Abstract
Please cite this paper as: Kersun et al. (2013) A prospective study of chemotherapy immunologic effects and predictors of humoral influenza vaccine responses in a pediatric oncology cohort. Influenza and Other Respiratory Viruses 7(6), 1158–1167.
Background: Pediatric oncology patients represent a cohort of individuals uniquely at risk of complications from influenza, yet less likely to respond to the vaccine. It is not yet clear how to best protect this vulnerable population.
Methods: We performed a prospective analysis of 177 pediatric oncology patients to define the predictors of influenza vaccine responses. Each variable was examined over three time points and a repeated measure analysis was performed.
Results: Patients with ALL vaccinated during induction phase had superior influenza vaccine responses than those subjects vaccinated during post‐induction or maintenance phases (P = 0·0237). Higher aggregate HAI titer responses were associated with a higher baseline B‐cell count (P = 0·0240), and higher CD4 and CD8 influenza‐specific T‐cell responses, suggesting prior antigen exposure is a significant contributor. The solid tumor cohort had equivalent responses during all time frames of chemotherapy.
Discussion: The optimal protection from influenza of pediatric patients on chemotherapy should include vaccination, but it is clear that not all patients produce high titers of antibodies after vaccination. This study identified biomarkers that could be used to individualize vaccine approaches. Immunologic predictors might have a role in targeting resources, as B‐cell counts predicted of vaccine responses among the patients with ALL.
Keywords: ALL, antibody, B cell, ELISPOT, influenza, vaccine
Introduction
Influenza continues to represent an important pathogen in the United States. Significant immune compromise represents one of the more common high‐risk conditions and pediatric cancer patients have demonstrated a high rate of complications from influenza. 1 , 2 , 3 , 4 , 5 A significant concern regarding pediatric patients with malignancy is that concomitant infection with the influenza virus can prolong hospitalization and delay chemotherapy. 5 From a public health perspective, immune compromised patients can shed the influenza virus for a prolonged period and develop resistance to antiviral agents, thus representing a high‐risk reservoir for other children in the hospital. 6 , 7 For all these reasons, understanding the variables that govern vaccine responses is of critical importance in this vulnerable population.
The current guidelines for the administration of the influenza vaccine in pediatric oncology patients have recommended waiting until patients are on maintenance or intermittent chemotherapy to initiate vaccination. 8 , 9 Given these data, it is surprising that vaccination rates of children on chemotherapy as well as other high‐risk children are quite low. 10 , 11 During the 2009 H1N1 pandemic, there was pressure to identify populations who would most benefit from vaccination due to the limited availability of vaccine initially. This reignited the interest in stratifying high‐risk patients. A recent Cochrane report noted the challenges in comparing studies or performing a meta‐analysis. 12 While a titer of 1:40 is considered protective in healthy populations, it is not necessarily protective for immune compromised patients. Nevertheless, titers of >1:40 are considered seroprotective. A more specific measure of vaccine responses is to report a fourfold increase in titer as seroconversion. It is not possible to directly compare studies that report seroconversion with those that report seroprotection. Nevertheless, recent studies have improved our understanding of the effects of chemotherapy on vaccine responses. A cohort of predominantly pediatric ALL patients was studied to define the impact of influenza vaccination. Children off therapy for at least 6 months had serologic and clinical responses comparable with controls. 13 In a recent study of breakthrough influenza after vaccination, 10% of patients required an ICU admission after vaccination and 5% of patients died. 4 We had previously estimated a breakthrough rate of 15% using an indirect analysis. 14 In all the studies of influenza vaccine responses in children on chemotherapy, the seroconversion rates have varied from 21 to 60%. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26
One factor that drives vaccination efforts is the seasonal availability of the vaccine. We previously performed a study that examined vaccination of patients with ALL during induction, post‐induction or maintenance chemotherapy. 14 In that study, we demonstrated that the influenza vaccine response was significantly better when patients with ALL were vaccinated during induction phase. We found early vaccination elicited the same response as late vaccination in patients with solid tumors. This study was performed to examine laboratory predictors of the influenza vaccine response.
Methods
Study design
Patients with acute lymphoblastic leukemia (ALL) (n = 110) and solid tumors receiving cyclical chemotherapy (n = 67) seen at The Children’s Hospital of Philadelphia were approached regarding participation beginning with the 2006–2007 season and ending with the 2009–2010 season. Inactivated influenza vaccine was given to all patients, with naïve, young vaccine recipients receiving two doses and repeat vaccinees receiving one dose, according to the Advisory Committee on Immunization Practices. 27 Eighty‐nine patients had HAI titers previously reported. 14 The demographic information for each group is given in Table 1. Laboratory studies were collected on the day of vaccination and at 2 months, 4 months, and 1‐year post‐vaccination. Clinical data were also recorded at each time point. This study was designed as a prospective study with predefined analyses and was approved by the IRB.
Table 1.
Demographic information
| Number of Patients | Age mean (std) | Age median (IQR) | Male n (%) | White n (%) | |
|---|---|---|---|---|---|
| ALL | 125 | 7·81 (5·15) | 6·08 (7·25) | 64 (51·2) | 97 (83·7) |
| ALL SR* | 65 | 5·70 (2·83) | 5·17 (3·00) | 33 (50·8) | 52 (83·9) |
| ALL HR** | 52 | 10·48 (5·75) | 10·99 (11·20) | 26 (50·0) | 40 (87·0) |
| ALL relapse† | 8 | 7·68 (7·78) | 5·63 (13·30) | 5 (62·5) | 5 (62·5) |
| Sarcoma + Brain tumor | 72 | 10·32 (5·70) | 10·67 (8·92) | 43 (59·7) | 52 (78·8) |
| Sarcoma | 54 | 11·15 (5·30) | 12·33 (6·59) | 33 (61·1) | 38 (76·0) |
| Sarcoma chemotherapy†† | 52 | 10·91 (5·20) | 12·00 (6·84) | 31 (59·6) | 38 (79·2) |
| Sarcoma biologics‡ | 2 | 17·38 (0·77) | 17·38 (1·08) | 2 (100) | 0 (0·0) |
| Brain tumor | 18 | 7·81 (6·56) | 6·42 (11·25) | 10 (55·6) | 14 (87·5) |
| Standard chemotherapy‡‡ | 10 | 5·85 (6·45) | 2·75 (6·54) | 7 (70·0) | 9 (100) |
| Alternative management§ | 8 | 10·26 (6·21) | 9·11 (7·54) | 3 (37·5) | 5 (71·4) |
*Cooperative Group Trials for Standard Risk ALL.
**Cooperative Group Trials for High‐Risk ALL.
†Cooperative Group or local institutional protocols for relapsed ALL.
††Cooperative Group Trials for Ewing’s Sarcoma, Rhabdomyosarcoma and Osteosarcoma.
‡Cooperative Group Developmental Therapeutic Studies.
‡‡Cooperative Group or local institutional protocols for CNS tumors.
§Irinotecan, cis‐retinoic acid, bevacizumab, vinblastine.
Immunologic assays
Hemagglutination inhibition assays were carried out using the season‐specific HA antigen. 28 Flow cytometry and functional analyses were performed as previously described. 29 , 30 , 31
Statistical methods
Statistical analyses were performed using sas software version 9.2 for windows (SAS Institute Inc., Cary, NC, USA). We defined HAI titers at baseline and defined patients who had at least a fourfold increase at any subsequent time point as Responders. The ALL group was divided into three cohorts depending on the stage of their chemotherapy at the time of vaccination; induction, post‐induction, and maintenance. The solid tumor group was divided into three cohorts depending on the length of time they had been on chemotherapy on the day they were vaccinated; <1 month, 1–3 month, and >3 month. We defined the geometric mean titer at each time point. The geometric mean titers were calculated using the standard formula: n‐th root of (X 1)(X 2)...(X n). The 95% confidence intervals of the geometric mean titers were calculated by taking the antilog of the 95% confidence intervals of the arithmetic means of the log‐transformed values. The comparison of the study’s endpoints measured repeatedly over time was carried out using the mixed effects models and/or the generalized estimating equations (GEE) method. The longitudinal assessments of the outcomes were statistically tested using a repeated measures model with the following three main effects: The overall group differences, the overall changes over time, and the interaction effect. Baseline measurements for both groups were used as covariates to adjust for potential group differences at baseline. The independent t‐test or the Mann–Whitney test was used for the comparisons between the ALL and the Solid Tumor groups, and the Kruskal–Wallis test was used for comparing data between the three stages of chemotherapy and the three solid tumor cohorts. Data are also presented descriptively using mean and standard deviation (SD) for continuous variables and frequencies (%) for categorical variables. Statistical significance was defined at a P value <0·05.
Results
Vaccine responses
To compare the effect of different chemotherapy regimens on the response to the inactivated influenza vaccine, we divided our cohort into patients with ALL and those patients with sarcomas or brain tumors receiving cyclical chemotherapy (Figure 1). The patients with sarcomas and brain tumors were combined because of the similar nature of their chemotherapy and are referred to as the “solid tumor” group. Patients were enrolled and vaccinated according to the availability of the vaccine and not the stage of chemotherapy, thus providing a real‐life diversity in the timing of vaccine administration. Patients were stratified at the time of analysis according the phase of chemotherapy to compare responses. We calculated the responder (seroconversion) frequency for patients with ALL and solid tumors. A Responder was defined as a fourfold increase in titer to at least one serotype in the vaccine compared with baseline. Approximately, half of all patients responded to at least one serotype (Figure 1A). There were no statistically significant differences in responder status comparing the ALL and solid tumor groups. We further examined seroconversion in a serotype‐specific manner (Figure S1) and found no significant differences between the serotypes. In addition, we calculated the geometric mean titer at each time point (Figure 1B, C, D). No statistically significant difference was seen comparing individual time points in the ALL and solid tumor groups. The GEE was used to identify differences between the two cohorts, evaluating both the changes over time and the group variable. In this analysis, vaccine responses were significantly better in the solid tumor group for responses to H1N1 compared with the ALL cohort (P = 0·0017). These data also demonstrate the limited durability of the humoral response to the vaccine. The variability of the responses I more easily appreciated in the dot plots shown in Figure S1.
Figure 1.
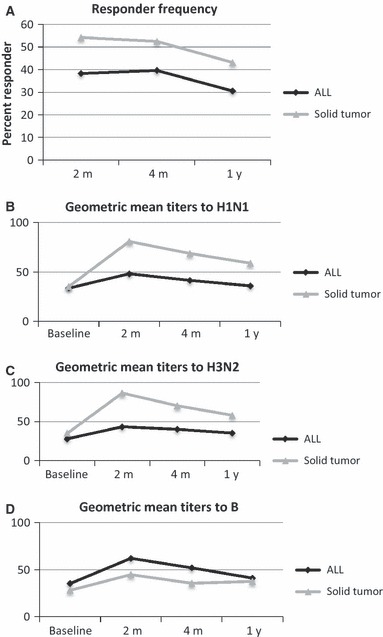
A comparison of influenza vaccine antibody responses in patients with ALL and solid tumors. (A) The responder frequency was defined by identifying individuals with a fourfold increase from baseline in any of the three serotypes. The differences between the two groups (ALL and Solid Tumor) are not significant. The Geometric Mean Titer was calculated at each time point for each group and displayed for the H1N1 (B), H3N2 (C) and influenza B (D) serotypes. The GEE method found that for all three serotypes there was a time effect (P < 0·0001 for all), reflecting differences across the time points. Significant group effects were found for H1N1 and H3N2 with P values of 0·0060 and 0·0135, respectively). Only H1N1 demonstrated a significant group*time effect with P = 0·0017.
ALL B cells
To examine the cumulative effect of chemotherapy on vaccine responses, we stratified our ALL cohort according to whether they received the vaccine during induction, during post‐induction, or while on maintenance chemotherapy. In our previous study of a subset of 89 of these patients with ALL, we found that the most robust responses to vaccination occurred when subjects were vaccinated during induction chemotherapy. 14 We reanalyzed the data using the entire cohort and found once again that vaccination during induction was still strongly associated with the best vaccine responses (Figure 2A). At the time of the peak humoral response (2 months), there was a significant difference between the three ALL cohorts with P = 0·0237.
Figure 2.
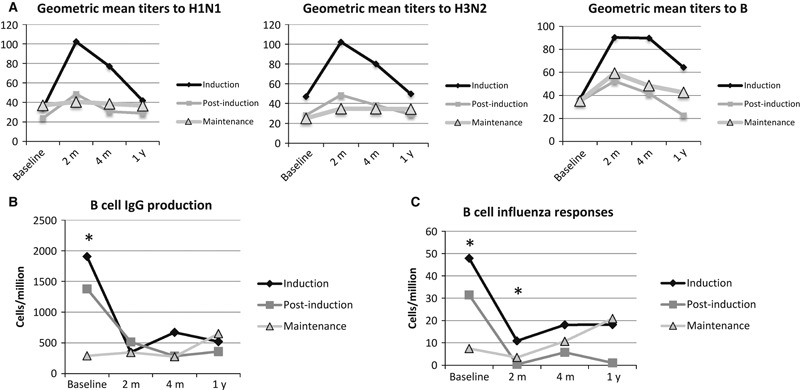
ALL chemotherapy has a cumulative effect on B‐cell function. The ALL group was divided into three cohorts depending on the stage of chemotherapy at the time of vaccination. We identified differences between the three cohorts using the Kruskal–Wallis test. The cohort vaccinated during induction had more robust responses to each serotype (A). We examined total IgG production after stimulation (B) in an ELISPOT analysis and found that the three cohorts differed at baseline (P = 0·0001). We examined influenza antibody production in an ELISPOT analysis (C) and found that there were significant differences at baseline and the 2‐month time point (P < 0·0001 and P = 0·0111, respectively). Asterisks indicate significance.
To better understand the basis of this association, we examined B‐cell function by measuring total IgG‐producing cells (Figure 2B) and influenza‐specific antibody production (Figure 2C) using a B‐cell ELISPOT. In patients with ALL, the baseline number of total IgG and influenza‐specific IgG antibody secreting cells differed between the three cohorts, reflecting the different amounts of time on chemotherapy (Figure 2). Both total IgG and influenza‐specific antibody secreting cells were diminished at the 2‐month time point for those enrolled during induction and post‐induction. Those enrolled during maintenance exhibited some recovery of B‐cell function at the 1‐year time point, although this was not statistically significantly different. To better characterize the effects of chemotherapy on B cells, we examined the distribution of B‐cell subsets at each time point. Total B cells, naïve B cells, non‐switched memory (marginal zone‐like) B cells, and switched memory B cells were defined using flow cytometry (Figure 3). All B‐cell subsets significantly differed at baseline between the induction, post‐induction, and maintenance cohorts. Similar to the B‐cell ELISPOT functional results, the B cells declined at the 2‐month time point for both the induction cohort and post‐induction cohort. The maintenance cohort exhibited improvement at the 1‐year time point, although there appear to be differences in kinetics of repletion between the different subsets with the switched memory B cells exhibiting a slower recovery than the naïve B cells.
Figure 3.
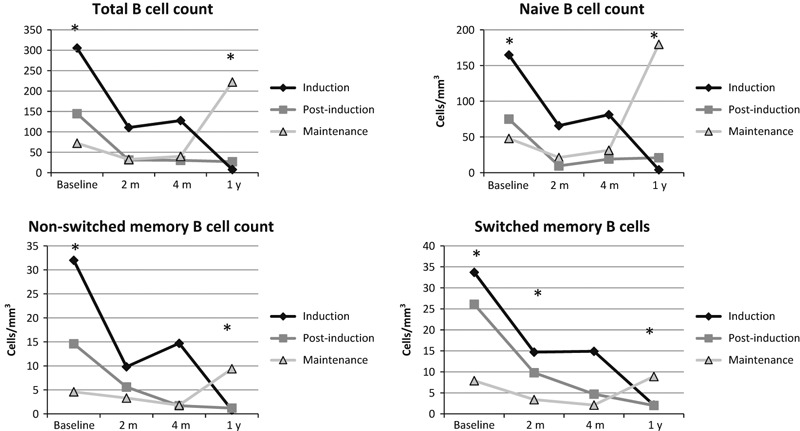
B‐cell subsets differ between the three ALL cohorts. The ALL group was divided into three cohorts depending on the stage of chemotherapy on the day of vaccination. We assessed B‐cell subset counts using flow cytometry and compared the subset counts using the Kruskal–Wallis test. The asterisks indicate statistical significance. Total B‐cell counts (CD19+) differed between cohorts on the day of vaccination and the 1‐year time point (P = 0·0002, P = 0·0035). Similarly, naïve B cells differed at those two points (P = 0·0002 and P = 0·0038) and non‐switched memory B cells differed at the same two time points (P < 0·0001 and P = 0·0065). Switched memory B cells differed at baseline, the 2‐month and 1‐year time points (P = 0·0002, P = 0·0250, and P = 0·0150). The children on maintenance chemotherapy on the day of vaccination demonstrate some recovery in their B‐cell counts at the 1‐year time point.
Solid tumor B cells
The phased chemotherapy used for patients with ALL might impact the immune system differently than the cyclical chemotherapy used for solid tumors. We therefore performed comparable analyses in patients with solid tumors. We divided the patients into those who had less than 1 month of chemotherapy, 1–3 months of chemotherapy, and >3 months of chemotherapy at the time of vaccination. There were no statistically significant differences in HAI titers between those vaccinated at <1 m, 1–3 m, or >3 m of chemotherapy (Figure S4). Similarly, there were no differences in B cell total IgG or influenza‐specific IgG responses across the three cohorts (Figure S5). We then examined the B‐cell subsets and found that there were inconsistent differences at baseline. The solid tumor chemotherapy had a rapid effect on the B‐cell compartment (Figure 4).
Figure 4.
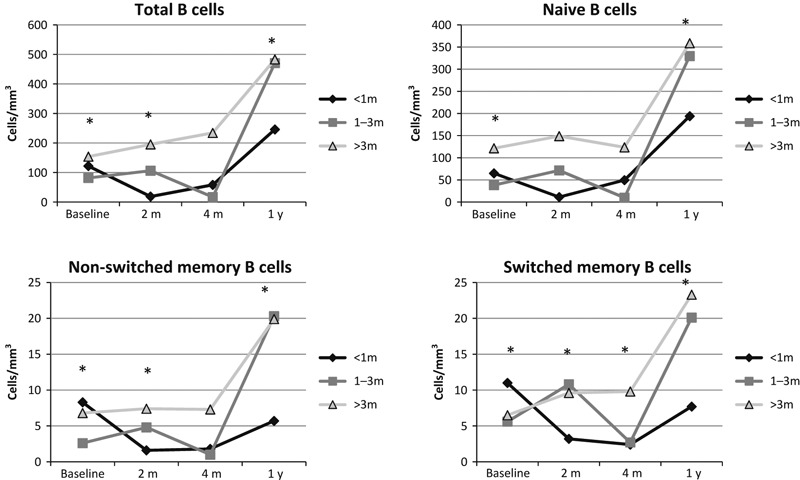
B‐cell subsets differ between the three solid tumor cohorts. The solid tumor group was divided into three cohorts depending on the length of time they had been on chemotherapy on the day they were vaccinated. We used the Kruskal–Wallis test to identify time points where the three cohorts differed significantly. The asterisks indicate statistical differences. There were differences between the three cohorts at baseline and the 1‐year time point as was true in the ALL cohorts and again there was improvement for those subjects off chemotherapy at the 1‐year time point. The differences at baseline are significant for all four subsets with total, naïve, non‐switched memory, and switched memory P values of <0·0001, 0·0007, <0·0001, and 0·00005, respectively. The 1‐year time points were significantly different for total, naïve, non‐switched memory, and switched memory B cells with P values of 0·0009, 0·0011, 0·0011, and 0·0026, respectively.
ALL T cells
To examine other immunologic variables that could impact on vaccine responses, we characterized the effect of chemotherapy on T‐cell function and T‐cell counts in ALL. We utilized the same three cohorts as the previous analyses: induction, post‐induction, and maintenance chemotherapy at the time of enrollment and vaccination. The most significant finding is that unlike the B‐cell compartment which appeared to exhibit signs of quantitative recovery at the 1‐year time point for those vaccinated in maintenance (i.e., those who had been off chemotherapy the longest), no clear sign of quantitative recovery was seen in the T‐cell compartment (Figure 5). Only CD4 reverted memory and CD8 reverted memory T cells were different at baseline between the three ALL cohorts, suggesting these populations were most impacted by the length of time on chemotherapy. For the CD4 reverted memory T cells, the maintenance cohort had the highest numbers, while in the CD8 reverted memory T‐cell population, the induction cohort had the highest numbers.
Figure 5.
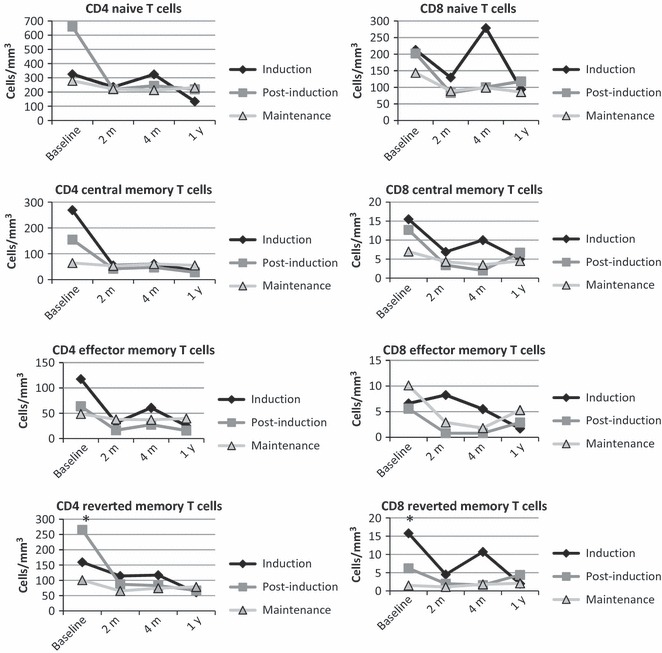
T‐cell subsets are relatively preserved in patients with ALL. The ALL group was divided into three cohorts depending on the stage of chemotherapy on the day of vaccination. We assessed T‐cell subset counts using flow cytometry and compared the subset counts using the Kruskal–Wallis test. The only significant differences (indicated with asterisks) were observed at baseline for the reverted CD4 and CD8 T‐cell subsets with P = 0·0073 and P = 0·0055, respectively.
To assess T‐cell function, we analyzed the proliferation of T cell after stimulation with viral antigen or in response to phytohemagglutinin. There were no differences in proliferation in response to PHA or influenza across the three ALL cohorts (Figure S2). A T‐cell ELISPOT was used to define cytokine responses to either a global stimulus (PMA and ionomycin) or HA antigen. The CD4 influenza‐specific response after vaccination can be clearly seen in the induction cohort, while the effect is much less robust in the other two cohorts. There were no statistically significant differences in the response to PMA and ionomycin (Figure S3). Similarly, CD8 influenza‐specific responses did not significantly differ between the three cohorts.
Solid tumor T cells
The solid tumor population exhibited very different B‐cell effects from chemotherapy compared with the ALL population. We therefore analyzed T cells in the solid tumor population. The solid tumor population was again stratified according to time on chemotherapy at the time of enrollment and vaccination. There were no statistically significant differences in T‐cell responses between the three cohorts (Figures S7, S8).
Associations with HAI vaccine responses
With these data suggesting that there were substantial differences in antibody production in patients with ALL depending on the time on chemotherapy, we wished to define the laboratory associations with antibody responses after vaccination. We analyzed a differential HAI aggregate titer by taking the sum of the 2‐month post‐vaccine HAI titers (H1N1, H3N2, B) and subtracting the sum of the baseline HAI titers for each subject. This measure of antibody response to the vaccine was used in a Spearman’s correlation analysis with baseline CD19 lymphocyte count, switched memory B‐cell count, CD3 count, CD4 count, total IgG production, influenza‐specific IgG production, CD4 influenza‐specific responses (Table 2). The total B‐cell count on the day of vaccination was associated with the antibody responses to the vaccine. When individual serotypes were examined, only influenza B titers were significantly associated with the baseline B‐cell count (P = 0·0031). In addition, evidence of prior antigen experience (CD4 and CD8 influenza‐specific responses) was also associated with the antibody response. We applied the same analysis to the solid tumor cohort (Table S1). None of the baseline variables demonstrated any association with the antibody responses to the vaccine.
Table 2.
HAI Titer Association with Baseline Variables*
| Spearman ρ | P Value | |
|---|---|---|
| ALL Δ HAI titer | ||
| CD19 count | 0·23027 | 0·0240 |
| Switched memory B‐cell count | 0·19076 | 0·0626 |
| CD3 count | 0·08690 | 0·5166 |
| CD4 count | .04425 | 0·7415 |
| Total IgG (ELISPOT) | 0·10381 | 0·3302 |
| Influenza IgG (ELISPOT) | 0·13297 | 0·2115 |
| PMA ionomycin (ELISPOT) | 0·12260 | 0·2818 |
| CD4 influenza responses (ELISPOT) | 0·29377 | 0·0086 |
| CD8 influenza responses (ELISPOT) | 0·40607 | 0·0002 |
| ALL absolute HAI titer | ||
| CD19 Count | 0·07770 | 0·4220 |
| Switched memory B‐cell count | 0·04885 | 0·6140 |
| CD3 count | 0·09821 | 0·4327 |
| CD4 count | 0·06765 | 0·5894 |
| Total IgG (ELISPOT) | 0·04322 | 0·6616 |
| Influenza IgG (ELISPOT) | 0·19735 | 0·0436 |
| PMA ionomycin (ELISPOT) | 0·21774 | 0·0371 |
| CD4 influenza responses (ELISPOT) | 0·40737 | <0·0001 |
| CD8 influenza responses (ELISPOT) | 0·35082 | 0·0006 |
*The Spearman’s correlation analysis was performed using the baseline variables defined in the left column and the outputs of either the aggregate delta HAI (the sum of all three serotypes at the 2‐month time point minus the sum of the three serotypes at baseline) or the total aggregate HAI at the 2‐month time point. Significant associations are bolded.
Protection from Infection
This study was not designed to determine the incidence of infection in vaccinated patients, and therefore, we did not perform systematic surveillance for influenza among the study subjects; however, we found that there were 15 cases of influenza with a positive PCR test subsequent to vaccine administration in the same season. This gives an estimated breakthrough infection rate of 8·5%. This case rate includes the time frame of the pandemic H1N1, for which the standard vaccine had limited efficacy. We therefore defined the case rate among 123 patients vaccinated in 2006–2007 and found 5 cases of infection detected by PCR for a case rate of 4·1%. These must be considered minimal estimates because not all children with fever or respiratory symptoms were tested for influenza.
Conclusion
The purpose of this study was to identify correlates of influenza vaccine antibody responses. These data could be used to identify patients unlikely to respond to the vaccine who could be provided alternative types of protection such as oseltamivir. 32 , 33 Current guidelines recommend influenza vaccination during maintenance chemotherapy; however, this is not always possible due to the timing of the vaccine production and delivery. 8 Identification of a biomarker could be useful in stratifying patients for vaccination or alternative approaches.
This study also represents a careful delineation of the immunologic consequences of chemotherapy. In analyzing the state of the immune system during induction, post‐induction or maintenance chemotherapy for ALL, the largest difference observed was the effect on the B‐cell compartment. This is consistent with a study that evaluated T‐ and B‐cell counts in children with ALL undergoing chemotherapy. 34 As the patients experienced more cycles of chemotherapy, all B‐cell subsets quantitatively declined. Paralleling the decline in B‐cell counts was a decline in B cells capable of secreting antibody as measured by total IgG production and influenza‐specific IgG production. In contrast to the ALL cohort, the effect of solid tumor chemotherapy appeared to be very rapid, with no evidence of a cumulative effect. Additionally, B‐cell function did not parallel B‐cell counts in the solid tumor cohort. These data collectively suggest that the immune compromise in pediatric oncology patients is distinct depending on the chemotherapeutic agents. However, in both ALL and solid tumor groups, evidence of immunologic recovery was observed at the 1‐year time point, although it was not clear that recovery was complete.
This study did not formally define clinical vaccine efficacy with active surveillance. A Cochrane analysis identified nine studies of pediatric chemotherapy patients vaccinated with the standard inactivated influenza vaccine and none of the nine studies evaluated clinical efficacy. 35 Even among healthy children, efficacy data are limited. 36 Additional caveats of this study include the heterogeneity of the types of malignancy and the chemotherapy protocols. Larger studies may be able to stratify patients for these potential confounders. Nevertheless, this study represents the best data to date on the efficacy of the influenza vaccine in this vulnerable population.
The importance of this study was to identify practical correlates of antibody responses to the influenza vaccine. The baseline (day of vaccination) B‐cell count was associated with subsequent antibody production in patients with ALL. Previous antigenic experience, long known to enhance vaccine responses, was also strongly associated with subsequent antibody production in response to the influenza vaccine. 37 The CD8‐specific influenza responses suggest that this was wild type infection not previous vaccination.
Overall, this study identified substantial effects of chemotherapy on the adaptive immune system. These laboratory‐identified effects would be predicted to have medically significant consequences to the patients. Impaired vaccine responses are only one measurable consequence, but the identified deficits might also contribute to the pattern of infection. A direct product of this study is the identification of a potential biomarker in the ALL group and additional data supporting a strategy of early vaccination. Optimizing vaccination protocols to improve protection from this common yet preventable infection could lead to fewer/shorter hospital admissions and a lower in hospital transmission rates.
Addendum
All authors participated in the preparation of the manuscript. Leslie S. Kersun and Anne Reilly were responsible for patient recruitment, review of entry criteria and performed some of the clinical analyses for the manuscript. Susan Coffin designed the study and the clinical reporting forms. She reviewed all data. Jean Boyer and Eline T. Luning Prak designed and oversaw the running of many of the cellular the assays. Kenyetta McDonald, Xiaoling Hou, and Abbas F. Jawad performed statistical analyses. Kathleen E. Sullivan designed and oversaw the execution of the study. The final manuscript was approved by all authors.
Supporting information
Figure S1. Serotype‐specific responses are demonstrated in the bar graphs below.
Figure S2. CFSE was used to measure proliferation in response to PHA or to influenza proteins.
Figure S3. A T cell ELISPOT was used to examine global responses (PMA and ionomycin) or to a cocktail of influenza peptides or whole protein.
Figure S4. The solid tumor group was divided into three cohorts depending on the number of months of cumulative chemotherapy they had received on the day of vaccination.
Figure S5. A B cell ELISPOT was used to define differences between the three solid tumor cohorts.
Figure S6. T cell subsets were analyzed in the solid tumor group after stratification for the length of time on chemotherapy.
Figure S7. T cell ELISPOT responses were compared between the three solid tumor cohorts.
Figure S8. T cell proliferation was analyzed using CFSE in the three solid tumor cohorts, stratified according to the time on chemotherapy.
Table S1. Spearman Correlation Analysis of baseline variables and HAI titers.
Supporting info item
Acknowledgements
The authors would like to acknowledge the expert technical contributions of Kelly Maurer, the staff at the Human Immunology Core facility, Yang‐Zhu Du, and Noah Goodman. We also wish to acknowledge the nurses and physicians caring for these patients as well as Dan Schullery for his organizational contributions. This manuscript was supported by funding from the National Institutes of Health [NO1‐AI‐50 024 to KES] and the Wallace Chair of Pediatrics [to KES].
References
- 1. Potter MN, Foot AB, Oakhill A. Influenza A and the virus associated haemophagocytic syndrome: cluster of three cases in children with acute leukaemia. J Clin Pathol 1991; 44:297–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kempe A, Hall CB, MacDonald NE et al. Influenza in children with cancer. J Pediatr 1989; 115:33–39. [DOI] [PubMed] [Google Scholar]
- 3. Feldman S, Webster RG, Sugg M. Influenza in children and young adults with cancer: 20 cases. Cancer 1977; 39:350–353. [DOI] [PubMed] [Google Scholar]
- 4. Kersun LS, Coffin SE, Leckerman KH, Ingram M, Reilly AF. Community acquired influenza requiring hospitalization: vaccine status is unrelated to morbidity in children with cancer. Pediatr Blood Cancer 2010; 54:79–82. [DOI] [PubMed] [Google Scholar]
- 5. Tasian SK, Park JR, Martin ET, Englund JA. Influenza‐associated morbidity in children with cancer. Pediatr Blood Cancer 2008; 50:983–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weinstock DM, Gubareva LV, Zuccotti G. Prolonged shedding of multidrug‐resistant influenza A virus in an immunocompromised patient. N Engl J Med 2003; 348:867–868. [DOI] [PubMed] [Google Scholar]
- 7. Klimov AI, Rocha E, Hayden FG, Shult PA, Roumillat LF, Cox NJ. Prolonged shedding of amantadine‐resistant influenzae A viruses by immunodeficient patients: detection by polymerase chain reaction‐restriction analysis. J Infect Dis 1995; 172:1352–1355. [DOI] [PubMed] [Google Scholar]
- 8. Kroger AT, Atkinson WL, Marcuse EK, Pickering LK. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55:1–48. [PubMed] [Google Scholar]
- 9. General recommendations on immunization – recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports. Centers for Disease Control. 2011; 60:1–64. [PubMed] [Google Scholar]
- 10. Crawford NW, Heath JA, Buttery JP. Immunisation practices of paediatric oncologists: an Australasian survey. J Paediatr Child Health 2007; 43:593–596. [DOI] [PubMed] [Google Scholar]
- 11. Porter CC, Poehling KA, Hamilton R, Frangoul H, Cooper WO. Influenza immunization practices among pediatric oncologists. J Pediatr Hematol Oncol 2003; 25:134–138. [DOI] [PubMed] [Google Scholar]
- 12. Goossen GM, Kremer LC, van de Wetering MD. Influenza vaccination in children being treated with chemotherapy for cancer. Cochrane Database Syst Rev 2009; 2:CD006484. [DOI] [PubMed] [Google Scholar]
- 13. Esposito S, Cecinati V, Scicchitano B et al. Impact of influenza‐like illness and effectiveness of influenza vaccination in oncohematological children who have completed cancer therapy. Vaccine 2010; 28:1558–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reilly A, Kersun LS, McDonald K, Weinberg A, Jawad AF, Sullivan KE. The efficacy of influenza vaccination in a pediatric oncology population. J Pediatr Hematol Oncol 2010; 32:e177–e181. [DOI] [PubMed] [Google Scholar]
- 15. Monkman K, Mahony J, Lazo‐Langner A, Chin‐Yee BH, Minuk LA. The pandemic H1N1 influenza vaccine results in low rates of seroconversion for patients with hematological malignancies. Leuk Lymphoma 2011; 52:1736–1741. [DOI] [PubMed] [Google Scholar]
- 16. Matsuzaki A, Suminoe A, Koga Y, Kinukawa N, Kusuhara K, Hara T. Immune response after influenza vaccination in children with cancer. Pediatr Blood Cancer 2005; 45:831–837. [DOI] [PubMed] [Google Scholar]
- 17. Hsieh YC, Lu MY, Kao CL et al. Response to influenza vaccine in children with leukemia undergoing chemotherapy. J Formos Med Assoc (Taiwan) 2002; 101:700–704. [PubMed] [Google Scholar]
- 18. Porter CC, Edwards KM, Zhu Y, Frangoul H. Immune responses to influenza immunization in children receiving maintenance chemotherapy for acute lymphoblastic leukemia. Pediatr Blood Cancer 2004; 42:36–40. [DOI] [PubMed] [Google Scholar]
- 19. Carr S, Allison KJ, Van De Velde LA et al. Safety and immunogenicity of live attenuated and inactivated influenza vaccines in children with cancer. J Infect Dis 2011; 204:1475–1482. [DOI] [PubMed] [Google Scholar]
- 20. Yen TY, Jou ST, Yang YL et al. Immune response to 2009 pandemic H1N1 influenza virus A monovalent vaccine in children with cancer. Pediatr Blood Cancer 2011; 57:1154–1158. [DOI] [PubMed] [Google Scholar]
- 21. Bate J, Yung CF, Hoschler K et al. Immunogenicity of pandemic (H1N1) 2009 vaccine in children with cancer in the United Kingdom. Clin Infect Dis: an official publication of the Infectious Diseases Society of America 2010; 51:e95–e104. [DOI] [PubMed] [Google Scholar]
- 22. Shahgholi E, Ehsani MA, Salamati P, Maysamie A, Sotoudeh K, Mokhtariazad T. Immunogenicity of trivalent influenza vaccine in children with acute lymphoblastic leukemia during maintenance therapy. Pediatr Blood Cancer 2010; 54:716–720. [DOI] [PubMed] [Google Scholar]
- 23. Chisholm JC, Devine T, Charlett A, Pinkerton CR, Zambon M. Response to influenza immunisation during treatment for cancer. Arch Dis Child 2001; 84:496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chisholm J, Howe K, Taj M, Zambon M. Influenza immunisation in children with solid tumours. Eur J Cancer 2005; 41:2280–2287. [DOI] [PubMed] [Google Scholar]
- 25. Gross PA, Lee H, Wolff JA, Hall CB, Minnefore AB, Lazicki ME. Influenza immunization in immunosuppressed children. J pediatr 1978; 92:30–35. [DOI] [PubMed] [Google Scholar]
- 26. Steinherz PG, Brown AE, Gross PA et al. Influenza immunization of children with neoplastic diseases. Cancer 1980; 45:750–756. [DOI] [PubMed] [Google Scholar]
- 27. Committee on Infectious Diseases . Recommendations for prevention and control of influenza in children, 2011‐2012. Pediatrics 2011; 128:813–825. [DOI] [PubMed] [Google Scholar]
- 28. Levin MJ, Song LY, Fenton T et al. Shedding of live vaccine virus, comparative safety, and influenza‐specific antibody responses after administration of live attenuated and inactivated trivalent influenza vaccines to HIV‐infected children. Vaccine 2008; 26:4210–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sutter JA, Kwan‐Morley J, Dunham J et al. A longitudinal analysis of SLE patients treated with rituximab (anti‐CD20): factors associated with B lymphocyte recovery. Clin Immunol 2008; 126:282–290. [DOI] [PubMed] [Google Scholar]
- 30. Stadtmauer EA, Vogl DT, Luning Prak E et al. Transfer of influenza vaccine‐primed costimulated autologous T cells after stem cell transplantation for multiple myeloma leads to reconstitution of influenza immunity: results of a randomized clinical trial. Blood 2011; 117:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jawad AF, Prak EL, Boyer J et al. A Prospective Study of Influenza Vaccination and a Comparison of Immunologic Parameters in Children and Adults with Chromosome 22q11.2 Deletion Syndrome (DiGeorge Syndrome/Velocardiofacial Syndrome). J Clin Immunol 2011; 31:927–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chik KW, Li CK, Chan PK et al. Oseltamivir prophylaxis during the influenza season in a paediatric cancer centre: prospective observational study. Hong Kong Med J 2004; 10:103–106. [PubMed] [Google Scholar]
- 33. Vu D, Peck AJ, Nichols WG et al. Safety and tolerability of oseltamivir prophylaxis in hematopoietic stem cell transplant recipients: a retrospective case‐control study. Clin Infect Dis: an official publication of the Infectious Diseases Society of America 2007; 45:187–193. [DOI] [PubMed] [Google Scholar]
- 34. Caver TE, Slobod KS, Flynn PM et al. Profound abnormality of the B/T lymphocyte ratio during chemotherapy for pediatric acute lymphoblastic leukemia. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 1998; 12:619–622. [DOI] [PubMed] [Google Scholar]
- 35. Goossen GM, Kremer LC, van de Wetering MD. Influenza vaccination in children being treated with chemotherapy for cancer. Cochrane Database Syst Rev 2009; 15:CD006484. [DOI] [PubMed] [Google Scholar]
- 36. Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta‐analysis. Lancet Infect Dis 2012; 12:36–44. [DOI] [PubMed] [Google Scholar]
- 37. Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev 2008; 16:CD004879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Serotype‐specific responses are demonstrated in the bar graphs below.
Figure S2. CFSE was used to measure proliferation in response to PHA or to influenza proteins.
Figure S3. A T cell ELISPOT was used to examine global responses (PMA and ionomycin) or to a cocktail of influenza peptides or whole protein.
Figure S4. The solid tumor group was divided into three cohorts depending on the number of months of cumulative chemotherapy they had received on the day of vaccination.
Figure S5. A B cell ELISPOT was used to define differences between the three solid tumor cohorts.
Figure S6. T cell subsets were analyzed in the solid tumor group after stratification for the length of time on chemotherapy.
Figure S7. T cell ELISPOT responses were compared between the three solid tumor cohorts.
Figure S8. T cell proliferation was analyzed using CFSE in the three solid tumor cohorts, stratified according to the time on chemotherapy.
Table S1. Spearman Correlation Analysis of baseline variables and HAI titers.
Supporting info item


