Abstract
Background
To ascertain the full mortality of influenza and other respiratory viruses, the testing of community autopsy specimens is essential.
Methods
Respiratory virus PCR and culture were performed on 2418 fresh unfrozen respiratory samples collected from 1611 coronial cases where the death was either unknown or infection was suspected, from July 2007 to June 2011, to detect the common respiratory viruses in children and adults, using standardized microbiological testing.
Results
The respiratory virus positive rate was 8·3% (134 cases) with a peak of 28% (42 of 151 cases) in children under 10 years of age. Influenza virus was the commonest respiratory virus (50 cases, 3%), followed by respiratory syncytial virus (RSV) (30 cases, 2%). All tested respiratory viruses were found in children, most commonly adenovirus, enterovirus and RSV, and influenza A and RSV predominated in those over 60 years, but coinfection was uncommon. Almost all influenza cases occurred when influenza was widely circulating in the community but few were diagnosed pre‐mortem. Influenza and RSV detection was associated with bronchitis or bronchiolitis in 7 (9%) of the 80 cases and caused pneumonia in 14 (0·8%) deaths overall.
Conclusions
Our prospective review of respiratory viruses using standardized testing found a single lower respiratory tract autopsy specimen for respiratory virus PCR would detect most community infections at the time of death.
Keywords: Autopsy, coronial, culture, influenza, PCR, respiratory virus
The proper investigation of deaths and testing of post‐mortem specimens is important to assess the impact of serious infectious diseases. It captures cases that may not have been suspected pre‐mortem, which is particularly difficult with influenza‐like illnesses where the presentation is syndromic, and cases that have not been fully investigated prior to death. In addition, it allows a better ascertainment of the role of any detected infectious agents in the death of that patient.1 Post‐mortem diagnosis of hospitalized deaths, however, is hindered by the falling hospital autopsy rate and the inaccuracy of death certificate diagnoses2 and may not be representative of deaths that occur in the community. The investigation of deaths in the community for respiratory viruses such as influenza is therefore of potential value in assessing the full impact of these infections.1 The most appropriate means of achieving this is to access the coronial autopsies as these cases represent community deaths with often little medical intervention or investigation prior to death.
Although influenza is known to be a significant cause of mortality during winter in temperate climates, most deaths due to influenza go undiagnosed. Recent initiatives to address this are the US Centers for Disease Control and Prevention (CDC) Emerging Infections Program Unexplained Deaths Program, established in 1995, and the Medical Examiner Infectious Disease Death Surveillance Program, established in 1999.2 A small number of studies have looked at sudden unexpected deaths in infancy,3, 4, 5 and there have been limited investigations of other respiratory viruses such as respiratory syncytial virus (RSV)6 and adenoviruses7 in pediatric autopsies. Some have relied on relatively insensitive immunofluorescence (IF) testing5 rather than the more sensitive polymerase chain reaction (PCR) testing.8 We are aware of only one limited pilot PCR‐based systematic study of coronial autopsy tissues from Hong Kong.9
Therefore, to better understand the population at risk of death from influenza and other respiratory viruses in Western Australia (WA), we undertook a PCR study of all coronial autopsy cases in WA from June 2007 until July 2011 where the cause of death was initially unknown or an infection‐related etiology was suspected. This included the pre‐pandemic, H1N1 09 influenza A pandemic, and post‐pandemic periods.
Material and methods
PathWest Laboratory Medicine WA (PWLM) performs all the microbiological investigations for the Western Australian State Mortuary and also provides all the respiratory serological testing for the state. Nucleic acid detection tests for influenza were performed exclusively by PWLM prior to 2009, after which limited testing was performed in private sector laboratories. However, positive influenza samples are routinely referred to PWLM for subtyping, so that all or nearly all influenza positive patients are recorded by PWLM. Nucleic acid detection tests for respiratory viruses other than influenza are performed only at PWLM but antigen detection by IF is performed more widely. Samples positive for influenza A by IF are routinely referred to PWLM, but samples positive for influenza B and other respiratory viruses may not.
Following the detection of influenza A in three pediatric sudden deaths within several weeks in 2007, an enhanced microbiological surveillance of autopsy cases was commenced. Deaths referred to the state coroner where the cause of death was unknown or an infectious etiology was suspected had respiratory tract samples collected for microbiological examination. Specimens were received by the microbiology laboratory without delay as the mortuary and microbiology services are colocated. Virus PCR and culture were performed to detect the common respiratory viruses in children and adults, using standardized microbiological testing of autopsy cases over the study period.
Specimens for bacteriology were processed according to standard laboratory operating procedures. Briefly, tissue samples were macerated with a scalpel blade, then Gram stain microscopy was performed followed by inoculation onto non‐selective (blood agar and chocolate blood agar) and selective (MacConkey and colistin nalidixic acid agar) solid media and incubated at 35°C in 5% CO2 for 48 hour. Growth of bacterial pathogens was identified using standard biochemical methods. For virus studies, respiratory tissues were homogenized in viral transport medium, centrifuged for 20 minute at 2150 g, and the pellets discarded. The supernatants were cultured for enterovirus, rhinovirus A and B, influenza A and B, adenovirus, and RSV using Madin–Darby canine kidney and human diploid fibroblast cells and the viral nucleic acid purified using either a Qiagen QiaAmp Viral RNA mini kit or Ambion MagMAX Viral RNA isolation kit. Both kit protocols were modified to incorporate heated elution.10 PCR testing for influenza A and B virus, parainfluenza virus 1–3, human metapneumovirus, and RSV detection during the study period included nested and real‐time methods (Table 1).11, 12 Prior to August 2007, a nested reverse‐transcriptase influenza A and B virus PCR assay that targeted the matrix gene was used,11 so that routine subtyping of influenza A viruses was not performed, but subsequently PCR assays targeting the hemagglutinin (HA) genes of the circulating H1 and H3 influenza A subtypes were added.10 All assays included checks for extraction efficiency and PCR inhibitor removal and were validated according to the criteria established by the National Pathology Accreditation Advisory Committee and approved by the National Association of Testing Authorities.
Table 1.
Primers and probes used for respiratory virus PCR
| Respiratory virusa | Primer/probe 5′–3′ nucleotide sequenceb | Ampliconc (base pairs) |
|---|---|---|
| Respiratory syncytial virus A | F CAACTTCTGTCATCCAGCAAA | 77 |
| R TGCACATCATAATTAGGAGTATCAAT | ||
| Pb CACCATCCAACGGAGC (FAM) | ||
| Respiratory syncytial virus B | F ATTCAACGTAGTACAGGAGATAATA | 74 |
| R CCACATAGTTTGTTTAGGTGTTT | ||
| Pb TGACACTCCCAATTAT (FAM) | ||
| Parainfluenza virus 1 | F GCAAAGAGARAATGCRGATCTAG | 67 |
| R AGCTCCGAGACATGCAGGAT | ||
| Pb TCCATATGTCTGAAGCAA (FAM) | ||
| Parainfluenza virus 2 | F ATTCCAGATGCTCGATCAACTATG | 65 |
| R TCYTCAGCTAATGCT TCRAARGC | ||
| Pb AGC ACY TCT CCT CTG (FAM) | ||
| Parainfluenza virus 3 | F CGCGCTCCWTTYATCTGTATC | 59 |
| R TTGCCTGGTGCGAACTCA | ||
| Pb TCAGAGATCCYATACATG (FAM) | ||
| Human metapneumovirus | F ATCATCAGGYAAYATYCCACAAA | 81 |
| R TATTAARGCACCTACACATAATAA | ||
| Pb CAGCACCAGACACACC (FAM) |
Influenza A and B serology was performed using a complement fixation test (CFT) employing sheep red blood cells and in‐house produced antigen. Briefly, the patient samples were diluted, inactivated and titrated, then incubated in the presence of influenza A or B antigen and complement at 4°C overnight, before the addition of sensitized sheep red blood cells and incubated at 37°C on a rotating platform (280 rpm) for 30–40 minute until full lysis of the red blood cells in the complement control. The samples were then centrifuged at 377 g for 3 minute at 4°C and then inspected for red blood cell lysis. The end point was recorded as the highest dilution with no red blood cell lysis.
One author (DMM) reviewed the forensic pathology reports, which included clinical history, macroscopic and histological pathology findings, hematology, clinical chemistry, and microbiological results to verify the cause of death and identify comorbidities. Permission to access the information for research purposes was obtained from the WA State Coroner.
Results
There were 50 404 deaths from July 2007 to June 2011 recorded in WA,13 with 7 216 (14%) undergoing coronial autopsy. Of these, 1 611 (22%) coronial autopsies underwent microbiological sampling due to either unknown cause of death or a suspected infectious etiology. There was a winter peak of cases undergoing microbiological sampling. A total of 3870 samples, 2418 from respiratory sites, were received from these cases. The commonest age‐group represented was 60–69 years (Figure 1A), with a male predominance (999 cases, 62%) for all age‐groups to 89 years.
Figure 1.
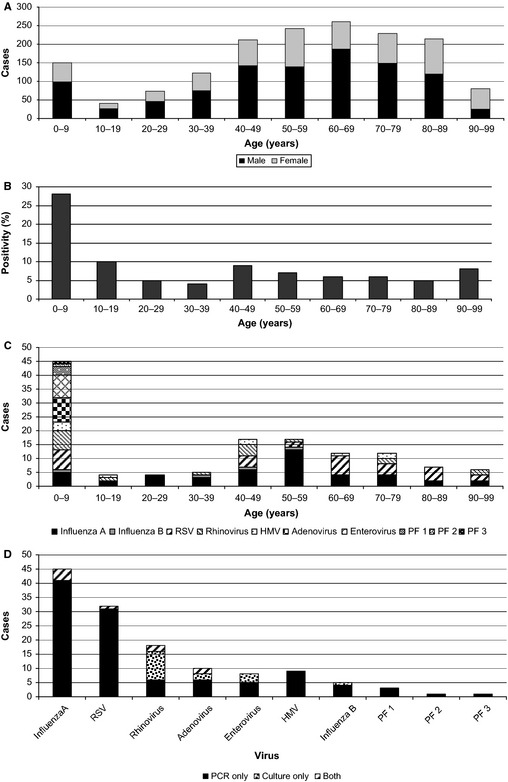
Case numbers and respiratory virus detections. (A) Cases sampled by age group. (B) Respiratory virus% case positivity (PCR and/or culture) by age. (C) Respiratory virus detections by age. (D) Total respiratory virus detections by PCR and/or culture. RSV, respiratory syncytial virus; HMV, human metapneumovirus; PF, parainfluenza virus.
Of the 1611 deaths, 1578 had both virus culture and PCR performed, 28 cases had only PCR performed and five cases had only culture performed. The respiratory sites sampled were lung (2079 samples), trachea (219 samples), bronchus (54 samples), nose and throat swabs (66 samples), and sputum (one sample). A total of 134 cases (8·3%) had a respiratory virus detected, either by PCR only (93 cases, 5·8%), culture only (22 cases, 1·4%), or both (19 cases, 1·2%), with the highest detection rate of 28% (42 of 151 cases) in the 0–9‐year age‐group (Figure 1B).
All of the respiratory viruses tested for were detected in children aged 0–9 years, whereas influenza A virus and RSV predominated in adults, accounting for 26 (60%) and 6 (14%) of the virus detections, respectively, in the 43 adults aged 20–59 years, and 12 (32%) and 18 (49%) in the 37 adults over 60 years (Figure 1C). Coinfections were uncommon and found almost exclusively in young children; there was one case each of dual infection (adenovirus and rhinovirus), triple infection (adenovirus, rhinovirus, and RSV), and quadruple infection (adenovirus, RSV, enterovirus, and parainfluenza 1 virus) in the 0–9‐year age–group, and one adult had a dual human metapneumovirus and influenza A virus infection. The commonest viruses overall were influenza A and RSV (Figure 1D). All but one case of influenza and all cases of RSV were detected by PCR alone. Influenza virus was detected from all sites in 22 (85%) of the 26 cases with multiple samples collected.
The forensic pathology reports from the 50 influenza and 30 RSV cases were reviewed together with the bacteriology findings to ascertain the cause of death and better define the role of these viruses in each case. The mean age for influenza detection was 47 years, peaking in the 50–59‐year age‐group (Figure 2A) with a male predominance (Table 2). Interestingly, the mean age and male predominance varied with the influenza type and subtype with a younger mean age and male predominance for H1N1pdm09 (44 years) and A/untyped (38 years) compared with seasonal H3N2 (60 years). There were too few cases of other influenza viruses to interpret. The influenza type and subtype detected from autopsy specimens were similar to the proportion found from routine diagnostic testing for the same period (Figure 3) with no obvious predilection for certain types or subtypes. The overall mean age for RSV detection was older at 55 years (Table 2) with a peak in the 60–69 age‐group (Figure 2B), and it also displayed a male predominance. Of the 50 influenza cases, bacterial pneumonia and diffuse alveolar damage were present in 26 (52%) and 9 (18%) cases, respectively, and bronchitis or bronchiolitis was found in a further 5 (10%) cases. The remaining 15 (30%) cases died of non‐infective causes. In contrast, 8 (27%) of the 30 RSV cases had bacterial pneumonia, 5 (17%) had diffuse alveolar damage, 2 (1%) had bronchitis, and 15 (50%) died without evidence of infection.
Figure 2.
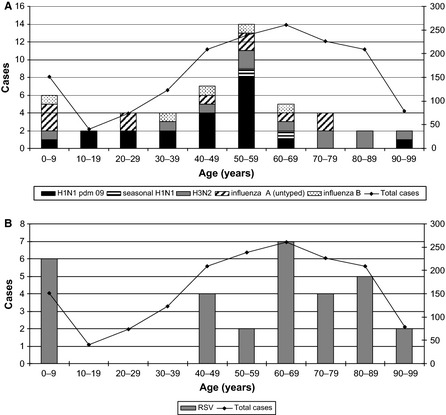
Influenza type and subtype (A) and respiratory syncytial virus (B) detections by age compared to total number of cases. RSV, respiratory syncytial virus.
Table 2.
Characteristics and clinical outcomes of influenza virus and RSV autopsy cases
| Characteristics and pathological findings | Influenza A | Influenza B | Influenza total | RSV | |||
|---|---|---|---|---|---|---|---|
| Untyped | H1N1 pdm 09 | Seasonal H1N1 | H3N2 | ||||
| Cases | |||||||
| Age, years, mean (range) | 38 (2–67) | 44 (6–92) | 61 (58–65) | 60 (8–91) | 42 (0·5–60) | 47 (0·5–91) | 55 (0·4–96) |
| Gender (M:F) | 9:2 | 16:5 | 1:1 | 6:5 | 3:2 | 35:15 | 18:12 |
| Total | 11 | 21 | 2 | 11 | 5 | 50 | 30 |
| Clinical outcome | |||||||
| Pneumonia | |||||||
| Diffuse alveolar damage | 1 | 6 | 1 | 1 | 9 | 5 | |
| Bacterial | 6 | 11 | 1 | 5 | 3 | 26 | 8 |
| Bronchitis/bronchiolitis | |||||||
| Viral | 1 | 1 | 1 | 3 | 2 | ||
| Bacterial | 1 | 1 | 2 | 0 | |||
| Acute myocardial infarction | 1 | 1 | 2 | 4 | 3a | ||
| Acute cardiac failure | 2a | ||||||
| Otherb | 1 | 1 | 12a | ||||
| Unknown | 3 | 1 | 1 | 5 | 4 | ||
| Comorbidities | |||||||
| Coronary heart disease | 3 | 5 | 2 | 5 | 2 | 17 | 15 |
| Diabetes mellitus | 1 | 1 | 1 | 1 | 2 | 6 | 2 |
| Chronic obstructive pulmonary disease | 1 | 4 | 5 | 3 | |||
| Obesity | 2 | 1 | 3 | 1 | |||
| Asthma | 1 | 1 | |||||
| Renal failure | 4 | ||||||
| Cirrhosis | 3 | ||||||
| Bacteriology | |||||||
| Staphylococcus aureus | 2 | 7 | 1 | 2 | 3 | 15 | 2 |
| Streptococcus pneumoniae | 4 | 1 | 2 | 7 | 3 | ||
| β‐hemolytic streptococcic | 3 | 3 | 1 | 7 | 5 | ||
| Haemophilus influenzae | 1 | 2 | 2 | 5 | 6 | ||
| Other Gram‐negative rodsd | 2 | 1 | 3 | 3 | |||
Six cases had respiratory and non‐respiratory contributors to death.
Two cases each of trauma, drug overdose, uncontrolled bleeding; single cases of diabetic ketoacidosis, choking, systemic amyloidosis, seizures, subdural hematoma, multi‐organ failure.
Streptococcus pyogenes (four cases), Streptococcus agalactiae (seven cases), Streptococcus dysgalactiae (one case).
Klebsiella sp. (two cases), Pseudomonas aeruginosa (one case), Serratia marcescens (one case), Aeromonas sp. (one case).
Figure 3.
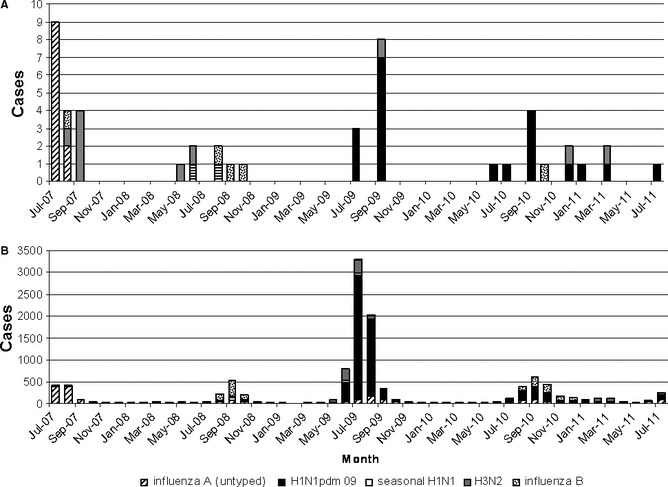
Detection of influenza types and subtypes from (A), autopsy tissues and (B), routine specimens.
Comorbidities were identified in over half of influenza cases and almost all RSV cases. Coronary heart disease was the most common, often in combination with diabetes, chronic obstructive pulmonary disease, or renal failure, and acute myocardial infarction was found in 7 (8·8%) cases. Histological evidence of a primary viral pneumonia was found in 9 (18%) influenza cases, most of which were H1N1pdm09 cases and 5 (17%) RSV cases. Histological and microbiological evidence of bacterial pneumonia was found in 26 (52%) influenza cases, most commonly due to Staphylococcus aureus, and 8 (27%) RSV cases, mainly due to Haemophilus influenzae.
A review of the state public pathology laboratory information system found that six of the 50 influenza cases had undergone influenza virus testing prior to death. Of the five cases undergoing PCR testing, four cases were positive 2–15 days before death, one of which was also culture positive, and one case was PCR negative on upper respiratory tract sampling 11 days before death. Interestingly, this case had a CFT titer of 1:80 3 days before death and influenza A(H1N1)pdm09 was detected from lung and tracheal tissue by PCR at autopsy 4 days after death. The sixth case was tested serologically and produced a CFT titer of >1:320 4 days before death.
Discussion
This prospective study of routine PCR testing of selected coronial autopsy specimens greatly increases our understanding of the contribution of respiratory viruses to deaths in hospitals and the community. This has previously been proposed as being particularly valuable for deaths in younger and/or previously healthy patients,2 but we have shown that it has an important role in all age‐groups. Routine testing of autopsy tissues could also help detect novel outbreaks and new serious disease threats.2, 8
Our prospective study of respiratory viruses using PCR from all coronial cases in WA where an infectious etiology was suspected or cause of death was unknown over 4 years including the influenza pre‐pandemic, pandemic, and post‐pandemic periods is the largest such study to date. Previously, respiratory virus autopsy studies have concentrated on childhood deaths and been performed retrospectively on frozen or formalin‐fixed paraffin‐embedded tissues or nose and throat swabs.2, 4, 6, 7, 8, 9, 14, 15 We used fresh unfrozen respiratory samples and included all age‐groups to better assess the prevalence of these viruses at the time of death and to overcome some of the factors that reduce detection rates. For example, the use of upper respiratory tract samples has been shown to underestimate the presence of influenza virus in primary influenza pneumonia16 and formalin fixation is known to lead to a loss of PCR sensitivity due to the cross‐linking of the nucleic acids and significant RNA degradation due to ribonucleases.8, 17 This may account for the low virus yield in some previous studies that have led the authors to conclude that routine autopsy sampling for viruses is not warranted.9 Furthermore, the colocation of our laboratory with the state mortuary meant that there was minimal delay in specimen processing. Therefore, we believe that we optimized the processes for detection of respiratory viruses in this series. We detected a respiratory virus in 134 (8·3%) of 1611 cases and in 42 (28%) of 151 cases <10 years of age. As expected, we found PCR to be more sensitive than virus culture, such that testing for influenza by PCR on a single lung tissue specimen detected 47 (94%) of 50 influenza cases. The minority (22 cases, 16%) of cases that were culture positive but PCR negative may have been due to sampling error due to heterogeneous distribution of virus in the respiratory specimens or viral RNA degradation prior to extraction, because extraction and inhibitor controls were included with each PCR assay.
All viruses tested for were found in the 0–9‐year age‐group with narrowing of the virus spectrum to mainly influenza virus and RSV in adulthood. We found adenovirus, enterovirus, and parainfluenza viruses exclusively in those less than 10 years of age, whereas influenza virus, RSV, human metapneumovirus, and rhinoviruses were present in the children and adults. Coinfection of influenza A virus and other respiratory viruses has been associated with increased disease severity18 but we found this to be uncommon, being present in only one adult and in three young children. Two of these children had a combination of RSV and adenovirus, both of which have been previously found to be important contributors to severe pediatric pneumonia6, 7 and the combination of the two has been associated with more severe disease.19
Thompson et al.20 have estimated over 40 000 influenza‐associated deaths and almost 10 000 RSV‐associated deaths annually in those over 65 years in the United States but there is little PCR autopsy data on the contribution of seasonal influenza and RSV to adult deaths.21, 22, 23 Few (five cases) of our influenza cases were diagnosed prior to death even though the study period encompassed an influenza pandemic. The CDC recommends influenza diagnostic testing to aid clinical judgment and help guide treatment decisions.24 An increase in influenza diagnoses has occurred in Australia following the 2009 pandemic, potentially preventing some deaths. In 2008, there were 9223 laboratory‐confirmed influenza cases notified in Australia, which jumped to 59 090 cases in 2009, then settled to 13 419 in 2010.25 We found influenza virus the most common virus detected in adults less than 60 years of age; however, we were surprised that RSV was substantially more common than influenza in adults over 60 years. This may reflect the relative sparing of the elderly during the 2009 pandemic26 and the high influenza vaccination rates of the elderly in Australia.27 The relative frequency of detection of influenza A and RSV in our study, especially in those 60 years and above, reflects the ubiquity of these viruses circulating in the community and the increased propensity to cause deaths in the elderly, especially in those residing in long‐term care facilities.28 The rise in all‐cause mortality over the winter months in temperate climates, thought predominantly due to influenza and RSV,20, 28, 29 correlates with our peaks in detection of these viruses through the winter months.
We looked at the two most common respiratory viruses detected, influenza and RSV, to ascertain any differences between case demographics, the causes of death, comorbidities, or secondary bacterial infections. We reviewed the bacteriology and forensic pathology reports to ascertain whether influenza and RSV directly attributed to the death, potentially contributed to death through secondary bacterial infection or exacerbation of another serious disease, or represented coincidental infection. Although bacterial pneumonia was the commonest cause of death in those with influenza or RSV, 14 (17·5%) deaths, due mainly to influenza A H1N1pdm09 and RSV, had acute viral lung injury. This equates to 0·8% of the overall cohort (1611 cases).
The association of influenza with bacterial pneumonia is well known,30, 31 being described for S. pneumoniae,32 S. aureus,23, 33, 34 β‐hemolytic streptococci,35 and H. influenzae 1, 36 but we also found this with RSV. Tamme et al.37 found that influenza A(H1N1)pdm09 was the sole cause of death for a third of their 21 cases, and it was the direct cause of death in 14% of influenza A(H1N1)pdm09 cases in the CDC Unexplained Deaths Program,2 similar to our figure of 18%, while a higher figure of 66% of influenza A(H1N1)pdm09 deaths was reported in England.1 Autopsy studies of both seasonal22 and influenza A(H1N1)pdm09 cases1, 2, 36, 38 have shown necrotizing bronchiolitis and diffuse alveolar damage with or without hemorrhage. There have been fewer autopsy studies involving influenza B but one case series found similar changes of bronchiolitis and alveolitis with hemorrhage.23 The histopathology of RSV deaths has shown airway obstruction due to inflammatory cell debris and pneumonic consolidation with syncytial giant cells.14, 39
Of interest, influenza and RSV infection were detected in a number of cases who died of non‐infective causes. Coronary heart disease was the commonest comorbidity in our influenza and RSV cases, comprising over half of those identified, with acute myocardial infarction identified in 4 (24%) of 17 influenza cases and 3 (20%) of 15 RSV cases with coronary heart disease. Influenza may trigger cardiovascular events by causing an acute inflammatory state leading to rupture of atherosclerotic plaques,40 and an autopsy study of nearly 35 000 subjects found that influenza increased the risk of death due to acute myocardial infarction by a factor of 1·30.29 More often we found severe atherosclerotic coronary arterial disease without acute thrombosis. Coronary heart disease, diabetes, obesity, and chronic pulmonary disorders are recognized comorbidities of influenza deaths.1, 36 However, we cannot conclude that these deaths were contributed to by influenza and RSV. The detection of influenza and RSV at autopsy following the traumatic deaths is likely to represent asymptomatic or clinically mild coincidental infection, as medical review was not sought prior to death and almost all cases occurred during the expected winter epidemic periods.
A limitation of this study is our inability to ascertain the contribution of the respiratory viruses to the non‐infective causes of death. Other limitations include the spectrum of viruses assessed, although the most significant lower respiratory tract viruses were included; the unknown influenza vaccination status of the cohort, although a vaccine was not available for influenza A(H1N1)pdm09 during the 2009 season; and lack of information on neuraminidase inhibitor therapy, but due to the few cases tested for influenza in life, it is unlikely many received such therapy.
This study demonstrates that routine respiratory virus testing by PCR at autopsy can contribute to the assessment of the overall impact of influenza and other respiratory viruses. In addition, coronial autopsy studies can help define the role of respiratory viruses in the deaths of those from the community without pre‐existing medical illnesses compared with hospitalized cohorts. This can be achieved with minimal additional testing, as illustrated by the fact that only a single respiratory specimen at autopsy for influenza PCR during the yearly influenza season will detect most of these infections at death.
Competing interest
The authors have no competing interests.
Acknowledgement
The authors acknowledge Simon Williams for the establishment of the WA mortuary cases influenza detection database.
Speers et al (2013) Influenza and respiratory syncytial virus are the major respiratory viruses detected from prospective testing of pediatric and adult coronial autopsies. Influenza and Other Respiratory Viruses 7(6), 1113–1121.
All work has been carried out at PathWest Laboratory Medicine WA, Queen Elizabeth II Medical Centre.
References
- 1. Lucas S. Predictive clinicopathological features derived from systematic autopsy examination of patients who died with A/H1N1 influenza infection in the UK 2009–10 pandemic. Health Technol Assess 2010; 14:83–114. [DOI] [PubMed] [Google Scholar]
- 2. Lees CH, Avery C, Asherin R et al Pandemic (H1N1) 2009–associated deaths detected by unexplained death and medical examiner surveillance. Emerg Infect Dis 2011; 17:1479–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bajanowski T, Wiegand P, Cecchi R et al Detection and significance of adenoviruses in cases of sudden infant death. Virchows Arch 1996; 428:113–118. [DOI] [PubMed] [Google Scholar]
- 4. Bajanowski T, Rolf B, Jorch G, Brinkmann B. Detection of RNA viruses in sudden infant death (SID). Int J Legal Med 2003; 117:237–240. [DOI] [PubMed] [Google Scholar]
- 5. Weber MA, Hartley JC, Ashworth MT, Malone M, Sebire NJ. Virological investigations in sudden unexpected deaths in infancy (SUDI). Forensic Sci Med Pathol 2010; 6:261–267. [DOI] [PubMed] [Google Scholar]
- 6. Bustamante‐Calvillo ME, Velazquez FR, Cabrera‐Munoz L et al Molecular detection of respiratory syncytial virus in postmortem lung tissue samples from Mexican children deceased with pneumonia. Pediatr Infect Dis J 2001; 20:495–501. [DOI] [PubMed] [Google Scholar]
- 7. Ou Z‐Y, Zeng Q‐Y, Wang F‐H et al Retrospective study of adenovirus in autopsied pulmonary tissue of pediatric fatal pneumonia in South China. BMC Infect Dis 2008; 8:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Denison AM, Blau DM, Jost HA et al Diagnosis of influenza from respiratory autopsy tissues: detection of virus by real‐time reverse transcription‐PCR in 222 cases. J Mol Diagn 2011; 13:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nicholls JM, Peiris JSM, Chan KH, Poon LM, Beh SLP. Occult respiratory viral infections in coronial autopsies: a pilot project. Hong Kong Med J 2009; 15:S13–S15. [PubMed] [Google Scholar]
- 10. Chidlow G, Harnett G, Williams S, Levy A, Speers D, Smith DW. Duplex real‐time RT‐PCR assays for the rapid detection and identification of Pandemic (H1N1) 2009, seasonal influenza viruses A/H1, A/H3 and B. J Clin Microbiol 2010; 48:862–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang W, Evans DH. PCR detection and differentiation of Influenza viruses A, B, and C strains; in Persing DH, Smith TF, Tenover FC. et al (eds): Diagnostic Molecular Microbiology, Principles and Applications, 1st edn Washington, DC: ASM Press, 1993; 374–382. [Google Scholar]
- 12. Whiley DM, Sloots TP. A 5′‐nuclease real‐time reverse transcriptase‐polymerase chain reaction assay for the detection of a broad range of influenza A subtypes, including H5N1. Diagn Microbiol Infect Dis 2005; 53:335–337. [DOI] [PubMed] [Google Scholar]
- 13. Australian Bureau of Statistics . Deaths, Australia, 2011. Available at http://www.abs.gov.au/ausstats/abs@.nsf/mf/3302.0 (Accessed 6 May 2013).
- 14. Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol 2007; 20:108–119. [DOI] [PubMed] [Google Scholar]
- 15. Gill JR, Sheng ZM, Ely SF et al Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med 2010; 134:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mulrennan S, Tempone SS, Ling IT et al Pandemic influenza (H1N1) 2009 pneumonia: CURB‐65 score for predicting severity and nasopharyngeal sampling for diagnosis are unreliable. PLoS ONE 2010; 5:e12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McKinney MD, Moon SJ, Kulesh DA, Larsen T, Schoepp RJ. Detection of viral RNA from paraffin‐embedded tissues after prolonged formalin fixation. J Clin Virol 2009; 44:39–42. [DOI] [PubMed] [Google Scholar]
- 18. Goka E, Vallely P, Mutton K, Klapper P. Influenza A dual and multiple infections with other respiratory viruses and risk of hospitalisation and mortality. Influenza Other Respi viruses 2012. doi: 10.1111/irv.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chidlow GR, Laing IA, Harnett GB et al Respiratory viral pathogens associated with lower respiratory tract disease among young children in the highlands of Papua New Guinea. J Clin Virol 2012; 54:235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thompson WW, Shay DK, Weintraub E et al Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–186. [DOI] [PubMed] [Google Scholar]
- 21. Levenson RM, Kantor OS. Fatal pneumonia in an adult due to respiratory syncytial virus. Arch Intern Med 1987; 147:791–792. [PubMed] [Google Scholar]
- 22. Taubenberger JK, Morens DM. The pathology of influenza virus infections. Annu Rev Pathol 2008; 3:499–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paddock CD, Liu L, Denison AM et al Myocardial injury and bacterial pneumonia contribute to the pathogenesis of fatal influenza B virus infection. J Infect Dis 2012; 205:895–905. [DOI] [PubMed] [Google Scholar]
- 24. Influenza symptoms and the role of laboratory diagnostics. Centers for Disease Control and Prevention. Available at http://www.cdc.gov/flu/professionals/diagnosis/labrolesprocedures.htm (Accessed 20 February 2013).
- 25. NNDSS Annual Report Writing Group . Australia's notifiable disease status, 2010: annual report of the National Notifiable Diseases Surveillance System. Commun Dis Intell 2012; 36:1–69. [DOI] [PubMed] [Google Scholar]
- 26. Trauer JM, Bandaranayake D, Booy R et al Seroepidemiologic effects of influenza A(H1N1)pdm09 in Australia, New Zealand, and Singapore. Emerg Infect Dis 2013; 19:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Macroepidemiology of Influenza Vaccination (MIV) Study Group . The macroepidemiology of influenza vaccination in 56 countries, 1997‐2003. Vaccine 2005; 23:5133–5143. [DOI] [PubMed] [Google Scholar]
- 28. Murata Y, Falsey AR. Respiratory syncytial virus infection in adults. Antivir Therapy 2007; 12:659–670. [PubMed] [Google Scholar]
- 29. Madjid M, Miller C, Zarubaev V et al Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy‐confirmed coronary heart disease death: results from 8 years of autopsies in 34,892 subjects. Eur Heart J 2007; 28:1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brundage JF, Shanks GD. Deaths from bacterial pneumonia during the 1918–19 influenza pandemic. Emerg Infect Dis 2008; 14:1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morens DM, Taubenburger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198:962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 2006; 19:571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Robertson L, Caley JP, Moore J. Importance of Staphylococcus aureus in pneumonia in the 1957 epidemic of influenza A. Lancet 1958; 2:233–236. [DOI] [PubMed] [Google Scholar]
- 34. Murray RJ, Robinson JO, White JN et al Community‐acquired pneumonia due to pandemic A(H1N1)2009 influenzavirus and methicillin resistant Staphylococcus aureus co‐infection. PLoS ONE 2010; 5:e8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scaber J, Saeed S, Ihekweazu C, Efstratiou A, McCarthy N, O'Moore E. Group A streptococcal infections during the seasonal influenza outbreak 2010/2011 in South East England. Euro Surveill 2011; 16. pii=19780. [PubMed] [Google Scholar]
- 36. Shieh WJ, Blau DM, Denison AM et al 2009 pandemic influenza A (H1N1): pathology and pathogenesis of 100 fatal cases in the United States. Am J Pathol 2010; 177:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tamme K, Minajeva A, Adamson V, Ristmagi K, Poder J, Lutsar I. Clinical and pathological findings of fatal 2009‐2010 pandemic influenza A (H1N1) infection in Estonia. Medicina (Kaunas) 2012; 48:48–56. [PubMed] [Google Scholar]
- 38. Prasad HB, Puranik SC, Kadam DB et al Retrospective analysis of necropsy findings in patients of H1N1 and their correlation to clinical features. J Assoc Physicians India 2011; 59:498–500. [PubMed] [Google Scholar]
- 39. Neilson KA, Yunis EJ. Demonstration of respiratory syncytial virus in an autopsy series. Pediatr Pathol 1990; 10:491–502. [DOI] [PubMed] [Google Scholar]
- 40. Meier CR, Jick SS, Derby LE, Vasilakis C, Jick H. Acute respiratory‐tract infections and risk of first‐time acute myocardial infarction. Lancet 1998; 351:1467–1471. [DOI] [PubMed] [Google Scholar]


