Abstract
Background
The role of maternal 25-hydroxyvitamin D [25(OH)D] in fetal development is uncertain and findings of observational studies are inconsistent. Most studies have assessed 25(OH)D only once in pregnancy, but the tracking of an individual’s 25(OH)D during pregnancy is unknown.
Objective
We determined the tracking of serum 25(OH)D from early to late pregnancy, and factors which influence this.
Design
The Southampton Women’s Survey is a prospective mother-offspring birth cohort study. Lifestyle, diet and 25(OH)D status were assessed at 11 and 34 weeks gestation. A Fourier transformation was used to model seasonal variation in 25(OH)D for early and late pregnancy, separately, and the difference between measured and seasonally modelled 25(OH)D calculated to generate a season-corrected 25(OH)D. Tracking was assessed using Pearson’s correlation coefficient, and multivariate linear regression used to determine factors associated with change in season-corrected 25(OH)D.
Results
1753 women had 25(OH)D measured in both early and late pregnancy. There was a moderate correlation between season-corrected 25(OH)D measurements at 11 and 34 weeks gestation (r=0.53, p<0.0001, n=1753). Vitamin D supplementation was the strongest predictor of tracking: compared with women who never used supplements, discontinuing supplementation after 11 weeks was associated with reduction in season-corrected 25(OH)D (β=−7.3nmol/l, p<0.001), whereas commencing (β=12.6nmol/l, p<0.001) or continuing (β=6.6nmol/l, p<0.001) supplementation were associated with increases. Higher pregnancy weight gain was associated with reduction in season-corrected 25(OH)D (β=−0.4nmol/l per kg, p=0.015), whereas greater physical activity (β=0.4nmol/l per hour/week, p=0.011) was associated with increases.
Conclusions
There is moderate tracking of 25(OH)D status through pregnancy; factors such as vitamin D supplementation, weight gain and physical activity are associated with changes in season-corrected 25(OH)D from early to late gestation. These findings have implications for study design and analysis and approaches to intervention studies and clinical care.
Keywords: Moon, Crozier, Dennison, Davies, Robinson, Inskip, Godfrey, Cooper, Harvey
Introduction
Tracking describes the stability of a measurement relative to the population distribution over time. As such, if a biological marker is known to track highly, one measurement can be used to predict future measurements, and therefore inform the need for interventions to prevent high or low levels. Many studies have investigated the role of serum 25-hydroxyvitamin D [25(OH)D] in a wide range of clinical outcomes (1, 2); the majority of observational investigations have used only a single measurement of 25(OH)D, and yet the tracking of 25(OH)D is not currently well understood. High correlation between 25(OH)D status in samples obtained in the same month at one to five year intervals has been demonstrated in adults (3-5). However, it is well recognised that at latitudes far from the equator, 25(OH)D displays seasonal variation at the population level (6, 7). To our knowledge, there are no data in any population group demonstrating the tracking of an individual’s 25(OH)D measurement within the population distribution after taking account of seasonal variation. This might be of particular relevance during pregnancy when delineation of trimester specific effects has major practical relevance.
There is a wealth of observational data assessing the relationships between maternal serum 25(OH)D and obstetric complications and offspring development (2, 8). These studies have not consistently demonstrated that a higher serum 25(OH)D in pregnancy is associated with improved clinical outcomes for either the mother or offspring. However, the timing of maternal 25(OH)D measurement varies between data sets from early gestation to delivery and across seasons, and this adds complexity to the comparison of results from different studies. Despite there being few data available from intervention studies demonstrating clinical benefits of antenatal vitamin D supplementation (2, 8), many national guidelines recommend vitamin D supplementation to all women in pregnancy (9-11). As such, knowledge regarding the tracking of 25(OH)D during pregnancy might enable clearer interpretation and comparison of studies with inconsistent findings for the same outcome, and may influence the development of future supplementation polices. We therefore assessed the tracking of 25(OH)D from early to late pregnancy in a prospective mother-offspring birth cohort study, the Southampton Women’s Survey (SWS). Additionally we explored maternal factors which might influence 25(OH)D tracking.
Methods
The Southampton Women’s Survey
Details of the study have previously been published (12), but briefly, the SWS is a population based prospective mother-offspring birth cohort study based in Southampton, UK (latitude 50.9°N). Non-pregnant women aged 20-34 years were recruited into the study between April 1998 and October 2002 (n=12583) and asked to inform the research centre if they became pregnant. The SWS was conducted according to the guidelines laid down in the Declaration of Helsinki, and the Southampton and South West Hampshire Research Ethics Committee approved all procedures (276/97 and 307/97). Written informed consent was obtained from all participating women. The SWS is registered in CLOSER, birthcohort.net and the UK MRC cohort directory.
Maternal data
At the pre-pregnancy interview, details of maternal parity, highest educational attainment and ethnicity were obtained, and height and weight were measured. For women who became pregnant, assessments were performed at 11 (early pregnancy) and 34 (late pregnancy) weeks gestation. Information on smoking status, alcohol intake, exercise participation and dietary supplement usage was obtained from an interview-administered health and lifestyle questionnaire. Information collected regarding vitamin D supplementation included brand of supplements used and frequency of usage. The manufacturer’s information was used to determine the vitamin D content of the supplements. A validated 100 item food frequency questionnaire was used to assess dietary intake (13), and from this dietary vitamin D intake was determined by multiplying the frequency of consumption of a portion of each food by its vitamin D content according to the UK food-composition tables or manufacturers’ composition data.
25-hydroxyvitamin D analysis
Non-fasted venous blood samples were obtained at 11 and 34 weeks gestation and an aliquot of maternal serum stored at −80°C. The early pregnancy 25(OH)D samples were all analysed in a single batch in 2013, and the late pregnancy samples similarly in a single batch in 2008.
From the early pregnancy samples, serum 25(OH)D was analysed using high-performance liquid chromatography and tandem mass spectrometry: serum samples had an internal standard added, followed by protein denaturation by the addition of zinc sulphate and methanol. The internal standard and both 25(OH)D2 and 25(OH)D3 were then extracted into hexane, which was dried, and reconstituted in mobile phase. The extracts were analysed by liquid chromatography with detection by tandem mass spectrometry (Waters, Milford, MA, USA). From the late pregnancy samples, serum 25(OH)D concentrations were analysed by radioimmunoassay (Diasorin, Minnesota, USA). This assay measures both 25(OH)D2 and 25(OH)D3. Total 25(OH)D was calculated from the sum of 25(OH)D2 and 25(OH)D3 for both early and late pregnancy. The laboratories that undertook both analyses are members of the Vitamin D EQA scheme (DEQAS) and both assays met the requirements for this. The intra- and inter-assay coefficients of variation for both methods were <10%.
Statistical analysis
Maternal characteristics for women with and without serum 25(OH)D status in pregnancy were compared using t-tests, Mann-Whitney and χ2 tests for normally distributed, non-normally distributed and categorical outcomes, respectively. Fourier transformations were used to model the seasonal variation in loge[25(OH)D] for early and late pregnancy. The difference of the measured 25(OH)D from the seasonally modelled 25(OH)D for the exact date of sampling was calculated for each participant, to generate a season-corrected 25(OH)D. Tracking of both loge[25(OH)D] and season-corrected 25(OH)D from early to late pregnancy were assessed using the Pearson’s correlation coefficient (14). A Bland-Altman plot was also used to assess agreement between season-corrected 25(OH)D in early and late pregnancy (15). Maternal factors which were associated with the change in season-corrected 25(OH)D were assessed using simple linear regression, and predictors with p<0.20 were included in a multiple linear regression model. Finally, we assessed differences in maternal characteristics according to vitamin D supplement usage using ANOVA and χ2 test.
All analysis was performed in Stata v13. A p value of <0.05 was considered to be statistically significant (Statacorp, College Station, Texas, USA).
Results
3158 women participating in the SWS delivered a liveborn singleton infant. Serum 25(OH)D status was assessed in 2019 (64.0%) and 2328 (73.7%) women in early and late pregnancy, respectively. 1753 women had 25(OH)D measured in both early and late pregnancy. Characteristics of these women are shown in Table 1. In comparison with women delivered a live birth in the study but who did not have 25(OH)D measured in both early and late pregnancy, those included in this analysis were younger, of higher parity and less likely to have smoked in early pregnancy (Table 1).
Table 1.
Characteristics of the women including in the analysis, and where available in comparison to women from the Southampton Women’s Survey for whom serum 25(OH)D was not measured in both early and late pregnancy
| 25(OH)D measured in both early and late pregnancy |
25(OH)D not measured in early and late pregnancy |
P1 | ||
|---|---|---|---|---|
| n | 1753 | 1405 | ||
| Maternal age at delivery (years), mean (SD) | 30.4 (3.7) | 31.0 (4.0) | <0.001 | |
| White ethnicity (%) | 96.8 | 94.0 | <0.001 | |
| Education to degree level or higher (%) | 22.2 | 21.7 | 0.74 | |
| Nulliparous (%) | 48.3 | 54.6 | <0.001 | |
| Pre-pregnancy BMI (kg/m2), median (IQR) | 24.2 (21.9-27.4) | 24.1 (21.8-27.3) | 0.28 | |
| Current smoker (%) | Early pregnancy | 13.9 | 19.2 | <0.001 |
| Late pregnancy | 13.5 | |||
| Consumed alcohol in last 14 weeks (%) | Early pregnancy | 80.1 | ||
| Late pregnancy | 77.9 | |||
| Moderate/Strenuous exercise (hours per week), median (IQR) | Early pregnancy | 1.25 (0.25-3.25) | ||
| Late pregnancy | 0.75 (0.13, 2.25) | |||
| Weight gain early to late pregnancy (kg), mean (SD) | 10.7 (4.3) | |||
| Dietary vitamin D intake (iu/day), median (IQR) | Early pregnancy | 129 (91-169) | ||
| Late pregnancy | 135 (98-178) | |||
| Vitamin D supplementation usage (%) | Early pregnancy | 37.3 | ||
| Late pregnancy | 22.2 | |||
| Serum 25(OH)D (nmol/l), median (IQR) | Early pregnancy | 61 (43-81) | ||
| Late pregnancy | 59 (41-84) |
p values were determined using t-tests, Mann-Whitney and χ2 tests for normally distributed, non-normally distributed and categorical outcomes, respectively.
Seasonal modelling of 25(OH)D in pregnancy
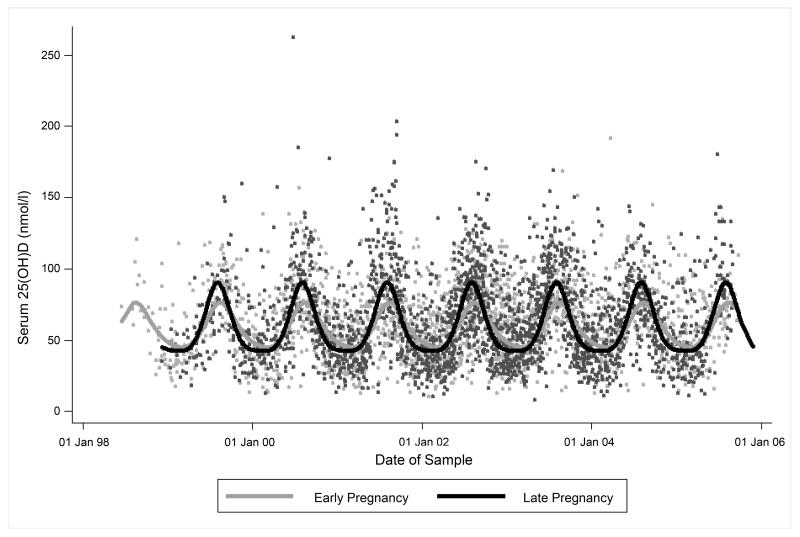
In both early pregnancy and late pregnancy, 25(OH)D displayed statistically significant seasonal variation (Figure 1). The Fourier series model explained 17% (p<0.0001) and 30% (p<0.0001) of the variance in 25(OH)D in early and late pregnancy, respectively. The mean difference between measured 25(OH)D and that modelled by the Fourier series for date of sampling in early pregnancy was 4.6nmol/l (SD 23.3nmol/l, range −61.2 to 146.0 nmol/l) and in late pregnancy was 4.8nmol/l (SD 25.7nmol/l, range −66.2 to 182.7nmol/l).
Figure 1.
Seasonal variation in maternal serum 25-hydroxyvitamin D status in early and late pregnancy (n=1753). Fourier transformations were used to model the seasonal variation in loge[25(OH)D] for early and late pregnancy.
Tracking of 25(OH)D status from early to late pregnancy
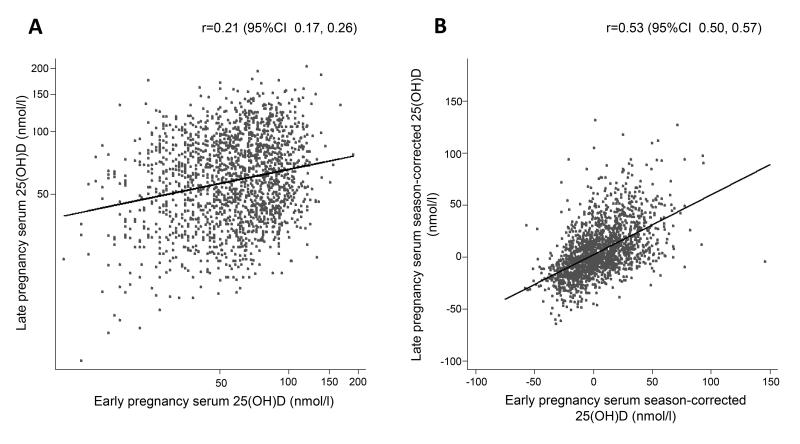
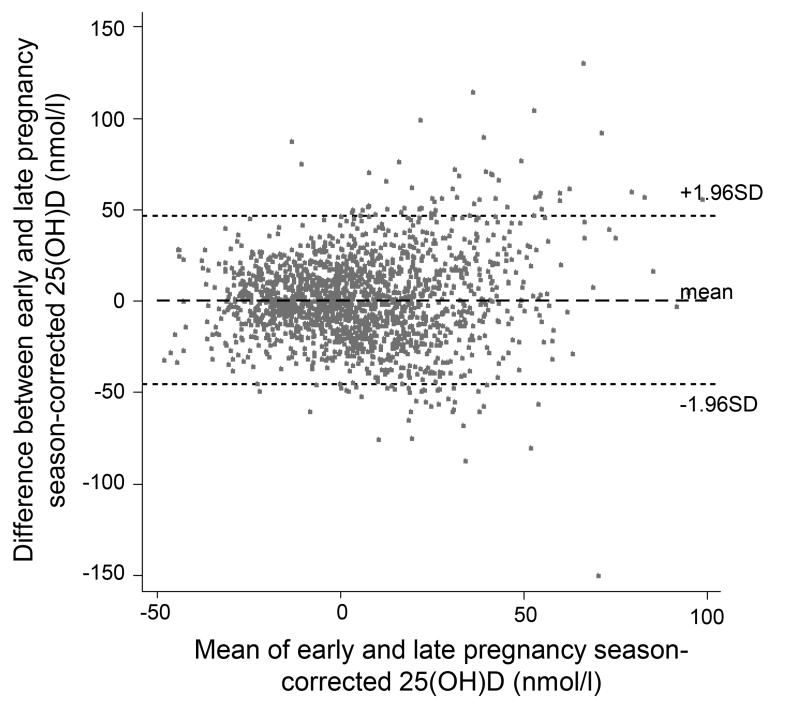
The correlation coefficient between measured 25(OH)D in early and late pregnancy was low (r=0.21, 95% CI 0.17, 0.26; Figure 2). However, season-corrected 25(OH)D was more highly correlated from EP to LP (r=0.53, 95% CI 0.50, 0.57; Figure 2). Figure 3 illustrates the agreement between the season-corrected 25(OH)D in early and late pregnancy using a Bland-Altman plot.
Figure 2.
Correlation between the early and late pregnancy (A) serum 25(OH)D (B) season-corrected serum 25(OH)D (n=1753). Correlations were determined using the Pearson’s correlation coefficient.
Figure 3.
Bland-Altman plot demonstrating the agreement between early and late pregnancy season-corrected serum 25(OH)D (n=1753).
Maternal determinants of change in season-corrected 25(OH)D
A number of maternal factors were associated with the change in season-corrected 25(OH)D (Table 2), but only timing of maternal vitamin D supplementation use, exercise in LP and pregnancy weight gain remained significantly associated in multivariate analysis (Table 2). Thus compared with women who never took supplements, discontinuation of vitamin D supplements after early pregnancy blood sampling was negatively associated with change in season-corrected 25(OH)D (β=−7.3nmol/l, p<0.001), whereas continuing (β=12.6nmol/l, p<0.001) or starting supplementation (β=6.6nmol/l, p<0.001) were positively associated.
Table 2.
Associations between maternal demographic and lifestyle factors and change in season-corrected serum 25(OH)D during pregnancy.
| Δ[season-corrected serum 25(OH)D] (nmol/l) | |||||||
|---|---|---|---|---|---|---|---|
| Univariate1 | Multivariate2 | ||||||
| n | β (95% CI) | p | n | β (95% CI) | p | ||
| Age at delivery (years) | 1752 | −0.0 (−0.3, 0.3) | 0.86 | ||||
| Ethnicity (Other vs White) | 1753 | 4.3 (−1.9, 10.5) | 0.17 | 1705 | 2.4 (−4.0, 8.7) | 0.46 | |
| Education to degree level or higher (yes vs no) | 1750 | 2.6 (−0.1, 5.3) | 0.06 | 1705 | 1.8 (−0.8, 4.5) | 0.18 | |
| Parity (Multiparous vs Nulliparous) | 1752 | −0.7 (−2.9, 1.5) | 0.54 | ||||
| Pre-pregnancy BMI (kg/m2) | 1741 | 0.1 (−0.2, 0.3) | 0.52 | ||||
| Smoking (yes vs no) | Early pregnancy | 1740 | −1.7 (−4.9, 1.5) | 0.30 | |||
| Late pregnancy | 1738 | −2.1 (−5.3, 1.2) | 0.21 | ||||
| Alcohol consumption (yes vs no) | Early pregnancy | 1736 | −2.0 (−4.8, 0.8) | 0.15 | 1705 | −2.1 (−4.9, 0.6) | 0.13 |
| Late pregnancy | 1738 | −0.1 (−2.6, 2.7) | 0.96 | ||||
| Moderate/Strenuous Exercise (Hours/week) | Early pregnancy | 1732 | 0.1 (−0.1, 0.4) | 0.25 | |||
| Late pregnancy | 1738 | 0.4 (0.1, 0.8) | 0.01 | 1705 | 0.4 (0.1, 0.7) | 0.01 | |
| ΔEarly to late pregnancy | 1717 | 0.9 (−0.1, 0.3) | 0.46 | ||||
| Weight gain early to late pregnancy (kg) | 1708 | −0.4 (−0.7, −0.1) | 0.003 | 1705 | −0.4 (−0.7, −0.1) | 0.003 | |
| Vitamin D supplement use (compared to never in pregnancy) | Early pregnancy only | 1722 | −7.4 (−10.3, −4.5) | <0.001 | 1705 | −7.3 (−10.1, −4.4) | <0.001 |
| Late pregnancy only | 11.9 (6.8, 17.0) | <0.001 | 12.6 (7.5, 17.8) | <0.001 | |||
| Early and late pregnancy | 6.5 (3.5, 9.5) | <0.001 | 6.6 (3.6, 9.7) | <0.001 | |||
P values were determined by univariate linear regression models of season-corrected 25(OH)D on maternal characteristics.
All associations from univariate analysis with p≤0.2 were included in the multivariable model. Statistically significant associations are in bold type.
Women who either never started or discontinued taking supplements during pregnancy were younger, less well educated, more likely to smoke in early pregnancy and less likely to be in their first pregnancy compared with women who continued supplementation throughout pregnancy (Table 3).
Table 3.
Maternal characteristics according to vitamin D supplementation usage in pregnancy
| Vitamin D supplementation use | |||||
|---|---|---|---|---|---|
| Never | Early Pregnancy Only |
Late Pregnancy Only |
Early and Late Pregnancy |
p between groups1 |
|
| N (%)2 | 1018 (59.1) | 327 (19.0) | 84 (4.9) | 293 (17.0) | |
| Early pregnancy season-corrected serum 25(OH)D (nmol/l), mean (SD) |
−0.1 (22.3) | 9.4 (21.2) | 1.6 (20.1) | 18.5 (23.0) | <0.0001 |
| Late pregnancy season-corrected serum 25(OH)D (nmol/l), mean (SD) |
0.0 (21.8) | 2.0 (22.2) | 13.6 (27.8) | 25.0 (28.7) | <0.0001 |
| Maternal age at delivery (years), mean (SD) | 30.3 (3.8) | 30.2 (3.6) | 30.3 (3.4) | 31.2 (3.5) | 0.002 |
| White Ethnicity (%) | 96.7 | 97.3 | 100 | 96.6 | 0.37 |
| Education to degree level or higher (%) | 17.3 | 22.6 | 28.6 | 36.9 | <0.001 |
| Nulliparous (%) | 40.6 | 50.5 | 61.9 | 70.0 | <0.001 |
| Pre-pregnancy BMI (kg/m2), median (IQR) | 24.4 (22.2-27.8) | 24.3 (22.0-27.2) | 24.0 (22.2-27.8) | 23.7 (21.2-25.2) | <0.001 |
| Smoked in early pregnancy (%) | 18.0 | 11.3 | 3.6 | 4.8 | <0.001 |
Differences in maternal characteristics according to vitamin D supplement usage were assessed using ANOVA and χ2 test for continuous and categorical data, respectively. Pre-pregnancy BMI was not normally distributed, therefore statistical significance was determined using the log of this variable.
Data on supplement usage in pregnancy was not available for 31 of the 1753 women included in the tracking analysis. These women were similar in age, ethnicity, educational achievement, parity, BMI and smoking status to those included in this table (p>0.05 for all).
Discussion
In this large prospective cohort study, we have demonstrated that there is moderate tracking of serum 25(OH)D status from early to late pregnancy, and that the change in deviation from modelled seasonal average of an individual may be influenced by vitamin D supplementation, weight gain, physical activity and dietary vitamin D intake. To our knowledge, no previous studies have investigated the longitudinal tracking of 25(OH)D status either during pregnancy or between seasons in other population groups.
There are several small studies, including between 10 and 40 women, which have attempted to describe the effect of pregnancy on 25(OH)D status (16-18), the findings of which are contradictory. However the interpretation of these studies is limited by recruitment of all women during the same season, or insufficient account being taken of the season of blood sampling. Furthermore, none of these studies considered tracking at the individual level. In non-pregnant adults, three studies have demonstrated high levels of 25(OH)D tracking when measured in the same month over several years (3-5), although Hofmann et al. found that the correlation coefficient reduced with increasing number of years between sampling (5).
Our finding could have an important clinical use: in combination with population data across seasons, a single measurement of 25(OH)D could be used to identify women who are at risk of low levels of 25(OH)D at other stages of pregnancy. Thus appropriate counselling regarding the need for vitamin D supplementation could be provided.
We observed that changes in supplement use strongly influenced 25(OH)D stability relative to the population distribution. Women who either did not use vitamin D supplementation or stopped supplementation following early pregnancy were younger, less well educated, more likely to smoke, less likely to be in their first pregnancy and had a higher pre-pregnancy BMI. Previous cross-sectional studies have identified that younger age (19), higher BMI or weight (20-22), smoking (6, 20-22) and lower educational achievement (20) increase the risk of vitamin D deficiency in pregnancy, whereas higher parity is protective (22). Although the majority of women in this study were pregnant prior to publication of the UK Department of Health guidelines that suggest all women should receive vitamin D supplementation in pregnancy (9), similar demographic factors have also been associated with reduced likelihood of folic acid supplementation during pregnancy (23, 24). These findings therefore highlight a group of women who might require additional health education during early pregnancy.
We additionally found that greater weight gain during pregnancy and less exercise in late pregnancy were associated with downward tracking of 25(OH)D. Adiposity is negatively associated with 25(OH)D in non-pregnant populations, and this is hypothesised to result from sequestration of 25(OH)D within adipose tissue (25). Indeed, vitamin D supplementation studies have demonstrated that the incremental rise in 25(OH)D is lower in obese than non-obese individuals (26, 27), whereas, conversely, weight loss is associated positively with change in 25(OH)D (28-30). Thus, while we cannot be certain that the greater weight gain represents increased fat mass as opposed to feto-placental tissues, it is likely that the downward tracking of 25(OH)D reflects a higher volume of dilution. Furthermore this finding suggests that women who gain greater weight in pregnancy might require higher supplementation doses to prevent vitamin D deficiency. This needs to be established in intervention studies.
The strength of this study is in the detailed phenotyping and large number of women included. However, there are a number of limitations which should be considered in its interpretation. Firstly, the women who participated in SWS but did not have blood sampling in both early and late pregnancy were older, and were more likely to be of non-white ethnicity, in their first pregnancy and to smoke in early pregnancy. These women were therefore at higher risk of vitamin D deficiency, and our findings are likely to be of particular relevance to this group. It is also likely that inclusion of such women would have increased the number at the lower end of the 25(OH)D distribution and thus yielded greater statistical power. Therefore, although strictly there may be limitations to generalisability, there is no reason to suppose that the associations observed would have been materially affected. Our findings would suggest that women who are at high risk of vitamin D deficiency should be informed that they are likely to remain vitamin D deficient throughout pregnancy unless approaches to increasing 25(OH)D status are implemented. Secondly, the women were recruited over a 4 year period. The Fourier transformation used to model the seasonal variation in 25(OH)D assumes that this is the same for each year. However, given year to year differences in weather, it is unlikely that the pattern is identical every year. Nonetheless, the effect of season was highly statistically significant and accounted for 17-30% of the variation in 25(OH)D. Secondly, 25(OH)D was measured using different assays in early and late pregnancy using stored frozen serum samples. However, each model was generated based only on a single assay in a laboratory that is a member of the DEQAS scheme, and it is therefore unlikely that the use of different assays in early and late pregnancy would affect the rank change or the tracking coefficient. Furthermore, it has previously been shown that storage of serum at −80°C does not affect the stability of 25(OH)D (31). Nonetheless, graphical representation of the seasonal models of 25(OH)D does suggest a higher summer time peak and a more flattened winter trough in late than early pregnancy. We cannot be certain whether this is a pregnancy-induced effect or due to assay differences, and indeed care should be taken translating these findings to other populations. Finally, we did not obtain information on time spent outdoors or holidays abroad and how this changed during pregnancy.
In conclusion, in this study, we have shown the moderate tracking of 25(OH)D status during pregnancy, which could be used to predict the likelihood of an individual developing low levels of 25(OH)D during pregnancy. High quality randomised controlled trials are now needed to demonstrate whether antenatal vitamin D supplementation can affect clinical outcomes (1, 32). If so, a number of maternal characteristics have been associated with both 25(OH)D status and the use of supplementation currently exist and should be considered in the provision of public health advice.
Acknowledgements
We thank Mrs G Strange and Mrs R Fifield for helping prepare the manuscript. CC and NCH are joint senior authors. This work was supported by grants from the Medical Research Council, British Heart Foundation, Arthritis Research UK, National Institute for Health Research (NIHR) Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton NHS Foundation Trust, and NIHR Musculoskeletal Biomedical Research Unit, University of Oxford. The work leading to these results was supported by the European Union’s Seventh Framework Programme (FP7/2007-2013), projects EarlyNutrition and ODIN under grant agreements numbers 289346 and 613977.
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- BMI
body mass index
- SWS
Southampton Women’s Survey
Footnotes
Disclosures
KMG has acted as a consultant to Abbott Nutrition and Nestle Nutrition, and has received reimbursement for speaking at an Abbott Nutrition Conference on Pregnancy Nutrition and Later Health Outcomes, at a Nestle Nutrition Institute Workshop and at a workshop funded by the International Life Sciences Institute (ILSI Europe). He is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone. No other authors declare a conflict of interest.
References
- 1.Harvey NC, Cooper C. Vitamin D: some perspective please. BMJ. 2012;345:e4695. doi: 10.1136/bmj.e4695. [DOI] [PubMed] [Google Scholar]
- 2.Harvey N, Holroyd C, Ntani G, Javaid M, Cooper P, Moon R, Cole Z, Tinati T, Godfrey K, Dennison E, et al. Vitamin D supplementation in pregnancy: a systematic review. Health Technol Assess. 2014;18(45) doi: 10.3310/hta18450. doi: 10.3310/hta18450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol. 2010;171(8):903–8. doi: 10.1093/aje/kwq005. doi: 10.1093/aje/kwq005. [DOI] [PubMed] [Google Scholar]
- 4.Sonderman JS, Munro HM, Blot WJ, Signorello LB. Reproducibility of serum 25-hydroxyvitamin d and vitamin D-binding protein levels over time in a prospective cohort study of black and white adults. Am J Epidemiol. 2012;176(7):615–21. doi: 10.1093/aje/kws141. doi: 10.1093/aje/kws141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofmann JN, Yu K, Horst RL, Hayes RB, Purdue MP. Long-term Variation in Serum 25-Hydroxyvitamin D Concentration among Participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 2010;19(4):927–31. doi: 10.1158/1055-9965.EPI-09-1121. doi: 10.1158/1055-9965.epi-09-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crozier SR, Harvey NC, Inskip HM, Godfrey KM, Cooper C, Robinson SM. Maternal vitamin D status in pregnancy is associated with adiposity in the offspring: findings from the Southampton Women’s Survey. Am J Clin Nutr. 2012;96(1):57–63. doi: 10.3945/ajcn.112.037473. doi: ajcn.112.037473 [pii];10.3945/ajcn.112.037473 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mavroeidi A, O’Neill F, Lee PA, Darling AL, Fraser WD, Berry JL, Lee WT, Reid DM, Lanham-New SA, Macdonald HM. Seasonal 25-hydroxyvitamin D changes in British postmenopausal women at 57 degrees N and 51 degrees N: a longitudinal study. J Steroid Biochem Mol Biol. 2010;121(1-2):459–61. doi: 10.1016/j.jsbmb.2010.03.038. doi: 10.1016/j.jsbmb.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 8.Moon R, Harvey N, Cooper C. ENDOCRINOLOGY IN PREGNANCY: Influence of maternal vitamin D status on obstetric outcomes and the foetal skeleton. European Journal of Endocrinology. 2015 doi: 10.1530/EJE-14-0826. doi: 10.1530/eje-14-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institute for Health and Clincial Excellence Antenatal care (NICE Clinical Guideline 62) 2010 www.guidance.nice.org.uk/cg62. [PubMed]
- 10.Paxton GA, Teale GR, Nowson CA, Mason RS, McGrath JJ, Thompson MJ, Siafarikas A, Rodda CP, Munns CF. Vitamin D and health in pregnancy, infants, children and adolescents in Australia and New Zealand: a position statement. Med J Aust. 2013;198(3):142–3. doi: 10.5694/mja11.11592. [DOI] [PubMed] [Google Scholar]
- 11.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. doi: 10.1210/jc.2011-0385. doi: jc.2011-0385 [pii];10.1210/jc.2011-0385 [doi] [DOI] [PubMed] [Google Scholar]
- 12.Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJ, Cooper C. Cohort profile: The Southampton Women’s Survey. Int J Epidemiol. 2006;35(1):42–8. doi: 10.1093/ije/dyi202. doi: dyi202 [pii];10.1093/ije/dyi202 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson S, Godfrey K, Osmond C, Cox V, Barker D. Evaluation of a food frequency questionnaire used to assess nutrient intakes in pregnant women. Eur J Clin Nutr. 1996;50(5):302–8. [PubMed] [Google Scholar]
- 14.Twisk JW, Kemper HC, Mellenbergh GJ. Mathematical and analytical aspects of tracking. Epidemiol Rev. 1994;16(2):165–83. doi: 10.1093/oxfordjournals.epirev.a036149. [DOI] [PubMed] [Google Scholar]
- 15.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. [PubMed] [Google Scholar]
- 16.Zhang JY, Lucey AJ, Horgan R, Kenny LC, Kiely M. Impact of pregnancy on vitamin D status: a longitudinal study. Br J Nutr. 2014:1–7. doi: 10.1017/S0007114514001883. doi: 10.1017/s0007114514001883. [DOI] [PubMed] [Google Scholar]
- 17.More C, Bhattoa HP, Bettembuk P, Balogh A. The effects of pregnancy and lactation on hormonal status and biochemical markers of bone turnover. Eur J Obstet Gynecol Reprod Biol. 2003;106(2):209–13. doi: 10.1016/s0301-2115(02)00237-3. [DOI] [PubMed] [Google Scholar]
- 18.Cross NA, Hillman LS, Allen SH, Krause GF, Vieira NE. Calcium homeostasis and bone metabolism during pregnancy, lactation, and postweaning: a longitudinal study. Am J Clin Nutr. 1995;61(3):514–23. doi: 10.1093/ajcn/61.3.514. [DOI] [PubMed] [Google Scholar]
- 19.Xiao JP, Zang J, Pei JJ, Xu F, Zhu Y, Liao XP. Low maternal vitamin D status during the second trimester of pregnancy: a cross-sectional study in Wuxi, China. PLoS One. 2015;10(2):e0117748. doi: 10.1371/journal.pone.0117748. doi: 10.1371/journal.pone.0117748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandevijvere S, Amsalkhir S, Van Oyen H, Moreno-Reyes R. High prevalence of vitamin D deficiency in pregnant women: a national cross-sectional survey. PLoS One. 2012;7(8):e43868. doi: 10.1371/journal.pone.0043868. doi: 10.1371/journal.pone.0043868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneuer FJ, Roberts CL, Guilbert C, Simpson JM, Algert CS, Khambalia AZ, Tasevski V, Ashton AW, Morris JM, Nassar N. Effects of maternal serum 25-hydroxyvitamin D concentrations in the first trimester on subsequent pregnancy outcomes in an Australian population. Am J Clin Nutr. 2014;99(2):287–95. doi: 10.3945/ajcn.113.065672. doi: 10.3945/ajcn.113.065672. [DOI] [PubMed] [Google Scholar]
- 22.Andersen LB, Abrahamsen B, Dalgard C, Kyhl HB, Beck-Nielsen SS, Frost-Nielsen M, Jorgensen JS, Barington T, Christesen HT. Parity and tanned white skin as novel predictors of vitamin D status in early pregnancy: a population-based cohort study. Clin Endocrinol (Oxf) 2013;79(3):333–41. doi: 10.1111/cen.12147. doi: 10.1111/cen.12147. [DOI] [PubMed] [Google Scholar]
- 23.Forster DA, Wills G, Denning A, Bolger M. The use of folic acid and other vitamins before and during pregnancy in a group of women in Melbourne, Australia. Midwifery. 2009;25(2):134–46. doi: 10.1016/j.midw.2007.01.019. doi: 10.1016/j.midw.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Langley-Evans SC, Langley-Evans AJ. Use of folic acid supplements in the first trimester of pregnancy. J R Soc Promot Health. 2002;122(3):181–6. doi: 10.1177/146642400212200315. [DOI] [PubMed] [Google Scholar]
- 25.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 26.Dong Y, Stallmann-Jorgensen IS, Pollock NK, Harris RA, Keeton D, Huang Y, Li K, Bassali R, Guo DH, Thomas J, et al. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J Clin Endocrinol Metab. 2010;95(10):4584–91. doi: 10.1210/jc.2010-0606. doi: 10.1210/jc.2010-0606. [DOI] [PubMed] [Google Scholar]
- 27.Aguirre Castaneda R, Nader N, Weaver A, Singh R, Kumar S. Response to vitamin D3 supplementation in obese and non-obese Caucasian adolescents. Horm Res Paediatr. 2012;78(4):226–31. doi: 10.1159/000343446. doi: 10.1159/000343446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinehr T, de Sousa G, Alexy U, Kersting M, Andler W. Vitamin D status and parathyroid hormone in obese children before and after weight loss. Eur J Endocrinol. 2007;157(2):225–32. doi: 10.1530/EJE-07-0188. doi: 10.1530/eje-07-0188. [DOI] [PubMed] [Google Scholar]
- 29.Mason C, Xiao L, Imayama I, Duggan CR, Bain C, Foster-Schubert KE, Kong A, Campbell KL, Wang CY, Neuhouser ML, et al. Effects of weight loss on serum vitamin D in postmenopausal women. Am J Clin Nutr. 2011;94(1):95–103. doi: 10.3945/ajcn.111.015552. doi: 10.3945/ajcn.111.015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rock CL, Emond JA, Flatt SW, Heath DD, Karanja N, Pakiz B, Sherwood NE, Thomson CA. Weight loss is associated with increased serum 25-hydroxyvitamin D in overweight or obese women. Obesity (Silver Spring) 2012;20(11):2296–301. doi: 10.1038/oby.2012.57. doi: 10.1038/oby.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colak A, Toprak B, Dogan N, Ustuner F. Effect of sample type, centrifugation and storage conditions on vitamin D concentration. Biochem Med (Zagreb) 2013;23(3):321–5. doi: 10.11613/BM.2013.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harvey NC, Javaid K, Bishop N, Kennedy S, Papageorghiou AT, Fraser R, Gandhi SV, Schoenmakers I, Prentice A, Cooper C. MAVIDOS Maternal Vitamin D Osteoporosis Study: study protocol for a randomized controlled trial. Trials. 2012;13(1):13. doi: 10.1186/1745-6215-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]