Abstract
Please cite this paper as: Mahmud et al. (2012) Outbreaks of influenza‐like illness in long‐term care facilities in Winnipeg, Canada. Influenza and Other Respiratory Viruses 10.1111/irv.12052
Background Outbreaks of influenza‐like illness (ILI) are common in long‐term care facilities (LTCFs) and result in significant morbidity and mortality among residents.
Objectives We describe patterns of reported ILI outbreaks in LTCFs in Winnipeg, Canada, and examine LTCF and outbreak characteristics that influence the clinical outcomes of these outbreaks.
Methods We analyzed the electronic records of all ILI outbreaks reported by LTCFs in Winnipeg from 2003 to 2011. Outbreak duration, ILI attack rates among staff and residents, and residents’ death rates were calculated by presumed viral etiology, staff vaccination rates, type of influenza chemoprophylaxis used, and time to notification to public health.
Results Of a total of 154 reported outbreaks, most (N = 80) were attributed to influenza, and these outbreaks tended to have higher attack and death rates among LTCF residents compared with outbreaks caused by other respiratory viruses (12) or those of unknown etiology (62). About 92% of residents and 38% of staff of the average LTCFs were vaccinated. Chemoprophylaxis was used in 57·5% of influenza outbreaks. Regardless of presumed viral etiology, outbreaks reported within 3 days of onset ended sooner and had lower attack and mortality rates among residents.
Conclusions Influenza‐like illness outbreaks still occur among highly immunized LTCF residents, so in addition to vaccination of staff and residents, it is important to maintain competent infection control practices. Early identification and notification to public health authorities and possibly early initiation of control measures could improve clinical outcomes of ILI outbreaks.
Keywords: Epidemiology, influenza‐like illness outbreaks, long‐term care facilities
Introduction
Outbreaks of influenza and other respiratory pathogens are not uncommon in long‐term care facilities (LTCFs) and could result in significant morbidity and mortality among their residents. 1 , 2 The often crowded environment of LTCFs is conducive to rapid spread of respiratory pathogens, 3 and their residents are typically at higher risk of severe outcomes when infected by these pathogens. 4 However, there are very few published studies that examined the patterns of occurrence and outcomes of outbreaks of influenza‐like illness (ILI) in LTCFs. 5
Long‐term care facilities vary in their ability to respond to ILI outbreaks. 6 It is possible that certain aspects of the LTCF response to the initial cases of an outbreak (e.g., the promptness of initiation of control measures) may influence the outbreak’s clinical outcomes. Identifying these factors may aid in improving the effectiveness of the management of ILI outbreaks, which could translate into improved health outcomes.
In this article, we describe patterns and trends of reported ILI outbreaks in LTCFs within the Winnipeg Health Region (WHR) from 2003 to 2011 and examine LTCF and outbreak characteristics and other aspects of the public health response to the outbreaks that could influence the clinical outcomes of these outbreaks and the effectiveness of outbreak control measures.
Methods
The data analyzed in this report were obtained from the electronic records of the Public Health Program of the WHR. In the Province of Manitoba, ILI outbreaks in LTCFs are reportable to public health authorities. 7 For this purpose, ILI is defined as acute respiratory illness characterized by cough and fever and one or more of sore throat, arthralgia, myalgia, and prostration. 7 Within the LTCF setting, an ILI outbreak is defined as two or more cases of ILI occurring within 7 days and with evidence of spread. 7 Disease management and control measures in response to LTCF ILI outbreaks are guided by regional and provincial guidelines and protocols. These measures include prompt notification of public health authorities, submission of nasopharyngeal swabs for rapid influenza testing, implementation of infection control measures, and antiviral chemoprophylaxis if appropriate. 7
Information available on each outbreak included the name of the LTCF affected by the outbreak, number of residents and staff, influenza vaccination rates for both residents and staff, results of laboratory testing (rapid testing, viral cultures, and more recent PCR), use of chemoprophylaxis, and the outbreak clinical outcomes (e.g., number of cases and deaths occurring among residents during the outbreak period). The present analysis includes all ILI outbreaks reported by LTCFs between September 2003 and August 2011.
Statistical analysis
For each outbreak, we measured the following outcomes: outbreak duration, ILI attack rates among residents and staff during the outbreak, and the death rate among residents. Duration of the outbreak was measured from the date of onset of the index case (“onset of outbreak”) until the date of onset of the last reported case. The resident (staff) attack rate was calculated by dividing the number of reported cases among residents (staff) by the total number of LTCF residents (staff) at the time of the outbreak. The death rate during the outbreak was calculated by dividing the number of reported deaths (from any cause) among residents by the total number of LTCF residents. Rates of hospitalization due to ILI were not calculated because reliable information on number of hospitalizations was not available.
These measures were calculated for all outbreaks and for subsets of outbreaks defined by certain LTCF and outbreak characteristics including presumed viral etiology, staff vaccination rates, type of influenza chemoprophylaxis used, and time to outbreak notification to public health. Time to notification was measured from the date of the onset of the outbreak to the date public health was notified about the outbreak. For the purposes of the present analyses, public health notification was deemed “delayed” if it did not occur within 3 days of the outbreak onset. This cutoff point was chosen because the median time to notification for all outbreaks included in this analysis was 3 days.
The statistical significance of the differences in clinical outcomes by LTCF and outbreak characteristics was assessed using t‐tests (for differences between means) and Kruskall–Wallis tests (for differences between medians). To adjust for potential mutual confounding between the assessed characteristics (e.g., delayed notification could be associated with increased use of chemoprophylaxis), we modeled the effects of these covariates on the geometric mean of the (non‐normally distributed) outcome variables (outbreak duration and resident attack rates) using generalized equation estimation (GEE) linear regression analysis. Generalized equation estimation was used to account for the correlated nature of the data resulting from clustering of outbreaks by LTCFs. All analyses were completed using Strata version 11 (College Station, TX, USA).
Results
During the study period, a total of 154 ILI outbreaks were reported by 37 different LTCFs representing 95% of the 39 LTCFs operating in the WHR. The number of outbreaks reported per facility ranged from 1 to 15 (median = 3). Testing for influenza was performed using rapid antigen assays, viral culture, and PCR for 131 (85%), 96 (62%) and 27 (18%) outbreaks, respectively. At least one individual tested positive for influenza during 63 (48%) of those outbreaks with available rapid testing results. The corresponding figures were 43 (45%) outbreaks for viral cultures and 13 (48%) for viral PCR. Information on laboratory testing was not available for 5 (3%) outbreaks.
Using all available results, the number of outbreaks attributed to influenza A was as follows: 69 (45% of all reported outbreaks), influenza B: 7 (5%), para‐influenza: 7 (5%), respiratory syncytial virus: 4 (3%), rhinovirus: 1 (<1%), and multiple viruses: 4 (3%). In the remaining 62 (40%) outbreaks, the cause of the outbreak was not identified. Whenever two or more viruses were detected (N = 4), either influenza A (N = 3) or influenza B (N = 1) was one of the detected viruses. Overall, influenza A was detected in 72 outbreaks (47%) and influenza B in another 8 (5%) outbreaks.
The largest number of ILI outbreaks in LTCFs was reported during the 2008/2009 flu season (N = 40; Figure 1), which overlapped with the first “wave” of the H1N1 pandemic (April–August, 2009). Influenza A outbreaks were reported during every season (except the 2009/2010 season), whereas influenza B outbreaks were reported only during the 2004/2005 and 2007/2008 seasons. The largest number of influenza A outbreaks was reported during the 2010/2011 (21) and the 2004/2005 seasons (20). As expected, most outbreaks occurred during the winter and spring months (Figure 1). Influenza outbreaks, which comprised most outbreaks reported between December and February, were almost never observed between July and October.
Figure 1.
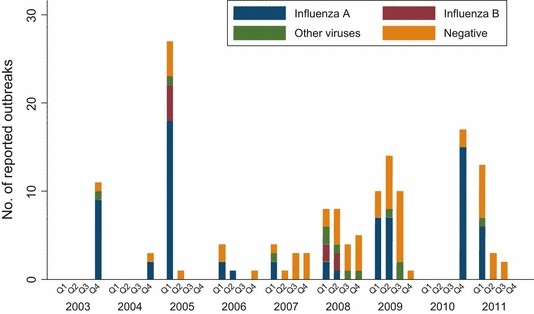
Number of reported influenza‐like illness (ILI) outbreaks by etiology and calendar year and quarter.
Table 1 shows the characteristics of the reported outbreaks by viral etiology. For outbreaks attributed to influenza A and B, the median attack rate was around 7·2% among residents and 3·3% among staff. Outbreaks attributed to other respiratory viruses or to unknown etiology had attack rates that were generally 30–50% lower than the attack rates observed in influenza outbreaks (P = 0·019 for residents and 0·045 for staff). For outbreaks attributed to influenza A or B, the average death rate among residents was 4·4/1000 and was higher for outbreaks attributed to influenza B (6·7/1000), and much lower for outbreaks with unknown etiology (1·1/1000). However, of the 70 deaths reported for all outbreaks, the majority (67%) occurred during influenza A outbreaks.
Table 1.
Characteristics of reported influenza‐like illness outbreaks by etiology
| Influenza A | Influenza B | Influenza | Other viruses | Unknown | All outbreaks | |
|---|---|---|---|---|---|---|
| Total no. of Outbreaks (% of all outbreaks) | 72 (46·8) | 8 (5·2) | 80 (52·0) | 12 (7·8) | 62 (40·3) | 154 |
| Residents | ||||||
| Median no. of residents per LTCF (Q1–Q3) | 150·0 (100·0–213·0) | 143·0 (109·0–155·0) | 150·0 (100·0–213·0) | 174·0 (105·0–216·0) | 155·0 (116·0–230·0) | 151·0 (110·5–216·5) |
| Median vaccination rate/100 residents (Q1–Q3) | 92·0 (88·0–95·1) | 91·0 (91·0–96·0) | 92·0 (88·2–95·1) | 90·5 (90·0–93·0) | 93·0 (89·0–95·8) | 92·0 (89·0–95·0) |
| Median attack rate/100 residents (Q1–Q3) | 7·4 (3·6–13·4) | 6·4 (1·7–9·7) | 7·2 (3·4–11·9) | 4·4 (2·8–7·7) | 4·4 (1·4–10·5) | 6·8 (2·6–11·1) |
| Mean death rate/1000 residents (SD) | 4·1 (7·0) | 6·7 (10·7) | 4·4 (7·4) | 2·4 (3·9) | 1·1 (3·1) | 2·9 (6·0) |
| Staff | ||||||
| Median no. of staff per LTCF (Q1–Q3) | 199·0 (128·8–305·0) | 200·0 (135·0–218·8) | 200·0 (130·0–300·0) | 227·5 (142·5–875·0) | 227·0 (150·0–489·0) | 200·0 (131·0–350·0) |
| Median vaccination rate/100 staff (Q1–Q3) | 38·0 (26·0–58·0) | 49·5 (41·0–52·0) | 40·0 (27·0–58·0) | 32·5 (27·9–37·8) | 37·9 (28·0–52·8) | 38·0 (28·0–55·0) |
| Median attack rate/100 staff (Q1–Q3) | 3·5 (0·6–7·4) | 3·1 (1·1–8·4) | 3·3 (0·7–7·3) | 0·8 (0·0–2·7) | 1·0 (0·0–5·2) | 1·7 (0·0–6·0) |
| Outbreak | ||||||
| No. of outbreaks for which chemoprophylaxis used (%) | 41 (56·9) | 5 (62·5) | 46 (57·5) | 0 (0·0) | 0 (0·0) | 46 (29·9) |
| Median duration of outbreak, days (Q1–Q3) | 16·0 (14·0–22·8) | 19·5 (14·0–45·3) | 16·0 (14·0–22·8) | 20·0 (15·8–29·5) | 21·0 (13·0–30·8) | 18·0 (14·0–26·3) |
| Median time to notification, days (Q1–Q3) | 2·0 (0·8–6·0) | 3·0 (1·8–6·0) | 2·0 (1·0–5·8) | 3·0 (0·0–9·8) | 3·0 (1·0–8·0) | 3·0 (1·0–7·0) |
| Median post‐notification duration, days (Q1–Q3) | 12·0 (11·0–17·5) | 15·0 (10·0–38·0) | 12·5 (11·0–17·8) | 17·5 (12·5–21·0) | 15·5 (11·3–22·5) | 14·0 (11·0–20·0) |
LTCF, long‐term care facility; Q1–Q3, first‐third quartile; SD, standard deviation.
Generally, vaccination rates among residents (median: 92%) were significantly higher than the staff vaccination rates (38%; Table 1). There was also less variability among LTCFs in residents’ vaccination rates with rates ranging from 75% to 100%. Staff vaccination rates ranged from 11% to 90%. The correlation between staff and residents’ vaccination rates was weak (Spearman correlation coefficient = 0·16; P = 0·076). Antiviral chemoprophylaxis was used in 57% and 63% of outbreaks attributed to influenza A and B, respectively (Table 1). Amantadine was used exclusively until the 2003/2004 season, whereas oseltamivir has been used exclusively since the 2005/2006 season.
The median outbreak duration was 18 days (range: 3–53 days), with no statistically significant differences between different etiologies. In most cases, public health authorities were notified within a relatively short period of time (median = 3 days). Notification took place within 7 days in 75% of the outbreaks and within 15 days in 90% of outbreaks. On average, outbreaks reported within 3 days of onset ended sooner (by about 9 days; P < 0·001), regardless of the presumed viral etiology (Table 2). Early notification was also associated with lower attack (P = 0·003) and mortality rates (P = 0·002) among residents, especially if the outbreak was not attributed to influenza.
Table 2.
Outcomes of reported influenza‐like illness outbreaks by etiology and reporting time, staff vaccination uptake, and use of chemoprophylaxis
| Influenza A | Influenza B | Influenza | Other viruses | Unknown | All outbreaks | |
|---|---|---|---|---|---|---|
| Duration of outbreak, median (Q1–Q3) | ||||||
| Outbreak reported in ≤3 days | 14·0 (12·0–19·3) | 15·0 (13·0–17·0) | 14·0 (12·0–17·8) | 18·0 (12·0–20·0) | 15·0 (11·0–21·0) | 14·0 (12·0–20·0) |
| Outbreak reported in >3 days | 22·0 (18·0–29·0) | 37·5 (22·0–53·0) | 22·0 (18·0–29·5) | 28·0 (21·0–34·5) | 31·5 (22·3–40·3) | 26·0 (21·0–35·0) |
| Staff vaccine uptake ≤38% | 16·0 (14·0–24·3) | 22·0 (13·0–53·0) | 16·0 (14·0–25·0) | 16·0 (16·0–16·0) | 17·0 (12·0–27·0) | 16·0 (13·5–24·0) |
| Staff vaccine uptake >38% | 16·0 (12·0–23·0) | 17·0 (17·0–17·0) | 16·5 (12·3–22·8) | 23·5 (18·3–32·5) | 27·5 (16·0–38·0) | 20·0 (14·0–28·8) |
| No chemoprophylaxis | 16·0 (14·0–24·5) | 22·0 (22·0–22·0) | 16·0 (14·0–24·0) | 20·0 (15·8–29·5) | 21·0 (13·0–30·8) | 20·0 (14·0–28·0) |
| Oseltamivir used | 16·0 (13·0–24·3) | 17·0 (13·0–53·0) | 16·0 (13·0–25·0) | – | – | 16·0 (13·0–25·0) |
| Amantadine used | 17·0 (14·0–21·8) | – | 17·0 (14·0–21·8) | – | – | 17·0 (14·0–21·8) |
| Residents’ attack rate (%), median (Q1–Q3) | ||||||
| Outbreak reported in ≤3 days | 7·0 (2·8–10·9) | 7·0 (4·4–9·2) | 7·0 (3·0–10·3) | 2·9 (0·9–3·4) | 3·4 (1·4–7·9) | 4·8 (2·1–9·0) |
| Outbreak reported in >3 days | 10·4 (5·4–18·4) | 1·3 (0·6–18·2) | 10·0 (4·5–18·2) | 7·5 (4·5–13·4) | 7·8 (1·6–11·5) | 8·1 (4·4–14·6) |
| Staff vaccine uptake ≤38% | 7·0 (4·3–13·2) | 5·0 (1·1–12·3) | 7·0 (3·0–11·7) | 4·2 (4·2–4·2) | 4·9 (2·3–10·5) | 6·6 (3·0–11·0) |
| Staff vaccine uptake >38% | 8·9 (3·4–13·4) | 8·0 (8·0–8·0) | 8·8 (3·4–12·9) | 4·6 (1·8–7·5) | 6·9 (1·9–10·8) | 7·1 (2·7–11·2) |
| No chemoprophylaxis | 10·0 (4·3–15·2) | 7·0 (0·6–18·2) | 9·5 (4·3–15·9) | 4·4 (2·8–7·7) | 4·4 (1·4–10·5) | 6·4 (2·5–11·2) |
| Oseltamivir used | 7·0 (2·0–11·7) | 5·9 (2·1–9·2) | 6·9 (2·0–11·3) | – | – | 6·9 (2·0–11·3) |
| Amantadine used | 6·7 (3·8–9·8) | – | 6·7 (3·8–9·8) | – | – | 6·7 (3·8–9·8) |
| Residents’ death rate (/1000), mean (SD) | ||||||
| Outbreak reported in ≤3 days | 3·3 (6·4) | 5·9 (13·2) | 3·6 (7·2) | 0·8 (1·9) | 0·3 (1·6) | 2·1 (5·6) |
| Outbreak reported in >3 days | 5·4 (7·8) | 8·1 (7·0) | 5·6 (7·7) | 4·0 (4·8) | 2·3 (4·2) | 4·1 (6·3) |
| Staff vaccine uptake ≤38% | 5·0 (8·1) | 8·9 (11·7) | 5·6 (8·6) | 0·0 (.) | 1·5 (3·9) | 4·0 (7·4) |
| Staff vaccine uptake >38% | 3·7 (6·2) | 0·0 (.) | 3·6 (6·1) | 3·2 (4·2) | 1·0 (3·0) | 2·7 (5·2) |
| No chemoprophylaxis | 3·2 (6·2) | 3·8 (6·6) | 3·3 (6·2) | 2·4 (3·9) | 1·1 (3·1) | 1·9 (4·4) |
| Oseltamivir used | 3·3 (6·2) | 8·5 (13·0) | 4·2 (7·7) | – | – | 4·2 (7·7) |
| Amantadine used | 6·9 (9·0) | – | 6·9 (9·0) | – | – | 6·9 (9·0) |
| Staff attack rate (%), median (Q1–Q3) | ||||||
| Outbreak reported in ≤3 days | 2·8 (0·3–7·7) | 3·1 (2·2–4·0) | 3·1 (0·5–7·3) | 0·0 (0·0–1·4) | 0·5 (0·0–3·0) | 1·3 (0·0–4·9) |
| Outbreak reported in >3 days | 3·7 (0·8–7·2) | 6·4 (0·0–12·8) | 3·7 (0·7–7·4) | 1·3 (0·0–5·9) | 2·3 (0·9–6·0) | 3·1 (0·8–6·6) |
| Staff vaccine uptake ≤38% | 4·0 (0·5–7·0) | 3·5 (0·8–10·6) | 4·0 (0·5–7·0) | 0·0 (0·0–0·0) | 2·1 (0·0–8·8) | 3·2 (0·0–7·1) |
| Staff vaccine uptake >38% | 2·7 (0·6–9·4) | – | 2·7 (0·6–9·4) | 0·8 (0·0–1·8) | 0·9 (0·0–5·3) | 1·3 (0·3–5·9) |
| No chemoprophylaxis | 4·3 (0·8–9·0) | 7·9 (3·1–12·8) | 4·3 (0·8–9·9) | 0·8 (0·0–2·7) | 1·0 (0·0–5·2) | 1·6 (0·0–6·1) |
| Oseltamivir used | 1·3 (0·3–5·7) | 2·2 (0·0–4·0) | 1·7 (0·3–4·8) | – | – | 1·7 (0·3–4·8) |
| Amantadine used | 3·3 (0·8–8·7) | – | 3·3 (0·8–8·7) | – | – | 3·3 (0·8–8·7) |
Q1–Q3, first–third quartile; SD, standard deviation.
Generally, there were no significant differences in duration of influenza outbreaks or residents’ attack rates when comparing outbreaks reported for LTCFs where staff vaccination rates were >38% (the median staff vaccination rate) and those with 38% or lower staff vaccination rates (Table 2). Attack rates among staff were slightly lower in outbreaks reported from LTCFs with higher staff vaccination rates, but the difference was not statistically significant (P = 0·579). The average death rate among residents for all influenza outbreaks (3·6/1000) was slightly lower in outbreaks reported for LTCFs where staff vaccination rates were >38% than for those with staff vaccination rate ≤38% (5·6/1000), but the difference was not statistically significant (P = 0·4617). Similarly, use of antiviral chemoprophylaxis in influenza outbreaks was not associated with shorter outbreak duration or with lower mortality. However, chemoprophylaxis was associated with lower residents’ attack rates (median ∼6·8% compared with 9·5% for outbreaks where chemoprophylaxis was not used).
Using multivariate GEE linear regression models, delayed notification was associated with 60% (95% CI 30–100%; P < 0·001) increase in the duration of the typical outbreak regardless of LTCF size (as measured by resident and staff census), staff vaccination rate, or presumed etiology; and none of these latter factors had a detectable independent effect on outbreak duration (Table 3). When analyses were limited to influenza outbreaks (and additional adjustment was made for use of chemoprophylaxis), the effect of delayed notification was associated with 50% (95% CI: 20–80%) increase in the duration of the typical outbreak, and again, none of the other variables (including chemoprophylaxis) had a detectable independent effect on outbreak duration. Delayed notification was also associated with 80% (95% CI 30–150%; P = 0·001) and 50% (95% CI 0–140%; P = 0·076) increase in the residents’ attack rate of the typical outbreak and the typical influenza outbreak, respectively (Table 3). Use of chemoprophylaxis was associated with small statistically non‐significant reduction in the average residents’ attack rate (−10%; 95% CI −40 to 50%; P = 0·762).
Table 3.
Relative risk estimates (95% confidence intervals) from generalized estimating equation (GEE) models of the association between listed variables and outbreak duration and residents’ attack rates
| Outbreak duration, geometric mean | Residents’ attack rate, geometric mean | |
|---|---|---|
| All outbreaks | ||
| Outbreak reported in >3 days | 1·6 (1·3–2·0) | 1·8 (1·3–2·5) |
| Staff vaccine uptake ≤38% | 1·1 (0·9–1·4) | 1·2 (0·9–1·6) |
| No. of residents | 1·0 (1·0–1·0) | 1·0 (1·0–1·0) |
| No. of staff | 1·0 (1·0–1·0) | 1·0 (1·0–1·0) |
| Etiology | ||
| Influenza A | Ref | Ref |
| Influenza B | 1·2 (0·8–1·8) | 0·6 (0·2–1·3) |
| Other viruses | 1·3 (1·0–1·8) | 0·6 (0·4–0·8) |
| Unknown | 1·0 (0·8–1·3) | 0·7 (0·5–0·9) |
| Influenza outbreaks only | ||
| Outbreak reported in >3 days | 1·5 (1·2–1·8) | 1·5 (1·0–2·4) |
| Staff vaccine uptake ≤38% | 1·0 (0·8–1·3) | 1·5 (1·0–2·3) |
| No. of residents | 1·0 (1·0–1·0) | 1·0 (1·0–1·0) |
| No. of staff | 1·0 (1·0–1·0) | 1·0 (1·0–1·0) |
| Chemoprophylaxis used | 1·0 (0·7–1·3) | 0·9 (0·6–1·5) |
Discussion
We found that almost all LTCFs in the WHR were frequently affected by ILI outbreaks during the study period. Yet, it is likely the frequency of these outbreaks was underestimated in this study because it is unlikely that all outbreaks were reported to public health authorities. 8 Using both active surveillance and retrospective chart audits, Loeb et al. 1 identified 46 outbreaks in five nursing homes in metropolitan Toronto over 3 years. However, our figures are comparable with those reported from systems based on passive surveillance. 5
More than half of the reported outbreaks in this analysis were attributed to influenza, and these outbreaks tended to have higher attack and death rates among LTCF residents compared with outbreaks caused by other respiratory viruses or those of unknown etiology. In a review of 207 published reports about infectious disease outbreaks in LTCFs around the world, the most common etiologic agent was influenza, accounting for 24% of reported outbreaks. 9 For the reported influenza outbreaks, the median attack rate among residents was 35% with a case fatality rate of 6·5%. 9 These figures are much higher than those observed in our analysis, likely because severe influenza outbreaks are more likely to be reported in the scientific literature. It is also possible that the attack rates were lower in our analysis due to incomplete identification or reporting of symptomatic cases or deaths during outbreaks.
We also found that, on average, outbreaks reported within 3 days of onset ended sooner regardless of the size of the LTCF, viral etiology, staff vaccination rate, or use of chemoprophylaxis. This is consistent with the results of a study investigating outbreaks of lower respiratory infections in nursing homes in France, which found higher attack rate, hospitalization rate, and case fatality rate among residents when outbreaks were reported three or more days after disease onset. 5 Among aged‐care facilities participating in an active surveillance system for the detection of respiratory illness outbreaks in Sydney, Australia, those which implemented prevention and control measures within 7 days of onset of the first case had attack rates of 14% and 3% in the first two outbreaks after implementation, 8 much lower than the attack rate of 45% among similar facilities in eastern Australia. 10
We found that residents’ vaccination rates were high across the board (median = 92%), whereas staff vaccination rates were generally much lower (median = 38%) and varied significantly between LTCFs (from 11% to 90%). Because of the consistently high residents’ vaccination rate, it was not possible to reliably assess the role of this factor in determining outbreak outcomes. Obviously, despite consistently high rates of vaccination among LTCF residents, many ILI outbreaks still occurred. This is consistent with the results of many other studies 1 , 5 , 8 , 10 , 11 and suggests reduced vaccine effectiveness among LTCF residents. Mismatch between vaccine strains and circulating influenza strains may have contributed to lower vaccine effectiveness in this population, 12 as we observed that outbreaks were more common in seasons when there was a vaccine mismatch, for example, the 2004/2005 season. 13
Further, staff vaccination did not appear to have an important role in determining outbreak outcomes. This does not rule out an important role for staff vaccination in preventing the occurrence of influenza outbreaks. Staff are often implicated in the introduction and transmission of influenza in LTCFs. 8 However, direct evidence for the effectiveness of staff vaccinations in preventing influenza outbreaks among LTCF residents is not strong. Recently, the results of a Cochrane review indicated that vaccination of healthcare workers who work with the elderly has no effect on the incidence of laboratory‐confirmed influenza, pneumonia, or deaths from pneumonia among LTCF residents. 14 Although three studies in that review reported reduced ILI and resident all‐cause mortality in facilities with higher staff vaccination rates, the authors attributed the effects to residual confounding by factors that might be associated with increased vaccine uptake among staff such as improved infection control measures that affect the incidence of all respiratory pathogens and not just influenza. 14 On the other hand, several observational studies have found that higher rates of staff and resident vaccination may be associated with lower incidence of ILI and mortality among residents. 15 , 16 Very few studies specifically examined the effect of staff vaccination on the occurrence of ILI outbreaks in LTCFs. 17
Except for slightly lower attack rates among residents, use of chemoprophylaxis in outbreaks attributed to influenza A or B was not associated with significantly better outcomes. The strength of these associations may have been reduced by confounding due to the fact that chemoprophylaxis is typically used in more severe outbreaks which by definition are longer in duration and have higher attack rates. In one randomized controlled trial, the use of oseltamivir to prevent influenza among residents of LTCFs was 90% effective in preventing laboratory‐confirmed influenza. 18 This is consistent with the results of a study where oseltamivir was used in all nursing homes with ongoing influenza transmission, and earlier intervention was found to result in better outbreak control, 17 and other results suggesting that starting chemoprophylaxis within 5 days of the onset of an influenza A outbreak is associated with faster resolution and with lower incidence and case fatality rates. 19 This hypothesis could not be tested directly in our analysis because of lack of information on when chemoprophylaxis was started. It is possible that in the context of high vaccination rates among residents, chemoprophylaxis adds additional benefits only if started during the early phases of an outbreak when a larger number of susceptible individuals could be potentially protected by chemo‐prophylaxis. This hypothesis is worth testing in larger studies because of the implication that the benefits of delayed administration of prophylaxis may not justify the associated health risks and costs.
Limitations
Although we included all outbreaks reported to public health from virtually all LTCFs operating in Winnipeg, these analyses were limited by small numbers and by the inability to control for potential confounding by characteristics of the virus causing the outbreak (e.g., strain virulence and vaccine strain match), LTCF factors (e.g., effectiveness and speed of implementation of other outbreak control measures such as isolation and respiratory hygiene), and by residents’ characteristics such as socioeconomic status and levels of comorbidity and overall functional status. For instance, it is possible that LTCFs that tend to notify public health early on are also more successful in implementing other outbreak control measures or are more likely to have healthier residents. If this is the case, early notification could be simply a marker of a better functioning LTCF. Due to the lack of information on these factors, it was not possible to account for their potential confounding effects. Nonetheless, a causal interpretation of these findings is plausible. Early notification permits early identification of the etiological agent and the institution of appropriate and specific interventions such as chemoprophylaxis. Finally, we were unable to assess the causes of delayed notification due to the lack of information on the likely complex management issues that may influence the decision to notify public health.
Conclusions
Our study demonstrates that influenza outbreaks do still occur among highly immunized LTCF residents, so in addition to vaccination of staff and residents, it is important to maintain high levels of competency in infection control, detection, and notification among staff. 20 Early identification and notification to public health authorities and possibly early initiation of control measures could improve clinical outcomes of ILI outbreaks.
Funding
SMM was supported by the Mona and Allen Copp Award from the Cancer Care Manitoba Foundation and by an establishment grant from the Manitoba Health Research Council. SMM is the Great‐West Life, London Life and Canada Life Junior Investigator of the Canadian Cancer Society (grant no. 2011‐700644).
Disclaimers
The interpretation and conclusions contained herein do not necessarily represent those of the Winnipeg Regional Health Authority.
Acknowledgement
We are grateful to the Communicable Disease Coordinators of the Population and Public Health Program of the Winnipeg Regional Health Authorities who collected the data used in this study.
References
- 1. Loeb M, McGeer A, McArthur M, Peeling RW, Petric M, Simor AE. Surveillance for outbreaks of respiratory tract infections in nursing homes. Can Med Assoc J 2000; 162:1133–1137. [PMC free article] [PubMed] [Google Scholar]
- 2. Tamblyn S. Recognizing and controlling respiratory disease outbreaks in long‐term care facilities. CMAJ 1997; 157:1257. [PMC free article] [PubMed] [Google Scholar]
- 3. Lofgren E, Fefferman N, Naumov Y, Gorski J, Naumova E. Influenza seasonality: underlying causes and modeling theories. J Virol 2007; 81:5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dolin R. Influenza – interpandemic as well as pandemic disease. N Engl J Med 2005; 353:2535–2537. [DOI] [PubMed] [Google Scholar]
- 5. Vaux S, Poujol I, Bonmarin I, Lévy‐Bruhl D, Desenclos J‐C. Surveillance of lower respiratory tract infections outbreaks in nursing homes in France. Eur J Epidemiol 2009; 24:149–155. [DOI] [PubMed] [Google Scholar]
- 6. Stevenson CG, McArthur MA, Naus M, Abraham E, McGeer AJ. Prevention of influenza and pneumococcal pneumonia in Canadian long‐term care facilities: how are we doing? Can Med Assoc J 2001; 164:1413–1419. [PMC free article] [PubMed] [Google Scholar]
- 7. Manitoba Health . Communicable disease management protocols: seasonal influenza. Winnipeg, Manitoba, 2011. Available at http://www.gov.mb.ca/health/publichealth/cdc/protocol/influenza1.pdf (Ac‐cessed 15 December 2011).
- 8. Rosewell A, Chiu C, Lindley R et al. Surveillance for outbreaks of influenza‐like illness in the institutionalized elderly. Epidemiol Infect 2010; 138:1126. [DOI] [PubMed] [Google Scholar]
- 9. Utsumi M, Makimoto K, Quroshi N, Ashida N. Types of infectious outbreaks and their impact in elderly care facilities: a review of the literature. Age Ageing 2010; 39:299–305. [DOI] [PubMed] [Google Scholar]
- 10. Guy RJ, Di Natale R, Kelly HA et al. Influenza outbreaks in aged‐care facilities: staff vaccination and the emerging use of antiviral therapy. Med J Aust 2004; 180:640. [DOI] [PubMed] [Google Scholar]
- 11. Morens DM, Rash VM. Lessons from a nursing home outbreak of influenza A. Infect Control Hosp Epidemiol 1995; 16:275–280. [DOI] [PubMed] [Google Scholar]
- 12. Durando P, Iudici R, Alicino C et al. Adjuvants and alternative routes of administration towards the development of the ideal influenza vaccine. Hum Vaccin Immunother 2011; 7:29–40. [DOI] [PubMed] [Google Scholar]
- 13. Skowronski DM, De Serres G, Dickinson J et al. Component‐specific effectiveness of trivalent influenza vaccine as monitored through a sentinel surveillance network in Canada, 2006–2007. J Infect Dis 2009; 199:168–179. [DOI] [PubMed] [Google Scholar]
- 14. Thomas RE, Jefferson T, Lasserson TJ. Influenza vaccination for healthcare workers who work with the elderly. Cochrane Database Syst Rev 2010; 2:CD005187. [DOI] [PubMed] [Google Scholar]
- 15. Lemaitre M, Meret T, Rothan‐Tondeur M et al. Effect of influenza vaccination of nursing home staff on mortality of residents: a cluster‐randomized trial. J Am Geriatr Soc 2009; 57:1580–1586. [DOI] [PubMed] [Google Scholar]
- 16. Cai S, Temkin‐Greener H. Influenza vaccination and its impact on hospitalization events in nursing homes. J Am Med Dir Assoc 2011; 12:493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Monto AS, Rotthoff J, Teich E et al. Detection and control of influenza outbreaks in well‐vaccinated nursing home populations. Clin Infect Dis 2004; 39:459. [DOI] [PubMed] [Google Scholar]
- 18. Peters PH Jr, Gravenstein S, Norwood P et al. Long‐term use of oseltamivir for the prophylaxis of influenza in a vaccinated frail older population. J Am Geriatr Soc 2001; 49:1025–1031. [DOI] [PubMed] [Google Scholar]
- 19. Rubin MS, Nivin B, Ackelsberg J. Effect of timing of amantadine chemoprophylaxis on severity of outbreaks of influenza A in adult long‐term care facilities. Clin Infect Dis 2008; 47:47–52. [DOI] [PubMed] [Google Scholar]
- 20. Smith PW, Bennett G, Bradley S et al. SHEA/APIC Guideline: infection prevention and control in the long‐term care facility. Infect Control Hosp Epidemiol 2008; 29:785–814. [DOI] [PMC free article] [PubMed] [Google Scholar]


