Abstract
Objective Seasonal vaccination has been consistently shown to significantly reduce morbidity and mortality because of influenza epidemics, even in healthy, working adults. Here we report the results of the yearly licensing studies of the past 11 influenza seasons (1997–2007) with a trivalent, inactivated whole virus vaccine with an aluminum phosphate adjuvant system.
Methods Sixty healthy volunteers per age group (18–60 years and 60 years and older) were enrolled to receive vaccination each year, thus, a total of 1080 subjects were studied. Serum antibody titers were measured by hemagglutination inhibition (HI).
Results: The vaccine met the criteria for licensing each year, meaning seroprotection (achievement of an HI titer of >1:40 in >70% of subjects); seroconversion, i.e. a >4‐fold increase in HI antibody titer, or reaching a titer of >1:40, in >40% of subjects; and an increase in geometric mean titers by >2·5‐fold. Side effects were rare and mild. The same method was used to produce a pre‐pandemic vaccine against influenza A (H5N1), which has been shown to be safe and immunogenic in humans.
Conclusions We conclude that the method presented is safe, effective and may serve as a useful approach to seasonal and pandemic vaccine production even in less well‐developed countries by means of technological transfer.
Keywords: Influenza, licensing, seasonal, vaccine
Introduction
Influenza is among the leading causes of respiratory infection and it represents a significant public health burden. Elderly people and patients with underlying health conditions are at increased risk of complications of influenza, including hospitalization and death. 1 During epidemics, the hospitalization rate for the elderly and people with chronic health problems may increase two to fivefold compared with non‐epidemic periods. 1 Seasonal vaccination has been consistently shown to significantly reduce morbidity and mortality because of influenza epidemics, even in healthy, working adults. 2
It has been suggested that an influenza pandemic can occur in the near future, and that an influenza A (H5N1) virus might be the cause of the next pandemic. 3 However, pandemic vaccine development has progressed slowly. Experts suggest that validated methods for seasonal (interpandemic) vaccine production could be useful and easier to be utilized for pandemic vaccines, as facilities are already available for production. 4 , 5
Influenza vaccine development and production has a half‐century long history in Hungary. Between 1961 and 1995 the vaccine against seasonal influenza was produced at the Hungarian National Institute of Public Health. The production technology has continuously been improved, but the basic idea of the method remained unchanged. The vaccine contains whole, inactivated virus, with an adjuvant system. In 1995, Omninvest Ltd (Budapest, Hungary) received a national marketing authorization for influenza vaccine, by using essentially the same method.
Here we report the 11‐year experience with a seasonal, inactivated whole virus vaccine with an aluminum phosphate adjuvant system. The unique parts of the method are the concentration and purification of the virus and the adjuvant system. The same method was used to produce a pandemic mock up vaccine against H5N1, which has been shown to be safe and immunogenic in humans after just one dose. 6
Methods
Vaccine
We report the essence of the method used each year in Hungary for the past 11 years for seasonal vaccination. 7 The vaccine was produced as described previously. 6 Briefly, the virus strains were influenza A (H3N2), A (H1N1) and B [for the 2006/07 season: Influenza A/New Caledonia/20/99(H1N1), Influenza A/Wisconsin/67/2005 (H3N2), and Influenza B/Malaysia/2506/2004. Strains for previous seasons were as recommended each year by the WHO]. The vaccine is a hens’ egg grown, formaldehyde‐inactivated, whole virus vaccine, which contains a minimum of 15 μg of hemagglutinin (HA)/dose/strain (as determined by single radial immunodiffusion test), in one dose (0·5 ml) in 1·0 ml ampoules. The HA content was determined by single radial immunodiffusion test, in 0·5 ml ampoules as described previously. 8 Purity was evaluated by endotoxin content (determined by chromogenic endotoxin assay, utilizing a modified limulus amoebocyte lysate and a synthetic color‐producing substrate to detect endotoxin presence), which was measured to be present in concentrations less than 0·05 IU/dose, and the amount of ovalbumin determined by ELISA, which was less than 5 ng/dose. Both values are much lower than the concentrations considered acceptable by the European Pharmacopoeia (Ph. Eur.), which are 100 IU/human dose and 1000 ng/human dose, respectively. 9 Aluminum phosphate (AlPO4) was used as adjuvant, in the amount of 0·31 mg Al/ampoule and mertiolate was added as preservative in the amount of 0·1mg/ml, meeting the requirements of Ph. Eur. 9
The final composition of the vaccine for 0·5 ml is: 0·31 mg/dose Al (as AlPO4), NaCl (1·66 mg), potassium dihydrogen phosphate (0·31 mg), Disodium phosphate dihydrate (0·19 mg), thiomersal (0·05 mg), KCl (0·04 mg) to 0·5 ml sterile water.
The method fulfills all criteria for Good Manufacturing Practice (GMP) and it has been validated by meeting the requirements of the European Committee for Medicinal Products for Human Use (CHMP) related to interpandemic influenza vaccines each year since 1995, and by having been administered in humans in Hungary in a total of more than 15 million subjects since 1995. 10 , 11
Subjects
Sixty healthy volunteers per age group (age of 18–60 years and 60 years and older) were enrolled to receive annual vaccination each year. As recommended by the WHO, in 1999 and 2003 the seasonal influenza vaccines were produced with the same strains as the previous year, and therefore, no licensing studies were required, meaning that a total of 1080 subjects were studied. Negative urine or serum pregnancy test was required for women of childbearing potential. Also, in female subjects of childbearing potential, use of an acceptable contraception method was required and the subject was not to become pregnant for the duration of the study. Acceptable contraception included implants, injectables, combined oral contraceptives, intrauterine devices, sexual abstinence, or a vasectomized partner. Exclusion criteria included diagnosed immunodeficiency, history of Guillain–Barré syndrome severe concomitant disease states (e.g. uncontrolled diabetes, autoimmune disease, malignancy) that may affect the immune reactivity of the individual, use of immunosuppressive medication (corticosteroid nasal sprays were permitted), medical or psychiatric condition that precluded subject compliance with the study protocol, receiving an inactivated vaccine 14 days prior to the study, use of live attenuated vaccines within 60 days of study, use of investigational agents within 30 days prior to the study, receipt of blood products or immunoglobulins in the past 6 months, acute febrile illness 1 week before vaccination, pregnancy or nursing, known allergies to any component of the vaccine, including thiomersal, history of allergy to eggs or egg products.
The sample size was chosen to exceed the requirements of 50 patients per group by the European guidelines for yearly influenza vaccine trials. 11 All patients signed a written informed consent. The study protocol was approved by the National Institute of Pharmacy, Budapest, Hungary, and the Central Ethics Committee for Clinical Pharmacology of the Medical Research Council, Budapest, Hungary. The study sponsor was the National Public Health and Medical Officer Service, Budapest, Hungary, and Omninvest Ltd, Budapest, Hungary. The funding source had no role in the conduct of the study or the preparation of this report.
Laboratory tests
Serum antibody titers were measured by hemagglutination inhibition (HI) following standard procedures. 12 , 13
All serological tests were performed at a central laboratory (Department of Virology, National Center for Epidemiology, Budapest, Hungary).
Immunogenicity assessment
Hemagglutination inhibition antibody titers were determined at baseline and on day 21 after vaccination. HI titers were used to calculate seroconversion rates, seroprotection rates, and increase in geometric mean titers (GMT). Immunogenicity was assessed according to the criteria of the European Agency for the Evaluation of Medicinal Products (EMEA) and the European Centre for Disease Protection and Control (ECDC) related to interpandemic and pre‐pandemic influenza vaccines. 11 , 15 In order to confirm protective immunogenicity in adult patients, one of the following three requirements have to be met: (i) seroprotection, i.e. achievement of an HI titer of ≥1:40 in >70% of subjects; (ii) seroconversion, i.e. a ≥4‐fold increase in HI antibody titer, or reaching a titer of ≥1:40, in >40% of subjects; and (iii) an increase in GMT by >2·5‐fold. For patients >60 years of age, the following criteria were used: (i) seroconversion, i.e. a ≥4‐fold increase in HI antibody titer, or reaching a titer of ≥1:40, in >30% of subjects; (ii) seroprotection, i.e. achievement of an HI titer of ≥1:40 in 60% of subjects; and (iii) an increase in GMT by >2‐fold. 11 , 15 The above guidelines have also been proposed in a draft guideline by the US Food and Drug Administration (FDA), with the exception of the GMT increase criterion. 15 Efficacy was assessed by comparing the rates of laboratory confirmed influenza cases between the vaccinated and unvaccinated population each season.
Procedures
Baseline evaluations included demographic data, medical history and physical examination, recording pre‐existing conditions, concomitant medications, vital signs (blood pressure, pulse rate). In case of female subjects of childbearing age, a pregnancy test was performed. Blood samples were taken from the cubital vein to test for specific antibodies against the virus strains by HI. The purpose of the day 0 serological examination was to test for the presence of such antibodies prior to treatment.
After a physical examination and blood collection, 0·5 ml of the vaccine was administered at one side into the deltoid muscle by a deep intramuscular injection. The injection was not repeated. On day 21, medical history and the list of any medications used during the days since the last visit were taken, physical examination was performed, and blood samples were taken from the cubital vein to test for specific antibodies against the virus strains by HI.
Statistical analysis
We assessed the occurrence of the following reactions in the 3 days after vaccination in accordance with guidance for interpandemic vaccines: injection site induration of more than 5 cm for more than 3 days; injection site ecchymosis; body temperature of more than 38·0°C – i.e. oral temperature of greater than 37·5°C – for 24 hours or more; malaise; and shivering. 16
We gave HI titers below the limit of detection an arbitrary intermediate value of one in two. The geometric mean of duplicate results for each specified time was used for the calculation. Geometric mean titers of antibody and their confidence intervals were computed by transforming the results to a logarithmic scale, assuming asymptotic normality conditions were satisfied on the scale and converting back to the original scale. The HI endpoints were the GMT, as well as the variables recommended for interpandemic influenza vaccines: post‐vaccination seropositivity rate (% of subjects with titers ≥64, which is higher than the titer of 1:40 required by the CHMP and FDA); the post‐to‐pre‐vaccination GMT ratio; and the proportion of people seroconverting, meaning displaying an at least fourfold titer increase post‐vaccination and post‐vaccination titers of at least 1:64, which again, is higher than the CHMP and FDA requirement of 1:40. 11 , 14 , 15 We used Fisher’s exact test and the non‐parametric Kruskal–Wallis test as appropriate to test for differences between groups. A P value of <0·05 was considered significant.
Results
Immunogenicity
Immunogenicity findings of adult and elderly subjects are summarized in 1, 2 for the years of 1997, 1998, 2000, 2001, 2002, 2004, 2005, 2006 and 2007. As recommended by the WHO, in 1999 and 2003 the seasonal influenza vaccines were produced with the same strains as the previous year. Therefore, no licensing studies were required.
Table 1.
Immunogenicity findings of the trivalent seasonal influenza vaccine FluvalAB in adult subjects (18–60 years)
| Year | Strain | Seropositivity (CHMP criterion: >70%) | Seroconversion (CHMP criterion: >40%) | GMT increase (CHMP criterion: >2·5) | CHMP requirement met |
|---|---|---|---|---|---|
| 1997 | A(H1N1) A(H3N2) B | 84% 85% 75% | 41% 46% 43% | 3·0 3·4 3·3 | Yes |
| 1998 | A(H1N1) A(H3N2) B | 86% 82% 86% | 56% 50% 68% | 4·7 3·6 4·2 | Yes |
| 2000 | A(H1N1) A(H3N2) B | 84% 70% 72% | 72% 56% 62% | 3·5 3·3 3·0 | Yes |
| 2001 | A(H1N1) A(H3N2) B | 84% 84% 79% | 47% 46% 49% | 3·4 3·1 3·4 | Yes |
| 2002 | A(H1N1) A(H3N2) B | 88% 90% 80% | 62% 66% 54% | 3·3 3·9 3·9 | Yes |
| 2004 | A(H1N1) A(H3N2) B | 72% 76% 86% | 46% 54% 48% | 3·4 2·6 3·6 | Yes |
| 2005 | A(H1N1) A(H3N2) B | 84% 78% 80% | 44% 42% 48% | 3·1 3·0 3·2 | Yes |
| 2006 | A(H1N1) A(H3N2) B | 88% 82% 77% | 43% 47% 45% | 2·8 3·5 3·0 | Yes |
| 2007 | A(H1N1) A(H3N2) B | 76% 80% 76% | 52% 52% 50% | 5·4 4·0 3·9 | Yes |
Table 2.
Immunogenicity findings of the trivalent seasonal influenza vaccine FluvalAB in elderly persons (>60 years)
| Year | Strain | Seropositivity (CHMP criterion: >60%) | Seroconversion (CHMP criterion: >30%) | GMT increase (CHMP criterion: >2) | CHMP requirement met |
|---|---|---|---|---|---|
| 1997 | A(H1N1) A(H3N2) B | 52% 68% 58% | 32% 38% 31% | 2·9 2·8 3·1 | Yes |
| 1998 | A(H1N1) A(H3N2) B | 62% 70% 78% | 36% 44% 58% | 2·9 2·7 4·0 | Yes |
| 2000 | A(H1N1) A(H3N2) B | 68% 70% 74% | 44% 52% 56% | 2·6 2·8 2·8 | Yes |
| 2001 | A(H1N1) A(H3N2) B | 67% 69% 70% | 40% 38% 41% | 2·9 2·8 2·7 | Yes |
| 2002 | A(H1N1) A(H3N2) B | 72% 82% 88% | 38% 36% 44% | 3·1 3·2 3·9 | Yes |
| 2004 | A(H1N1) A(H3N2) B | 72% 70% 82% | 38% 32% 38% | 3·3 3·1 3·2 | Yes |
| 2005 | A(H1N1) A(H3N2) B | 72% 70% 82% | 32% 36% 42% | 2·4 2·7 3·5 | Yes |
| 2006 | A(H1N1) A(H3N2) B | 75% 78% 70% | 37% 40% 35% | 2·7 3·1 2·8 | Yes |
| 2007 | A(H1N1) A(H3N2) B | 68% 68% 68% | 42% 50% 42% | 3·0 3·1 2·9 | Yes |
The pre‐ and post‐vaccination HA antibody titers for both age groups are shown in 1, 2 for each virus strain in the season of 2007.
Figure 1.
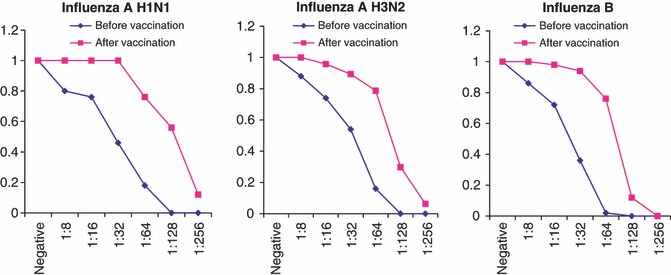
Reverse cumulative distribution curve for HI antibody titers to homologous H1N1, H3N2 and B vaccine strains 21 days after vaccination in the age group 18–60 years.
Figure 2.
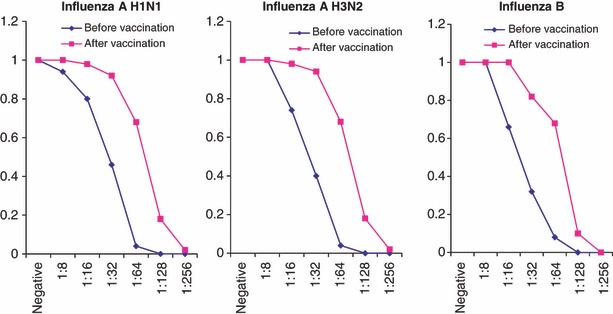
Reverse cumulative distribution curve for HI antibody titers to homologous H1N1, H3N2 and B vaccine strains 21 days after vaccination in the age group over 60 years.
The vaccines met at least one independent CHMP criterion for licensure for every influenza season. In adult subjects, all three licensing criteria were met each year.
In elderly subjects, all three criteria were met each year, with the exception of 1997, when seropositivity for influenza A (H1N1) and influenza B were only close to the 60% criterion (52 and 58%, respectively). Nonetheless, the criteria for seroconversion and GMT increase were met each year, including 1997, meaning that the vaccine fulfilled CHMP licensing criteria for interpandemic influenza vaccines in every season. Thus, the requirement for licensure, e.g. meeting at least one independent criterion, was fulfilled each year.
Efficacy
The efficacy of the vaccine produced in Hungary 1962–2007 was 25–92%, which is in line with the international findings of efficacy. 17 , 18 The lowest rate was found in 1978, which is due to the fact that the epidemic was caused by the A/USSR/90/77(H1N1) strain. This subtype returned unexpectedly, after 20 years, and, therefore, the vaccine produced based on the yearly WHO recommendation did not contain it. The efficacy rate was especially low, 3·3% in the population younger than 20 years of age. However, a 41·7% efficacy rate was found in persons older than 20 years. This is likely explained by the fact that the population over 20 years of age had been exposed to that strain before, and, therefore, could have had some protection against it, which was boosted with the vaccine by non‐specific immunostimulation. The effects of repeated annual vaccinations were not studied in the present work. However, we do have some data suggesting that repeated vaccinations enhance the immune response, or even cross reactive immunity. 19
Safety
Each year, in ≤1·67% of the participants, adverse reactions in the form of local pain or erythema at the injection site occurred within the first 48 hours; these reactions disappeared within 1 day. No other local reactions, such as injection site induration, swelling, warmth, or ecchymosis, were noted. No systemic reaction (fever, malaise, headache, shivering) was detected. No serious adverse events were observed in the study population. This is supported by the yearly reaction reports from the Department of Viral Vaccine Control, National Center for Epidemiology, Budapest, Hungary, which published 0–5 reactions/year, from more than 1 million vaccinations yearly. 20
Discussion
Influenza disease is an underestimated public health problem. Epidemics spread rapidly from country to country and may affect as many as 500 million people all over the world in each year. The disease, particularly influenza A may kill the patients and the new influenza viruses which appeared in 1957 (Asian influenza) and 1968 (Hong Kong) are estimated to have caused at least 3 000 000 deaths in the world.
Seasonal influenza continues to have a huge annual impact in the United States, accounting for tens of millions of illnesses, hundreds of thousands of excess hospitalizations, and tens of thousands of excess deaths. Vaccination remains the mainstay for the prevention of influenza. 21 Children, adults <65 years of age, and the elderly all receive substantial health benefits from vaccination. In addition, vaccination appears to be cost‐effective, if not cost saving, across the age spectrum. 2 , 21
Here we present the essence of the production and the results of the yearly licensing studies of an inactivated whole virus seasonal influenza vaccine with an aluminum phosphate adjuvant system, which has been used for vaccination in Hungary for 11 years and over 15 million subjects in humans. The same method has been used to produce a vaccine against influenza A (H5N1), which demonstrated safety and efficacy in humans. 6 Experts suggest that the adaptation of existing technologies for seasonal influenza vaccine production would be the most straightforward approach to produce pandemic vaccines, because these technologies are commercially available and licensing would be relatively simple. 4 , 5 , 21 However, the trials published so far on H5N1 vaccines have reported very low immunogenicity or used different methods than used for the production of seasonal vaccines. 22 , 23 , 24 , 25 There has also been pandemic experience with the above described method during the 1968 pandemic in Hungary. 17 In 1968, the number of influenza cases in Hungary was lower than in the preceding and subsequent years, which is possibly explained by the fact that in 1968 the number of vaccinations was several folds higher than in any other year of the decade. 26
Inactivated influenza vaccines were first licensed in 1941 and they have been produced in Hungary since 1961. The original method was developed by Takatsy. 27 The essence of the method is based on biological purification and concentration, achieved by dialysis.
In Hungary, approximately 1·5 million doses of seasonal influenza vaccines are produced each year with the industrial adaptation of the method described in this study. Besides the fact that influenza vaccine production in Hungary is independent of the world wide suppliers of vaccines and uses a different approach, the importance of the above also lies in the finding that the same method can be applied to produce a vaccine against an influenza A (H5N1) virus, which is highly pathogenic and considered to be the potential cause of the next influenza pandemic. 6 We recently reported the first clinical trial with 146 patients having received an inactivated whole virus H5N1 vaccine. It was found that the vaccine produced essentially with the above method was safe and immunogenic in humans inducing seroconversion in 63·7% of the study subjects without side effects other than injection site pain. 6 Moreover, as opposed to other reports with H5N1 vaccines, it was found to be immunogenic after only one injection. 6 , 22 , 23 , 24 , 25 Furthermore, we also tested the pre‐pandemic H5N1 vaccine produced with the above method in children, and confirmed its immunogenicity and safety. 28 Therefore, the vaccine has been officially offered to be part of the WHO pre‐pandemic vaccine stockpile. 29
In Hungary, a ‘cold capacity’ has been developed, which, using the virus strain supplied by the WHO, enables the production of the first 500 000 doses of pandemic vaccine in 8 weeks. In another 9 weeks, the production of the 4·5 million doses needed for a pandemic in Hungary can be produced, using the above method.
There is some evidence that whole‐virus vaccines are more immunogenic than split or subunit vaccines, but this needs substantiating by further studies. As far as seasonal influenza vaccinations, the vaccine we studied provided results compatible with non‐adjuvanted split or subvirion vaccines as they all meet CHMP licensing requirements. In the case of pandemic influenza vaccines, non‐adjuvanted split and subvirion vaccines performed poorly in clinical trials. 22 , 23 We feel that developing an effective adjuvant system is of importance, as H5 vaccines appear to be particularly poor immunogens and there is evidence that an adjuvant may be needed to boost their effect. 30 We report the use of a simple effective method of creating an aluminum phosphate‐based adjuvant system complying with all principles and detailed guidelines of CHMP on adjuvants in vaccines for human use. 31 , 32 We previously carried out experiments with a different, aluminum hydroxide‐based adjuvant system, which we found much less effective and stable than the above described aluminum phosphate‐based gel system in laboratory and animal studies (Jankovics et al., unpublished data). A potential explanation of this is the observation that the pH of the aluminum hydroxide system changes more after autoclaving compared with aluminum phosphate systems, and that the absorption of influenza viruses to the adjuvant largely depends on the pH. We are currently conducting a large clinical trial, in which 3·5 and 6 μg of HA are used in seasonal flu vaccinations, as opposed to the conventionally used 15 μg.
We consistently detected a very low rate of side effects each year. In general, whole, inactivated virus vaccines are thought to be more reactogenic than split virion or subunit vaccines, but the few side effects seen with the present vaccine may be explained at least in part by the purity of the vaccine. The endotoxin content of 0·05 IU/dose and the amount of ovalbumin of less than 5 ng/dose in the present vaccine are much smaller than the allowed amounts of 100 IU/dose and 1000 ng/human dose, respectively, accepted by standards. 9 The present vaccine has been approved and used in children, and a similarly low‐rate of side effects has been observed.
In summary, we report a method of vaccine production that is different from those used by worldwide manufacturers and yearly licensing studies for seasonal influenza vaccines that demonstrate that the vaccine is safe and effective. This method has successfully been used to produce a pre‐pandemic vaccine using a virus strain with pandemic potential.
Conflict of interest
None declared. All authors are government employees.
Funding
The study sponsor was the National Public Health and Medical Officer Service, Budapest, Hungary, and Omninvest Ltd, Budapest, Hungary. The funding source had no role in the conduct of the study or the preparation of this report.
References
- 1. Barker WH. Excess pneumonia and influenza associated hospitalizations during influenza epidemics in the United States. Am J Public Health 1986; 76:761–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nichol KL, Lind A, Margolis KL et al. The effectiveness of vaccination against influenza in healthy, working adults. N Engl J Med 1995; 333:889–893. [DOI] [PubMed] [Google Scholar]
- 3. Fedson DS. Vaccine development for an imminent pandemic: why we should worry, what we must do. Hum Vaccin 2006; 2:38–42. [DOI] [PubMed] [Google Scholar]
- 4. Dennis C. Flu‐vaccine makers toil to boost supply. Nature 2006; 440:1099. [DOI] [PubMed] [Google Scholar]
- 5. Fauci AS. Seasonal and pandemic influenza preparedness: science and countermeasures. J Infect Dis 2006; 194(Suppl 2):S73–S76. [DOI] [PubMed] [Google Scholar]
- 6. Vajo Z, Kosa L, Visontai I, Jankovics M, Jankovics I. Inactivated whole virus influenza A (H5N1) vaccine. Emerg Infect Dis 2007; 13:807–808.Available from http://www.cdc.gov/eid/content/13/5/06‐1248.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. License number: OGYI‐T‐8998/01, National Institute of Pharmacology , Budapest, Hungary, 1995.
- 8. Wood JM, Schild GC, Newman RW, Seagroatt V. An improved single‐radial‐immunodiffusion technique for the assay of influenza haemagglutinin antigen: application for potency determinations of inactivated whole virus and subunit vaccines. J Biol Stand 1977; 5:237–247. [DOI] [PubMed] [Google Scholar]
- 9. European Directoriate for the Quality Medicines . European Pharmacopia, 5.8 CD, 7/2007, Strasbourg, France: European Directoriate for the Quality Medicines; pp. 3406–3407. [Google Scholar]
- 10. European Committee for Proprietary Medicinal Products . Guideline on Dossier Structure and Content for Pandemic Influenza Vaccine Marketing Authorisation Application (CPMP/VEG/4717/03). Brussels: European Agency for the Evaluation of Medicinal Products, April 5, 2004. [Google Scholar]
- 11. European Committee for Proprietary Medicinal Products . Note for Guidance on Harmonization of Requirements for Influenza Vaccines, March 1997 (CPMP/BWP/214/96). Brussels: European Agency for the Evaluation of Medicinal Products, March 12, 1997. [Google Scholar]
- 12. Kendal AP, Pereira MS, Skehel JJ, (eds). Concepts and Procedures for Laboratory Based Influenza Surveillance. Atlanta: Centers for Disease Control, 1982. [Google Scholar]
- 13. Klimov A, Cox N. Serologic Diagnosis of Influenza Virus Infections by Hemagglutination Inhibition. Influenza Laboratory Course, Atlanta: Centers for Disease Control, 7.1‐5, 2003. [Google Scholar]
- 14. Giesecke J, Sellwood C, Van‐Tam J. Expert Advisory Groups on Human H5N1 Vaccines. Brussels: European Centre for Disease Control and Prevention; 2007, p. 47. [Google Scholar]
- 15. US Food and Drug Administration . Draft Guidance on Clinical Data Needed to Support the Licensure of Pandemic Influenza Vaccines. http://www.fda.gov/cber/gdlns/panfluvac.htm [Google Scholar]
- 16. Stephenson I, Nicholson KG, Wood JM, Zambon MC, Katz JM. Confronting the avian influenza threat: vaccine development for a potential pandemic. Lancet Infect Dis 2004; 4:499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jankovics I. The influenza viruses: past, present and future. Lege Artis Med 1996; 6:62–68. [PubMed] [Google Scholar]
- 18. Meiklejohn G. Viral respiratory disease at Lowry Air Force Base in Denver, 1952–1982. J Infect Dis 1983; 148:775–783. [DOI] [PubMed] [Google Scholar]
- 19. Fazekas G, Martosne‐Mendi R, Zimonyi F et al. 3rd. Cross‐reactive Immunity Induced by Fluval H5N1, a Reverse Genetic‐derived A/Vietnam/1194/04 (NIBRG‐14) Influenza Vaccine WHO Meeting on Influenza Vaccines with Broad Spectrum and Long Lasting Immune Responses. Geneva, Switzerland: WHO, 2007. [Google Scholar]
- 20. Department of Viral Vaccine Control . Yearly Reaction Reports. Budapest, Hungary: National Center for Epidemiology. [Google Scholar]
- 21. Nichol KL, Treanor JJ. Vaccines for seasonal and pandemic influenza. J Infect Dis 2006; 194(Suppl 2):S111–S118. [DOI] [PubMed] [Google Scholar]
- 22. Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immungenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med 2006; 354:1343–1351. [DOI] [PubMed] [Google Scholar]
- 23. Bresson JL, Perronne C, Launay O et al. Safety and immunogenicity of an inactivated split‐virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet 2006; 367:1657–1664. [DOI] [PubMed] [Google Scholar]
- 24. Lin J, Zhang J, Dong X et al. Safety and immunogenicity of an inactivated adjuvanted whole‐virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet 2006; 368:991–997. [DOI] [PubMed] [Google Scholar]
- 25. Leroux‐Roels I, Borkowski A, Vanwolleghem T et al. Antigen sparing and cross‐reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 2007; 18:370. [DOI] [PubMed] [Google Scholar]
- 26. Yearly Statistic Reports. Budapest, Hungary: National Center for Epidemiology. [Google Scholar]
- 27. Takatsy G. Purified precipitated virus obtained by a new simple method. Acta Med Acad Sci Hung 1952; 3:185–191. [PubMed] [Google Scholar]
- 28. Vajo Z, Kosa L, Szilvasy I et al. Safety and immunogenicity of a prepandemic influenza A (H5N1) vaccine in children. Pediatr Infect Dis J (In press). [DOI] [PubMed] [Google Scholar]
- 29. WHO Announcement, July 13th, 2007: http://www.who.int/mediacentre/news/statements/2007/s14/en/index.html [Google Scholar]
- 30. Wood JM. Developing vaccines against pandemic influenza. Philos Trans R Soc Lond B Biol Sci 2001; 356:1953–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guideline on Adjuvants in Vaccines for Human Use (CHMP/VEG/134716/2004), p. 8. http://www.emea.europa.eu/pdfs/human/vwp/13471604en.pdf . [Google Scholar]
- 32. EC Guide to Good Manufacturing Practice (GMP), Volume 4, basing upon the Directive 2001/83/EC of the European Parliment and the Council of 6 November 2001 relating to medicinal product for human use Official Journal; L 311:67–128. [Google Scholar]


