Abstract
Please cite this paper as: He et al. (2013) Amaryllidaceae alkaloids inhibit nuclear‐to‐cytoplasmic export of ribonucleoprotein (RNP) complex of highly pathogenic avian influenza virus H5N1. Influenza and Other Respiratory Viruses 7(6), 922–931.
Background Few drugs are currently licensed to treat influenza A infection, and new therapies are needed, especially for highly pathogenic strains. Traditional medicinal plants, such as Lycoris radiata, are a potential source of new antiviral agents.
Objective To test 15 Amaryllidaceae alkaloids isolated from the bulbs of L. radiata in vitro for antiviral activities against influenza virus type A, A/Chicken/GuangDong/178/2004 (H5N1, 178).
Methods Antiviral activities of the compounds were tested in time‐of‐addition assays, hemagglutination inhibition (HI) assays, neuraminidase (NA) activity assays, and viral entry inhibition assays using H5N1‐HIV pseudoviruses. Effects of the compounds on localization and activity of the viral ribonucleoprotein (RNP) were determined by immunofluorescence and an RNP minigenome assay, respectively.
Results Among the alkaloids, lycorine (AA1), hippeastrine (AA2), hemanthamine (AA3) and 11‐hydroxy vittatine (AA4) exhibited antiviral activities, with EC90 values of 0·52, 82·07, 4·15, and 13·45 μm, respectively. These compounds did not affect the function of the outer membrane proteins or the viral entry process and viral RNP activity. As AA1 and AA3 exhibited stronger antiviral activities, they were further analyzed. Intracellular nucleoprotein (NP) localization showed that AA1 and AA3 inhibited the RNP complex in the nucleus at an early stage of a single‐round and multi‐round of replication.
Conclusion Four Amaryllidaceae alkaloids were first determined that could exert anti‐influenza activities after virus entry into cells. Furthermore, AA1 and AA3 could inhibit nuclear‐to‐cytoplasmic export of the RNP complex of virus replication. Thus, these compounds may be developed further as anti‐influenza drug candidates.
Keywords: Amaryllidaceae alkaloid, H5N1 influenza A virus, vRNP export
Background
Avian influenza virus (AIV) is a negative‐sense, segmented, single‐stranded RNA virus that belongs to the Orthomyxoviridae family. 1 , 2 , 3 Globally, AIVs occasionally cause infection in humans and their livestock, such as poultry, pigs, and other wild animals with harmful outcomes. Some of the highly pathogenic AIV (HPAIV) strains, in particular H5N1, have occasionally infected humans with high mortality rates exceeding 60%. 4 , 5 , 6 As a consequence of high rates of antigenic drift and shift, reassortant strains and novel mutants have emerged, and some have acquired the ability to cross‐species barriers and become pathogenic in their new hosts. 7
Efficacy and practicality of timely vaccinations remain problematic in the worldwide effort to combat influenza, 8 , 9 and strains resistant to drugs (e.g., amantadine) often appear. 10 Thus far, there are four antiviral agents approved by the US Food and Drug Administration (FDA) to treat influenza virus infection, which can be divided to two groups. One is comprised of the M2 ion‐channel inhibitors, such as amantadine and rimantadine, which interfere with viral uncoating within the host cells. The M2 inhibitors are effective only against influenza virus A and are associated with several toxic effects on the digestive and nervous systems. 11 The other group, consisting of such drugs as oseltamivir and zanamivir, are neuraminidase (NA) inhibitors. 9 , 10 , 12 Although oseltamivir and zanamivir have high antiviral activities, they have low bioavailability and adverse effects such as nausea and vomiting. 13 , 14 In light of these issues, new classes of drugs are urgently needed for suitable long‐term use.
Traditional medicinal plants are a potential source of new antiviral agents. The bulbs of Lycoris radiata have long been used as a traditional Chinese medicine in the treatment of laryngeal complications, furuncles, carbuncles, and suppurative wounds. 15 , 16 , 17 Previous phytochemical and pharmacological studies revealed that the major chemical constituents of this plant are alkaloids, which have demonstrated antiviral, antitumor, antimalarial, and acetylcholinesterase‐inhibitory activities. 12 , 18 , 19
To obtain the specific anti‐AIV compounds, the total alkaloids from L. radiata were separated and purified using chromatographic methods by our group. Fifteen alkaloids were isolated and identified on the basis of extensive spectroscopic analysis. Among them, four compounds lycorine (AA1), hippeastrine (AA2), hemanthamine (AA3), and 11‐hydroxy vittatine (AA4) exhibited inhibitory activities against an H5N1 strain 178 in Mardin‐Darby canine kidney (MDCK) cells. In the current study, we investigated the anti‐AIV activities of these Amaryllidaceae alkaloids and the mode of action of the active compounds. Infection experiments in vitro indicated that AA1 and AA3 could inhibit nuclear‐to‐cytoplasm export of the RNP complex in single‐round and multi‐round replication assays, which could be a possible mechanism of their antiviral activities. Therefore, these compounds may be developed as anti‐influenza drug candidates.
Methods
Plant materials
The bulbs of L. radiata were collected from Lanxi City, Zhejiang Province of China, and were identified by Min‐jian Qin of the Department of Pharmacognosy, China Pharmaceutical University, Nanjing, P. R. China. A voucher specimen (No. 2006101201) was deposited in the herbarium of China Pharmaceutical University.
Isolation of Amaryllidaceae alkaloids and compounds
The isolation procedures for alkaloids from L. radiata were previously described. The alkaloid fraction was subjected to repeated column chromatography over silica gel, Sephadex LH‐20, and prep‐HPLC to yield lycorine (AA1), hippeastrine (AA2), hemanthamine (AA3), 11‐hydroxy vittatine (AA4), lycorenine, dihydrolycorine, lycoramine, 9‐O‐demethylhomolycorine, galanthamine, galanthine, O‐methyl‐lycorenine, O‐demethylycoramine, demethymaritidine, ungeremine, and trisphaeridine. The chemical structures were determined by spectroscopic analyses and comparison with published data. The purity of the compounds was determined by high‐performance liquid chromatography (HPLC; purity >90%) and nuclear magnetic resonance (NMR) spectroscopy. The structures of compounds AA1–AA4 are displayed in Figure 1. All these compounds for bioassay were dissolved in DMSO.
Figure 1.
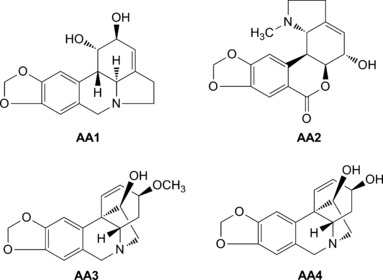
Chemical structures of alkaloids AA1–AA4.
Oseltamivir carboxylate Ro64‐0802 (GS4071) was kindly provided by Hoffmann‐La Roche.
Cells and viruses
MDCK cells (kindly supplied by Ze‐jun Li, Shanghai Veterinary Research Institute, CAAS) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; Cell Culture Bioscience, Hampshire, Tasmania, Austria). The H5N1 influenza virus strain 178 (GenBank Accession No. AY737296‐737300) was isolated from a chicken in Guangdong, China, by the MOA Key Laboratory for Animal Vaccine Development, P.R. China. All experiments involving the H5N1 influenza virus were carried out in BSL‐3 facilities.
Cytotoxicity assay
The cytotoxicity and cell‐based antiviral activity of Amaryllidaceae alkaloids and oseltamivir were evaluated using the WST‐1 assay. 20 For cytotoxicity analysis, MDCK cells in 96‐well plates (cell plates, Corning, NY, USA) were incubated with serial two‐fold dilutions of Amaryllidaceae alkaloids (containing <0·5% DMSO) in DMEM (100 μl). After incubation for 72 h, 10 μl of WST‐1 solution (CCK‐8, Dojindo Chemicals, Kumamoto, Japan) was added to each well of the plate. Cells were incubated with 0·01 MOI of virus (100 μl) at 37°C for 1 h and then removed by washing with PBS three times, followed by the addition of serial twofold dilutions of Amaryllidaceae alkaloids (each concentration was lower than the 50% cytotoxic concentration, CC50) in 100 μl per well. After incubation for 72 h, 10 μl of the WST‐1 solution was added to each well of the plate. The optical density (OD)/absorbance was measured 4 h later by scanning at 450 and 630 nm as reference using a Biotek microplate reader (Molecular Devices, Winooski, VT, USA). Three independent experiments were carried out, and each experiment was performed in triplicate. The percentage of viable cells was compared with untreated controls and plotted against the concentration of the four compounds. Linear regression analysis was performed using Microsoft Excel software to calculate the CC50. The HA titer was used to test the 50% and 90% effective concentration (EC50, EC90). The selectivity index (SI) for each Amaryllidaceae alkaloid in Table 1 was calculated by dividing the CC50 by the EC50 (CC50/EC50). 21
Table 1.
Anti‐influenza virus effects of Amaryllidaceae alkaloids by treatment of cells after infection
| Compound | Classification | CC50 (μm) | EC50 (μm) | SI | |
|---|---|---|---|---|---|
| AA1 | Lycorine type | 20·9 ± 0·07 | EC50 EC90 | <0·46 0·52 ± 0·57 | >45 |
| AA2 | Lycorenine type | >317 | EC50 EC90 | 47·5 ± 0·37 82·07 ± 0·33 | >6·7 |
| AA3 | Crinine type | 50 ± 0·12 | EC50 EC90 | 1·48 ± 0·003 4·15 ± 0·052 | 34 |
| AA4 | Crinine type | >278 | EC50 EC90 | 6·7 ± 0·038 13·45 ± 0·57 | >41 |
| Oseltamivir | Neuraminidase inhibitor | >10 | EC50 EC90 | 0·015 ± 0·56 0·625 ± 0·11 | >666·7 |
CC50: mean (50%) value of cytotoxic concentration.
EC50: mean (50%) value of effective concentration.
EC90: mean (90%) value of effective concentration.
SI: CC50/EC50.
Antiviral assay
To detect antiviral activity against H5N1, 15 compounds were tested in pre‐treatment, simultaneous treatment and post‐treatment. The active compounds were chose for the further experiments. To further explore the mechanism of the antiviral activity of the compounds, seven different protocols for delivery were used. MDCK cells were first seeded in 96‐well plates at 1 × 104 cells/well and grown for 24 h. Various protocols were then carried out as follows: (1) As a negative control, the supernatant was replaced with four compounds (AA1: 0·52 μm; AA2: 82·07 μm; AA3: 4·15 μm; AA4: 13·45 μm, 100 μl) for 72 h. (2) As a positive control, after virus adsorption (0·01 MOI) three washes with PBS and incubation with DMEM. To confirm whether the compounds were competing with the virus in binding to the influenza virus receptor, virus mixed with the compounds were incubated at 4°C for 1 h and then inoculated onto cells at 37°C for 1 h, removed and washed, and cultivated the culture medium with or without drug (protocol 4 or 3). (5) For post‐infection treatment, virus was absorbed for 1 h, and then the supernatant was replaced with drug‐DMEM. (6) To determine whether the compounds were competing with the virus in binding to receptor of host cells, virus and various compounds were added together to cells at 37°C for 1 h, and then the supernatant was replaced with or without drug for 72 h (protocol 7 or 6). The oseltamivir (0·625 μm, 100 μl) was added after virus entry as drug control group. The HA titer was tested with hemagglutinin agglutination assay after supernatants of the above seven protocols were collected at different time points (12, 24, 36, 48, 60, and 72 h) to test.
Construction of polI‐fluc plasmid and viral polymerase activity assay
The human RNA polymerase I promoter and the mouse RNA polymerase I terminator were obtained from plasmid pDL obtained from the Key Laboratory of Animal Disease Control and Prevention, Ministry of Agriculture of P.R. China. The primers used for amplification contained EcoRI and PstI sites. The polI vector was generated by insertion of the promoter and terminator into the pGEMT vector. The luciferase genes were amplified from the plasmid pGL3 control vector (Promega, Madison, WI, USA) by PCR amplification. The luciferase gene fluc was cloned in the antisense orientation and flanked by non‐coding regions from segment 5 of influenza virus A/PR/8/34, at the Esp3I sites of polI‐pro‐fluc‐ter to generate the polI‐fluc plasmid. The full‐length cDNAs of PB2, PB1, PA, and NP were then cloned into pDL, which had been digested with Esp3I.
The viral RNP complex plays an important role in viral generation, replication, and pathogenicity. 22 , 23 To determine whether the Amaryllidaceae alkaloid compounds inhibited virus replication via the RNP, four expression plasmids for PB2, PB1, PA, and NP (50 ng each) from virus strain 178 were co‐transfected into 293T cells (12‐well plates) together with polI‐fluc (10 ng, expressing firefly luciferase) and an internal control plasmid pRL‐TK (5 ng, expressing Renilla luciferase, Promega) at 37°C, 5% CO2. At 8 h after transfection, the supernatants were replaced with 500 μl media containing the Amaryllidaceae alkaloid compounds at indicated concentrations (AA1:4·67 μm; AA2:15·20μm; AA3:4·90μm; AA4:16·70μm). At 24 h after transfection, cells were collected for detecting luciferase activities (Promega) using a Genius Pro luminometer.
Time‐of‐addition assay
Pre‐treatment, simultaneous treatment, and post‐treatment of MDCK cells relative to virus infection were used to determine which stages of the viral life cycle were affected by the compounds. The four Amaryllidaceae alkaloid compounds were assayed for virus inhibition in triplicate. After 72 h of incubation in all antiviral assays, the HA titer was determined as above. Monolayers of MDCK cells were grown in 96‐well plates and inoculated with influenza virus at 0·01 MOI (100 μl). The plates were incubated for 1 h at 4°C to ensure the viruses would replicate synchronously (time 0 = after 1‐h virus adsorption period at 4°C) with or without the compounds (100 μl) at different indicated time intervals for the adsorption period (Figure 5). The cultures were then frozen, and the virus yields were determined by HA titer.
Hemagglutination inhibition (HI) assay
The HI assay was carried out as described by Ehrhardt et al. 10 Briefly, serial twofold dilutions (25 μl) of the four Amaryllidaceae alkaloids compounds were prepared and mixed with an equal volume of influenza virus suspension (4 HA units/25 μl). After incubation for 1 h at 4°C, 0·5% (v/v) chicken erythrocytes (50 μl) in PBS were added, and the four samples were incubated for 30 min at room temperature.
NA activity assay
The NA activity was measured in a fluorescence‐based assay using the Neuraminidase Inhibitors Screen kit (Beyotime Institute of Biotechnology, Hai men city, Jiang su Province, China). Ten microliters of a solution of purified N1‐type NA was added to 70 μl of detection buffer, and then 10 μl of a test compound (serial twofold dilutions) was added at 37°C for 2 min, followed by addition of 10 μl of NA substrate at 37°C for 30 min. The fluorescence intensity was measured at an excitation wavelength of 340 nm and an emission wavelength of 535 nm using a microplate reader (GENios Pro, Tecan, Mannedorf, Seestrasse, Switzerland).
Determination of inhibitory activity of test compounds on H5N1 pseudovirus entry
Pseudotyped virus was produced by triple transfection with 2 μg of a plasmid encoding HA of A/Qinghai/59/2005(H5N1), 2 μg of a plasmid encoding NA of A/Thailand/Kan353/2004 (N1‐type), and 3 μg of the HIV backbone plasmid (pNL4‐3.luc.R‐E‐), which contains a luciferase reporter gene and defective viral Env and Vpr genes. 24 The three plasmids were kindly supplied by Shu‐wen Liu (School of Pharmaceutical Sciences, Southern Medical University, Guangzhou, China). At 48 h after transfection using the calcium phosphate precipitation method, 25 the supernatant was harvested and stored at −70°C. The titer of the pseudotyped virus was determined using an ELISA kit (Retro‐Tek, Buffalo, NY, USA) to measure the HIV p24 core structural protein p24. To test the compounds for inhibition of viral entry, MDCK cells were seeded in 96‐well plates (1 × 104/well) for 24 h, and pseudotype particles (1 ng/well) were pre‐incubated with different compounds (serial twofold dilutions, 100 μl) at various concentrations for 30 min at 37°C. The mixture was then transferred to the cells and incubated for an additional 48 h. Before harvesting, cells were washed with PBS three times, and luciferase activity was measured using a luciferase kit (Promega) and a microplate luminometer (Genios Pro).
AIV intracellular RNP localization
To prepare the cells for confocal microscopy to examine the stage of viral infection targeted by the compounds, MDCK cells were plated in DMEM and used in the exponential growth phase. Cells (1 × 106) in 1000 μl were exposed to virus strain 178 at an MOI of 1 and incubated at 4°C for 1 h to allow virus binding. For AA1 and AA3 (1000 μl) treatment, the virus was incubated with the protective Amaryllidaceae alkaloid compounds (AA1, AA3) at 4·67 and 4·90 μm for different times single replication (1, 2, 4, 6, 8, and 12 h p.i.) and multi‐round replication (24, 48 h p.i). The positive control drug LY294002 (Sigma, San Francisco, CA, USA) at 30 μm was removed at 12, 24, 48 h p.i. The cells were fixed with 4% paraformaldehyde for 10 min, permeabilized with 0·25% Triton for 15 min, washed with PBS three times, and incubated with an anti‐AIV NP (Perfect Biotechnology, Beijing, China) monoclonal antibody at 37°C for 1 h. After washing with PBS twice, the cells were incubated with an Alexa Fluor 488‐conjugated goat anti‐mouse IgG (Zhongshan Goldenbridge Biotechnology, Beijing, China) for 40 min in the dark. The cells were washed again and incubated with DAPI for 10 min in the dark to stain nuclei. Specific staining and structure of the cells were observed using a confocal laser scanning microscope (Olympus, Shinjuku, Tokyo, Japan).
Results
Antiviral efficiency and cytotoxicity assay of Amaryllidaceae alkaloids
The replication of influenza virus was inhibited by four active compounds AA1–AA4 after virus entry. The other eleven compounds were not possessed antiviral activity in pre‐treatment, simultaneous treatment, and post‐treatment. We tested the active compounds in detail to explore the antiviral activity. SI values were calculated to classify the selected molecules as inactive (SI < 2), weak (SI = 2–10), moderate (SI = 10–50), or strong (SI > 50) inhibitors (Table 1). The EC90 values of oseltamivir and AA1–AA4 compounds were 0·625 ± 0·11, 0·52 ± 0·57, 82·07 ± 0·33, 4·15 ± 0·052, and 13·45 ± 0·57, respectively.
Inhibitory effects of Amaryllidaceae alkaloids after virus entry into cells
To test the ability of the Amaryllidaceae alkaloids to prevent the attachment of influenza virus to MDCK cells, we added the compounds prior to (pre‐treatment) or simultaneously with virus infection of the cells. 26 With pre‐treatment, the HA titer was not inhibited (data not shown). The results showed that the antiviral effects of the compounds against influenza were not due to the prevention of virus attachment or alteration of the cell receptor. With simultaneous treatment, the results also showed that the antiviral effects against influenza virus were not targeted to the viral surface proteins. The virus replication was inhibited after treated with four Amaryllidaceae alkaloid compounds, AA1 and AA3 exhibited higher antiviral activity.
The HA titer was tested to determined the effect on release of total virus particles from infected cells by treating infected cells with the Amaryllidaceae alkaloid compounds over multiple replication cycles with seven different protocols as shown in Figure 2. When cultured with DMEM‐containing compounds (protocol 4, 5, 7), the HA titer was strong inhibited that no HA titer was detected (AA1, AA3, AA4), no HA titer was detected when added AA2 before 48 h. The result demonstrated that AA1, AA2, AA3, AA4 exerted its effect at a step after virus entry into cells, and the activity of AA2 was lower than other compounds. (Figure 3 A–D). However, the results were very different when comparing between cultures in which the compound was removed or not over the cultivation period. For protocol 3 and 6 in which the cells were incubated in drug‐free DMEM post‐infection, the viral HA titer was similar to that of the positive control (protocol 2). These results demonstrated that the primary effect of the four compounds were by inhibition of the virus after entry into cells, while the antiviral activity of AA2 was lower than other compounds that HA titer was detected after 48 h p.i.
Figure 2.
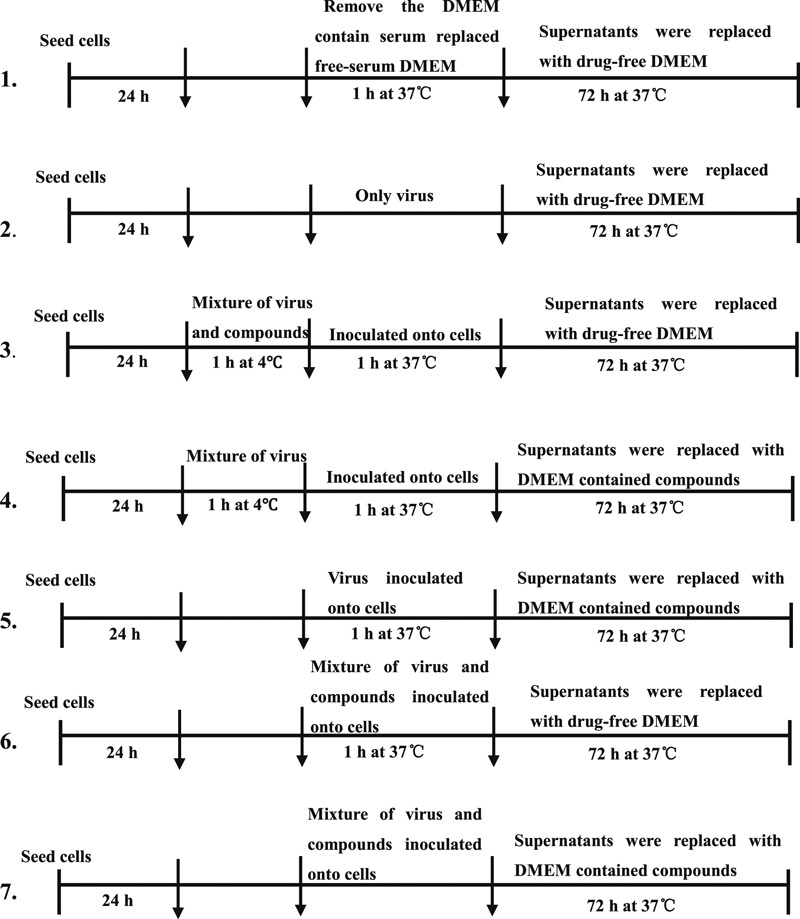
Experimental design of protocols for testing effect of the alkaloid compounds by HA assay at different time points. Virus mixtures contained 0·01 MOI. 1, negative control; 2, virus only as positive control; 3, mixture of virus at 0·01 MOI and different compounds (AA1:0·52 μm; AA2:82·07 μm; AA3:4·15 μm; AA4:13·45 μm, Oseltamivir: 0·625 μm, 100 μl) were incubated at 4°C for 1 h, inoculated onto cells 1 h and then the media were replaced with drug‐free DMEM for 72 h; 4, same as 3, except the replacement media DMEM contained compounds; 5, after adsorption 1 h, the culture containing Amaryllidaceae alkaloids compounds; 6, mixture of virus at 0·01 MOI and Amaryllidaceae alkaloids compounds were added to cells at 37°C for 1 h, and then replaced with drug‐free DMEM for 72 h; 7, same as 6, except the replacement media contained drugs. Supernatants from the above seven protocols were collected at different time points (12, 24, 36, 48, 60 and 72 h) to test the HA titer.
Figure 3.
HA titers of different Amaryllidaceae alkaloid compounds (A, B, C, D corresponding to AA1, AA2, AA3, AA4 and different times of addition were tested. The different protocols (1–7) are illustrated in Figure 2 and described in Methods. Values are shown the means ± SD of the three independent experiments and standardized.
AA1–AA4 do not affect viral RNP activity in vitro
The viral RNP complex has important roles in viral generation, replication, and pathogenicity. 22 , 23 To study the mechanisms underlying the inhibition of influenza virus strain 178 replication by the compounds, the activities of RNP combinations of PB2, PB1, PA, and NP from influenza virus strain 178 were determined by measuring luciferase activities in a minigenome assay. As shown in Figure 4, there were slightly higher luciferase activities with AA1 or AA2, but no change was observed when cultured with AA3, and AA4 showed a slightly but not significantly lower activity (∼90% activity) when compared with no treatment. These results demonstrated that the activity of RNP was not directly inhibited by the four compounds.
Figure 4.
Polymerase activities of combinations of RNP proteins from virus strain 178. 293T cells were transfected in triplicate with the luciferase reporter plasmid polI‐Luc and internal control plasmid Renilla, together with plasmids expressing PB2, PB1, PA and NP from the 178 virus strain. At 8 h p.i. the supernatants were removed and replaced with Optimem containing various concentrations of Amaryllidaceae alkaloids compounds (AA1:4·67 μm; AA2:15·20 μm; AA3:4·90 μm; AA4:16·70 μm, 500 μl) for 24 h, and cell lysates were analyzed to measure firefly and Renilla luciferase activities. Values are shown the means ± SD of the three independent experiments and standardized to those of the influenza virus strain 178 only measured at 37°C (100%).
Effect of time of addition of compounds on HA titer
As the results above showed that no HA titer was detected by treatment of the cells with AA1 and AA3 after infection, time‐of‐addition experiments were carried out to explore in further detail the effects of these two compounds on viral replication. The results of this experiment are summarized in Figure 5. Monolayers of MDCK cells were grown in 24‐well plates, and 0·01 MOI of virus was incubated for 1 h at 4°C to ensure synchronous replication. The administration of AA was according to indicated time intervals, and drug‐free DMEM was replaced after incubation until 72 h. The supernatants were harvest at the end of the incubation period, and the HA titer was tested.
Figure 5.
Effect of the time‐of‐addition of AA1:0·52 μm; AA3:4·15 μm, 100 μl at various times during the replication cycle of influenza virus. Time 0 = post 1 h adsorption period at 4°C. Each value represents the mean ± SEM of three separate assays.
Several concentrations of these compounds were tested to verify that the drugs did not cause cellular injury or toxicity and to optimize the dose for effective inhibition of influenza virus. In Figure 5, the inhibitory effect for AA1 (0·52 μm) against virus was observed after a single round of replication (12 h p.i.), and about 50% of the virus was inhibited when added at 24–72 h. For AA3 (4·15 μm), significant inhibition was observed between 0 and 4 h p.i. The inhibitory activity was not significant when the AA1 or AA3 compound was added at time intervals 5 min–1 h, 15 min–1 h, 30 min–1 h, 1–2 h, 2–4 h, or 4–8 h. The data suggested the two compounds could affect virus replication, and the antiviral activity of the AA1 compound was more significant during a single round of replication.
AA1 and AA3 do not inhibit hemagglutination activity
To verify the results above that the two alkaloids inhibited virus replication after entry, we also evaluated whether the compounds inhibited hemagglutination of influenza virus. None of the four compounds inhibited viral attachment onto cRBCs, indicating that they did not block viral attachment by affecting the HA viral surface protein (data not shown).
AA1 and AA3 do no exhibit inhibitory activities on N1‐type neuraminidase
To determine whether the NA protein is affected by the alkaloids, the activity of purified N1‐type NA was used to test the effect of the two compounds AA1 (4·67 μm) and AA3 (4·90 μm), which demonstrated stronger antiviral activities among the four compounds tested, using a neuraminidase inhibitor screening kit. However, these compounds did not exhibit significant inhibitory activity on the N1‐type neuraminidase. The result suggested that the NA protein was not targeted for inhibition of influenza A virus by AA1 or AA3 (data not shown).
AA1 and AA3 do not inhibit entry of H5N1 pseudoviruses
To further confirm whether the AA1 or AA3 compounds inhibited H5N1 influenza A virus entry, we produced H5N1 pseudoviruses by triple transfection with A/Qinghai/59/2005(H5N1) HA and A/Thailand/Kan353/2004 NA plasmids with an HIV backbone plasmid (pNL4‐3.luc.R‐E‐). The results showed that neither AA1 nor AA3 could prevent virions carrying HA and NA proteins from binding and internalizing into MDCK cells (data not shown).
Amaryllidaceae alkaloids inhibited NP export
To evaluate the effects of AA1 and AA3 on intracellular localization of the viral NP, indirect immunofluorescence staining was performed using an anti‐NP antibody. In infected but no AA‐treated cells (−AA), the nucleocapsid protein (NP, green), which is the main component of the RNP complex, showed a diffuse distribution in the cytoplasm after 8 h p.i in single‐round replication and multi‐round replication (Figure 6A,B), which was compared with positive drug group (LY294002). LY294002 is a derivative of quercetin, a member of flavonoids that could suppress Akt phosphorylation. 27 , 28 , 29
Figure 6.
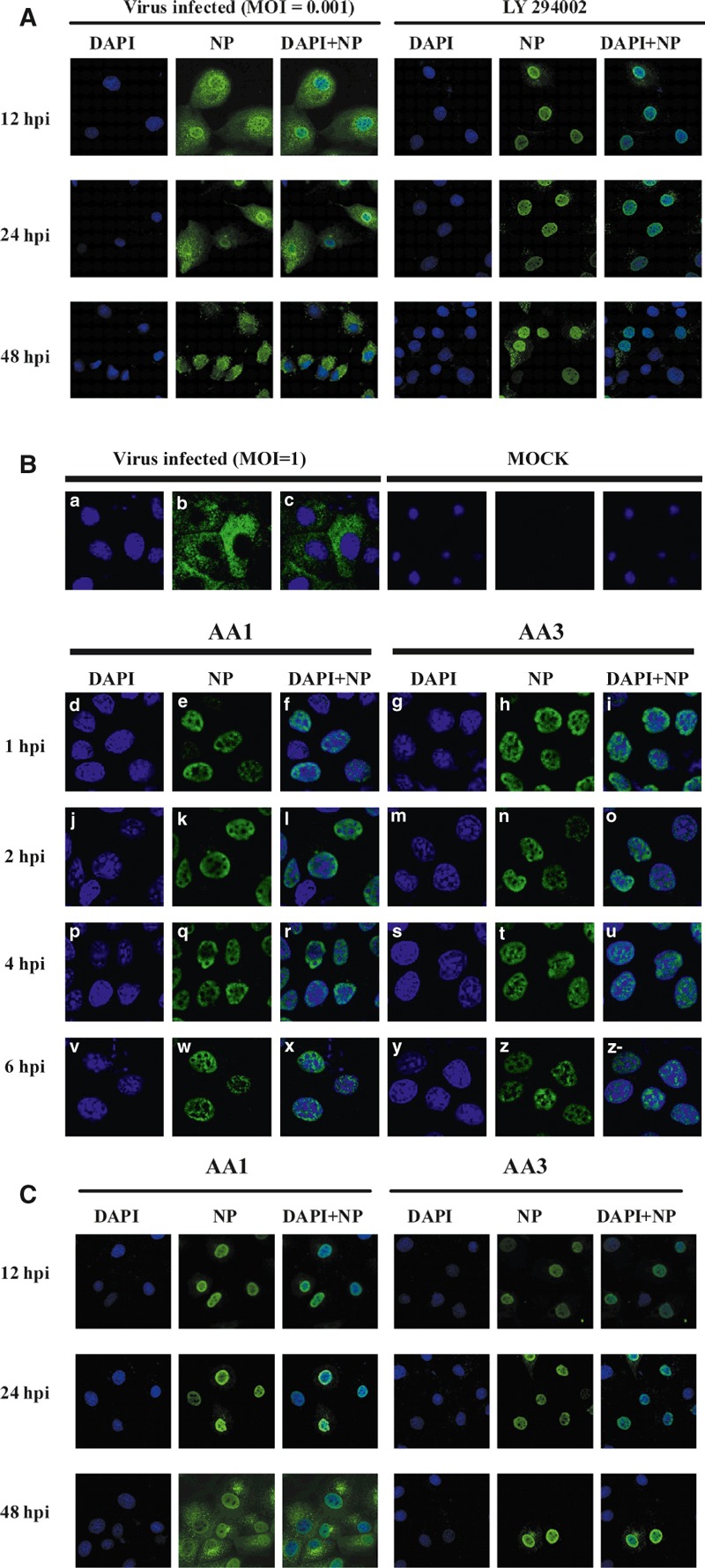
178 H5N1 HPAIV (MOI = 1) and MDCK cells were either untreated or were treated with AA1 or AA3 as follows: (−) AA, normal infection with no AA treatment as control (Figure 6A,B); The location of NP was tested in single replication (8 h p i), cells treated with AA1 (4·67 μm), AA3 (4·90 μm) after 1 h of virus adsorption at 4°C. Infected cells were then incubated in medium with AA1 (d, e, f, j, k, l, p, q, r, v, w, x) or AA3 (g, h, I, m, n, o, s, t, u, y, z, z‐) at different time intervals (1, 2, 4 and 6 h p.i.) until 8 h p.i., and the intracellular amount and localization of viral RNPs (green, e, h, k, n, q, t, w, z), as well as the nuclei (blue, d, g, j, m, p, s, v, y), DAPI + NP (f, I, l, o, r, u, x, z‐) were detected by immunofluorescence. The location of NP protein at muti‐round replication (24 and 48 h p i) was tested in Figure 6C.
In Figure 6A, there were virus‐infected group and the positive drug group at 12, 24, and 48 h p.i. The distribution of NP protein was migrated to the cytoplasm, but the protein was still in the nucleus with treatment of LY294002.
The previous experiments demonstrated the strong inhibition by compounds in single replication of virus. The single replication was tested in detail. In AA‐treated cells after virus adsorption (1 or 2 h p.i.), abundant RNP complexes assembled in the nucleus (Figure 6d–o). After treatment of cells with the compounds for 4 or 6 h, the RNPs were stayed in nucleus (Figure 6p–z), which was opposite to the control (Figure 6a–c). It should be noted that the treatment of infected cells at different time intervals affected the number of cells positive for NP export. Consistent with results above indicating that the compounds did not affect virus entry, these results suggested that AA1 and AA3 affected migration of the RNP complex from the nucleus to cytoplasm at the early and middle stages of the replication cycle (0–6 h). The same results as the treatment of AA1 or AA3 at 12 and 24 h p.i. The NP protein was exported to cytoplasm with the treatment of AA1 at 48 h p i, and this compound delayed the protein export from nucleus. With treatment of AA3, NP protein still in nucleus by 48 h p.i indicated this compound could block the protein export (Figure 6C).
Discussion
Associated with significant morbidity and mortality, H5N1 avian influenza viruses pose a serious public health threat, and the search for novel drugs against influenza is ongoing. Although many plant extracts have been tested and some research laboratories have found that many Lycoris alkaloids exhibit anti‐viral activity, the anti‐influenza activities of compounds from L. radiata have not been reported. In our study, we screened 15 compounds obtained from bulbs of L. radiata used in traditional Chinese medicine and found four compounds (lycorine, AA1; hippeastrine, AA2; hemanthamine, AA3; 11‐hydroxyvittatine, AA4) that exhibited anti‐influenza activity, with EC90 values of 0·52, 82·07, 4·15, and 13·45 μm, respectively. 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 The pre‐treatment and simultaneous treatment with these compounds relative to time of infection did not result in significant antiviral activity. However, post‐infection treatment with the compounds showed significant inhibitory effects. These data suggested that the four compounds may interfere with viral particle assembly. AA1 and AA3 exhibiting stronger antiviral activities were chosen for further study in MDCK cell‐based assays.
The influenza virus particles contain three proteins, the surface glycoproteins HA and NA and the M2 ion‐channel protein, which are derived from the host cellular membrane. To determine whether the Amaryllidaceae alkaloid compounds could inhibit the function of HA, NA, and M2, time‐of‐addition assays, HI assays, NA activity assay, and pseudotype virus infection assay were used. Results of the HI assays indicated that the compounds did not affect viral attachment through the HA protein. In NA activity assays, the two compounds also did not inhibit the activity of NA protein. In experiments using the pseudotyped virus, which is widely used to screen drugs and resistant viruses in HIV studies, neither AA1 nor AA3 could prevent virions entry. However, post‐infection treatment with AA1 and AA3 resulted in significant inhibition. By detailed analysis of the effect of time of addition of the compounds, the data indicated that both compounds significantly inhibited the virus during a single round of replication after virus entry.
Recently, the antiviral activity of lycorine (AA1) was demonstrated against the severe acute respiratory syndrome coronovirus (SARS‐CoV), 39 human enterovirus 71, 40 and cancer cells. AA1 and AA3 have also been shown to have high antiretroviral activities (e.g., against HIV‐1). 41 , 42 Lycorine has been reported as the immunosuppressor and against apoptosis‐resistant cancer cells. 43 , 44 , 45 , 46 , 47 However, as mentioned above, the anti‐influenza activities of these compounds from L. radiata have never been reported. Here, we found that these compounds could inhibit influenza virus replication, which was more effective when added earlier during infection. While none of these compounds affected the activity of the RNP complex, which is important to viral generation and replication, their migration from nuclear to cytoplasm was blocked in single and multiple replications with the treatment of AA3, and the compound AA1 delayed the export of NP protein from nuclear. Thus, the results suggested that the viral inhibitory activity of the compounds involved inhibiting the export of the RNP complex. There are many factors (such as Hsc70 protein, NF‐κB, and caspase pathway) that can inhibit export of the RNP complex from nuclear to cytoplasm, and the underlying mechanism will require further study. In addition, the chemical structures of the four compounds that exhibited antiviral activity and compound AA2 were lower than other three compounds.
In conclusion, this study for the first time demonstrated the anti‐influenza activities of four L. radiata compounds. AA1 and AA3 in particular showed strong activities against influenza A virus in vitro. They did not target the outer membrane proteins, nor the activity of the RNP complex and internalization. Instead, they retained their antiviral activity by block in vRNP nuclear export, and AA1 retained its antiviral activity for up to 24 h, while AA3 retained this for even longer. The mode of action of AA1 and AA3 on viruses in the host cell observed in this study implies that they may potentially be developed as antiviral agents against influenza A virus. Further studies are needed to explore the viral and cellular protein/pathway targets of these compounds.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81001375, 81273391), Natural Science Foundation of Guangdong Province (No.10251064201000004), Program for National Broiler Industry (nycytx‐42‐G3‐03), Innovative Research Team in Chinese Universities (No.IRT0723), Program for New Century Excellent Talents in University (Grant No. NCET‐06‐0752) and the Science and Technology Projects of Guangdong Province (No. 20100206).
References
- 1. Palese P. Influenza: old and new threats. Nat Med 2004; 10(Suppl 12):S82–S87. [DOI] [PubMed] [Google Scholar]
- 2. Palese P, Shaw ML. “Orthomyxoviridae: the viruses and their replication”. In: Knipe DM, Howley PM, Griffin DE. et al. (eds) Fields Virol 2007:1647–1689. Philadelphia: Wolters Kluwer; Lippincott Williams & Wilkins. [Google Scholar]
- 3. Palese P, Wang TT. H5N1 influenza viruses: facts, not fear. Proc Natl Acad Sci U S A. 2012 Feb 14; 109:2211–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wainright PO, Perdue ML, Brugh M, Beard CW. Amantadine resistance among hemagglutinin subtype 5 strains of avian influenza virus. Avian Dis 1991; 35:31–39. [PubMed] [Google Scholar]
- 5. Bahlky H. Avian influenza: the tip of the iceberg. Ann Thorac Med 2009; 3:154–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suzuki Y. The highly pathogenic avian influenza H5N1‐initial molecular signals for the next influenza pandemic. Chang Gung Med J 2009; 32:258–263. [PubMed] [Google Scholar]
- 7. Cannell JJ, Zasloff M, Giovannucci E. On the epidemiology of influenza. Virology J 2008; 5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hayden F. Developing new antiviral agents for influenza treatment: what does the future hold? Clin Infec Dis 2009; 48:S3–S13. [DOI] [PubMed] [Google Scholar]
- 9. Jefferson T, Di Pietrantonj C, Debalini MG, Rivetti A, Demicheli V. Inactivated influenza vaccines: methods, policies, and politics. J Clin Epidemiol 2009; 62:677–686. [DOI] [PubMed] [Google Scholar]
- 10. Lackenby A, Thompson CI, Democratis J. The potential impact of neuraminidase inhibitor resistant influenza. Curr Opin Infec Dis 2008; 21:626–638. [DOI] [PubMed] [Google Scholar]
- 11. Linhares RE, Wigg MD, Lagrota MH, Nozawa CM. The in vitro antiviral activity of isoprinosine on simian rotavirus (SA‐11). Braz J Med Biol Res 1989; 22:1095–1103. [PubMed] [Google Scholar]
- 12. Cheng PKC, Leung TWC, Ho ECM et al. Oseltamivir‐ and amantadine‐resistant influenza viruses A (H1N1). Emerg Infec Dis 2009; 15:966–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kitching A, Roche A, Balasegaram S, Heathcock R, Maguire H. Oseltamivir adherence and side effects among children in three London schools affected by influenza A (H1N1) v, May 2009 an internet‐based cross‐sectional survey. Eurosurveillance 2009; 14:1–4. [DOI] [PubMed] [Google Scholar]
- 14. Dutkowski R. Oseltamivir in seasonal influenza: cumulative experience in low‐ and high‐risk patients. J Antimicrob Chemother 2010; 65(Suppl 2):ii11–ii24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kretzing S, Abraham G. Dose‐dependent emetic effects of the Amaryllidaceous alkaloid lycorine in beagle dogs. Toxicon 2011; 57:117–124. [DOI] [PubMed] [Google Scholar]
- 16. Dewick PM. The biosynthesis of C5‐C25 terpenoid compounds. Nat Prod Rep 2002; 19:181–222. [DOI] [PubMed] [Google Scholar]
- 17. Jin Z. Amaryllidaceae and Sceletium alkaloids. Nat Prod Rep 2003; 20:606–614. [DOI] [PubMed] [Google Scholar]
- 18. Jin Z. Amaryllidaceous and Sceletium alkaloids. Nat Prod Rep 2007; 24:886–905. [DOI] [PubMed] [Google Scholar]
- 19. Zupkó I, Réthy B, Hohmann J, Molnár J, Ocsovszki I, Falkay G. Antitumor activity of alkaloids derived from Amaryllidaceae species. In Vivo 2009; 23:41–48. [PubMed] [Google Scholar]
- 20. Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, Ueno K. A combined assay of cell viability and in vitro cytotoxicity with a highly water‐soluble tetrazolium salt, neutral red and crystal violet. Biol Pharm Bull 1996; 19:1518–1520. [DOI] [PubMed] [Google Scholar]
- 21. DeLean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose‐response curves. Am J Physiol 1978; 235:E97–E102. [DOI] [PubMed] [Google Scholar]
- 22. Li C, Hatta M, Watanabe S, Neumann G, Kawaoka Y. Compatibility among polymerase subunit proteins is a restricting factor in reassortment between equine H7N7 and human H3N2 influenza viruses. J Virol 2008; 82:11880–11888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leung BW, Chen H, Brownlee GG. Correlation between polymerase activity and pathogenicity in two duck H5N1 influenza viruses suggests that the polymerase contributes to pathogenicity. Virology 2010; 401:96–106. [DOI] [PubMed] [Google Scholar]
- 24. Liu SW, Li RM, Zhang R et al CL‐385319 inhibits H5N1 avian influenza A virus infection by blocking viral entry. Eur J Pharmacol 2011; 660:460–467. [DOI] [PubMed] [Google Scholar]
- 25. Zheng B, Mittal SK, Graham FL, Prevec L. The E1 sequence of bovine adenovirus type 3 and complementation of human adenovirus type 5 E1A function in bovine cells. Virus Res 1994; 31:163–186. [DOI] [PubMed] [Google Scholar]
- 26. Kwon H‐J, Kim H‐H, Yoon SY. In Vitro inhibitory activity of Alpinia katsumadai extracts against influenza virus infection and hemagglutination. Virology J 2010; 7:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shin Y‐K, Liu Q, Tikoo S‐K, Babiuk L‐A, Zhou Y. Effect of the phosphatidylinositol 3‐kinase/Akt pathway on influenza A virus propagation. J Gen Virol 2007; 88:942–950. [DOI] [PubMed] [Google Scholar]
- 28. Wu M‐S, Yen HR, Chang CW, et al. Mechanism of action of the suppression of influenza virus replication by Ko‐Ken Tang through inhibition of the phosphatidylinositol 3‐kinase/Akt signaling pathway and viral RNP nuclear export. J Ethnopharmacol 2011; 134:614–623. [DOI] [PubMed] [Google Scholar]
- 29. Li C, Liu VW, Chan DW, Yao KM, Ngan HY. LY294002 and metformin cooperatively enhance the inhibition of growth and the induction of apoptosis of ovarian cancer cells. Int J Gynecol Cancer 2012; 22:15–22. [DOI] [PubMed] [Google Scholar]
- 30. Lamb J. The Connectivity Map: a new tool for biomedical research. Nat Rev Cancer 2007; 7:54–60. [DOI] [PubMed] [Google Scholar]
- 31. Evidente A, Rosaria cicala M, Giudicanni I, Randazzo G, Riccio R. 1H and 13C NMR analysis if lycorine and α‐dihydrolycorine. Phytochemistry 1983; 22:581–584. [Google Scholar]
- 32. Viladomat F, Giovanna R, Bastida J. Alkaloids from Brunsvigia orientalis . Phytochemistry 1996; 43:1379–1384. [Google Scholar]
- 33. August W. Frahm, Ahmed A. Ali, Mahmoud A. Ramadan. 13C nuclear magnetic resonance spectra of Amaryllidaceae alkaloids. Magn Reson Chem 1985; 23:804–808. [Google Scholar]
- 34. Masaru KiHRA, Konishi K, Lai XU, Shigeru Kobayashi. Alkaloidal constituents of flowers of lycoris radiate HERB (Amaryllidaceae). Chem Pharm Bull 1991; 39:1848–1853. [Google Scholar]
- 35. Evidente A. Identification of 1 1‐Hydroxwittatine in Sternbergialutea lutea. J Nat Prod 1986; 49:168–169. [Google Scholar]
- 36. Evidente A, Andolfi A, Shawky E. (‐)‐Amarbellisine, a lycorine‐type alkaloid from Amaryllis belladonna L. growing in Egypt. Phytochemistry 2004; 65:2113–2118. [DOI] [PubMed] [Google Scholar]
- 37. Latvala A. Alkaloids of Galanthus elwesii . Phytochemistry 1995; 39:1229–1240. [Google Scholar]
- 38. Wagner J, Huong L. Alkaloids from Hippeastrum Equestre Herb‐5. Circular Dichroism Studies. Tetrahedron 1996; 52:6591–6600. [Google Scholar]
- 39. Li SY, Chen C, Tan XH. Identification of natural compounds with antiviral activities against SARS‐associated coronavirus. Antiviral Res 2005; 67:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu JG, Zhang LF. Lycorine reduces mortality of human enterovirus 71‐infected mice by inhibiting virus replication. Virology J 2011; 8:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Szlávik L, Gyuris A, Minárovits J, Forgo P, Molnár J, Hohmann J. Alkaloids from Leucojum vernum and antiretroviral activity of Amaryllidaceae alkaloids. Planta Med 2004; 70:871–873. [DOI] [PubMed] [Google Scholar]
- 42. CDC . Transmission of influenza A viruses between animals and people, the virus and its spread. 2005; October 17.
- 43.Dickneite; Gerhard (Marburg‐Cappel, DE) Schorlemmer; Hans‐UAAich (Weimar, DE) Sedlacek; Hans‐Harald (Marburg, DE). Use of Lycorine as an immunosuppressor. 4699912 October 13, 1987.
- 44. Yui S, Mikami M, Kitahara M, Yamazaki M. The inhibitory effect of lycorine on tumor cell apoptosis induced by polymorphonuclear leukocyte‐derived calprotectin. Immunopharmacology 1998; 40:151–162. [DOI] [PubMed] [Google Scholar]
- 45. Ancuceanu RV, Istudor V. Pharmacologically active natural compounds for lung cancer. Altern Med Rev 2004; 9:402–419. [PubMed] [Google Scholar]
- 46. Van Goietsenoven G, Andolfi A. Amaryllidaceae alkaloids belonging to different structural subgroups display activity against apoptosis‐resistant cancer cells. J Nat Prod 2010; 73:1223–1227. [DOI] [PubMed] [Google Scholar]
- 47. Evidente A, Kireev AS, Jenkins AR, Romero AE, Kornienko A. Biological evaluation of structurally diverse amaryllidaceae alkaloids and their synthetic derivatives: discovery of novel leads for anticancer drug design. Planta Med 2009; 75:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]


