Abstract
Background
Allergic asthma is a globally respiratory inflammatory disease. Influenza virus is a respiratory pathogen that causes yearly epidemics and results in high rates of morbidity and mortality. Patients with allergic asthma had a more severe symptom and a higher mortality when they were infected with influenza virus. Hence, influenza vaccination is recommended for patients with asthma.
Objectives
We evaluated the efficacy and effects of influenza vaccination on allergic asthma in a mouse model.
Methods
Ovalbumin‐immunized mice were inoculated with inactivated influenza virus A/Puerto Rico/8/34 (PR8) as vaccines and morbidity or mortality and allergic asthma features of these mice were analyzed.
Results
Mice inoculated with inactivated PR8 induced high levels of anti‐PR8 IgG2a and upregulation of Toll‐like receptor (TLR) 7. Vaccinated allergic mice were healthy when they were challenged with live influenza virus while none of non‐vaccinated allergic mice survived. Furthermore, inactivated influenza virus vaccine induced neither extra airway inflammation nor asthma features such as IgE, airway hyper‐reactivity, and eosinophilia in allergic mice. Particularly, decreased frequency of immune cell infiltrated airways and Th2 cytokines IL‐4 and IL‐6 production in the bronchoalveolar lavage fluid were noted in vaccinated allergic mice. These results suggested that inactivated influenza virus vaccine is efficient to protect allergic mice from further influenza infection, and it does not exacerbate but reduces IL‐4 and IL‐6 of allergic asthma.
Conclusion
Influenza vaccination is essential and efficient for allergic subjects to protect influenza virus infection.
Keywords: asthma, IL‐4, IL‐6, PR8, vaccine
Introduction
Allergic asthma is a respiratory inflammatory disease characterized by reversible airway obstruction, airway hyper‐responsiveness, airway inflammation, and mucus hypersecretion in atopic patients. The common hallmarks of allergic asthma are the production of allergen‐specific IgE and the increased presence of eosinophils and T helper 2 (Th2) cells in the airway. Th2 cells play a major role in the development of asthma by producing cytokines such as interleukin (IL)‐4, IL‐5, and IL‐13 to enhance IgE class switching, mast cell production, goblet cell hyperplasia, mucus hypersecretion and the maturation, activation, and accumulation of eosinophils.1, 2 In addition, increased activity of Th17 or Th9 as well as decreased regulatory T cells represents additional mechanisms to contribute to asthma which may skew the system toward an increased Th2 response.3
Due to the great improvement of detection techniques including the development of highly sensitive and specific molecular diagnosis, there is considerable evidence that respiratory viral infection is linked to the initial development as well as exacerbations of asthma.4, 5 Many different viruses are associated with these episodes, particularly respiratory syncytial virus, influenza viruses, parainfluenza viruses, and rhinoviruses.3, 6 Despite extensive association of common types of respiratory viruses with asthma, there is no evidence yet to prove viral infection is a cause of asthma, but suggests that there may be common susceptibilities to both viral infection and asthma.3, 7, 8, 9, 10 Influenza viruses, an orthomyxovirus, are single‐stranded negative sense RNA viruses with three major types A, B, and C, and multiple subtypes. Upon infection of host cells by influenza virus, viral RNAs are sensed by pattern recognition receptors (PRRs), such as Toll‐like receptor 7 (TLR7).11, 12 TLR7 is important not only for the activation of the innate antiviral response but also for the induction of adaptive immunity.13 The recognition of influenza viruses by plasmacytoid dendritic cells through TLR7 results in their activation of costimulatory molecules and production of IFN‐α,11, 12 which is a critical cytokine for establishing an antiviral state and bridging the innate and adaptive immune systems.14 In addition, whole inactivated influenza virus also activates dendritic cells through the engagement of TLR7.15, 16 Of note, influenza A virus infection represents a significant public health threat, particularly in the case of children, the elderly, and those with underlying diseases, all of whom are at a significantly increased risk of disease complications and death following influenza virus infection.17, 18, 19 In fact, atopy itself may have more severe respiratory viral infection and associated wheezing, particularly in rhinovirus infection,20, 21 suggesting that virus–allergy interaction is at work in at least some asthmatics.20, 22, 23, 24
While yearly influenza vaccination is widely recommended for patients with asthma, the efficacy of protection of influenza infection and the benefits of influenza vaccination in preventing asthma exacerbations are unclear. In this study, we examined the efficacy and effects of influenza vaccination on an ovalbumin (OVA)‐immunized mouse model of allergic asthma by using inactivated influenza virus vaccine. To rule out the possible effects induced by commercial vaccine formulation such as excipients, chicken egg components, and formaldehyde, we chose UV‐inactivated virus as the vaccine. Our results demonstrated that influenza vaccinated OVA‐immunized mice got protection from influenza virus infection while non‐vaccinated mice did not. Moreover, influenza vaccination did not induce extra airway inflammation but reduced cell infiltration to airways and Th2 cytokines IL‐4 and IL‐6 production in the bronchoalveolar lavage fluid (BALF) of OVA‐immunized and challenged mice.
Materials and methods
Mice
Female BALB/c mice, 6–8 weeks old, were obtained from the National Laboratory Animal Center and maintained in the Animal Center of the College of Medicine, National Taiwan University. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Taiwan University. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of National Taiwan University College of Medicine and College of Public Health (Permit Number: 20100436).
Allergic asthma model
The protocol for establishing mouse model of allergic asthma was modified by our previous report.25 Briefly, BALB/c mice were immunized intraperitoneally with a total volume of 200 μl containing 50 μg of ovalbumin (OVA) (Grade V; Sigma Chemical Co., St. Louis, MO, USA) emulsified in 2 mg of aluminum hydroxide (AlumImmject; Pierce Chemical, Rockford, IL, USA) on day 0. On days 7, 14, and 21, mice were boosted with 25 μg of OVA in 2 mg of aluminum hydroxide. On days 42 and day 43, mice were intranasally challenged with 100 μg of OVA and then euthanized for following immunological assay (Figure 3A).
Figure 3.
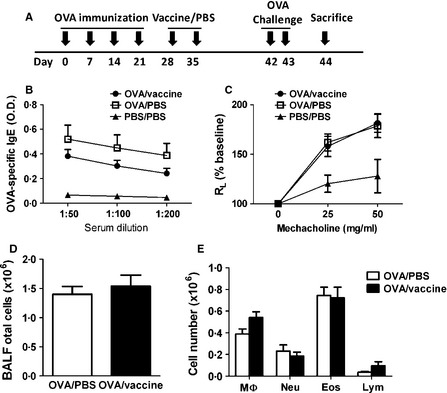
Inactivated influenza vaccination did not change serum levels of anti‐OVA IgE, airway hyper‐reactivity, and inflammatory cell infiltration of allergic asthma mice. (A) Experimental protocol. Mice were immunized with OVA/Alum on days 0, 7, 14, and 21, inoculated with inactivated PR8 influenza virus or PBS on days 28 and 35, and then challenged with intranasally OVA on days 42 and 43. Mice were euthanized on day 44. (B) Serum levels of anti‐OVA IgE were measured by ELISA. (C) Airway function was measured by lung resistance (RL). (D) BAL total cell numbers and (E) the absolute numbers of macrophages (MΦ), neutrophils (Neu), eosinophils (Eos), and lymphocytes (Lym) were obtained by counting cells on a cytocentrifuged preparation. The absolute numbers were calculated by multiplying the respective frequencies by the absolute number of BAL cells. N = 9 mice per group.
PR8 influenza virus preparation
The laboratory‐adapted A/Puerto Rico/8/1934 (H1N1) [PR8] influenza virus was chosen in this study. Mice are the most widely used in the preclinical research. However, most inbred mouse strains are resistant to disease following infection with most primary human influenza virus isolates.26 For this reason, influenza virus strains that have previously been adapted through serial passage in mice are most often used. In particular, the majority of influenza virus research in mice employs either BALB/c or C57BL/6 strains in conjunction with the laboratory‐adapted A/Puerto Rico/8/1934 (H1N1) [PR8] or A/WSN/1933 (H1N1) [WSN] influenza viruses.27, 28 In addition, PR8 could induce a strong immune response in mouse.29 Hence, we chose laboratory‐adapted PR8 influenza virus to perform the study. Influenza virus PR8 were cultured in Madin–Darby canine kidney (MDCK) cells and purified by sucrose gradient (4°C, 20 000 rpm for 2 hours). MDCK cells were maintained in Dulbecco's modified Eagle's medium (DMEM) plus 10% fetal bovine serum. The virus titer was determined by standard plaque assay on MDCK cells and hemagglutination assay. Our result showed that 1 hemagglutination unit (HAU) was equal to 105 PFU in our virus stock. For preparation of inactivated influenza vaccine, PR8 virus stocks were exposed to ultraviolet light (UV) for 30 minutes. The loss of infectivity of UV‐inactivated PR8 virus was confirmed by plaque assay (data not shown).
Measurement of serum PR8‐specific IgG2a and IgG1
Serum PR8‐specific IgG2a and IgG1 were measured by enzyme‐linked immunosorbent assay (ELISA). Briefly, plates were coated with purified UV‐inactivated PR8 virus at 105 PFU/ml in carbonate buffer (pH 9·6) at 4°C for overnight. Microtiter wells were blocked with 1% BSA in PBS for 1 hour, followed by the addition of 1:500 diluted sera. Plates were washed with PBS‐Tween followed by the addition of horseradish peroxidase (HRP)‐conjugated goat anti‐mouse IgG2a (1:2000) or IgG1 (1:1000) (BD Biosciences, San Diego, CA, USA). The microtiter plates were incubated for another hour, and immunoreactivity was detected by measuring the optical density (O.D.) at 450 nm after exposure for 15 minutes to tetramethylbenzidine (TMB) substrate (Clinical Science Products, Mansfield, MA, USA). Known positive and negative samples were included in each assay.
Measurement of serum OVA‐specific IgE
OVA‐specific IgE was measured by enzyme‐linked immunosorbent assay (ELISA). Briefly, OVA at 10 μg/mL in carbonate buffer (pH 9·6) was coated onto ELISA plates at 4°C for overnight, and blocked with 1% BSA for 1 hour. Diluted sera (1:200) were added and incubated for 2 hours at room temperature. The microtiter plates were washed with PBS‐Tween followed by the addition of horseradish peroxidase (HRP)‐conjugated goat anti‐mouse IgE (1:2000) (BD Biosciences). The microtiter plates were incubated for another hour, and immunoreactivity was detected by measuring the optical density (O.D.) at 450 nm after exposure for 15 minutes to TMB substrate (Clinical Science Products). Known positive and negative samples were included in each assay.
Quantitative RT‐PCR analysis for TLR‐7
Eighteen hours after intramuscular influenza virus vaccine inoculation, total RNA was isolated from muscle tissue using Trizol reagent (Invitrogen Life Technologies). Total RNA was reverse transcribed to cDNA with random primers using the Reverse Transcription System kit following manufacturer's instructions (Thermo Scientific Fermentas, Rockford, IL, USA). PCR was performed in triplicate on a 7500 Fast Real‐Time PCR System (Applied Biosystems). β‐actin served as internal controls and H2O served as a negative control. SYBR Green DNA‐binding dye was used in the amplification reactions included oligonucleotide primers for TLR‐7 gene and β‐actin. Fluorescence signals were analyzed during each of 35 cycles (denaturation 1 minute at 95°C, annealing 1 minute at 55°C, and extension 1 minute at 72°C). TLR7 mRNA expression values were normalized to internal controls, β‐actin. The primer sequences used for amplification of mouse TLR7 are forward: 5′‐CCA TTT TGA AAG AAA ACT GA‐3′, reverse: 5′‐CTC TGG GAT ATT TAC TTT AA‐3′.
Determination of the airway function
Airway function was measured by measuring the changes of lung resistance in response to aerosolized methacholine (Sigma‐Aldrich) using the Buxco Pulmonary Mechanics System (Buxco Electronics, Wilmington, NC, USA) as described previously.30 Data were expressed as the lung resistance (RL) in the ratio of RL after PBS nebulization of three independent experiments.
Analysis of cellular composition and cytokine production in the BALF
Bronchoalveolar lavage fluid was collected as previously described to analyze cellular components and cytokine production in the lung.25 Briefly, mice were sacrificed, and tracheas were immediately lavaged three times via a 20‐gauge catheter with 1 ml of HBSS (free of ionized calcium and magnesium). Supernatants of the first BALF were collected for cytokine production assays. Cell numbers in the BALF were counted using a standard hemocytometer. Differential cell counts were performed by counting at least 400 cells in cytocentrifuged preparations (Shandon, Pittsburgh, PA, USA), stained with Liu's stain and then differentiating them by standard morphological criteria. Quantification of IFN‐γ, IL‐4, IL‐5, IL‐6, and eotaxin in the BALF was performed by using commercially ELISA kits (Duoset; R&D, Minneapolis, MN, USA).
Histopathology
Lungs were excised and immediately fixed with 10% buffered formalin solution for 2 days at room temperature. Paraffin‐embedded tissue blocks were then cut into 5‐μm slices for routine hematoxylin and eosin (H&E) staining. Airway infiltration was scored as the frequency of infiltrative airways by counting the numbers of infiltrative airways and dividing by the total numbers of airways in each slice.
Statistical analysis
Results are expressed as the mean ±standard error of the mean (SEM). All graphing and statistical analyses were performed using the Prism graphing program (GraphPad Software, San Diego, CA, USA). P‐values were calculated using a two‐tailed unpaired Mann–Whitney test except Figure 2E. The survive curve in Figure 2E was evaluated using log‐rank test. Significance levels were set at a P‐value of 0·05.
Figure 2.
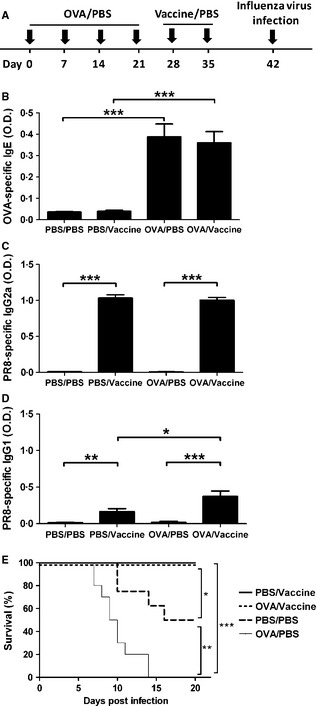
Inactivated influenza virus vaccine protected allergic mice from influenza infection. (A) Experimental protocol. Mice were immunized with OVA/Alum or PBS on days 0, 7, 14, and 21, inoculated with inactivated PR8 influenza virus or PBS on days 28 and 35, and challenged with live influenza virus on day 42. (B) Sera were collected on day 27 and OVA‐specific IgE were measured by ELISA. (C) Sera were collected on day 41 and PR8‐specific IgG2a were measured by ELISA. (D) Sera were collected on day 41 and PR8‐specific IgG1 were measured by ELISA. (E) Survival of mice challenged with live influenza virus was measured. Mice were observed for 20 days after influenza infection. N = 10 mice per group. *P < 0·05; **P < 0·01; ***P < 0·005.
Results
Intramuscular injection of inactivated PR8 influenza virus induced high levels of IgG2a and expression of TLR7
At first, we determined whether UV‐inactivated PR8 influenza virus could induce immune responses in mice and the best route of inoculation. As shown in Figure 1A, UV‐inactivated PR8 influenza virus could induce anti‐PR8 IgG2a in mice receiving intramuscular, intranasal, and subcutaneous inoculation. Of note, mice receiving intramuscular inoculation of inactivated PR8 influenza virus had the highest serum levels of anti‐PR8 IgG2a. There were no increased inflammatory cells in lung of mice receiving intramuscular and subcutaneous inoculation of inactivated PR8 influenza virus. However, cell numbers in BALF were significantly increased in mice receiving intranasal injection (Figure 1B). Previous study showed that the immunogenicity of the whole inactivated vaccine against influenza is dependent on MyD88‐dependent TLR7 signaling.16 We then detected the TLR7 expression and found that mice intramuscularly inoculated with inactivated PR8 virus expressed high levels of TLR7 (Figure 1C). These results demonstrated that UV‐inactivated PR8 influenza virus was immunogenic in mice and intramuscular inoculation of virus vaccine induced the strongest immune response.
Figure 1.
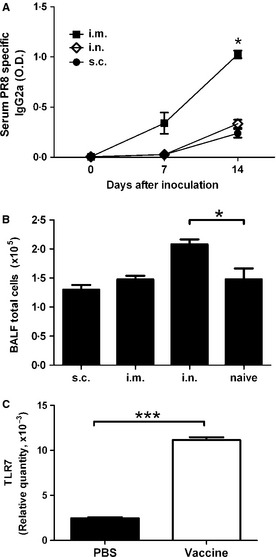
Intramuscular inoculation of inactivated PR8 influenza virus (10 HAU/mouse) induced the highest production of anti‐PR8 IgG2a and upregulated TLR7 expression. (A, B) Mice were inoculated with inactivated PR8 influenza virus on days 0 and 7 by different routes. (A) Serum anti‐PR8 IgG2a was measured on days 0, 7 and 14 by ELISA. (B) BAL total cells were counted. i.m., intramuscular; i.n., intranasal; s.c. subcutaneous. N = 4–5 mice per group. (C) Mice were received intramuscular injection of PBS or inactivated PR8 influenza virus. After 18 hours, the muscles near the site of injection were isolated and expression of TLR‐7 was detected by quantitative RT‐PCR. Relative mRNA was calculated by normalizing the values of the TLR7 to that of β‐actin. *P < 0·05; ***P < 0·005.
Inactivated influenza vaccine protected allergic mice from influenza virus infection
To investigate the efficacy of influenza vaccination on allergic subjects, allergic mice were inoculated with UV‐inactivated PR8 influenza virus, intranasally challenged with live influenza virus and determined morbidity or mortality of mice. Allergic mice were mice immunized with OVA/alum once a week for 4 weeks (Figure 2A) and were confirmed by high production of serum OVA‐specific IgE (Figure 2B). Vaccinated mice was confirmed by producing high levels of serum anti‐PR8 IgG2a (Figure 2C). Normal and allergic mice vaccinated with or without inactivated PR8 virus were challenged with live PR8 virus, and the survival of these mice was determined. As shown in Figure 2E, normal mice without vaccination were found dead beginning on day 10 post‐live PR8 virus challenge and 50% of mice were dead 20 days post‐infection (PBS/PBS group). Allergic mice without vaccination were found dead beginning on day 7 post‐live PR8 virus infection and all dead after 14 days of infection (OVA/PBS group). In contrast, vaccinated allergic mice and vaccinated normal mice were all alive and healthy at least 20 days after live PR8 virus challenge (OVA/vaccine and PBS/vaccine groups). These results suggested that allergic mice were more vulnerable to influenza virus infection than non‐allergic mice. Fortunately, vaccination can protect allergic mice from death of influenza virus infection. To understand whether a strong OVA/alum immunized Th2‐associated immune response would affect the Th1/Th2 anti‐influenza virus immune response after vaccination, we measured anti‐PR8 IgG2a (Th1 immune response) and IgG1 (Th2 immune response) of vaccinated allergic and normal mice. As shown in Figure 2C and D, serum levels of anti‐PR8 IgG2a were not different in vaccinated allergic and normal mice. Compared to the levels of anti‐PR8 IgG2a, vaccinated mice produced far lower levels of anti‐PR8 IgG1. However, vaccinated allergic mice had a higher level of anti‐PR8 IgG1 than vaccinated normal mice (OVA/vaccine and PBS/vaccine groups). These results suggested that a strong OVA/alum immunized Th2‐associated immune response enhanced anti‐influenza virus Th2‐associated IgG1 production but did not change the Th1‐associated anti‐influenza virus IgG2a production.
Inactivated influenza vaccination reduced allergic IL‐4 and IL‐6 production in the BALF of allergic mice
To investigate whether influenza vaccination affect allergic immune responses, we analyzed asthma features of vaccinated allergic mice and compared with that of PBS‐injected allergic mice. OVA‐immunized mice were inoculated with inactivated influenza vaccine. Seven days after last vaccine inoculation, mice were challenged with intranasal OVA and their asthma features were measured (Figure 3A). Serum levels of OVA‐specific IgE in vaccinated mice were slightly lower than non‐vaccinated mice but not significant (Figure 3B). There were no differences in the airway hyper‐responsiveness and airway inflammation in vaccinated allergic mice and control mice (Figure 3C–E). However, decreased frequency of airways infiltrated with immune cells was observed in vaccinated allergic mice (Figure 4A and B). BALF cytokines IL‐4 and IL‐6 production were significantly decreased in vaccinated allergic mice compared to control mice while no difference in IL‐5 and eotaxin production between two groups were observed (Figure 5A–D). In addition, IFN‐γ production in vaccinated allergic mice was as low as PBS‐injected allergic mice (Figure 5E).
Figure 4.
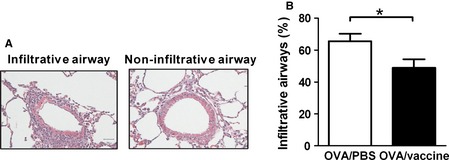
Inactivated influenza vaccination reduced the frequency of infiltrative airways of allergic asthma mice. Mice were immunized with OVA/Alum, inoculated with inactivated PR8 influenza virus or PBS, and challenged with intranasally OVA. Mice were euthanized on day 44 as shown in Figure 3A. (A) Representative H&E stain of lung tissue. (B) The percentages of infiltrative airways in mice. N = 9 mice per group. *P < 0·05.
Figure 5.
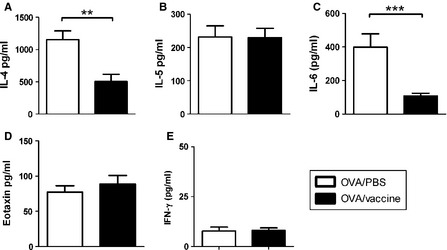
Inactivated influenza vaccination reduced IL‐4 and IL‐6 in the BALF of allergic asthma mice. Mice were immunized with OVA/Alum, inoculated with inactivated PR8 influenza virus or PBS, and challenged with intranasally OVA. Mice were euthanized on day 44 as shown in Figure 3A. BALF cytokines (A) IL‐4, (B) IL‐5, (C) IL‐6, (D) eotaxin, and (E) IFN‐γwere determined by ELISA. N = 9 mice per group. **P < 0·01; ***P < 0·005.
Discussion
In this study, we determined the efficacy and effects of inactivated influenza virus vaccine on an OVA‐immunized mouse model of allergic asthma. Our results demonstrated that mice inoculated with inactivated influenza virus vaccine induced high levels of anti‐PR8 IgG2a and upregulation of TLR7. Vaccinated allergic mice were healthy while non‐vaccinated allergic mice were all dead after subsequent influenza virus challenge. Inactivated influenza virus vaccine neither induced airway inflammation nor changed asthma features such as IgE, airway hyper‐responsiveness, and eosinophilia in allergic mice. Of note, decreased frequency of airways infiltrated with immune cells was observed in vaccinated allergic mice. Th2 cytokines IL‐4 and IL‐6 production in the BALF of allergic mice inoculated with inactivated influenza virus was decreased. These results suggested that UV‐inactivated influenza virus vaccine is efficient in protecting allergic mice from influenza infection, and it does not exacerbate but reduce some features of allergic asthma.
A growing number of epidemiologic studies have shown that certain respiratory viral infections such as respiratory syncytial virus, rhinovirus, and influenza virus are associated with increased atopy to common allergens and an overall increased risk of asthma.7, 8, 9, 17, 20, 21, 23 In the recent study on H1N1 influenza virus, Kloepfer et al.4 reported that increased susceptibility to H1N1 infection and greater hospitalization rates for those with asthma were observed and that H1N1 accounted for 23% of episodes of loss of asthma control during the peak viral season. Previous studies demonstrated that respiratory viral Th1 responses did not down regulate Th2 immune response but can positively regulate Th2 responses and exacerbate allergic Th2‐type diseases.7, 9, 17 Moreover, respiratory viral infection in atopic patients initiates an atopy‐dependent cascade that amplifies and sustains airway inflammation and then drives cumulative airway tissue damage.8, 23 Here, our study showed that allergic mice were more vulnerable to influenza infection like allergic patients, while vaccination can protect them from infection. A survey study in pediatric patients shows that influenza vaccination is associated with fewer asthma exacerbations. After controlling for several potential confounding variables, administration of influenza vaccine is associated with a protective effect against indicators of asthma exacerbations.5 Hence, these findings reinforce the influenza vaccination is essential and efficient for allergic subjects to prevent further infection.
Chirkova et al.'s31 study shows that intranasal administration with live attenuated influenza vaccine during the remission phase of bronchial asthma did not enhance allergic inflammatory changes in the lung, OVA‐specific IgE, and IL‐4 production by spleen lymphocytes. In addition, after additional OVA exposure, histological and immunological changes in these mice were the same as in the control group. Using intranasal instillation of inactivated influenza virus before allergen sensitization, Minne et al.32 show that vaccination significantly reduced the serum levels of total and OVA‐specific IgE as well as allergen induced airway hyper‐reactivity. Moreover, only slight and short‐term local inflammatory responses are shown in intranasal instillation of inactivated influenza virus, whereas immunogenic efficacy of vaccine is still maintained.33 These results, coupled with our data herein, suggest that influenza vaccination is safe and does not exacerbate but reduce when encountering subsequent contact of asthma sufferers with allergen.
While Th2 cells promote airway inflammation in asthma, it has been proposed that Th1 cells, which secrete IFN‐γ, protect against allergic disease by dampening the activity of Th2 effector cells, which secrete IL‐4, IL‐5 and IL‐6. The immune balance controlled by Th1 and Th2 is crucial for immunoregulation, and its imbalance causes various immune diseases including allergic asthma.2, 34 In this study, we found BALF Th2 cytokines IL‐4 and IL‐6 were significantly decreased in vaccinated allergic mice. However, no increased production of IFN‐γ was observed. In addition, we found a strong OVA/alum immunized Th2‐associated immune response did affect following immune responses against influenza virus to produce more IgG1. However, the increased anti‐PR8 IgG1 neither enhance allergic immune response nor reduce the efficacy of vaccination. Hence, the decreased IL‐4 in vaccinated allergic mice was not due to the counter balance by Th1 cytokine. IL‐4, which induces differentiation of Th2 cell and class switching of B cell, plays an important role in allergic asthma.1 IL‐6, a proinflammatory cytokine, prevents Th1 differentiation by upregulating suppressor of cytokine signaling 1 (SOCS1) expression in activated CD4+ T cells, thereby interfering with signal transducer and activator of transcription 1 (STAT1) phosphorylation induced by IFN‐γ35 In addition, IL‐6 induces the production of IL‐4 in activated CD4+ T cells, which promotes the differentiation of these cells into effector Th2 cells;36 these observations suggest that decrease in both IL‐4 and IL‐6 in vaccinated mice in this study may be due to the decrease in IL‐6 which then leads to the decrease of IL‐4.
Many pathogens, including bacteria and viruses, have been shown to be recognized by Toll‐like receptors (TLRs) of innate immune cells. Influenza virus is recognized by TLR7.11, 12 In this study, we found that high levels of TLR7 expression in mice inoculated with inactivated PR8 virus. This is consistent with previous study that the immunogenicity of the whole inactivated vaccine against influenza is dependent on MyD88‐dependent TLR7 signaling.16 In addition, administration of TLR7 agonists reduces serum IgE, IL‐4, airway hyper‐reactivity, and airway inflammation in murine models of allergic airway disease.37, 38, 39, 40 These protective effects of TLR7 agonists in allergic asthma are mediated through type I interferons,37 IL‐12 and IL‐10,40 or TGF‐β.39 Taken together, inactivated influenza virus vaccination induces the expression of TLR7, which then reduces IL‐4 in allergic asthma.
In summary, our findings clearly indicate that vaccination with inactivated influenza virus can protect allergic mice from influenza infection. In contrast to exacerbation, influenza vaccination seems to downregulate the allergic airway inflammatory responses maybe through TLR7 signaling.
Jian et al (2013) Inactivated influenza virus vaccine is efficient and reduces IL‐4 and IL‐6 in allergic asthma mice. Influenza and Other Respiratory Viruses 7(6), 1210–1217.
References
- 1. Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol 2008; 8:218–230. [DOI] [PubMed] [Google Scholar]
- 2. Chuang YH, Yang YH, Wu SJ, Chiang BL. Gene therapy for allergic diseases. Curr Gene Ther 2009; 9:185–191. [DOI] [PubMed] [Google Scholar]
- 3. Holtzman MJ. Asthma as a chronic disease of the innate and adaptive immune systems responding to viruses and allergens. J Clin Invest 2012; 122:2741–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kloepfer KM, Olenec JP, Lee WM et al Increased H1N1 Infection Rate in Children with Asthma. Am J Respir Crit Care Med 2012; 185:1275–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ong BA, Forester J, Fallot A. Does influenza vaccination improve pediatric asthma outcomes? J Asthma 2009; 46:477–480. [DOI] [PubMed] [Google Scholar]
- 6. Glezen WP. Asthma, influenza, and vaccination. J Allergy Clin Immunol 2006; 118:1199–1206. [DOI] [PubMed] [Google Scholar]
- 7. Jackson DJ, Johnston SL. The role of viruses in acute exacerbations of asthma. J Allergy Clin Immunol 2010; 125:1178–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Al‐Garawi AA, Fattouh R, Walker TD et al Acute, but not resolved, influenza A infection enhances susceptibility to house dust mite‐induced allergic disease. J Immunol 2009; 182:3095–3104. [DOI] [PubMed] [Google Scholar]
- 9. Dahl ME, Dabbagh K, Liggitt D, Kim S, Lewis DB. Viral‐induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nat Immunol 2004; 5:337–343. [DOI] [PubMed] [Google Scholar]
- 10. Stein RT, Martinez FD. Respiratory syncytial virus and asthma: still no final answer. Thorax 2010; 65:1033–1034. [DOI] [PubMed] [Google Scholar]
- 11. Lund JM, Alexopoulou L, Sato A et al Recognition of single‐stranded RNA viruses by Toll‐like receptor 7. Proc Natl Acad Sci USA 2004; 101:5598–5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7‐mediated recognition of single‐stranded RNA. Science 2004; 303:1529–1531. [DOI] [PubMed] [Google Scholar]
- 13. Koyama S, Ishii KJ, Kumar H et al Differential role of TLR‐ and RLR‐signaling in the immune responses to influenza A virus infection and vaccination. J Immunol 2007; 179:4711–4720. [DOI] [PubMed] [Google Scholar]
- 14. Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol 2002; 14:432–436. [DOI] [PubMed] [Google Scholar]
- 15. Geeraedts F, Bungener L, Pool J, ter Veer W, Wilschut J, Huckriede A. Whole inactivated virus influenza vaccine is superior to subunit vaccine in inducing immune responses and secretion of proinflammatory cytokines by DCs. Influenza Other Respi Viruses 2008; 2:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Geeraedts F, Goutagny N, Hornung V et al Superior immunogenicity of inactivated whole virus H5N1 influenza vaccine is primarily controlled by Toll‐like receptor signalling. PLoS Pathog 2008; 4:e1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Doherty PC, Turner SJ, Webby RG, Thomas PG. Influenza and the challenge for immunology. Nat Immunol 2006; 7:449–455. [DOI] [PubMed] [Google Scholar]
- 18. Papadopoulos NG, Christodoulou I, Rohde G et al Viruses and bacteria in acute asthma exacerbations–a GA(2) LEN‐DARE systematic review. Allergy 2011; 66:458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nguyen‐Van‐Tam JS, Openshaw PJ, Hashim A et al Risk factors for hospitalisation and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May‐September 2009). Thorax 2010; 65:645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jackson DJ, Evans MD, Gangnon RE et al Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med 2012; 185:281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carroll KN, Gebretsadik T, Minton P et al Influence of maternal asthma on the cause and severity of infant acute respiratory tract infections. J Allergy Clin Immunol 2012; 129:1236–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olenec JP, Kim WK, Lee WM et al Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol 2010; 125:1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Subrata LS, Bizzintino J, Mamessier E et al Interactions between innate antiviral and atopic immunoinflammatory pathways precipitate and sustain asthma exacerbations in children. J Immunol 2009; 183:2793–2800. [DOI] [PubMed] [Google Scholar]
- 24. Holt PG, Rowe J, Kusel M et al Toward improved prediction of risk for atopy and asthma among preschoolers: a prospective cohort study. J Allergy Clin Immunol 2010; 125:653–659. [DOI] [PubMed] [Google Scholar]
- 25. Chuang YH, Wang TC, Jen HY, Yu AL, Chiang BL. alpha‐Galactosylceramide‐induced airway eosinophilia is mediated through the activation of NKT cells. J Immunol 2011; 186:4687–4692. [DOI] [PubMed] [Google Scholar]
- 26. Itoh Y, Shinya K, Kiso M et al In vitro and in vivo characterization of new swine‐origin H1N1 influenza viruses. Nature 2009; 460:1021–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bouvier NM, Lowen AC. Animal Models for Influenza Virus Pathogenesis and Transmission. Viruses 2010; 2:1530–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quan FS, Steinhauer D, Huang C, Ross TM, Compans RW, Kang SM. A bivalent influenza VLP vaccine confers complete inhibition of virus replication in lungs. Vaccine 2008; 26:3352–3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Surls J, Nazarov‐Stoica C, Kehl M, Casares S, Brumeanu TD. Differential effect of CD4 + Foxp3 + T‐regulatory cells on the B and T helper cell responses to influenza virus vaccination. Vaccine 2010; 28:7319–7330. [DOI] [PubMed] [Google Scholar]
- 30. Chang CJ, Yang YH, Liang YC et al A novel phycobiliprotein alleviates allergic airway inflammation by modulating immune responses. Am J Respir Crit Care Med 2011; 183:15–25. [DOI] [PubMed] [Google Scholar]
- 31. Chirkova T, Petukhova G, Korenkov D, Naikhin A, Rudenko L. Immunization with live influenza viruses in an experimental model of allergic bronchial asthma: infection and vaccination. Influenza Other Respi Viruses 2008; 2:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Minne A, Jaworska J, Gerhold K et al Intranasal delivery of whole influenza vaccine prevents subsequent allergen‐induced sensitization and airway hyper‐reactivity in mice. Clin Exp Allergy 2007; 37:1250–1258. [DOI] [PubMed] [Google Scholar]
- 33. Minne A, Huaux F, Jaworska J, Rha RD, Hamelmann E, Vanbever R. Safety evaluation of pulmonary influenza vaccination in healthy and “asthmatic” mice. Vaccine 2008; 26:2360–2368. [DOI] [PubMed] [Google Scholar]
- 34. Elser B, Lohoff M, Kock S et al IFN‐gamma represses IL‐4 expression via IRF‐1 and IRF‐2. Immunity 2002; 17:703–712. [DOI] [PubMed] [Google Scholar]
- 35. Diehl S, Anguita J, Hoffmeyer A et al Inhibition of Th1 differentiation by IL‐6 is mediated by SOCS1. Immunity 2000; 13:805–815. [DOI] [PubMed] [Google Scholar]
- 36. Diehl S, Chow CW, Weiss L et al Induction of NFATc2 expression by interleukin 6 promotes T helper type 2 differentiation. J Exp Med 2002; 196:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xirakia C, Koltsida O, Stavropoulos A et al Toll‐like receptor 7‐triggered immune response in the lung mediates acute and long‐lasting suppression of experimental asthma. Am J Respir Crit Care Med 2010; 181:1207–1216. [DOI] [PubMed] [Google Scholar]
- 38. Meng L, He X, Zhu W et al TLR3 and TLR7 modulate IgE production in antigen induced pulmonary inflammation via influencing IL‐4 expression in immune organs. PLoS ONE 2011; 6:e17252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Van LP, Bardel E, Gregoire S et al Treatment with the TLR7 agonist R848 induces regulatory T‐cell‐mediated suppression of established asthma symptoms. Eur J Immunol 2011; 41:1992–1999. [DOI] [PubMed] [Google Scholar]
- 40. Sel S, Wegmann M, Bauer S, Garn H, Alber G, Renz H. Immunomodulatory effects of viral TLR ligands on experimental asthma depend on the additive effects of IL‐12 and IL‐10. J Immunol 2007; 178:7805–7813. [DOI] [PubMed] [Google Scholar]


