Abstract
Abstract Sinovac Biotech started to develop prototype pandemic influenza H5N1 vaccines in March 2004. On 2 April 2008, Sinovac’s inactivated, aluminium‐adjuvanted, whole‐virion prototype pandemic influenza A (H5N1) vaccine (PanFlu™) was granted production licensure by the China regulatory authority State Food and Drug Administration. The whole‐virion H5N1 vaccine was manufactured in embryonated hens’ eggs using the reassortant strain NIBRG‐14 (A/Vietnam/1194/2004‐A/PR/8/34) as vaccine virus. It showed good safety, immunogenicity and cross‐reactivity in immunologically naïve adults. In primed adults, the vaccine induced a strong booster response. Plasma from a vaccinated individual showed a beneficial effect following passive immunotherapy of an H5N1 human infection case. This article reviews the process, status and results of clinical evaluation of Sinovac’s whole‐ and split‐virion H5N1 vaccines by focusing on the whole‐virion vaccine.
Keywords: Immunogenicity, passive immunotherapy, safety, split‐virion H5N1 vaccine, whole‐virion H5N1 vaccine
Introduction
Since the first human infection with the highly pathogenic avian influenza virus H5N1 was reported in Hong Kong in 1997, concerns of a potential human influenza pandemic caused by the virus have been growing. 1 The virus has become widespread in many regions since 2003, and caused 385 cases and 243 deaths in humans according to WHO’s latest release. 2 , 3 Vaccine development is considered as a critical priority for preparedness against a potential influenza pandemic.
Sinovac Biotech launched the development of its prototype pandemic influenza H5N1 vaccines by collaborating with the Chinese Center for Disease Control and Prevention in March 2004. It was taken as an important part of the Chinese National Pandemic Preparedness Plan and supported by the China Ministry of Science and Technology and China Ministry of Health.
Sinovac’s inactivated, aluminium‐adjuvanted, whole‐virion prototype pandemic influenza A (H5N1) vaccine (PanFlu™, Sinovac Biotech, Beijing, China) is the only licensed H5N1 vaccine in China at present. It was granted production licensure by the China regulatory authority State Food and Drug Administration (FDA) on 2 April 2008, after the completion of a phase II clinical trial at the end of 2007.
Sinovac has been focusing on the development of whole‐virion vaccine, and simultaneously the development of a split‐virion vaccine. At present, Sinovac’s whole‐virion H5N1 vaccine has undergone clinical evaluation for safety and immunogenicity in adults and is under evaluation in adolescents and elders. The split‐virion vaccine has undergone safety observation in adults, adolescents, older adults and children, and is undergoing the assessment of immunogenicity and safety in adolescents and children. The dose ranges of whole‐ and split‐virion vaccines are 1·25–15 and 5–30 μg respectively. Table 1 summarizes the clinical trials completed or under evaluation.
Table 1.
The clinical trials of Sinovac’s H5N1 vaccines completed or under evaluation
| Vaccine | Phase | Dose (μg) | Participant enrolled | Design | Purpose | Status | Reference |
|---|---|---|---|---|---|---|---|
| Whole virion | I | 1·25, 2·5, 5, 10 | 120 Adults | Randomized, double‐blind, placebo‐controlled | Safety, immunogenicity | Completed | [6] |
| I | 1·25, 2·5, 5, 10 | 57 or 88 Adults* | Non‐randomized, single‐blind | Antibody persistence, booster response | Completed | [7] | |
| Ib | 15 | 10 Adults | Open‐labelled | Safety | Completed | Unpublished Wu J, et al. | |
| 5, 10, 15 | 28 Adolescents | ||||||
| 5, 10, 15 | 30 Elders | ||||||
| II | 5, 10, 15 | 402 Adults | Randomized, double‐blind | Immunogenicity, safety, cross‐reactivity | Completed | Unpublished Wu J, et al. | |
| IIb | 5 | 70 Adolescents | Randomized, double‐blind, | Immunogenicity, safety | Under evaluation | Unpublished | |
| IIb | 10 | 70 Elders | Non‐randomized, Open‐labelled | Immunogenicity, safety | Under evaluation | Unpublished | |
| Split virion | I | 5, 10, 15, 30 | 40 Adults | Open‐labelled | Safety | Completed | |
| 5, 10, 15, 30 | 40 Adolescents | Unpublished | |||||
| 5, 10, 15, 30 | |||||||
| 5, 10, 15, 30 | 40 Elders | ||||||
| 40 Children | |||||||
| II | 10, 15, 30 | 210 Adolescents | Randomized, double‐blind, | Immunogenicity, safety | Under evaluation | Unpublished | |
| II | 10, 15 | 140 Children | Randomized, double‐blind, | Immunogenicity, safety | Under evaluation | Unpublished |
*Participants were from two‐dose primed population in previous phase I trial enrolling 120 adults. Fifty‐seven and 88 adults at 6 and 12 months after the second dose respectively.
Vaccine manufacture
The reassortant strain NIBRG‐14 (A/Vietnam/1194/2004‐A/PR/8/34), which was prepared by the UK National Institute for Biological Standards and Control and recommended by WHO and EU Committee for Medicinal Products for Human Use (CHMP) for the production of H5N1 vaccines, 4 , 5 was used as a vaccine virus to manufacture Sinovac’s H5N1 vaccines. The H5N1 antigen was produced on a pilot scale in embryonated hens’ eggs. Two H5N1 vaccines including whole‐ and split‐virion vaccines were developed by Sinovac, both of which were formulated with aluminium hydroxide to contain 0·25 mg aluminium/dose. The vaccines were manufactured in compliance with the Chinese Pharmacopoeia (III, 2005). The temporary validity period (expiry dating) was 24 months.
Safety
The safety results of the whole‐virion H5N1 vaccine in adults are summarized in Table 2. The whole‐virion H5N1 vaccine was firstly evaluated in adults in a phase I trial enrolling 120 participants with 24 of them receiving placebo. 6 The results showed that the four vaccine doses (1·25, 2·5, 5 and 10 μg) were well tolerated with no serious adverse events reported. Most of the reported local and systemic reactions were graded as mild and transient and resolved within 72 h.
Table 2.
Safety profile of whole‐virion H5N1 vaccine in adults
| 1·25 μg (Phase I) | 2·5 μg (Phase I) | 5 μg (Phases I + II) | 10 μg (Phases I + II) | 15 μg (Phases Ib + II) | Sum | |
|---|---|---|---|---|---|---|
| n | 24 | 24 | 124 | 226 | 110 | 508 |
| Total | 66·7 (16) | 45·8 (11) | 32·3 40) | 31·9 (72) | 45·5 (50) | 37·2 (189) |
| Mild | 58·3 (14) | 33·3 (8) | 25·8 (32) | 19·9 (45) | 31·8 (35) | 26·4 (134) |
| Moderate | 8·3 (2) | 12·5 (3) | 6·5 (8) | 9·3 (21) | 11·8 (13) | 9·3 (47) |
| Severe | 0·0 (0) | 0·0 (0) | 0·0 (0) | 2·7 (6) | 1·8 (2) | 1·6 (8) |
| Local | 66·7 (16) | 33·3 (8) | 23·4 (29) | 22·6 (51) | 30·0 (33) | 27·0 (137) |
| Systemic | 25·0 (6) | 41·7 (10) | 20·2 (25) | 24·3 (55) | 30·9 (34) | 25·6 (130) |
| First vaccination | 62·5 (15/24) | 45·8 (11/24) | 28·2 (35/124) | 29·2 (66/226) | 40·0 (44/110) | 33·7 (171/508) |
| Second vaccination | 29·2 (7/24) | 20·8 (5/24) | 10·8 (13/120) | 7·2 (16/221) | 18·1 (19/105) | 12·1 (60/494) |
Data are incidences of adverse reactions (number of participants reporting adverse reactions/number of participants receiving vaccine).
The most common local and systemic reactions were pain at injection site and fever respectively.
The incidence of adverse reactions did not occur in a dose‐dependant manner. A higher incidence of adverse reactions was reported in participants receiving the 1·25 μg dose than other doses and placebo, which was largely due to a higher frequency of pain at the injection site in this group.
In phases Ib and II trials, the safety of the whole‐virion H5N1 vaccine was further evaluated in a larger population including adults, adolescents and older adults (unpublished data Wu J, et al.). A dose of 15 μg was assessed initially in a small group of 10 adults. After the 15 μg dose was well tolerated in adults in the phase Ib trial, the phase II trial evaluating 5, 10 and 15 μg doses was conducted in 402 adults. The results showed that all the doses were well tolerated without immediate allergic reactions or serious adverse events. There were 34% (136/402) of participants who reported adverse reactions, all of which resolved within 72 h. Pain at the injection site and fatigue were the most common local and systemic reactions respectively. The incidence of adverse reactions did not show dose‐dependency.
The safety of the whole‐virion H5N1 vaccine at a dose of 5, 10 or 15 μg was also evaluated in adolescents and older adults in a phase Ib trial. The three doses were well tolerated in older adults with only one participant reporting moderate fever in the 10 μg group. In adolescents, however, there were six of ten and five of eight participants reporting adverse reactions, of whom two and one participants respectively reported severe adverse reactions after the first dose of 10‐ and 15‐μg vaccines. It seemed that the incidence and severity of adverse reactions were not acceptable in adolescents. However, it is too early to draw a conclusion that the whole‐virion H5N1 vaccine is less safe in adolescents due to the small sample size, and further study may be needed to assess the safety profile of the whole‐virion vaccine in adolescents.
In phases I, Ib and II trials of whole‐virion vaccine, it was found that the incidences of adverse reactions in adults were remarkably lower after the second dose than those after the first dose as shown in Table 2.
The safety of the booster (third) dose of whole‐virion vaccine was evaluated in 57 adults 12 months after the second dose. 7 The results showed that the third dose was well tolerated with no serious adverse events reported. Only 5·3% (three of 57) of participants reported adverse reactions. By comparison with the first and second doses (33·7% and 12·1% respectively), the incidence of adverse reactions after the third dose was significantly lower.
The safety of split‐virion vaccine including 5, 10, 15 and 30 μg doses was evaluated in 160 participants including adults, adolescents, older adults and children. No serious adverse events were reported. There were 31·9% (51/160) of participants who reported adverse reactions, all of which were transient. Most of the adverse reactions were mild (34/160, 21·3%) or moderate (14/160, 8·8%). The incidences of adverse reactions in adults, adolescents, older adults and children were 35%, 32·5%, 20% and 40% respectively. Pain at the injection site and fever were the most common local and systemic reactions respectively. The incidences of adverse reactions showed dose‐dependency. The results indicated that the split‐virion H5N1 vaccine was well tolerated.
Immunogenicity
Immunogenicity in an unprimed population
Immunogenicity of the whole‐virion H5N1 vaccine has been assessed in adults in phases I and II clinical trials where all participants received two priming doses at the regimens of days 0–28 or 0–14. The phase I trial showed that all four doses (1·25, 2·5, 5 and 10 μg) induced haemagglutination‐inhibition (HI) and microneutralization (MN) antibody responses after the first dose. The second dose significantly boosted the response. A dose–response relationship was seen across the four vaccination groups. Haemagglutination‐inhibition geometric mean titres (GMTs) of 13·9–57·4 and MN GMTs of 15·7–45·1 were developed in the four groups 14 days after the second dose. The highest immune response of 78% HI seroconversion and seroprotection rates and a 11·5 post‐to‐pre‐vaccination GMT ratio was elicited after two doses of 10 μg vaccine, meeting all three EU CHMP licensure criteria for HI GMT ratio (>2·5), seroconversion rate (>40%) and seroprotection rate (>70%). 4 , 6
A larger phase II trial conducted in 402 participants with 100 or 101 participants per group, confirmed that the aluminium‐adjuvanted, whole‐virion H5N1 vaccine had good immunogenicity in adults (unpublished data). After two doses of 10 μg vaccine, an immune response (12·4 GMT ratio, 78% seroconversion and seroprotection rates) was induced, which met all three EU CHMP criteria. An obvious dose–response relation was seen. A higher immune response was induced in participants receiving the 15‐μg dose. For the licensure of H5N1 vaccine, the US FDA has released more stringent criteria for HI seroconversion rate (lower limit of two‐sided 95% CI ≥40%) and seroprotection rate (lower limit of two‐sided 95% CI ≥70%). 8 After the second dose, the 10‐μg vaccine induced immune response meeting US FDA criterion for seroconversion rate and the 15‐μg vaccine met the two US FDA criteria. In addition, it was found that the days 0–28 regimen induced a higher immune response than days 0–14 regimen did.
Antibody persistence
To detect antibody persistence after priming vaccination with the whole‐virion H5N1 vaccine, blood samples from volunteers who had received two doses of this vaccine in previous phase I trial were collected 6 and 12 months after two priming doses. 7 Haemagglutination‐inhibition and MN assays showed that both the antibody levels declined noticeably. Six months after the second dose, 14·8% (13/88) and 15·9% (14/88) of participants remained HI and MN titres ≥1:40 respectively. Twelve months after the second dose, 8·8% (5/57) and 14·0% (8/57) of participants retained HI and MN titres ≥1:40 respectively. Another study on non‐adjuvanted, split‐virion H5N1 vaccine has also shown that both HI and MN titres declined substantially after two‐dose priming. 9 These results suggested that antibody titres after priming vaccinations of H5N1 vaccines were not very persistent and that a booster (third) dose was needed to maintain a seroprotective titre. The antibody persistence after two priming doses is shown in Figure 1.
Figure 1.
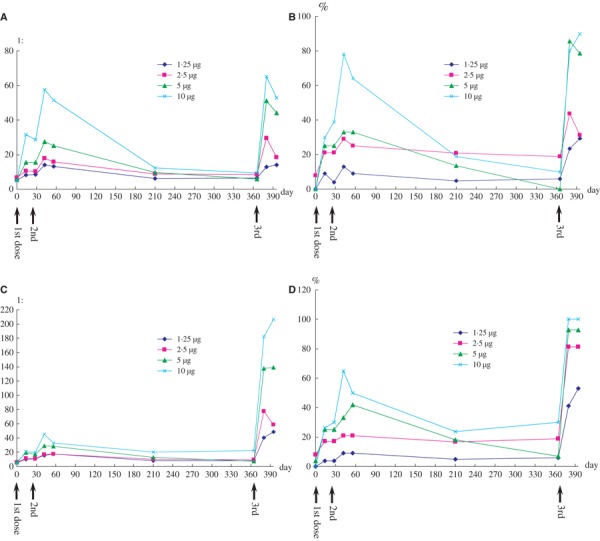
Kinetics of immune responses after the first, second and third doses of whole‐virion H5N1 vaccine. Geometric mean titres (A) and seroprotection rates (B) as determined by haemagglutination‐inhibition assay, and geometric mean titres (C) and proportions of participants developing titre ≥1:40 (D) as determined by microneutralization assay. n = 94 from days 0 to 56; n = 88 at day 210; n = 57 from day 365 forward.
Booster response
A booster (third) dose of the whole‐virion H5N1 vaccine given 12 months after the second dose significantly boosted the immune response in primed adults. 7 Thirty days after the third dose, 29%, 31%, 79% and 90% of participants developed HI titre ≥1:40, and 53%, 81%, 93% and 100% of participants developed MN titre ≥1:40 in 1·25, 2·5, 5 and 10 μg groups respectively. Both 5‐ and 10‐μg doses met all EU licensure criteria. The kinetics of immune responses after the first, second and third doses are shown in Figure 1.
Cross‐reactivity
The vaccine virus is a reassortant of the A/Vietnam/1194/2004 strain, which phylogenetically belongs to clade 1. Cross‐reactivity assays were performed against three heterologous H5N1 drifted strains including IBCDC‐RG2 (A/Indonesia/5/2005‐A/PR/8/34), NIBRG‐23 (A/turkey/Turkey/1/2005‐A/PR/8/34) and IBCDC‐RG5 (A/Anhui/1/2005‐A/PR/8/34), which belong to clade 2.1, clade 2.2 and clade 2.3 respectively. 5 The three heterologous strains were reassortants derived by reverse genetics and recommended by WHO as H5N1 candidate vaccine viruses. Both HI and MN assays showed strong cross‐reactive responses against the recombinant Indonesia and Anhui strains. After two doses of 10 μg vaccine, 65% of participants had developed HI titre ≥1:40, and 98% and 87% of participants had developed MN titre ≥1:40 against recombinant Indonesia and Anhui strains respectively (unpublished data). However, the cross‐reactive response against the recombinant Turkey strain was relatively lower.
Passive immunotherapy
Passive immunotherapy for H5N1 infection using post‐vaccination anti‐H5N1 plasma has shown positive and exciting result. 10 In December 2007, a family cluster of two individuals infected with H5N1 virus was identified in Jiangsu Province, China. The 24‐year‐old index case died, and the second case, his 52‐year‐old father, survived after receiving early antiviral treatment and transfusion of post‐vaccination anti‐H5N1 plasma. The plasma was donated by a volunteer 280 days after she received two doses of the whole‐virion H5N1 vaccine at days 0–28 regimen in the phase I clinical trial. The plasma showed 1:40 MN titre against homologous NIBRG‐14 and 1:20 against the second case’s strain A/Jiangsu/2/2007. The low MN titres were mainly due to the long interval between the second vaccination and the bleeding when the antibody titre was undergoing decline.
Although it was difficult to distinguish exactly the effects of antiviral agents and plasma on the treatment of the patient, the fact that the patient still had a worsened respiratory status after 4 days treatment with antiviral agents and that the transfusions of anti‐H5N1 plasma resolved the patient’s fever, suggesting that post‐vaccination plasma might be a desirable alternative for treatment of H5N1 infection.
Problem and prospect
The past a few years have witnessed remarkable achievements of pandemic influenza H5N1 vaccine development. Many promising H5N1 vaccines including whole‐virion, split‐virion, adjuvanted and non‐adjuvanted vaccines have been developed and clinically evaluated, 6 , 11 , 12 , 13 some of which have been granted production licensure in different regions. All these achievements arm us with a powerful, if not sufficient, defence against the next human influenza pandemic.
Even if several H5N1 vaccines are available for deployment, the demand for influenza vaccines will far outstrip the manufacturing capacity during a pandemic. Production capacity expansion and antigen‐sparing strategy are encouraged. We have shown that whole‐virion H5N1 vaccine elicited a satisfactory immune response using less antigen than subunit or split‐virion vaccines did. Other studies have reported good immunogenicity of pandemic influenza candidate vaccines adjuvanted with oil‐in‐water emulsions, resulting in significant antigen sparing. 13 , 14 , 15 Further study may be necessary to evaluate the immunogenicity of whole‐virion H5N1 vaccine adjuvanted with an oil‐in‐water emulsion, which is hoped that it will be further antigen sparing.
Cross‐reactivity against genetically and antigenically distinct strains should be considered for the development of pandemic candidate vaccines. Previous trials have reported the cross‐reactivity of pandemic candidate vaccines. 13 , 16 , 17 Our whole‐virion H5N1 vaccine has shown good cross‐reactivity against the heterologous Indonesia and Anhui recombinant strains. However, there are few data about the protection efficacy and cross‐protection, and it is impossible to study the protection efficacy and cross‐protection in humans during the interpandemic period. The direct evidence from virus challenge by homologous and heterologous wild‐type H5N1 viruses in animals will help us understand the protection efficacy and cross‐protection of pandemic candidate vaccines.
References
- 1. Claas EC, Osterhaus AD, Van Beek R et al. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 1998; 351:472–477. [DOI] [PubMed] [Google Scholar]
- 2. WHO . H5N1 Avian Influenza: Timeline of Major Events, 2008. Available at: http://www.who.int/entity/csr/disease/avian_influenza/timeline2008_04_09.pdf (accessed 14 April 2008). [Google Scholar]
- 3. WHO . Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO, 2008. Available at: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2008_06_19/en/index.html (accessed 4 July 2008). [Google Scholar]
- 4. European Committee for Proprietary Medicinal Products . Guideline on Dossier Structure and Content for Pandemic Influenza Vaccine Marketing Authorisation Application (CPMP/VEG/4717/03), 2004. Available at: http://www.emea.europa.eu/pdfs/human/vwp/471703en.pdf (accessed 28 April 2008). [Google Scholar]
- 5. WHO . Antigenic and Genetic Characteristics of H5N1 Viruses and Candidate H5N1 Vaccine Viruses Developed for Potential Use as Pre‐pandemic Vaccines, 2007. Available at: http://www.who.int/csr/disease/avian_influenza/guidelines/summaryH520070403.pdf (accessed 26 March 2008). [Google Scholar]
- 6. Lin JT, Zhang JS, Dong XP et al. Safety and immunogenicity of an inactivated adjuvanted whole‐virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet 2006; 368:991–997. [DOI] [PubMed] [Google Scholar]
- 7. Lin JT, Li CG, Wang X et al. Antibody persistence after two‐dose priming and booster response to a third dose of an inactivated, adjuvanted whole‐virion H5N1 vaccine. J Infect Dis 2009; in press. [DOI] [PubMed] [Google Scholar]
- 8. US Food and Drug Administration . Guidance for Industry: Clinical Data Needed to Support the Licensure of Pandemic Influenza Vaccines, 2007. Available at: http://www.fda.gov/cber/gdlns/panfluvac.htm (accessed 4 January 2008). [Google Scholar]
- 9. Zangwill KM, Treanor JJ, Campbell JD, Noah DL, Ryea J. Evaluation of the safety and immunogenicity of a booster (third) dose of inactivated subvirion H5N1 influenza vaccine in humans. J Infect Dis 2008; 197:580–583. [DOI] [PubMed] [Google Scholar]
- 10. Wang H, Feng ZJ, Shu YL et al. Probable limited person‐to‐person transmission of highly pathogenic avian influenza A (H5N1) virus in China. Lancet 2008; 371:1427–1434. [DOI] [PubMed] [Google Scholar]
- 11. Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med 2006; 354:1343–1351. [DOI] [PubMed] [Google Scholar]
- 12. Bresson JL, Perronne C, Launay O et al. Safety and immunogenicity of an inactivated split‐virion influenza A/Vietnam/1194/2004 (H5N1) Vaccine: phase I randomized trial. Lancet 2006; 367:1657–1664. [DOI] [PubMed] [Google Scholar]
- 13. Leroux‐Roels I, Borkowski A, Vanwolleghem T et al. Antigen sparing and cross‐reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 2007; 370:580–589. [DOI] [PubMed] [Google Scholar]
- 14. Nicholson KG, Colegate AE, Podda A et al. Safety and antigenicity of non‐adjuvanted and MF59‐adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 2001; 357:1937–1943. [DOI] [PubMed] [Google Scholar]
- 15. Almar RL, Keitel WA, Patel SM et al. Safety and immunogenicity of nonadjuvanted and MF59‐adjuvanted influenza A/H9N2 vaccine preparations. Clin Infect Dis 2006; 43:1135–1142. [DOI] [PubMed] [Google Scholar]
- 16. Stephenson I, Bugarini R, Nicholson KG et al. Cross‐reactivity to highly pathogenic avian influenza H5N1 viruses after vaccination with nonadjuvanted and MF59‐adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a potential priming strategy. J Infect Dis 2005; 191:1210–1215. [DOI] [PubMed] [Google Scholar]
- 17. Höschler K, Gopal R, Andrews N et al. Cross‐neutralisation of antibodies elicited by an inactivated split‐virion influenza A/Vietnam/1194/2004 (H5N1) vaccine in healthy adults against H5N1 clade 2 strains. Influenza Other Respir Viruses 2008; 1:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]


