with indeterminacy, cell proliferation, and the meristem while down-regulating genes associated with leaf differentiation and patterning.
The up-regulation of KNOX gene expression early in leaf development (at the P0-P1 stage) is important. In tomato (and many other species), KNOX gene activity promotes leaflet formation (Janssen et al., 1998; Bharathan et al., 2002). Additionally, KNOX activity is associated with meristem identity (Vollbrecht et al., 1991) and in leaves has stage-specific effects. In young tomato leaf primordia, KNOX activity prolongs the process of leaf initiation, consistent with the changes in gene expression we observe associated with an indeterminate fate and antagonistic with differentiation (Shani et al., 2009). Again, such changes in gene expression are consistent with increases in leaflet serration and leaf complexity we observe induced during the shade-avoidance response (Figs. 1 and 2; Table III).
With respect to hormones, we observe increased expression of AUXIN RESISTANT, INDOLE-3-ACETIC ACID INDUCIBLEs, and AUXIN RESPONSE FACTORs (ARFs) as well as increased cytokinin oxidase homolog transcript levels in the P1 samples exposed to acute shade (Supplemental Data Set S4). Auxin-induced cytokinin breakdown has been shown to lead to a transient arrest in leaf development in Arabidopsis (Carabelli et al., 2007), and shade avoidance-induced up-regulation of auxin-related transcripts has been observed in the shoot apex and young leaf primordia (Nito et al., 2015). However, unlike the inhibited blade outgrowth observed in Arabidopsis, shade induces increased laminar outgrowth in tomato (Chitwood et al., 2012a), suggesting alternative regulation of cytokinin during shade avoidance in tomato. We observe increased cytokinin responsiveness through increased expression of cytokinin-inducible WOODEN LEG and numerous HISTIDINE-CONTAINING PHOSPHOTRANSMITTER and ARABIDOPSIS RESPONSE REGULATOR homolog transcripts and cell division markers, consistent with the increased expression in KNOX and other meristem identity genes described above. KNOX gene activity and cytokinin are known to affect SAM size (Vollbrecht et al., 2000; Giulini et al., 2004; Leibfried et al., 2005), providing a basis for the shade-induced increases in SAM and P1 volumes we observe (Fig. 4F).
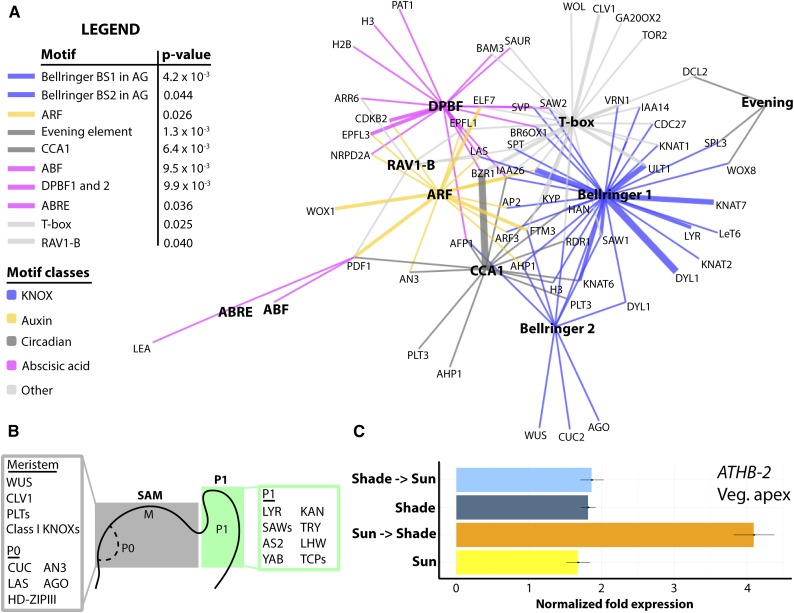
To better understand the gene regulatory networks that underlie acute shade-induced increases in SAM cell proliferation, growth, and indeterminacy, promoters of differentially expressed genes were analyzed for overrepresented motifs. Many promoters of differentially expressed genes contain multiple types of significantly enriched motifs (Fig. 6A). BELLRINGER (a class I KNOX gene transcriptional regulator) and ARF binding sites are among the enriched motifs, congruent with KNOX and auxin response genes that are differentially expressed upon exposure to acute shade in the P1. Interestingly, EVENING ELEMENT and CIRCADIAN CLOCK ASSOCIATED1 binding sites are overrepresented, which reflects the gating of the shade-avoidance response by circadian rhythms (Salter et al., 2003; Nozue et al., 2011). We also observe an enrichment of ABA motifs, reflecting the increased expression of ABA-related transcripts (e.g. LATE EMBRYOGENESIS ABUNDANT and ABA-RESPONSIVE ELEMENT BINDING PROTEIN homologs) in the shade P1 and increased ABA levels in shaded tomato leaves (Cagnola et al., 2012).
Figure 6.
Enriched motifs in promoters of select genes acutely up-regulated in shade P1 and ATHB2 expression. A, Provided are P values for the enrichment of different motifs, with color indicating different motif classes. The network shows select developmental regulators (terminal nodes) that harbor enriched motifs (hubs, boldface). Promoters of many developmental regulators contain motifs of many classes. Classes of motifs are as follows: blue, KNOX; yellow, auxin; dark gray, circadian; magenta, abscisic acid (ABA); and light gray, other. Edge thickness is proportional to the number of motifs present in the promoters of the respective genes. B, Select genes with significantly increased expression in respective tissues, regardless of light treatment (SAM, gray; and P1, green). As expected, numerous regulators of indeterminacy are up-regulated in the SAM, whereas transcripts promoting leaf differentiation and patterning are expressed at higher levels in the P1. Many of the genes associated with indeterminacy in the SAM are differentially expressed upon acute shade treatment in the P1 (see Fig. 7E). C, ATHB2 is rapidly, and transiently, up-regulated after exposure to low R:FR light treatment for 28 h in vegetative apices. This differential expression is absent in LCM SAM and P1 samples. Vegetative apices contain P5 to P7 leaf primordia that are 1 mm in length, demonstrating that differential expression is restricted to relatively mature leaves. Error bars indicate sd. Yellow, constant sun; dark blue, constant shade; orange, sun-to-shade swap; and cyan, shade-to-sun swap.
Shade Avoidance in the SAM Is Ephemeral
Our own results (Fig. 4, A–C) and previous work demonstrate that the shade-avoidance response is a rapid and transient phenomenon (Sessa et al., 2005). If instead of microdissecting SAM and P1 tissue shifted to shade for only 28 h, samples are collected from constant sun and shade conditions (Fig. 5C), transcriptional responses to shade are largely abolished. The absence of a prominent transcriptional shade-avoidance response under constant light conditions reveals that, molecularly, shade avoidance in the tomato SAM and P1 is ephemeral. The differences in shade-avoidance response under transient versus constant shade conditions are easily visualized by hierarchical clustering (Fig. 5, D and E). When plants are exposed to only 28 h of shade, expression patterns of differentially expressed genes separate by tissue and light treatment (Fig. 5D), whereas under constant light conditions, expression patterns are mainly explained by tissue type alone (Fig. 5E). No significant differential gene expression between constant high and low R:FR conditions in the SAM is detected. In the P1, 13 genes are up-regulated in constant shade conditions, including GIBBERELLIN REQUIRING2, a GA biosynthetic gene homolog, and the KNOX gene LeT6 (sufficient to produce leaflets), suggesting that only a minimal shade-avoidance response sustains growth differences between light conditions (Supplemental Data Set S6).
Analysis of genes differentially expressed between the SAM and P1 under constant light conditions (i.e. differential expression by organ type, not light treatment) reveals the same dichotomy between regulators of indeterminacy and differentiation acutely up-regulated in the shade P1 after 28 h (Fig. 6B; Supplemental Data Set S7). WUS, CLV1, LeT6, three class I KNOX family members, and PLT are among the genes up-regulated in the SAM relative to the P1 that are significantly enriched for transcription, cell division, and epigenetics-related GO terms (Fig. 6B; Supplemental Data Set S8). LYR/JAG, SAWs, and TCPs, as well as photosynthetic GO terms, define genes with increased P1 expression (Fig. 6B; Supplemental Data Set S9). The association of known developmental regulators of indeterminacy and differentiation with SAM and P1 samples, respectively, attests to the accuracy of our laser microdissection. It also demonstrates the significant overlap between genetic programs associated with the promotion of indeterminacy in the SAM with P1 samples acutely exposed to shade for 28 h, consistent with the increases in leaf serration and complexity we observe during the shade-avoidance response (Figs. 1 and 2; Table III).
Shade Avoidance and Heteroblasty Increase Leaf Complexity through Independent Pathways
Conspicuously absent from the acute (or constant) transcriptional shade-avoidance response we describe in the tomato SAM and P1 are canonical shade-avoidance response genes, such as ARABIDOPSIS THALIANA HOMEOBOX PROTEIN2 (ATHB2; Supplemental Data Sets S3 and S4; Carabelli et al., 1993; Devlin et al., 2003). Most of these genes are expressed at sufficient levels that a differential expression call is possible. Consistent with previous studies, we do observe increased expression of ATHB2 in shade in the vegetative apex by quantitative reverse transcription-PCR (which includes leaf primordia up to P5–P7 and 1 mm in length, as opposed to the SAM and P1 captured by laser microdissection), suggesting that, indeed, tomato exhibits a canonical shade-avoidance response that is restricted to mature organs (Fig. 6C; Steindler et al., 1999). Of course, the most tantalizing hypothesis is that, in mature organs, the canonical molecular shade-avoidance pathway cell-autonomously responds to changes in light quality. Yet unknown non-cell-autonomous signals would then be transmitted to the SAM, where developmental changes in initiating organs take place (Lake et al., 2001; Thomas et al., 2004).
The association of classical developmental pathways regulating P0 specification with the shade-avoidance response is unexpected but intriguing, because the phenotypic effects of both pathways are well characterized. Across diverse eudicots, increased expression of KNOX genes (including LeT6, up-regulated in shade P1) and decreased expression of their negative regulators PHAN, SAWs, and LYR/JAG leads to increased leaf complexity and/or serrations (Janssen et al., 1998; Kim et al., 2003; Kimura et al., 2008; David-Schwartz et al., 2009). A prediction from our data is that shade leaves would be more complex. Indeed, this is the case in domesticated tomato (Fig. 2; Table III).
Shade is not the only modulator of leaf complexity and the degree of serration, however. Tomato leaves emerging from successive nodes are increasingly complex and serrated (Fig. 2; Table III; Chitwood et al., 2012a, 2012b, 2014). Such graded morphological change reflects the temporal status of the vegetative meristem as it transitions from vegetative, to adult, to reproductive fates (Ashby, 1948; Diggle, 2002). Recent work in the Brassicaceae revealed that heteroblastic increases in leaf serration are mediated through temporal licensing by small RNAs of CUP-SHAPED COTYLEDONS (CUCs) through protein interactions with TCPs (Rubio-Somoza et al., 2014). In tomato, the CUC2 homolog GOBLET is required for the proper patterning of serrations and leaflet formation (Berger et al., 2009), and the TCP family member LANCEOLATE regulates the differentiation of leaf margins (Ori et al., 2007). In response to acute shade, only GOBLET shows increased expression in the P1, and CUC and TCP family members are not as represented as KNOX genes and other regulators of indeterminacy (Supplemental Data Sets S3 and S4). Despite both shade and heteroblasty mediating increases in leaf complexity and serration, do independent molecular pathways modulate their effects on leaf morphology? This is an especially important question considering the once prevailing hypothesis that changes in shade leaf morphology manifested via alterations in the heteroblastic progression through prolonged juvenility (Goebel, 1908; Allsopp, 1954; Jones, 1995).
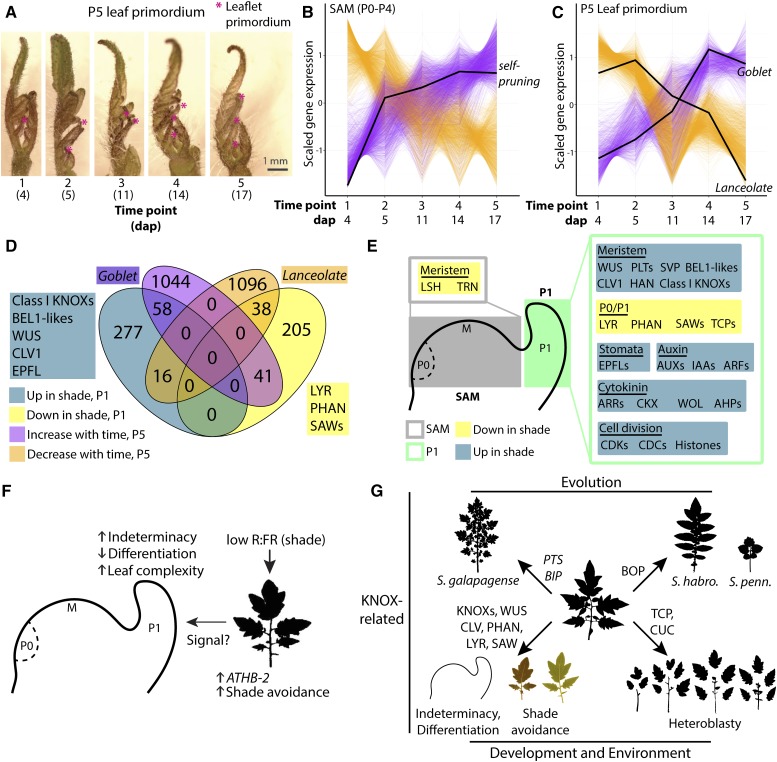
To determine if increases in leaf complexity in shade and the heteroblastic series are modulated by independent pathways at the gene expression level, we performed RNA-Seq analysis of hand-dissected (1) SAMs (consisting of the SAM proper plus four leaf primordia) and (2) primordium P5 at sequential times over a period of 17 DAP, sampling successive leaf primordia at the same stage of development over the heteroblastic series (Fig. 7A). We note that the hand-dissected P5 used to measure heteroblastic effects does not correspond with the P1 laser microdissected for the analysis of shade avoidance, and the subsequent results should be interpreted with this in mind. However, this misses a key point: whereas shade avoidance is measured for a consistent developmental stage (P1), measurements of heteroblasty compare successively emerging leaves (i.e. different DAP) at the same developmental time point (P5).
Figure 7.
Shade avoidance and heteroblasty both modulate leaf complexity but are transcriptomically distinct processes. A, Representative images of P5 leaf primordia that were dissected and analyzed for gene expression using RNA-Seq. At five time points at the indicated DAP, equivalently staged leaf primordia (P5) and SAM (P0–P4) were dissected. Sampling leaf primordia at the same stage of development separates heteroblastic changes in gene expression (i.e. successively emerging leaves) from the development of individual leaves. Note the increases in leaf complexity of successive leaves, indicated by magenta asterisks. B and C, Scaled expression patterns of genes significantly positively (magenta) or negatively (orange) correlated with the heteroblastic series in the SAM (meristem plus P0–P4; B) and P5 (C). Black lines show the expression patterns of SELF-PRUNING in the SAM and GOBLET and LANCEOLATE in the P5. D, Venn diagram of genes that are up-regulated (dark blue) or down-regulated (yellow) upon acute shade exposure in the P1 and genes that are positively (magenta) or negatively (orange) correlated with heteroblasty in the P5. E, Sets of genes up-regulated upon acute shade treatment (blue) or down-regulated (yellow) in the SAM (gray) and P1 (green). F, Model for shade-induced developmental change in the SAM. Low R:FR activates the canonical shade-avoidance response in mature organs through the increased expression of upstream regulators such as ATHB2. A hypothetical signal from mature leaves would increase the expression of positive regulators of indeterminacy and effect patterning differences in the P1 (such as increased leaf complexity and serration). G, Shade is only one of many factors modulating leaf complexity, including heteroblasty (modulated by CUCs and TCPs) and evolution (modulated by BOP and KNOX-like and BEL-like genes). Shade-induced leaf complexity most closely associates with indeterminacy factors regulating meristem maintenance and leaf differentiation.
Those genes with expression significantly correlated with the heteroblastic series were analyzed further (Fig. 7, B and C). In the SAM, we detect 2,171 genes with significantly increasing and 1,797 genes with decreasing expression over the heteroblastic series (Supplemental Data Set S10). SELF-PRUNING, a TERMINAL FLOWER1/FLOWERING LOCUS T homolog, shows increasing expression over the heteroblastic series (Fig. 7B). Significantly enriched GO terms among genes increasing with the heteroblastic series include small RNA regulation, transcription factor, and photosynthetic terms (Supplemental Data Set S11), consistent with the transition to reproductive development, whereas translation dominates genes with decreasing expression over the heteroblastic series (Supplemental Data Set S12).
In the P5, we detect 1,142 genes with significantly increasing and 1,959 genes with decreasing expression in successive leaves of the same developmental stage (Supplemental Data Set S13). Increasing GOBLET and decreasing LANCEOLATE expression is consistent with the increasing leaf complexity seen in successive leaves (Fig. 7C; Ori et al., 2007; Berger et al., 2009). Like the SAM, in the P5, genes with significantly increasing expression over the heteroblastic series include GO terms related to small RNA regulation and transcription factor activity (Supplemental Data Set S14). Decreasing gene expression in the P5 is associated overwhelmingly with photosynthetic activities (Supplemental Data Set S15).
Because only 54 genes are differentially expressed in the SAM upon acute shade exposure (Supplemental Data Set S3), we instead focused our efforts on analyzing the overlap between differential expression in the P1 upon exposure to acute shade (Supplemental Data Set S4) and changes in P5 gene expression over the heteroblastic series (Supplemental Data Set S13). A majority of genes differentially expressed in acute shade (75.9%) and across the heteroblastic series (93.3%) are not shared between experiments (Fig. 7D). GOBLET increases in expression both upon acute shade and across the heteroblastic series (consistent with increased leaf complexity for both), but LANCEOLATE is only significantly associated with heteroblasty. Most importantly, significantly increased expression of the KNOX family members WUS, CLV1, and EPFL homologs and decreased expression of PHAN/AS1, SAW, and LYR, are restricted exclusively to the acute shade condition rather than heteroblasty (Fig. 7D).
We conclude that the increases in leaf complexity observed in shade-avoiding tomato plants and across the heteroblastic series (Fig. 7, B and C) are largely molecularly independent, with KNOX and other indeterminacy regulators mediating the former and possibly CUC and TCP homologs mediating the latter. Such a result is consistent with the acute responses to shade observed both morphologically (Fig. 4) and in gene expression (Fig. 5), which argue against the heterochronic regulation of development. Furthermore, our results are consistent with classic morphological studies in Cucurbita spp. demonstrating that shade does not modulate the heteroblastic progression of leaf forms (Jones, 1995), contrary to the prevailing hypotheses of the time (Goebel, 1908; Allsopp, 1954). Although both heteroblasty and shade avoidance affect leaf complexity and serrations (Fig. 2; Table III), they do so through different associated changes in gene expression.
DISCUSSION
The shade-avoidance response, which allows plants to competitively intercept light and more efficiently use resources when faced with crowding, is inherently morphological in its nature, modifying internode, petiole, and leaf lengths, branching and tillering, the timing of the reproductive transition, and root architecture (Smith and Whitelam, 1997; Steindler et al., 1999; Casal, 2012). In tomato, shade increases leaf complexity and serrations (Figs. 1 and 2; Table III) in addition to leaf length, width, and area, laminar outgrowth, and stomatal patterning (Figs. 3 and 4). It is important to distinguish the developmental origins of such plasticity. Do shade-induced morphological changes occur beginning with the inception of a leaf, or later? Are the morphological changes heterochronic in nature, modulated through the timing of the heteroblastic series, or do they alter leaf morphology independently? Answering such questions is necessary if a developmental understanding of the plasticity induced by the shade-avoidance response is to be achieved.
In tomato, simulated foliar shade alters SAM architecture, demonstrating that shade affects the P0 (Fig. 4F). Underlying change in SAM size is a strong, transcriptional response to acute shade exposure, especially in the P1 leaf primordium (Fig. 5). Rather than the canonical shade-avoidance pathway involved in perceiving and responding to low R:FR light quality in mature leaves, the transcriptional response in the P1 to shade is largely developmental in nature. Shade increases the expression of genes promoting indeterminacy and cell proliferation or maintaining stem cell identity (such as KNOX, WUS, and CLV1 homologs and genes modulating the cytokinin response) while down-regulating genes involved in leaf differentiation (such as PHAN and TCP homologs; Fig. 7E). The shade-induced expression of canonically responding genes such as ATHB2 is restricted to mature organs (Fig. 6C), implying possible non-cell-autonomous signals instructing changes in the patterning of leaf primordia (Fig. 7F), such as increased leaf complexity and serrations (Figs. 1 and 2; Table III).
For over a century, shade-induced changes in leaf morphology have been speculated to be heteroblastic in nature, the morphological changes in leaves arising from successive nodes influenced by nutrition and the amount of available photosynthate (Goebel, 1908; Allsopp, 1954). This hypothesis has recently been revisited, as sugar has been identified as an endogenous signal modulating the vegetative phase transition (Yang et al., 2013; Yu et al., 2013). Yet, classical morphological work in Cucurbita spp. morphologically separates shade- and heteroblasty-related morphological changes in leaf primordia (Jones, 1995). Our results support the hypothesis that shade-induced morphological change is not mediated through changes in the heteroblastic series. (1) Both morphologically (Fig. 4) and molecularly (Fig. 5), shade avoidance is ephemeral rather than ongoing, the latter expected if changes in temporal development had occurred. (2) Measuring the molecular heteroblastic progression in the SAM (Fig. 7B) and P5 (Fig. 7C), it is evident that increases in leaf complexity across the heteroblastic series are accompanied by gene expression changes in TCP and CUC pathways (Rubio-Somoza et al., 2014) rather than the KNOX family members regulating shade avoidance (Fig. 7D). These results are similar to recent findings that KNOX activity underlies both shade- and water-induced increases in leaf complexity in North American lake cress (Rorippa aquatica [Brassicaceae]; Nakayama et al., 2014). The confusion between similar changes in leaves in response to the heteroblastic series and shade avoidance is purely morphological (Fig. 2), reflecting the contributions of distinct molecular pathways (Fig. 7D) to the same trait (Table III).
The study of plant morphology, how it arises and its potential functions, touches upon all attributes of plant science: development, physiology, genetics, evolution, systematics, and environmental response (Kaplan, 2001). Tomato leaf complexity is modulated by all of these factors (Fig. 7G). Our results put the shade-induced increases in leaf complexity of tomato leaves in a broader evolutionary, developmental, and environmental perspective. Shade avoidance most closely aligns with genes responsible for differentiating leaf primordia from the meristem and patterning leaf complexity, in particular LeT6 (Janssen et al., 1998; Kim et al., 2003; David-Schwartz et al., 2009), and is distinct from heteroblastic changes mediated by TCPs and CUCs (Ori et al., 2007; Efroni et al., 2008; Berger et al., 2009; Rubio-Somoza et al., 2014) or evolutionary changes mediated by BOP (Ichihashi et al., 2014) or other KNOX-related pathways (PETROSELINUM and BIPINNATA; Kimura et al., 2008). The morphology of any tomato leaf is the result of contributions of separate developmental, genetic, and environmental effects (Chitwood et al., 2014; Chitwood and Topp, 2015), modulated by distinct molecular pathways.
MATERIALS AND METHODS
Phenotypic Analysis
Tomato seeds (Solanum lycopersicum ‘M82’) were washed in 50% (v/v) bleach for approximately 2 min, rinsed, and placed onto water-soaked paper towels in Phytatrays (Sigma) in the dark for 3 d at room temperature. Phytatrays were then placed into high R:FR (sun) conditions as described below for another 3 d. Seedlings were then transplanted into Sunshine Mix soil (Sun Gro) in 9 × 4 subdivided trays (11 × 22 inches).
Half the transplanted seedlings were placed into high R:FR (simulated sun) and the other half into low R:FR (simulated shade) conditions. Temperature was adjusted to 22°C and photoperiod to a 16/8-h light/dark cycle. Lighting consisted of alternating fluorescent (F48T12CWHO) and far-red (F48T12FRHO; peak emission, 750 nm; Interlectric) bulbs. High R:FR was achieved by blocking far-red irradiance with sleeves, whereas all bulbs (both normal fluorescent and far-red) transmitted light in the low R:FR treatment. Shade cover was placed perpendicularly over bulbs in the low R:FR treatment to adjust overall light intensity to match that of the high R:FR condition. The photosynthetically active radiation level for both simulated shade and sun treatments was approximately 110 μmol, and the R:FR under simulated shade and sun conditions was approximately 0.5 and 1.5, respectively.
At 20 DAP, half of the seedlings from each treatment were swapped into the other condition. Measurements were taken every 2 d, starting at 20 DAP and continuing until 34 DAP. Seventeen individuals were measured for each time point for each of four conditions (constant sun, constant shade, sun-to-shade swap, and shade-to-sun swap). At each time point for each condition, plants were randomly selected for analysis.
For leaf area and red-to-green ratio measurements, leaves were arranged under nonreflective glass. Olympus SP-500 UZ cameras were mounted on copy stands (Adorama; 36-inch Deluxe Copy Stand) and controlled remotely by computer using Cam2Com software (Sabsik). ImageJ (Abramoff et al., 2004) was used to threshold, extract, and measure leaf area from photographs. The red-to-green ratio of leaves was measured from the abaxial side. Average red, green, and blue values (RGB, a digital color model) of pixels in the leaf were recorded by selecting leaves using an appropriate tolerance value to separate them from the white background in Photoshop (Adobe).
For pavement cell and stomatal measurements, dental impression (Provil Novo Light Standard Fast; Pearson Dental Supplies) was applied to terminal leaflets using an application gun and allowed to dry before archiving. Fingernail polish (Sally Hanson; Double Duty) was applied to impressions, allowed to dry completely, removed from the impressions, and floated on microscope slides with water. Water was removed, and the nail polish remained affixed to the slide. Micrographs of samples were taken using a standard compound microscope. For each individual impression, two micrographs were taken to ensure representative measures. For each micrograph, four pavement cells were traced using Bamboo Tablets (Wacom) in ImageJ (Abramoff et al., 2004) and the area was recorded. For stomata, Bamboo Tablets were used to quickly place dots in ImageJ over the feature of interest, followed by custom macros that would count and record the number of features. Pseudoreplication was averaged.
To measure palisade cell size, terminal leaflet tissue was cleared using a 3:1 solution of ethanol:acetic acid for 4 h, followed by 70% (v/v) ethanol for 1 h, and 95% (v/v) ethanol solution overnight. The following day, leaflets were mounted between two slides in 95% (v/v) ethanol and images were taken with a standard compound microscope. Two micrographs were taken from each sample, and for each, the area of four palisade cells was measured. Bamboo Tablets were used to trace and measure the area in ImageJ (Abramoff et al., 2004). Pseudoreplication was averaged.
ANOVA models were fitted for leaf area, palisade and pavement cell size, and stomatal density using the aov() function in R (R Core Team, 2013). Traits were appropriately transformed so that linear values over time were attained. ANOVA models were fitted for the constant sun and sun-to-shade swap comparisons as well as for the constant shade and shade-to-sun swap comparisons, and the significance of time, light, and light × time factors was determined. Relative growth rate was calculated over 2-d time spans using the formula (ln(W2) – ln(W1))/(T2 – T1) (Evans, 1972), where W1 and W2 are trait values at times T1 and T2.
Morphometric analysis was carried out on leaflets grown under simulated sun and foliar shade treatments similar to those described above. Experimental procedures to obtain leaflet outlines are detailed in previous publications, including Chitwood et al. (2012a, 2012b) describing leaflets from Solanum arcanum, Solanum habrochaites, and Solanum pimpinellifolium accessions and Chitwood et al. (2014) describing leaflets from S. lycopersicum ‘M82’, S. pennellii LA0716, and ILs. LDA on previously calculated aspect ratio, roundness, circularity, and solidity values, as well as the first three PCs calculated for EFDs (Chitwood et al., 2014), were performed using the lda function from the MASS package in R (Venables and Ripley, 2002). LDA was performed to maximize the discrimination of plants from different light treatments as a function of different traits for leaflets found across the leaf series. Scaling values defining the contributions of traits and leaflets to the discrimination of light treatments from which a plant originates were calculated from scaled values for traits (using the scale function), such that overall trait magnitude did not influence the derived LDA scaling values. The predict function (stats package) and table function (base package) were used (dependent on MASS) to predict the light treatment a plant originated from based on the calculated linear discriminant space. A Mann-Whitney U test (wilcox.test function in R) was used to detect differences in linear discriminant values between light treatments.
Laser-Capture Microdissection and Amplification
Tomato plants were grown as described above in either high or low R:FR conditions. Samples either remained in high and low R:FR conditions for the constant sun and constant shade experiment, or high R:FR plants were swapped into low R:FR conditions 28 h before harvest. Vegetative apices were harvested 19 DAP and were prepared using standard histological procedures as described previously (Belmonte et al., 2013). Briefly, under RNase-free conditions, apices were fixed in ice-cold 3:1 ethanol:acetic acid solution, vacuum infiltrated for 1 h, and fixed overnight rotating at 4°C. Samples were then rinsed three times with 70% (v/v) ethanol, dehydrated in a graded ethanol series from 70% (v/v) to 100% ethanol, and then infiltrated with a xylene series from a 1:3 xylene:ethanol mixture to pure xylene. Samples were incubated with paraffin chips in xylene before numerous paraffin exchanges over 3 d at 60°C. Samples were embedded in disposable rings and blocks and stored for no more than 2 weeks at 4°C before sectioning. Apices were transverse sectioned at 10 μm, one apex per previously RNase-treated polyethylene naphthalate membrane slide, and left to dry overnight at room temperature. Slides were subsequently deparaffinized twice in xylene, 1 min each time.
Approximately four SAMs were microdissected per biological replicate. Approximately 100,000 μm2 of 10-μm-thick sections were captured for both SAM (containing the dome of the SAM and the P0) and P1 samples. All tissue was collected from sections proceeding (in an apical-to-basal direction) from the first appearance of the SAM or P1 to their junction. SAM and P1 tissue was collected from the same vegetative apices, such that most SAM replicates have a sister P1 replicate, derived from the same apices. Microdissected tissue was harvested into RNA extraction buffer (RNAqueous-Micro; Ambion) and stored at −80°C until further use.
RNA was isolated following the manufacturer’s instructions with an additional treatment of the samples on the RNA purification column with RNase-free DNase (1:4 dilution of DNase I in RDD buffer; Qiagen). RNA levels were quantified (Quant-iT RiboGreen RNA Assay Kit; Invitrogen) using an ND-3330 Fluorospectrometer (Nano-Drop). Total RNA was analyzed by microcapillary electrophoresis (RNA 6000 Pico Chip, Agilent 2100 BioAnalyzer; Agilent Technologies) before linear amplification (Ovation Pico WTA System; NuGEN Technologies).
Laser-Capture Microdissection Gene Expression Analysis
Library preparation for RNA-Seq was performed as in our earlier publication (Kumar et al., 2012) except for the following changes: for second-strand synthesis, we added 10 μL of complementary DNA (cDNA; 100–250 ng), 0.5 μL of random primers, and 0.5 μL of deoxyribonucleotide triphosphate. Sample was mixed and heated at 80°C for 2 min, 60°C for 10 s, 50°C for 10 s, 40°C for 10 s, 30°C for 10 s, and 4°C for at least 2 to 5 min. We then added the following (on ice): 5 μL of 10× DNA polymerase I buffer, 31.5 μL of water, and 2.5 μL of DNA polymerase I, followed by incubation at 16°C for 2.5 h. From this point onward, the published RNA-Seq protocol (Kumar et al., 2012) was followed, except for the fragmentation time, which in this case was 20 min.
edgeR (Robinson et al., 2010) was used for differential gene expression analysis. Transcripts for which there were 2 or fewer cpm in less than four replicates were not considered further. General linearized models were first used to estimate light and tissue effects under constant sun and constant shade conditions (McCarthy et al., 2012), but light was not found to be a significant factor. Therefore, pairwise comparisons between light treatments for a given tissue were made using an exact test based on conditional maximum likelihood methods. For consistency, pairwise methods were used to call differential expression in the constant sun and 28-h shade experiments as well.
PCA was performed on all replicates using any gene with differential expression between tissues or light in either the constant sun/constant shade or constant sun/28-h shade experiments using the prcomp() function and visualized using ggplot2 (Wickham, 2009) in R (R Core Team, 2013). Hierarchical clustering was performed on scaled expression values of genes differentially expressed by tissue or light treatment. A distance matrix using the dist() function was calculated followed by hierarchical clustering using the Ward method with the hclust() function. Results were visualized using the geom_tile() function from ggplot2 (Wickham, 2009). Promoter enrichment analysis was performed using custom scripts in R with motifs represented in the Arabidopsis Gene Regulatory Information Server AtTFDB database (http://arabidopsis.med.ohio-state.edu/AtTFDB/; Davuluri et al., 2003; Palaniswamy et al., 2006). Analysis was performed on 1,000 bp upstream of the ATG translation start site. The abundance of promoter elements in differentially expressed genes was compared with motif abundance in promoters of all considered genes using Fisher’s exact test allowing for a single mismatch using the Biostrings package (Pages et al., 2013). For select developmental regulators that (1) were differentially expressed and (2) possessed significantly enriched motifs in their promoter, a network was visualized using Gephi (Bastian et al., 2009).
Heteroblasty Gene Expression Analysis
Tomato plants were grown as described above in a growth chamber at 22°C with 70% relative humidity and a daylength of 16 h. P5 leaf primordia and SAMs (consisting of the SAM proper plus four leaf primordia) were dissected carefully using a razor blade and harvested into RNase-free tubes cooled with liquid nitrogen. This sampling was performed for the following time points: 1 (4 DAP), 2 (5 DAP), 3 (11 DAP), 4 (14 DAP), and 5 (17 DAP). Our experimental design samples successive leaves at the same developmental stage (P5 leaf primordia), measuring effects of the heteroblastic series but not leaf ontogeny.
Heteroblasty RNA-Seq was performed using BrAD-seq, a strand-specific digital gene expression method (Townsley et al., 2015). As briefly as possible, tissues were processed and lysed as described by Kumar et al. (2012) with the following modifications: (1) wash volumes of Washing Buffer A, Washing Buffer B, and Low Salt Buffer were 300 μL each, and buffers were chilled on ice prior to use; and (2) mRNA elution was done into 16 μL of 10 mm Tris-HCl, pH 8, containing 1 mm β-mercaptoethanol. Instead of cDNA, mRNA was fragmented using magnesium ions at elevated temperature, and priming of the cDNA synthesis reaction was carried out with 3′ adaptor polyT priming oligonucleotide in the presence of 5× Thermo Scientific RT Buffer. cDNA synthesis was carried out, and cDNA subsequently was cleaned and size selected using Ampure XP beads. After 5 min, samples were placed on a magnetic tray, supernatant was removed, and pellets were washed twice with 80% (v/v) ethanol. Residual ethanol was removed, and samples were allowed to air dry. Dry pellets were rehydrated by the addition of 4 μL of 10 μm 5′ breath-capture adapter. A 6-μL volume of reaction mix containing Escherichia coli DNA polymerase I was added to the hydrated pellet and mixed by pipetting, and the preenrichment library synthesis reaction was allowed to proceed at room temperature for 15 min.
The preenrichment libraries were washed and size selected using Ampure XP beads present from the previous step and allowed to stand prior to placing on the magnetic tray. Supernatant was removed, and pellets were washed twice with 80% (v/v) ethanol. Pellet was resuspended and placed on a magnetic tray. Supernatant was transferred without beads to fresh strip tubes and stored at −20°C prior to enrichment by PCR. Samples were amplified in a thermocycler using the following program: 98°C for 30 s; 11 cycles of 98°C for 10 s, 65°C for 30 s, and 72°C for 30 s; 72°C for 5 min; and 10°C hold. Each library sample was run on a 1% (w/v) agarose gel for size and quantity reference. The remaining 8 μL of enriched library sample was cleaned and size selected using Ampure XP beads and washed twice with 80% (v/v) ethanol, similar to previous wash steps. The libraries were eluted from the pellet with 10 mm Tris, pH 8, and quantified and pooled as described by Kumar et al. (2012). The 50-bp single-end sequencing was carried out at the Vincent J. Coates Genomic Sequencing Facility at the University of California, Berkeley.
Gene expression analysis was carried out as described above for the laser-capture microdissection data set to arrive at normalized, log2(x+1)-transformed read count values. Replicates were assigned values of 1 to 5 corresponding to the time point at which they were sampled, and the Pearson correlation coefficient and P value were calculated. P values were multiple test adjusted to control false discovery rate using the Benjamini-Hochberg method. GO enrichment terms for genes up- or down-regulated over the heteroblastic series in the SAM (P0–P4) and P5 were determined at a 0.05 false discovery rate cutoff value using the goseq package in Bioconductor (Young et al., 2010). Venn diagrams were created using VennDiagram (Chen and Boutros, 2011). Unless otherwise specified, all analyses were carried out in R (R Core Team, 2013) and graphics were visualized using ggplot2 (Wickham, 2009).
The quality-filtered, barcode-sorted, and trimmed short-read data set was deposited to the National Center for Biotechnology Information Short Read Archive under Bioproject accession number SRP061929. RNA-Seq reads from the constant sun-shade experiment were deposited under accession numbers SRR2141260 and SRR2141262 to SRR2141279. Threads from the transient shift shade experiment were deposited under accession numbers SRR2141280 to SRR2141298, SRR2141300, SRR2141302 to SRR2141306, and SRR2141314 to SRR2141316. Reads from the heteroblasty experiment were deposited under accession numbers SRR2141325 to SRR2141369.
Supplemental Data
The following supplemental materials are available.
Supplemental Data Set S1. Morphometric data for leaflets from domesticated tomato and wild relatives grown under simulated sun and foliar shade conditions.
Supplemental Data Set S2. Scaling values for Linear Discriminant Analyses (LDAs) separating leaflet morphology by light treatment.
Supplemental Data Set S3. Differential gene expression in the SAM between constant sun and 28-h shade treatments.
Supplemental Data Set S4. Differential gene expression in the P1 between constant sun and 28-h shade treatments.
Supplemental Data Set S5. Overrepresented Gene Ontology terms for genes up-regulated in the shade P1.
Supplemental Data Set S6. Differential gene expression in the P1 between constant sun and constant shade treatments.
Supplemental Data Set S7. Differential gene expression between the SAM and P1.
Supplemental Data Set S8. Overrepresented Gene Ontology terms for genes up-regulated in the SAM.
Supplemental Data Set S9. Overrepresented Gene Ontology terms for genes up-regulated in the P1.
Supplemental Data Set S10. Correlation of gene expression with the heteroblastic series in the SAM.
Supplemental Data Set S11. Overrepresented Gene Ontology terms for genes positively correlated with the heteroblastic series in the SAM.
Supplemental Data Set S12. Overrepresented Gene Ontology terms for genes negatively correlated with the heteroblastic series in the SAM.
Supplemental Data Set S13. Correlation of gene expression with the heteroblastic series in the P5.
Supplemental Data Set S14. Overrepresented Gene Ontology terms for genes positively correlated with the heteroblastic series in the P5.
Supplemental Data Set S15. Overrepresented Gene Ontology terms for genes negatively correlated with the heteroblastic series in the P5.
Supplementary Material
Glossary
- R:FR
ratio of red light to far-red light
- EFDs
elliptical Fourier descriptors
- IL
introgression line
- PCA
principal component analysis
- PC
principal component
- LDA
linear discriminant analysis
- LD1
linear discriminant 1
- DAP
days after planting
- SAM
shoot apical meristem
- RNA-Seq
RNA sequencing
- GO
Gene Ontology
- ABA
abscisic acid
- cDNA
complementary DNA
Footnotes
This work was supported by the National Science Foundation (grant no. IOS–0820854 to N.R.S. and J.N.M.).
Articles can be viewed without a subscription.
References
- Abramoff MD, Magelhaes PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics International 11: 36–42 [Google Scholar]
- Allsopp A. (1954) Juvenile stages of plants and the nutritional status of the shoot apex. Nature 173: 1032–1035 [Google Scholar]
- Ashby E. (1948) Studies in the morphogenesis of leaves. I. An essay on leaf shape. New Phytol 47: 152–176 [Google Scholar]
- Bastian M, Heymann S, Jacomy M (2009) Gephi: an open source software for exploring and manipulating networks. International AAAI Conference on Weblogs and Social Media. https://gephi.org/ (August 24, 2013)
- Belmonte MF, Kirkbride RC, Stone SL, Pelletier JM, Bui AQ, Yeung EC, Hashimoto M, Fei J, Harada CM, Munoz MD, et al. (2013) Comprehensive developmental profiles of gene activity in regions and subregions of the Arabidopsis seed. Proc Natl Acad Sci USA 110: E435–E444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger Y, Harpaz-Saad S, Brand A, Melnik H, Sirding N, Alvarez JP, Zinder M, Samach A, Eshed Y, Ori N (2009) The NAC-domain transcription factor GOBLET specifies leaflet boundaries in compound tomato leaves. Development 136: 823–832 [DOI] [PubMed] [Google Scholar]
- Bharathan G, Goliber TE, Moore C, Kessler S, Pham T, Sinha NR (2002) Homologies in leaf form inferred from KNOXI gene expression during development. Science 296: 1858–1860 [DOI] [PubMed] [Google Scholar]
- Cagnola JI, Ploschuk E, Benech-Arnold T, Finlayson SA, Casal JJ (2012) Stem transcriptome reveals mechanisms to reduce the energetic cost of shade-avoidance responses in tomato. Plant Physiol 160: 1110–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabelli M, Possenti M, Sessa G, Ciolfi A, Sassi M, Morelli G, Ruberti I (2007) Canopy shade causes a rapid and transient arrest in leaf development through auxin-induced cytokinin oxidase activity. Genes Dev 21: 1863–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabelli M, Sessa G, Baima S, Morelli G, Ruberti I (1993) The Arabidopsis Athb-2 and -4 genes are strongly induced by far-red-rich light. Plant J 4: 469–479 [DOI] [PubMed] [Google Scholar]
- Casal JJ. (2012) Shade avoidance. The Arabidopsis Book 10: e0157, doi/10.1199/tab.0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Boutros PC (2011) VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinformatics 12: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood DH, Headland LR, Filiault DL, Kumar R, Jiménez-Gómez JM, Schrager AV, Park DS, Peng J, Sinha NR, Maloof JN (2012a) Native environment modulates leaf size and response to simulated foliar shade across wild tomato species. PLoS One 7: e29570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood DH, Headland LR, Kumar R, Peng J, Maloof JN, Sinha NR (2012b) The developmental trajectory of leaflet morphology in wild tomato species. Plant Physiol 158: 1230–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood DH, Kumar R, Headland LR, Ranjan A, Covington MF, Ichihashi Y, Fulop D, Jiménez-Gómez JM, Peng J, Maloof JN, et al. (2013) A quantitative genetic basis for leaf morphology in a set of precisely defined tomato introgression lines. Plant Cell 25: 2465–2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood DH, Ranjan A, Kumar R, Ichihashi Y, Zumstein K, Headland LR, Ostria-Gallardo E, Aguilar-Martínez JA, Bush S, Carriedo L, et al. (2014) Resolving distinct genetic regulators of tomato leaf shape within a heteroblastic and ontogenetic context. Plant Cell 26: 3616–3629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood DH, Topp CN (2015) Revealing plant cryptotypes: defining meaningful phenotypes among infinite traits. Curr Opin Plant Biol 24: 54–60 [DOI] [PubMed] [Google Scholar]
- Cho E, Zambryski PC (2011) Organ boundary1 defines a gene expressed at the junction between the shoot apical meristem and lateral organs. Proc Natl Acad Sci USA 108: 2154–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnops G, Neyt P, Raes J, Petrarulo M, Nelissen H, Malenica N, Luschnig C, Tietz O, Ditengou F, Palme K, et al. (2006) The TORNADO1 and TORNADO2 genes function in several patterning processes during early leaf development in Arabidopsis thaliana. Plant Cell 18: 852–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Schwartz R, Koenig D, Sinha NR (2009) LYRATE is a key regulator of leaflet initiation and lamina outgrowth in tomato. Plant Cell 21: 3093–3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davuluri RV, Sun H, Palaniswamy SK, Matthews N, Molina C, Kurtz M, Grotewold E (2003) AGRIS: Arabidopsis Gene Regulatory Information Server, an information resource of Arabidopsis cis-regulatory elements and transcription factors. BMC Bioinformatics 4: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin PF, Yanovsky MJ, Kay SA (2003) A genomic analysis of the shade avoidance response in Arabidopsis. Plant Physiol 133: 1617–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle P. (2002) A developmental morphologist’s perspective on plasticity. Evol Ecol 16: 267–283 [Google Scholar]
- Donnelly PM, Bonetta D, Tsukaya H, Dengler RE, Dengler NG (1999) Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev Biol 215: 407–419 [DOI] [PubMed] [Google Scholar]
- Efroni I, Blum E, Goldshmidt A, Eshed Y (2008) A protracted and dynamic maturation schedule underlies Arabidopsis leaf development. Plant Cell 20: 2293–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GC. (1972) Relative growth rate. In The Quantitative Analysis of Plant Growth. University of California Press, Berkeley, p 292 [Google Scholar]
- Giulini A, Wang J, Jackson D (2004) Control of phyllotaxy by the cytokinin-inducible response regulator homologue ABPHYL1. Nature 430: 1031–1034 [DOI] [PubMed] [Google Scholar]
- Goebel K. (1900) Organography of Plants. I. General Organography. Hafner Publishing, New York [Google Scholar]
- Goebel K. (1908) Einleitung in die Experimentelle Morphologie der Pflanzen BG Teubner, Leipzig, Germany [Google Scholar]
- Hales S. (1727) Vegetable Staticks or, an Account of Some Statistical Experiments on the Sap in Vegetables. W and J Innys, London [Google Scholar]
- Ichihashi Y, Aguilar-Martínez JA, Farhi M, Chitwood DH, Kumar R, Millon LV, Peng J, Maloof JN, Sinha NR (2014) Evolutionary developmental transcriptomics reveals a gene network module regulating interspecific diversity in plant leaf shape. Proc Natl Acad Sci USA 111: E2616–E2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata H, Ukai Y (2002) SHAPE: a computer program package for quantitative evaluation of biological shapes based on elliptic Fourier descriptors. J Hered 93: 384–385 [DOI] [PubMed] [Google Scholar]
- Janssen BJ, Lund L, Sinha N (1998) Overexpression of a homeobox gene, LeT6, reveals indeterminate features in the tomato compound leaf. Plant Physiol 117: 771–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CS. (1995) Does shade prolong juvenile development? A morphological analysis of leaf shape changes in Cucurbita argyrosperma subsp. sororia (Cucurbitaceae). Am J Bot 82: 346–359 [Google Scholar]
- Kaplan DR. (2001) The science of plant morphology: definition, history, and role in modern biology. Am J Bot 88: 1711–1741 [PubMed] [Google Scholar]
- Kerstetter RA, Poethig RS (1998) The specification of leaf identity during shoot development. Annu Rev Cell Dev Biol 14: 373–398 [DOI] [PubMed] [Google Scholar]
- Kim GT, Yano S, Kozuka T, Tsukaya H (2005) Photomorphogenesis of leaves: shade-avoidance and differentiation of sun and shade leaves. Photochem Photobiol Sci 4: 770–774 [DOI] [PubMed] [Google Scholar]
- Kim M, McCormick S, Timmermans M, Sinha N (2003) The expression domain of PHANTASTICA determines leaflet placement in compound leaves. Nature 424: 438–443 [DOI] [PubMed] [Google Scholar]
- Kimura S, Koenig D, Kang J, Yoong FY, Sinha N (2008) Natural variation in leaf morphology results from mutation of a novel KNOX gene. Curr Biol 18: 672–677 [DOI] [PubMed] [Google Scholar]
- Kozuka T, Horiguchi G, Kim GT, Ohgishi M, Sakai T, Tsukaya H (2005) The different growth responses of the Arabidopsis thaliana leaf blade and the petiole during shade avoidance are regulated by photoreceptors and sugar. Plant Cell Physiol 46: 213–223 [DOI] [PubMed] [Google Scholar]
- Kuhl FP, Giardina CR (1982) Elliptic Fourier features of a closed contour. Comput Graph Image Process 18: 236–258 [Google Scholar]
- Kumar R, Ichihashi Y, Kimura S, Chitwood DH, Headland LR, Peng J, Maloof JN, Sinha NR (2012) A high-throughput method for Illumina RNA-Seq library preparation. Front Plant Sci 3: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake JA, Quick WP, Beerling DJ, Woodward FI (2001) Plant development: signals from mature to new leaves. Nature 411: 154. [DOI] [PubMed] [Google Scholar]
- Leibfried A, To JPC, Busch W, Stehling S, Kehle A, Demar M, Kieber JJ, Lohmann JU (2005) WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 438: 1172–1175 [DOI] [PubMed] [Google Scholar]
- MacAlister CA, Park SJ, Jiang K, Marcel F, Bendahmane A, Izkovich Y, Eshed Y, Lippman ZB (2012) Synchronization of the flowering transition by the tomato TERMINATING FLOWER gene. Nat Genet 44: 1393–1398 [DOI] [PubMed] [Google Scholar]
- McCarthy DJ, Chen Y, Smyth GK (2012) Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res 40: 4288–4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Nakayama N, Seiki S, Kojima M, Sakakibara H, Sinha N, Kimura S (2014) Regulation of the KNOX-GA gene module induces heterophyllic alteration in North American lake cress. Plant Cell 26: 4733–4748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nito K, Kajiyama T, Unten-Kobayashi J, Fujii A, Mochizuki N, Kambara H, Nagatani A (2015) Spatial regulation of the gene expression response to shade in Arabidopsis seedlings. Plant Cell Physiol 56: 1306–1319 [DOI] [PubMed] [Google Scholar]
- Nozue K, Harmer SL, Maloof JN (2011) Genomic analysis of circadian clock-, light-, and growth-correlated genes reveals PHYTOCHROME-INTERACTING FACTOR5 as a modulator of auxin signaling in Arabidopsis. Plant Physiol 156: 357–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori N, Cohen AR, Etzioni A, Brand A, Yanai O, Shleizer S, Menda N, Amsellem Z, Efroni I, Pekker I, et al. (2007) Regulation of LANCEOLATE by miR319 is required for compound-leaf development in tomato. Nat Genet 39: 787–791 [DOI] [PubMed] [Google Scholar]
- Pages H, Aboyoun P, Gentleman R, DebRoy S (2013) Biostrings: String Objects Representing Biological Sequences, and Matching Algorithms. R package version 2.28.0
- Palaniswamy SK, James S, Sun H, Lamb RS, Davuluri RV, Grotewold E (2006) AGRIS and AtRegNet: a platform to link cis-regulatory elements and transcription factors into regulatory networks. Plant Physiol 140: 818–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poethig RS. (1990) Phase change and the regulation of shoot morphogenesis in plants. Science 250: 923–930 [DOI] [PubMed] [Google Scholar]
- Poethig RS. (2010) The past, present, and future of vegetative phase change. Plant Physiol 154: 541–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2013) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna [Google Scholar]
- Remmler L, Rolland-Lagan AG (2012) Computational method for quantifying growth patterns at the adaxial leaf surface in three dimensions. Plant Physiol 159: 27–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland-Lagan AG, Remmler L, Girard-Bock C (2014) Quantifying shape changes and tissue deformation in leaf development. Plant Physiol 165: 496–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Somoza I, Zhou CM, Confraria A, Martinho C, von Born P, Baena-Gonzalez E, Wang JW, Weigel D (2014) Temporal control of leaf complexity by miRNA-regulated licensing of protein complexes. Curr Biol 24: 2714–2719 [DOI] [PubMed] [Google Scholar]
- Salter MG, Franklin KA, Whitelam GC (2003) Gating of the rapid shade-avoidance response by the circadian clock in plants. Nature 426: 680–683 [DOI] [PubMed] [Google Scholar]
- Sessa G, Carabelli M, Sassi M, Ciolfi A, Possenti M, Mittempergher F, Becker J, Morelli G, Ruberti I (2005) A dynamic balance between gene activation and repression regulates the shade avoidance response in Arabidopsis. Genes Dev 19: 2811–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani E, Burko Y, Ben-Yaakov L, Berger Y, Amsellem Z, Goldshmidt A, Sharon E, Ori N (2009) Stage-specific regulation of Solanum lycopersicum leaf maturation by class 1 KNOTTED1-LIKE HOMEOBOX proteins. Plant Cell 21: 3078–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H, Whitelam GC (1997) The shade avoidance syndrome: multiple responses mediated by multiple phytochromes. Plant Cell Environ 20: 840–844 [Google Scholar]
- Steindler C, Matteucci A, Sessa G, Weimar T, Ohgishi M, Aoyama T, Morelli G, Ruberti I (1999) Shade avoidance responses are mediated by the ATHB-2 HD-zip protein, a negative regulator of gene expression. Development 126: 4235–4245 [DOI] [PubMed] [Google Scholar]
- Thomas PW, Woodward FI, Quick WP (2004) Systemic irradiance signaling in tobacco. New Phytol 161: 193–198 [Google Scholar]
- Townsley BT, Covington MF, Ichihashi Y, Zumstein K, Sinha NR (2015) BrAD-seq: Breath Adapter Directional sequencing. A streamlined, ultra-simple and fast library preparation protocol for strand specific mRNA library construction. Front Plant Sci 6: 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukaya H, Kozuka T, Kim GT (2002) Genetic control of petiole length in Arabidopsis thaliana. Plant Cell Physiol 43: 1221–1228 [DOI] [PubMed] [Google Scholar]
- Venables WN, Ripley BD (2002) Modern Applied Statistics with S, Ed 4 Springer, New York [Google Scholar]
- Vollbrecht E, Reiser L, Hake S (2000) Shoot meristem size is dependent on inbred background and presence of the maize homeobox gene, knotted1. Development 127: 3161–3172 [DOI] [PubMed] [Google Scholar]
- Vollbrecht E, Veit B, Sinha N, Hake S (1991) The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature 350: 241–243 [DOI] [PubMed] [Google Scholar]
- Wickham H. (2009) ggplot2: Elegant Graphics for Data Analysis. Springer, New York [Google Scholar]
- Yang L, Xu M, Koo Y, He J, Poethig RS (2013) Sugar promotes vegetative phase change in Arabidopsis thaliana by repressing the expression of MIR156A and MIR156C. eLife 2: e00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MD, Wakefield MJ, Smyth GK, Oshlack A (2010) Gene Ontology analysis for RNA-seq: accounting for selection bias. Genome Biol 11: R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Cao L, Zhou CM, Zhang TQ, Lian H, Sun Y, Wu J, Huang J, Wang G, Wang JW (2013) Sugar is an endogenous cue for juvenile-to-adult phase transition in plants. eLife 2: e00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Nakazawa M, Takase T, Manabe K, Kobayashi M, Seki M, Shinozaki K, Matsui M (2004) Overexpression of LSH1, a member of an uncharacterised gene family, causes enhanced light regulation of seedling development. Plant J 37: 694–706 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.