Abstract
Over the last decades advancements have improved survival and outcomes of severely burned patients except one population, elderly. The Lethal Dose 50 (LD50) burn size in elderly has remained the same over the past three decades, and so has morbidity and mortality, despite the increased demand for elderly burn care. The objective of this study is to gain insights on why elderly burn patients have had such a poor outcome when compared to adult burn patients. The significance of this project is that to this date, burn care providers recognize the extreme poor outcome of elderly, but the reason remains unclear. In this prospective translational trial, we have determined clinical, metabolic, inflammatory, immune, and skin healing aspects. We found that elderly have a profound increased mortality, more premorbid conditions, and stay at the hospital for longer, p < 0.05. Interestingly, we could not find a higher incidence of infection or sepsis in elderly, p > 0.05, but a significant increased incidence of multi organ failure, p < 0.05. These clinical outcomes were associated with a delayed hypermetabolic response, increased hyperglycemic and hyperlipidemic responses, inversed inflammatory response, immune-compromisation and substantial delay in wound healing predominantly due to alteration in characteristics of progenitor cells, p < 0.05. In summary, elderly have substantially different responses to burns when compared to adults associated with increased morbidity and mortality. This study indicates that these responses are complex and not linear, requiring a multi-modal approach to improve the outcome of severely burned elderly.
Keywords: Elderly, Burn, Inflammasome, Inflammation, Hypermetabolism, Morbidity, Mortality, Pathophysiologic response, Stem cell, Skin healing
Highlights
-
•
The outcome of elderly burn management is low with reasons that remain unclear.
-
•
Elderly have a higher mortality, more premorbid conditions and a higher incidence of multi organ failure.
-
•
Elderly stay at the hospital for longer time.
-
•
The incidence of infection or sepsis is not higher than young adult.
-
•
Elderly show delayed hyper-metabolic response, increased hyperglycemic and hyperlipidemic responses.
-
•
Elderly present inversed inflammatory response.
-
•
Elderly show substantial delay in wound healing, predominantly due to alteration in characteristics of progenitor cells.
Despite advancements in treatment of severely burned patients, the death rate is still high in elderly. In this project, we investigate the reason behind this poor outcome. Our report highlights some of the deficiencies that we have observed in elderly patients and compare them to the young adults. Elderly have late immune responses which are necessary to fight the disease. Their body lacks some of the essential stem cells which are essential for skin healing. By learning the major deficiencies that come with this age group, we will be able to help elderly who have been subjected to burn injury.
1. Introduction
A severe burn is a profound and devastating injury, associated with significant morbidity and mortality (Jeschke et al., 2008a, Jeschke et al., 2011) and occurs in more than two million people in North America each year (Bringham and McLoughlin, 1996). According to the World Health Organization, there are an estimated 330,000 deaths per year worldwide related to thermal injury ((WHO) OWH, 2002). Over the last three to four decades clinical and therapeutic advancements in burn care, such as early excision and grafting, adequate nutrition, and implementation of critical care bundles, have markedly increased the Lethal Dose 50 (LD50) for pediatric and adult burn patients (Kraft et al., 2012), and therefore have significantly improved outcomes of burn patients. However, this is not the case in burn patients over 65 years of age. The LD50 burn size in elderly has remained the same over the past three decades, and so has morbidity and mortality, despite the increased demand for elderly burn care.
In general, elderly, who represent the fastest growing population, have been shown to be particularly susceptible to burn injuries (Pham et al., 2009). This is due to thinning skin, decreased sensations, mental alterations, or other contributing factors (Pham et al., 2009, Gerstein et al., 1993). The higher risk of suffering from a burn injury in elderly population along with the rapid growth of this population will change the burn treatment paradigms in the near future. As previously mentioned, little or almost no progress has been made that improves elderly outcomes post-burn over the last two decades with the LD50, remaining at around 35% total body surface area (TBSA) burn. Speculations about the factors contributing to poor outcomes in elderly have been: pre-existing medical conditions (Lundgren et al., 2009), failure of the immune system to fight of post-burn infections along with altered inflammatory and immune responses (Rani and Schwacha, 2012), skin thinning leading to deeper burns (Albornoz et al., 2011), and generally decreased metabolic resources and capacity (Grimble, 2003). All these factors are associations and not causally related and the exact physiological and biological mechanisms leading to worse outcomes are unknown.
Despite the recognized poor outcome of elderly patients, the reason why this patient population is doing so poorly remains unclear. Therefore, the objective of this study is to further elucidate on the mechanisms behind this.
2. Materials & Methods
In this study, we first looked at our entire cohort of patients from 1995 to 2015 (n = 2796). Subsequently, we focused our analysis on patients admitted from 2006 to April 2015 (n = 1461) since we wanted to reduce the effect of time or treatment changes on burn outcomes. In general, burn patients that were admitted to our burn center with thermal injuries were eligible for enrollment. Demographic data were collected on all patients. Patients who required surgery were asked for consent for blood and tissue collection. These procedures were approved by the Research Ethics Board of Sunnybrook Health Sciences Centre (Study #194-2010). All patients received standard of care according to our clinical protocols, including early excision and grafting, early nutrition, adequate ventilation, and adequate antibiotic coverage as previously published. If needed, all patients received insulin for glucose control during their stay in the burn unit. Insulin dosage was titrated on a sliding scale pursuant to the patient's blood glucose levels and corresponding needs.
Patients were divided into adults, between 18 to < 65 years of age, and elderly ≥ 65 years of age. We used the current WHO definition of elderly, aware of the critique for using this age, but we hypothesized that this definition was the best cut-off age for elderly at this time. Clinical data as well as tissue and serum was obtained at various time points and processed according to established protocols.
2.1. Clinical Outcomes Including Mortality
Patients' clinical outcome markers were recorded prospectively during rounds and entered into a database. Outcomes included mortality during hospitalization; infections, pneumonia, and septic episodes defined by the American Burn Association guidelines (Greenhalgh et al., 2007) organ function. It also included: demographic data, height, weight, burn size, length of stay, heart rate, blood pressure, number of required surgeries, time between surgeries, nutritional intake, ventilation, and presence or absence of inhalation injury (Jeschke et al., 2008a, Jeschke et al., 2011, Jeschke et al., 2013, Kraft et al., 2013).
2.2. Metabolic Responses
Metabolic outcomes were compared for adults vs. elderly. We determined insulin sensitivity via oral glucose tolerance testes (OGTT) which was conducted when a patient was 95% healed. Standard OGTT with intake of 75 g of glucose and subsequent glucose, insulin, and c-peptide measurements were conducted over a period of 2 h (Jeschke et al., 2013) and calculated according to well established protocols. We calculated insulin sensitivity indices (ISI), ISI Matsuda, ISI quantitative insulin sensitivity check index, and homeostasis model assessment as previously described (Jeschke et al., 2013). Furthermore, resting energy expenditure (REE) was assessed weekly until D/C. The REE was measured with a Sensor Medics 2900 metabolic measurement cart.
Protein lysates from fat obtained at first OR (less than 10 days) and last OR (greater than 10 days) were extracted using a RIPA lysis buffer and separated on SDS-PAGE gel prior to immunoblotting. ATF6, GRP78 (BiP) and CHOP antibodies were from Cell Signaling Technology (MA, USA), NLRP3 antibody was from Millipore (Darmstadt, Germany), MPO antibody was from R&D systems (MN, USA) and β-actin antibody was Thermo Fisher (MA, USA).
2.2.1. Immunohistochemistry
Fat tissues were embedded in paraffin and sectioned prior to staining with Hematoxylin Eosin (H&E) or incubation with Myeloperoxidase (MPO) antibody followed by Diaminobenzidine (DAB) staining. Imaging was performed using an optical microscope (Leica Microsystems, Wetzlar, Germany) with 200 × objective lenses.
2.2.2. Serum Fatty Acids Composition
Serum fatty acids composition from patients were analyzed by gas chromatography–mass spectrometry (GC–MS) performed by the Analytical Facility for Bioactive Molecules (AFBM) platform at the hospital for Sick Children, Toronto, Ontario, Canada. The respective concentrations of each fatty acid were added to determine the total fatty acid concentration and the percentage for each fatty acid.
2.3. Inflammatory and Immunological Responses
Inflammatory responses were determined in serum and adipose tissue. Serum cytokine profiling was conducted twice weekly. Adipose tissue inflammatory markers, cytokines and NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome activation, were determined in adipose tissue that was collected as discarded tissue at surgeries. Tissue or systemic inflammation was determined by standard biochemical techniques such as western blotting, using the Bio-Plex Suspension Array System (Bio-Rad, Hercules, CA) for the measurement of 17 different cytokines, flow cytometry, and ELISA (Jeschke et al., 2007, Jeschke et al., 2008a, Gauglitz et al., 2008a, Gauglitz et al., 2008b, Gauglitz et al., 2009, Gauglitz et al., 2010a, Gauglitz et al., 2010b).
2.3.1. Harvesting of Stromal Vascular Fractions From White Adipose Tissue (WAT)
Specimens were collected at surgery and immediately transferred to the laboratory where they were digested with collagenase (Sigma, St. Louis, MO) at 1 mg/ml in RPMI1640 in a shaking incubator for 2 h at 37 °C. The digest was then strained through sterile gauze to remove particulates, and the cell fraction was collected by centrifugation. The cell pellets were washed multiple times with Hank's Balanced Salt Solution (HBSS), re-suspended in HBSS, and red blood cells were removed by density centrifugation with Lympholyte H (Cedarlane, Burlington, ON), according to the manufacturer's protocol. The cell suspension was then passed through a 100 μm strainer (BD Biosciences, Mississauga, ON), and cells were counted.
2.3.2. Flow Cytometry
All monoclonal antibodies used for flow cytometric analysis were obtained from Becton Dickinson Immunocytometry Systems, eBioscience (San Diego, CA), and BioLegend (San Diego, CA). Each blood sample was labeled with the following 3-color antibody combinations to determine the co-expression of corresponding antigens on monocytes: APC-conjugated mouse anti-human CD45 and PE-conjugated mouse anti-human CD14; and SVF derived leukocytes were stained with mAbs (APC-Cy7-conjugated mouse anti-human CD45, PE-Cy7-conjugated mouse anti-human CD3, PE-conjugated mouse anti-human CD14, APC-conjugated mouse anti-human IL-1β) on ice for 25 min, followed by washing. Prepared blood and SVF samples were both analyzed using BD LSR II Special Order System (BD Biosciences, San Jose, CA, USA). After adjustment of the fluorochrome compensation electronically, 200,000 events per sample were acquired and analyzed. FMO controls were used to set up the gating of specific cell populations.
2.4. Wound Healing, Dermal and Epidermal Responses
2.4.1. Immunoblotting
Whole cell lysates were harvested from cells in the dermal component of burned skin in Reporter Gene Lysis Buffer containing protease and phosphatase inhibitor cocktails (Roche, Mississauga, ON, Canada). Protein concentrations were measured using the Bicinchoninic Acid (BCA) assay. After resolving proteins by SDS-polyacrylamide gel electrophoresis, the proteins transferred and the blot hybridized with the following antibodies: β-catenin from BD Biosciences (San Jose, CA, USA), pSmad2 from Cell Signaling (Beverly, MA, United States) and β-actin antibody was from Thermo Fisher (MA, USA).
2.4.2. Cell Isolation and Culturing From Skin
Stem cell pool was assessed at surgical interventions when burned skin and wound were collected from discarded tissue. From this, skin cells were liberated from the dermal compartment of burned skin by one hour incubation and treatment with digestion cocktail containing Clostridium histolyticum collagenase type 1 (2.5 mg/ml, Bioshop, Canada), trypsin EDTA (0.05%, wisent), and dispase (1:20, wisent). Cells were then cultured in DMEM containing 10% FBS and 1% Ab/Am at 37 °C in 5% CO2/95% air atmosphere incubator with medium changes every other day. Cells were utilized for experimental work between passages 2–4.
2.4.3. Skin Cell and Tissue Staining
Human cells derived from burned skin (8 × 10 (Bringham and McLoughlin, 1996) cells/well) were seeded into 8-well cell culture slides (BD Falcon) in DMEM (10% FBS, 1% Ab/Am) for 24 h. Cells were fixed with 4% PFA for 30 min at room temperature and permeabilized with 0.25% TritonX-100 in PBS for 10 min and blocked in PBS containing 1% BSA for 30 min at room temperature. Cells were incubated with primary antibodies, containing monoclonal mouse anti-human ki-67 antibody (1:200, Dako), rabbit anti-human OCT4 antibody (#2890, 1:200, Cell Signaling), overnight at 4 °C in a humidified chamber. The cells were then incubated in the dark for 1 h at room temperature with secondary antibody, containing biotinylated anti-rabbit IgG (1:500, Vector Laboratories), and then Strapdavidion 594 (1:5000, Vector Laboratories) for 20 min at room temperature in the dark. After incubation with appropriate antibodies/probes, samples were mounted with Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, California, United States) and imaged with a fluorescence microscope. Skin sections for histological analysis were collected and fixed in 10% formalin. Samples were embedded in paraffin, sectioned and stained with respective antibodies. Deparaffinized sections were incubated with primary antibodies. Hematoxylin was used to stain the nuclei. Antibodies were obtained from the following sources; anti-Stro-1 (#2037638, 1:1000, Millipore), anti-ki-67 antibody (Dako, 1:200), anti-Oct-4, (#2890, 1:200, Cell Signaling), Biotynalated anti-rabbit IgG (1:500, Vector Laboratories), Strapdavidion 488, 594 (1:5000 Vector Laboratories). Western HRP chemiluminescence (ThermoScientific, Rockford, IL) Phosphatase and protease inhibitor cocktails were purchased from Sigma-Aldrich (St. Louis, MO). Antibiotic and antimycotic preparation, and trypsin were obtained from Wisent (St Bruno, QC, Canada). For quantifications, three different fields in the dermal component of burned skin have been randomly chosen and the ratio of cells positive for each marker divided to the total number of cells in each histological field. The average of ratios was considered for each subject.
2.5. Statistical Analysis
Student's unpaired t-test was used to compare all results, with Welch's correction where appropriate. Data is presented as means and standard error of the mean (SEM) for continuous variables, frequency and percentages for categorical variables. Statistical comparisons were conducted using SPSS 20 and figures were generated using GraphPad Prism 5.0 software or Microsoft Excel. Significance was accepted at a p values less than 0.05.
3. Results
3.1. Clinical Outcomes
3.1.1. Patient Cohort From 1995–2015
From 1995 to April 2015, we have admitted 2796 burn patients to the Ross Tilley Burn Centre (RTBC). Average burn size was 15% TBSA, with 8% TBSA third degree burn, 15% of the patients had an inhalation injury; length of stay per percent burn was 2.5 days. Mortality for all patients was 10.6% (297/2796); mortality for over 20% TBSA burn was 33.5% (238/710). By stratifying patients into adults (n = 2310) and elderly (n = 486) we see that the burn size is similar in both groups (adults: 15% TBSA, elderly: 17% TBSA). Elderly had a significantly longer stay when compared to adults (adults: 2.4 days/percent burn, elderly: 3.2 days/percent burn), p < 0.001. Significantly different was furthermore overall mortality (adults: 7% (155/2310), elderly: 29% (142/486)) (Kaplan Meier Survival Curve Fig. 1A) as well as mortality for patients with burns over 20% burns of their body (adults: 24% (135/566) vs. elderly: 72% (103/144)), p < 0.0001 (Fig. 1B). The results of this large cohort of patients indicate that elderly in fact have a substantially higher mortality associated with longer length of stay in the hospital when compared to adult burn patients.
Fig. 1.
Kaplan Meier survival curves. (A) Survival curve for adults vs. elderly from 1995 to 2015 for all patients and all burn sizes. (B) Survival curve for burn patients with burns over 20% total body surface area (TBSA) adults vs. elderly from 1995 to April 2015. (C) Survival curve for adults vs. elderly from 2006 to April 2015 for all patients all burn sizes. (D) Survival curve for burn patients with burns over 20% TBSA adults vs. elderly from 2006 to April 2015. * Significant difference between adults vs. elderly p < 0.05.
3.1.2. Patient Cohort From 2006–2015
For previously mentioned reasons, we focused this study on the time period from 2006 to April 2015; during this time period 1461 patients were admitted to RTBC. Demographics, clinical markers, and incidence of various morbidities are shown in Table 1. Burn size was similar between adult and elderly but third degree burn and Baux score was significantly higher in elderly, as well as length of stay normalized to percent burn, p < 0.05 (Table 1). The incidence of inhalation injury was similar in both groups. There were no significant differences in ethnic background between the two groups. We determined pre-existing medical conditions in these two patient populations (Table 1) and found that elderly had significantly more pre-existing cardiovascular, respiratory, endocrine, and renal comorbidities when compared to adults. Alcoholism, drug abuse, liver, and lipid associated comorbidities were significantly lower in elderly, p < 0.05 (Table 1).
Table 1.
Demographics and outcomes of patients admitted from 2006–2015.
| All patients n = 1461 | Patients < 65 n = 1225 | Patients > 65 n = 236 | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age years (Median) | 44.76 | 41.04 | 76.22 | < 0.0001 |
| Gender male n (%) | 1051 (72) | 905 (74) | 146 (62) | 0.002 |
| Total TBSA (Median) (%) | 13.6 | 13.2 | 15.1 | NS |
| 2nd degree TBSA (%) | 6.3 | 6.7 | 4.7 | 0.002 |
| 3rd degree TBSA (%) | 6.8 | 6.2 | 10.2 | 0.0003 |
| Baux score | 63 | 57 | 94 | < 0.0001 |
| Inhalation injury n (%) | 229 (16) | 187 (15) | 40 (17) | NS |
| Burn mechanism | ||||
| Flame n (%) | 829 (57) | 665 (54) | 158 (67) | NS |
| Scald n (%) | 397 (27) | 337 (28) | 62 (26) | NS |
| Electrical n (%) | 95 (7) | 92 (8) | 3 (1) | NS |
| Chemical n (%) | 40 (3) | 36 (3) | 4 (2) | NS |
| Other n (%) | 100 (7) | 95 (8) | 9 (4) | NS |
| Ethnicity | ||||
| Caucasian n (%) | 889 (61) | 723 (59) | 165 (70) | NS |
| Asian n (%) | 209 (14) | 188 (15) | 21 (9) | NS |
| African American n (%) | 56 (4) | 47 (4) | 9 (4) | NS |
| Other n (%) | 307 (21) | 267 (22) | 41 (17) | NS |
| Pre-admission comorbidities | ||||
| CVS n (%) | 340 (23) | 135 (11) | 205 (47) | < 0.0001 |
| Respiratory n (%) | 144 (10) | 94 (8) | 50 (12) | 0.016 |
| Endocrine n (%) | 148 (11) | 77 (6) | 71 (16) | < 0.0001 |
| Renal n (%) | 31 (2) | 16 (1.4) | 15 (3) | 0.0047 |
| Alcohol n (%) | 212 (15) | 186 (15) | 26 (6) | < 0.0001 |
| Drug abuse n (%) | 168 (12) | 163 (13) | 5 (1) | < 0.0001 |
| Liver n (%) | 29 (2) | 26 (2) | 3 (0.7) | 0.0496 |
| Cholesterol n (%) | 102 (7) | 57 (5) | 45 (10) | < 0.0001 |
| CVA n (%) | 29 (2) | 14 (1) | 15 (3) | 0.001 |
| Clinical markers | ||||
| Escharotomy n (%) | 188 (13) | 153 (12) | 35 (15) | NS |
| Length of stay (days) | 18 | 17 | 20 | 0.004 |
| Length of stay/TBSA (survivors) (days/%) | 2.53 | 2.42 | 3.22 | < 0.05 |
| Number of ORs average (n) | 2.1 | 2.1 | 2.1 | NS |
| Morbidities | ||||
| Denver 2 score daily average | 0 | 1 | 3 | < 0.001 |
| Denver 2 score daily maximum | 0 | 3.4 | 4.8 | < 0.05 |
| Apache II score | 5.1 | 4.4 | 9.2 | < 0.0001 |
| Renal failure n (%) | 36 (3) | 31 (3) | 5 (2) | NS |
| ACS n (%) | 5 (0.3) | 5 (0.4) | 0 (0) | NS |
| Upper/lower GI bleed n (%) | 19 (1.3) | 12 (1) | 7 (3) | 0.03 |
| Pulmonary embolism n (%) | 9 (0.6) | 8 (0.6) | 1 (0.4) | NS |
| Deep venous thrombosis n (%) | 12 (1) | 10 (0.8) | 2 (0.8) | NS |
| ARDS n (%) | 63 (4) | 52 (4) | 11 (4) | NS |
| Ventilation n (%) | 900 (62) | 765 (62) | 135 (57) | NS |
| Pneumonia n (%) | 222 (15) | 181 (15) | 41 (15) | NS |
| Bacteremia n (%) | 288 (20) | 248 (20) | 40 (17) | NS |
| Sepsis n (%) | 130 (9) | 98 (8) | 32 (14) | NS |
| Burn wound infection n (%) | 403 (28) | 329 (27) | 74 (31) | NS |
| Cellulitis n (%) | 298 (20) | 250 (21) | 48 (20) | NS |
| Mortality n (%) | 94 (6) | 49 (4) | 45 (19) | < 0.0001 |
In terms of clinical outcome data, we found that the amount of surgeries and escharotomies were similar between groups. Of notable interest was the significantly increased length of stay in elderly when compared to adults, p < 0.05 (Table 1). We hypothesized that elderly have greater comorbidities but we only were able to find a few significantly different ones; namely Denver 2 score median, Apache II, and GI bleed were significantly different, p < 0.05 (Table 1). Contrary to our hypothesis, there were no significant or striking differences between adults and elderly for complications including ARDS, pneumonia, sepsis, and wound infection (Table 1).
There was a considerable difference in mortality, while only 4% of adults died after the burn injury 19% died in the elderly group after burn, p < 0.05 (Table 1); this is shown in the Kaplan Meier survival curve (Fig. 1C). This difference was even more prominent for burns over 20% TBSA where elderly had over 70% mortality and adults less than 15%, p < 0.05 (Kaplan Meier survival curve Fig. 1D).
3.2. Metabolic Responses
3.2.1. Resting Energy Expenditure
Resting energy expenditure (REE) expressed as percent predicted, indicated that adult and elderly patients are hypermetabolic after burn injury (Fig. 2A). Adult burn patients are more hypermetabolic the first week after burn but then steadily decrease their metabolic needs. Elderly patients on the other hand, demonstrate an inverse metabolic response. Elderly are less hypermetabolic during the initial phase after burn but steadily increased their metabolic needs and at > 4 weeks post-burn elderly had a significantly increased REE compared to adults, p < 0.05 (Fig. 2A).
Fig. 2.
Metabolic measurements. (A) Resting energy expenditure expressed as percent predicted was significantly increased in elderly patients > 4 weeks after burn when compared to adults. * Significant difference between adults vs. elderly, p < 0.05. (B) 6 AM average glucose levels. Elderly have a significantly greater area under the curve (AUC) at 6 AM glucose levels compared to adults. (C) Daily average glucose levels. Elderly have a significantly greater AUC when compared to adults. (D) Daily max glucose levels were also significantly higher when compared to adults. (E) No differences could be detected between elderly and adults in daily min glucose levels. (F) Significantly elderly required more insulin than adults.
3.2.2. Glucose and Lipid Metabolism
We measured daily average glucose, 6 AM, minimal (min) and maximal (max) as well as insulin levels. Elderly have higher daily average glucose levels early after burn and again during the later phase after burn when compared to adults, p < 0.05 (Fig. 2). Mean daily 6 AM (Fig. 2B) and mean daily max glucose levels (Fig. 2D) were significantly higher in the elderly 7–20 days post-burn, while daily min glucose levels did not differ between groups (Fig. 2E). The signal that elderly is more insulin resistant was confirmed by the percent of patients treated with insulin over time (Fig. 2F). A greater percent of elderly was treated with insulin when compared to adults, p < 0.05, indicating a substantial hyperglycemic response in elderly burn patients. Furthermore, it appears that glucose variability is greater in elderly when compared to adults; increased glucose variability has been shown to be associated with poorer outcomes (data not shown).
To determine whether increased hyperglycemia in elderly patients was due to increased peripheral or central insulin resistance or lack of insulin production, we conducted oral glucose tolerance tests (OGTT) on 14 adult burn patients and 7 elderly burn patients at the time the patients were 95% healed; these patients were matched for burn size. We found that elderly burn patients present with an abnormal β-cell function; demonstrated in lower indices for IGI, HOMA2% β and a 48% decrease in insulin (AUC μU mL− 1 min: 5339 ± 3288 vs. 2790 ± 1735, Fig. S1 C and D) and a significant decrease by 40% in c-peptide (AUC ng mL− 1 min: 969 ± 368 vs. 579 ± 301; p < 0.05, Fig. S1 E and F). Abnormal β-cell function resulted in a 17% increase in glucose (AUC mg dL− 1 min: 17,686 ± 3777 vs. 20,660 ± 2996, Fig. S1 A and B). The other indices were found not to be significantly different. Table 2 illustrates that elderly have significantly lower insulinogenic index and lower HOMA2 index when compared to adults, p < 0.05 (Table 2). Fig. S1 depicts time curves and area under the curve for glucose, c-peptide and insulin during OGTT.
Table 2.
Glucose and insulin indices.
| Normal | Adults | Elderly | p | |
|---|---|---|---|---|
| Days to OGTT | N/A | 24 ± 11 | 23 ± 10 | 0.798 |
| Age years | N/A | 34 ± 13 | 68 ± 7 | < 0.001 |
| BMI | N/A | 26 ± 4 | 27 ± 3 | 0.526 |
| TBSA total (%) | N/A | 30 ± 7 | 30 ± 8 | 0.967 |
| TBSA 3rd (%) | N/A | 18 ± 13 | 17 ± 11 | 0.823 |
| QUICKI | ≥ 0.357 | 0.363 ± 0.044 | 0.368 ± 0.04 | 0.823 |
| ISI | ≥ 3.0 | 8 ± 6 | 8 ± 5 | 0.932 |
| IGI | ≥ 0.4 | 0.9 ± 0.7 | 0.2 ± 0.1 | < 0.05 |
| HOMA2-IR | ≤ 1 | 1.2 ± 0.7 | 1.0 ± 0.6 | 0.524 |
| HOMA2% β | 100% | 116 ± 33 | 82 ± 33 | < 0.05 |
| HOMA2% S | 100% | 126 ± 93 | 146 ± 80 | 0.651 |
Data expressed as mean ± SD. Glucose (mg/dL); insulin (μU/mL); and c-peptide (ng/mL).
QUICKI, quantitative insulin sensitivity check index; ISI, whole body insulin sensitivity index (Matsuda et al.); IGI, insulinogenic index (index of β-cell function); HOMA, homeostasis model assessment of insulin resistance; β, β-cell function; and S, insulin sensitivity.
Confirming previous studies we found that both burned adults and burned elderly have increased lipolysis associated with increased levels of circulating fatty acids after burn (Herndon et al., 1994, Wolfe et al., 1987). By dividing patients into adult and elderly, we found that elderly have significantly increased lipolysis expressed as increased free fatty acids when compared to adults, p < 0.05 (Fig. 2S A and B). We further performed a qualitative and quantitative analysis of free fatty acid species (Non-Esterified Fatty Acids) in the serum of adult and elderly burned patients (n = 10 per group), approximately 10 days post-injury (adult 11.6 ± 3.4 days vs. elderly 10.4 ± 4.8 days). We found that Docopentaneoic, Cis-7-Hexadecenoic, Myristic, Palmitoleic, Stearic and Vaccenic acids were significantly increased in elderly when compared to adults as well as control non-burned patients, p < 0.05 (Fig. S2). Consequently, the sum of these fatty acids indicated that the levels of circulating fatty acids (FA) was greatly elevated in elderly patients compared to adults burned and control (158.7 ± 31.3 ng/μl vs. 96.1 ± 13.1 ng/μl, p = 0.02). In contrast the omega 3 FA Eicosenoic was significantly decreased in elderly when compared to adults, p < 0.01. We also quantified the levels of circulating serum proteins, which determine fatty acids bioavailability (van der Vusse, 2009), and noticed a significant decrease in burned patients, but there was no difference between adult and elderly (data not shown). Finally, we analyzed these fatty acids by families and the percentage for each family is depicted (Fig. S2 C). Interestingly, elderly patients demonstrated a unique pattern in the percentage of the different fatty acids families compared to control and adult patients. In this population, saturated fatty acids were more abundant (p = 0.02), whereas polyunsaturated (p = 0.001) and omega 3 (p = 0.001) and 6 (p = 0.007) fatty acids were under-represented.
3.2.3. Metabolic Bio-Molecular Markers
Using a multiplex platform we measured a panel of 10 metabolic markers over time for adults and elderly burn patients. When comparing various time intervals post-burn injury, elderly patients had lower expression of c-peptide and GLP-1 during the acute phase (Fig. S3) while their Pancreatic Polypeptide (PP), Peptide YY (PYY) decreased p < 0.05 (Fig. S3). PP a peptide produced in the pancreas auto-regulates pancreatic activity and a decrease reflects the hypermetabolic state. Peptide YY is a short 36-amino acid peptide released by cells in the ileum and colon in response to feeding, increases with nutrition decreases with starvation, indicating the hypermetabolic demand of the burn. This bio panel confirms the glucose and OGTT results, indicating that elderly have a decreased production of insulin and GI associated peptides.
3.2.4. Molecular Biological Metabolic Markers
We hypothesized that elderly have an increased inflammatory and ER stress response in the adipose tissue when compared with adults. However, we found the opposite. While adults and elderly both increased adipose ER stress after burn, elderly burn patients had a significantly decreased ER stress response in the adipose tissue when compared with adult burn patients, p < 0.05 (Fig. 3A–D). While adults had increased expression of ER stress markers, e.g. BiP, ATF-6, CHOP, and XBP1s elderly showed a dampened ER stress with significantly decreased ER stress markers (Fig. 3A–D). Furthermore, we determined pJNK-JNK and NLRP3 and found that both adults and elderly have similar expression patterns with significantly increased levels at early and late time points (Fig. 3E–F); but there was no significant difference between adults and elderly.
Fig. 3.
Metabolic bio-molecular markers. (A–D) Adipose ER stress markers. Adults and elderly both increased adipose ER stress after burn, elderly burn patients had a significantly decreased ER stress response in the adipose tissue when compared with adult burn patients. (E–F) pJNK-JNK and NLRP3 and found that both adults and elderly have similar expression patterns with significantly increased levels at early and late time points with no significant difference between adults and elderly. (G–I) MPO in adipose tissue. Elderly have significantly lower MPO expression in adipose at 0–3 days and 14–20 days after burn when compared with adults indicating less inflammatory infiltration of the adipose tissue. n = 5 to 8 per group. Data is represented as mean ± SEM. * Significant difference between adults vs. elderly, p < 0.05.
Lastly, we determined the expression of the neutrophil marker Myeloperoxidase (MPO) in adipose tissue and found that elderly have significantly lower MPO expression in adipose at 0–3 days and 14–20 days after burn when compared with adults, indicating less neutrophils infiltration of the adipose tissue, p < 0.05 (Fig. 3G–I).
3.3. Inflammatory and Immune Responses
We hypothesized that elderly have an altered immune and inflammatory response. We first determined a panel of 6 cytokines and chemokines over time. Based on pilot studies we found that elderly have a distinct profile with an early hypo-inflammation followed by a hyper-inflammation after 2 weeks post-burn (data not shown). Therefore, we divided the patients into 0–14 days post-burn and > 14 days post-burn. As depicted in Fig. 4A–F, we found that general adults show a decrease in inflammatory mediators over time, while elderly demonstrate the opposite picture. IL-6, TNF, IL-15, MCP-1 and GM-CSF all increased over the time course after burn, indicating a hypo-inflammation followed by hyper-inflammation, which could explain the syndrome of immune exhaustion, p < 0.05 (Fig. 4A–F).
Fig. 4.
Inflammatory and immune profile in adult and elderly burned patients. (A–F) Adults show a decrease in inflammatory mediators over time, while elderly demonstrate the opposite picture; IL-6, TNF, IL-15, MCP-1 and GM–CSF all increased over the time course after burn indicating a hypo-inflammation followed by hyper-inflammation. (G–J) Elderly had significantly reduced numbers of CD14 +/HLA-DR + monocytes (G). Comparing septicemia patients exclusively, the impaired phenotype previously observed was upheld (H). Despite a relatively steady impairment in CD14 +/HLA-DR + monocytes expression in adults, elderly patients had a notable surge in at 30 + days post-injury. Lastly, severely burned (> 30% TBSA) elderly patients showed a correlation between HLA-DR + expression and length of stay (I–J). n for analysis of inflammatory cytokine: adult = 94 and elderly = 36. n for HLA-DR flow cytometry: healthy = 11, adult = 37 and elderly 15. Data is represented as mean ± SEM. * Significant difference between adults vs. elderly, p < 0.05.
This was corroborated by looking at immune markers. Monocyte HLA-DR is a well-established indicator of immune status and indictor of septicemia and mortality in critically ill patients (Cazalis et al., 2013, Demaret et al., 2013). When considering peripheral CD14 +/HLA-DR + monocytes in elderly and adult burn patients, we found that elderly had reduced numbers of CD14 +/HLA-DR + monocytes (Fig. 4G). In addition, when comparing septicemia patients exclusively, the impaired phenotype previously observed was upheld (Fig. 4H). Despite a relatively steady impairment in CD14 +/HLA-DR + monocytes expression in adults, elderly patients had a notable surge in at 30 + days post-injury. Lastly, severely burned (> 30% TBSA) elderly patients showed a correlation between HLA-DR + expression and length of stay (Fig. 4I–J). Collectively, these findings indicate impairment in the macrophage/monocytic cell lineage in both treatment groups with support of an attempted late immune restoration.
3.3.1. Inflammasome
Analysis of the White adipose tissue (WAT) of burned patients within the first 4-days post-injury shows that elderly patients have less macrophages (27 vs. 19%) and IL-1β + cells in the CD14 + macrophage fraction (3.0 vs. 2.2%, ns). These findings did not reach statistical significance likely due to the small sample size in this sub-study (representative images Fig. 4S A–B). When extending this analysis to the fraction of CD14 +, macrophages that are double-positive for IL-1β and FLICA (caspase-1), both adults and elderly were higher than healthy controls (t(19) = 3.752, p < 0.01; t(12) = 2.841, p < 0.05, Fig. 4S C–D) despite a significant reduced level in elderly compared to young adults. Both length of stay and Baux score correlated with the percentage of IL-1β +, CD14 + cells (r2 = 0.54, p < 0.001; r2 = 0.57, p = 0.001, Fig. 4S E–F). When relating adipose tissue inflammation to plasma cytokine levels (< 7 days post-injury), a positive correlation was also found between percentage of IL-1β + cells and IL-1α (p < 0.05) and IP-10 (p < 0.001), exclusive to elderly. Collectively, this suggests that early analysis of inflammation in excised tissue, or site of injury, is dampened in the entire elderly patient cohort, which is consistent with the systemic cytokine profile detected in patient plasma.
3.4. Wound Healing, Dermal and Epidermal Responses
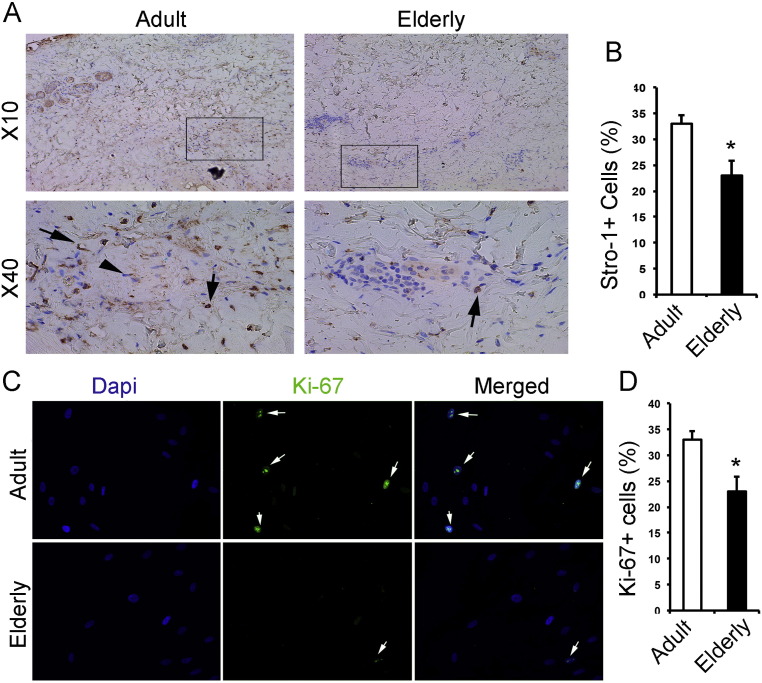
A substantial difference between elderly and adults is a significantly longer hospital stay for elderly when compared to adults. We hypothesized that the longer length of hospital stay is due to a delay in wound healing which is associated with an alteration of characteristics of skin stem cells. It has been shown that Mesenchymal Stem Cells (MSCs) have an important role in enhancing wound healing (Shumakov et al., 2003). As such, we assessed the effects of burn injury on the progenitor pool of young and elderly patients and examined the other characteristics of cells contributing into skin healing. Stro-1 is a well-established marker of mesenchymal progenitor cells which predominantly contribute in granulation tissue formation during skin healing. Moreover, Octamer-Binding Transcription Factor 4 (OCT-4) is a transcription factor which is associated with stem cell self-renewal. We analyzed the expression of these markers in the dermal part of skin of both adult and elderly burn patients. Stro-1 was significantly decreased (Fig. 5A–B) in the skin of elderly compared to young adult burn patients, p < 0.05. Additionally, cells harvested from the skin of elderly and adults burn patients also showed a decreased expression of Oct-4 (Fig. 5S), an embryonic stem cell marker, p < 0.05, indicating an alteration in the skin stem cell abundance in the skin of elderly burn patients.
Fig. 5.
Deficient characteristics of skin progenitor cells in elderly patients. (A–B) Representative immunohistochemistry staining is showing that skin tissue elderly patients has less Stro-1 + cells. (C–D) Individual signals of 4,6-diamidino-2-phenylindole (DAPI) and anti-Ki-67 (green) from isolated skin cells of young adults and elderly burned patients are shown on alongside a merged view of DAPI. (E–F) Representative photomicrographs of a scratch assay performed with young adult skin mesenchymal cells and elderly's skin mesenchymal cells after 48 h. Migration was quantified by counting the number of cells migrating into the scratch zone. (G) Western blot showing lower activation (quantified in I) of Wnt/β-catenin signaling in the burned skin of elderly in compare with young adult. (H) Western blot showing lower activation (quantified in J) of TGF-β/Smad2 pathway in the burned skin of elderly in compare with young adult. Bar graphs show the means and standard deviation (n = 3–5). Three representative lysates of 3 subjects have been shown here. Data is represented as mean ± SEM. * Significant difference between adults vs. elderly, p < 0.05 and ** indicates p < 0.01.
Stem cells, which undergo rapid activation and proliferation within hours after injury, exhibit a remarkable age-specific decline in performance, as observed by diminished proliferation capacity when isolated from different organs of aged animals (Teta et al., 2005, Geiger et al., 2014, Kuang et al., 2015). As elderly burn patients have defects in wound healing and that cell proliferation is an important phenomenon in early wound healing, we suspected defects in the proliferation capacity of mesenchymal stem cells in the skin of burn patients. In order to asses this, the proliferation of mesenchymal cells harvested from the burned skin of young adult and elderly burn patients were assessed based on the expression profile of Ki-67. Cells obtained from elderly burn patients showed significantly decreased proliferation (Fig. 5C–D) in comparison to adult patients, p < 0.05.
Cell motility and migration play critical roles during wound healing (Schneider et al., 2010, Amini-Nik et al., 2014). To further understand the underlying mechanism of delayed healing in elderly, we examined the migration characteristics of mesenchymal cells isolated from skin of burn patients. Compared to the cells isolated from the burn skin of young adults, elderly cells showed a profoundly reduced migration by almost 50% (Fig. 5E–F). Wnt/β-catenin as well as TGF-β/Smad 2 signaling plays an essential role during skin healing (Amini Nik et al., 2007, Poon et al., 2009). These two pathways regulate proliferation, migration and maturation of different cell types during different phases of skin healing. We, therefore, examined the level of activation of these two important pathways in the burn skin. Immunoblotting of dermal components of burned skin indicated that elderly patients exhibited a significant lower activation of Wnt/β-catenin as well as TGF-β/Smad 2 signaling pathways in comparison with the skin of young adults (Fig. 5G–J), p < 0.05. Taken together, these findings suggest that the impaired wound healing observed in elderly burn patients associated with reduced stem cell pool, a diminished self-renewal capacity of progenitor cells, a deficient migration of MSC and an altered activation of essential signaling pathways for skin healing. This might be the underlying reason of increased length of stay in hospital for this group of patients due to longer healing requirement.
4. Discussion
These studies were conducted to obtain insights into the pathophysiologic response to burn in elderly patients. We aimed to determine physiologic and biochemical differences between adults and elderly in order to uncover potential mechanisms to why elderly have a profoundly higher mortality rate. Over the last decades, changes in the care of burned children and adults have improved outcomes significantly, however mortality in elderly has not improved and the LD50 remained steady at 35% TBSA burn. This lack of progress is of great importance in light of a substantially growing elderly population. We hypothesized that the response to burn injury must be different in elderly when compared to adults. In this present study we determined metabolic, inflammatory, and immune responses, as well as skin regeneration to identify possible mechanistic insights why elderly do so poorly.
We hypothesized that elderly have a higher mortality after a burn associated with increased incidence of sepsis and infections, increased hypermetabolism, increased inflammation, and increased incidence of organ failure. To our surprise, we could not confirm this hypothesis in its entirety. We were able to confirm in a large cohort that elderly patients with a like size burn have a significantly higher mortality when compared to adults. However, our hypothesis that elderly have an increased incidence of sepsis, bacteremia, pneumonia, cellulitis, and burn wound infection was not proved; there was no difference between adults and elderly. Furthermore, there was no difference in the incidence of renal failure, ACS, pulmonary embolism, DVT, or ARDS. The only significant differences we were able to detect was an increased Apache II score, Denver 2 score, increased lower GI bleed, and as mentioned above, mortality. Apache II and Denver 2 are organ function and multi organ failure scores and they indicate that elderly have an increased incidence of organ failure. Increased incidence of multi organ failure is associated with increased mortality (Kraft et al., 2014) and could be a possible explanation why mortality is increased in elderly but given the complexity of the post-burn response this seems almost too simplistic. We therefore investigated other possible causes contributing to increased mortality in elderly burn patients.
We hypothesized that elderly have an augmented inflammatory response associated with an impaired immune response leading to immune paralysis after a severe burn. We could not confirm our hypothesis, but we found that during the early phases after burn elderly were hypo-inflammatory with significantly decreased inflammatory markers in serum and adipose tissue. We found that serum IL-6, MCP-1, and IL-15, as well as adipose neutrophil infiltration, and inflammasome activity are all significantly decreased in elderly during early time points after burn when compared to adults. However, this hypo-inflammatory and immune-senescent response then changed into a hyper-inflammatory response, which is in contrast to adult burn patients. While adults were hyper-inflammatory during the early phases after burn and became less inflammatory at the end of their hospital course, elderly were hypo-inflammatory during early phases changing to a hyper-inflammatory state during later time points. It is not entirely clear what the underlying mechanisms are causing this inverse response. We speculate that this could be due to an unresolved stress and hypermetabolic response, for example by persistent danger associated molecular pattern (DAMP's) or stress hormones, which induces the immune system and inflammation but in a delayed fashion (Diao et al., 2014, Lamkanfi et al., 2009). Supporting this hypothesis are the findings that elderly have almost no neutrophil migration into adipose tissue, MPO expression, as well as significantly decreased CD14 + IL-1β cells. These results suggest that this population of patients is unable to recruit sufficient inflammatory cells to the site of injury. Such defect is expected to have severe consequences as inflammatory cells are required to eliminate dead cells by phagocytosis, as well as stimulate wound healing and prevent potential infections. These results confirm the notion that elderly have an immune-senescent state and decreased immune responses compared to adults. The challenge is to figure out how to modulate this hypo-inflammatory followed by hyper-inflammatory response to change the trajectory from morbidity to recovery in elderly patients.
Another discovery of importance was the metabolic response to burns in elderly. Elderly and adults do not seem to be different in terms of hypermetabolism measured by predicted REE during the early phases after burn (Jeschke et al., 2008b). Adults, show a decrease in their metabolic need after 2–3 weeks. However, elderly show the opposite, they increase their metabolic requirements which indicate a persistent and increased hypermetabolic response during the later phase after burn. This was reflected in glucose and lipid metabolism. Glucose levels were significantly higher, despite the greater need of insulin, and had a greater variability when compared to adults. Based on the OGTT results and the metabolic profile results, it appears that this is due to a decreased function of the pancreas and β- cell production of insulin. OGTT results indicate that elderly are similarly insulin resistant but the significantly decreased insulinogenic index and decreased HOMA2 index indicate a substantially impaired insulin production by the pancreas. These results are supported by the metabolic Luminex data which showed decreased c-peptide, PP, PYY, and glucagon in elderly after burn. Therefore one would expect that elderly would require higher insulin doses during the acute hospitalization to control glucose level. This is in fact exactly what we found when we determined glucose levels and insulin requirements over time. The clinical relevance of hyperglycemia and high glucose variability is that they have been previously shown to be associated from a metabolic aspect with an increased morbidity and mortality after burn (Jeschke et al., 2010). It is currently not known whether hyperglycemia and insulin resistance can cause organ damage via reactive oxygen species or increased inflammation or whether hyperglycemia and IR are symptoms of attenuated organ function leading poor outcome. We can speculate based on the organ function and multi organ failure scores that elderly have worse organ function compared to adults and therefore high glucose and lack of insulin are a symptom of poor organ function and not the cause. It still remains unclear why elderly have poorer organ function when compared to adults, and we suggest that at the cellular level, mitochondria are dysfunctional or impaired resulting in organ failure. This could be either due to pre-existing medical conditions (co-morbidities) or pre-existing organ dysfunction or due to a lack of a resource to adequately respond to stress or trauma. However, it seems imperative to include mitochondria analyses in future studies in elderly.
The exact function and role of fat and the adipose tissue on the hypermetabolic and inflammatory response are essentially unknown. Only recently, studies started to recognize that the fat plays an important role in various metabolic and inflammatory conditions, e.g. diabetes, DAMPs, inflammasome activation, to name a few (Talukdar et al., 2011, Solinas et al., 2006). We have recently shown that after burn injuries in children, elevated triglycerides contribute to multi organ failure and increased risk for death via unknown mechanisms (Kraft et al., 2013). Additionally we found that free fatty acids were significantly elevated after burn. In the present study, we found that elderly patients have a significantly altered fatty acid profile when compared to adults. These differences could explain some of the differences we observed when comparing elderly to adults. Lipolysis and free fatty acids have been linked to insulin resistance by several mechanisms. While fatty acids can directly cause insulin resistance, they are also inducers of mitochondrial alterations and ROS production, ER stress and inflammation, which in turn cause and worsen insulin resistance (Kim et al., 2008). We found worsened glucose metabolism, augmented hyperglycemia and increased insulin resistance in elderly, and at the same time we found increased FFA in elderly. Interestingly, while fatty acids, in particular saturated fatty acids, can directly induce ER stress, we observed a disconnection between fatty acids levels and ER stress in elderly patients, suggesting that elderly may be unable to activate the Unfolded Protein Response (UPR). In fact, since the function of the UPR is to restore homeostasis in response to stress, it seems possible that this lack of response is responsible for the worsened conditions observed in elderly patients.
When fatty acids were clustered and analyzed as distinct families, the elderly population showed many alterations. In particular, elderly patients presented higher levels of saturated and monounsaturated fatty acids. Interestingly, for several decades, saturated fatty acids have been associated with numerous ailments and immuno-metabolic conditions including diabetes (Fu et al., 2011). The elevation in the percentage of MUFAs was common between the adult and elderly burned patients and was in large part attributable to the increased release of oleic acid, which is the most abundant MUFA in the circulation. Interestingly, oleate has been linked with insulin resistance and its interconnection with inflammation (Shi et al., 2006). In contrast with the Saturated Fatty Acids (SFAs) and Mono Unsaturated Fatty Acids (MUFAs), the Poly Unsaturated, the omega3 and omega 6 fatty acids were significantly decreased in the elderly population. The decreased percentage of Poly Unsaturated Fatty Acids (PUFAs) was in large proportion caused by lower percentage of the two essential fatty acids alpha-Linolenic and Linoleic Acids. Consumption of these two essential fatty acids as well as omega 3 is associated with lower inflammation, improved insulin sensitivity and better prognosis in critically ill patients (Simopoulos, 1991, Riserus et al., 2009, Martin and Stapleton, 2010). All together, the fatty acids profile in elderly burned population indicate that lowering lipolysis and circulating Non-Esterified Fatty Acids (NEFAs) may represent an attractive strategy to dampen the consequences of thermal injury in the elderly population. Furthermore, in addition to lowering plasma NEFAs, restoring the balance in the fatty acid population with a nutritional intervention aiming at lowering the percentage of SFAs and MUFAs while increasing PUFAs, in particular the two essential fatty acids and the omega3 fatty acids may provide additional benefits on the conditions of the patients.
When normalized for burn size, elderly had a significantly longer hospital stay of approximately 1 day per percent burn, when compared to adults. Our data on characteristics of skin progenitor cells highlight another line of alteration that we observed in elderly patients in compared with young adults. Aging is a complex phenomenon which is associated with a progressive weakening in homeostatic and regenerative capacities of different tissues. Our data suggest that elderly patients are not only deficient in the tissue-specific stem cells but also the niche and systemic cues that regulate stem cell activity are perturbed. As noted, failure in initiation of an inflammatory cascade in elderly burn patients is one the hallmark of our study. In post-natal life, the inflammatory reaction is a foreseeable consequence of skin injury (Bielefeld et al., 2013, Amini-Nik et al., 2011). Several experimental studies demonstrated that inflammation is vital in the formation of skin homeostasis following injury (Abdullahi et al., 2014). Although an optimal level of inflammatory responses is necessary for healing, failure to start inflammatory responses have been attributed to the deficient healing. In the early phase of wound healing, some cytokines which are mainly released by platelets (Singer and Clark, 1999, Schultz et al., 2011) entice inflammatory cells like neutrophils and monocytes into the site of injury. Cytokines such as TGF-β and platelet-derived growth factor (PDGF) have essential role in this attraction. Moreover, macrophages release TGF-β and other cytokines, and thereby enhance the migration of mesenchymal and epithelial cells into the wound (Singer and Clark, 1999, Mahdavian Delavary et al., 2011), both are accompanied with the activation of Wnt/β-catenin signaling pathway (Bielefeld et al., 2011). Since we observe a lower activation of these two essential pathways in the burned skin of elderly patients (ie: Wnt/β-catenin and TGF-β signaling pathways) suggest that failure in initiation of an inflammatory cascade is the prominent underlying mechanism in the disturbed healing observed in elderly. Nevertheless, as mentioned, aging is a multifaceted phenomenon and hence alteration in stem cells, their niche, systemic cues that regulate stem cell activity, the gender of patients (Hardman and Ashcroft, 2008) converge together, leading to a deficient skin healing in elderly burn patients compared with young adults. We would like to mention that it is not entirely clear to what extend pre-existing co-morbidities contribute to poor outcomes. As expected we found that elderly have significantly more premorbid conditions when compared to adults. A pre-existing premorbid condition may cause chronic stress or physiological alterations that may affect cells or systems and decrease their ability to adequately respond. Pre-morbid conditions have to be included as they are widely present in elderly. But as the population is expected to substantially grow over the next decades, patients with premorbid conditions will follow the same trend. Therefore we need to account for these conditions.
This paper is to highlight a great number of abnormal markers in this category of patients. The future essential step is to control for the health status elderly and compare the two groups. Nevertheless, this translational study is the first to highlight a great number of abnormal markers to explain why elderly have such a poor outcome and advance the science in an area that has not progressed for the last 2–3 decades. We have found that elderly have a significantly greater mortality after burn when compared to adults with like size burn sizes. We were able to identify several pathophysiologic responses that are associated with the detrimental outcomes. It appears that elderly are at higher risk to develop multi organ failure, are unable to decrease their metabolic needs over time, express profound alterations in glucose and fat metabolism, have an impaired and reversed immune-response with the inability to adequately respond to stress, and lastly have a significantly impaired capacity for dermal and epidermal regeneration due to a decreased stem cell pool and dysfunctional stem cells. All of these contributors are pieces of a complex clinical picture and the next steps are now to further extract these pathophysiological pathways to elucidate more cellular responses and mechanisms particularly in compare with non-burnt elderly in order to develop novel intervention improving the outcomes of severely burned elderly.
The following are the supplementary data related to this article.
Supplemental Fig. 1.
Oral Glucose Tolerance Test (OGTT) glucose, insulin, and c-peptide. Oral Glucose Tolerance test were performed on patients and glucose (A), insulin (C) and c-Peptide (E) quantified. The respective AUC for these markers are shown in (B), (D) and (F). n for adults = 55 and for elderly = 29* Significant difference between adults vs. elderly, p < 0.05.
Supplemental Fig. 2.
Lipid profile of adult and elderly burned patients. Serum free fatty acid species were analyzed by GC–MS and a heat map was created to illustrate their concentration sin the respective groups of patients (A). The sum of the FFAs and the percentage of fatty acids subfamilies are shown in B and C respectively. n for control = 5, for adults = 10 and for elderly = 10. Data expressed as absolute values or as mean ± SEM. * Significant difference between control vs. adult, p < 0.05. §Significant difference between control vs. elderly, p < 0.05. φ Significant difference between adults vs. elderly, p < 0.05. Using double symbols means p < 0.001.
Supplemental Fig. 3.
Metabolic profile over time. Adult and elderly have increased c-Peptide and GLP-1 levels, as well as decreased (Pancreatic Peptide) PP, (Peptide YY) PYY, Leptin, GIP and Ghrelin levels (A–J). Data is represented as mean ± SEM. Significant difference between adults vs. elderly, *p < 0.05 and **p < 0.01.
Supplemental Fig. 4.
Inflammasome in WAT. Elderly patients have less macrophages (A) and Interleukin-1 beta (IL-1β +) cells in the CD14 + macrophage fraction (B–C), p > 0.05. In the fraction of CD14 + macrophage that are double-positive for IL-1β and FLICA (caspase-1), both adults and elderly were significantly higher than healthy controls (D). Both length of stay and Baux score correlated with the percentage of IL-1β +, CD14 + cells (E–F). n for adult = 18 and for elderly = 11.
Supplemental Fig. 5.
Altered expression of Oct-4 transcription factor in the mesenchymal cells isolated from skin of elderlies in compare with young adults. (A–B) Representative fluorescence images of cells from burned human skin of elderly patients show reduced number of cells positive for Oct4 (green). DAPI (4,6-diamidino-2-phenylindole) was used to identify the nuclei (blue). Arrows indicate cells positive for nuclear Oct4. (n = 3 for each group).
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.07.040.
Acknowledgments
We thank Miss Andrea Datu for proofreading of the manuscript.
Footnotes
Conflicts of interest and source of funding: This study was supported by the Canadian Institutes of Health Research # 123336 and CFI Leader's Opportunity Fund: Project # 25407 NIH RO1 GM087285-01.
References
- (WHO) OWH . 2002. A Graphical Overview of the Global Burden of Injuries. The Injury Chart Book. (Geneva) [Google Scholar]
- Abdullahi A., Amini-Nik S., Jeschke M.G. Animal models in burn research. Cell. Mol. Life Sci. 2014;71:3241–3255. doi: 10.1007/s00018-014-1612-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albornoz C.R., Villegas J., Sylvester M., Pena V., Bravo I. Burns are more aggressive in the elderly: proportion of deep burn area/total burn area might have a role in mortality. Burns. 2011;37:1058–1061. doi: 10.1016/j.burns.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Amini Nik S., Ebrahim R.P., Van Dam K., Cassiman J.J., Tejpar S. TGF-beta modulates beta-catenin stability and signaling in mesenchymal proliferations. Exp. Cell Res. 2007;313:2887–2895. doi: 10.1016/j.yexcr.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Amini-Nik S., Glancy D., Boimer C., Whetstone H., Keller C., Alman B.A. Pax7 expressing cells contribute to dermal wound repair, regulating scar size through a beta-catenin mediated process. Stem Cells (Dayton, Ohio) 2011;29:1371–1379. doi: 10.1002/stem.688. [DOI] [PubMed] [Google Scholar]
- Amini-Nik S., Cambridge E., Yu W. Beta-catenin-regulated myeloid cell adhesion and migration determine wound healing. J. Clin. Invest. 2014;124:2599–2610. doi: 10.1172/JCI62059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeld K.A., Amini-Nik S., Whetstone H. Fibronectin and beta-catenin act in a regulatory loop in dermal fibroblasts to modulate cutaneous healing. J. Biol. Chem. 2011;286:27687–27697. doi: 10.1074/jbc.M111.261677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeld K.A., Amini-Nik S., Alman B.A. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cell. Mol. Life Sci. 2013;70:2059–2081. doi: 10.1007/s00018-012-1152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringham P.A., McLoughlin E. Burn incidence and medical care use in the United States: estimates, trends and data sources. J. Burn Care Rehabil. 1996;17:95–107. doi: 10.1097/00004630-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Cazalis M.A., Friggeri A., Cave L. Decreased HLA-DR antigen-associated invariant chain (CD74) mRNA expression predicts mortality after septic shock. Crit. Care (Lond. Engl.) 2013;17:R287. doi: 10.1186/cc13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaret J., Walencik A., Jacob M.C. Inter-laboratory assessment of flow cytometric monocyte HLA-DR expression in clinical samples. Cytometry B Clin. Cytom. 2013;84:59–62. doi: 10.1002/cyto.b.21043. [DOI] [PubMed] [Google Scholar]
- Diao L., Marshall A.H., Dai X. Burn plus lipopolysaccharide augments endoplasmic reticulum stress and NLRP3 inflammasome activation and reduces PGC-1alpha in liver. Shock (Augusta, Ga) 2014;41:138–144. doi: 10.1097/SHK.0000000000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S., Yang L., Li P. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–531. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauglitz G.G., Finnerty C.C., Herndon D.N., Mlcak R.P., Jeschke M.G. Are serum cytokines early predictors for the outcome of burn patients with inhalation injuries who do not survive? Crit. Care. 2008;12:R81. doi: 10.1186/cc6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauglitz G.G., Song J., Herndon D.N. Characterization of the inflammatory response during acute and post-acute phases after severe burn. Shock. 2008;30:503–507. doi: 10.1097/SHK.0b013e31816e3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauglitz G.G., Herndon D.N., Kulp G.A., Meyer W.J., III, Jeschke M.G. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J. Clin. Endocrinol. Metab. 2009;94:1656–1664. doi: 10.1210/jc.2008-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauglitz G.G., Toliver-Kinsky T.E., Williams F.N. Insulin increases resistance to burn wound infection-associated sepsis. Crit. Care Med. 2010;38:202–208. doi: 10.1097/CCM.0b013e3181b43236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauglitz G.G., Halder S., Boehning D.F. Post-burn hepatic insulin resistance is associated with endoplasmic reticulum (ER) stress. Shock. 2010;33:299–305. doi: 10.1097/SHK.0b013e3181b2f439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger H., Denkinger M., Schirmbeck R. Hematopoietic stem cell aging. Curr. Opin. Immunol. 2014;29:86–92. doi: 10.1016/j.coi.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Gerstein A.D., Phillips T.J., Rogers G.S., Gilchrest B.A. Wound healing and aging. Dermatol. Clin. 1993;11:749–757. [PubMed] [Google Scholar]
- Greenhalgh D.G., Saffle J.R., Holmes JHt. American Burn Association consensus conference to define sepsis and infection in burns. J. Burn Care Res. 2007;28:776–790. doi: 10.1097/BCR.0b013e3181599bc9. [DOI] [PubMed] [Google Scholar]
- Grimble R.F. Inflammatory response in the elderly. Curr. Opin. Clin. Nutr. Metab. Care. 2003;6:21–29. doi: 10.1097/00075197-200301000-00005. [DOI] [PubMed] [Google Scholar]
- Hardman M.J., Ashcroft G.S. Estrogen, not intrinsic aging, is the major regulator of delayed human wound healing in the elderly. Genome Biol. 2008;9:R80. doi: 10.1186/gb-2008-9-5-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon D.N., Nguyen T.T., Wolfe R.R. Lipolysis in burned patients is stimulated by the beta 2-receptor for catecholamines. Arch. Surg. 1994;129:1301–1304. doi: 10.1001/archsurg.1994.01420360091012. (discussion 4–5) [DOI] [PubMed] [Google Scholar]
- Jeschke M.G., Mlcak R.P., Finnerty C.C. Burn size determines the inflammatory and hypermetabolic response. Crit. Care. 2007;11:R90. doi: 10.1186/cc6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke M.G., Chinkes D.L., Finnerty C.C. Pathophysiologic response to severe burn injury. Ann. Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke M.G., Mlcak R.P., Finnerty C.C. Gender differences in pediatric burn patients: does it make a difference? Ann. Surg. 2008;248:126–136. doi: 10.1097/SLA.0b013e318176c4b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke M.G., Kulp G.A., Kraft R. Intensive insulin therapy in severely burned pediatric patients: a prospective randomized trial. Am. J. Respir. Crit. Care Med. 2010;182:351–359. doi: 10.1164/rccm.201002-0190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke M.G., Gauglitz G.G., Kulp G.A. Long-term persistence of the pathophysiologic response to severe burn injury. PLoS One. 2011;6:e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke M.G., Finnerty C.C., Emdad F. Mild obesity is protective after severe burn injury. Ann. Surg. 2013;258:1119–1129. doi: 10.1097/SLA.0b013e3182984d19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.A., Wei Y., Sowers J.R. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 2008;102:401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft R., Herndon D.N., Al-Mousawi A.M., Williams F.N., Finnerty C.C., Jeschke M.G. Burn size and survival probability in paediatric patients in modern burn care: a prospective observational cohort study. Lancet. 2012;379:1013–1021. doi: 10.1016/S0140-6736(11)61345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft R., Herndon D.N., Finnerty C.C., Hiyama Y., Jeschke M.G. Association of postburn fatty acids and triglycerides with clinical outcome in severely burned children. J. Clin. Endocrinol. Metab. 2013;98:314–321. doi: 10.1210/jc.2012-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft R., Herndon D.N., Finnerty C.C., Shahrokhi S., Jeschke M.G. Occurrence of multiorgan dysfunction in pediatric burn patients: incidence and clinical outcome. Ann. Surg. 2014;259:381–387. doi: 10.1097/SLA.0b013e31828c4d04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang W., Xu X., Lin J. Functional and molecular changes of MSCs in aging. Curr. Stem Cell Res. Ther. 2015 doi: 10.2174/1574888x10666150211162933. (Epub Ahead of print) [DOI] [PubMed] [Google Scholar]
- Lamkanfi M., Mueller J.L., Vitari A.C. Glyburide inhibits the cryopyrin/Nalp3 inflammasome. J. Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren R.S., Kramer C.B., Rivara F.P. Influence of comorbidities and age on outcome following burn injury in older adults. J. Burn Care Res. 2009;30:307–314. doi: 10.1097/BCR.0b013e318198a416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdavian Delavary B., van der Veer W.M., van Egmond M., Niessen F.B., Beelen R.H. Macrophages in skin injury and repair. Immunobiology. 2011;216:753–762. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Martin J.M., Stapleton R.D. Omega-3 fatty acids in critical illness. Nutr. Rev. 2010;68:531–541. doi: 10.1111/j.1753-4887.2010.00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham T.N., Kramer C.B., Wang J. Epidemiology and outcomes of older adults with burn injury: an analysis of the National Burn Repository. J. Burn Care Res. 2009;30:30–36. doi: 10.1097/BCR.0b013e3181921efc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon R., Nik S.A., Ahn J., Slade L., Alman B.A. Beta-catenin and transforming growth factor beta have distinct roles regulating fibroblast cell motility and the induction of collagen lattice contraction. BMC Cell Biol. 2009;10:38. doi: 10.1186/1471-2121-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani M., Schwacha M.G. Aging and the pathogenic response to burn. Aging Dis. 2012;3:171–180. [PMC free article] [PubMed] [Google Scholar]
- Riserus U., Willett W.C., Hu F.B. Dietary fats and prevention of type 2 diabetes. Prog. Lipid Res. 2009;48:44–51. doi: 10.1016/j.plipres.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L., Cammer M., Lehman J. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell. Physiol. Biochem. 2010;25:279–292. doi: 10.1159/000276562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz G.S., Davidson J.M., Kirsner R.S., Bornstein P., Herman I.M. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19:134–148. doi: 10.1111/j.1524-475X.2011.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Kokoeva M.V., Inouye K., Tzameli I., Yin H., Flier J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumakov V.I., Onishchenko N.A., Rasulov M.F., Krasheninnikov M.E., Zaidenov V.A. Mesenchymal bone marrow stem cells more effectively stimulate regeneration of deep burn wounds than embryonic fibroblasts. Bull. Exp. Biol. Med. 2003;136:192–195. doi: 10.1023/a:1026387411627. [DOI] [PubMed] [Google Scholar]
- Simopoulos A.P. Omega-3 fatty acids in health and disease and in growth and development. Am. J. Clin. Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- Singer A.J., Clark R.A. Cutaneous wound healing. N. Engl. J. Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Solinas G., Naugler W., Galimi F., Lee M.S., Karin M. Saturated fatty acids inhibit induction of insulin gene transcription by JNK-mediated phosphorylation of insulin-receptor substrates. Proc. Natl. Acad. Sci. U. S. A. 2006;103:16454–16459. doi: 10.1073/pnas.0607626103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S., Olefsky J.M., Osborn O. Targeting GPR120 and other fatty acid-sensing GPCRs ameliorates insulin resistance and inflammatory diseases. Trends Pharmacol. Sci. 2011;32:543–550. doi: 10.1016/j.tips.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teta M., Long S.Y., Wartschow L.M., Rankin M.M., Kushner J.A. Very slow turnover of beta-cells in aged adult mice. Diabetes. 2005;54:2557–2567. doi: 10.2337/diabetes.54.9.2557. [DOI] [PubMed] [Google Scholar]
- van der Vusse G.J. Albumin as fatty acid transporter. Drug Metab. Pharmacokinet. 2009;24:300–307. doi: 10.2133/dmpk.24.300. [DOI] [PubMed] [Google Scholar]
- Wolfe R.R., Herndon D.N., Peters E.J., Jahoor F., Desai M.H., Holland O.B. Regulation of lipolysis in severely burned children. Ann. Surg. 1987;206:214–221. doi: 10.1097/00000658-198708000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]