Abstract
Objective
To examine the effects of diets varying in carbohydrate and glycemic index (GI) on changes in body composition, resting metabolic rate (RMR) and metabolic adaptation during and after weight loss.
Methods
Adults with obesity (n = 91) were randomized to one of four provided-food diets for 17 wk. Diets differed in percentage energy from carbohydrate (55% or 70%) and GI (low or high), but were matched for protein, fiber and energy. Body weight, body composition, RMR, and metabolic adaptation (measured RMR – predicted RMR) were measured during weight loss and subsequent weight stability.
Results
No effect of dietary carbohydrate content or GI on body weight loss or percentage of weight lost as fat mass was observed. Measured RMR was significantly lower (−226 kJ/d [95%CI: −314 kJ/d, −138 kJ/d] P < 0.001) than predicted RMR following weight loss, but this difference was attenuated after 5 wk weight stability. Metabolic adaptation did not differ by dietary carbohydrate content or GI, and was not associated with weight regain 12 mo later.
Conclusion
Moderate-carbohydrate and low-GI diets did not preferentially reduce fat mass, preserve lean mass, or attenuate metabolic adaptation during weight loss compared to high-carbohydrate and high-GI diets.
Keywords: Metabolic adaptation, Body composition, Body fat, Diet composition, Energy expenditure
Introduction
Obesity rates remain at epidemic levels in part because weight regain following weight loss is common (1). One frequently cited hypothesis for weight regain is that there is a ‘metabolic adaptation’ to weight loss (2, 3), which can be defined as a reduction in resting metabolic rate (RMR) greater than can be accounted for by weight loss alone (4). Several groups have observed this phenomenon during active weight loss (5–9), but whether metabolic adaptation can be influenced by dietary factors, persists after weight stabilizes, or contributes to weight regain is controversial (4, 10, 11).
Dietary carbohydrate quantity and glycemic index (GI) are among the factors thought to influence metabolic adaptation. Some studies (12–14), but not others (15–19), have suggested that low-carbohydrate (< 45% energy from carbohydrate) or low-GI diets attenuate reductions in RMR during and following weight loss, with postulated mechanisms including altered substrate availability and endocrine-mediated effects on anabolic and catabolic pathways (12). In addition, some (16, 20–22), but again, not other (13, 19,23–25) studies have suggested that low-carbohydrate or low-GI diets may promote a preferential loss of fat mass (FM) and preservation of fat free mass (FFM) during weight loss, which would also attenuate reductions in RMR. However, few studies have directly assessed the effects of dietary carbohydrate content and GI on metabolic adaptation and change in body composition with weight loss while carefully controlling dietary composition. Moreover, whether high-carbohydrate (≥ 65% total energy) diets adversely affect these outcomes is unclear.
To address these gaps, and to determine the effects of dietary GI and the relative proportions of dietary energy from carbohydrate versus fat on changes in body weight, body composition and metabolic adaptation during and after weight loss, we designed and provided protein-matched diets containing moderate or high amounts of carbohydrate (and therefore moderate or low in fat) with low or high GI. We hypothesized that dietary carbohydrate composition would not influence body weight, body composition or metabolic adaptation when potentially confounding dietary factors were controlled.
Methods
Study population
Men and postmenopausal women (45–65 y; BMI 28–38 kg/m2) were recruited from the Boston, MA metro area. Exclusion criteria included abnormal thyroid, liver, and kidney function tests, LDL cholesterol ≤ 100 mg/dL, fasting triglycerides ≥ 400 mg/dL, chronic illness, diabetes, and taking medication for elevated blood lipids. The study was conducted at the Jean Mayer United States Department of Agriculture Human Nutrition Research Center on Aging at Tufts University between 2000 and 2004 with approval by the Tufts University Health Sciences Institutional Review Board. All participants gave written, informed consent prior to participating, and received a stipend.
Study design
The primary study objective was to assess the effects of altering dietary carbohydrate-to-fat ratio and GI on body weight, and biomarkers of cardiometabolic health in an adult population with overweight and obesity. This report concerns the study’s secondary objectives, specifically the effects of dietary carbohydrate composition on body composition and metabolic adaptation during and following weight loss. The moderate- and high-carbohydrate diets used reflect common recommendations at the time of study conception to consume low-fat diets for weight loss and cardiometabolic health. Though high-carbohydrate weight loss diets have somewhat fallen out of favor in the intervening years, investigating the metabolic effects of these diets remains of interest given concerns over the long-term health effects of low-carbohydrate diets (12), which are likely to grow in lieu of emerging evidence linking gut bacteria metabolites of common low-carbohydrate diet components to inflammatory bowel and cardiovascular diseases (26).
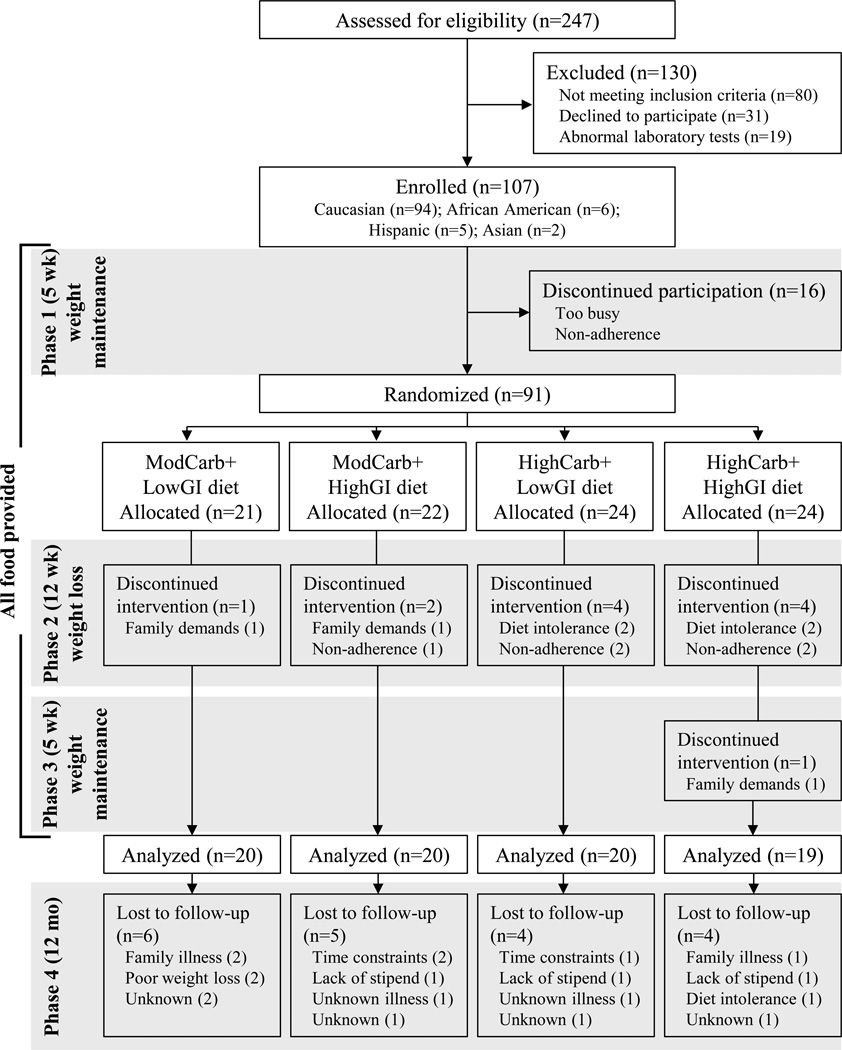
The study design included three controlled-diet phases totaling 22 wk, followed by a 12-mo ad libitum-diet follow-up period (Figure 1). All food and energy-containing beverages were provided to participants throughout the first three phases, and volunteers were instructed to abstain from dietary supplement use. The provided diets were matched for dietary protein, fiber and energy density, and differed only in carbohydrate-to-fat ratio and GI (Table 1).
Figure 1.
CONSORT diagram and study design. GI, glycemic index; HighCarb, 70% energy from carbohydrate; ModCarb, 55% energy from carbohydrate.
Table 1.
Volunteer characteristics at the end of the weight maintenance run-in diet (Phase 1), and diet composition during active weight loss (Phase 2) and relative weight stability (Phase 3).
| ModCarb+ HighGI |
HighCarb+ HighGI |
ModCarb+ LowGI |
HighCarb+ LowGI |
|
|---|---|---|---|---|
| Volunteer characteristics1 | ||||
| Age (y) | 55 ± 5 | 56 ± 5 | 57 ± 8 | 56 ± 5 |
| M/F (n) | 10/10 | 9/10 | 10/10 | 10/10 |
| Height (cm) | 168 ± 7 | 168 ± 7 | 169 ± 12 | 168 ± 7 |
| Body weight (kg) | 89.1 ± 11.1 | 94.0 ± 9.7 | 95.7 ± 13.7 | 92.9 ± 13.6 |
| BMI (kg/m2) | 32.2 ± 3.4 | 33.4 ± 2.6 | 33.6 ± 4.2 | 32.3 ± 3.4 |
| Body fat (%) | 39.5 ± 7.7 | 41.6 ± 6.5 | 41.3 ± 9.1 | 40.7 ± 7.0 |
| RMR (kJ/d) | 6760 ± 1042 | 6974 ± 1013 | 7058 ± 1088 | 6798 ± 938 |
| RQ | 0.83 ± 0.03 | 0.84 ± 0.06 | 0.83 ± 0.03 | 0.83 ± 0.04 |
| Diet composition2 | ||||
| Energy (kJ/d)3 | 8079 | 8205 | 8192 | 8192 |
| Carbohydrate (%) | 54 | 70 | 54 | 68 |
| Fat (%) | 29 | 14 | 31 | 16 |
| Protein (%) | 16 | 16 | 16 | 15 |
| Fiber (g/ 1000 kcal) | 14 | 14 | 14 | 15 |
| Glycemic index | 80 | 86 | 51 | 59 |
| Glycemic load | 193 | 283 | 124 | 188 |
| Diet adherence4 | ||||
| Phase 2 (%) | 79 | 77 | 94 | 72 |
| Phase 3 (%) | 79 | 73 | 88 | 67 |
1 kcal = 4.186 kJ. Carb, carbohydrate; GI, glycemic index; Mod, moderate; RMR, resting metabolic rate; RQ, respiratory quotient.
Mean ± SD. 1-way ANOVA; no between-group differences.
Prescribed intake; macronutrient composition and fiber content measured by chemical analysis. GI calculated with white bread as reference.
During Phase 2.
Diet adherence assessed by study staff, and was defined as the percentage of individuals having ≤ 1 occurrence/wk of incomplete consumption of the provided study foods.
Phase 1 was a 5-wk weight maintenance phase in which weight maintenance energy needs were determined by adjusting provided energy intake to maintain stable weight. Mean Phase 1 energy intake was 12.2 MJ/d with 48% energy provided as carbohydrate, 16% as protein and 36% as fat. Following Phase 1, participants were randomized by the study statistician to their Phase 2 dietary assignment using computer-generated randomization. The four diets differed in carbohydrate content (55%, ModCarb or 70%, HighCarb of total energy) and dietary GI (< 60, LowGI or ≥ 80, HighGI), and were provided for 12 wk at 67% of the weight maintenance energy intake determined in Phase 1. Participants were allowed to increase their energy intake during Phase 2 by requesting additional, randomization-appropriate foods from the metabolic kitchen if too hungry to be adherent. Phase 3 was a 5-wk weight maintenance phase during which food was provided according to randomization. Energy intake during Phase 3 was prescribed to support weight maintenance at the new, lower body weight, and was predicted from body weight and energy intake measured at the end of Phase 2, with adjustment for self-reported physical activity. Phase 4 was a 12-mo follow-up period during which participants selected and prepared their own meals after being provided with instructions on following the diet to which they were randomized.
Participants reported to the center 3–5 d/wk during Phase 1, and 3 d/wk during Phases 2 and 3 to be weighed, return study materials, pick up study foods, and eat a meal under staff supervision. Participants were required to return all empty food containers and any uneaten foods for documentation of leftover food items. Diet adherence was assessed by dietary staff, and non-adherence was defined as incomplete consumption of provided foods. During Phase 4, interaction with study personnel was limited to quarterly visits and monthly phone calls with a nutritionist.
Diet composition was analyzed using the Nutrition Data System for Research v 4.0.4 (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN) and verified by chemical analysis of each diet (Covance Laboratories, Madison, WI). GI was lowered without creating differences in macronutrient composition primarily by substituting low-GI foods for higher GI foods that had similar macronutrient proportions. Pilot-testing confirmed lower postprandial glycemia following consumption of low-GI versus high-GI experimental meals. Glycemic load and GI (white bread as reference) were recently updated using the Nutrition Data System for Research 2010.
Study outcomes
Participants were blinded to their randomization during Phases 1–3, and outcomes were measured by trained staff who were also blinded. Pre-study height was measured using a wall-mounted stadiometer. Fasting body weight was measured during each study visit using a calibrated digital scale.
Body density was measured in duplicate at the end of Phases 1 and 2, and once at the end of Phase 3 by air displacement plethysmography (BOD POD; Life Measurement Instruments, Concord, CA) according to standard procedure (27). FM and FFM were calculated from measured body density (27). The CV for duplicate measures of body fatness was 1.8% and 1.7% at the end of Phases 1 and 2, respectively.
RMR was measured in duplicate at the end of Phases 1 and 2, and once at the end of Phase 3 by indirect calorimetry using a portable metabolic cart (Deltatrac metabolic monitor; SensorMedics, Anaheim, CA). Participants were instructed to fast for ≥ 12 hr, and avoid vigorous exercise for ≥ 24 hr prior to measurements. Measurements were completed over 40 min following a 30 min rest period, and under thermo-neutral conditions. The final 30 min of data were used to calculate RMR using Weir’s equation (28). The CV for duplicate measures was 3.4% and 3.0% at the end of Phases 1 and 2, respectively.
Metabolic adaptation was calculated as the difference between RMR measured at the end of each phase and the predicted RMR for that phase (i.e., measured RMR – predicted RMR). Predicted RMR for each phase was calculated by entering FM and FFM measured at the end of that phase into a regression model developed from baseline age, sex, FM, FFM, and RMR (29).
Statistical analysis
Sample size calculations indicated 20 participants per group was required to detect a 3% difference in body weight by carbohydrate-content and GI-level at α = 0.05 and power = 0.80. Using previously published work from our group (15), this sample size was determined sufficient for detecting a between-group RMR difference of 105 kcal/d (α = 0.008, power = 0.80), an effect size consistent with previous findings on the effects of low-glycemic impact diets on RMR (13).
All outcomes were assessed for normality. Repeated measures ANCOVA was used to test for main effects of carbohydrate- and GI-level, and their interaction over time on body weight. Body composition, RMR and respiratory quotient (RQ) were analyzed by 2-factor (carbohydrate and GI) ANCOVA within Phase 2, and Phases 2 and 3 combined. End of Phase 1 value (i.e. baseline) was entered as a covariate in ANCOVA models. Metabolic adaptation was analyzed by 2-factor (carbohydrate and GI) ANOVA. Pearson’s correlation was used to examine associations.
Missing data for two study completers without Phase 3 RMR measurements were imputed using multiple imputation (30). Two individuals randomized to HighCarb+LowGI were classified as outliers based on having measured Phase 3 RMR change scores > 3 times the interquartile range above the 75th percentile in that group. Including outliers did not alter the statistical significance of any analyses, and RMR and RQ results are presented with outliers excluded. Only study completers were included in analyses because the aims of this analysis concerned the effects of diet adherence and not diet randomization.
SPSS version 20.0 was used for analyses. Values are reported as mean ± SD or mean difference (M [95% CI]) unless otherwise noted. All tests were two-sided and considered statistically significant at P < 0.05.
Results
Seventy-nine of 91 randomized participants completed all three controlled-diet phases (Figure 1 and Table 1). The sex (P = 0.34), age (P = 0.65), and BMI (P = 0.42) of study drop-outs did not differ from completers.
Diet adherence, defined as the percentage of individuals within each group with ≤ 1 occurrence/wk of incomplete consumption, was ≥ 77% within the full cohort during Phases 2 and 3, and did not differ by carbohydrate content (P ≥ 0.19), by GI (P ≥ 0.55), or across groups (P ≥ 0.36) during either phase (Table 1). To further evaluate adherence, the energy deficit required to elicit the measured Phase 2 weight loss was estimated using group means and the National Institutes of Health body weight simulator (31). The 4.0 MJ/d prescribed energy deficit was 1.2 MJ/d (ModCarb+HighGI), 1.5 MJ/d (HighCarb+HighGI), 0.8 MJ/d (ModCarb+LowGI), and 0.8 MJ/d (HighCarb+LowGI) lower than the simulator-estimated energy deficit. The non-significant differences in weight loss (see below) indicate these values were not different across groups.
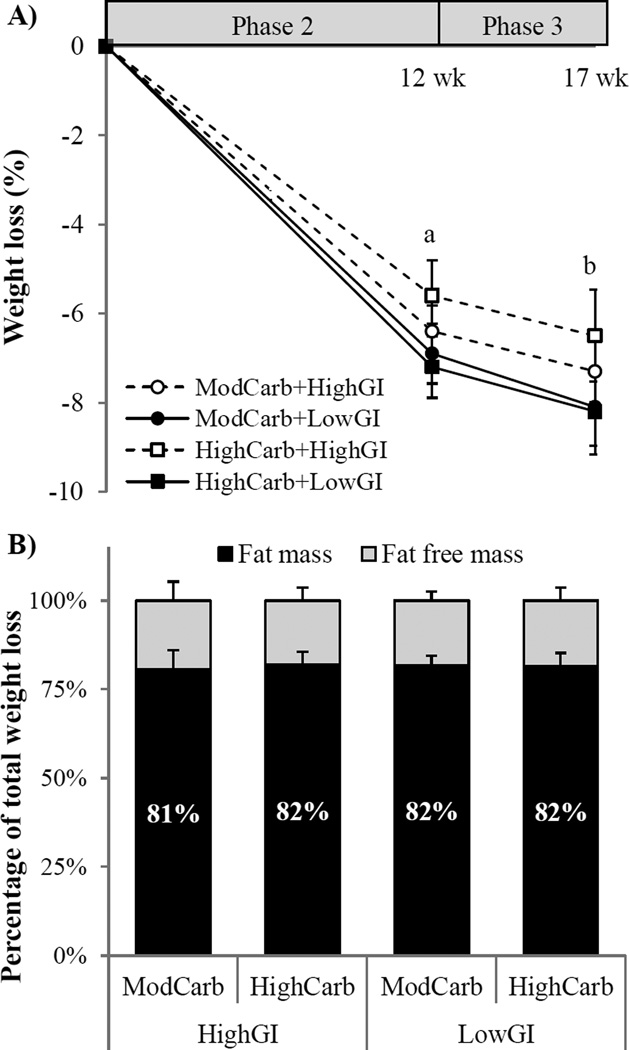
The combined cohort lost 7.5% ([95% CI: −8.4%, −6.6%] P < 0.001) of initial body weight (Figure 2a), with losses occurring primarily during Phase 2 (−6.1 kg ([95% CI: −6.8 kg to −5.4 kg], P < 0.001). A modest additional mean weight loss of 0.9 kg ([95% CI −1.3 kg to −0.5 kg], P < 0.001) was documented during Phase 3. Total weight loss did not differ by carbohydrate content (P = 0.60), by GI (P = 0.52), or across groups (P-interaction = 0.69; Table 2). At the end of Phase 3, the proportion of total weight loss attributable to FM and FFM did not differ by carbohydrate content (P = 0.94), by GI (P = 0.70), or across groups (P-interaction = 0.97) (Figure 2b).
Figure 2.
A) Weight loss and B) percentage of total weight loss attributable to fat mass and fat free mass while consuming provided-food diets differing in glycemic index (GI), and percent energy from carbohydrate (55%, ModCarb and 70%, HighCarb) for 17 wk (n = 79). Values are mean ± SEM. Weight loss analyzed by repeated measures ANCOVA, body composition by 2-factor ANOVA. a,bMain effect of time; asignificant decrease from baseline (P < 0.001), bsignificant difference from Phase 2 end (P < 0.001). No diet effects (main effects or interactions) for any comparisons.
Table 2.
Changes in body composition and resting metabolism, and metabolic adaptation while consuming diets differing in carbohydrate content and glycemic index during active weight loss (Phase 2) and relative weight stability (Phase 3).1
| Main effects | ||||||
|---|---|---|---|---|---|---|
| ModCarb+ HighGI |
HighCarb+ HighGI |
ModCarb+ LowGI |
HighCarb+ LowGI |
GI-level (low v. high) |
Carb-content (moderate v. high) |
|
| Weight (kg)2 | ||||||
| ΔPhase 2 | −5.8 [−7.1, −4.6] | −5.3 [−6.9, −3.7] | −6.6 [−8.0, −5.2] | −6.8 [−8.3, −5.2] | −0.8 [−2.1, 0.4] | −0.3 [−1.5, 1.0] |
| ΔTotal | −6.6 [−8.1, −5.1] | −6.1 [−8.1, −4.1] | −7.8 [−9.6, −5.9] | −7.6 [−9.7, −5.6] | −1.1 [−2.8, 0.6] | −0.5 [−2.1, 1.3] |
| Fat mass (kg)3 | ||||||
| ΔPhase 2 | −4.6 [−5.7, −3.5] | −4.7 [−5.9, −3.5] | −4.9 [−6.0, −3.8] | −5.1 [−6.5, −3.8] | −0.2 [−1.3, 0.9] | 0.1 [−1.0, 1.2] |
| ΔTotal | −5.8 [−7.2, −4.4] | −4.9 [−6.6, −3.3] | −6.4 [−7.8, −5.0] | −6.1 [−7.9, −4.4] | −0.7 [−2.0, 0.7] | −0.7 [−2.1, 0.7] |
| Fat free mass (kg)3 | ||||||
| ΔPhase 2 | −1.2 [−2.0, −0.5] | −0.6 [−1.3, 0.1] | −1.7 [−2.3, −1.1] | −1.6 [−2.2, −1.0] | −0.7 [−1.3, −0.1]a | −0.3 [−0.9, 0.3] |
| ΔTotal | −0.8 [−1.5, −0.1] | −1.1 [−1.9, −0.4] | −1.4 [−2.0, −0.8] | −1.5 [−2.2, −0.7] | −0.4 [−1.0, 0.2] | 0.2 [−0.4, 0.8] |
| RMR (kJ/d)3 | ||||||
| ΔPhase 2 | −473 [−703, −243] | −410 [−615, −209] | −427 [−590, −260] | −527 [−716, −343] | −33 [−213, 151] | 21 [−167, 205] |
| ΔTotal | −389 [−640, −138] | −373 [−624, −126] | −460 [−666, −255] | −494 [−737, −251] | −96 [−331, 138] | 8 [−226, 243] |
| RMR, measured – predicted (kJ/d)4 | ||||||
| Phase 2 | −243 [−414, −71] | −193 [−385, 0] | −155 [−314,0] | −310 [−536, −84] | −13 [−184, 163] | 50 [−121, 226] |
| Phase 3 | −159 [−327, 13] | −109 [−356, 138] | −176 [−377, 29] | −251 [−498, −4] | −75 [−293, 138] | 13 [−205, 230] |
| RQ3 | ||||||
| ΔPhase 2 | −0.01 [−0.02, 0.01] | −0.01 [−0.04, 0.01] | −0.02 [−0.04, 0.00] | −0.01 [−0.03, 0.01] | −0.01 [−0.02, 0.00] | −0.01 [−0.02, 0.01] |
| ΔTotal | 0.00 [−0.03, 0.03] | −0.01 [−0.03, 0.02] | −0.02 [−0.04, 0.00] | −0.01 [−0.03, 0.02] | −0.01 [−0.03, 0.01] | −0.01 [−0.03, 0.01] |
1 kcal = 4.186 kJ
Values are mean [95% CI] (n = 77–79). ΔPhase 2, change from baseline to end of Phase 2; ΔTotal, change from baseline to end of Phase 3; GI, glycemic index; FFM, fat free mass; FM, fat mass; HighCarb, 70% energy from carbohydrate; ModCarb, 55% energy from carbohydrate; RMR, resting metabolic rate; RQ, respiratory quotient.
3-factor (carbohydrate, GI and time) repeated measures ANCOVA. Main effect of time (P < 0.05); effects of diet were not statistically significant.
2-factor (carbohydrate and GI) ANCOVA.
2-factor (carbohydrate and GI) ANOVA. Regression equation for computing predicted RMR developed from baseline data (R2 = 0.82, P < 0.001): Predicted-RMRPhase i (kcal/d) = 6.36*FMPhase i + 19.33*FFMPhase i + 1.98*age − 60.3*sex + 265.38; M = 0, F = 1
Main effect of GI-level; P = 0.02
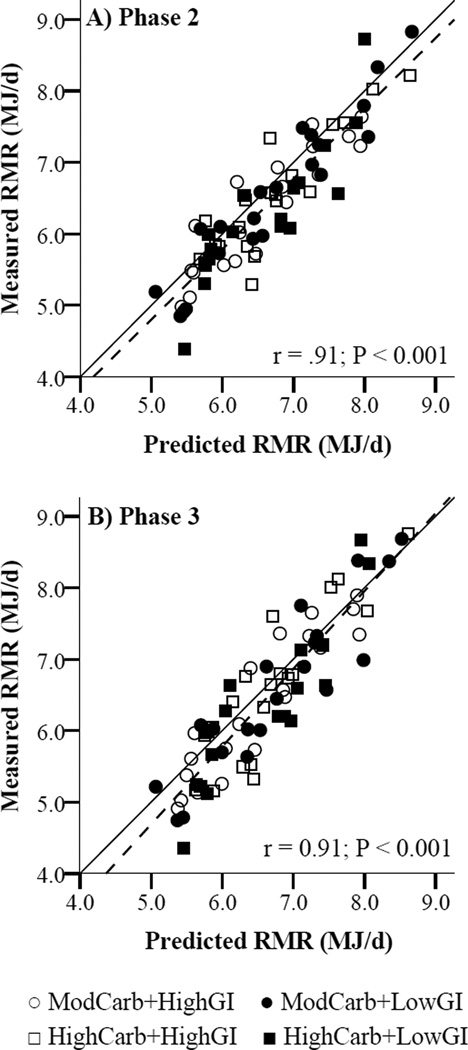
At the end of Phase 2 (active weight loss), measured RMR was 6.5% [95% CI: −7.7%, −5.3%] lower than baseline in the combined cohort, and was 226 kJ/d ([95% CI: −314 kJ/d, −138 kJ/d] P < 0.001) lower than predicted RMR. Neither the decrease in measured RMR during Phase 2, including after adjusting for weight change, nor the difference between measured and predicted RMR at the end of Phase 2, differed by carbohydrate content, by GI, or across groups (Table 2). Measured and predicted RMR were strongly correlated at the end of Phase 2 (r = .91, P < 0.001; Figure 3a). The difference between measured and predicted RMR was not correlated with Phase 2 weight loss (r = −.04, P = 0.72). RQ decreased 0.1 units ([95% CI −0.02, 0.00], P = 0.01) during Phase 2 in the combined cohort, but the decrease did not differ by carbohydrate content (P = 0.50), by GI (P = 0.16), or across groups (P-interaction = 0.53; Table 2).
Figure 3.
Measured versus predicted resting metabolic rate (RMR) at the end of A) Phase 2 and B) Phase 3 (n = 77; Pearson’s correlations). Solid line, measured = predicted; dashed line, regression line between measured and predicted. GI, glycemic index; HighCarb, 70% energy from carbohydrate; ModCarb, 55% energy from carbohydrate.
At the end of Phase 3, measured RMR remained 6.2% lower ([95% CI: −7.9%, −4.5%] P < 0.001) than baseline in the combined cohort, and 172 kJ/d ([95% CI: −280 kJ/d, −63 kJ/d] P = 0.002) lower than predicted RMR whereas RQ did not differ from baseline (P = 0.16). The difference between measured and predicted RMR was not statistically significant after controlling for weight change during Phase 3 (P = 0.11), and was not correlated with total weight loss (r = .05, P = 0.69). Neither the total measured change in RMR or RQ during Phases 2 and 3, nor the difference in measured and predicted RMR at the end of Phase 3 differed by carbohydrate content, by GI, or across groups (Table 2). Measured and predicted RMR were strongly correlated at the end of Phase 3 (r = .91, P < 0.001; Figure 3b). Re-analyzing Phase 2 and Phase 3 body weight, body composition, RMR and metabolic adaptation data using only adherent volunteers did not alter the statistical significance of any comparison.
Body weight change during Phase 4 was measured in 60 participants. Attrition during Phase 4 did not differ according to diet (χ2 = 0.67, P = 0.88), but men (n = 13) tended to be more likely to drop-out than women (n = 6; χ2 = 3.63, P = 0.06). Relative to Phase 4 completers, participants not completing Phase 4 lost slightly less weight during the controlled intervention (−6.0% ± 3.2% vs. −8.1% ± 4.1%, P = 0.05), but did not experience a greater relative decline in RMR (−5.8% ± 3.2% vs. −6.5% ± 6.2%, P = 0.72) or exhibit greater metabolic adaptation (−209 ± 477 kJ/d vs. −293 ± 448 kJ/d, P = 0.84).
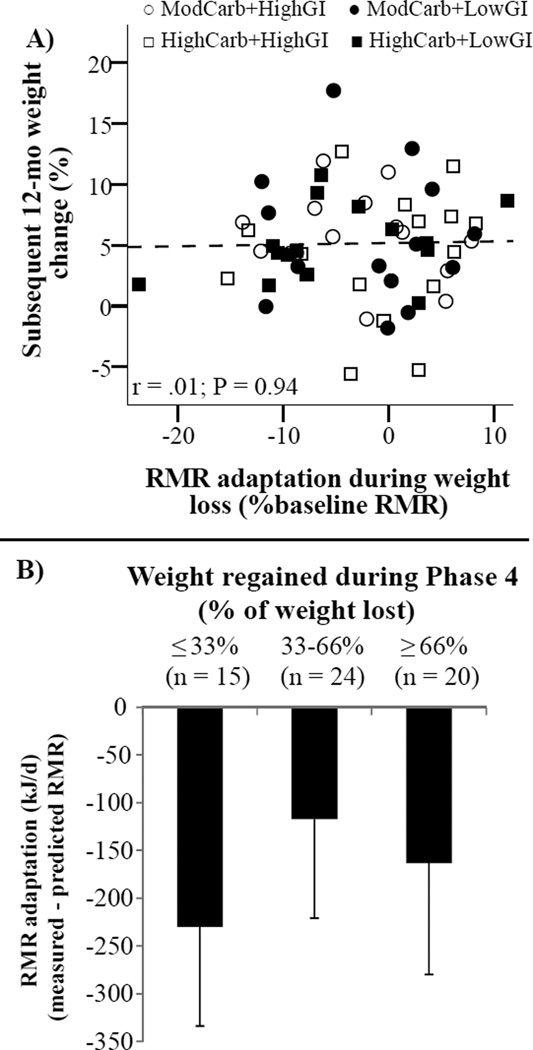
Within the cohort of Phase 4 completers, there was an average weight regain of 4.3 kg [95% CI: 3.3 kg, 5.3 kg], equivalent to 58% of the weight lost during Phases 2 and 3. Weight regain did not differ by carbohydrate content (P = 0.34), by GI (P = 0.92), or across diet groups (P-interaction = 0.54), including after adjusting for prior weight loss. Therefore, the full cohort was combined to examine relationships between changes in RMR and weight regain. Neither measured RMR at the end of Phase 3 (r = .13, P = 0.27), percent change in RMR during Phases 2 and 3 (r = −.19, P = 0.15), nor metabolic adaptation measured at the end of Phase 3 and expressed as a percent of baseline RMR (r = .01, P = 0. 94; Figure 4a) was correlated with weight change during Phase 4. There were no differences in metabolic adaptation measured at the end of Phase 3 when compared across tertiles of subsequent weight change during Phase 4 (P = 0.77, Figure 4b).
Figure 4.
Adaptation of resting metabolic rate (RMR) and subsequent 12-mo weight change (n = 60). A) Association between RMR adaptation measured at the end of Phase 3 and subsequent 12-mo weight change during Phase 4 (Pearson’s correlation). B) RMR adaptation by tertile of weight regain during Phase 4. Differences between tertiles were not statistically significant; 1-way ANOVA, P = 0.77. Bars are mean – SEM. Glycemic index (GI); HighCarb, 70% energy from carbohydrate; ModCarb, 55% energy from carbohydrate.
Discussion
The major findings of this study were that when potentially confounding dietary factors were controlled: 1) moderate-carbohydrate and low-GI diets relative to high-carbohydrate and high-GI diets did not show differences for preserving FFM or attenuating metabolic adaptation during weight loss, and 2) that metabolic adaptation tended to dissipate with weight stability following weight loss and did not predict subsequent weight regain. Collectively, these findings indicate that although adaptive variations in energy expenditure can be measured, they are unrelated to dietary carbohydrate content and GI within the levels of carbohydrate and GI tested in this study.
One major finding was that neither dietary GI nor the percentage dietary energy from carbohydrate impacted the quality of weight loss (FFM relative to FM losses). Meta-analyses have suggested that low-carbohydrate diets may help preserve FFM relative to FM loss during negative energy balance (21, 32). However, low-carbohydrate diets are commonly high in protein, which has also been associated with preservation of FFM (21, 33), thereby confounding many previous studies in this area. By providing all food to our subjects and designing the diets to be matched for confounding factors such as dietary protein, the results of this study pertain specifically to the ratio of fat to carbohydrate energy at high and moderate carbohydrate levels, and GI. Concerning GI, our current results are consistent with the majority of the evidence base (22, 34–36), and with the few previous studies that have isolated effects of GI by providing complete low-GI and high-GI diets matched for percent energy from carbohydrate, fat and protein (25, 35, 36).
Study results also indicated that moderate-carbohydrate and low-GI diets relative to high-carbohydrate and high-GI diets did not differentially effect metabolic adaptation. Previous work suggested that reducing dietary carbohydrate and GI (12, 13) may mitigate reductions in RMR through mechanisms related to substrate availability, and hormonal and autonomic activity. However, our current findings are consistent with previous findings from our group and others that do not substantiate a benefit for low-GI diets in attenuating metabolic adaptation (15, 16, 34). Reasons for the incongruent results are unclear, but the inability to reliably separate the effects of a diet’s carbohydrate content and glycemic impact from confounding factors such as differences in protein and/or fiber intake between groups (12, 13,15, 16), or a reliance on self-reported dietary intake (34), may be important. Our findings begin to resolve these issues, demonstrating that when these confounders are controlled for, carbohydrate quantity and GI, within the levels of carbohydrate and GI tested in this study, do not appear to influence RMR adaptation during weight loss. However, by design, our results cannot determine whether GI would attenuate metabolic adaptation at low-carbohydrate intakes.
This study also provided data relevant to the question of whether metabolic adaptation during weight loss increases susceptibility to subsequent weight regain. Consistent with most previous studies (5–9), we found that RMR did decrease to a greater extent during weight loss than anticipated from FM and FFM losses. However, following 5 wk of relative weight stability there was no measurable metabolic adaptation after controlling for acute weight change. Moreover, as noted in some (37–39), but not all (40) studies, individual variability in RMR following weight loss relative to baseline RMR did not predict weight change over the subsequent 12 mo period, and no difference in metabolic adaptation between weight re-gainers and those who maintained weight loss was observed. One possible explanation for conflicting results in previous studies is that metabolic adaptation may only be seen during the dynamic phase of weight loss. For experimental validity it may be important to have an extended period of energy balance prior to RMR measurements to prevent the influence of acute effects of overeating or undereating (38). Clearly, energy expenditure is adaptive to acute changes in energy balance, but based on the results of this study and other comparable investigations (38), there appears to be minimal sustained metabolic adaptation response to weight loss once weight has stabilized.
Several study strengths and weaknesses should be acknowledged. Important strengths include the tight control of diet composition and intake over an extended period, and duplicate measurements of body composition and RMR which were used to improve accuracy. However, weight changes were not, by design, large, and study results may not reflect changes occurring with greater body weight losses. Further, the 2-comparment model used to assess body composition does not account for differential changes in organ masses during weight loss which could influence metabolic adaptation calculations (5). Though volunteers were instructed to maintain habitual activity levels, we could not verify adherence to this instruction which precludes determining whether changes in physical activity patterns differed between groups or affected study outcomes. Additionally, body weight simulator estimations suggested that actual energy intake was somewhat greater than prescribed. This could indicate a moderate degree of diet non-adherence that could have attenuated between-group differences in diet composition. However, subjects were provided additional randomization-appropriate food if hungry, and to what extent the estimated difference in intake was due to consumption of additional provided foods, non-adherence to diet or activity instruction, or variability associated with model assumptions could not be determined. Attrition during the follow-up period could bias results, though RMR adaptation in study drop-outs did not differ from study completers, lessening the risk of bias. This study did not include low carbohydrate levels, which may be of additional interest. Finally, though we found no evidence for effect modification by sex in any analysis, this study was not adequately powered to address sex differences.
Conclusion
Neither low-GI relative to high-GI diets nor moderate-carbohydrate relative to high-carbohydrate diets showed differences with respect to effects on changes in body composition or resting metabolism during weight loss when confounding dietary factors were tightly controlled in a study providing all food for 22 weeks, and individual variability in metabolic adaptation following weight loss did not predict weight regain over 12 months. These findings demonstrate that adaptive variations in energy expenditure can be measured, but are unrelated to dietary carbohydrate content and GI within the levels of carbohydrate and GI tested in this study.
What is already known about this subject?
Reduced carbohydrate and low-glycemic index diets have been suggested to enhance fat mass loss during weight loss, and deter weight regain by attenuating reductions in energy expenditure during weight loss, but these proposed effects are uncertain because previous study designs were potentially confounded.
What does this study add?
When confounding dietary factors were controlled, moderate-carbohydrate (55% total energy) and low-GI diets did not preferentially reduce fat mass, preserve lean mass, or attenuate metabolic adaptation during weight loss compared to high-carbohydrate (70% total energy) and high-glycemic index diets.
Acknowledgements
We thank the study volunteers, and Metabolic Research Unit staff at the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University. Any opinions, findings, conclusions, or recommendations expressed in this article are those of the authors and do not necessarily reflect the views of the US Department of Agriculture.
Funding: This work was supported by grant R01-057477 from the National Institutes of Health and contracts 53-3K06-5-10 and 8050-15000-097-01S from the United States Department of Agriculture. JPK was supported by the Science, Mathematics, and Research Transformation Defense Education Program.
Abbreviations
- GI
glycemic index
- FM
fat mass
- FFM
fat free mass
- HighCarb
70% of total energy from carbohydrate
- ModCarb
55% of total energy from carbohydrate
- RMR
resting metabolic rate
- RQ
respiratory quotient
Footnotes
Disclosure: The authors declare no conflict of interest.
Clinical trial registration: ClinicalTrials.gov #NCT02379897
References
- 1.Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: A meta-analysis of US studies. Am J Clin Nutr. 2001;74:579–584. doi: 10.1093/ajcn/74.5.579. [DOI] [PubMed] [Google Scholar]
- 2.Major GC, Doucet E, Trayhurn P, Astrup A, Tremblay A. Clinical significance of adaptive thermogenesis. Int J Obes (Lond) 2007;31:204–212. doi: 10.1038/sj.ijo.0803523. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond) 2010;34(Suppl 1):S47–S55. doi: 10.1038/ijo.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller MJ, Bosy-Westphal A. Adaptive thermogenesis with weight loss in humans. Obesity. 2013;21:218–228. doi: 10.1002/oby.20027. [DOI] [PubMed] [Google Scholar]
- 5.Bosy-Westphal A, Kossel E, Goele K, Later W, Hitze B, Settler U, et al. Contribution of individual organ mass loss to weight loss-associated decline in resting energy expenditure. Am J Clin Nutr. 2009;90:993–1001. doi: 10.3945/ajcn.2008.27402. [DOI] [PubMed] [Google Scholar]
- 6.Camps SG, Verhoef SP, Westerterp KR. Weight loss, weight maintenance, and adaptive thermogenesis. Am J Clin Nutr. 2013;97:990–994. doi: 10.3945/ajcn.112.050310. [DOI] [PubMed] [Google Scholar]
- 7.Dulloo AG, Jacquet J. Adaptive reduction in basal metabolic rate in response to food deprivation in humans: A role for feedback signals from fat stores. Am J Clin Nutr. 1998;68:599–606. doi: 10.1093/ajcn/68.3.599. [DOI] [PubMed] [Google Scholar]
- 8.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: A randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 10.Flatt JP. Issues and misconceptions about obesity. Obesity. 2011;19:676–686. doi: 10.1038/oby.2011.7. [DOI] [PubMed] [Google Scholar]
- 11.Weinsier R, Hunter G, Schutz Y. Metabolic response to weight loss. Am J Clin Nutr. 2001;73:655–658. doi: 10.1093/ajcn/73.3.655. [DOI] [PubMed] [Google Scholar]
- 12.Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, Garcia-Lago E, et al. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. 2012;307:2627–2634. doi: 10.1001/jama.2012.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereira MA, Swain J, Goldfine AB, Rifai N, Ludwig DS. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA. 2004;292:2482–2490. doi: 10.1001/jama.292.20.2482. [DOI] [PubMed] [Google Scholar]
- 14.Baba NH, Sawaya S, Torbay N, Habbal Z, Azar S, Hashim SA. High protein vs high carbohydrate hypoenergetic diet for the treatment of obese hyperinsulinemic subjects. Int J Obes Relat Metab Disord. 1999;23:1202–1206. doi: 10.1038/sj.ijo.0801064. [DOI] [PubMed] [Google Scholar]
- 15.Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: A 1-y randomized controlled trial. Am J Clin Nutr. 2007;85:1023–1030. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- 16.Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Dallal GE, et al. Long term effects of energy-restricted diets differing in glycemic load on metabolic adaptation and body composition. Open Nutr J. 2007;85:1023–1030. [PMC free article] [PubMed] [Google Scholar]
- 17.de Jonge L, Bray GA, Smith SR, Ryan DH, de Souza RJ, Loria CM, et al. Effect of diet composition and weight loss on resting energy expenditure in the POUNDS LOST study. Obesity. 2012;20:2384–2389. doi: 10.1038/oby.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luscombe ND, Clifton PM, Noakes M, Parker B, Wittert G. Effects of energy-restricted diets containing increased protein on weight loss, resting energy expenditure, and the thermic effect of feeding in type 2 diabetes. Diabetes Care. 2002;25:652–657. doi: 10.2337/diacare.25.4.652. [DOI] [PubMed] [Google Scholar]
- 19.Racette SB, Schoeller DA, Kushner RF, Neil KM, Herling-Iaffaldano K. Effects of aerobic exercise and dietary carbohydrate on energy expenditure and body composition during weight reduction in obese women. Am J Clin Nutr. 1995;61:486–494. doi: 10.1093/ajcn/61.3.486. [DOI] [PubMed] [Google Scholar]
- 20.Bazzano LA, Hu T, Reynolds K, Yao L, Bunol C, Liu Y, et al. Effects of low-carbohydrate and low-fat diets: A randomized trial. Ann Intern Med. 2014;161:309–318. doi: 10.7326/M14-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: A meta-regression. Am J Clin Nutr. 2006;83:260–274. doi: 10.1093/ajcn/83.2.260. [DOI] [PubMed] [Google Scholar]
- 22.McMillan-Price J, Petocz P, Atkinson F, O'Neill K, Samman S, Steinbeck K, et al. Comparison of 4 diets of varying glycemic load on weight loss and cardiovascular risk reduction in overweight and obese young adults: A randomized controlled trial. Arch Intern Med. 2006;166:1466–1475. doi: 10.1001/archinte.166.14.1466. [DOI] [PubMed] [Google Scholar]
- 23.de Souza RJ, Bray GA, Carey VJ, Hall KD, LeBoff MS, Loria CM, et al. Effects of 4 weight-loss diets differing in fat, protein, and carbohydrate on fat mass, lean mass, visceral adipose tissue, and hepatic fat: Results from the POUNDS LOST trial. Am J Clin Nutr. 2012;95:614–625. doi: 10.3945/ajcn.111.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: The A to Z weight loss study: A randomized trial. JAMA. 2007;297:969–977. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 25.Juanola-Falgarona M, Salas-Salvado J, Ibarrola-Jurado N, Rabassa-Soler A, Diaz-Lopez A, Guasch-Ferre M, et al. Effect of the glycemic index of the diet on weight loss, modulation of satiety, inflammation, and other metabolic risk factors: A randomized controlled trial. Am J Clin Nutr. 2014;100:27–35. doi: 10.3945/ajcn.113.081216. [DOI] [PubMed] [Google Scholar]
- 26.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fields DA, Goran MI, McCrory MA. Body-composition assessment via air-displacement plethysmography in adults and children: A review. Am J Clin Nutr. 2002;75:453–467. doi: 10.1093/ajcn/75.3.453. [DOI] [PubMed] [Google Scholar]
- 28.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johannsen DL, Knuth ND, Huizenga R, Rood JC, Ravussin E, Hall KD. Metabolic slowing with massive weight loss despite preservation of fat-free mass. J Clin Endocrinol Metab. 2012;97:2489–2496. doi: 10.1210/jc.2012-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gadbury GL, Coffey CS, Allison DB. Modern statistical methods for handling missing repeated measurements in obesity trial data: Beyond LOCF. Obes Rev. 2003;4:175–184. doi: 10.1046/j.1467-789x.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 31.Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378:826–837. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santos FL, Esteves SS, da Costa Pereira A, Yancy WS, Jr, Nunes JP. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048–1066. doi: 10.1111/j.1467-789X.2012.01021.x. [DOI] [PubMed] [Google Scholar]
- 33.Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: A meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96:1281–1298. doi: 10.3945/ajcn.112.044321. [DOI] [PubMed] [Google Scholar]
- 34.Abete I, Parra D, Martinez JA. Energy-restricted diets based on a distinct food selection affecting the glycemic index induce different weight loss and oxidative response. Clin Nutr. 2008;27:545–551. doi: 10.1016/j.clnu.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Raatz SK, Torkelson CJ, Redmon JB, Reck KP, Kwong CA, Swanson JE, et al. Reduced glycemic index and glycemic load diets do not increase the effects of energy restriction on weight loss and insulin sensitivity in obese men and women. J Nutr. 2005;135:2387–2391. doi: 10.1093/jn/135.10.2387. [DOI] [PubMed] [Google Scholar]
- 36.Solomon TP, Haus JM, Kelly KR, Cook MD, Filion J, Rocco M, et al. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am J Clin Nutr. 2010;92:1359–1368. doi: 10.3945/ajcn.2010.29771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amatruda JM, Statt MC, Welle SL. Total and resting energy expenditure in obese women reduced to ideal body weight. J Clin Invest. 1993;92:1236–1242. doi: 10.1172/JCI116695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinsier RL, Nagy TR, Hunter GR, Darnell BE, Hensrud DD, Weiss HL. Do adaptive changes in metabolic rate favor weight regain in weight-reduced individuals? An examination of the set-point theory. Am J Clin Nutr. 2000;72:1088–1094. doi: 10.1093/ajcn/72.5.1088. [DOI] [PubMed] [Google Scholar]
- 39.Weinsier RL, Nelson KM, Hensrud DD, Darnell BE, Hunter GR, Schutz Y. Metabolic predictors of obesity. Contribution of resting energy expenditure, thermic effect of food, and fuel utilization to four-year weight gain of post-obese and never-obese women. J Clin Invest. 1995;95:980–985. doi: 10.1172/JCI117807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasman WJ, Saris WH, Westerterp-Plantenga MS. Predictors of weight maintenance. Obes Res. 1999;7:43–50. doi: 10.1002/j.1550-8528.1999.tb00389.x. [DOI] [PubMed] [Google Scholar]