Abstract
Background
Asthma was the most common chronic condition among adults hospitalized for 2009 pandemic influenza A (H1N1) (pH1N1).
Objectives
We describe the epidemiology and factors for severe outcomes among adults with asthma who were hospitalized or died from pH1N1 in California.
Methods
We reviewed California Department of Public Health pH1N1 reports from April 23, 2009 through August 11, 2009. Reports were included if the patient had pH1N1 (or non‐subtypeable influenza A) infection by polymerase chain reaction in an adult (age ≥ 18 years) with asthma who was hospitalized or died. Patients were classified as having intermittent or persistent asthma on the basis of regular medications. Risk factors associated with severe outcomes (i.e., intensive care unit admission or death) vs those with less severe outcomes were assessed by chi‐square tests and logistic regression.
Results
Among 744 identified patients, 170 (23%) had asthma (61% intermittent, 39% persistent). 132 of 142 (93%) patients had other chronic medical conditions. Severe outcomes occurred in 54 of 162 (33%), more commonly among those with renal disease (64% versus 31%; P = 0.04) and chest radiograph infiltrates (54% versus 11%; P < 0.01), less commonly among those who received antivirals within 48 hours of symptom onset (22% versus 44%; P = 0.02). In multivariable analysis, chest radiograph infiltrates were associated with severe outcomes (adjusted odds ratio 9·38, 95% confidence interval 3·05–28·90).
Conclusions
One third of adults with asthma who died or were hospitalized with pH1N1 experienced severe outcomes. Early empiric antiviral therapy should be encouraged, especially among asthma patients.
Keywords: pandemic H1N1, adults with asthma, death, hospitalizations
Introduction
Asthma affects 7·7% of the U.S. adult population, approximately 17·5 million persons.1 These patients are known to be at greater risk of influenza‐related complications.2 Among patients hospitalized in the United States during April–June 2009 with pandemic influenza A(H1N1) (pH1N1), asthma was the most commonly reported underlying chronic medical condition, affecting 28%.3 However, severe outcomes among adult patients with asthma with pH1N1 are still not well described.
In the United States, the pH1N1 virus, a novel influenza virus, was first detected in California in April 2009.4 The California Department of Public Health (CDPH) along with 61 local health jurisdictions initiated surveillance for all confirmed and probable cases of disease caused by pH1N1. Mandatory reporting requirements changed in July 2009 to include all deaths and hospitalizations but excluded outpatients and changed again in August 2009 to include all patients admitted to the intensive care unit (ICU) and deaths only.
Our study objectives were to describe the characteristics of adult asthma patients in California who were hospitalized or died with pH1N1 and to determine the prevalence of severe outcomes and factors associated with these outcomes before the pH1N1 vaccine became available.
Methods
We reviewed CDPH pH1N1 reports submitted to CDPH during April 23–August 11, 2009, the time period when all hospitalizations were notifiable. Reports were completed by hospital clinicians and infection control practitioners (or by county health officials if a pH1N1‐related death occurred outside a hospital) and were mandatory under Title 17 of the California Code of Regulations.5 Medical records, including history and physical examinations, discharge and transfer summaries, medication lists, laboratory results, and radiology reports were used to supplement information obtained from reports, including identification of asthma patients, and incorporated into a final database for analysis.
Reports were included if the patient had pH1N1 (or non‐subtypeable for human subtypes H1 and H3 influenza A) viral infection by polymerase chain reaction in a California resident aged ≥18 years with a history of asthma according to the medical chart who was hospitalized or had died. Using a modified version of the National Asthma Education and Program Expert Panel Report 3 guidelines, asthma severity was defined by assuming that regular asthma medications were a reflection of clinician assessment of symptoms and subsequent treatment, that is, asthma severity defined by symptoms prior to treatment. Patients were therefore classified as having either intermittent, mild persistent, moderate persistent, or severe persistent asthma on the basis of regular medications used before onset of acute influenza noted on admission (Table 1).6 If a patient was labeled as having asthma but no asthma medications were listed in their chart, they were included in the study but not classified by asthma severity. For the bivariate and multivariate analyses, a case or severe outcome was defined as a patient with an ICU stay or death with or without hospitalization, while a control or non‐severe outcome was defined as a patient with a non‐ICU hospitalization without death.
Table 1.
A modification of the National Asthma Education and Prevention Program Expert Panel Report 3 classification of asthma severity on the basis of day and nighttime symptoms and subsequent treatment6, a
| Classification | Day symptoms | Nighttime awakenings | Treatment |
|---|---|---|---|
| Intermittent | ≤2 days/week | ≤2 × /month | Short‐acting B‐agonists |
| Mild persistent | >2 days/week | 3–4 × /month | Plus steroid inhalers |
| Moderate persistent | Daily | >1 × /week | Plus long‐acting B‐agonists |
| Severe persistent | All day | Nightly | Plus oral steroids |
Regular treatment medications were used to classify patients.
Age‐group categories 18–49 years and ≥50 years were used to be consistent with historic influenza recommendations for only vaccinating healthy adults aged ≥50 years. Race–ethnicity was classified according to report categories, including Hispanic, non‐Hispanic white, non‐Hispanic black, and Asian–Pacific Islander. Obesity was defined by CDC as a body mass index of ≥30 in those aged ≥20 years (patients aged < 20 years were not evaluated for obesity).7 Women of childbearing years, defined by census as ages 15–44, were evaluated for pregnanacy.8 Chronic medical conditions were defined by the Centers for Disease Control and Prevention (CDC) in their interim recommendations for the usage of antiviral medications for pH1N1,9 with the presence of renal disease determined on the basis of documentation in the report regardless of cause.
Only data from asthma patients with a known outcome status were used in the bivariate and logistic regression analyses. Using SAS 9.2 by SAS Institute Inc., Cary, NC, we performed chi‐square and Fisher's exact tests to analyze multiple risk factors, including demographics (i.e., age, sex, and race/ethnicity), asthma severity, obesity, pregnancy status, presence of chronic medical conditions, presence of an infiltrate on chest radiographs (as noted in the reports), and antivirals administered within 48 hours of symptom onset (those who did not receive antivirals were excluded). For our bivariate analysis, we assessed potential risk factors for a severe outcome among asthma patients who died or were hospitalized. We then performed a multivariable logistic regression including all demographic factors a priori and any risk factor that was significant at a P < 0.05 level in the bivariate analysis.
Results
The CDPH received 1088 reports of pH1N1 among California patients who were hospitalized or died during the study period (Figure 1). Among these, 744 (68%) were adults, including 170 (23%) who had a past medical history of asthma as noted in their medical chart; among patients with asthma, 162 (95%) had known outcome severity. Fifty‐four (33%) had a severe outcome (i.e., admitted to the ICU or died). The remainder 108 (67%) had a non‐severe outcome and were hospitalized only. Among those with a severe outcome, two (4%) died before reaching the hospital, 19 (35%) died in the hospital, 29 (54%) were admitted to the ICU but survived, and 4 (7%) were admitted to the ICU with unknown survival.
Figure 1.
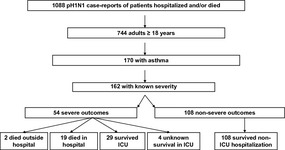
2009 pandemic influenza A(H1N1) case classification on the basis of outcome severity as noted in reports received by the California Department of Public Health during April 23–August 11, 2010.
Among the 170 patients with asthma who were hospitalized with or died from pH1N1, 114 (67%) were aged 18–49 years; 56 (33%) were aged ≥50 years (Table 2). One hundred and seven (63%) were female. Race/ethnicity data were available for 139 patients. Among these, 59 (42%) were non‐Hispanic white. Asthma severity categorization was possible for 132 patients. Eighty‐one (61%) were classified as having intermittent asthma, and 51 (39%) had persistent asthma. Among the 142 patients for whom complete information on chronic medical conditions was available, 132 (93%) had at least one such condition aside from their underlying asthma. Height and weight data were available for 71 patients; 48 (68%) were obese. Among 47 women of childbearing age, 13 (28%) were pregnant, two of whom died. Eleven of 170 (6%) had renal disease, described as renal insufficiency in one patient, end‐stage renal disease in 4, and unspecified in 6. Among these patients with renal disease, six also had cardiac disease and three were diabetic. Among 148 patients for whom information was available, infiltrates on chest radiograph were observed in 81 (55%). Among 136 patients for which information was available, only 45 (33%) received antivirals within 48 hours of symptom onset as recommended by CDC.
Table 2.
Characteristics of California asthma patients who were hospitalized or who died with 2009 pandemic influenza A (H1N1) infection
| Characteristics | n/N (%) |
|---|---|
| Age (years) | |
| 18–49 years | 114/170 (67) |
| ≥50 years | 56/170 (33) |
| Sex | |
| Female | 107/170 (63) |
| Race/ethnicity | |
| White, non‐Hispanic | 59/139 (42) |
| Hispanic | 39/139 (28) |
| Black, non‐Hispanic | 24/139 (17) |
| Asian–Pacific Islander | 14/139 (10) |
| Other | 3/139 (2) |
| Asthma classification | |
| Intermittent | 81/132 (61) |
| Mild persistent | 31/132 (23) |
| Moderate persistent | 9/132 (7) |
| Severe persistent | 11/132 (8) |
| Obesity (age ≥ 20 years) | |
| BMI ≥ 30 | 48/71 (68) |
| Pregnancy | |
| Females age 15–44 years | 13/47 (28) |
| Chronic medical condition | |
| Any | 132/142 (93) |
| Cancer | 4/170 (2) |
| Cardiac diseases | 34/157 (22) |
| Chronic obstructive pulmonary disease | 31/170 (18) |
| Diabetes | 26/170 (15) |
| HIV | 5/170 (3) |
| Neuromuscular diseases | 21/159 (13) |
| Renal diseases | 11/170 (6) |
| Infiltrates on chest radiograph | 81/148 (55) |
| Antivirals given within 48 hours | 45/136 (33) |
BMI, body mass index; HIV, human immunodeficiency virus infection.
Our bivariate analyses demonstrated no significant differences in the proportion of severe outcomes by age, sex, race/ethnicity, or asthma classification (Table 3). Three factors did demonstrate a statistically significant association with a severe outcome. Forty‐one (54%) patients with infiltrates on chest radiograph had a severe outcome, but only seven (11%) patients without infiltrates had a severe outcome (P value < 0.01). Those who received antiviral therapy within 48 hours of symptom onset were less likely to suffer a severe outcome than those who were not administered antivirals within 48 hours of symptom onset (P value = 0.02). Those with renal disease were more likely to suffer a severe outcome than those without renal disease (P value = 0.04).
Table 3.
Prevalence of severe outcomesa by selected characteristics among asthma patients who were hospitalized or who died with 2009 pandemic influenza A (H1N1) infection
| Characteristics | Severe outcome n/N (%) | Unadjusted OR | P value | 95% CI |
|---|---|---|---|---|
| Age (years) | ||||
| 18–49 | 40/108 (37) | 1·68 | 0·16 | 0·82–3·46 |
| ≥50 | 14/54 (26) | |||
| Sex | ||||
| Female | 30/102 (29) | 0·63 | 0·17 | 0·32–1·22 |
| Male | 24/60 (40) | |||
| Race/ethnicity | ||||
| White, non‐Hispanic | 22/55 (40) | |||
| Hispanic | 11/38 (29) | 0·61 | 0·27 | 0·25–1·48 |
| Black, non‐Hispanic | 7/23 (30) | 0·66 | 0·43 | 0·23–1·86 |
| Asian–Pacific Islander, non‐Hispanic | 6/12 (50) | 1·50 | 0·53 | 0·43–5·25 |
| Asthma classification | ||||
| Intermittent | 24/76 (32) | |||
| Mild persistent | 10/30 (33) | 1·08 | 0·86 | 0·44–2·66 |
| Moderate persistent | 5/9 (56) | 2·71 | 0·15 | 0·67–10·99 |
| Severe persistent | 3/11 (27) | 0·81 | 0·77 | 0·20–3·34 |
| Obesity (BMI ≥ 30 in age ≥ 20 years) | ||||
| Present | 16/46 (35) | 0·40 | 0·09 | 0·14–1·15 |
| Absent | 12/21 (57) | |||
| Pregnancy (female aged 15–44 years) | ||||
| Present | 5/13 (38) | 1·53 | 0·72 | 0·39–5·95 |
| Absent | 9/31 (29) | |||
| Any chronic condition | ||||
| Present | 43/128 (34) | 0·06 | ||
| Absent | 0/8 (0) | |||
| Cancer | ||||
| Present | 1/4 (25) | 0·66 | >0·99 | 0·07–6·50 |
| Absent | 53/158 (34) | |||
| Cardiac diseases | ||||
| Present | 10/31 (32) | 0·89 | 0·80 | 0·38–2·08 |
| Absent | 41/118 (35) | |||
| Chronic obstructive pulmonary disease | ||||
| Present | 9/31 (29) | 0·78 | 0·57 | 0·33–1·84 |
| Absent | 45/131 (34) | |||
| Diabetes | ||||
| Present | 8/26 (31) | 0·87 | 0·76 | 0·35–2·15 |
| Absent | 46/136 (34) | |||
| HIV | ||||
| Present | 2/5 (40) | 1·35 | >0·99 | 0·22–8·31 |
| Absent | 52/157 (33) | |||
| Neuromuscular | ||||
| Present | 11/21 (52) | 2·39 | 0·06 | 0·94–6·07 |
| Absent | 41/130 (32) | |||
| Renal diseases | ||||
| Present | 7/11 (64) | 3·87 | 0·04 | 1·08–13·87 |
| Absent | 47/151 (31) | |||
| Infiltrates on chest radiograph | ||||
| Present | 41/76 (54) | 9·71 | <0·01 | 3·93–23·99 |
| Absent | 7/65 (11) | |||
| Antivirals | ||||
| Administered within 48 hours | 10/45 (22) | 0·37 | 0·02 | 0·15–0·89 |
| Not administered within 48 hours | 37/85 (44) | |||
OR, odds ratio; CI, confidence interval; HIV, human immunodeficiency virus infection.
Defined as ICU stay or death.
On the basis of bivariate analyses, the variables renal diseases, infiltrates on chest radiograph, and antivirals administered within 48 hours of symptom onset were included in our logistic regression model along with age, sex, and race/ethnicity. Asthma classification and other chronic medical conditions did not meet our inclusion criteria and were therefore not included in the model.
Age, sex, and race/ethnicity did not demonstrate an association with severe outcomes in the logistic regression analysis (Table 4). In the multivariable logistic regression analysis, odds of a severe outcome were significantly higher among patients with infiltrates on chest radiograph compared with those without infiltrates (adjusted OR 9·38, 95% confidence interval 3·05–28·90). Neither the variables renal disease nor antivirals administered within 48 hours of symptom onset maintained their statistical significance. However, the adjusted OR for antivirals administered within 48 hours of symptom onset was small, and the sample size among those with renal disease was only 11, with a lower confidence interval boundary close to 1·0.
Table 4.
Logistic regression model: association between selected characteristics and a severe outcome among asthma patients who were hospitalized or who died with 2009 pandemic influenza A (H1N1) infectiona
| Characteristics | Severe outcome no. (%) | Adjusted OR | 95% CI |
|---|---|---|---|
| Age (years) | |||
| 18–49 | 40/108 (37) | Reference | |
| ≥50 | 14/54 (26) | 0·41 | 0·13–1·27 |
| Sex | |||
| Female | 30/102 (29) | Reference | |
| Male | 24/60 (40) | 2·4 | 0·76–7·67 |
| Race/ethnicity | |||
| White, non‐Hispanic | 22/55 (40) | Reference | |
| Hispanic | 11/38 (29) | 0·65 | 0·18–2·32 |
| Black, non‐Hispanic | 7/23 (30) | 0·63 | 0·11–3·54 |
| Asian–Pacific Islander | 6/12 (50) | 1·27 | 0·26–6·19 |
| Renal diseases | |||
| Absent | 47/151 (31) | Reference | |
| Present | 7/11 (64) | 6·21 | 0·95–40·63 |
| Infiltrates on chest radiograph | |||
| Absent | 7/65 (11) | Reference | |
| Present | 41/76 (54) | 9·38 | 3·05–28·90 |
| Antivirals | |||
| Not administered within 48 hours | 37/85 (44) | Reference | |
| Administered within 48 hours | 10/45 (22) | 0·43 | 0·14–1·33 |
We included all demographic factors a priori and any risk factor that was significant at a P < 0.05 level in the bivariate analysis.
Discussion
Asthma affects 13% of the California adult population, approximately 5 million persons.10 We determined that 23% of adults who were hospitalized or died with pH1N1 in California had underlying asthma. Among these patients, one‐third experienced a severe outcome, defined as an ICU stay or death with or without hospitalization, confirming findings from other studies that indicate asthma with pH1N1 is a particularly serious combination.11 Chest radiograph infiltrates were present in a majority of severe illnesses and, with an adjusted odds ratio of 9·38, statistically associated with a severe outcome in the logistic regression analysis. Either viral pneumonia or a bacterial coinfection might explain these infiltrates as seen in previous pandemics where the role of viral–bacterial copathogenesis has been described among deceased patients.12, 13
Our finding that pulmonary involvement was associated with a severe outcome confirms findings of other studies among patients without asthma. In a study of persons infected with pH1N1 from the United Kingdom, the mortality rate among those with pneumonia was 2·6 times higher than those without pneumonia.14 In a CDC study of respiratory specimens from 77 confirmed fatal pH1N1 cases, 29% were coinfected with an identified bacterium.13 While it is well known that influenza alone can cause pneumonia and the causes of infiltrates among patients in our study were undefined, treatment with antibiotics is warranted given the possibility of bacterial pneumonia. Our findings, in addition to data from CDC demonstrating frequent bacterial coinfections associated with mortality, support the Infectious Diseases Society of America 2009 guidelines that bacterial coinfections and need for antibiotics should be considered in patients with influenza.15
Whereas CDC recommended empiric antiviral treatment for groups at high risk (e.g., patients with asthma) as soon as possible, preferably within 48 hours of onset of suspected influenza respiratory symptoms,16 studies indicate that these recommendations were not universally followed.3 Our study revealed that only 43% of asthma patients received antiviral medications within the recommended period. Although the proportion of severe outcomes decreased from 44% to 22% with timely antiviral treatment, it was not a statistically significant finding on multivariable analysis, possibly due to our small sample size. However, Jain et al.3 did find that patients who received antivirals within 48 hours of symptom onset were less likely to experience severe outcomes than those who did not.
Although the presence of renal disease as a risk factor for severe outcome among patients with asthma did not maintain statistical significance, a confidence interval with a lower bound close to 1·0 in such a limited sample is possibly an intriguing finding. Published literature indicates that critically ill patients with pH1N1 are at an increased risk of renal failure.17 Although the mechanism remains unknown, a similar effect may be occurring in patients with pre‐existing renal disease with subsequent worsening of renal function. Lack of sufficient creatinine clearance reserve would thus put them at greater risk of total organ failure and therefore severe outcomes than patients without renal disease. Further research on this association between renal disease and severe outcomes in the presence of influenza is warranted while controlling for associated renal comorbidities such as cardiac disease and diabetes that are known risk factors for severe influenza.
Our study had certain limitations. Results are from early in the pandemic when clinician awareness was limited and a vaccine was not yet available; results, particularly use of antiviral treatment, should be viewed in this light. The database lacked information regarding how patients were diagnosed with asthma. We were unable to classify asthma severity on the basis of optimal treatment required to control symptoms (i.e., severity during treatment), as recommended by the American Thoracic Society, but rather classified asthma severity based on chart review where physician assessment of symptoms led to initiation of treatment (i.e., severity before treatment).18 Our patients might therefore have been misclassified as having less severe asthma. Further, lack of access to medical care and medications might have caused potential patients to be missed, and unfilled asthma prescriptions might have led to misclassification of asthma severity. This could explain why asthma classification was not associated with a severe outcome. Finally, most patients had at least one additional chronic medical condition besides asthma, including diabetes, renal disease, and cardiac disease, conditions that increase the risk of severe outcomes. Physicians may also be more apt to test for influenza in asthma patients with comorbidities. Thus, the independent effect of asthma on outcome severity of influenza needs to be further evaluated.
Clinicians need to be aware that asthma patients comprise a large portion of persons hospitalized for pH1N1, one‐third of whom experienced a severe outcome. Primary care physicians, in partnership with their pulmonology colleagues, should implement all possible preventive measures such as optimizing asthma medications and administering influenza and pneumococcal vaccinations. In both outpatient and inpatient settings, asthma patients, regardless of severity classification, suffering from acute respiratory illness suspected of being influenza should be treated with early empiric antiviral therapy. Further, antibiotics should be considered if infiltrates on chest radiographs are present.
Author contributions
Dr Mortensen, principal investigator, had full access to the data and vouches for the integrity of the data and the accuracy of the data analysis. Dr Louie made substantial contributions to conception and design, or acquisition of data, or analysis, and interpretation of data; drafted the submitted article or revised it critically for important intellectual content; and provided final approval of the version to be published. Dr Pertowski contributed to conception and design of study and analysis and interpretation of data; reviewed and revised drafts. Ms Cadwell involved in data management and analysis; interpretation of data; critical review of manuscript. Dr Weiss contributed to conception and design of study and analysis and interpretation of data; reviewed and revised drafts. Ms Acosta managed collection and entry of data, contributed to design of study and analysis and interpretation of data; reviewed and revised drafts. Dr Matyas contributed to conception and design of study and analysis and interpretation of data; reviewed and revised drafts.
Disclosures and conflict of interests
The authors declare that there are no conflict of interests.
Funding sources
This study did not receive any funding.
Acknowledgements
The investigators thank David B. Callahan, MD, National Center for Environmental Health, Centers for Disease Control, for his subject matter expertise.
Mortensen et al (2013) Epidemiology and outcomes of adults with asthma who were hospitalized or died with 2009 pandemic influenza A (H1N1) – California, 2009. Influenza and Other Respiratory Viruses 7(6), 1343–1349.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1. National Center for Health Statistics . FastStats. Centers for Disease Control and Prevention. 2011, Available at http://www.cdc.gov/nchs/fastats/asthma.htm (accessed 27 January 2011).
- 2. Centers for Disease Control and Prevention . People at high risk of developing flu‐related complications. Centers for Disease Control and Prevention 2010, Available at http://www.cdc.gov/flu/about/disease/high_risk.htm (accessed 9 December 2010).
- 3. Jain S, Kamimoto L, Bramley AM et al Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med 2009; 361:1935–1944. [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention . The 2009 H1N1 Pandemic: Summary Highlights, April 2009‐April 2010. Centers for Disease Control and Prevention, 2010. Available at http://www.cdc.gov/h1n1flu/cdcresponse.htm (accessed 20 May 2011). [Google Scholar]
- 5. Health and Human Services Agency . Title 17. State of California. Available at http://www.cdph.ca.gov/HealthInfo/Documents/Reportable_Diseases_Conditions.pdf. (accessed 27 January 2011).
- 6. National Institutes of Health . National asthma education and prevention program expert panel report 3: Guidelines for the diagnosis and management of asthma. 08–5846, 2007.
- 7. Centers for Disease Control and Prevention . Defining Overweight and Obesity. Centers for Disease Control and Prevention, 2010. Available at http://www.cdc.gov/obesity/defining.html (accessed 26 April 2011). [Google Scholar]
- 8. U.S. Census Bureau . Population Profile of the United States: 2000 (Internet Release), 2000. Chapter 4‐1. Available at http:www.census.gov/population/www/pop-profile/files/2000/chap04.pdf (accessed 16 August 2001).
- 9. Centers for Disease Control and Prevention . Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009–2010 season. Centers for Disease Control and Prevention, 2009. Available at http://www.cdc.gov/H1N1flu/recommendations.htm (accessed 5 January 2010). [Google Scholar]
- 10. UCLA Center for Health Policy Research . California Health Interview Survey – CHIS, 2007. Available at http://www.chis.ucla.edu/about.html (accessed 26 January 2011).
- 11. Campbell A, Rodin R, Kropp R et al Risk of severe outcomes among patients admitted to hospital with pandemic (H1N1) influenza. CMAJ 2010; 182:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morens DM, Tuabenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198:962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention . Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic A (H1N1) ‐ United States, May–August 2009. MMWR Morb Moral Wkly Rep 2009; 58:1071–1074. [PubMed] [Google Scholar]
- 14. Nguyen‐Van‐Tam JS, Openshaw PJM, Hashim A et al Risk factors for hospitalization and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May–September 2009). Thorax 2010; 65:645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harper SA, Bradley JS, Englund JA et al Seasonal influenza in adults and children – diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention . Interim guidance on antiviral recommendations for patients with novel influenza A (H1N1) virus infection and their close contacts. Centers for Disease Control and Prevention, 2009. Available at http://www.dzsp21.com/H1N1%20News/Resources/Interim%20Guidance%20on%20Antiviral%20Recommendations%20for%20Patients%20with%20Novel%20H1N1%20Infection%20and%20Their%20Close%20Contacts.pdf (accessed 27 January 2011). [Google Scholar]
- 17. Sood MM, Rigatto C, Zarychanski R et al Acute kidney injury in critically ill patients infected with 2009 pandemic influenza A(H1N1): report from a Canadian province. Am J Kidney Dis 2010; 55:848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reddel HK, Taylor DR, Bateman ED et al An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations. Am J Respir Crit Care Med 2009; 180:59–99. [DOI] [PubMed] [Google Scholar]


