Abstract
Background
The aim of this study was to investigate the feasibility and clinical value of transvaginal surgical treatment for cesarean scar pregnancy (CSP-II).
Material/Methods
This study was a retrospective analysis of 25 CSP-II patients who received transvaginal surgical treatments. These patients were admitted in our hospital between January 2010 and June 2012.
Results
All surgical treatments were successful without overt complications. The average operation time was 61.5 minutes, the average intraoperative blood loss was 60.5 ml, the average hospital stay was 9.4 days and the average time that blood β-human chorionic gonadotropin (β-HCG) returned to normal range was 15 days. In all 25 patients, the cesarean scar mass located at the anterior wall of the lower uterine segment disappeared by B-ultrasound examination within 1 or 2 weeks after surgery. Postoperatively, the normal menstrual period started again with an average time of 28.9 days. No menstruation-related abnormalities, such as menstrual dripping or an abnormal amount of blood, were reported after surgery.
Conclusions
Transvaginal surgery for CSP-II is a novel surgical approach. It has several advantages, including a thorough one-time treatment lesion clearance, short operation time, minimized trauma, minimal intraoperative blood loss, quick reduction of blood β-HCG, and rapid menstruation recovery. It is a simple and feasible surgical approach of great clinical value and few treatment-related complications.
MeSH Keywords: Gynecology, Obstetric Surgical Procedures, Pregnancy
Background
Cesarean scar pregnancy (CSP) is a rare type of ectopic pregnancy, in which the gestational sac is implanted in a scar of a previous cesarean section; 6.1% of ectopic pregnancies after cesarean section have been reported to be CSPs, with an incidence of 1 in 1800–3000 pregnancies [1]. There are 2 types of CSPs. Type I is caused by implantation of the amniotic sac on the scar with progression toward either the uterine cavity or the cervicoisthmic space. Type II is caused by implantation deeply into a previous CS, which is defect with infiltrating growth into the uterine myometrium and bulging from the uterine serosal surface. Type II may result in uterine rupture and severe bleeding during the first trimester of pregnancy [2]. Since 2010 in our hospital, 25 CSP-II patients underwent transvaginal surgery to remove ectopic pregnancy; all patients had a satisfactory therapeutic outcome while remaining fertile. Our experiences were reviewed and shared below.
Material and Methods
Patient demographic features
Twenty-five CSP-II patients were admitted after cesarean section in our hospital between January 2012 and June 2014. The age of patients ranged between 22 years and 42 years of age, with an average age of 30.5 years. The time between the last previous cesarean section and current CSP-II ranged from between 5 months and 6 years, with an average time period of 4.6 years. Fifteen out of 25 patients had 1 cesarean section previously, and the other 10 had 2 cesarean sections previously. The lower uterine segment transverse and longitudinal incisions were performed on 18 and 7 patients, respectively.
The study protocol was approved by the Ethics Committees of the Affiliated Hospital of HeBei University, Baoding, and all participants provided written informed consent.
The Features of CSP-II
The clinical manifestations of the study included amenorrhea, irregular vaginal bleeding, lower abdominal pain, and bleeding after curettage. All patients had a history of amenorrhea, ranging between 46 days and 120 days, with an average of 60 days. Nine out of 25 patients had vaginal bleeding, with bleeding time ranging from between 0 and 34 days, and an average bleeding time of 16.6 days. Eight out of 25 patients had lower abdominal pain, which manifested mainly as paroxysmal pain. Four out of 25 patients were transferred from other hospitals due to either a misdiagnosed intrauterine pregnancy or massive bleeding caused by uterine curettage and postoperative residues. Two patients had amenorrhea lasting for over a month, followed by uterine curettage and persistent vaginal bleeding for 24 days afterwards with a normal amount of menstruation; CSP-II was considered by repeated B-ultrasound examinations and patients were transferred to our hospital for further treatment. Another 2 patients also had amenorrhea lasting for over a month followed by uterine curettage; massive vaginal bleeding occurred with rapid blood loss of around 400 mL. After being transferred to our hospital, the abnormal signals in the uterus were detected by ultrasound and considered postoperative residues. Therefore, secondary uterine curettage was performed; however, massive vaginal bleeding occurred again on the third day after the operation, with rapid blood loss of around 800 mL and 3 episodes of dizziness and fainting. Thus, the patient underwent urgent surgery.
Supplementary examinations
Supplementary examinations included blood β-human chorionic gonadotropin (β-HCG) and transvaginal B-ultrasound examination. Ten patients had an elevated blood β-HCG level (range: 2,043–186,754 mU/mL) with an average level of 45,326 mU/mL. Transvaginal B-ultrasound examinations were performed to detect a gestational sac-like mass detected at the cesarean scar located at the lower uterine segment: maximum diameters in patients ranged between 1.5 cm and 5.8 cm, with an average of 3.7 cm. A beating heart tube was detected in 5 patients; disordered or uneven signals were detected at the lower uterine segment along with rich blood flow signals. The anterior wall of the uterus muscle where masses attached turned into thinner layers, which measured only 1.5 to 12 mm at its thinnest region. The not homogeneous echogenic mass was only detected in 1 patient who presented to our hospital with a large amount of intrauterine blood, and a smaller blood flow signal; thus it was misdiagnosed as postoperative residues (Table 1).
Table 1.
Demographics and clinical characteristics of patients of cesarean scar pregnancy II.
| Case | Age (years) | Gravidity | Parity | Gestational age (days) | Duration of vaginal bleeding (days) | Diameter of embryo sac (mm) | Thickness of myometrium from serosa (mm) | Blood β-HCG level (mIU/ml) |
|---|---|---|---|---|---|---|---|---|
| 1 | 22 | 1 | 1 | 47 | 5 | 2.6 | 11 | 12.335 |
| 2 | 23.3 | 1 | 1 | 55 | 7 | 4.1 | 10 | 2.921 |
| 3 | 24.1 | 2 | 1 | 76 | 24 | 4.9 | 6 | 59.411 |
| 4 | 25.2 | 2 | 1 | 52 | 11 | 3.7 | 4 | 42.506 |
| 5 | 25.9 | 2 | 1 | 48 | 8 | 3.2 | 4 | 8.900 |
| 6 | 26 | 1 | 1 | 49 | 6 | 2.1 | 4 | 46.69 |
| 7 | 26.1 | 3 | 1 | 46 | 0 | 1.5 | 12 | 2.043 |
| 8 | 26.3 | 2 | 1 | 49 | 12 | 3.4 | 8 | 26.465 |
| 9 | 26.5 | 1 | 1 | 51 | 19 | 3.6 | 9 | 0.604 |
| 10 | 27 | 2 | 1 | 77 | 25 | 4.9 | 6 | 101.216 |
| 11 | 27.2 | 3 | 1 | 61 | 22 | 4.9 | 6 | 55.202 |
| 12 | 28.4 | 3 | 2 | 106 | 49 | 5.5 | 1.5 | 159.404 |
| 13 | 28.6 | 1 | 1 | 89 | 22 | 5.3 | 4 | 78.595 |
| 14 | 28.5 | 2 | 1 | 48 | 6 | 2.4 | 9 | 115.409 |
| 15 | 29.8 | 1 | 1 | 47 | 9 | 3 | 8 | 7.544 |
| 16 | 30.6 | 2 | 1 | 50 | 5 | 3.2 | 10 | 32.754 |
| 17 | 30.8 | 4 | 2 | 120 | 68 | 5.8 | 1.7 | 186.754 |
| 18 | 32.9 | 2 | 1 | 58 | 4 | 3.8 | 12 | 40.425 |
| 19 | 34.5 | 2 | 1 | 55 | 16 | 3.2 | 10 | 44.307 |
| 20 | 36.4 | 1 | 1 | 57 | 20 | 3.9 | 12 | 28.667 |
| 21 | 38.8 | 4 | 1 | 64 | 30 | 4.3 | 9 | 53.628 |
| 22 | 39.6 | 2 | 1 | 47 | 10 | 3.4 | 10 | 9.454 |
| 23 | 40.4 | 3 | 1 | 49 | 8 | 3.4 | 8 | 40.462 |
| 24 | 41.6 | 2 | 1 | 48 | 9 | 3.2 | 8 | 22.904 |
| 25 | 42 | 3 | 1 | 51 | 10 | 3.4 | 7 | 24.543 |
Preoperative preparation
Liver and kidney function tests were performed; other routine examinations were completed prior to surgery, such as complete blood cell count, urinalysis, bleeding and coagulation tests, and electrocardiography to confirm there were no contraindications to surgery. Vaginal cleaning was conducted and oral mifepristone was administrated to patients with high blood β-HCG. Six patients out of 25 were administrated oral mifepristone. One patient with β-HCG of 78 697 mU/mL experienced a reduction in vaginal bleeding 1 day after administration of oral mifepristone (50 mg BID); however, repeated blood β-HCG testing showed no significant reduction. Another patient had 2 cesarean sections; the CSP-II was found only 6 months since the last cesarean section. Considering the surgical risk, oral mifepristone (50 mg BID) was administrated for 2 days; however, the blood β-HCG significantly increased in the retest. Surgery was performed to terminate pregnancy.
Operative procedure
All surgeries were performed under general anesthesia. Patients were placed in a lithotomy position, and the operation field was sterilized. The bladder was emptied by a metal catheter. The vagina and cervix were exposed by a vaginal retractor. The anterior vaginal fornix was exposed by a cervical clamp which was clamped to the cervical upper lip and pulled downwards, before 0.3 mg epinephrine dissolved into 500 mL physiological saline was injected into the cervico-vaginal gap. The pressure from the injected physiological saline separated the bladder and cervix. A transverse incision was made 2 cm superior to the clamped site; the bladder was surgically pushed away through the cervico-vaginal gap to the bladder-peritoneal fold, where the peritoneum was punctured in order to place a vaginal retractor. Usually, the uterine isthmus was mildly bulged and the uterine serosa displayed a purplish-blue color; the thin and tender area was detected by a probe and considered as a scar pregnancy lesion. A transverse incision was made at the cesarean section scar tissue of the uterine isthmus, where the visible dark red blood clots associated with pregnancy tissue could be visualized; sometimes the villi were visible as well.
The scar and pregnancy tissue was removed in the following order. First, the pregnancy tissues were removed by bending pliers or small oval forceps through the incision, followed by suction to clear the uterine cavity. Next, the incision was closed by a continuous lock stitch with 2-0 threads under the guidance of the detecting probe. The bladder was then examined before peritoneal suture to ensure there was no active bleeding. Finally, the vaginal wall was fixed by a continuous lock stitch with 2-0 threads and then placed with 3 pieces of iodine oil gauze; this was removed 24 hours later after the operation. An indwelling catheterized bladder was placed to observe urine output (Figures 1–4).
Figure 1.
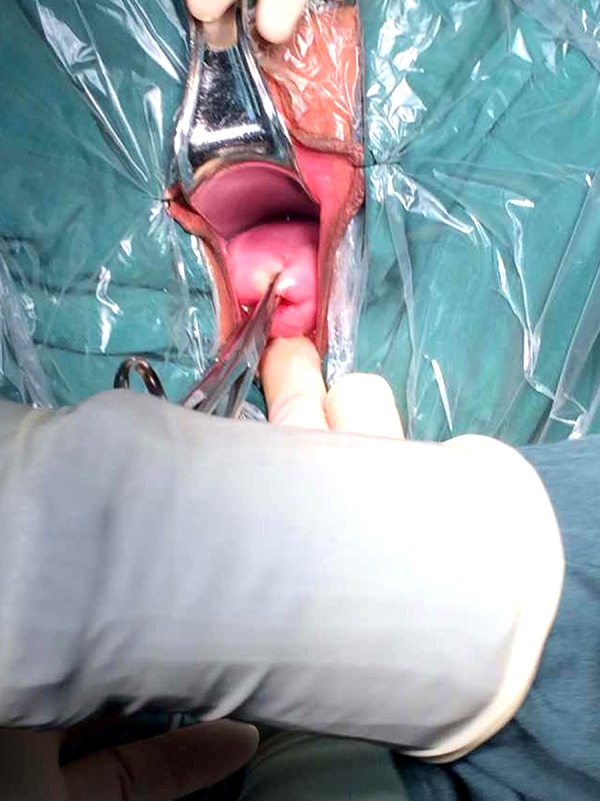
Exposed vesicocervical gap.
Figure 2.
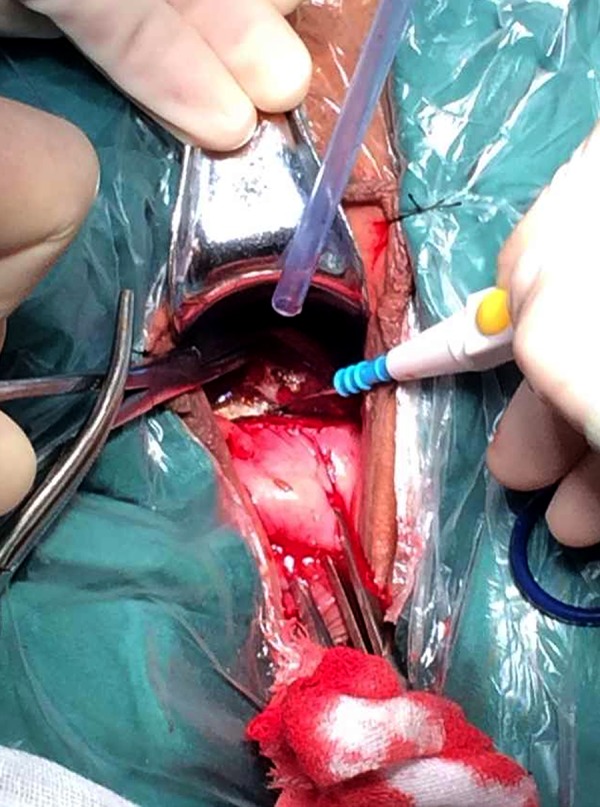
Vaginal fornix mucosa seen before incision, exposing the pregnancy mass scar in the bladder-cervix gap.
Figure 3.
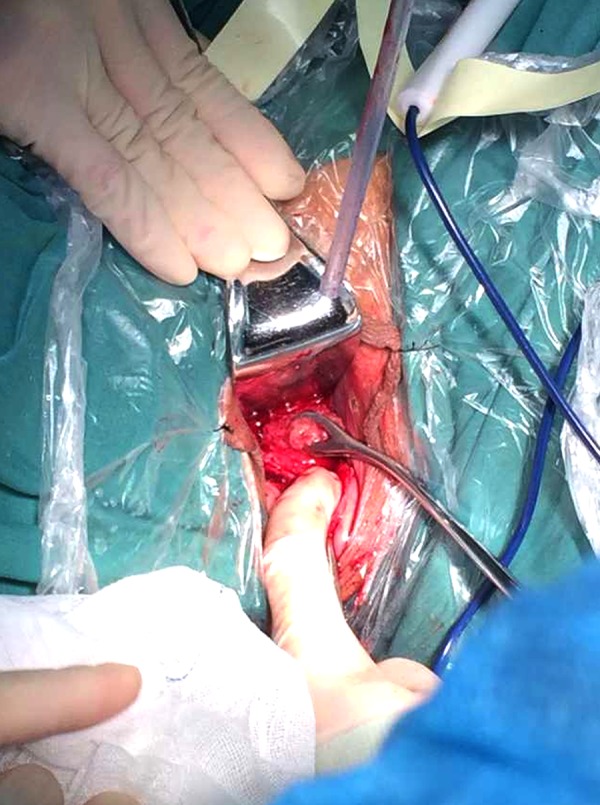
By cutting the gestational sac and removing the villi through the uterine cavity, the suction curettage decidua can be used to prevent profuse postoperative vaginal bleeding.
Figure 4.
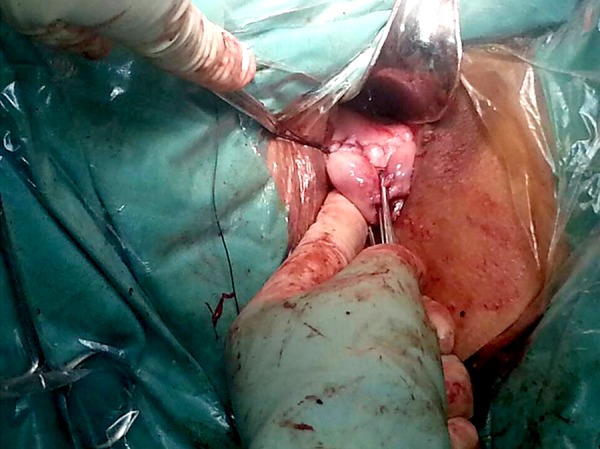
Suturing the uterine wall and the vaginal mucosa.
Postoperative management
Postoperative management included several steps. First, iodine oil gauze pads were removed from the vagina 24 hours after surgery. Next, the indwelling bladder catheterization was placed in the patients for 1 to 2 days and then the infection was prophylactically treated. Regular monitoring of blood β-HCG was performed, in which the blood β-HCG was tested every 3 days with recorded fluctuations; for instance, the postoperative blood β-HCG was not significantly reduced, increased, or decreased <20% 3 days after operation. All of these outcomes indicated the possibility of villi residues and a persistent pregnancy scar. In those patients who had a relatively high blood β-HCG level, oral mifepristone could be administrated if the postoperative blood β-HCG level did not significantly reduce after the procedure. No severe complications were observed in any of the patients, such as bladder perforation or massive pulmonary embolization. None of the patients died as a result of massive bleeding.
Follow-up by telephone interview or outpatient department visit
The patients were recommended to have their blood β-HCG levels tested once a week until levels returned to normal. Ultrasound examination was recommended every 2 weeks to monitor both the mass size and alterations on the anterior uterine wall until the mass disappeared completely. The mean ±SD follow-up period was 16.3±4.6 months (range 6–24 months). Seven women (28%) wanted to become pregnant in the future, and during the follow-up period, no pregnancies were reported.
Results
Both the intraoperative procedure and postoperative recovery of patients went smoothly. The success rate is 100%. The purple-blue bulge, fresh villi-like tissue, and obsolete yellow implanted tissue could be visualized at the anterior wall of the lower uterine segment. Removed gestational tissue weighed around 8 to 40 grams per patient; all of the removed tissue proved to be uterine muscle scar tissue and villous gestational villi tissue by pathological inspection. Operation time ranged between 25 and 90 minutes, with an average time of 61.5 minutes; intraoperative blood loss ranged between 15 mL and 150 mL, with an average loss of 60.5 mL. Length of hospital stay ranged between 4 and 16 days, with the average stay of 9.4 days; and time for blood β-HCG levels returning to normal ranged between 6 days and 31 days, with an average time of 15 days. The cesarean scar mass of the lower uterine wall anterior segment disappeared by the time of B-ultrasound examination in 5 patients after surgery and before discharge. The other 20 patients were followed up by B-ultrasound examination at the outpatient department; their masses disappeared in the second week after discharge. All patients had normal menstruation after surgery, with the average postoperative menstrual period of 28.9 days. There were no reported menstruation abnormalities such as menstrual dripping or abnormal amount of blood.
Surgical complications
There were no surgery- or anesthesia-related complications. No vaginal incision infections, poor healing, bladder trauma, vesicovaginal fistulas, or persistent ectopic pregnancies were reported
Discussion
Amenorrhea and a painless and small amount of vaginal bleeding are the main manifestations of CSP-II. All patients described in the study had amenorrhea. Twenty-one out of 25 patients had a small amount of painless vaginal bleeding, with some patients experiencing mild or moderate lower abdominal pain; other patients experienced no discomfort and had an accidently discovered CSP-II by routine examination. Therefore, the CSP-II misdiagnosis rate was usually high, and misdiagnosed as abortion, cervical pregnancy, or gestational tissue residue after uterine curettage, or trophoblastic disease. Four patients were misdiagnosed in the present study. Vaginal color-ultrasound examination with high resolution is an important tool for CSP-II diagnosis, with a sensitivity of 86.4% (95% CI: 0.763–0.905). Ultrasound CSP-II diagnosis criteria proposed by Godin [3] Fylstra [4] and Vial Y [2] included no diagnosed intrauterine pregnancy or cervical pregnancy, gestational sac was detected at either the uterine isthmus or the anterior uterine wall, and myometrial tissue depression was detected between the gestational sac and bladder wall. Complicated cases can be confirmed by MRI, hysteroscopy, or laparoscopy. In order to reduce the misdiagnosis rate, a combined transvaginal and trans-abdominal ultrasound examination was proposed by Maymon et al. [5] to thoroughly inspect the uterus and accurately measure the distance between the gestational sac and bladder wall. Pavlova et al. [6] used 3-dimensional ultrasound examination for monitoring uterine neovascularization before and after CSP-II treatment; they suggested that this superior technology could better reveal vascularization around the gestational sac and intrauterine membrane as compared with the conventional color-ultrasound test. In the current study, gestational sac-like masses were detected in the cesarean scar of the lower uterine segment of 25 patients. The B-ultrasound not only helped with diagnosis, but also provided information such as mass location, size, and thickness of the mass attached to the uterine wall, for assisting with the evaluation of associated surgical difficulty and risk.
CSP-II may result in either uterine rupture or uncontrollable massive bleeding; therefore, timely termination of the pregnancy is necessary once diagnosis is confirmed. Unified criteria is lacking for CSP-II diagnosis due to its rare incidence rate [7]. Presently, the most common treatments include medication alone [1,8,9], interventional therapy [10,11], uterine curettage [12,13], and either transabdominal or laparoscopic scar removal [14,15]. Methotrexate (MTX) treatment alone or uterine artery embolization for CSP-II treatment were not optimal treatments. Their disadvantages include a long course of treatment, delayed recovery of patients, severe chemotherapy complications, a tardy disappearance of scar mass, slow blood β-HCG recovery, and menstruation dripping. Our previous studies indicated that the MTX treatment cure rate was 83.3% with an adverse complication rate of 41.7%; notably, 33.3% patients had menstrual dripping after operation. Uterine artery embolization in combination with chemotherapy could reduce the risk of acute massive hemorrhage complicated by ectopic embolization. The massive hemorrhage or uterine rupture might occur during treatment or follow-up treatment causing a need for hysterectomy. Though conservative medical therapy could be successful, the villi implantation-related scar defect might lead to CSP-II recurrence or uterine rupture. Uterine curettage not only results in remaining scar residue, but also induces the uterine perforation at the scar tissue, fatal massive bleeding, or other severe complications, even under guidance of B-ultrasound. Thus, most clinicians refuse to perform curettage as the only treatment. Since 1978, when Larsen et al. [16] first reported successful CSP-II treatment by local lesion removal, transabdominal or laparoscopic scar removal have been reported by accumulated papers. Local lesion removal has been accepted as a safe and effective CSP-II treatment. The local lesion removal surgery could thoroughly clear the lesion by resecting micro channels within the affected uterine muscles, fixing scar tissue, and reducing risk of CSP-II reoccurrence. Meanwhile, quick blood β-HCG reduction and rapid recovery of patients have additional merits. However, transabdominal surgery is associated with large trauma; laparoscopic operation has a relatively higher risk and requires precise hospital equipment and surgeon experience, which have restricted broad application in primary hospitals.
In recent decades, new techniques for minimally invasive surgery have experienced numerous developments and changes [17]. Minimally invasive surgery has a well-established role in the modern era of surgery, yielding less postoperative pain, a shorter hospital stay, faster recovery, and better esthetic results [18,19]. Recently, transvaginal surgery, as a new minimally invasive surgical approach, has been increasingly reported [20,21]. This approach yields access to the abdominal cavity without any incisions in the abdominal wall (scarless surgery), and the natural orifices (mouth, urethra, anus, and soon) serve as the gateway to the peritoneal cavity.
Transvaginal surgery for removing local lesions is a novel minimally invasive therapy for CSP-II. It has several advantages such as one-time thorough lesion clearance, maintaining patient fertility, reducing hospital stay, rapid patient recovery, and no adverse effect on menstruation. In addition, transvaginal surgery has minimal surgical trauma, simple operation, less equipment requirements, reduced operation cost, lower risk, and less intraoperative or postoperative complications than other therapies. These all benefited its broad application in primary hospitals where transvaginal surgery has been adopted for other diseases. In the current study, all of the 25 patients had successful surgery, without intraoperative or postoperative complications; average hospital stay was 9.5 days. The scar tissue mass at the anterior uterine wall disappeared within 2 weeks after operation. Normal menstruation started from between 23 to 38 days after operation, with an average time of 29.7 days. No menstruation dripping or abnormal amount occurred in any patients.
In summary, from our 25 patients, we found that injection of dissolved epinephrine (0.3 mg epinephrine: 500 mL physiological saline) at the cervix reduced intraoperative hemorrhage, and injection of physiological saline at the bladder cervical gap separated the bladder and the uterus; the bladder was pushed upwards bilaterally by the surgeon to avoid surgical trauma on the bladder, uterine track, or formation of vesicovaginal fistula. Thorough gestational tissue clearance by suction after tissue removal avoided CSP-II recurrence by the continuous lock stitch under guidance of detecting a probe for fixing on the anterior uterine wall. Entire uterine wall layers should be carefully sutured to prevent a uterine incision diverticulum or scar microchannel formation. This may be done to reduce the risk of CSP-II recurrence, preserve fertility, and reduce impact on future pregnancy and postoperative monitoring on blood β-HCG, the villi residues, or persistent scar pregnancy if postoperative blood β-HCG was not significantly reduced, increased, or decreased <20% 3 days after the operation. In those who had a relatively high level of preoperative blood β-HCG or unsatisfactory postoperative blood β-HCG reduction, oral mifepristone could be administrated. It is not recommended to use prophylactic joint chemotherapy before surgery.
Conclusions
Transvaginal surgery for CSP-II is a new surgical approach, with advantages of short operation time, thorough lesion clearance, minimal trauma, rapid patient recovery, and few complications. It is a worthwhile CSP-II treatment approach with a satisfactory level of feasibility. However, since it is a novel surgical approach, both accumulated patient experiences and surgical techniques are necessary before broad application.
Footnotes
Source of support: Departmental sources
Conflict of interests
All authors declare that they have no conflict of interests.
References
- 1.Cok T, Kalayci H, Ozdemir H, et al. Transvaginal ultrasound-guided local methotrexate administration as the first-line treatment for cesarean scar pregnancy: Follow-up of 18 cases. J Obstet Gynaecol Res. 2015;41:803–8. doi: 10.1111/jog.12627. [DOI] [PubMed] [Google Scholar]
- 2.Vial Y, Petignat P, Hohlfeld P. Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol. 2000;16:592–93. doi: 10.1046/j.1469-0705.2000.00300-2.x. [DOI] [PubMed] [Google Scholar]
- 3.Godin PA, Bassil S, Donnez J. An ectopic pregnancy developing in a previous caesarian section scar. Fertil Steril. 1997;67:398–400. doi: 10.1016/S0015-0282(97)81930-9. [DOI] [PubMed] [Google Scholar]
- 4.Fylstra DL, Pound-Chang T, Miller MG, et al. Ectopic pregnancy within a cesarean delivery scar: a case report. Am J Obstet Gynecol. 2002;187:302–4. doi: 10.1067/mob.2002.125998. [DOI] [PubMed] [Google Scholar]
- 5.Maymon R, Svirsky R, Smorgick N, et al. Fertility performance and obstetric outcomes among women with previous cesarean scar pregnancy. J Ultrasound Med. 2011;30:1179–84. doi: 10.7863/jum.2011.30.9.1179. [DOI] [PubMed] [Google Scholar]
- 6.Pavlova E, Gunev D, Diavolov V, Slavchev B. Cesarean scar ectopic pregnancy: diagnosis with 2D, three-dimensional (3D) ultrasound and 3D power doppler of a case and review of the literature. Akush Ginekol (Sofiia) 2013;52:43–52. [PubMed] [Google Scholar]
- 7.Ng BK, Lim PS, Ahmad S, et al. Cesarean scar pregnancy: What can we offer? Taiwan J Obstet Gynecol. 2015;54:208–10. doi: 10.1016/j.tjog.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 8.Peng P, Gui T, Liu X, et al. Comparative efficacy and safety of local and systemic methotrexate injection in cesarean scar pregnancy. Ther Clin Risk Manag. 2015;11:137–42. doi: 10.2147/TCRM.S76050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng M, Li L, Ding Y, et al. Exploration of the successful treatment algorithms used in 23 cases of early live cesarean scar pregnancy. Gynecol Obstet Invest. 2015;79:139–44. doi: 10.1159/000368400. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y, Li Y, Xi R, et al. An application of uterine artery chemoembolization in treating cesarean scar pregnancy. Int J Clin Exp Med. 2015;8:2570–77. [PMC free article] [PubMed] [Google Scholar]
- 11.Kong D, Dong X, Qi Y. Ultrasonography-guided multidrug stratification interventional therapy for cesarean scar pregnancy. Arch Gynecol Obstet. 2015;292:143–48. doi: 10.1007/s00404-014-3602-0. [DOI] [PubMed] [Google Scholar]
- 12.Zhu X, Deng X, Wan Y, et al. High-intensity focused ultrasound combined with suction curettage for the treatment of cesarean scar pregnancy. Medicine (Baltimore) 2015;94:e854. doi: 10.1097/MD.0000000000000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qian ZD, Huang LL, Zhu XM. Curettage or operative hysteroscopy in the treatment of cesarean scar pregnancy. Arch Gynecol Obstet. 2015 doi: 10.1007/s00404-015-3730-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Wu X, Xue X, Wu X, et al. Combined laparoscopy and hysteroscopy vs. uterine curettage in the uterine artery embolization-based management of cesarean scar pregnancy: a cohort study. Int J Clin Exp Med. 2014;7:2793–803. [PMC free article] [PubMed] [Google Scholar]
- 15.He Y, Wu X, Zhu Q, et al. Combined laparoscopy and hysteroscopy vs. uterine curettage in the uterine artery embolization-based management of cesarean scar pregnancy: a retrospective cohort study. BMC Womens Health. 2014;14:116. doi: 10.1186/1472-6874-14-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen JV, Muller EJ. Obstetric care in a rural population. S Afr Med J. 1978;54:1137–40. [PubMed] [Google Scholar]
- 17.Olweny EO, Best SL, Tracy CR, Cadeddu JA. New technology and applied research: what the future holds for LESS and NOTES. Arch Esp Urol. 2012;65:434–43. [PubMed] [Google Scholar]
- 18.Masoomi H, Mills S, Dolich MO, et al. Comparison of outcomes of laparoscopic versus open appendectomy in adults: data from the Nationwide Inpatient Sample (NIS), 2006–2008. J Gastrointest Surg. 2011;15:2226–31. doi: 10.1007/s11605-011-1613-8. [DOI] [PubMed] [Google Scholar]
- 19.Trastulli S, Cirocchi R, Listorti C, et al. Laparoscopic vs. open resection for rectal cancer: a meta-analysis of randomized clinical trials. Colorectal Dis. 2012;14:e277–96. doi: 10.1111/j.1463-1318.2012.02985.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee CL, Wu KY, Su H, et al. Hysterectomy by transvaginal natural orifice transluminal endoscopic surgery (NOTES): a series of 137 patients. J Minim Invasive Gynecol. 2014;21:818–24. doi: 10.1016/j.jmig.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Wang CJ, Huang HY, Huang CY, Su H. Hysterectomy via transvaginal natural orifice transluminal endoscopic surgery for nonprolapsed uteri. Surg Endosc. 2015;29:100–7. doi: 10.1007/s00464-014-3639-y. [DOI] [PubMed] [Google Scholar]


