Abstract
Background
Acute respiratory infections are an important cause of morbidity and mortality worldwide, with a major burden of disease in developing countries. The relative contribution of viruses in acute lower respiratory infections (ALRI) is, however, poorly documented in Lao PDR.
Objective
The objective of this study is to investigate the etiology of ALRI in patients of all ages in two hospitals of Laos.
Methods
Multiplex PCR/RT‐PCR methods were used to target 18 major common respiratory viruses. Between August 2009 and October 2010, samples from 292 patients presenting with ALRI were collected.
Results and conclusion
Viruses were detected in 162 (55%) samples. In 48% (140/292) of the total ALRI cases, a single virus was detected while coinfections were observed in 8% (22/292) of the samples. The most frequent viruses were rhinovirus/enterovirus (35%), human respiratory syncytial virus (26%), and influenza viruses (13%). Parainfluenza viruses were detected in 9%, adenovirus in 6%, human metapneumovirus in 4%, coronaviruses (229E, NL63, OC43, HKU1) in 4%, and bocavirus in 3% of ALRI specimens. Most viral infections occurred in patients below 5 years of age. The distribution of viruses varied according to age‐groups. No significant correlation was observed between the severity of the disease and the age of patients or the virus species. This study provides the description of viral etiology among patients presenting with ALRI in Lao PDR. Additional investigations are required to better understand the clinical role of the different viruses and their seasonality in Laos.
Keywords: Acute lower respiratory infection, Lao People's Democratic Republic, respiratory viruses, South‐East Asia
Introduction
Acute respiratory infections are a leading cause of morbidity and mortality worldwide.1 They represent around 2 million deaths per year, especially in infants.2 The burden of these infections is particularly important in developing countries.3 During the last decade, South East Asia received much attention from the international scientific community due to the emergence of respiratory viruses with pandemic potential (SARS‐CoV, avian influenza A/H5N1 virus).4
Respiratory infections can be caused by numerous viruses, including influenza viruses, parainfluenza viruses, human respiratory syncytial virus (HRSV), human metapneumo‐virus (HMPV), human coronaviruses (HCoV), adenoviruses, human bocavirus, and human enteroviruses. Molecular techniques have become more and more popular to detect these viruses. Multiplex reverse transcription–polymerase chain reaction (RT‐PCR) has been shown to be a sensitive tool and allows identification of a majority of respiratory viruses, as well as coinfections.5, 6, 7
In Lao PDR, the etiology of respiratory infections is still poorly documented. To improve the clinical management of the patients, limit unnecessary antibiotic use, and prevent opportunistic secondary infections, it appears important to develop surveillance and tools to assess the etiology of acute respiratory infections in this country.8, 9
The purpose of this study was to describe during a limited period of time the viral etiology of acute lower respiratory infections (ALRI) in patients hospitalized in two Lao hospitals by using a set of five multiplex RT‐PCR/PCR targeting 18 common respiratory viruses.
Materials and methods
Study design
This study was part of the Surveillance and Investigation of Epidemic Situations in South‐East Asia (SISEA) Project. This project was implemented by the International Network of Pasteur Institutes in Asia to improve the surveillance and management of epidemic situations in the region.
The study included children and adults hospitalized for ALRI. For patients below 5 years of age, ALRI was defined as cough or dyspnea on admission with a duration of symptoms <14 days and polypnea at more than 50 breaths/min for children aged below 1 year and more than 40 breaths/min for children between 1 and 5 years old. In infants and young children, fever is sometimes absent and was therefore not considered as an inclusion criteria.
For the children between 5 and 15 years old, the inclusion criteria in addition to the cough, was fever >38°C on admission (or history of fever) with a history of symptoms of <14 days. For the adults (>15‐years‐old), ALRI definition consisted of cough and fever for <14 days and one of the following symptoms of lower respiratory infection: dyspnea, chest pain, and abnormal auscultatory findings.
Patients with known tuberculosis (TB), known acquired immunodeficiency such as HIV/AIDS, and cancer were excluded.
Severity was assessed according to the World Health Organization criteria.10
The surveillance of ALRI was conducted in two Laotian hospitals: Setthathirath Hospital in Vientiane capital (between August 2009 and October 2010) and in the Provincial Hospital of Luang Prabang province (between January and June 2010).
In Setthathirath Hospital, the patients enrolled in the study were from all ages and were recruited from three wards: pediatric, intensive care unit, and internal medicine. In Luang Prabang Provincial Hospital, only children below 5 years of age hospitalized in the pediatric ward were included.
Sample collection
For each patient who met the ALRI case definition, nasopharyngeal and throat swabs were collected and immediately placed into a sterile tube containing viral transport medium (VTM).11 The samples were transported at 4°C to the National Center for Laboratory and Epidemiology. They were then aliquoted and stored at −80°C prior to testing.
RNA and DNA extraction
Nucleic acids were extracted using Qiagen Viral RNA Mini Kit (Qiagen, CA, USA). For each sample, 140 μl of VTM was processed according to the manufacturer's instructions, eluted in 60 μl of Qiagen AVE buffer, and then stored at −80°C until testing by multiplex PCR/RT‐PCR.
Multiplex PCR/RT‐PCR
Five multiplex PCR/RT‐PCR were used to screen for eighteen common respiratory viruses: influenza A (IA); influenza B (IB); influenza C (IC); HMPV; HRSV; parainfluenza viruses 1‐4 (PIV1‐4); human rhinovirus (HRhV); enteroviruses; severe acute respiratory syndrome‐associated coronavirus (SARS‐CoV); HCoVs OC43, 229E, HKU1, and NL63; human bocavirus, and adenoviruses. These tests were performed as described by Buecher et al.6, 7, 12
Influenza A viruses subtyping
Influenza A strains were subtyped according to the method developed by the two French National Influenza Centres (Northern and Southern France) and described in the WHO information for laboratory diagnosis of pandemic (H1N1) 2009 virus.13, 14
Clinical data
Hospital physicians filled out a standard case report form for each participant, including information on patient's medical history, clinical features, treatment, laboratory and radiological results, and status at the time of discharge.
Medical records and chest x‐rays were retrospectively reviewed by an expert pulmonologist.
Severity was assessed according to the World Health Organization criteria.10
Clinical data were anonymized and entered into a database by two persons who did not have knowledge of virus identities.
Ethical statement
The protocol was approved by the National Ethical Committee of Lao PDR and by the Clinical Research Committee (CoRC) of Institut Pasteur in Paris.
Samples were collected after an informed written consent was obtained from the patients or their guardians/parents (in children below 15 years of age).
Statistical analysis
Proportions were compared using a chi‐squared or contingency table randomization test as appropriate. P‐values < 0·05 were considered significant. The Mann–Whitney U‐test was applied to compare continuous or ordinal measures. Analyses were performed using stata/SE version 11.1 (StataCorp., College Station, TX, USA) and R statistical software (R 2.8.1, R Foundation for Statistical Computing, Vienna, Austria).
For analysis purposes, the following pathogens were grouped together: HCoV OC43, HCoV HKU1, HCoV 229E, and HCoV NL63 (HCoVs); influenza A, influenza B, and influenza C viruses (influenza); parainfluenza viruses 1‐4 (PIVs); rhinoviruses and enteroviruses (rhinoviruses/enteroviruses).
Results
Patient characteristics
Between August 2009 and October 2010, 292 nasopharyngeal swabs were collected including 27 samples from Luang Prabang Provincial Hospital and 265 samples from Setthathirath Hospital (Vientiane). Of the 292 patients, 71% were hospitalized in pediatric ward, 24% in internal medicine department, and 5% in intensive care unit. The median duration of hospitalization was 4 days.
Of the study population, 145 (49·7%) were male. The median age of the patients was 2·2 years (range: 12 days–86 years) with 187 patients (64%) below 5 years of age. The different age‐groups are described in Figure 1.
Figure 1.
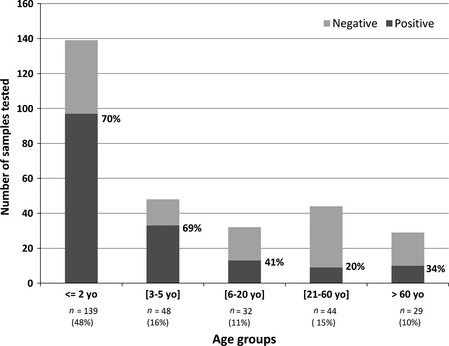
Positivity rate for respiratory viruses detection by age‐group. N represents the number of cases in each age‐group.% in bracket represents the proportion of patients included in the age‐group in comparison with the total population.
To allow comparisons with statistical significance, the 5 age‐groups were merged into two main groups: infants and children aged ≤5 years (N = 187, 64%), and adults and children above 5 years of age (N = 105, 36%).
Prevalence of respiratory viruses
A total of 186 viruses were detected in 162 [55·5%; CI 95 = (50–61%)] of the 292 samples tested. The most common viruses were rhinoviruses/enteroviruses [35%; CI 95 = (28–42%)], HRSV [26%; CI 95 = (20–33%)], influenza viruses [12%; CI 95 = (8–17%)], PIVs [9%; CI 95 = (5–13%)], and adenoviruses [6%; CI 95 = (3–9%)]. Human metapneumovirus and HCoV were identified in 4% [CI 95 = (1–6%)] and bocavirus in 3% [CI 95 = (1–6%)] of the samples. Severe acute respiratory syndrome‐associated coronavirus was never detected (Figure 2).
Figure 2.
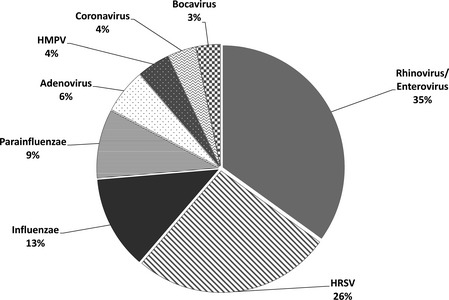
Proportion of each virus/group of viruses detected in respiratory samples.
Influenza A viruses were detected in 9·1% of the samples (17 cases, all 2009 pandemic H1N1 virus), influenza B in 2·7% (five cases), and influenza C in only one case. PIV‐1 and PIV‐3 represented the majority of the PIVs detected (35·3% each). Multiple viral infections were observed in 22 [7·5%; CI 95 = (5–11%)] patients including 20 dual infections and two infections with three different viruses (Table S1). Among these multiple viral infections, the most frequent pathogens were rhinoviruses, HRSV, and adeno‐viruses.
Relation of viral etiology with age
A majority of ALRI were observed among children aged ≤5 years (187/292). The positivity rate was also the highest (i.e., 70%) in children aged ≤5 (Figure 1).
Human respiratory syncytial virus was found in patients aged from 15 days to 31 years but was more frequent in young children aged ≤5 years (P = 0·0097) (Figure 3). Influenza viruses were detected in all age‐groups (median age = 12·3 years; range: 3·6 months–66 years), but were more frequent in the age‐group >5 years (P < 10 E‐6). Rhinoviruses were mainly detected in young patients and in few occasions in elderly (median age = 1·2 years; range: 12 days – 86 years). Bocavirus was exclusively detected in children below 5 years of age (range = 6 months – 3·5 years). For the other viruses, there was no difference statistically significant between both the age‐groups.
Figure 3.
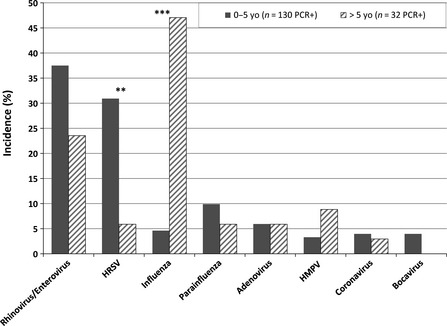
Incidence of respiratory virus infections in two main age‐groups. **P < 0·05, ***P < 0·001.
Seasonal distribution
The monthly distribution of the viral respiratory infections as well as of the rhinoviruses/enteroviruses and HRSV is shown in Figure 4. The average number of samples collected monthly was 19·5 (range: 8–32). We did not observe a clear seasonality for the ALRI associated with a respiratory virus. Rhinovirus was detected all year round. Human respiratory syncytial virus circulation seemed more important during the rainy season, between July and October. The prevalence of the other viruses was low during the study period, ranging from 0 to 5 positive samples per month for each virus. These numbers appeared too low to allow the description of seasonal patterns. However, PIVs were detected only between March and August 2010 with a peak in May.
Figure 4.
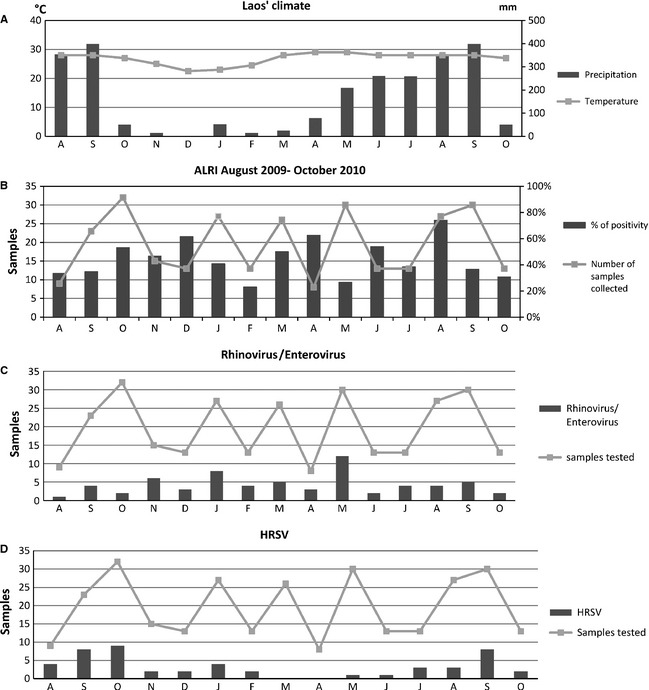
Climate in Lao PDR (A), seasonal occurrences of viral respiratory infections (B), seasonality of rhinovirus/enteroviruses (C), and HRSV (D) between August 2009 and October 2010.52
Coinfections were observed all year long, but with only one or two cases per month. As for PIVs, there was a peak in May 2010, which was most probably only a bias corresponding to a higher number of samples collected during that month.
Clinical characteristics
The clinical classification of the 162 patients who tested positive is summarized in the Table 1. Bronchitis and exacerbation of asthma were the most frequent clinical presentations recorded in patients who tested positive for respiratory viruses (59/162, 36%). Bronchiolitis and pneumonia with or without pleurisy were identified in respectively 20% and 19% of the patients with viral infections. The expert pulmonologist could not review 39 clinical records, which were excluded from the clinical analysis and labeled as unspecified ALRI. There was no significant difference in clinical presentation between patients with single infections and those with multiple viral infections. A total of 64 viruses were detected in the 59 patients presenting with bronchitis, 39 viruses in the 33 patients who experienced a bronchiolitis, and 37 viruses in the 31 patients with pneumonia. In bronchiolitis, the most common viruses detected were HRSV (16 patients) and rhinovirus (15 patients). In patients presenting with bronchitis and pneumonia, all the respiratory viruses tested were detected with an approximately similar frequency. The proportion of patients from whom no virus was detected was higher in patient hospitalized for pneumonia (46%) than in those presenting with bronchitis or asthma (37%) or bronchiolitis (23%). Bronchiolitis was diagnosed almost exclusively in patients aged <2 years (31 of 33), whereas pneumonia was more frequent in patients aged >5 years.
Table 1.
Clinical characteristics of patients who tested positive by multiplex PCR/RT‐PCR
| Clinical diagnosis | Virus (%)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rhinoviruses/Enteroviruses (n = 65) | HRSV (n = 49) | Influenza viruses (n = 23) | Parainfluenza viruses (n = 17) | Adenoviruses (n = 11) | HMPV (n = 8) | Coronaviruses (n = 7) | Bocavirus (n = 6) | Total | |
| Bronchitis or asthma (n = 59) | 23 (35) | 19 (39) | 10 (43) | 4 (24) | 4 (36) | 2 (25) | 2 (29) | 0 | 64 (34%) |
| Bronchiolitis (n = 33) | 15 (23) | 16 (33) | 0 | 2 (12) | 1 (9) | 1 (12) | 1 (14) | 3 (50) | 39 (21%) |
| Pneumonia ± Pleurisy (n = 31) | 11 (17) | 7 (14) | 9 (39) | 3 (18) | 3 (27) | 1 (12) | 1 (14) | 2 (33) | 37 (20%) |
| Unspecified ALRI (n = 39) | 16 (25) | 7 (14) | 4 (17) | 8 (47) | 3 (27) | 4 (50) | 3 (43) | 1 (17) | 46 (25%) |
HRSV, human respiratory syncytial virus; HMPV, human metapneumovirus; ALRI, acute lower respiratory infections.
The percentage corresponds, for each virus, to the proportion of positive detected in each clinical group. The sum for a column is 100%.
Severity
Three deaths occurred during the study period. For two of them, no virus was identified, and for one patient, a rhinovirus was detected. These patients were respectively 5, 18 and 60 years old.
Of the 162 patients for which respiratory viruses were detected, 39 (24%) presented symptoms of severity. Among these, 45 viruses were identified. The most common virus observed was the rhinovirus (21), followed by HRSV (7), influenza viruses (6), and bocavirus (4).
Discussion
In this study, we report for the first time in Lao PDR the viral etiologies in patients hospitalized for ALRIs. We identified 186 respiratory viruses in 162 (55%) patients of all ages using 5 multiplex PCR/RT‐PCR. Rhinovirus and HRSV were the viruses the most frequently detected, representing 35% and 26% of the total number of viruses observed, respectively. These results are consistent with other studies conducted in the region previously.6, 15, 16 The majority of the patients included in the study were aged <5 years (64%), and 48% were <2 years old.
Human respiratory syncytial virus is frequently defined as the predominant virus associated with hospitalizations for ALRI in children aged ≤5 years.17, 18, 19 However, in our study, we detected more rhinoviruses (HRhVs) and enteroviruses (35%) than HRSV (26%) among the 292 patients included. Even if HRhVs are typically associated with the common cold,20 recent studies suggest that these viruses may also be associated with more severe illness, including lower respiratory disease and asthma exacerbations.21, 22 In this study, HRhV was detected in respiratory specimens from 33% of patients with bronchitis or asthma, in 25% of patients with bronchiolitis, and in 16% of those presenting with pneumonia. Rhinoviruses/enteroviruses were often implicated in coinfections (73% of all the coinfections detected). However, the clinical significance of the detection of a HRhV by a highly sensitive RT‐PCR method has been questioned as these viruses can also be detected in asymptomatic children.23, 24 Rhinoviruses and enteroviruses seem to circulate all year round, without clear seasonality.
Human respiratory syncytial virus was the second most common virus detected in this study with a total of 49 cases (26% of patients with a positive RT‐PCR result). This virus is recognized as the leading cause of hospitalizations in children aged ≤5 years for respiratory illness in industrialized countries.25, 26, 27 Similarly to other countries, we demonstrated a substantial burden of HRSV‐associated ALRI in Lao PDR. The infants and children aged <5 years were significantly more frequently infected, and then the incidence of HRSV infections decreased with age, probably because of the development of anti‐HRSV immunity which is boosted during each subsequent reinfection.28, 29, 30 During the study period, the peak of HRSV activity occurred from June to October, which corresponds to the rainy season (Figure 4). Similar observations were also reported in neighboring countries.17, 25, 31
The overall incidence of influenza viruses infection was relatively low (12%), and the majority of the cases detected (69·6%) were among patients older than 5 years with a median age of 12·3 years. These results are in line with those of a preliminary study on influenza‐like illness in Lao PDR in which the incidence of influenza was 10·4%.32 The influenza A virus strains detected during the study period were exclusively 2009 pandemic H1N1 viruses.
As expected and as reported in other studies, the serotypes 1 and 3 were the most frequent PIVs detected in Laos.12, 17, 33 However, PIV‐4 was also identified only in four cases and accounted for 24·5% of all the PIVs detected. PIV‐4 is usually uncommon,17, 34, 35 but has been identified in severe respiratory illnesses,5, 36 and its role is probably more important than originally thought.37, 38
The overall prevalence of HMPV was 4%, which is comparable to the results reported in Greece,39 the USA,40 or Thailand.41 The detection rate of the human bocavirus, another recently discovered respiratory virus, was 3%. This prevalence appears to vary largely between countries: 0·4% in Cambodia6 where a similar study was conducted, 3·9% in Thailand,42 16% in Vietnam,17 and 24·5% in China.16 Bocavirus is often implicated in coinfections.12, 19, 43 In this study, 66% of the bocavirus strains detected were observed during multiple infections.
Human coronaviruses were detected in 4% of the ALRI patients in Lao PDR, which is comparable to other countries (China: 5%; Vietnam: 8%, Cambodia: 8%).6, 16, 17 HCoV‐OC43, HCoV‐NL63, and HCoV‐229E were identified only in patients aged <4 years while the only case of HCoV‐HKU1 infection was observed in a 75 years old patient hospitalized for pneumonia.
Of the 162 patients infected by a respiratory virus, we detected 13·6% of coinfections. The majority of these multiple infections were identified in patients <5 years of age (90%) and associated a rhinovirus (15/22). As rhinoviruses were detected all year round, coinfection cases were also observed regularly each month. Respiratory virus coinfections being frequent,5, 19, 44 it demonstrates the usefulness of the multiplex RT‐PCR approach, which allows the detection of the most important viruses in only few reactions while multiple infections are often undetected in viral culture or by direct immunofluorescence. Nevertheless, the difficult question of the clinical significance of these multiple infections remains unanswered.
In our study, we did not see any association between coinfection and severity of the disease, which is in line with other reports,5, 19, 24, 45 but this has been subjected to much controversy.46, 47, 48
We also did not find any significant association between any virus and disease severity.
In this study, bronchitis and pneumonia were the most frequent clinical presentations observed among all the age‐groups of patients hospitalized for ALRI. Bronchiolitis was observed almost exclusively in patients <2 years of age (40/43 cases). The viruses that were most frequently detected in patients <2 years of age with bronchiolitis were HRSV (15) and HRhVs (14), which is consistent with previous observations.49, 50, 51 Fifty‐seven percent of pneumonia occurred in children below 5 years of age. A better understanding of the roles of the different viruses is of great importance as pneumonia is responsible for approximately 19% of all deaths in children aged <5 years, of which more than 70% take place in sub‐Saharan Africa and South‐East Asia.1 The two main viruses observed in pneumonia in Lao PDR were HRhVs and influenza A viruses.
Even if Lao PDR is significantly less populated than neighboring Thailand, Vietnam, and China, the viral etiologies observed in Laotian patients hospitalized with ALRI demonstrate some similarities to those of other South‐East Asian countries. However, this study has several limitations. Indeed, it was conducted in only two sites (Vientiane Capital and Luang Prabang), and the second site was included only during the last 6 months of the study. Moreover, our sample size is limited, especially when we stratify by age and viral etiology. Finally, it was difficult to collect sputum, particularly in young children. Thus, identification of bacteria was not possible.
This study aimed at determining the main viral etiologies of patients hospitalized in Lao PDR with ALRI. Rhinoviruses, HRSV, and influenza virus were the more common viruses detected in the patients. Bronchitis and pneumonia accounted for the majority of the hospitalizations for ALRI. These data are consistent with those of the literature. This study demonstrated also the usefulness of multiplex PCR/RT‐PCR to detect viral infections and to expand our knowledge of respiratory infections in such country where the data are still sparse. Although the low numbers of some viruses do not allow drawing clear conclusions and considering that bacterial infections cannot be dismissed, this study provides some important preliminary data that can be used for other more focused surveys in a larger population, for instance to better describe the seasonality of the respiratory viruses. The frequency of viral infection should be taken into account by pediatricians to avoid unnecessary use of antibiotics.
Supporting information
Table S1. Multiple infections detected in 292 patients presenting with ALRI.
Acknowledgements
This study was supported by Surveillance and Investigation of Epidemic Situations in South‐East Asia (SISEA) project, a grant from the French Agency for Development (AFD). We gratefully thank Dr Anne Mornand for her precious expertise in pediatric pulmonology and review of the clinical data and Dr Jean‐Jacques Bernatas for the coordination of the project. We thank the team of the Virology Unit at Institut Pasteur in Cambodia for their assistance in implementing the multiplex PCR/RT‐PCR techniques in Lao PDR.
Sentilhes A‐C et al (2013) Respiratory virus infections in hospitalized children and adults in Lao PDR . Influenza and Other Respiratory Viruses 7(6), 1070–1078.
References
- 1. Rudan I. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ 2008; 86:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Williams BG, Gouws E, Boschi‐Pinto C, Bryce J, Dye C. Estimates of world‐wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis 2002; 2:25–32. [DOI] [PubMed] [Google Scholar]
- 3. Mizgerd JP. Lung infection–a public health priority. PLoS Med 2006; 3:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coker RJ, Hunter BM, Rudge JW, Liverani M, Hanvoravongchai P. Emerging infectious diseases in southeast Asia: regional challenges to control. Lancet 2011; 377:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bellau‐Pujol S, Vabret A, Legrand L et al Development of three multiplex RT‐PCR assays for the detection of 12 respiratory RNA viruses. J Virol Methods 2005; 126:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buecher C, Mardy S, Wang W et al Use of a multiplex PCR/RT‐PCR approach to assess the viral causes of influenza‐like illnesses in Cambodia during three consecutive dry seasons. J Med Virol 2010; 82:1762–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang W, Ren P, Sheng J et al Simultaneous detection of respiratory viruses in children with acute respiratory infection using two different multiplex reverse transcription‐PCR assays. J Virol Methods 2009; 162:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nascimento‐Carvalho CM, Ribeiro CT, Cardoso MR et al The role of respiratory viral infections among children hospitalized for community‐acquired pneumonia in a developing country. Pediatr Infect Dis J 2008; 27:939–941. [DOI] [PubMed] [Google Scholar]
- 9. Scott JA, Brooks WA, Peiris JS, Holtzman D, Mulholland EK. Pneumonia research to reduce childhood mortality in the developing world. J Clin Invest 2008; 118:1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. WHO . Cough or Difficult Breathing. Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Illnesses with Limited Resources. Geneva, Switzerland: World Health Organization, 2005. [Google Scholar]
- 11. Horm VS, Gutierrez RA, Nicholls JM, Buchy P. Highly pathogenic influenza A(H5N1) virus survival in complex artificial aquatic biotopes. PLoS One 2012; 7:e34160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choi EH, Lee HJ, Kim SJ et al The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000–2005. Clin Infect Dis 2006; 43:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. WHO . WHO Information for Laboratory Diagnosis of Pandemic (H1N1) 2009 Virus in Humans – Revised. Geneva, Switzerland: World Health Organization, 2009. Available at http://www.who.int/csr/resources/publications/swineflu-WHO_Diagnostic_RecommendationsH1N1_20090521.pdf. [Google Scholar]
- 14. Duchamp MB, Casalegno JS, Gillet Y et al Pandemic A(H1N1)2009 influenza virus detection by real time RT‐PCR: is viral quantification useful? Clin Microbiol Infect 2010; 16:317–321. [DOI] [PubMed] [Google Scholar]
- 15. Yoshida LM, Suzuki M, Yamamoto T et al Viral pathogens associated with acute respiratory infections in central Vietnamese children. Pediatr Infect Dis J 2010; 29:75–77. [DOI] [PubMed] [Google Scholar]
- 16. Wang W, Cavailler P, Ren P et al Molecular monitoring of causative viruses in child acute respiratory infection in endemo‐epidemic situations in Shanghai. J Clin Virol 2010; 49:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Do AH, van Doorn HR, Nghiem MN et al Viral etiologies of acute respiratory infections among hospitalized Vietnamese children in Ho Chi Minh City, 2004–2008. PLoS One 2011; 6:e18176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Olsen SJ, Thamthitiwat S, Chantra S et al Incidence of respiratory pathogens in persons hospitalized with pneumonia in two provinces in Thailand. Epidemiol Infect 2010; 138:1811–1822. [DOI] [PubMed] [Google Scholar]
- 19. Tripp R, Bezerra PGM, Britto MCA et al Viral and atypical bacterial detection in acute respiratory infection in children under five years. PLoS One 2011; 6:e18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hayden FG. Rhinovirus and the lower respiratory tract. Rev Med Virol 2004; 14:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miller EK, Lu X, Erdman Dean D et al Rhinovirus‐associated hospitalizations in young children. J Infect Dis 2007; 195:773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Louie JK, Roy‐Burman A, Guardia‐LaBar L et al Rhinovirus associated with severe lower respiratory tract infections in children. Pediatr Infect Dis J 2009; 28:337–339. [DOI] [PubMed] [Google Scholar]
- 23. Cowling B, Fry AM, Lu X et al Human rhinovirus infections in rural Thailand: epidemiological evidence for rhinovirus as both pathogen and bystander. PLoS One 2011; 6:e17780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singleton RJ, Bulkow LR, Miernyk K et al Viral respiratory infections in hospitalized and community control children in Alaska. J Med Virol 2010; 82:1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fry AM, Chittaganpitch M, Baggett HC et al The burden of hospitalized lower respiratory tract infection due to respiratory syncytial virus in rural Thailand. PLoS One 2010; 5:e15098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hall CB, Weinberg GA, Iwane MK et al The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360:588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nair H, Nokes DJ, Gessner BD et al Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta‐analysis. Lancet 2010; 375:1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mufson MA, Orvell C, Rafnar B, Norrby E. Two distinct subtypes of human respiratory syncytial virus. J Gen Virol 1985; 66(Pt 10):2111–2124. [DOI] [PubMed] [Google Scholar]
- 29. Reiche J, Schweiger B. Genetic variability of group A human respiratory syncytial virus strains circulating in Germany from 1998 to 2007. J Clin Microbiol 2009; 47:1800–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bhattarakosol P, Pancharoen C, Mungmee V, Thammaborvorn R, Semboonlor L. Seroprevalence of anti‐RSV IgG in Thai children aged 6 months to 5 years. Asian Pac J Allergy Immunol 2003; 21:269–271. [PubMed] [Google Scholar]
- 31. Arnott A, Vong S, Mardy S et al A study of the genetic variability of human respiratory syncytial virus (HRSV) in Cambodia reveals the existence of a new HRSV group B genotype. J Clin Microbiol 2011; 49:3504–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vongphrachanh P, Simmerman JM, Phonekeo D et al An early report from newly established laboratory‐based influenza surveillance in Lao PDR. Influenza Other Respir Viruses 2010; 4:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Glezen WP, Frank AL, Taber LH, Kasel JA. Parainfluenza virus type 3: seasonality and risk of infection and reinfection in young children. J Infect Dis 1984; 150:851–857. [DOI] [PubMed] [Google Scholar]
- 34. Akinloye OM, Rönkkö E, Savolainen‐Kopra C et al Specific viruses detected in Nigerian children in association with acute respiratory disease. J Trop Med 2011; 2011:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schmidt AC, Schaap‐Nutt A, Bartlett EJ et al Progress in the development of human parainfluenza virus vaccines. Expert Rev Respir Med 2011; 5:515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lindquist SW, Darnule A, Istas A, Demmler GJ. Parainfluenza virus type 4 infections in pediatric patients. Pediatr Infect Dis J 1997; 16:34–38. [DOI] [PubMed] [Google Scholar]
- 37. Ren L, Gonzalez R, Xie Z et al Human parainfluenza virus type 4 infection in Chinese children with lower respiratory tract infections: a comparison study. J Clin Virol 2011; 51:209–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aguilar JC, Perez‐Brena MP, Garcia ML, Cruz N, Erdman DD, Echevarria JE. Detection and identification of human parainfluenza viruses 1, 2, 3, and 4 in clinical samples of pediatric patients by multiplex reverse transcription‐PCR. J Clin Microbiol 2000; 38:1191–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gioula G, Chatzidimitriou D, Melidou A, Exindari M, Kyriazopoulou‐Dalaina V. Contribution of human metapneumovirus to influenza‐like infections in North Greece, 2005‐2008. Euro Surveill 2010; 15: pii: 19499. [DOI] [PubMed] [Google Scholar]
- 40. Williams John V, Edwards Kathryn M, Weinberg Geoffrey A et al Population‐based incidence of human metapneumovirus infection among hospitalized children. J Infect Dis 2010; 201:1890–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Samransamruajkit R, Thanasugarn W, Prapphal N, Theamboonlers A, Poovorawan Y. Human metapneumovirus in infants and young children in Thailand with lower respiratory tract infections; molecular characteristics and clinical presentations. J Infect 2006; 52:254–263. [DOI] [PubMed] [Google Scholar]
- 42. Fry Alicia M, Lu X, Chittaganpitch M et al Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis 2007; 195:1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sloots T, McErlean P, Speicher D, Arden K, Nissen M, Mackay I. Evidence of human coronavirus HKU1 and human bocavirus in Australian children. J Clin Virol 2006; 35:99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Auburn H, Zuckerman M, Broughton S, Greenough A, Smith M. Detection of nine respiratory RNA viruses using three multiplex RT‐PCR assays incorporating a novel RNA internal control transcript. J Virol Methods 2011; 176:9–13. [DOI] [PubMed] [Google Scholar]
- 45. Pierangeli A, Gentile M, Di Marco P et al Detection and typing by molecular techniques of respiratory viruses in children hospitalized for acute respiratory infection in Rome, Italy. J Med Virol 2007; 79:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aberle JH, Aberle SW, Pracher E, Hutter H‐P, Kundi M, Popow‐Kraupp T. Single versus dual respiratory virus infections in hospitalized infants. Pediatr Infect Dis J 2005; 24:605–610. [DOI] [PubMed] [Google Scholar]
- 47. Bharaj P, Sullender WM, Kabra SK et al Respiratory viral infections detected by multiplex PCR among pediatric patients with lower respiratory tract infections seen at an urban hospital in Delhi from 2005 to 2007. Virol J 2009; 6:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McNamara PS, Flanagan BF, Smyth RL, Hart CA. Impact of human metapneumovirus and respiratory syncytial virus co‐infection in severe bronchiolitis. Pediatr Pulmonol 2007; 42:740–743. [DOI] [PubMed] [Google Scholar]
- 49. Pavia AT. Viral infections of the lower respiratory tract: old viruses, new viruses, and the role of diagnosis. Clin Infect Dis 2011; 52(Supplement 4):S284–S289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Khadadah M, Essa S, Higazi Z, Behbehani N, Al‐Nakib W. Respiratory syncytial virus and human rhinoviruses are the major causes of severe lower respiratory tract infections in Kuwait. J Med Virol 2010; 82:1462–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Calvo C, García‐García ML, Blanco C, Pozo F, Flecha IC, Pérez‐Breña P. Role of rhinovirus in hospitalized infants with respiratory tract infections in Spain. Pediatr Infect Dis J 2007; 26:904–908. [DOI] [PubMed] [Google Scholar]
- 52. Anonymous . Vientiane climate guide to the average weather & temperatures, 2011.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Multiple infections detected in 292 patients presenting with ALRI.


