Abstract
Background
The annual differences in the seasonal influenza vaccine and the circulating strains make it necessary to assess influenza vaccine effectiveness (VE) yearly. We assessed the effectiveness of the trivalent inactivated influenza vaccine for the 2010–2011 and 2011–2012 influenza seasons among children in Guangzhou, China.
Methods
We conducted a 1:2 matched case–control study based on date of birth (±7 days), gender, and area of residence. The influenza cases from surveillance sites in Guangzhou were laboratory‐confirmed during the 2010–2012 seasons. The controls were randomly selected from children aged 6–59 months in the Children's Expanded Programmed Immunization Administrative Computerized System. The influenza vaccination information for both cases and controls was retrieved from this system.
Results
We analyzed the vaccination information for 1255 influenza cases and 2510 matched controls in 2 influenza seasons in Guangzhou, China. We found that the VE for vaccination during the 2010–2011 and 2011–2012 seasons of virus circulation was 73·2% (95% confidence interval (CI), 52·2–85·0%) and 52·9% (95% CI, 42·1–61·7%), respectively. The VE decreased from 68·9% (95% CI, 57·5–77·2%) in the period between January and March to 48·4% (95% CI, 33·8–59·7%) in the period between April and June.
Conclusions
This post‐licensing study of VE found moderate protection against influenza for vaccinated children aged 6–59 months. Although the influenza vaccine strains for the 2010–2011 and the 2011–2012 seasons were the same, our study indicated that annual vaccination is recommended even for those who received the vaccine during the previous season.
Keywords: Case–control studies, children, China, seasonal influenza, vaccine
Introduction
Influenza causes more morbidity than any other vaccine‐preventable illness; for every 1000 children <5 years of age in the United States, influenza leads to an estimated 0·7–0·9 hospitalizations, 50–95 outpatient visits, 6–27 emergency department visits, and 30–90 courses of antibiotics annually.1, 2 Annual immunization is the primary public health measure for prevention of influenza virus infection.
Guangzhou, located on the Tropic of Cancer, is the largest trading city in southern China. There are 312 583 children <5 years of age (60 000 births per year), comprising 2·5% of the total population of Guangzhou (12·7 million). Routine influenza virus surveillance from Guangzhou shows that the influenza season usually peaks from March to July, and the seasonal influenza vaccine usually becomes available before October.3 While available only in the private sector, influenza vaccination has become more acceptable over time.4 According to the Vaccine Management System of the Guangzhou Center for Disease Control and Prevention (GZCDC), which distributes vaccines to all hospitals and health communities in the city, there were 273 636 and 312 515 doses administered in the 2010–2011 and 2011–2012 seasons, respectively, compared with 147 632 and 201 628 doses in the 2008–2009 and 2009–2010 seasons.
In China, the seasonal influenza vaccine is administered annually to children over 6 months of age; the vaccination is paid for by their guardians. One dose of influenza vaccine is recommended for children aged 6–35 months who received 2 doses of the vaccine in the previous season. Two doses of the influenza vaccine are recommended for children aged 6–35 months who received ≤1 dose of the vaccine in the previous season; the second dose should be given 1 month after the first dose. And one dose of vaccine is recommended annually for children more than 3 years of age (The Instructions of Seasonal Influenza Vaccination in China, 2010–2011). The recommended compositions for the influenza virus vaccines used in the 2010–2011 and 2011–2012 northern hemisphere influenza seasons were the same: A/California/7/2009 (H1N1)‐like virus, A/Perth/16/2009 (H3N2)‐like virus, and B/Brisbane/60/2008‐like virus (Recommendations for Influenza Vaccination Composition, WHO, 2012).
The differences in the vaccine and the circulating strains make it necessary to assess influenza vaccine effectiveness (VE) annually.5, 6 Post‐licensing evaluations of seasonal influenza VE have been reported in different populations in Japan, Australia, Europe, and the United States.7, 8, 9, 10, 11, 12 However, little information is known concerning China. In this study, we assessed the effectiveness of the trivalent inactivated influenza vaccine against laboratory‐confirmed influenza for the 2010‐2011 and 2011–2012 influenza seasons among children in China.
Methods
Study subjects
The laboratory‐confirmed cases came from nineteen sentinel surveillance hospitals that reported the total number of consultations and patients presenting with influenza‐like illness (ILI) to the GZCDC weekly. For the purposes of this study, ILI was defined as a history of body temperature ≥38°C accompanied by a cough or sore throat symptoms (National Influenza Surveillance Plan, 2010, China's Ministry of Health). After the informed consent was signed, participating general practitioners collected data on the patients' birth date, gender, and area of residence. We restricted patients in our study to those aged 6–59 months. Nose and throat swabs were offered to patients presenting within 3 days of symptom onset. Samples from patients with ILI meeting our case definition and who reported to surveillance sites in Guangzhou were subjected to laboratory testing. The presence of the influenza virus in the swab samples was detected by real‐time polymerase chain reaction (RT‐PCR) and/or isolation of the virus in cell cultures, as described previously.3 Influenza cases were confirmed by (i) RNA of influenza virus, including seasonal H1N1, H3N2, and B or (ii) isolation of the virus.
During the influenza seasons under study, we matched 2 controls without ILI symptoms to each case by birth date, gender, and residence (community or village). The controls were randomly selected from children in the Children's Expanded Programmed Immunization (EPI) Administrative Computerized System, which was designed to manage the immunization records of children aged <7 years in Guangzhou in 1997. The EPI system allows healthcare workers to easily record, retrieve, and analyze all children's vaccination information 13; registration of vaccination information in the system is required. A list of potential controls with a sequence number for each case subject was then created from the EPI system. After identification of a potential control, physicians from the GZCDC were called to confirm that the child did not have ILI during the influenza seasons under study. If the potential control declined to participate and/or had a prior history of influenza in the study season, the control candidate with the next closet date of birth to the case subject was enrolled.
Vaccination status
Influenza vaccination status was categorized into vaccinated (fully or partially vaccinated only for children aged 6–35 months) or unvaccinated.
Children aged 6–35 months: Children were considered fully vaccinated if during the influenza seasons included in the study period, they received ≥2 age‐appropriate doses of seasonal influenza vaccine 4 or more weeks apart, with the second dose given ≥2 weeks before acute respiratory symptom onset. Children were also considered fully vaccinated if they received ≥2 doses in the previous season and 1 dose in the study season. Children were counted as partially vaccinated if they received 1 dose in the study season or ≥2 doses in the season immediately prior to the study season (The Instructions of Seasonal Influenza Vaccination in China, 2010–2011) (Figure 1). In our analysis, both fully and partially vaccinated children were regarded as vaccinated. Unless otherwise stated, the reported VE refers to both fully and partially vaccinated children.
Figure 1.
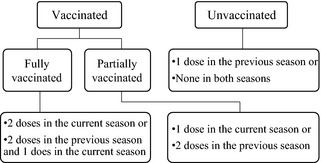
Influenza vaccination status in children aged 6–35 months.
Children aged 36–59 months: Children were counted as vaccinated if they received 1 age‐appropriate dose in the current season, and others were considered as not vaccinated.
For enrollees, only vaccinations received at least 15 days before the onset of influenza were considered valid. Because the influenza vaccine had the same composition in both seasons, we also estimated a combined VE for the 2010–2012 seasons. Regarding the influenza virus surveillance data in Guangzhou (unpublished), the influenza virus circulated from January to June in both the 2010–2011 and the 2011–2012 seasons. We evaluated the VE for the total year (from the previous September 1 to August 31) and for the period of virus circulation (from January 1 to June 30). The VE for the period between January and March and the VE for the period between April and June were also analyzed to evaluate the possibility of waning protection. To examine whether the influenza vaccination continued to benefit recipients a year later, the VE for vaccination in both seasons was also compared with that of the current season.
Statistical analysis
VE was calculated as one minus the odds ratio (OR) × 100%, where the odds of influenza among the vaccinated subjects was compared with the odds of influenza among the unvaccinated subjects. Simple descriptive statistics such as the means, standard deviations, and proportions were used when appropriate. Student t‐tests and chi‐square‐tests were used to analyze the group differences. Conditional logistic regression was used to model the association between influenza vaccination and laboratory‐confirmed influenza. Household registration (local or floating child) and vaccination age were included as potential confounders in the multivariate analysis. SPSS statistical software (version 16.0, SPSS, Inc., Chicago, IL, USA) was used for data validation and statistical analysis. P ≤ 0·05 was regarded as a statistically significant difference. Study approval was obtained from the GZCDC ethics committee.
Results
We included 209 (66·3% of 315) and 1046 (66·9% of 1564) laboratory‐confirmed influenza cases during the 2010–2011 and 2011–2012 seasons, respectively (Figure 2). A total of 624 (33·2% of 1879) cases were excluded due to missing vaccination records or loss during follow‐up. The included cases did not differ from all cases with respect to age, gender, or area of residence (P = 0·572, P = 0·646, and P = 0·826, respectively).
Figure 2.
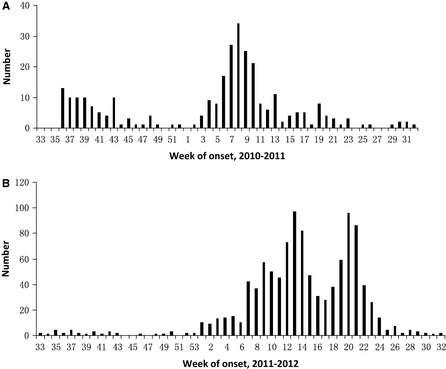
Distribution of influenza cases by onset week in 2010–2012. (A) Distribution of influenza cases by onset week in 2010–2011. (B) Distribution of influenza cases by onset week in 2011–2012.
A total of 1255 matched sets of cases and controls (3765 study subjects) were included during the 2 consecutive influenza seasons in the study. The median age of influenza onset was 32·0 months. Of the 303 cases who received the influenza vaccination, 52·7% and 47·3% were reported from January 1 to March 31 and from April 1 to June 30, respectively.
A higher percentage of the controls received full or partial influenza vaccination than did the cases (Table 1).
Table 1.
Demographic characteristics of study subjectsa
| 2010–2011 season | 2011–2012 season | |||||
|---|---|---|---|---|---|---|
| Number of influenza cases/total 209 (%) | Number of controls/total 418 (%) | P value | Number of influenza cases/total 1046 (%) | Number of controls/total 2092 (%) | P value | |
| Gender | ||||||
| Male | 137 (65·6) | 274 (65·6) | 1·000 | 640 (61·2) | 1280 (61·2) | 1·000 |
| Area | ||||||
| Urban | 70 (33·5) | 140 (33·5) | 1·000 | 519 (49·6) | 1038 (49·6) | 1·000 |
| Rural | 70 (33·5) | 140 (33·5) | 267 (25·5) | 534 (25·5) | ||
| Household register | ||||||
| Local | 178 (86·0) | 305 (73·0) | 0·000 | 830 (79·3) | 1484 (70·9) | 0·000 |
| Age of onset | ||||||
| 6–35 months | 133 (63·6) | 265 (63·4) | 0·953 | 586 (56·0) | 1172 (56·0) | 1·000 |
| 36–59 months | 76 (36·4) | 153 (36·6) | 460 (44·0) | 920 (44·0) | ||
| Age of vaccinationb( ± s(n)) | ||||||
| 21·9 ± 10·5 (26) | 21·1 ± 10·8 (59) | 0·729 | 24·2 ± 12·0 (208) | 25·1 ± 12·9 (647) | 0·340 | |
| Vaccination status | ||||||
| 6–35 months | ||||||
| Fully vaccinated | 14 (10·5) | 59 (22·3) | 0·001c | 67 (11·4) | 280 (23·9) | <0·001c |
| Partially vaccinated | 9 (6·8) | 39 (14·7) | 0·005c | 96 (16·4) | 195 (16·6) | 0·132c |
| Vaccinatedd | 23 (17·3) | 98 (27·0) | <0·001c | 163 (27·8) | 475 (40·5) | <0·001c |
| Unvaccinated | 110 (82·7 | 167 (63·0) | 423 (72·2) | 697 (59·5) | ||
| 36–59 months | ||||||
| Vaccinated | 3 (3·9) | 16 (10·5) | 0·093c | 45 (9·8) | 172 (18·7) | <0·001c |
| Unvaccinated | 171 (81·8) | 137 (89·5) | 415 (90·2) | 748 (81·3) | ||
| 6–59 months | ||||||
| Vaccinated | 26 (12·4) | 114 (27·3) | <0·001c | 208 (19·9) | 647 (30·9) | <0·001c |
| Unvaccinated | 183 (87·6) | 304 (72·7) | 838 (80·1) | 1445 (69·1) | ||
Chi‐square tests were used to analyze group differences.
Only included children received influenza vaccines. Two‐sample t‐test was used to analyze group difference.
Fully/partially vaccinated versus unvaccinated.
Vaccinated (fully or partially vaccinated).
VE estimates during the period of virus circulation (January to June)
From January to June during the 2010–2011 season, the VE was 74·4% for full vaccination for children aged 6–35 months. During this period 2011–2012, the VE was 52·9% for any vaccination for children aged 6–59 months. Influenza vaccination seemed to provide more protection for children aged 36–59 months than for younger children. The VE for the A and B viruses in the 2010–2012 seasons was protective, with the exception of the VE for influenza B in the 2010–2011 season (Table 2).
Table 2.
Seasonal vaccine effectiveness during January to June by age and vaccination status (%, 95% confidence interval)
| 2010–2011 season | 2011–2012 season | |||
|---|---|---|---|---|
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| 6–35 months | ||||
| Fully vaccinated | 75·8 (47·9, 88·7) | 74·4 (44·6, 88·2) | 69·0 (56·5, 77·9) | 68·8 (56·0, 77·9) |
| Partially vaccinated | 71·4 (35·3, 87·3) | 69·3 (30·1, 86·5) | 23·5 (−2·5, 42·9) | 21·8 (−6·1, 42·4) |
| Combined | 73·9 (51·9, 85·8) | 72·3 (48·5, 85·1) | 49·8 (36·2, 60·5) | 49·5 (35·3, 60·6) |
| 36–59 months | ||||
| vaccinated | 76·7 (−10·6, 95·1) | 82·6 (−6·0, 97·1) | 56·9 (37·2, 70·4) | 58·2 (38·7, 71·4) |
| 6–59 months | ||||
| vaccinated | 71·5 (51·0, 83·4) | 73·2 (52·2, 85·0) | 50·2 (39·6, 59·0) | 52·9 (42·1, 61·7) |
| Virus subtype | ||||
| A | 70·2 (42·0, 84·7) | 73·5 (45·5, 87·1) | 44·5 (22·9, 60·0) | 46·5 (24·4, 62·2) |
| B | 98·6 (−1225·9, 100) | 98·6 (−2724·1, 100) | 60·8 (39·3, 74·7) | 66·8 (46·3, 79·5) |
Adjusted by household registration (local or floating child) and vaccination age.
The VE among children who were vaccinated only in the study season and who were vaccinated in both the prior and current seasons was 55·9% (95% CI, 43·8–65·3%) and 56·8% (95% CI, 43·3–67·1%), respectively. However, no significant difference between these two groups was found (P = 0·900).
During the 2010–2012 seasons, the VE was 68·9% (95% CI, 57·5–77·2%) during the period between January and March and then decreased to 48·4% (95% CI, 33·8–59·7%) during the period between April and June.
VE estimates during the whole epidemic year (previous September to August)
Similar VE was found between the period of virus circulation (January to June) and the epidemic year (the previous September to August). The overall VE in the 2010–2011 season was 60·8% for vaccination for children aged 6–59 months. In 2011–2012, the VE was 52·5% for vaccination for children aged 6–59 months (Table 3).
Table 3.
Seasonal vaccine effectiveness during September to August by age and vaccination status (%, 95% confidence interval)
| 2010–2011 | 2011–2012 | |||
|---|---|---|---|---|
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| Vaccinated | ||||
| 6–35 months | 57·5 (30·2, 74·1) | 57·2 (29·1, 74·1) | 47·1 (33·2, 58·1) | 47·8 (33·3, 59·2) |
| 36–59 months | 78·1 (−2·5, 95·3) | 82·6 (3·2, 96·9) | 58·3 (39·3, 71·3) | 60·1 (41·6, 72·8) |
| 6–59 months | 58·1 (34·0, 73·5) | 60·8 (36·7, 75·7) | 48·7 (37·9, 57·5) | 52·5 (41·8, 61·3) |
| Virus subtype | ||||
| A | 62·2 (31·5, 79·1) | 65·7 (35·5, 81·8) | 43·0 (21·1, 58·8) | 45·0 (22·5, 60·9) |
| B | 80·6 (−72·1, 97·8) | 84·2 (−53·5, 98·4) | 58·2 (36·3, 72·6) | 64·1 (43·0, 77·4) |
Adjusted by household register and vaccination age.
Discussion
We analyzed the vaccination information for 1255 laboratory‐confirmed influenza cases and 2510 matched controls in 2 influenza seasons in Guangzhou, China. We discovered that the VE for vaccination versus no vaccination during the season of virus circulation was 73·2% in the 2010–2011 season and 52·9% in the 2011–2012 season. This post‐licensing study of VE found that vaccination provided moderate protection against influenza for children aged 6–59 months.
The present study showed VE similar to those in our previous case–control study in 2009 and 2010 (51·8% and 57·8%, respectively), which was the first post‐licensing study of the influenza vaccine's effectiveness in China.4 Our estimated VE was consistent with reported findings in other countries. For example, Joshi found that in Olmsted County, Minnesota, United States, during the 5 seasons between 1999–2000 and 2006–2007, influenza vaccination provided over 70% protection for children aged <5 years.14 A case–control study conducted in Wisconsin, Michigan, Rochester, and Tennessee, United States, reported a VE of 69%.15
We also discovered that partial vaccination showed no protection in 2011–2012 season; this finding is similar to the findings of Eisenberg in the 2003–2004 and 2004–2005 influenza seasons7 and of Cochran in the 2003–2004, 2004–2005, and 2005–2006 influenza seasons in the United States.16
It has already been shown that the degree of antigenic matching between the circulating and vaccine strains affects VE.17 Belongia et al.18 reported that the influenza VE was <25% for mismatched subtypes, but 50–95% for well‐matched subtypes. Possible explanations are mismatches between the vaccine and circulating influenza strains5 and/or host factors such as immune senescence or impaired immunity. Our viral surveillance data (unpublished) showed that in Guangzhou, the influenza virus constituent ratios for the A (H1N1, H3N2) and B subtypes in 2010–2011 were 83·52% and 16·48%, respectively; the ratios in 2011–2012 were 48·25% and 51·75%, respectively. In the current study, the small sample size (19 cases) in the 2010–2011 season may have resulted in a statistical power too low to accurately calculate the VE for the B virus. The moderate VE we observed may be explained by a limited match between the circulating influenza A virus strains and the vaccine strain. However, due to the lack of further information from the study area or China, we cannot determine how well the circulating and vaccine strains matched.
In the field vaccine evaluation, we observed the possible waning of the protection of influenza vaccination within the period of virus circulation (January to June). The VE decreased from 68·9% during the period from January to March to 48·4% during the period from April to June. This may suggest that waning immunity contributed to the moderate VE observed.11 However, with the sample size available in this analysis, we could not verify this hypothesis. Several serological studies have reported the persistence of the live attenuated influenza vaccine; in one study of children vaccinated against the A/Hong Kong/68(H3N2) virus, the vaccine efficacy against the same strain slowly decreased, but remained at high levels 3 years after vaccination.19 However, other studies have demonstrated that post‐vaccination antibody titers decline over the course of a year.20, 21 Additionally, similar VE was found among children who were vaccinated only in the study season (55·9%) and who were vaccinated in both prior and current seasons (56·8%). In the current study, the influenza vaccine may not continue to benefit recipients a year after vaccination. These results indicate that the protective effects of the vaccine may wane a year post‐vaccination.
Our study has several limitations. First, approximately 30% of the case subjects were missing from the computerized EPI system. It is possible that the exclusion of these case subjects may reduce the generalizability of our findings. This may be due to children from neighboring cities who seek medical treatment in Guangzhou or the floating children lived in Guangzhou. Missing vaccination records may be more frequent among unvaccinated children, which can result in a negative bias to the results. However, according to the sample size calculations, based on Schlesselman's equation for matched case–control studies, the large sample size (especially in the 2011–2012 season) could be sufficient to provide insightful evidence on the influenza VE, and our findings could provide insightful evidence on the influenza VE. Second, in contrast to many other studies in which laboratory‐confirmed controls were reviewed,6, 8, 22 we used healthy controls that were randomly selected from the vaccination registration system and did not report ILIs. Because children aged <5 years are required to register in the vaccination registration system whenever they receive the vaccine, the system covers almost all of the pediatric population under 5 in Guangzhou, except for a portion of the floating children. We think our controls might be more representative sample of the target population than are the cases. However, in studies that used data from test‐negative control subjects, the VE estimates were consistently higher than those that used data from traditional control subjects.18, 23 Additionally, controls were matched to cases by the street on which the cases lived; thus, cases and controls may be comparable in their exposure to influenza, accessible of underlying medical condition, and family financial status. Third, we did not collect and estimate other potential confounders, for example, comorbidity, socioeconomic status, physical health status, and the severity of influenza cases. The collection of these factors may have been important because they may indicate children with underlying health problems and/or children who may be more likely to present to the hospital, which could result in positive or negative biases. Fourth, because the time at risk of controls (who did not get influenza at all during the season) is different to those of cases (who got influenza at a particular time), the case–control method to evaluate VE employed in this study may not be so accurate as in the prospective studies, in which controls would be those who did not get influenza at the time a case presented (i.e., could get influenza later in the season). Another limitation of this study is that the VE estimates based on cases from the influenza sentinel surveillance hospitals may not be representative of all of the influenza cases in Guangzhou.
The seasonal 2010–2012 influenza vaccine showed moderate VE for preventing ILI laboratory‐confirmed influenza infections overall and by influenza type during both seasons. Continued annual assessments of seasonal vaccine efficacy and effectiveness are necessary to better guide vaccination policies and influenza infection control efforts.
Funding
This work was supported by grants from the Guangdong Provincial Department of Science and Technology (2011B050300001 and 2012B091100045) and the Department of Guangzhou Science and Information Technology (2012J5100005).
Conflict of Interests
The authors have no other conflict of interests or funding to disclose.
Acknowledgements
We appreciate the participation of all enrollees in this study,, and special thanks to the public unit coordinators and nurses in the surveillance hospitals in Guangzhou. In the study, nineteen sentinel surveillance hospitals in Guangzhou reported the total number of consultations and patients presenting with influenza‐like illness (ILI): The First Affiliated Hospital of Sun Yat‐sen University, Guangzhou Women and Children Medical Center, Guangzhou Red Cross Hospital, Liwan Hospital of Guangzhou Medical University, Guangzhou First People's Hospital, Guangzhou Yuexiu District Children's hospital, The Third Affiliated Hospital of Sun Yat‐sen University, The Second Affiliated Hospital of Guangzhou Medical University, Baiyun First People's Hospital, Liwan People's Hospital, Guangzhou Development District Hospital, Huangpu Chinese Medicine Hospital, Panyu Center Hospital, Huadu People's Hospital, Conghua Center Hospital, Zengcheng People's Hospital, Nansha First People's Hospital, Yuexiu Second People's Hospital, and Guanghe Hospital.
Fu et al (2013) Seasonal influenza vaccine effectiveness among children, 2010–2012. Influenza and Other Respiratory Viruses DOI:10.1111/irv.12157 7(6), 1168–1174.
References
- 1. Neuzil KM, Mellen BG, Wright PF Jr, Mitchel EF, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med 2000; 342:225–231. [DOI] [PubMed] [Google Scholar]
- 2. Poehling KA, Edwards KM, Weinberg GA et al The underrecognized burden of influenza in young children. N Engl J Med 2006; 355:31–40. [DOI] [PubMed] [Google Scholar]
- 3. Li T, Liu W, Yang Z et al Surveillance analysis of influenza in Guangzhou, 2006. Dis Surv 2008; 23:478–480. [Google Scholar]
- 4. Yang Z, Dong Z, Fu C. Seasonal influenza vaccine effectiveness among children aged 6 to 59 months in southern China. PLoS One 2012; 7:e30424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Szilagyi PG, Fairbrother G, Griffin MR et al Influenza vaccine effectiveness among children 6 to 59 months of age during 2 influenza seasons: a case‐cohort study. Arch Pediatr Adolesc Med 2008; 162:943–951. [DOI] [PubMed] [Google Scholar]
- 6. Staat MA, Griffin MR, Donauer S et al Vaccine effectiveness for laboratory‐confirmed influenza in children 6‐59 months of age, 2005–2007. Vaccine 2011; 29:9005–9011. [DOI] [PubMed] [Google Scholar]
- 7. Eisenberg KW, Szilagyi PG, Fairbrother G et al Vaccine effectiveness against laboratory‐confirmed influenza in children 6 to 59 months of age during the 2003‐2004 and 2004‐2005 influenza seasons. Pediatrics 2008; 122:911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kelly H, Jacoby P, Dixon GA et al Vaccine effectiveness against laboratory‐confirmed influenza in healthy young children: a case‐control study. Pediatr Infect Dis J 2011; 30:107–111. [DOI] [PubMed] [Google Scholar]
- 9. Katayose M, Hosoya M, Haneda T et al The effectiveness of trivalent inactivated influenza vaccine in children over six consecutive influenza seasons. Vaccine 2011; 29:1844–1849. [DOI] [PubMed] [Google Scholar]
- 10. Kissling E, Valenciano M. Early estimates of seasonal influenza vaccine effectiveness in Europe among target groups for vaccination: results from the I‐MOVE multicentre case‐control study, 2011/12. Euro Surveill. 2012; 17:pii: 20146. [PubMed] [Google Scholar]
- 11. Valenciano M, Kissling E. Early estimates of seasonal influenza vaccine effectiveness in Europe: results from the I‐MOVE multicentre case‐control study, 2012/13. Euro Surveill 2013; 18:3. [PubMed] [Google Scholar]
- 12. Suzuki M, Yoshimine H, Harada Y et al Estimating the influenza vaccine effectiveness against medically attended influenza in clinical settings: a hospital‐based case‐control study with a rapid diagnostic test in Japan. PLoS One 2013; 8:e52103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fu C, Wang M, Liang J, He T, Wang D, Xu J. Effectiveness of Lanzhou lamb rotavirus vaccine against rotavirus gastroenteritis requiring hospitalization: a matched case‐control study. Vaccine 2007; 25:8756–8761. [DOI] [PubMed] [Google Scholar]
- 14. Joshi AY, Iyer VN, St SJ, Jacobson RM, Boyce TG. Effectiveness of inactivated influenza vaccine in children less than 5 years of age over multiple influenza seasons: a case‐control study. Vaccine 2009; 27:4457–4461. [DOI] [PubMed] [Google Scholar]
- 15. Treanor JJ, Talbot HK, Ohmit SE et al Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin Infect Dis 2012; 55:951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cochran LW, Black S, Klein NP, Dekker CL, Lewis E, Reingold AL. Vaccine effectiveness against laboratory‐confirmed influenza in infants: A matched case control study. Hum Vaccin 2010; 6:729–735. [DOI] [PubMed] [Google Scholar]
- 17. Heikkinen T, Heinonen S. Effectiveness and safety of influenza vaccination in children: European perspective. Vaccine 2011; 29:7529–7534. [DOI] [PubMed] [Google Scholar]
- 18. Belongia EA, Kieke BA, Donahue JG et al Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004‐2005 season to the 2006‐2007 season. J Infect Dis 2009; 199:159–167. [DOI] [PubMed] [Google Scholar]
- 19. Foy HM, Cooney MK, McMahan R. A Hong Kong influenza immunity three years after immunization. JAMA 1973; 226:758–761. [PubMed] [Google Scholar]
- 20. Ochiai H, Shibata M, Kamimura K, Niwayama S. Evaluation of the efficacy of split‐product trivalent A(H1N1), A(H3N2), and B influenza vaccines: reactogenicity, immunogenicity and persistence of antibodies following two doses of vaccines. Microbiol Immunol 1986; 30:1141–1149. [DOI] [PubMed] [Google Scholar]
- 21. Ambrose CS, Yi T, Walker RE, Connor EM. Duration of protection provided by live attenuated influenza vaccine in children. Pediatr Infect Dis J 2008; 27:744–748. [DOI] [PubMed] [Google Scholar]
- 22. Janjua NZ, Skowronski DM, De Serres G et al Estimates of influenza vaccine effectiveness for 2007‐2008 from Canada's sentinel surveillance system: cross‐protection against major and minor variants. J Infect Dis 2012; 205:1858–1868. [DOI] [PubMed] [Google Scholar]
- 23. Orenstein EW, De Serres G, Haber MJ et al Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol 2007; 36:623–631. [DOI] [PubMed] [Google Scholar]


